Abstract
Nitrogen heterocycles have drawn considerable attention due to of their significant biological activities. The marine fungi residing in extreme environments are among the richest sources of these basic nitrogen-containing secondary metabolites. As one of the most well-known universal groups of filamentous fungi, marine-derived Aspergillus species produce a large number of structurally unique heterocyclic alkaloids. This review attempts to provide a comprehensive summary of the structural diversity and biological activities of heterocyclic alkaloids that are produced by marine-derived Aspergillus species. Herein, a total of 130 such structures that were reported from the beginning of 2014 through the end of 2018 are included, and 75 references are cited in this review, which will benefit future drug development and innovation.
Keywords: Aspergillus, metabolite, marine, alkaloid, biological activity
1. Introduction
Heterocyclic alkaloids are one of the most challenging natural product classes to characterize, not only because of their structurally unique skeletons that arise from distinct amino acids, but also because of their potential bioactivities. These nitrogen heterocycles are among the most active molecules and they are currently in various phases of human clinical trials for treating various diseases [1,2,3]. The ocean is a rich underexploited source of novel and bioactive molecules, because extreme marine conditions, including low temperature, high pressure, reduced light, and the presence of predators, can cause marine organisms to develop machinery for the construction of a greater diversity of metabolites than terrestrial organisms [4,5,6,7,8]. Fungi living in the extreme environments that are typical of marine ecosystems are very sensitive to culture media and are more liable to produce novel metabolites than fungi living in less extreme environments. As one of the most well-known universal groups of filamentous fungi, marine-derived Aspergillus species have one of the main sources of new heterocyclic alkaloids in recent years. This mini-review attempts to provide a comprehensive summary of the structural diversity and biological activities of the nitrogen-containing secondary metabolites that are produced by marine-derived Aspergillus species.
A series of excellent reviews on various aspects of secondary metabolites that are derived from the genus Aspergillus have been published in the past five years (from 2014 to present) [9,10,11,12,13,14,15,16]. However, there is only one work specifically aimed at Aspergillus species from the marine environment. In 2018, K.W. Wang and P. Ding summarized the information on 232 new bioactive secondary metabolites from marine-derived Aspergillus species, which had been reported from 2006 to 2016, with classification on the basis of biological activity and chemical structure [12]. As part of our ongoing investigations of biological compounds from endophytic Aspergillus species that reside on the marine brown algae Leathesia nana (Chordariaceae) [17], a detailed and comprehensive literature survey disclosed that the previously published structures might not be adequately represented. To the best of our knowledge, a total of approximately 400 new compounds were isolated from marine-derived Aspergillus species from the beginning of 2014 to the end of 2018 (see Supplementary Materials), of which 130 could be classified as heterocyclic alkaloids. This review aims to provide an update on the recent discoveries of the heterocyclic alkaloids that are produced by marine-derived Aspergillus species.
The selection of original articles was of greatest importance because these papers had a direct impact on the findings and the final results. This review included all original articles registered with the relevant subject in the Web of Science Core Collection database between 2014 and 2018. The literature search was performed while using a previously reported search method [18,19]. The search strategy was as follows: “Title: (from Aspergillus); Refined by: Topic (marine) and Document types (article); Timespan: 2014–2018; Indexes: SCI-EXPANDED, CPCI-S”. Notably, the present work was preliminarily planned in April 2019, and the studies that were published or being submitted in the current year might not be accurately indexed in the Web of Science Core Collection database; thus, the timespan of the literature search was from 2014 to 2018. With this approach, 166 records were finally identified and were considered to cover most of the related studies.
After retrieving the records that were related to the field of natural product chemistry, 123 original articles were indexed in the Web of Science Core Collection database over a period of five years (from the beginning of 2014 to the end of 2018). During this period, 398 naturally occurring compounds were isolated and characterized from marine-derived Aspergillus species. The 130 nitrogen-heterocyclic compounds included accounted for 32.7% of all newly reported secondary metabolites. Supplementary Materials lists all 123 original articles and the structures of these 398 newly reported secondary metabolites. This critical review focuses on the structural diversity, biological activities, and sources of these newly reported heterocyclic alkaloids.
