Abstract
Bamboo leaves of Phyllostachys nigra (PN), Lophatherum gracile (LG), and Pleioblastus amarus (PA) are three common herbs in China. In this work, a new high performance liquid chromatography (HPLC) method for the simultaneous determination of seven compounds in bamboo leaves has been developed; and PN, LG, and PA leaves were analyzed. PN showed four times as much chlorogenic acid (CA) than the other two, and contained the most isoorientin (iso-ORI) and isovitexin (iso-VIT) as well. The PA presented the most orientin (ORI) and LG covered a majority of cynaroside (CYN). We measured the antioxidant activity by scavenging the stable 2,2-diphenyl-1-pyridinohydrazinyl (DPPH) free radicals, and found that Luteolin (inhibitory concentration (IC)50 = 0.42 µM, LUT) and CYN (IC50 = 0.43 µM) showed 2–3 times higher antioxidant activity than iso-ORI (IC50 = 0.81 µM), ORI (IC50 = 0.84 µM), and other related antioxidant standards such as trolox (IC50 = 0.97 µM) and ascorbic acid (IC50 = 0.93 µM, VC). Among extracts, PN and PA showed considerable antioxidant activity, which was related well with the contents of CA, iso-ORI, and iso-VIT (p < 0.05). This study firstly provides evidence for functional antioxidant compounds of bamboo leaves based on statistical analysis of the HPLC analysis and DPPH assay, and it lays a foundation for its further development or utilization.
Keywords: Phyllostachys nigra, Lophatherum gracile, Pleioblastus amarus, content, antioxidant activity
1. Introduction
China, the world’s leading bamboo producing country, is rich in bamboo resources and has more than 500 genus of more than 500 species of bamboo [1]. The “Chinese Traditional Medicine Resources Guide” lists more than 30 species of medicinal bamboo [2,3] including Lophatherum gracile (LG), Pleioblastus amarus (PA), and Phyllostachys nigra (PN), among others. The medical stems and leaves of Lophatherum gracile were usually called “Dan Zhu Ye”, the leaves of Pleioblastus amarus were commonly named “Ku Zhu Ye”, and “Zhu Ye” was often used to define the leaves of Pleioblastus amarus and other bamboo plants. All three types of bamboo leaves have an extremely long history of medicinal and edible use in China [3]. The LG are the only medicinal bamboo contained in the Pharmacopoeia of the People’s Republic of China (hereinafter referred to as the “Pharmacopoeia”). It was first recorded in “Materia Medica of Southern Yunnan” in the Ming Dynasty. “Compendium of Materia Medica” for the first time, described in detail the morphological characteristics of LG, “Pharmacopoeia” was included in 1963 [4,5]. It has the functions of clearing heat and purging fire, removing annoyance and quenching thirst, as well as anti-inflammatory, antibacterial, antiviral, diuretic, and other functions. It is used to treat fever and thirst, urination, short and astringent pain, and sore tongue [4,6]. PA first appeared in Han’s “Mingyi Bielu”, which has the functions of clearing heat and clearing eyes, removing annoyance and thirst, antibacterial, and lowering blood sugar, among others, and it can treat inflammation, diabetes, cardiovascular diseases, and so on [7,8]. PN firstly appeared in Han’s “Mingyi Bielu” and were recorded as “Dan Zhu Ye”. Ming “Materia medica in South Yunnan” began with “Dan Zhu Ye” as the stems and leaves of LG, which have very good antioxidant and antibacterial effects, as well as great effects on oral pathogens and acute and chronic liver diseases [9,10,11]. However, the above three types of bamboo leaves’ characteristics are so similar that are easy to be confused in use. For example, the bamboo leaves used in the classic famous recipe “Bamboo Gypsum Soup” were not clearly marked, and there currently are no studies on the differences between the three types of bamboo leaves [12]. LG also do not have clearly identification methods of their chemical components that are carried in Pharmacopoeia, so people with botanical experience are needed to distinguish them.
Flavonoids and phenolic acids are natural antioxidants found in plants, and have been reported on bamboo leaves [13,14]. Bamboo leaves contain a lot of flavonoids [15,16,17], such as chlorogenic acid (CA) [18], isoorientin (iso-ORI) [19] and orientin (ORI) [20], isovitexin (iso-VIT), Cynaroside (CYN) [21], Luteolin (LUT) [22,23], and Apigenin (API) [24]. These ingredients have effects on oxidation, diabetes, cardiovascular disease, inflammation, and so on. For example, it can be used for facial mask and skin cream [25], and can also be used for processing pickled foods and so on [26]. Antioxidants isolated from the bamboo leaves have also been approved as a food antioxidant by the Food Additive Standardization Committee of the People’s Republic of China and safely used in the development of food, health care products, medicine, and cosmetics in North America, Asia, and other regions. However, bamboo leaves are too similar to availably distinguish them without an integrated quality control method, and there has been no detailed statistical analysis carried out to date for the functional compounds in bamboo leaves.
In the past year, we have made efforts to explore the correlation between the content of compounds and the antioxidant activity of extracts from the medicinal bamboo leaves of China. Firstly, a new high performance liquid chromatography (HPLC) method for the simultaneous determination of seven compounds was developed. Further, we determined that 26 methanol extracts from the leaves of the bamboo PN, LG, and PA, which grow mainly in southern areas of China, showed obvious differences in composition and content between species. We report here the antioxidant potentials of six flavonoids, one phenolic acid and methanol extracts from bamboo leaves of LG, PN, and PA. Further, the correlation between the content of compounds and antioxidant activity of extracts was investigated by the multivariate statistical analysis method, an important statistical analysis method that has been widely used in medical research [27,28,29]. This study firstly provides evidence for functional antioxidant compounds of bamboo leaves based on statistical analysis of HPLC analysis and 2,2-diphenyl-1-pyridinohydrazinyl (DPPH) assay, and it lays a foundation for its further development or utilization.
2. Results
2.1. Validation of the HPLC Method
Referring to the chromatograms of HPLC-UV detection, PA (No. 8) was selected to validate the method, including linearity, limit of detection (LOD), limit of quantitation (LOQ), precision, repeatability, stability, and recovery test. To construct calibration curves, linearity was tested at seven different concentrations of standards. Regression equations and correlation coefficients revealed a good linear response for the developed method (r > 0.9996), as shown in Table 1. LOD and LOQ were from 0.11 to 1.04 µg·mL−1, and 0.06 to 0.35 µg·mL−1, respectively. The repeatability, as relative standard deviation (RSD), was assessed by five parallel samples and was in the range of 0.14–3.04%. Table 2 presented the results of precision, stability, and recovery test. Intraday variability measurements of standard solutions were used to determine the precision and injected in six copies (RSD < 3%). The stability was evaluated by measuring and comparing the peak area of one sample at 0, 1, 2, 4, 8, 12, and 24 h. The standard solutions, at three different contents (50%, 100%, and 150%), were added into known extracts in recovery test. Further, the mean recovery was calculated using the following formula:
| Recovery (%) = 100 × (amount found − original amount)/amount spiked. | (1) |
Table 1.
