Abstract
Background
The aim of this study was to investigate lead endocarditis-related tricuspid valve regurgitation, to identify underlying causes, and to report our surgical approaches to tricuspid valve endocarditis.
Methods
Between March 2010 and August 2016, medical records of a total of 43 patients (23 males, 20 females; mean age: 63.2±13.6 years; range 48 to 72 years) who underwent tricuspid valve surgery for severe tricuspid regurgitation caused by lead endocarditis, which was previously placed as an implantable cardiac electronic device were reviewed. We removed all systems including infected leads and generators, revised infected wounds and tissues, performed tricuspid valve surgery for lead endocarditis, and applied long-term intravenous antibiotic regimen for the culprit agent, as confirmed by the culture.
Results
Of 43 patients, 18 underwent tricuspid valve repair and 25 underwent tricuspid valve replacement for lead endocarditisrelated severe tricuspid valve regurgitation. During followup (range, 2 to 62 months), two patients required temporary mechanical support due to postoperative acute right heart failure, while eight patients died due to sepsis (n=6; 14%) and stroke (n=2; 4.6%) in the early postoperative period. The remaining patients showed significant improvement in signs and symptoms of heart failure.
Conclusion
Our study results suggest that incompetent experience and inaccurate decision for valve repair may result in delayed valve replacement and prolonged operation time.
Keywords: Implantable cardioverter defibrillator, lead endocarditis, permanent pacemaker, tricuspid valve surgery
Introduction
Right-sided endocarditis accounts for 5 to 10% of all infective endocarditis cases in the overall population, and it is more frequent in intravenous drug users.[1] Although tricuspid valve endocarditis (TVE) accounts for 2.5 to 3.1% of all cases of infective endocarditis in earlier series,[2] it seems to be increased up to 6 to 36% in the recent articles[3] with an increasing incidence of lead endocarditis (LE) in the literature.[4]
Although primary treatment of LE includes using specific intravenous antibiotics, the removal of all infected leads, generator systems, and tricuspid valve repair (TR) or tricuspid valve replacement (TVR) may be required, if the vegetation involves the circumferential tissue and tricuspid valve.[1] The Society of Thoracic Surgeons and European Society of Cardiology guidelines recommend debridement of the infected area, TR for native TVE (Class I recommendation), and the use of a mechanical or stented tissue valve, if repair is not feasible (Class IIa recommendation).[1,5]
In the present study, we aimed to investigate LE-related tricuspid valve regurgitation, to identify underlying causes, and to report our surgical approaches to TVE.
Patients and Methods
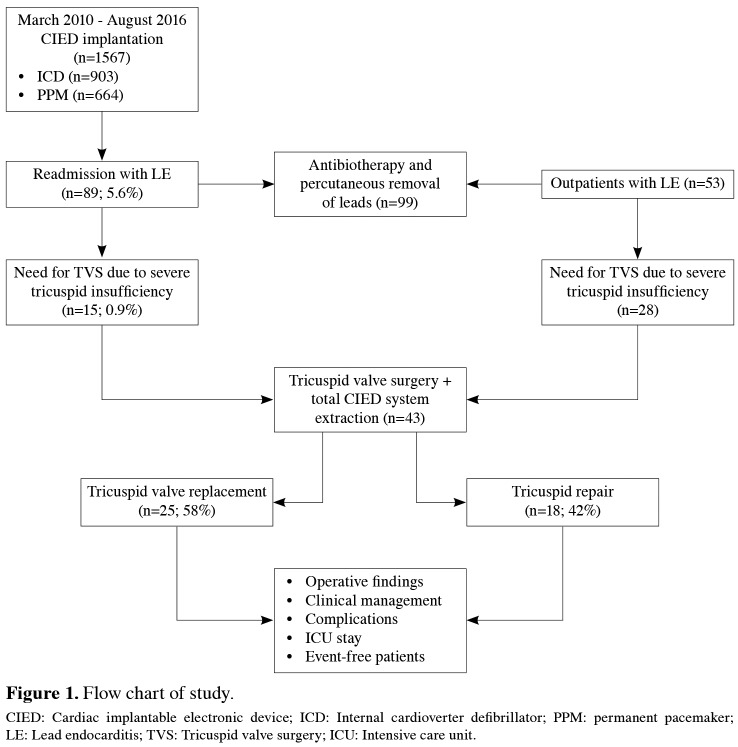
Between March 2010 and August 2016, a total of 142 consecutive patients with LE were screened. In 99 patients, clinical and echocardiographic improvement was achieved with effective antibiotherapy, and percutaneous removal of the infected system was performed in the operation room. We did not use percutaneous extraction, if the measured size of vegetation was over 20 mm. In the remaining 43 patients, failure of antibiotherapy, progression of lead vegetation and tricuspid regurgitation, and worsened right ventricular functions indicated the removal of system with concomitant tricuspid valve surgery. As a result, this study included a total of 43 patients (23 males, 20 females; mean age 63.2±13.6 years; range 48 to 72 years) who underwent tricuspid valve surgery for severe tricuspid regurgitation caused by LE, which was previously placed as an implantable cardiac electronic device. The flow chart of the study is given in Figure 1.