2. Structural Diversity
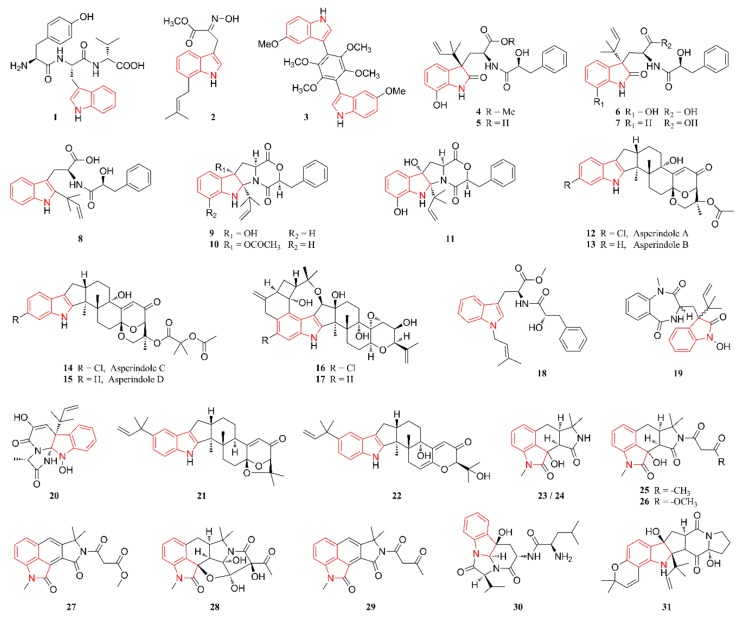
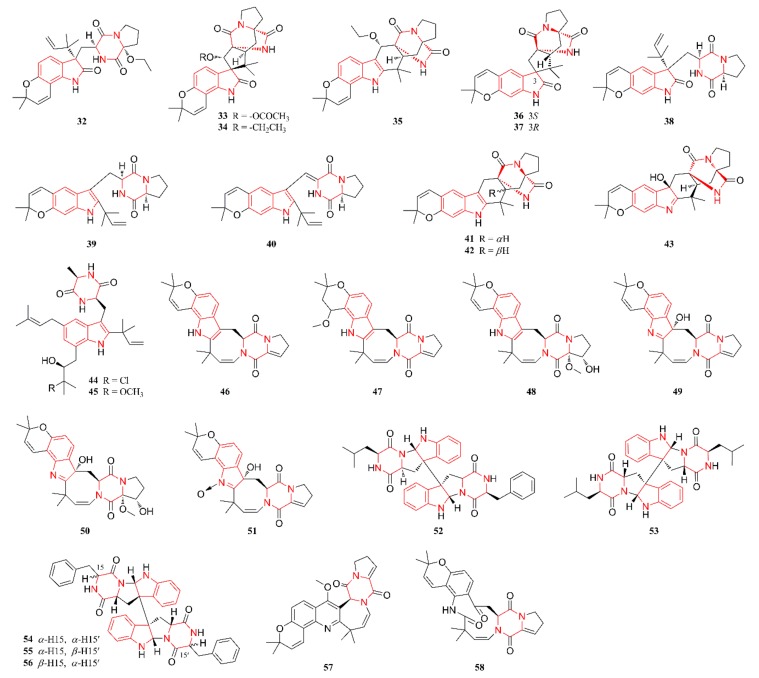
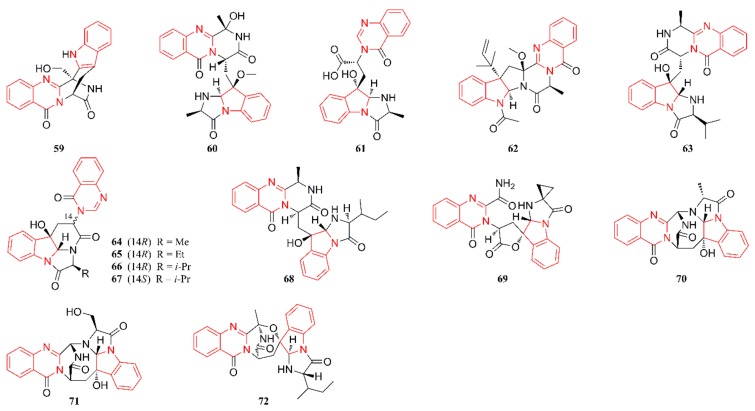
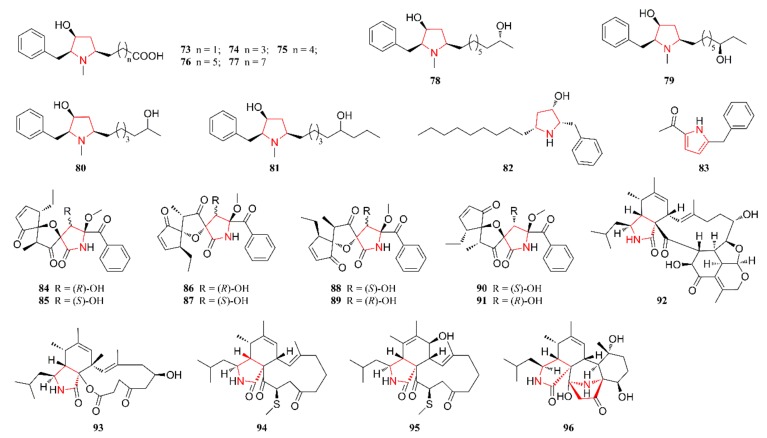
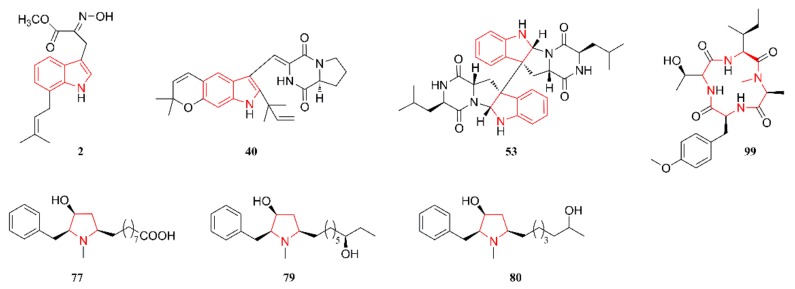
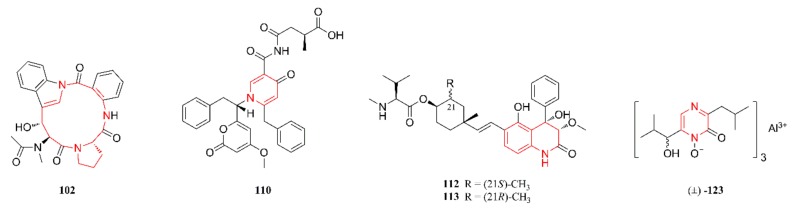
Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 present the structures of newly reported heterocyclic alkaloids (1–130) produced by marine-derived Aspergillus species from 2014 to 2018, in which the nitrogen-containing heterocyclic rings are marked in red. These heterocyclic alkaloids could be classified into six major categories: indole alkaloids (1–31), diketopiperazine alkaloids (32–58), quinazoline alkaloids (59–72), pyrrolidine alkaloids (73–96), cyclopeptide alkaloids (97–108), and other heterocyclic alkaloids (109–130) based on their structural patterns.
Figure 1.
Indole alkaloids produced by marine-derived Aspergillus species (1–31).
Figure 2.
Diketopiperazine alkaloids produced by marine-derived Aspergillus species (32–58).
Figure 3.
Quinazoline alkaloids produced by marine-derived Aspergillus species (59–72).
Figure 4.
Pyrrolidine alkaloids produced by marine-derived Aspergillus species (73–96).
Figure 5.
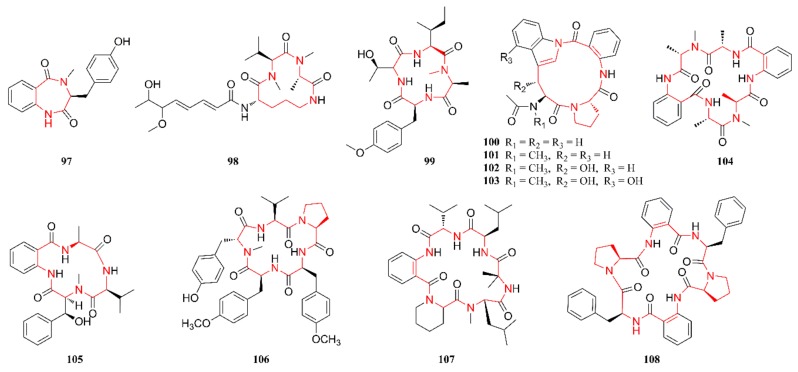
Cyclic peptide alkaloids produced by marine-derived Aspergillus species (97–108).
Figure 6.
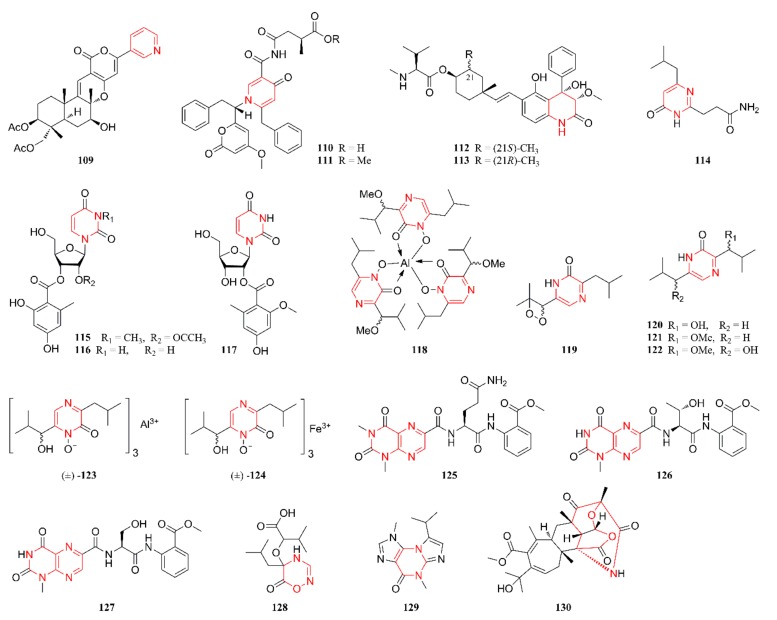
Other alkaloids isolated from marine-derived Aspergillus species (109–130).
2.1. Indole Alkaloids
Indole alkaloids serve as the active moiety in several clinical drugs, such as reserpine, and several well-known drugs, such as sumatriptan, tadalafil, fluvastatin, and rizatriptan, were designed on the basis of the indole framework [20]. The indole moiety is present in a wide range of marine natural products, especially fungal metabolites [8,21,22]. Figure 1 lists the structures of indole alkaloids produced by marine-derived Aspergillus species. Compound 1 was isolated from a culture broth of a gorgonian-originating fungal strain, A. sp. SCSIO 41501, and then characterized as a new linear peptide with three amino acid residues, d-Tyr, d-Val, and l-Trp [23]. Compound 2 was obtained from the coral-associated fungus A. terreus, whose structure featured an unusual (E)-oxime group, which is rare in natural products [24]. The structure of compound 3 was defined and characterized as a previously unreported bis-indolyl benzenoid and it was isolated from cultures of the marine sponge-derived fungus A. candidus KUFA0062 [25]. The C-3 position of the indole fragment in compounds 1–3 was substituted by methylene and phenyl groups, which were considered the same type of substituent. Chemical investigation of the algal-derived endophytic fungus A. alabamensis EN-547 led to the isolation of two new compounds, 4 and 5, possessing a rare diketomorpholine fragment [26]. The rice-based culture of a marine-associated fungal strain A. sp. MEXU 27854 was extensively chromatographed to produce five dioxomorpholine derivatives 6–10 [27]. The structure of compound 11, which was obtained from a marine sediment-derived Aspergillus sp. CMB-M081F, was identified and characterized as a dioxomorpholine derivative [28]. Compounds 12–15 were isolated from the fungal strain Aspergillus sp. from an unidentified colonial ascidian and then characterized as four new indole-diterpene alkaloids [29]. Compounds 16 and 17 were isolated and identified from the cultures of the endophytic fungus A. nidulans EN-330 that were collected from the marine red alga Polysiphonia scopulorum [30]. Interestingly, the structures of compounds 12 and 14–16 contain a halogen atom, which is rare in fungal secondary metabolites. Compound 18 represents the first example of an N-isopentenyl tryptophan methyl ester with a phenyl propanoic amide arm and it was identified from the marine sponge-derived fungus A. sp. SCSIO XWS03F03 [31]. Compound 19 was obtained through chromatographic separation of the crude organic extract of a sponge-associated fungal strain A. sp., whose structure was a tryptophan-derived indole alkaloid [32]. Compound 20 was characterized as a new polycyclic alkaloid, which was isolated by further chemical investigation of a coral-associated fungal strain A. versicolor LZD-14-1 [33]. Compounds 21 and 22 were identified and characterized as two new indole diterpenoids and they were isolated from the fermentation broth of the fungal strain A. flavus OUCMDZ-2205 [34]. Compounds 23–30 were reported as eight new cyclopiazonic acid (CPA)-type alkaloids, which are usually composed of three structural units: an indole, a tetramic acid unit, and a malonic acid unit. More precisely, compounds 23–29 were isolated from the culture of an epiphytic fungal strain of A. oryzae residing in marine sediments that were collected from Langqi Island, China, while compound 30 was obtained from the culture of a marine isopod-associated endophytic fungus, A. sp. Z-4, which was derived from the marine isopod Ligia oceanica [35,36,37]. Compound 31 was characterized as a new prenylated indole alkaloid, which was isolated from a coculture of the marine-derived fungi A. sulphureus KMM 4640 and Isaria felina KMM 4639 [38].