The results of linearity, LOD, LOQ, and repeatability. RSD, relative standard deviation. CA, chlorogenic acid; iso-ORI, isoorientin; VIT, vitexin; CYN, Cynaroside; LUT, Luteolin; API, Apigenin.
| Com. | Linear Range (µg·mL−1) |
Linear Regression Equation (Y = a X + b) 1 |
r | LOD (µg·mL−1) |
LOQ (µg·mL−1) |
Repeatability (RSD, %, n = 5) |
|---|---|---|---|---|---|---|
| CA | 1.15–73.80 | Y = 13.39 X + 8.37 | 0.9999 | 0.14 | 0.42 | 3.04 |
| iso-ORI | 1.85‒118.40 | Y = 29.14 X + 23.96 | 0.9999 | 0.08 | 0.17 | 2.86 |
| ORI | 0.85‒54.20 | Y = 17.18 X + 5.86 | 0.9999 | 0.08 | 0.12 | 2.91 |
| iso-VIT | 1.74–111.40 | Y = 26.87 X + 22.16 | 0.9999 | 0.06 | 0.11 | 2.71 |
| CYN | 0.32–20.24 | Y = 28.32 X + 2.35 | 1.0000 | 0.09 | 0.18 | 2.09 |
| LUT | 1.54–24.72 | Y = 39.79 X − 43.71 | 0.9998 | 0.19 | 0.58 | 0.14 |
| API | 1.28–10.22 | Y = 47.77 X − 16.63 | 0.9996 | 0.35 | 1.04 | 0.19 |
1 Y refers to peak area and X refers to the concentration of compound.
Table 2.
The results of the precision, stability, and recovery test.
| Com. | Concentration (µg·mL−1) | Intraday Precision (RSD, %) | Stability (RSD, %) | Recovery Test | ||||
|---|---|---|---|---|---|---|---|---|
| Initial (µg) | Added (µg) | Detected (µg) | Recovery (%) | RSD (%) | ||||
| CA | 4.612 | 0.14 | 0.73 | 5.45 | 2.72 | 8.37 | 107.02 | 0.86 |
| 18.45 | 0.28 | 5.45 | 11.22 | 105.79 | 2.30 | |||
| 73.80 | 1.13 | 8.18 | 14.19 | 106.88 | 0.53 | |||
| iso-ORI | 7.400 | 0.21 | 1.72 | 41.10 | 20.54 | 60.63 | 95.03 | 5.17 |
| 29.60 | 0.21 | 41.09 | 79.28 | 92.90 | 2.21 | |||
| 118.4 | 0.26 | 61.64 | 98.58 | 93.26 | 0.87 | |||
| ORI | 3.388 | 0.14 | 1.01 | 14.16 | 7.08 | 21.54 | 104.16 | 1.16 |
| 13.55 | 0.19 | 14.16 | 29.12 | 105.64 | 0.84 | |||
| 54.20 | 0.28 | 21.24 | 36.86 | 106.86 | 0.25 | |||
| iso-VIT | 6.962 | 0.10 | 2.18 | 20.77 | 10.38 | 31.12 | 99.71 | 2.59 |
| 26.85 | 0.21 | 20.75 | 41.92 | 101.92 | 1.01 | |||
| 111.4 | 0.28 | 31.11 | 53.55 | 105.30 | 0.05 | |||
| CYN | 1.265 | 0.22 | 2.01 | 1.26 | 0.63 | 1.92 | 103.30 | 2.61 |
| 5.060 | 0.20 | 1.26 | 2.52 | 99.24 | 1.10 | |||
| 20.24 | 0.39 | 1.89 | 3.15 | 99.59 | 0.92 | |||
| LUT | 1.545 | 1.07 | 2.58 | 0.45 | 0.22 | 0.69 | 107.40 | 1.56 |
| 6.180 | 0.41 | 0.44 | 0.92 | 107.39 | 0.54 | |||
| 24.72 | 0.28 | 0.67 | 1.18 | 109.26 | 0.41 | |||
| API | 0.6388 | 0.87 | 3.35 | 0.22 | 0.11 | 0.36 | 122.32 | 0.10 |
| 2.555 | 0.15 | 0.22 | 0.48 | 112.14 | 0.39 | |||
| 10.22 | 0.22 | 0.34 | 0.59 | 109.74 | 0.66 | |||
The recovery test results were in the range from 92.90% to 122.32%.
2.2. HPLC Analysis of Sample
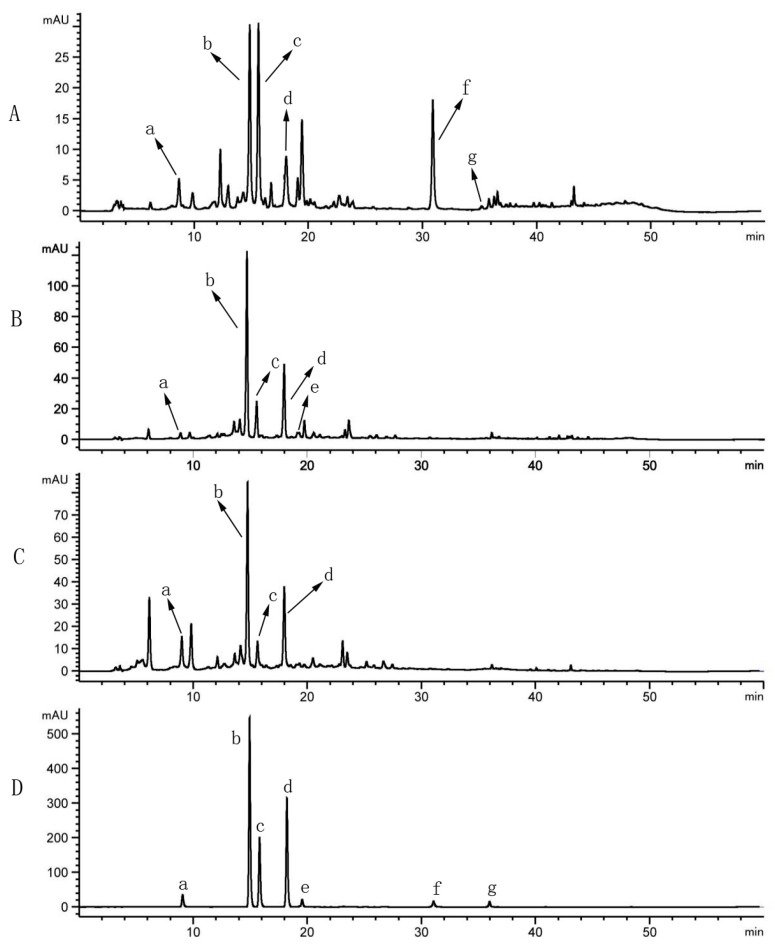
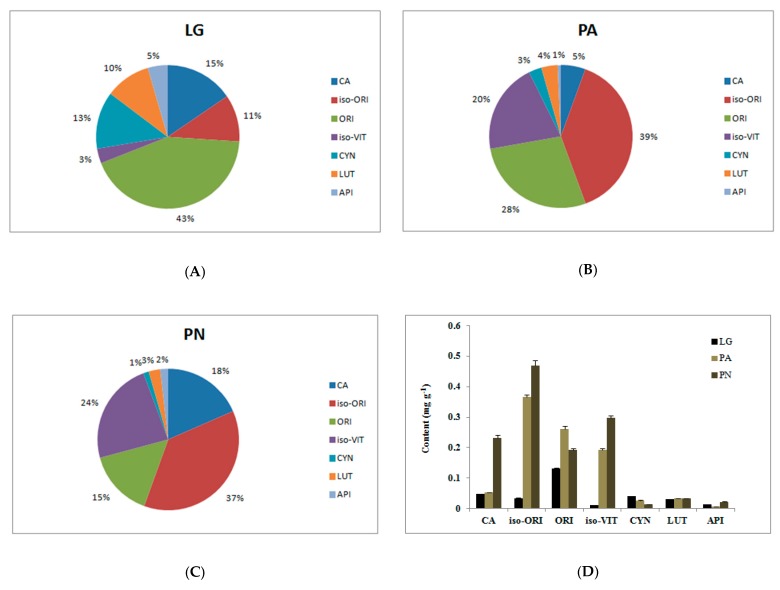
Quantitative analysis of seven components was performed on each specimen of bamboo leaves. The method specificity was assessed by comparing the consistency of the retention time between a sample and corresponding reference standard. Figure 1 showed a typical separation of Lophatherum gracile (LG), Pleioblastus amarus (PA), and Phyllostachys nigra (PN) leaf-extracts (A)–(C), and a standard mixture (D) under the optimized chromatographic conditions. All markers were well resolved from background peaks within the analysis time and the seven peaks in the chromatogram of LG, PA, and PN could be identified by the corresponding standards. As listed in Table 3 (results expressed as mean ± SD), PN indicated a four times higher content of CA than the other two, and contained the most iso-ORI and iso-VIT as well. PA presented the highest content of ORI and LG covered a majority of CYN. Figure 2 presented the average relative abundance of compounds in leaves of LG, PN, and PA.