Figure 1. Flow chart of study. CIED: Cardiac implantable electronic device; ICD: Internal cardioverter defibrillator; PPM: permanent pacemaker; LE: Lead endocarditis; TVS: Tricuspid valve surgery; ICU: Intensive care unit.
The study protocol was approved by the Ankara University, Faculty of Medicine, Ethics Committee. A written informed consent was obtained from each patient. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Of 43 patients, 22 had an implanted permanent pacemaker (PPM), while 21 had an implantable cardioverter defibrillator (ICD). Coronary angiography was performed prior to procedure in patients who were over 60 years, if the patient did not undergo coronary examination within the past one year. In patients with ischemic cardiomyopathy, coronary angiography was performed for the right coronary artery examination. Survival and follow-up records were obtained from the clinical records. Demographic and clinical characteristics, localizations of the generators and leads, laboratory findings, etiological agents as confirmed by cultures, operative procedures, and reasons for lead vegetation-related tricuspid valve regurgitation were recorded.
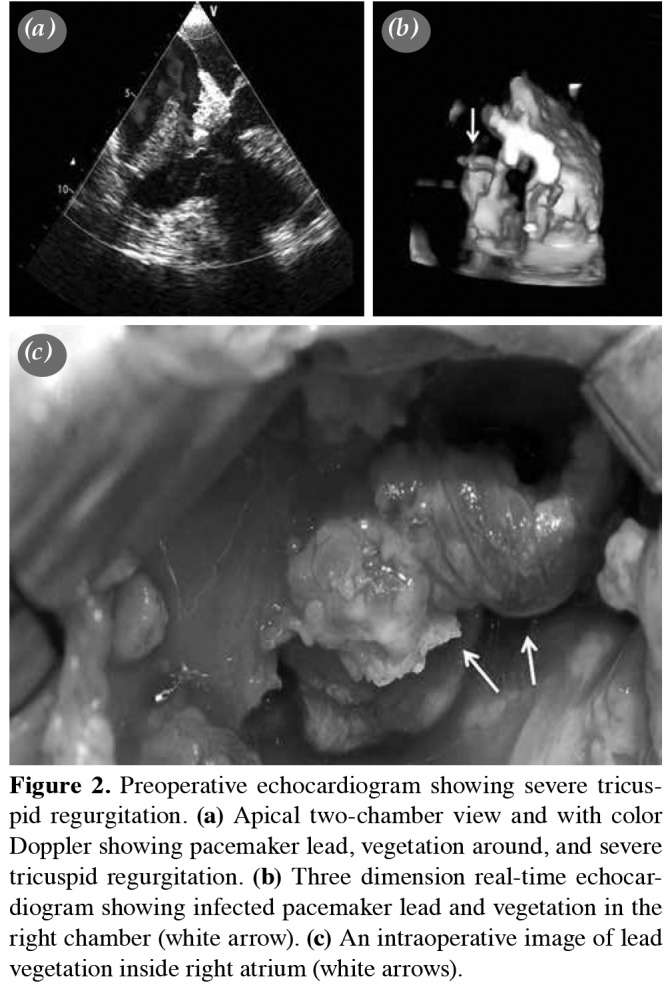
All patients underwent preoperative transthoracic echocardiogram (TTE), preoperative transesophageal echocardiogram (TEE), and intraoperative TEE to identify the main causes of the tricuspid lesions. Of note, TVE was defined by the existence of valve leaflet mutilation or vegetation, or both, or annular abscess. Strands were defined as fine, mobile, echodense structures, attached to the leads, min 1-2 mm wide, and a few mm long.[6] The vegetation size, leaflet destruction, subvalvular involvement, mechanisms of tricuspid valve regurgitation, pulmonary artery pressure, and functions and sizes of the ventricles were evaluated for all patients preoperatively. Both echocardiographic and direct visual examinations (Figure 2) for vegetation and mechanisms of valve dysfunction were analyzed to decide the most appropriate surgical technique.