2.2. Diketopiperazine Alkaloids
Diketopiperazine alkaloids are common metabolites of microorganisms that are widely distributed in filamentous fungi, especially in the genera Aspergillus and Penicillium of the phylum Ascomycota or sac fungi [39]. Interestingly, an indole fragment is typically present in the structures of these diketopiperazine alkaloids. Figure 2 lists the structures of diketopiperazine alkaloids that are produced by marine-derived Aspergillus species. Compounds 32–35 were isolated from a coculture of the marine sediment-derived fungi A. sulphureus KMM 4640 and Isaria felina KMM 4639 and identified as four new prenylated indole diketopiperazine alkaloids [38]. Compounds 36–43 were characterized as eight linearly fused prenylated indole diketopiperazines featuring an unusual pyrano[3,2-f]indole unit, which were isolated from the culture of a fungal strain, A. versicolor, residing in mud from the South China Sea [40]. Compounds 44 and 45 were isolated from the Antarctic marine-derived A. sp. SF-5976 obtained from an unidentified marine organism that was collected in the Ross Sea [41]. Compounds 46–51 were identified as six new prenylated indole diketopiperazines and they were isolated from a culture of the marine sediment-derived fungus A. versicolor HDN08-60 [42]. These compounds are characterized by a 6/6/5/8/6/5 hexacyclic ring system that possesses a hydrogenated azocine unit. Compounds 52–56 were reported as four new bis-indole diketopiperazine alkaloids characterized by the presence of two indole diketopiperazines in their structures. More specifically, compounds 52 and 53 were isolated from an organic extract of the sponge-derived fungal strains A. sp. SF-5280 and A. violaceofuscus, respectively, while compounds 54–56 were from a culture of a fungus residing in marine shrimp that were collected along the coast of Dinghai, China [43,44,45]. Compounds 57 and 58 were also isolated from a culture of the marine sediment-derived fungus A. versicolor HDN08-60, and their structures featured the presence of only a diketopiperazine fragment, but no indole ring. Structurally, compound 57 possesses an unprecedented skeleton of a 2,5-dihydro-1H-azepino[4,3-b]quinoline system, while 58 contains a novel 6/6/11/6/5 pentacyclic ring system. Moreover, the former compound is considered to be a derivative of the latter [42].
2.3. Quinazoline Alkaloids
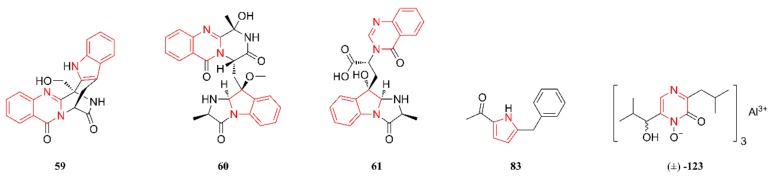
Figure 3 lists the structures of quinazoline alkaloids that are produced by marine-derived Aspergillus species. A quinazoline moiety was found in all these alkaloids, which might provide insights into the biogenetic relationships of quinazoline-containing indole alkaloids. Compounds 59–61 were characterized as two new quinazoline alkaloids and they were isolated from a culture of the deep-sea-derived fungus A. fumigatus SCSIO 41012 [46]. Compound 62 originated from a culture of the Australian marine sediment-derived A. sp. CMB-M081F, whose structure was elucidated by detailed spectroscopic analysis and biosynthetic considerations [28]. A solid-substrate culture of strain A. sp. F452 residing in submerged decaying wood was extensively chromatographed to produce five new quinazoline-containing alkaloids 63–67. Among them, compound 63 represents a new member of the fumiquinazoline class of alkaloids, which has been reported in a number of marine-derived Aspergillus, Acremonium, and Scopulariopsis fungal strains [47]. Compounds 68–72 were characterized as six new polycyclic alkaloids and they were isolated from a culture of a coral-associated fungus, A. versicolor LZD-14-1 [33].
2.4. Pyrrolidine Alkaloids
Figure 4 lists the structures of pyrrolidine alkaloids that are produced by marine-derived Aspergillus species. Bioactivity-guided chemical investigation of cultures of the marine sponge-associated fungal strain A. flocculosus 16D-1 facilitated the isolation of nine pyrrolidine alkaloids 73–81. The structures and configurations of these compounds were elucidated by detailed spectroscopic analysis, the modified Mosher’s method, and comparisons with literature data [48]. Compound 82 was isolated and characterized as a new hydroxypyrrolidine alkaloid from cultures of the marine sponge-associated fungus A. candidus KUFA 0062 [25]. Compound 83 was produced by a coculture of gorgonian-derived fungal strains of A. sclerotiorum and P. citrinum and characterized as a pyrrole analog [49]. Compounds 84–91 were identified and characterized as having a spiro-heterocyclic γ-lactam skeleton and they were isolated from a culture broth of the marine fish-associated endophytic fungi A. fumigatus [50]. Compounds 92–96 were characterized as four aspochalasin analogs and they were obtained from the intestines of the marine isopod Ligia oceanica, collected along the coast of Dinghai in Zhoushan, Zhejiang Province, China [51,52,53]. Aspochalasins are a small group of cytochalasans structurally featuring a macrocyclic ring system and perhydroisoindol-1-one unit with an isobutyl side chain. Among them, the structure of compound 92 includes a unique 5/6/6 tricyclic ring fused with the skeleton of aspochalasin [51]. Compounds 94 and 95 represent the first thiomethyl-substituted aspochalasin analogs [52]. Compound 96 is rare, in that it contains two nitrogen atoms in its molecular structure and an unusual skeleton that includes an azabicyclo moiety [53].
2.5. Cyclopeptide Alkaloids
Cyclopeptide alkaloids are mainly constructed from proteinogenic or nonproteinogenic amino acids that are joined together by amide bonds [54]. These alkaloids can be widely synthesized by both terrestrial and marine organisms. A diversity of cyclopeptide alkaloids with intriguing structures and possible pharmaceutical activities has been identified from marine fungi, a well-known producer [55]. To the best of our knowledge, twelve cyclic peptides (97–105) were published from 2014 to 2018. Figure 5 lists the structures of cyclopeptide alkaloids that are produced by marine-derived Aspergillus species. Compound 97 was isolated and characterized as a novel cyclic dipeptide with a skeleton of cyclo-(anthranilic acid-l-N-Me-Tyr) and it could also be considered a benzodiazepine alkaloid of the cyclopenin group [56]. Compounds 98 and 99 were isolated from the EtOAc extract of an endophytic fungal strain, A. violaceofuscus, residing in the interior of the marine sponge Reniochalina sp. collected from the Xisha Islands in the South China Sea. The structure of compound 98 was established as an aspochracin-type cyclic tripeptide, while that of 99 was elucidated as a cyclic tetrapeptide with the sequence cyclo-[l-Thr-l-O-Me-Tyr-l-N-Me-Ala-l-Ile] [44]. Compounds 100–104 were isolated from the fungal strain A. versicolor ZLN-60, which was obtained from marine sediment in the Yellow Sea in China. More precisely, the structures of compounds 100–103 were established as four cyclic peptides that possess a rare amide linkage between the carboxylic acid in anthranilic acid and the nitrogen in an indole moiety, while that of 104 was an anthranilic acid-containing hexapeptide [57,58]. Compound 105 was elucidated as a new cyclic tetrapeptide with a skeleton of cyclo[anthranilic acid-3(S)-OH-N-Me-Phe-d-Val-l-Ala] [59]. Compound 106 was isolated from a culture broth of the gorgonian-derived fungus A. terreus SCSGAF0162 and was characterized as a cyclic tetrapeptide with a skeleton of cyclo[l-Val-(N-Me-)-d-Tyr-(O-Me-)-l-Tyr-(O-Me-)-l-Tyr-l-Pro] [23]. Compound 107 was established as a new cyclohexapeptide with the sequence [cyclo (anthranilic acid-l-Val-d-Leu-l-Ala-N-methyl-l-Leu-d-pipecolic acid)] and it was isolated from the sponge-derived fungus A. similanensis KUFA 0013. Its amino acid sequence was the same as that of a previously reported compound (PF1171C), but the absolute configuration was different [60]. Compound 108 was isolated from the gorgonian-associated fungus A. versicolor TA01-14 that was collected from the South China Sea. Its structure was identified and characterized as a centrally symmetric cyclohexapeptide with a skeleton of cyclo [l-Phe-(anthranilic acid)-l-Pro-l-Phe-(anthranilic acid)-l-Pro] [61].