Figure 1.
HPLC chromatograms of sample solution and standards. Lophatherum gracile (LG, No. 2) leaf-extracts (A), Pleioblastus amarus (PA, No. 8) leaf-extracts (B), Phyllostachys nigra (PN, No. 21) leaf-extracts (C), and seven mixed reference standards (a, b, c, d, e, f, g) (D).
Table 3.
The content results of the seven characteristic compounds.
| No. | Botanical Name | Source | CA (µg·g−1) | iso-ORI (µg·g−1) | ORI (µg·g−1) | iso-VIT (µg·g−1) | CYN (µg·g−1) | LUT (µg·g−1) | API (µg·g−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L. gracile | Anhui | 16.9 ± 0.9 | 1.4 ± 0 | Nd | 2.2 ± 0 | Nd | 13 ± 0.1 | 10.8 ± 0 |
| 2 | L. gracile | Sichuan | 7.5 ± 0.2 | 7.1 ± 0.1 | 326 ± 3.8 | 3.9 ± 0 | 92.6 ± 0.4 | 3.3 ± 0 | 11.4 ± 0.2 |
| 3 | L. gracile | Zhejiang | 8.1 ± 0.3 | 3.7 ± 0.1 | 214.1 ± 5 | 0.3 ± 0 | 21.8 ± 1.5 | 0.6 ± 0 | 6.5 ± 0.1 |
| 4 | L. gracile | Guangdong | 61.5 ± 0.6 | 7.6 ± 0.2 | 242.2 ± 6.8 | 4.6 ± 0.2 | 20.3 ± 1.2 | 0.4 ± 0 | 7.1 ± 0.1 |
| 5 | L. gracile | Zhejiang | 26.3 ± 1 | 22.4 ± 0.7 | 2.6 ± 0 | 5.6 ± 0.1 | 17.7 ± 0.8 | 54.1 ± 0.3 | Nd |
| 6 | L. gracile | Zhejiang | 175.5 ± 3.4 | 83.3 ± 7.3 | 6 ± 0.2 | 4.2 ± 0.1 | 48.1 ± 2.9 | 60.5 ± 0.3 | 23.1 ± 0 |
| 7 | L. gracile | Hebei | 35.6 ± 0.7 | 103.1 ± 10.1 | 1.5 ± 0.1 | 51.3 ± 3.7 | 38.5 ± 2.6 | 87.7 ± 1.6 | 23.6 ± 0 |
| 8 | P. amarus | Hebei | 175.9 ± 16.4 | 1923 ± 32.1 | 579.6 ± 10.6 | 897.1 ± 14.8 | 62.6 ± 1.1 | 21.6 ± 0.2 | 13.3 ± 0.1 |
| 9 | P. amarus | Zhejiang | Nd | 61.9 ± 2 | 17.4 ± 0.4 | Nd | Nd | 17.8 ± 0.2 | 6.6 ± 0.6 |
| 10 | P. amarus | Guangdong | 195.1 ± 2.9 | 440.3 ± 17.3 | 168.4 ± 3.4 | 98.9 ± 3.4 | 13.2 ± 0.4 | 16.7 ± 0.2 | 7.5 ± 0.3 |
| 11 | P. amarus | Sichuan | Nd | 52.5 ± 1.1 | 326.5 ± 2.8 | 0.4 ± 0 | 17 ± 0.4 | 49 ± 0 | Nd |
| 12 | P. amarus | Sichuan | Nd | 340.7 ± 8 | 107.3 ± 0.6 | 26.7 ± 0.6 | 101.7 ± 2.4 | 49.5 ± 0.1 | 30 ± 0.1 |
| 13 | P. amarus | Jiangsu | 12.2 ± 1.5 | 310.6 ± 12 | Nd | 46.3 ± 1.2 | 8.6 ± 0.5 | 18.6 ± 0.2 | Nd |
| 14 | P. amarus | Sichuan | Nd | 78.8 ± 3.1 | 473.7 ± 57.1 | 16 ± 0.5 | Nd | Nd | Nd |
| 15 | P. amarus | Sichuan | 30.4 ± 0.1 | 102.8 ± 1.7 | 571.4 ± 12.1 | 1.4 ± 0.1 | 18.8 ± 0.3 | 53.8 ± 0.2 | Nd |
| 16 | P. amarus | Jiangxi | Nd | 307.8 ± 8.6 | 136.8 ± 4.9 | 838 ± 17.6 | 21.2 ± 0.2 | 13.5 ± 0.1 | 11.8 ± 0.1 |
| 17 | P. amarus | Sichuan | Nd | 35.2 ± 1.8 | 226.7 ± 7.5 | 2.5 ± 0 | 11.3 ± 0.3 | 49 ± 0 | Nd |
| 18 | P. nigra | Anhui | 339.6 ± 11.7 | 488.6 ± 2.5 | 180 ± 3 | 390.8 ± 3.3 | 0.5 ± 0 | 15.3 ± 0 | 18.4 ± 0.1 |
| 19 | P. nigra | Jiangxi | 442.1 ± 8.5 | 304 ± 7.2 | 119.1 ± 4.6 | 404.2 ± 5.8 | 5.1 ± 0.5 | 15.5 ± 0 | 14.1 ± 0.3 |
| 20 | P. nigra | Anhui | 449 ± 41.4 | 185.1 ± 6.1 | 49.5 ± 3.9 | 347.2 ± 19.9 | 15.3 ± 1.4 | 14.3 ± 0.6 | 12.9 ± 0.3 |
| 21 | P. nigra | Zhejiang | 195 ± 1.4 | 607.3 ± 6.4 | 288.3 ± 4.4 | 317.5 ± 2.3 | 9.4 ± 0.4 | 49.2 ± 0.1 | 26.4 ± 0 |
| 22 | P. nigra | Zhejiang | 116.7 ± 5.1 | 863.3 ± 37.6 | 224.1 ± 9.7 | 386.1 ± 8.1 | 12.1 ± 0.5 | 50.8 ± 0.1 | 32 ± 0.4 |
| 23 | P. nigra | Zhejiang | 122.6 ± 3.8 | 1065.4 ± 50.1 | 377.5 ± 7.9 | 480.8 ± 14.7 | 11.7 ± 0.1 | 49.8 ± 0.1 | 33.6 ± 0.9 |
| 24 | P. nigra | Sichuan | 32 ± 0.2 | 269.2 ± 26.2 | 15.2 ± 1.4 | 33.2 ± 2.9 | 5.7 ± 0.1 | 52 ± 0.2 | 25.5 ± 0.1 |
| 25 | P. nigra | Zhejiang | 255.7 ± 2.4 | 414.3 ± 13.1 | 269.3 ± 8.4 | 301.6 ± 10.6 | 65.6 ± 1 | 51.6 ± 0.1 | 28 ± 0.2 |
| 26 | P. nigra | Zhejiang | 134.7 ± 3.2 | 12.5 ± 0.2 | 202.3 ± 9.3 | 12.4 ± 0.2 | 2.5 ± 0.2 | 0.4 ± 0 | 7.6 ± 0.1 |
Figure 2.