Figure 2. Preoperative echocardiogram showing severe tricuspid regurgitation. (a) Apical two-chamber view and with color Doppler showing pacemaker lead, vegetation around, and severe tricuspid regurgitation. (b) T hree dimension real-time echocardiogram showing infected pacemaker lead and vegetation in the right chamber (white arrow). (c) An intraoperative image of lead vegetation inside right atrium (white arrows).
Clinical presentation of LE included fever, local signs of infection at the generator site, leukocytosis, and confirmed positive cultures which were obtained from the blood, generator site, or lead(s). All cultures were examined with the results of a further antibiotic susceptibility test, and the recommended antibiotic regimen was administered intravenously for four to six weeks, according to the culture results.
The patients also presented with clinical symptoms of right-sided congestive heart failure due to moderateto- severe tricuspid regurgitation. Postoperative right heart failure (RHF) was primary managed with medical treatment including vasopressors and inotropic support with diuresis. In refractory cases, mechanical supports, including conventional percutaneous femoral extracorporeal membrane oxygenation or femoral vein-pulmonary artery extracorporeal circuit without oxygenator, were used to improve the hemodynamic parameters and right ventricular functions.
The lead extraction under total cardiopulmonary bypass and TR or TVR on the beating heart were considered for all cases, and operative findings were recorded. An epicardial temporary pacing wire was inserted before right atriotomy in pacemaker-dependent patients.
In patients with adequate valvular involvement or destruction, our first choice was TR. After removal of the leads, we performed resection of the vegetation area and the leaflet margins were approximated with simple interrupted 5-0 prolene sutures. In our routine practice, if the annular dilatation is not responsible for valve regurgitation, we do not prefer any prosthetic tissue to achieve tricuspid valve competence. Therefore, we use a glutaraldehyde-treated pericardial patch, if a wide quadrangular leaflet resection, including the vegetations, is performed. In case of unsatisfactory final coaptation test or foresight of inability of TR, we perform TVR with a biological or mechanical prosthesis.
The median follow-up was 24 (range 2 to 62) months. Data including the complaints and physical examination findings, and early and late complications related to tricuspid valve surgery were obtained in every six months during follow-up. In addition, echocardiographic examinations, including the right heart dimensions and functions, tricuspid valve competence, pulmonary artery pressure, and left ventricular functions were performed in all scheduled visits.
Statistical analysis The statistical analysis was performed using the PASW for Windows version 17.0. software (SPSS Inc., Chicago, IL, USA). Continuous variables with normal distribution were expressed as mean ± standard deviation (SD) or median (min-max) values. Categorical variables were presented as number and frequency (%).
Results
Clinical and device characteristics of all patients are shown in Table 1. Twenty-two patients had infected PPM leads (14 VVI and 8 DDD), and 21 patients had infected ICD leads (10 CRT-D, seven DDDICD, 4 VVI-ICD) which caused severe tricuspid regurgitation. The median time from PPM or ICD placement to surgery for endocarditis was 32 months (range, 3 months to 6 years).