2.6. Other Heterocyclic Alkaloids
The remaining heterocyclic alkaloids (109–130) that were produced by marine-derived Aspergillus species are summarized in this section, and their structures are listed in Figure 6. Compounds 109–111 were identified and characterized as three new pyridine derivatives. In contrast, compound 109 was obtained from the EtOAc extract of cultures of the marine sponge-derived fungal strain A. similanensis KUFA 0013, while compounds 110 and 111 were identified from the cultures of the marine alga-derived fungus A. niger SCSIO Jcsw6F30 [60,62]. Compounds 112 and 113 were characterized as two new prenylated dihydroquinolone derivatives that were isolated from the mycelia of a gorgonian-derived Aspergillus fungus and they represent the first examples of prenylated dihydroquinolone derivatives containing an amino acid residue in the side chain [63]. Compounds 114 and 115 were isolated from the cultures of the marine sediment-derived epiphytic fungi A. versicolor and A. flavus KMM 4650, respectively, while compounds 116 and 117 were identified from the gorgonian-derived endophytic fungus A. versicolor [64,65,66]. Structurally, compounds 114–117 represent four pyrimidine derivatives, and compounds 115–117 are aromatic nucleosides. Compounds 118–122 were isolated from the fermentation broth of the marine coral-derived halotolerant A. ochraceus LCJ11-102 that was cultivated in nutrient-limited medium containing 10% NaI. Compounds 123 and 124 were isolated from a coculture of the marine gorgonian-derived P. citrinum SCSGAF 0052 and A. sclerotiorum [49,67]. These seven heterocyclic alkaloids were characterized as new pyrazinone alkaloids. The structures of compounds 125–127 were characterized as three open-chain peptides with an unusual skeleton of 1,3-dimethyllumazine-6-carboxylic acid, coupled to glutamine and anthranilic acid methyl ester [68,69]. In contrast, compound 125 was isolated from a culture of the mangrove-derived fungal strain A. sp. (33241) [68], while compounds 126 and 127 were obtained from cultures of the marine sediment-derived fungal strain A. terreus FA009 [69]. Compound 128 was identified as a novel oxadiazin derivative and it was also isolated from a coculture of the marine gorgonian-derived P. citrinum SCSGAF 0052 and A. sclerotiorum [49]. Compound 129 was isolated and identified from a static culture of the marine sediment-originated fungus A. sydowii SP-1 and possesses a 1H-imidazo[2,1-b]purin-4(5H)-one skeleton [70]. Compound 130 was elucidated as a novel hybrid polyketide-terpenoid with a unique skeleton of fused polycyclic fragments and it was isolated and identified from a crab collected from a Kueishantao hydrothermal vent in China [71].
3. Production Environment
Endophytic and epiphytic fungi have proven to be prolific sources of bioactive natural products with unique structures and potent pharmaceutical activity; such fungi harmoniously colonize the internal tissues of their hosts usually without causing obvious damage to the hosts [72,73]. Marine-derived fungi could be isolated from every possible marine habitat, such as marine sediments, marine invertebrates (sponges, corals, ascidians, and holothurians), and vertebrates (mainly fish), as well as marine plants (algae, driftwood, and mangrove plants) [74,75]. As shown in Table 1, a total of 44 heterocyclic alkaloids (11, 23–29, 31–43, 46–51, 57–62, 97, 100–104, 114, 115, 126, 127, and 129) originated from the fungi residing in marine sediments, which accounted for 33.8% of the 130 nitrogen-heterocyclic secondary metabolites. More precisely, the producing strains of 59–61, 97, and 114 originated from deep-sea sediment (deeper than 100 m) [46,56,64], while the other strains were collected from marine sediments at depths above 100 m or even tideland mud [28,35,36,40,42,57,65,69,70]. Unfortunately, the source of the producing strain of 31–35 (A. sulphureus KMM 4640 and I. felina KMM 4639) was not described [39]. To the best of our knowledge, an overwhelming majority of these fungal strains were isolated from marine invertebrates, including corals, sponges, crabs, shrimps, ascidians, and some isopods. For instance, a total of 24 heterocyclic alkaloids (1, 2, 20, 68–72, 83, 105, 106, 108, 112, 113, 116–124, and 128) were identified from the endophytic fungal strains of marine gorgonian species, including Melitodes squamata [23], Sarcophyton subviride [24], Pseudopterogorgia sp. LZD-14 [33], Muricella flexuosa [49], Echinogorgia aurantiaca [59], Carijoa sp. GX-WZ-2010001 [61], Muricella abnormaliz [63], and Dichotella gemmacea [66,67], and they accounted for 18.5% of the newly reported heterocyclic alkaloids. A total of 19 heterocyclic alkaloids (3, 18, 19, 52, 53, 73–82, 98, 99, 107, and 109) were identified from the fungi residing in marine sponges, including Epipolasis sp. [25], Tethya aurantium [32], Reniochalina sp. [44], Phakellia fusca [48], Rhabdermia sp. [60], and an unidentified species [31,43], and they accounted for 14.6% of the newly reported heterocyclic alkaloids. Six heterocyclic alkaloids (30 and 92–96) were also isolated from the endophytic fungi (A. sp. Z-4) residing in the marine isopod Ligia oceanica [37,51,52,53], while only one heterocyclic alkaloid (130) was identified from the endophytic fungi obtained from the marine crab Xenograpsus testudinatus [71]. Moreover, the producing strains of 21, 22, and 54–56 originated from marine shrimp or prawns [34,45], while those of compounds 12–15 were derived from an unidentified colonial ascidian that was collected at Shikotan Island in the Pacific Ocean [29]. In addition to these producing strains of marine invertebrate origin, only one fungal strain, which produced 84–91, was obtained from the marine fish Mugil cephalus, representing vertebrates [50]. It appears that the number of the fungal strains of marine plant origin that produce heterocyclic alkaloids is much less than that of marine invertebrate and vertebrate origin. For example, the producing strains of the heterocyclic alkaloids 4, 5, 16, 17, 110, and 111 originated from the fungi residing in marine algal species, including Ceramium japonicum [26], Polysiphonia scopulorum [30], and Sargassum sp. [58,62], while those of 63–67 were derived from submerged decaying wood at Jeju Island, Korea [47]. Moreover, the producing strain of 125 originated from the mangrove Bruguiera sexangula var. rhynchopetala that was collected in the South China Sea [68]. Interestingly, two heterocyclic alkaloids (44 and 45) were isolated from the fungi residing in an unidentified marine organism that was collected in the Ross Sea without any detailed description for species identification [41].
Table 1.
The producing strain, biological activities of these heterocyclic alkaloids (1–130).