The average relative abundance of compounds in leaves of Lophatherum gracile (LG) (A), Pleioblastus amarus (PA) (B), and Phyllostachys nigra (PN) (C), as well as the comparison of the content of the three specimens (D). CA, chlorogenic acid; iso-ORI, isoorientin; VIT, vitexin; CYN, Cynaroside; LUT, Luteolin; API, Apigenin.
2.3. DPPH Assay and Statistical Analysis
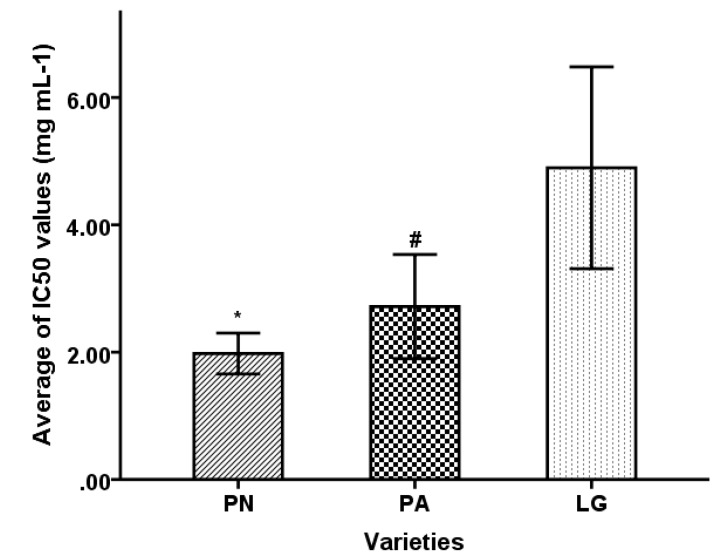
Before the DPPH assay, dry ointment yield of extracts was obtained. The antioxidant inhibitory concentration (IC)50 values of 26 extracts, 7 compounds, and 2 positive antioxidants were evaluated by scavenging DPPH free radical. These results were listed in Table 4 and Table 5. LUT (IC50 = 0.42 µM) and CYN (IC50 = 0.43 µM) showed 2–3 times higher antioxidant activity than iso-ORI (IC50 = 0.81 µM), ORI (IC50 = 0.84 µM), and other related antioxidant standards such as trolox (IC50 = 0.97 µM) and ascorbic acid (IC50 = 0.93 µM). Among extracts, LG yielded the weakest antioxidant activity, DPPH free radical scavenging IC50 values of LG varied from 2.10 to 10.17 mg·mL−1, PA and PN showed a considerable and stable ability to scavenge the DPPH free radical (1.25 to 5.07 mg·mL−1 and 1.59 to 14.64 mg·mL−1, respectively) as shown in Figure 3, and the activity of LG was significantly different from that of PA and PN by one-way analysis of variance (ANOVA) (p < 0.05).
Table 4.
The dry weight and antioxidant inhibitory concentration (IC)50 values of 26 leaf extracts.
| Sample No. | Botanical Name | Source | Dry Ointment Yield of Extracts (%) | IC50 Value (mg·mL−1) |
|---|---|---|---|---|
| 1 | L. gracile | Anhui | 5.15 ± 0.11 | 4.09 ± 0.84 |
| 2 | L. gracile | Sichuan | 6.80 ± 0.09 | 6.69 ± 0.21 |
| 3 | L. gracile | Zhejiang | 5.51 ± 0.07 | 4.51 ± 0.03 |
| 4 | L. gracile | Guangdong | 5.74 ± 0.03 | 2.10 ± 0.06 |
| 5 | L. gracile | Zhejiang | 6.13 ± 0.07 | 10.17 ± 0.06 |
| 6 | L. gracile | Zhejiang | 8.06 ± 0.10 | 4.89 ± 0.58 |
| 7 | L. gracile | Hebei | 5.25 ± 0.04 | 3.06 ± 0.07 |
| 8 | P. amarus | Hebei | 8.93 ± 0.04 | 1.25 ± 0.12 |
| 9 | P. amarus | Zhejiang | 3.02 ± 0.05 | 2.11 ± 0.06 |
| 10 | P. amarus | Guangdong | 8.64 ± 0.04 | 2.18 ± 0.01 |
| 11 | P. amarus | Sichuan | 6.08 ± 0.03 | 3.09 ± 0.18 |
| 12 | P. amarus | Sichuan | 10.74 ± 1.17 | 3.25 ± 0.56 |
| 13 | P. amarus | Jiangsu | 6.67 ± 0.18 | 3.46 ± 0.39 |
| 14 | P. amarus | Sichuan | 8.04 ± 0.64 | 3.67 ± 0.25 |
| 15 | P. amarus | Sichuan | 8.36 ± 0.05 | 3.89 ± 0.07 |
| 16 | P. amarus | Jiangxi | 16.20 ± 0.84 | 4.48 ± 0.08 |
| 17 | P. amarus | Sichuan | 7.15 ± 0.07 | 5.07 ± 0.58 |
| 18 | P. nigra | Anhui | 15.10 ± 0.19 | 2.72 ± 0.06 |
| 19 | P. nigra | Jiangxi | 13.04 ± 0.05 | 2.02 ± 0.08 |
| 20 | P. nigra | Anhui | 12.42 ± 0.04 | 1.80 ± 0.02 |
| 21 | P. nigra | Zhejiang | 8.90 ± 0.45 | 1.59 ± 0.09 |
| 22 | P. nigra | Zhejiang | 8.90 ± 0.45 | 1.65 ± 0.08 |
| 23 | P. nigra | Zhejiang | 8.90 ± 0.45 | 1.85 ± 0.03 |
| 24 | P. nigra | Sichuan | 8.90 ± 0.45 | 2.63 ± 0.07 |
| 25 | P. nigra | Zhejiang | 9.43 ± 0.11 | 1.90 ± 0.14 |
| 26 | P. nigra | Zhejiang | 9.37 ± 0.16 | 1.65 ± 0.03 |
Table 5.