Table 1. Demographics and preoperative clinical characteristics of patients.
| n | % | Mean±SD | |
| Age (year) | 63.2±13.6 | ||
| Gender | |||
| Male | 23 | 53 | |
| Generator location | |||
| Right prepectoral | 3 | 7 | |
| Left prepectoral | 40 | 93 | |
| Abdominal | 0 | 0 | |
| Comorbidities | |||
| Coronary artery disease | 26 | 60 | |
| Congestive heart failure | 21 | 48 | |
| Diabetes mellitus | 16 | 37 | |
| Chronic renal failure | 6 | 13 | |
| Liver dysfunction | 5 | 11 | |
| Others | 11 | 25 | |
| Clinical presentation | |||
| Fever | 40 | 93 | |
| Tricuspid regurgitant murmur | 32 | 74 | |
| Right heart failure | 18 | 41 | |
| Pulmonary septic emboli | 6 | 13 | |
| Median duration of symptoms (day) | 13 | ||
| Antibiotic prophylaxis at implantation | 100 | ||
| Location of vegetation | |||
| Auricular leads | 9 | 21 | |
| Ventricular leads | 6 | 14 | |
| Endocardium-tricuspid valve | 15 | 35 | |
| Both (lead and endocardium-tricuspid valve) | 13 | 30 | |
| Mobility of vegetation | |||
| Sessile | 31 | 72 | |
| Pedunculated | 12 | 28 | |
| Size of vegetation (mm) | 17.2±2.3 | ||
| SD: Standard deviation. | |||
All properties of vegetation are shown in Table 1. The most common vegetation location was endocardium or tricuspid valve (35%), and the mean size of vegetation was 17.2±2.25 mm. In addition, the reasons for tricuspid regurgitation are given in Table 2. The most common reason for tricuspid regurgitation was dysfunction in coaptation due to vegetation (n=15, 35%). Leaflet perforations were located on septal leaflet in all patients (n=7, 16%). Only in two of them, leaflet perforation was detected preoperatively with TTE. The remaining five patients were detected by intraoperative inspection.
Table 2. Operative findings, clinical management and outcomes.
| n | % | Median | Min-Max | |
| Mechanisms of tricuspid valve regurgitation | ||||
| Lead vegetation adherence to anterior papillary muscle | 11 | 26 | ||
| Lead vegetation impingement to septal leaflet | 10 | 23 | ||
| Lead vegetation related perforation | 7 | 16 | ||
| Vegetation related dysfunction in coaptation | 15 | 35 | ||
| Tricuspid valve replacement | ||||
| Mechanical | 3 | 7 | ||
| Bioprosthesis | 22 | 51 | ||
| Tricuspid valve repair | ||||
| Resection (with/without TRA) | 8 | 18,6 | ||
| Resection + pericardial patch (with/without TRA) | 3 | 7 | ||
| Lead extraction + TRA | 7 | 16,2 | ||
| Device extraction | 43 | 100 | ||
| Inotrope requirement | 15 | 34 | ||
| Median length of ICU stay (days) | 2 | 1-8 | ||
| Median length of follow-up of survivors (months) | 24 | 2-62 | ||
| Min: Minimum; Max: Maximum; TRA: Tricuspid ringed annuloplasty; ICU: Intensive care unit. | ||||
Laboratory findings and blood culture results are summarized in Table 3. The most common etiological agents were coagulase-negative Staphylococci (n=12, 27%) and methicillin-resistant Staphylococcus aureus (n=10, 23%). In one culture-negative patient, intraoperative inspection of vegetation was suspected as a fungal pathology, and the culture results of vegetation confirmed this suspicion (Candida Albicans). The fungal vegetation on the septal leaflet and PPM leads were removed completely. The affected part of the leaflet was repaired by a glutaraldehyde-treated pericardial patch in addition to ringed annuloplasty (TRA).
Table 3. Clinical characteristics.