| NO. | Producing Strain | Environmental Source | Biological Activities | Ref. |
|---|---|---|---|---|
| 1 | A. sp. SCSIO 41501 | the gorgonian Melitodes squamata collected from the South China Sea, Sanya, China | moderate antiviral activity against HSV-1 under non-cytotoxic concentrations against Vero cells | [23] |
| 2 | A. terreus | the coral Sarcophyton subviride collected from the coast of Xisha Island in the South China Sea | potent inhibition on LPS-induced NO production; nonsignificant inhibition on α-Glucosidase | [24] |
| 3 | A. candidus KUFA0062 | the marine sponge Epipolasis sp. collected at Similan Island National Park (15–20 m), Thailand | weak cytotoxic activity against eight cell lines; nonsignificant antibacterial activity | [25] |
| 4 | A. alabamensis EN-547 | the fresh inner tissue of marine alga Ceramium japonicum collected at Qingdao, China | moderate antimicrobial activities against E. coli, M. luteus, Ed. ictaluri and V. alginolyticus | [26] |
| 5 | A. alabamensis EN-547 | the fresh inner tissue of marine alga Ceramium japonicum collected at Qingdao, China | moderate antimicrobial activities against E. coli, M. luteus, Ed. ictaluri and V. alginolyticus | [26] |
| 6 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | nonsignificant cytotoxic activities | [27] |
| 7 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | no biological activity was tested | [27] |
| 8 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | nonsignificant cytotoxic activities | [27] |
| 9 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | no biological activity was tested | [27] |
| 10 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | no biological activity was tested | [27] |
| 11 | A. sp. CMB-M081F | the marine sediment collected at an intertidal depth of 1 m near Shorncliffe, Queensland, Australia | nonsignificant cytotoxic activities; potent inhibition on P-glycoprotein-mediated drug efflux | [28] |
| 12 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | potent cytotoxicity against 22Rv1, while moderate cytotoxicity against PC-3 and LNCaP | [29] |
| 13 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | no biological activity was tested | [29] |
| 14 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | nonsignificant cytotoxic activities against PC-3, LNCaP and 22Rv1 cell lines | [29] |
| 15 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | no biological activity was tested | [29] |
| 16 | A. nidulans EN-330 | The marine red alga P. scopulorum var. villum collected from Yantai coastline of north China | moderate antimicrobial activities against four human- and aqua-pathogens | [30] |
| 17 | A. nidulans EN-330 | The marine red alga P. scopulorum var. villum collected from Yantai coastline of north China | weak antimicrobial activities against four human- and aqua-pathogens | [30] |
| 18 | A. sp. SCSIO XWS03F03 | a sponge collected from the sea area Xuwen County, Guangdong, China | potent cytotoxic activity against HL-60 and LNCap cell lines | [31] |
| 19 | A. sp. | the sponge Tethya aurantium (Pallas 1766) collected at the entrance of Limski kanal (a depth of 20 m) | moderate selective activity against marine bacteria; nonsignificant cytotoxicity against L5178Y cells | [32] |
| 20 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; potent inhibitory activity against TrxR | [33] |
| 21 | A. flavus OUCMDZ-2205 | the prawn Penaeus vannamei collected in Lianyungang sea area, Jiangsu Province of China | moderate antibacterial and cytotoxic activities, as well as PKC-beta inhibition | [34] |
| 22 | A. flavus OUCMDZ-2205 | the prawn Penaeus vannamei collected in Lianyungang sea area, Jiangsu Province of China | moderate cytotoxicity; nonsignificant antibacterial activity and PKC-beta inhibition | [34] |
| 23 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | weak cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 24 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela, HL-60, and K562 cell lines | [36] |
| 25 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 26 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 27 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | weak cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 28 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela, HL-60, and K562 cell lines | [36] |
| 29 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela, HL-60, and K562 cell lines | [36] |
| 30 | A. sp Z-4 | the marine isopod Ligia oceanica collected in Zhoushan, Zhejiang, China | nonsignificant cytotoxicity against PC3 and HCT116 | [37] |
| 31 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 32 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 33 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 34 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 35 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 36 | A. versicolor | the mud of the South China Sea | moderate inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 37 | A. versicolor | the mud of the South China Sea | moderate inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 38 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 39 | A. versicolor | the mud of the South China Sea | moderate inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 40 | A. versicolor | the mud of the South China Sea | potent inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 41 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 42 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 43 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 44 | A. sp. SF-5976 | an unidentified marine organism collected in the Ross Sea | weak inhibitory activities against LPS-induced NO production in RAW 264.7 and BV2 cells | [41] |
| 45 | A. sp. SF-5976 | an unidentified marine organism collected in the Ross Sea | moderate inhibitory activities against LPS-induced NO production in RAW 264.7 and BV2 cells | [41] |
| 46 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 47 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 48 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 49 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 50 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 51 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 52 | A. sp. SF-5280 | an unidentified sponge collected at Cheju Island, Korea | moderate inhibitory effects against PTP1B activity | [43] |
| 53 | A. violaceofuscus | the inner part of marine sponge Reniochalina sp. collected from Xisha Islands in South China Sea | potent anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells | [44] |
| 54 | A. sp. DX4H | marine shrimp collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activities against PC3 cell line | [45] |
| 55 | A. sp. DX4H | marine shrimp collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activities against PC3 cell line | [45] |
| 56 | A. sp. DX4H | marine shrimp collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activities against PC3 cell line | [45] |
| 57 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | moderate cytotoxic activities and selective PTK inhibitory activities | [42] |
| 58 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HL-60, and K562 cell lines | [42] |
| 59 | A. fumigatus SCSIO 41012 | the deep-sea sediments (3614 m) collected from the Indian Ocean | potent antifungal and antibacterial activities | [46] |
| 60 | A. fumigatus SCSIO 41012 | the deep-sea sediments (3614 m) collected from the Indian Ocean | potent antibacterial activities | [46] |
| 61 | A. fumigatus SCSIO 41012 | the deep-sea sediments (3614 m) collected from the Indian Ocean | potent antibacterial activities | [46] |
| 62 | A. sp. CMB-M081F | the marine sediment collected at an intertidal depth of 1 m near Shorncliffe, Queensland, Australia | nonsignificant cytotoxic activities and inhibition on P-glycoprotein-mediated drug efflux | [28] |
| 63 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 64 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 65 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 66 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 67 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 68 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 69 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; potent inhibitory activity against TrxR | [33] |
| 70 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 71 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 72 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 73 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 74 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 75 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 76 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 77 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | potent inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 78 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 79 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | potent inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 80 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | potent inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 81 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 82 | A. candidus KUFA0062 | the marine sponge Epipolasis sp. collected at Similan Island National Park (15–20 m), Thailand | weak cytotoxic activity against eight cell lines; nonsignificant antibacterial activity | [25] |
| 83 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | moderate brine shrimp lethality; nonsignificant antibacterial and anti-biofilm activities | [49] |
| 84 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 85 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 86 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 87 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 88 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 89 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 90 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 91 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 92 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activity against PC3 cell line | [51] |
| 93 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activity against PC3 cell line | [51] |
| 94 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai, Zhejiang Province of China | moderate cytotoxic activities against PC3 and HCT116 cell lines | [52] |
| 95 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai, Zhejiang Province of China | no biological activity was tested | [52] |
| 96 | A. sp. Z-4 | the intestinal of the marine isopod Ligia oceanica | weak cytotoxic activities against PC3 cell line | [53] |
| 97 | A. sp. SCSIOW2 | the deep marine sediment (2439 m) collected in the South China Sea | weak inhibitory activity on NO production induced by lipopolysaccharide (LPS)/INF-γ | [56] |
| 98 | A. violaceofuscus | the inner part of marine sponge Reniochalina sp. collected from Xisha Islands in South China Sea | nonsignificant anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells | [44] |