The antioxidant IC50 values of compounds and the correlations between contents and IC50 values of extracts.
| No. | Compound | IC50 Value (mM) |
Correlation Coefficient r | ||
|---|---|---|---|---|---|
| PN | PA | LG | |||
| 1 | CA | 1.04 ± 0.04 | −0.023 | −0.508 ** | −0.169 |
| 2 | Iso-ORI | 0.81 ± 0.01 | −0.171 | −0.643 ** | −0.181 |
| 3 | ORI | 0.84 ± 0.02 | −0.501 ** | −0.120 | −0.133 |
| 4 | Iso-VIT | 14.5 ± 0.04 | −0.193 | −0.226 | −0.293 |
| 5 | CYN | 0.43 ± 0.00 | −0.164 | −0.228 | 0.169 |
| 6 | LUT | 0.42 ± 0.01 | 0.021 | 0.431 * | 0.139 |
| 7 | API | Nd | −0.026 | −0.264 | −0.491 * |
| 8 | Trolox | 0.97 ± 0.04 | -- | -- | -- |
| 9 | VC | 0.93 ± 0.02 | -- | -- | -- |
Nd, Not detected. --, None. * p < 0.05, ** p < 0.01.
Figure 3.
One-way analysis of variance (ANOVA) result of 2,2-diphenyl-1-pyridinohydrazinyl (DPPH) scavenging activity of bamboo leaves from PN, PA, and LG. * means the activity of LG was significantly different from that of PN (p < 0.05). # means the activity of LG was significantly different from that of PA (p < 0.05).
As a result of the correlation analysis (Table 5), contents of CA (r = −0.508, p < 0.01), iso-ORI (r = −0.643, p < 0.01), and LUT (r = 0.431, p < 0.05) were significantly correlated with the IC50 of PA extracts, the activity of PN extracts was found in relation to the content of ORI (r = −0.501, p < 0.01), and LG was associated with the content of API (r = −0.491, p < 0.05). Because of a significant correlation between the content of each component (p < 0.05), the stepwise regression method was used in our analysis. We found that the content of iso-ORI and total content of seven compounds jointly explained 53.4% of the variation in the activity of PA extracts, which means iso-ORI is the main active substance in PA. The main antioxidant compounds in PN is ORI (R2 = 21.9%), and the activity of LG extracts was influenced greatly by the contents of ORI, CYN, and API (R2 = 95.9%).
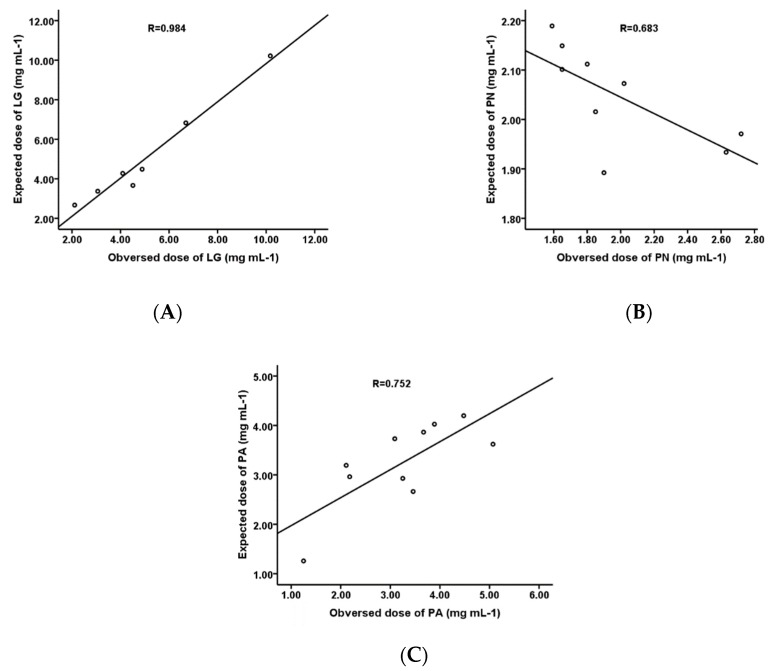
Besides, as shown in Figure 4, as a result of correlations between observed and expected antioxidant activities based on established multiple regression equation (Table 6), significant multiple correlation coefficients for DPPH free radical scavenging activities on LG (R = 0.984), PA (R = 0.752), and PN (R = 0.683) were found in relation to the contents of their main active compounds.
Figure 4.
The correlation between observed and expected antioxidant IC50 values on LG (A), PN (B), and PA (C). Antioxidant activities were expressed as a dose required for half the DPPH free radical.
Table 6.
The antioxidant IC50 values of compounds and the correlations between contents and IC50 values of extracts.
| Materials | Multiple Regression Equations 2 | IC50 Value(mM) |
|---|---|---|
| Lophatherum gracile | Y = 8.413 − 21.269 XORI + 104.946 XCYN − 383.105 XAPI | 5.07 ± 0.26 |
| Phyllostachys nigra | Y = 2.272 − 1.673 XORI | 3.25 ± 0.23 |
| Pleioblastus amarus | Y = 3.279 − 3.855 Xiso-ORI + 1.468 XTotal | 1.98 ± 0.06 |
2 Y refers to IC50 values of an extract to scavenging DPPH free radical, and X means the content of a compound or the total content of seven characteristic compounds.
3. Discussion
Bamboo leaf has been used as an antioxidant material to prevent food deterioration since ancient times. Studies indicated that flavonoids, polysaccharides, and phenolic acids might be the major active constituents [30,31]. Crude extracts and pure compounds from the leaves have been shown to possess multiple biological activities such as anti-inflammatory, antivirus, cardiovascular protection, cancer prevention, and particularly antioxidant activity [32]. However, as far as chemical constituents are concerned, pharmacological reports most often attribute some activities to various extracts rather than specific compounds. This makes it difficult to build a connection between the chemical constituents and pharmacological activities, resulting in the difficulty of identifying the major active compounds. Besides, bamboo leaves are various in varieties and confused in classification, and thus many scientific questions remain, which need to be answered through further laboratory research and clinical trials.
First, we developed a new HPLC method for the quantification of seven main active components in bamboo leaves simultaneously. Several chromatographic conditions using different elution systems were tested according to optimum resolution, peak shape, and efficiency of samples. Conditions were ensured with a Diamonsil Plus C18 column (4.6 × 250 mm, 5 µm) and a water/methanol mixture containing 0.1% (v/v) formic acid elution system for the detection of seven compounds. Formic acid (0.1%) was selected as the mobile phase additive to provide improved pH control, simplified preparation, and was found to achieve good peak intensity and resolution. Our preliminary experiments have measured individual maximum UV absorbance of CA, iso-ORI, ORI, iso-VIT, CYN, LUT, and API at 324, 350, 346, 336, 350, 350, 332, and 346 nm, respectively. To achieve optimal UV absorbance for all references, 350 nm was selected and demonstrated a high degree of sensitivity and precision. The method was applied successfully for the analysis and identification of the components of the three bamboo leaves. Then, the antioxidant activity of six flavonoids, one phenolic acid, and methanol extracts from bamboo leaves of LG, PN, and PA were detected by DPPH.