| n | % | Mean±SD | |
| Laboratory findings | |||
| White blood cell >8¥109/L | 31 | 72 | |
| Anemia (Hb<10.0 g/dL) | 16 | 37 | |
| Erythrocyte sedimentation rate | 41±14.3 | ||
| C-reactive protein | 51±23.4 | ||
| Procalcitonin | 1.2±0.4 | ||
| Etiologic agents | |||
| Staphylococci | |||
| Coagulase-negative staphylococci* | 12 | 27 | |
| Staphylococcus epidermidis | 8 | 18 | |
| Staphylococcus hominis | 3 | 6 | |
| Staphylococcus aureus* | 10 | 23 | |
| Viridans group streptococci | 3 | 6 | |
| Pseudomonas aeruginosa | 2 | 4 | |
| Serratia spp. | 2 | 4 | |
| Polymicrobial | 2 | 4 | |
| Culture negative | 1 | 2 | |
| SD: Standard deviation; * Methicillin-resistant. | |||
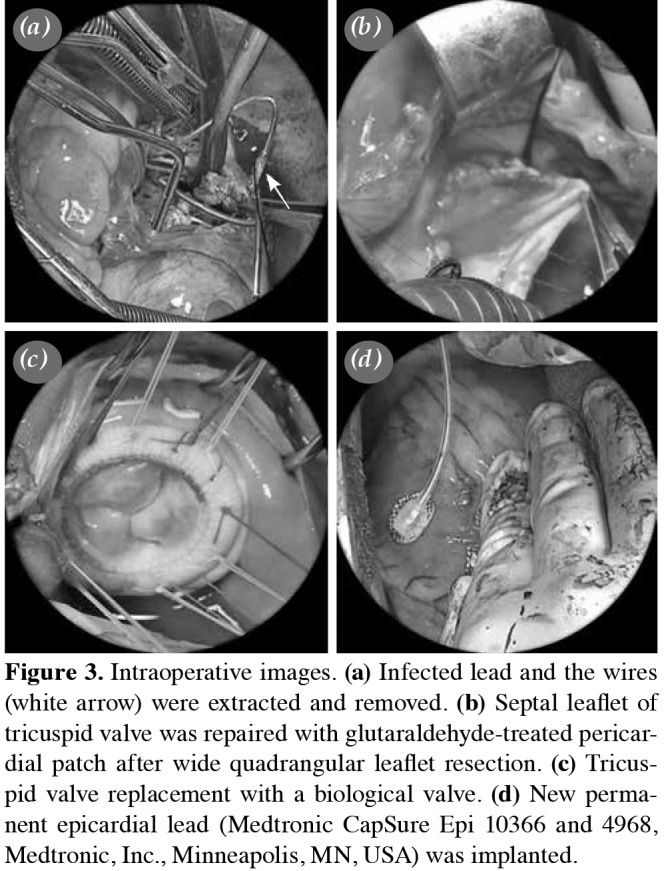
We spared the valvular structure and tended to perform TR with the resection of the infected tissue in all patients; however, only in 18 patients (42%) we were unable to perform TR. In three patients, an additional pericardial patch was required (Figure 3). In 14 patients, who had a diastolic tricuspid annular diameter of ≥40 mm, TRA was needed to achieve an optimum valve competence. Twenty-five patients (58%) underwent TVR (22 bioprosthesis and three mechanical valve) due to severe destruction, perforation or coaptation dysfunction of tricuspid valve. In eight patients, TVR was performed due to intraoperative failure of coaptation test after primary TR procedure. Mechanical prosthesis was only used due to the patient's preference.
Figure 3. Intraoperative images. (a) Infected lead and the wires (white arrow) were extracted and removed. (b) Septal leaflet of tricuspid valve was repaired with glutaraldehyde-treated pericar- dial patch after wide quadrangular leaflet resection. (c) Tricus- pid valve replacement with a biological valve. (d) New perma- nent epicardial lead (Medtronic CapSure Epi 10366 and 4968, Medtronic, Inc., Minneapolis, MN, USA) was implanted.
Postoperative complications are summarized in Table 2. Four of RHF events developed in the patients who underwent TVR. While two of them improved with inotropic support, two patients required temporary right heart mechanical support in the early postoperative period. In the TR group, two patients with RHF recovered with medical treatment. The main reasons for death were sepsis (n=6, 14%) and stroke (n=2, 4.6%). Carotid artery disease (n=1) and patent foramen ovale (n=1) were the main causes for stroke.
The median length of the intensive care unit stay was two (range, 1 to 8) days, and all patients had six weeks of antibiotherapy postoperatively. The median length of follow-up of survivors was 24 (range, 2 to 62) months. No patient had relapses during follow-up, while four patients were readmitted with RHF and five with acute renal failure within the first year of follow-up. Postoperative complication and survival data of TR and TVR groups are given in Table 4. Patients, in whom the PPM leads were removed, underwent permanent ventricular and atrial epicardial lead (Medtronic CapSure Epi 10366 and 4968, Medtronic, Inc., Minneapolis, MN, USA) implantation intraoperatively (Figure 3). The generator was placed in a new prepectoral pocket, which is usually created at the opposite side. The patients, in whom the ICD leads were removed, were referred to the Cardiology outpatient clinic after discharge for the decision of re-implantation of new ICD systems.