| 99 | A. violaceofuscus | the inner part of marine sponge Reniochalina sp. collected from Xisha Islands in South China Sea | potent anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells | [44] |
| 100 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| A. sp. BM-05 and BM-05ML | a brown algal species belonging to the genus Sargassum collected off Helgoland, North Sea, Germany | moderate cytotoxicities against K562, HCT116, A2780, and A2780CisR cell lines | [58] | |
| 101 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| 102 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | potent lipid-lowering effect; nonsignificant cytotoxic activities | [57] |
| 103 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| 104 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| 105 | A. terreus SCSGAF0162 | the gorgonian coral Echinogorgia aurantiaca in the South China Sea | nonsignificant antifouling activity towards larvae of the barnacle B. amphitrite | [59] |
| 106 | A. sp. SCSIO 41501 | the gorgonian Melitodes squamata collected from the South China Sea, Sanya, China | moderate antiviral activity against HSV-1 under non-cytotoxic concentrations against Vero cells | [23] |
| 107 | A. similanensis KUFA 0013 | the marine sponge Rhabdermia sp. collected in coral reef of Similan Islands, Phang Nga, Thailand | nonsignificant cytotoxic and antibacterial activities | [60] |
| 108 | A. versicolor TA01-14 | a gorgonian Carijoa sp. GX-WZ-2010001 collected in Weizhou coral reefs in the South China Sea | weak cytotoxic activity; nonsignificant brine shrimp lethality, antibacterial and antiviral activities, as well as AChE, Top I, and α-glucosacharase inhibition | [61] |
| 109 | A. similanensis KUFA 0013 | the marine sponge Rhabdermia sp. collected in coral reef of Similan Islands, Phang Nga, Thailand | weak cytotoxicity; nonsignificant antibacterial activities against four reference strains | [60] |
| 110 | A. niger SCSIO Jcsw6F30 | a marine alga Sargassum sp. collected in Yongxing Island, South China Sea | potent cytotoxic activity against TZM-bl cells; moderate anti-HIV-1 activity against HIV-1 SF162 | [62] |
| 111 | A. niger SCSIO Jcsw6F30 | a marine alga Sargassum sp. collected in Yongxing Island, South China Sea | no biological activity was tested | [62] |
| 112 | A. sp. XS20090B15 | the Muricella abnormaliz gorgonian collected from the Xisha Islands coral reef in South China Sea | nonsignificant antiviral activity against RSV virus-induced cytopathogenicity in Hep-2 cells | [63] |
| 113 | A. sp. XS20090B15 | the Muricella abnormaliz gorgonian collected from the Xisha Islands coral reef in South China Sea | potent antiviral activity against RSV virus-induced cytopathogenicity in Hep-2 cells | [63] |
| 114 | A. versicolor A-21-2-7 | the deep-sea sediment (3002 m) in South China Sea | no biological activity was tested | [64] |
| 115 | A. flavus KMM 4650 | Sakhalin Bay marine sediments (32 m, Sea of Okhotsk) | nonsignificant antimicrobial activity | [65] |
| 116 | A. versicolor | the inner part of gorgonian D. gemmacea collected from the Xisha Islands coral reef of the South China Sea | moderate antibacterial activities and brine shrimp lethality; nonsignificant cytotoxicities | [66] |
| 117 | A. versicolor | the inner part of gorgonian D. gemmacea collected from the Xisha Islands coral reef of the South China Sea | moderate antibacterial activities and brine shrimp lethality; nonsignificant cytotoxicities | [66] |
| 118 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | moderate antimicrobial activity against E. aerogenes; nonsignificant cytotoxic activities | [67] |
| 119 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | nonsignificant antimicrobial and cytotoxic activities | [67] |
| 120 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | nonsignificant antimicrobial and cytotoxic activities | [67] |
| 121 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | moderate antimicrobial activity against E. aerogenes; nonsignificant cytotoxic activities | [67] |
| 122 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | nonsignificant antimicrobial and cytotoxic activities | [67] |
| 123 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | potent brine shrimp lethality and cytotoxic activities; nonsignificant antibacterial and anti-biofilm activities | [49] |
| 124 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | moderate brine shrimp lethality and cytotoxic activities; nonsignificant antibacterial and anti-biofilm activities | [49] |
| 125 | A. sp. (33241) | the mangrove Bruguiera sexangula var. rhynchopetala collected in the South China Sea | nonsignificant antibacterial and cytotoxic activities | [68] |
| 126 | A. terreus FA009 | the marine sediment collected in Jeju Island, Korea | moderate enhancement effect on insulin sensitivity | [69] |
| 127 | A. terreus FA009 | the marine sediment collected in Jeju Island, Korea | moderate enhancement effect on insulin sensitivity | [69] |
| 128 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | weak brine shrimp lethality; nonsignificant cytotoxic, antibacterial and anti-biofilm activities | [49] |
| 129 | A. sydowii SP-1 | the marine sediment sample collected from site in the Antarctic Great Wall Station | weak antimicrobial activities against MRSA and MRSE | [70] |
| 130 | A. sp. WU 243 | the crab Xenograpsus testudinatus collected from a Kueishantao hydrothermal vent, Taiwan, China | no biological activity was tested | [71] |
4. Biological Activities
The biological activities of these heterocyclic alkaloids are detailed in Table 1. Anticancer and antimicrobial activities, as well as anti-inflammatory activity, were the three main indexes that were used to assess the pharmacological activity of these natural heterocyclic alkaloids. In this section, alkaloids with potent biological activities are the focus, and detailed descriptions are provided below.
4.1. Anticancer Activities
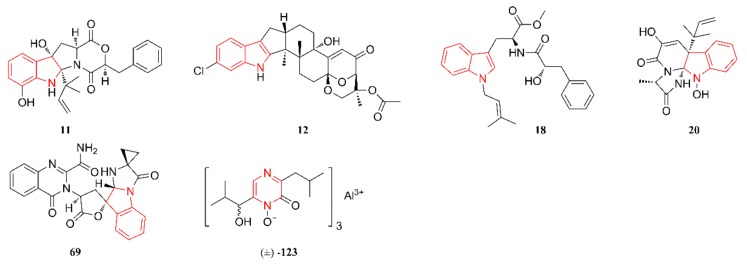
Figure 7 lists the anticancer heterocyclic alkaloids. Compound 11 at 20 μM was demonstrated to be a noncytotoxic inhibitor of P-glycoprotein-mediated drug efflux in multidrug-resistant (MDR) human colon cancer cells and it might be used to improve the prognosis for MDR cancer chemotherapy [28]. Compound 12 showed cytotoxicity against human PC-3, LNCaP, and 22Rv1 cells, with IC50 values of 69.4 µM, 47.8 µM, and 4.86 µM, respectively. The reference substance (Docetaxel) displayed IC50 values of 15.4 nM, 3.8 nM, and 12.7 nM, respectively. This compound was able to induce the apoptosis of 22Rv1 cells at low micromolar concentrations. Cell cycle progression analyses of 22Rv1 cells that were treated with 12 also revealed discrete G2/M-phase arrest [29]. Compound 18 exhibited potent cytotoxic activity against HL-60 and LNCap cells with IC50 values of 3.1 and 4.9 µM, respectively, but with no significant cytotoxicity against the rest of the tested cell lines (HepG2, Hela, A375, A549, HT29, SK-BR-3, and MCF-7) [31]. Compounds 20 and 69 exhibited significant inhibitory activities against thioredoxin reductase with IC50 values of 12.2 ± 0.7 and 13.6 ± 0.6 µM (the IC50 of the positive control curcumin was 25 µM), but weak toxicity against A549 cells (IC50 > 10 µM), which suggested that these two compounds might act as microenvironmental regulators of tumor progression and metastasis [33]. Compound 123 possesses selective cytotoxicity against U937 cells with an IC50 value of 4.2 μM and mild cytotoxicity against HeLa and MCF-7 cells, with IC50 values of 29.3 μM and 24.8 μM, respectively. The IC50 values of doxorubicin (positive control) towards U937, HeLa, and MCF-7 cells were 0.06 μM, 0.8 μM, and 23.1 μM, respectively [49].
Figure 7.
Anticancer heterocyclic alkaloids produced by marine-derived Aspergillus species.
4.2. Antimicrobial Activities
Figure 8 lists the antimicrobial heterocyclic alkaloids. Compounds 59 and 61 were tested for their antimicrobial activities. Compound 59 showed comparable or even higher antibacterial activity than the other tested compounds. This compound also showed excellent antifungal activity against F. oxysporum with an MIC of 1.5 µg/mL. Compound 60 exhibited high activity against S. aureus (16339 and 29213), with MIC values of 1.565 µg/mL and 0.78 µg/mL, respectively, while compound 61 exhibited significant activity against A. baumanii ATCC 19606 with an MIC of 6.25 µg/mL [46]. Although compounds 83 and 123 showed non-significant antimicrobial activity against two common bacterial strains (S. aureus and P. aeruginosa) and three marine-derived bacteria (P. nigrifaciens, B. amyloliquefaciens, and B. stearothermophilus), they increased the growth of S. aureus one-fold at 100 μg/mL, and 123 increased the biofilm formation of S. aureus 1.3-fold at 25 μg/mL [49].
Figure 8.
Antimicrobial heterocyclic alkaloids that are produced by marine-derived Aspergillus species.
4.3. Anti-Inflammatory Activities
Figure 9 lists the anti-inflammatory heterocyclic alkaloids. Compound 2 showed potent anti-inflammatory activity against NO production with an IC50 of 24.64 μM [24]. Compound 40 exhibited an excellent inhibition of iNOS with an IC50 of 5.39 μM, but weak activity against Raw 264.7 cells. The inhibitory effects might be the result of cell viability independent of concentration. Molecular docking studies with 40 and iNOS showed that it could adopt an extended conformation and fit well into the ligand binding site of mutant iNOS [40]. Compounds 53 and 99 were evaluated for their inhibitory activities against the production of cytokines in the serum of THP-1 by using the human inflammation cytometric bead array assay. The THP-1 cells that were pretreated with 53 and 99 showed a significant decrease in the LPS-induced expression of IL-10, with inhibitory rates of 78.1% and 84.3% (p < 0.01), respectively. Moreover, these two compounds did not show cytotoxicity against THP-1 cells after 24 h of treatment [44]. Compounds 77, 79, and 80 showed potent inhibitory activity against IL-6 production, with IC50 values of 0.11 μM, 0.19 μM, and 2.3 μM, respectively [48].
Figure 9.
Anti-inflammatory heterocyclic alkaloids produced by marine-derived Aspergillus species.
4.4. Other Biological Activities
Figure 10 lists other bioactive alkaloids. Compound 102 was found to possess potent lipid-lowering effects, but non-significant cytotoxicity [57]. Compound 110 exhibited significant HIV-1 inhibitory activities against SF162 infection in TZM-bl cells, with IC50 and CC50 values of 4.7 ± 0.4 and 35.0 ± 2.1 μM (selectivity index of 7.5), respectively, which might be beneficial for the development of heterocyclic alkaloids as anti-HIV agents [62]. Compound 112 showed strong toxicity towards brine shrimp with an LC50 value of 6.1 μM as compared with the positive control toosendanin (LC50 = 1.73 μM). Compound 113 possessed outstanding anti-RSV activity with an IC50 value of 42 nM, being approximately 500-fold stronger than that of the positive control ribavirin (IC50 = 20 μM), as well as a higher therapeutic ratio (TC50/IC50 = 520) [63]. Compound 123 also showed strong toxicity, with an LC50 value of 6.1 μM as compared with the positive control toosendanin (LC50 = 1.73 μM) [49].