According to Chinese Pharmacopoeia published in 2015, there is no standard assay for determining the contents of LG. In fact, HPLC fingerprints could be used to improve quality control. Assays of biological activities could be applied as well. In addition, studies on this medicinal plant were presently limited to the antioxidants and preservatives, even though ancient books report that the flesh can clear heat and remove vexation. More efforts should be made to fill the blank.
4. Materials and Methods
4.1. Reagents and Chemicals
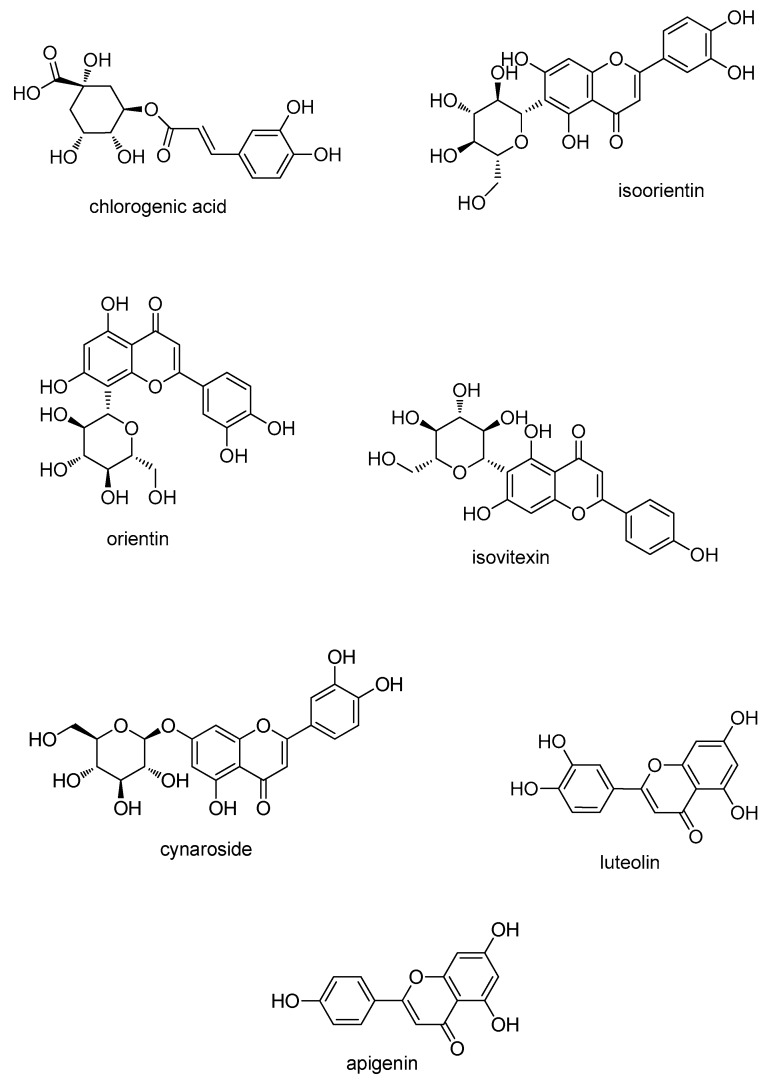
Chlorogenic acid, isoorientin, and apigenin were purchased from Shanghai Natural Biological Technology Co., Ltd. (Shanghai, China). Iso-vitexin, cynaroside, and luteolin were obtained from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China). Orientin was bought from Chengdu Push Biological Technology Co., Ltd. (Chengdu, China). The purity of all standards was more than 98%, and their chemical structures are listed in Figure 5. DPPH was gained from Sigma-Aldrich (St. Louis, MO, USA). Trolox was from Beyotime Institute of Biotechnology Co., Ltd. (Shanghai, China). Ascorbic acid (VC) was ordered from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Diamonsil Plus C18 column (4.6 × 250 mm, 5 µm) was used. All liquid chromatography solvents (methanol, acetonitrile) were of HPLC grade, obtained from Anhui Fulltime Specialized Solvent and Reagent Co., Ltd. (Anqing, China), and used after filtration by a 0.45 µm organic membrane. All other solvents were of analytical grade and used without further purification.
Figure 5.
Chemical structures of the seven characteristic compounds.
4.2. Plant Materials
Twenty-six commercial herbal samples (Table 7) of Phyllostachys nigra, Lophatherum gracile, and Pleioblastus amarus were collected via purchase from commercial Chinese markets and authenticated by Dr. Long Song from Shanghai University of Traditional Chinese Medicine. The air-dried samples were smashed into powder (65 mesh) and stored in a desiccator.
Table 7.
Information sheet of twenty-six commercial herbal samples.
| Botanical Name | Number | Source | Lot Number | Collection Date |
|---|---|---|---|---|
| P. amarus | 1 | Anguo Yikang Traditional Chinese Medicine Church, Hebei | XY170027 | 14 May 2017 |
| 2 | Anji, Zhejiang | XY170307 | 23 June 2017 | |
| 3 | Meizhou, Guangdong | XY170308 | 23 June 2017 | |
| 4 | Gaoxian, Sichuan | XY181247 | 26 March 2018 | |
| 5 | Yibin, Sichuan | XY181235 | 16 March 2018 | |
| 6 | Suqian, Jiangsu | XY181236 | 16 March 2018 | |
| 7 | Shuanghe, Sichuan | XY181248 | 26 March 2018 | |
| 8 | Meidong, Sichuan | XY181249 | 26 March 2018 | |
| 9 | Yudu, Jiangxi | XY170502 | 14 December 2017 | |
| 10 | Jiangan, Sichuan | XY181250 | 26 March 2018 | |
| L. gracile | 1 | Huoshan, Anhui | XY170499 | 10 December 2017 |
| 2 | Chengdu, Sichuan | XY170402 | 1 September 2017 | |
| 3 | Shanghai kangqiao traditional Chinese medicine decoction pieces co. LTD | XY170487 | 21 September 2017 | |
| 4 | Guangzhou, Guangdong | XY170489 | 5 November 2017 | |
| 5 | Wenzhou, Zhejiang | XY181230 | 12 March 2018 | |
| 6 | Lecheng, Zhejiang | XY181237 | 16 March 2018 | |
| 7 | Anguo Oriental Medicine City, Hebei | XY170311 | 18 June 2017 | |
| P. amarus | 1 | Yueshan, Anhui | XY170524 | 23 December 2017 |
| 2 | Jiujiang, Jiangxi | XY170526 | 25 December 2017 | |
| 3 | Anqing, Anhui | XY1700521 | 21 December 2017 | |
| 4 | Changxing, Zhejiang | XY170492 | 6 November 2017 | |
| 5 | Changxing, Zhejiang | XY181224 | 10 March 2018 | |
| 6 | Zhejiang | XY181233 | 15 March 2018 | |
| 7 | Qvzhou, Zhejiang (drying) | XY181242 | 18 March 2018 | |
| 8 | Qvzhou, Zhejiang (air drying) | XY170027 | 18 March 2018 | |
| 9 | Yibin, Sichuan | XY170307 | 16 March 2018 |
4.3. Instruments and Chromatographic Conditions
HPLC analyses were primarily performed using an Agilent Technologies 1260 infinity pump and a 1260 infinity II ultraviolet (UV) detector (Palo Alto, CA, USA). The chromatography system consisted of a 1260 infinity quaternion liquid. A microplate spectrophotometer (Epoch 2, Biotek Instruments, Winooski, VT, USA) was used in the assay of the scavenging activity on the DPPH radical.