Table 4. Postoperative complications and survival data.
| TR | TVR | Total | ||||
| n | % | n | % | n | % | |
| Mortality | 3 | 16,6 | 5 | 20 | 8 | 18,6 |
| Right heart failure | 2 | 11 | 4 | 16 | 6 | 13,9 |
| Sepsis | 3 | 16,6 | 3 | 12 | 6 | 13,9 |
| Acute heart failure | 3 | 16,6 | 4 | 16 | 7 | 16,2 |
| Hemorrhage | 1 | 5,5 | 3 | 12 | 4 | 9,3 |
| Hepatic dysfunction | 1 | 5,5 | 4 | 16 | 5 | 11,6 |
| Stroke | - | - | 2 | 8 | 2 | 4,6 |
| Prolonged intensive care unit stay | 4 | 22,2 | 8 | 32 | 12 | 27,9 |
| Event-free patient | 12 | 67 | 16 | 64 | 28 | 65,1 |
| ARF: Acute heart failure; TVR: Tricuspid valve replacement. | ||||||
Discussion
According to the review of the literature, the rate of LE is about 1 to 2%.[7] In published studies, the mortality rate was reported as 8% in patients in whom the device was completely removed and as over 46% in those in whom the device was not removed.[8] O verall 142 patients who had pacemaker or ICD lead infection were referred to our clinic; however, 43 of them required tricuspid valve surgery for severe tricuspid regurgitation-related device endocarditis.
Several studies have advocated the conservative approach for LE with antibiotics and pocket debridement.[9] However, the high rate of uncontrolled or relapsing bacteremia, even after prolonged medical therapy, makes hardware removal the preferred option. Reported mortality rates for LE without device extraction ranges from 31 to 66%, compared to 18% in patients, in whom the hardware is extracted, followed by prolonged antibiotic therapy.[10] In our study, complete device extraction was performed in all patients, and none had a subsequent relapse with a mortality rate of 18.6% (n=8). The most common reason for death was sepsis (n=6; 75%). Based on these observations and current literature,[11,12] we recommend complete hardware extraction. In pacemaker-dependent patients, our practice is to place an epicardial temporary pacing wire for intraoperative pacing. In patients who had endocarditis-related PPM leads, we placed a permanent epicardial lead system (Medtronic CapSure Epi 10366 and 4968, Medtronic, Inc., Minneapolis, MN, USA) prior to weaning from cardiopulmonary bypass. A new prepectoral pocket was created for the generator system, which is usually located at opposite side to prior one.
Scar formation, thrombus, or vegetation on the leads impairing valve closure, perforation or laceration of leaflets, and asynchrony resulting from abnormal right ventricle activation from a pacemaker are the main causes for tricuspid regurgitation after the lead placement. Kucukarslan et al.[13] evaluated 61 patients with either ICD or PPM, of whom 49% had tricuspid regurgitation prior to cardiac electronic device implantation, and showed a worsening from normal/ trivial to mild in five patients (16%) and from mild to moderate in three patients (10%) with no patients showing an increase from moderate to severe tricuspid valve regurgitation. The absence of echocardiographic evaluation of tricuspid valve before and after PPM or ICD lead implantation in 28 patients with LE referred to our clinic from other centers was a limitation for our study. However, 15 patients with LE, in whom PPM or ICD lead placement was performed in our center, underwent an echocardiographic evaluation of tricuspid valve before and after the lead implantation, and tricuspid regurgitation was shown to increase from trivial-to-mild in six and mild-to-moderate in nine patients. All the patients were admitted with severe tricuspid regurgitation due to LE and underwent tricuspid valve surgery after a median time of 32 (range, 3 to 72) months.