Figure 10.
Other bioactive heterocyclic alkaloids produced by marine-derived Aspergillus species.
5. Conclusions
This review summarized the findings, including the biological activities, on a total of 130 nitrogen-containing secondary metabolites that originate from marine-derived Aspergillus species reported from the beginning of 2014 through the end of 2018. All of the original literature in the Web of Science database, which we believe covers most of the newly reported naturally occurring heterocyclic alkaloids from specific sources, was searched. However, several works may not have been retrieved by the literature method used in this review. In the process of preparing this review, compound 23 was reported as speradine B and it was shown to possess an identical planar structure to that of speradine G (24), which was not explained in the original articles [35,36]. A careful comparison of the one-dimensional NMR data makes us boldly propose that these two compounds are a pair of diastereoisomers. Further, it is quite interesting that psychrophilin E (100) was reported as a new compound by two completely independent research teams in the same year [57,58]. Therefore, for natural product chemists, the research results should be published in a timely manner. At the end of this review, compounds with potent bioactivities were comprehensively described, which will be beneficial in future drug development and innovation.
Acknowledgments
All of the authors would like to thank American Journal Experts (AJE) for their professional language editing.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/1/54/s1, the structures of all 398 new metabolites produced by marine-derived Aspergillus species from 2014 to 2018, as well as references.
Author Contributions
Conceptualization, K.X. and X.-D.L.; writing—Original draft preparation, K.X.; writing—Review and editing, X.-L.Y. and C.L.; funding acquisition, K.X. and X.-D.L. All authors have read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81803375), the Agricultural Science and Technology Innovation Program (No. ASTIP-TRIC05), the Key Research and Development Program of Shandong Province (No. 2019GSF107091), the Fundamental Research Funds for the Central Non-Profit Scientific Institution (No. 1610232019006), the National Postdoctoral Program for Innovative Talents (No. BX201700247), and the China Postdoctoral Science Foundation (No. 2018M630804).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rida P.C., LiVecche D., Ogden A., Zhou J., Aneja R. The noscapine chronicle: A pharmaco-historic biography of the opiate alkaloid family and its clinical applications. Med. Res. Rev. 2015;35:1072–1096. doi: 10.1002/med.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirillo A., Catapano A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449–461. doi: 10.1016/j.atherosclerosis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Ferraz C.A.A., de Oliveira Júnior R.G., Picot L., da Silva Almeida J.R.G., Nunes X.P. Pre-clinical investigations of β-carboline alkaloids as antidepressant agents: A systematic review. Fitoterapia. 2019;137:104196. doi: 10.1016/j.fitote.2019.104196. [DOI] [PubMed] [Google Scholar]
- 4.Wang B.G., Gloer J.B., Ji N.Y., Zhao J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013;113:3632–3685. doi: 10.1021/cr9002215. [DOI] [PubMed] [Google Scholar]
- 5.Gogineni V., Schinazi R.F., Hamann M.T. Role of marine natural products in the genesis of antiviral agents. Chem. Rev. 2015;115:9655–9706. doi: 10.1021/cr4006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bideau F.L., Kousara M., Chen L., Wei L., Dumas F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017;117:6110–6159. doi: 10.1021/acs.chemrev.6b00502. [DOI] [PubMed] [Google Scholar]
- 7.Soldatou S., Baker B.J. Cold-water marine natural products, 2006 to 2016. Nat. Prod. Rep. 2017;34:585–626. doi: 10.1039/C6NP00127K. [DOI] [PubMed] [Google Scholar]
- 8.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2019;36:122–173. doi: 10.1039/C8NP00092A. [DOI] [PubMed] [Google Scholar]
- 9.Guo C.J., Wang C.C.C. Recent advances in genome mining of secondary metabolites in Aspergillus terreus. Front. Microbiol. 2014;5:717. doi: 10.3389/fmicb.2014.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaegashi J., Oakley B.R., Wang C.C.C. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2014;41:433–442. doi: 10.1007/s10295-013-1386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anyaogu D.C., Mortensen U.H. Heterologous production of fungal secondary metabolites in Aspergilli. Front. Microbiol. 2015;6:77. doi: 10.3389/fmicb.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K.W., Ding P. New bioactive metabolites from the marine-derived fungi Aspergillus. Mini-Rev. Med. Chem. 2018;18:1072–1094. doi: 10.2174/1389557518666180305160856. [DOI] [PubMed] [Google Scholar]
- 13.Frisvad J.C., Moller L.L.H., Larsen T.O., Kumar R., Arnau J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reese. Appl. Microbiol. Biotechnol. 2018;102:9481–9515. doi: 10.1007/s00253-018-9354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y., Wang B., Chen W.P., Cox R.J., He J.R., Chen F.S. Recent advances in reconstructing microbial secondary metabolites biosynthesis in Aspergillus spp. Biotechnol. Adv. 2018;36:739–783. doi: 10.1016/j.biotechadv.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Salvatore M.M., Nicoletti R., Salvatore F., Naviglio D., Andolfi A. GC-MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Mar. Chem. 2018;206:19–33. doi: 10.1016/j.marchem.2018.08.003. [DOI] [Google Scholar]
- 16.Romsdahl J., Wang C.C.C. Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018) Medchemcomm. 2019;10:840–866. doi: 10.1039/C9MD00054B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K., Guo C., Meng J., Tian H., Guo S., Shi D. Discovery of natural dimeric naphthopyrones as potential cytotoxic agents through ROS-mediated apoptotic pathway. Mar. Drugs. 2019;17:207. doi: 10.3390/md17040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soosaraei M., Khasseh A.A., Fakhar M., Hezarjaribi H.Z. A decade bibliometric analysis of global research on leishmaniasis in Web of Science database. Ann. Med. Surg. (Lond.) 2018;26:30–37. doi: 10.1016/j.amsu.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang H.T., Lin M.H., Hwang I.H., Chen T.J., Lin H.C., Hou M.C., Hwang S.J. Scientific publications in gastroenterology and hepatology in Taiwan: An analysis of Web of Science from 1993 to 2013. J. Chin. Med. Assoc. 2017;80:80–85. doi: 10.1016/j.jcma.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Singh T.P., Singh O.M. Recent progress in biological activities of indole and indole alkaloids. Mini-Rev. Med. Chem. 2018;18:9–25. doi: 10.2174/1389557517666170807123201. [DOI] [PubMed] [Google Scholar]
- 21.Li S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010;27:57–78. doi: 10.1039/B909987P. [DOI] [PubMed] [Google Scholar]
- 22.Netz N., Opatz T. Marine indole alkaloids. Mar. Drugs. 2015;13:4814–4914. doi: 10.3390/md13084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X., Nong X.H., Ren Z., Wang J., Liang X., Wang L., Qi S.H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017;58:1151–1155. doi: 10.1016/j.tetlet.2017.02.005. [DOI] [Google Scholar]
- 24.Liu M.T., Sun W.G., Wang J.P., He Y., Zhang J.W., Li F.L., Qi C.X., Zhu H.C., Xue Y.B., Hu Z.X., et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018;80:525–530. doi: 10.1016/j.bioorg.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Buttachon S., Ramos A.A., Inacio A., Dethoup T., Gales L., Lee M., Costa P.M., Silva A.M.S., Sekeroglu N., Rocha E., et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs. 2018;16:119. doi: 10.3390/md16040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S.Q., Li X.M., Li X., Chi L.P., Wang B.G. Two new diketomorpholine derivatives and a new highly conjugated ergostane-type steroid from the marine algal-derived endophytic fungus Aspergillus alabamensis EN-547. Mar. Drugs. 2018;16:114. doi: 10.3390/md16040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aparicio-Cuevas M.A., Rivero-Cruz I., Sanchez-Castellanos M., Menendez D., Raja H.A., Joseph-Nathan P., Gonzalez M.D., Figueroa M. Dioxomorpholines and derivatives from a marine-facultative Aspergillus species. J. Nat. Prod. 2017;80:2311–2318. doi: 10.1021/acs.jnatprod.7b00331. [DOI] [PubMed] [Google Scholar]