The separation was performed on a Diamonsil Plus C18 column (4.6 × 250 mm, 5 µm). The mobile phase was composed of 0.1% aqueous formic acid (A, pH 2.8) and acetonitrile (B), using a gradient elution of 10% B at 0–2 min, 10–30% B at 2–30 min, 30–70% B at 30–45 min, and 70–10% B at 55–60 min. The flow rate was set at 1.0 mL·min−1, with the temperature maintained at 30 °C. The injection volume was 10 µL and the detection wavelength was set at 350 nm.
4.4. Preparation of Solutions for HPLC Analysis
4.4.1. Standard Solutions and Calibration Curves
The stock solution of each standard was prepared by dissolving single analyte in pure methanol at an appropriate concentration (CA, CYN, LUT, and API are about 0.5 mg·mL−1, and iso-ORI, ORI, and iso-VIT are about 1 mg·mL−1), and the preparations of ORI, iso-VIT, and API need to be pre-dissolved with dimethyl sulfoxide (DMSO). They may be precipitated when stored in a refrigerator, which can be solved by warming and ultrasound.
Standard solutions of CA, iso-ORI, ORI, iso-VIT, CYN, LUT, and API, in appropriate concentration ranges, were prepared by mixing each stock solution, and diluted by 50% methanol to obtain appropriate concentrations for calibration curves. The analyte peak area values were plotted against the corresponding concentrations of the analyte (expressed as µg·mL−1).
4.4.2. Sample Solutions
Each powdered material (1.0 g) was accurately weighed and extracted with 25 mL of methanol under ultrasonication for 30 min followed by centrifugation. The supernatant solutions were filtered through a 0.22 µm filter membrane prior to injection.
4.5. Preparation of Solutions for DPPH Assay
Twenty-six extracts were prepared as the sample preparation for HPLC analysis. The seven characteristic compounds, as well as positive antioxidants including VC and trolox, were configured to be 20 mM with DMSO. All samples were evaluated by DPPH radical scavenging activity, and 50% methanol was used as the blank solution. For the DPPH assay, we have some modifications on the basis of reported methods [15]. Samples of 10 µL at various concentrations were added into 100 µL of DPPH solution (0.65 mM, methanol) and 140 µL of 50% methanol. After one hour of incubation at room temperature in the dark, the absorbance of mixtures (test samples, control samples, blank samples) was recorded at 517 nm. The scavenging rates (SR) were calculated based on the following formula:
| SR (%) = 100 × [1 − (Abs blank − Abs sample or control)/Abs blank], | (2) |
where Abs blank and Abs sample or control represent the absorbance of the blank sample, test sample, and control sample, respectively.
4.6. Statistical Analysis of Content and Antioxidant Activity
The correlation between the content of components and antioxidant activity of extracts was explored by reported multivariate regression analysis [33]. All statistical analyses were performed with the SPSS Statistics (SPSS vision 21, IBM, Camp Takajo, NY, USA).
Author Contributions
Y.D., T.Z., and X.-R.Y. conceived and designed the experiments. N.-H.M., J.G., and S.-H.X.C. performed the experiments. N.-H.M. and J.G. analyzed the data and wrote the original draft. Y.D. revised the manuscript and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by programs of the National Natural Science Foundation of China [grant numbers 81872981]; the National Scientific and Technological Major Special Project of China [Grant number 2019ZX09201004-002]; the projects sponsored by the development fund for Shanghai talents [grant number 2018105], Youth Talent Sail Plan from the Shanghai Committee of Science and Technology [grant number 18YF1423600]; Program of Shanghai Academic/Technology Research Leader [grant number 18XD1403700]; Project of the Shanghai Municipal Commission of Health and Family Planning [grant number 2017YQ072 and 201740152]; the Key project of Shanghai 3-year plan (ZY(2018-2020)-CCCX-2001-04) and Xinglin Young Talent Program [grant number 2017.10-2020.09]; and the Research Fund for the Doctoral Program of Shanghai [grant number B201703]; Shanghai Science and technology innovation project [17401901900].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Peng Z., Lu Y., Li L., Zhao Q., Feng Q., Gao Z., Lu H., Hu T., Yao N., Liu K., et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla) Nat. Genet. 2013;45:456–461. doi: 10.1038/ng.2569. [DOI] [PubMed] [Google Scholar]
- 2.Wang S.Y., Tang F., Yue Y.D. Research Progress of Chemical Constituents and Their Pharmacological Activities of Bamboo Plants. Chem. Ind. Forest Prod. 2013;33:149–156. doi: 10.3969/j.issn.0253-2417.2013.03.029. [DOI] [Google Scholar]
- 3.Geng B.J., Wang Z.P. Flora Reipublicae Popularis Sinicae. Volume 35. Beijing Science Press; Beijing, China: 1996. p. 260. [Google Scholar]
- 4.Cai H.Q., Zhan Z.L., Zheng L.X., Huang Z.H. Overview of Quality Criteria of Lophatheri Herba. ACTA Chin. Med. 2017;12:2430–2434. doi: 10.16368/j.issn.1674-8999.2017.12.632. [DOI] [Google Scholar]
- 5.Huang Z.H., Cai H.Q., Zheng L.X., Guo Y.L., Zhan Z.L. Textual research on the text and text of Chinese herbal medicine light bamboo leaf. J. Chin. Med. Mater. 2017;40:973–977. doi: 10.13863/j.issn1001-4454.2017.04.051. [DOI] [Google Scholar]
- 6.Pharmacopoeia Committee of the people’s Republic of China . Pharmacopoeia of the People’s Republic of China. Beijing Chemical Industry Press; Beijing, China: 2015. [Google Scholar]
- 7.Ren Y., Ma Y.S., Zhang Z.D., Qiu L.Y., Zhai H.H., Gu R.M., Xie Y.P. Total Alkaloids from Bamboo Shoots and Bamboo Shoot Shells of Pleioblastus amarus (Keng) Keng f. and Their Anti-Inflammatory Activities. Molecules. 2019;24:2699. doi: 10.3390/molecules24152699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Y., Dang Y.H., Zhang Z.D., Wan D.G. A study on the leaves of Phyllostachys pubescens. J. Chin. Med. Mater. 2016;39:1430–1432. doi: 10.13863/j.issn1001-4454.2016.06.057. [DOI] [Google Scholar]
- 9.Kyung L.P., Sung T.K., Min J.K., Hee K.O. Antioxidative Components and Anti-Oralmicrobial Effect of Bamboo (Phyllostachys nigra var. henonis Stapf) Leaves. J. Korean Soc. Food Sci. Nutr. 2016;45:1265–1272. doi: 10.3746/jkfn.2016.45.9.1265. [DOI] [Google Scholar]