The literature review reveals that TR in patients with TVE results in high rates of surgical cure,[14] better hemodynamic results,[15,16] and improved survival.[17,18] The minimal use of foreign materials, thus reducing the incidence of recurrent infection, and better long-term results are the main reasons to choose a repair technique in TVE. Based on our experience, TR is the first choice in surgical management of tricuspid regurgitation due to LE, although severe leaflet destruction and perforation, adhesive lesions of the subvalvular structure, and existence of multi-leaflet involvement are the limitations for performing an effective repair strategy in all patients, and only 42% patients (n=18) underwent TR procedures. Although it is not the primary pathology for tricuspid regurgitation, the overloading of the right ventricle may result in a tricuspid annular dilatation on admission. In such cases, if the vegetation is located only on leads and the leaflet destruction is minimal, the extraction of the lead and performing a TRA to achieve an annular stabilization may be sufficient for treatment.
Some authors reported that TR had better early survival and RHF rates compared to TVR,[19] while the others showed no significant difference between the two procedures.[20,21] These contradictory results can be explained by the early reports of TVR, indicating a high incidence of valve-related complications including reinfection, heart block, prosthetic thrombosis, and poor hemodynamic performance.[22] However, the development of bileaflet valves and low-profile porcine valves has greatly improved the prognosis of patients after TVR. Thus, patients, in whom the TR procedures fail or who have excessive involvement or severe destruction of valvular structure, should be considered for the TVR procedure with biological prosthesis. Arbulu et al.[23,24] firstly described the valvulectomy procedure in patients with TVE result in severe destruction of valvular structures. However, they showed that right ventricular dysfunction might develop approximately in one-third of patients due to pulmonary hypertension secondary to multiple pulmonary emboli. Since the most of our patients had cardiomyopathy with limited right ventricular functions and higher pulmonary artery pressure, we did not perform a valvulectomy procedure in our study population.
In cases with excessive involvement of tricuspid valve, including the perforation and attachment of lead or vegetation to leaflets and subvalvular structures (n=28, 65.1%), resection should be essential with or without TRA. However, the failure rates of TR procedures were higher in this group, and the majority of patients underwent TVR. Only five patients were able to be performed successful TR in this group, and the remaining 23 patients underwent TVR procedure. In patients with limited involvement of the valvular structures, TR may be more feasible. We had 15 patients, in whom the main pathology for tricuspid regurgitation was present with coaptation. Only a few cases required valvular structural interventions, and extraction of the lead and vegetation with or without TRA was adequate to achieve the valve competence in this group. In 13 of them, TR was successful and valve regurgitation regressed to trivial-to-mild in all patients, and only two patients underwent TVR due to failure of intraoperative coaptation test.
Intraoperative failure of coaptation test after TR and conversion to TVR causes an unexpected prolongation of operation time. Therefore, the experience of surgeon is of utmost importance to make an accurate decision according to the examination of valvular structures. In combination of severe valvular-subvalvular involvement and insufficient surgical experience of repair procedures, the surgeon should keep away from TR procedures and perform TVR to obtain the most optimal results.
Nonetheless, there are some limitations to this study. The number of the patients included to study was small to compare the results of surgical techniques; however, the literature review shows that there are no larger series of tricuspid valve surgery due to LE of implantable cardiac electronic devices. In addition, 28 patients were referred to our clinic from external centers, we were unable to document the echocardiographic records before the diagnosis of LE.