- 10.Moon-Hee C., Han-Gyo J., Ji H.Y., Sung H.K., Hyun-Jae S. Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2018;19:409. doi: 10.3390/ijms19020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji H.Y., Moon-Hee C., Chang-Su N., Sam S.C., Jae H.K., Sae K.K., Il J.C., Hyun-Jae S., Sung H.K. Bamboo Stems (Phyllostachys nigra variety henosis) Containing Polyphenol Mixtures Activate Nrf2 and Attenuate Phenylhydrazine-Induced Oxidative Stress and Liver Injury. J. Nutrients. 2019;11:114. doi: 10.3390/nu11010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S.H., Ji Z.X., Li J.Y. Syndrome analysis and clinical application of Lophatherum and Gypsum Decoction. China’s Naturop. 2018;26:47–49. doi: 10.19621/j.cnki.11-3555/r.2018.1231. [DOI] [Google Scholar]
- 13.Wei Q., Wang S., Tang F., Zhang H., Yu J., Yue Y. Simultaneous Determination of 13 Flavonoids in Bamboo Leaves by HPLC. Sci. Silvae Sin. 2015;8:81–87. doi: 10.11707/j.1001-7488.20150811. [DOI] [Google Scholar]
- 14.Tang Q., Shao M., Wang Y., Zhao H., Fan C., Huang X., Li Y., Ye W. Simultaneous Determination of 10 Bioactive Components of Lophatherum gracile Brongn by HPLC-DAD. Chromatogr. Sci. 2015;53:963–967. doi: 10.1093/chromsci/bmu160. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H.N., Chen M., Fan C.L., Wang Y., Li Y.L., Ye W.C. A new flavone C-glycoside from leaves of Lophatherum gracile. China, J. Chin. Mater. Med. 2014;39:247–249. doi: 10.4268/cjcmm20140216. [DOI] [PubMed] [Google Scholar]
- 16.Sun J., Tang F., Yue Y.D., Xun H., Guo X.F. Two new compounds from the dry leaves of Pleioblastus amarus (Keng) keng f. Asian Nat. Prod. Res. 2014;16:930–935. doi: 10.1080/10286020.2014.944513. [DOI] [PubMed] [Google Scholar]
- 17.Gong J., Xia D., Huang J., Ge Q., Mao J., Liu S., Zhang Y. Functional components of bamboo shavings and bamboo leaf extracts and their antioxidant activities in vitro. Med. Food. 2014;18:453–459. doi: 10.1089/jmf.2014.3189. [DOI] [PubMed] [Google Scholar]
- 18.Wang L.P., Guo D., Wang G., Zhou H.H. Advancement of Chlorogenic Acid in Traditional Chinese Medicine. Lishizhen Med. Mater. Med. Res. 2011;22:961–963. doi: 10.3969/j.issn.1008-0805.2011.04.080. [DOI] [Google Scholar]
- 19.Luo C., Li Y., Long J.G., Zhang Y.Q. Pharmacological Effects of Isoorientin. Space Med. Med. Eng. 2016;29:381–384. doi: 10.16289/j.cnki.1002-0837.2016.05.014. [DOI] [Google Scholar]
- 20.Wan S.Q., Liu L.Y., Liu M.S., Huang X.L. Study on Pharmacological Action Mechanism of Orientin. J. Med. Res. 2018;47:183–185. doi: 10.11969/j.issn.1673-548X.2018.06.043. [DOI] [Google Scholar]
- 21.Ying Z., Min Z., Ting R.Z., Jun W.W., Jun W., Ke H.L., Nan N.Z. Antioxidant and Anti-inflammatory Activities of Cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018;13:1501–1504. doi: 10.1177/1934578X1801301122. [DOI] [Google Scholar]
- 22.Al-Megrin W.A., Alkhuriji A.F., Yousef A.O.S., Metwally D.M., Habotta O.A., Kassab R.B., Abdel Moneim A.E., El-Khadragy M.F. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants (Basel) 2020;9:10. doi: 10.3390/antiox9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao Y.Z., Li Y.H., Jing S.L., Lin Z. Luteolin Attenuates Diabetic Nephropathy through Suppressing Inflammatory Response and Oxidative Stress by Inhibiting STAT3 Pathway. Exp. Clin. Endocr. Diab. 2020 doi: 10.1055/a-0998-7985. [DOI] [PubMed] [Google Scholar]
- 24.Sun X.Q., Li R.H., Tang T. Research Progress of Apigenin Antioxidant Effect. [(accessed on 1 September 2019)];China Mod. Dr. 2009 47:34–35. Available online: http://www.cnki.net. [Google Scholar]
- 25.Zeng M.F. Application Research of Several Natural Antioxidants in Cosmetics. [(accessed on 31 August 2019)];Synth. Mater. Aging Appl. 2014 43:73–77. Available online: https://wenku.baidu.com/view/c112f3d2fd0a79563d1e723f.html. [Google Scholar]
- 26.Pan J.J., She X.Y., Hu L.F., Shi L.W., Zhang H. The Application of Bamboo Leaf Extracts in Foods. [(accessed on 31 June 2019)];J. Bamboo Res. 2019 38:57–61. Available online: http://www.cqvip.com/QK/97864A/201902/7100180029.html. [Google Scholar]
- 27.Suzuki H., Tabata T., Koizumi H., Hohchi N., Takeuchi S., Kitamura T., Fujino Y., Ohbuchi T. Prediction of hearing outcomes by multiple regression analysis in patients with idiopathic sudden sensorineural hearing loss. Ann. Oto. Rhinol. Laryn. 2014;123:821–825. doi: 10.1177/0003489414538606. [DOI] [PubMed] [Google Scholar]
- 28.Cao H.H., Du R.F., Yang J.N., Feng Y. Correlation of dry granulation process parameters and granule quality based on multiple regression analysis. Acta Pharm. Sin. 2014;49:406–410. doi: 10.16438/j.0513-4870.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Iizuka A., Iijima O.T., Kondo K., Itakura H., Yoshie F., Miyamoto H., Kubo M., Higuchi M., Takeda H., Matsumiya T. Evaluation of Rhubarb using antioxidative activity as an index of pharmacological usefulness. J. Ethnopharmacol. 2004;91:89–94. doi: 10.1016/j.jep.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Wang C.Z., Zhang H.Y., Li W.J., Ye J.Z. Chemical constituents and structural characterization of polysaccharides from four typical bamboo species leaves. Molecules. 2015;20:4162–4179. doi: 10.3390/molecules20034162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong J., Huang J., Xiao G., Chen F., Lee B., Ge Q., You Y., Liu S., Zhang Y. Antioxidant Capacities of Fractions of Bamboo Shaving Extract and Their Antioxidant Components. Molecules. 2016;21:996. doi: 10.3390/molecules21080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao J.W., Yin J., Ge Q., Jiang Z.L., Gong J.Y. In vitro antioxidant activities of polysaccharides extracted from Moso Bamboo-Leaf. Int. J. Biol. Macromol. 2013;55:1–5. doi: 10.1016/j.ijbiomac.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Hamdi A., Viane J., Mahjoub M.A., Majouli K., Gad M., Kharbach M., Demeyer K., Marzouk Z., Heyden Y.V. Polyphenolic contents, antioxidant activities and UPLC-ESI-MS analysis of Haplophyllum tuberculatum A. Juss leaves extracts. Int. J. Biol. Macromol. 2017;106:1071–1079. doi: 10.1016/j.ijbiomac.2017.08.107. [DOI] [PubMed] [Google Scholar]