In conclusion, the experience of surgeon and the grade of valve destruction may significantly differ the success rates of these procedures. We consider that the insistent acts for tricuspid valve repair may result in severe valvular incompetence and prolonged operation time. Therefore, we suggest tricuspid valve replacement may be the primary surgical treatment choice of lead endocarditis in patients with excessive involvement of the valvular structures. Tricuspid valve repair should only be performed for cases, in which the surgeon has strong predictions about the success of procedure.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 2.Heydari AA, Safari H, Sarvghad MR. Isolated tricuspid valve endocarditis. e109-11Int J Infect Dis. 2009;13 doi: 10.1016/j.ijid.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Seratnahaei A, Leung SW, Charnigo RJ, Cummings MS, Sorrell VL, Smith MD. The changing 'face' of endocarditis in Kentucky: an increase in tricuspid cases. Am J Med. 2014;127:786–786. doi: 10.1016/j.amjmed.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J. 2004;147:582–586. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Byrne JG, Rezai K, Sanchez JA, Bernstein RA, Okum E, Leacche M, et al. Surgical management of endocarditis: the society of thoracic surgeons clinical practice guideline. Ann Thorac Surg. 2011;91:2012–2019. doi: 10.1016/j.athoracsur.2011.01.106. [DOI] [PubMed] [Google Scholar]
- 6.Victor F, De Place C, Camus C, Le Breton H, Leclercq C, Pavin D, et al. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart. 1999;81:82–87. doi: 10.1136/hrt.81.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conklin EF, Giannelli S Jr, Nealon TF Jr. Four hundred consecutive patients with permanent transvenous pacemakers. J Thorac Cardiovasc Surg. 1975;69:1–7. [PubMed] [Google Scholar]
- 8.Cacoub P, Leprince P, Nataf P, Hausfater P, Dorent R, Wechsler B, et al. Pacemaker infective endocarditis. Am J Cardiol. 1998;82:480–484. doi: 10.1016/s0002-9149(98)00365-8. [DOI] [PubMed] [Google Scholar]
- 9.Bracke FA, Meijer A, van Gelder LM. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data. Heart. 2001;85:254–259. doi: 10.1136/heart.85.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier A, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998;97:1796–1801. doi: 10.1161/01.cir.97.18.1796. [DOI] [PubMed] [Google Scholar]
- 11.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 12.Baddour LM, Bettman MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 13.Kucukarslan N, Kirilmaz A, Ulusoy E, Yokusoglu M, Gramatnikovski N, Ozal E, et al. Tricuspid insufficiency does not increase early after permanent implantation of pacemaker leads. J Card Surg. 2006;21:391–394. doi: 10.1111/j.1540-8191.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 14.Allen MD, Slachman F, Eddy AC, Cohen D, Otto CM, Pearlman AS. Tricuspid valve repair for tricuspid valve endocarditis: tricuspid valve "recycling". Ann Thorac Surg. 1991;51:593–598. doi: 10.1016/0003-4975(91)90317-j. [DOI] [PubMed] [Google Scholar]
- 15.Yee ES, Ullyot DJ. Reparative approach for right-sided endocarditis. Operative considerations and results of valvuloplasty. J Thorac Cardiovasc Surg. 1988;96:133–140. [PubMed] [Google Scholar]
- 16.Sons H, Dausch W, Kuh JH. Tricuspid valve repair in rightsided endocarditis. J Heart Valve Dis. 1997;6:636–641. [PubMed] [Google Scholar]
- 17.Dreyfus G, Serraf A, Jebara VA, Deloche A, Chauvaud S, Couetil JP, et al. Valve repair in acute endocarditis. Ann Thorac Surg. 1990;49:706–711. doi: 10.1016/0003-4975(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JR, Scott P, Nair RU. Conservative surgery in multiple cusp involvement in tricuspid valve endocarditis. Br Heart J. 1991;66:244–245. doi: 10.1136/hrt.66.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SK, Tang GH, Maganti MD, Armstrong S, Williams WG, David TE, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg. 2006;82:1735–1741. doi: 10.1016/j.athoracsur.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Moraca RJ, Moon MR, Lawton JS, Guthrie TJ, Aubuchon KA, Moazami N, et al. Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg. 2009;87:83–88. doi: 10.1016/j.athoracsur.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Marquis-Gravel G, Bouchard D, Perrault LP, Pagé P, Jeanmart H, Demers P, et al. Retrospective cohort analysis of 926 tricuspid valve surgeries: clinical and hemodynamic outcomes with propensity score analysis. Am Heart J. 2012;163:851–858. doi: 10.1016/j.ahj.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox BR, Murray GF, Starek PJ. The long-term outlook for valve replacement in active endocarditis. J Thorac Cardiovasc Surg. 1977;74:860–863. [PubMed] [Google Scholar]
- 23.Arbulu A, Holmes RJ, Asfaw I. Surgical treatment of intractable right-sided infective endocarditis in drug addicts: 25 years experience. J Heart Valve Dis. 1993;2:129–137. [PubMed] [Google Scholar]
- 24.Arbulu A, Holmes RJ, Asfaw I. Tricuspid valvulectomy without replacement. Twenty years' experience. J Thorac Cardiovasc Surg. 1991;102:917–922. [PubMed] [Google Scholar]