- 28.Khalil Z.G., Huang X.C., Raju R., Piggott A.M., Capon R.J. Shornephine A: Structure, chemical stability, and P-glycoprotein inhibitory properties of a rare diketomorpholine from an Australian marine-derived Aspergillus sp. J. Org. Chem. 2014;79:8700–8705. doi: 10.1021/jo501501z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanets E.V., Yurchenko A.N., Smetanina O.F., Rasin A.B., Zhuravleva O.I., Pivkin M.V., Popov R.S., von Amsberg G., Afiyatullov S.S., Dyshlovoy S.A. Asperindoles A-D and a p-terphenyl derivative from the ascidian-derived fungus Aspergillus sp. KMM 4676. Mar. Drugs. 2018;16:232. doi: 10.3390/md16070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P., Li X.M., Li X., Wang B.G. New indole-diterpenoids from the algal-associated fungus Aspergillus nidulans. Phytochem. Lett. 2015;12:182–185. doi: 10.1016/j.phytol.2015.03.017. [DOI] [Google Scholar]
- 31.Zhou R., Liao X.J., Li H.B., Li J., Peng P.J., Zhao B.X., Xu S.H. Isolation and synthesis of misszrtine A: A novel indole alkaloid from marine sponge-associated Aspergillus sp. SCSIO XWS03F03. Front. Chem. 2018;6:212. doi: 10.3389/fchem.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y.M., Debbab A., Wray V., Lin W.H., Schulz B., Trepos R., Pile C., Hellio C., Proksch P., Aly A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014;55:2789–2792. doi: 10.1016/j.tetlet.2014.02.062. [DOI] [Google Scholar]
- 33.Cheng Z.B., Liu D., Cheng W., Proksch P., Lin W.H. Versiquinazolines L-Q, new polycyclic alkaloids from the marine-derived fungus Aspergillus versicolor. RSC Adv. 2018;8:31427–31439. doi: 10.1039/C8RA06854B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun K.L., Li Y., Guo L., Wang Y., Liu P.P., Zhu W.M. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X., Xia Q.W., Zhao Y.Y., Zheng Q.H., Liu Q.Y., Chen L., Zhang Q.Q. Speradines B–E, four novel tetracyclic oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Heterocycles. 2014;89:1662–1669. doi: 10.1248/cpb.c14-00312. [DOI] [PubMed] [Google Scholar]
- 36.Hu X., Xia Q.W., Zhao Y.Y., Zheng Q.H., Liu Q.Y., Chen L., Zhang Q.Q. Speradines F–H, three new oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Chem. Pharm. Bull. 2014;62:942–946. doi: 10.1248/cpb.c14-00312. [DOI] [PubMed] [Google Scholar]
- 37.Wang P.M., Zhao S.Z., Liu Y., Ding W.J., Qiu F., Xu J.Z. Asperginine, an unprecedented alkaloid from the marine-derived fungus Aspergillus sp. Nat. Prod. Commun. 2015;10:1363–1364. doi: 10.1177/1934578X1501000812. [DOI] [PubMed] [Google Scholar]
- 38.Afiyatullov S.S., Zhuravleva O.I., Antonov A.S., Berdyshev D.V., Pivkin M.V., Denisenko V.A., Popov R.S., Gerasimenko A.V., von Amsberg G., Dyshlovoy S.A., et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018;71:846–853. doi: 10.1038/s41429-018-0072-9. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y.M., Liang X.A., Kong Y., Jia B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016;64:6659–6671. doi: 10.1021/acs.jafc.6b01772. [DOI] [PubMed] [Google Scholar]
- 40.Li H.Q., Sun W.G., Deng M.Y., Zhou Q., Wang J.P., Liu J.J., Chen C.M., Qi C.X., Luo Z.W., Xue Y.B., et al. Asperversiamides, linearly fused prenylated indole alkaloids from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2018;83:8483–8492. doi: 10.1021/acs.joc.8b01087. [DOI] [PubMed] [Google Scholar]
- 41.Kwon J., Lee H., Ko W., Kim D.C., Kim K.W., Kwon H.C., Guo Y.Q., Sohn J.H., Yim J.H., Kim Y.C., et al. Chemical constituents isolated from Antarctic marine-derived Aspergillus sp. SF-5976 and their anti-inflammatory effects in LPS-stimulated RAW 264.7 and BV2 cell. Tetrahedron. 2017;73:3905–3912. doi: 10.1016/j.tet.2017.05.060. [DOI] [Google Scholar]
- 42.Peng J.X., Gao H.Q., Li J., Ai J., Geng M.Y., Zhang G.J., Zhu T.J., Gu Q.Q., Li D.H. Prenylated indole diketopiperazines from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2014;79:7895–7904. doi: 10.1021/jo5010179. [DOI] [PubMed] [Google Scholar]
- 43.Cho K.H., Sohn J.H., Oh H. Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat. Prod. Res. 2018;32:214–221. doi: 10.1080/14786419.2017.1346642. [DOI] [PubMed] [Google Scholar]
- 44.Liu J.T., Gu B.B., Yang L.J., Yang F., Lin H.W. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018;6:226. doi: 10.3389/fchem.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J.Z., Hu Q., Ding W.J., Wang P.M., Di Y.N. New asymmetrical bispyrrolidinoindoline diketopiperazines from the marine fungus Aspergillus sp. DX4H. Nat. Prod. Res. 2018;32:815–820. doi: 10.1080/14786419.2017.1363752. [DOI] [PubMed] [Google Scholar]
- 46.Limbadri S., Luo X.W., Lin X.P., Liao S.R., Wang J.F., Zhou X.F., Yang B., Liu Y.H. Bioactive novel indole alkaloids and steroids from deep sea-derived fungus Aspergillus fumigatus SCSIO 41012. Molecules. 2018;23:2379. doi: 10.3390/molecules23092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao L., You M., Chung B.K., Oh D.C., Oh K.B., Shin J. Alkaloidal metabolites from a marine-derived Aspergillus sp. fungus. J. Nat. Prod. 2015;78:349–354. doi: 10.1021/np500683u. [DOI] [PubMed] [Google Scholar]
- 48.Gu B.B., Jiao F.R., Wu W., Jiao W.H., Li L., Sun F., Wang S.P., Yang F., Lin H.W. Preussins with inhibition of IL-6 expression from Aspergillus flocculosus 16D-1, a fungus isolated from the marine sponge Phakellia fusca. J. Nat. Prod. 2018;81:2275–2281. doi: 10.1021/acs.jnatprod.8b00662. [DOI] [PubMed] [Google Scholar]
- 49.Bao J., Wang J., Zhang X.Y., Nong X.H., Qi S.H. New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017;14:e1600327. doi: 10.1002/cbdv.201600327. [DOI] [PubMed] [Google Scholar]
- 50.Yamada T., Kimura H., Arimitsu K., Kajimoto T., Kikuchi T., Tanaka R. Absolute configuration of eight cephalimysins isolated from the marine-derived Aspergillus fumigatus. ChemistrySelect. 2017;2:10936–10940. doi: 10.1002/slct.201702256. [DOI] [Google Scholar]
- 51.Li X.Y., Ding W.J., Wang P.M., Xu J.Z. Two novel aspochalasins from the gut fungus Aspergillus sp. Z4. Mar. Drugs. 2018;16:343. doi: 10.3390/md16100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Zhao S.Z., Ding W.J., Wang P.M., Yang X.W., Xu J.Z. Methylthio-aspochalasins from a marine-derived fungus Aspergillus sp. Mar. Drugs. 2014;12:5124–5131. doi: 10.3390/md12105124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X.Y., Zhao Z.H., Ding W.J., Ye B., Wang P.M., Xu J.Z. Aspochalazine A, a novel polycyclic aspochalasin from the fungus Aspergillus sp. Z4. Tetrahedron Lett. 2017;58:2405–2408. doi: 10.1016/j.tetlet.2017.04.071. [DOI] [Google Scholar]
- 54.Wang X., Lin M., Xu D., Lai D., Zhou L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules. 2017;22:2069. doi: 10.3390/molecules22122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Y., Phat C., Hong S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides. 2017;95:94–105. doi: 10.1016/j.peptides.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X., Fang P.Y., Tang J.Q., Wu Z.Q., Li X.F., Li S.M., Wang Y., Liu G., He Z.D., Gou D.M., et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016;30:52–57. doi: 10.1080/14786419.2015.1033623. [DOI] [PubMed] [Google Scholar]
- 57.Peng J.X., Gao H.Q., Zhang X.M., Wang S., Wu C.M., Gu Q.Q., Guo P., Zhu T.J., Li D.H. Psychrophilins E-H and Versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014;77:2218–2223. doi: 10.1021/np500469b. [DOI] [PubMed] [Google Scholar]
- 58.Ebada S.S., Fischer T., Hamacher A., Du F.Y., Roth Y.O., Kassack M.U., Wang B.G., Roth E.H. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Aspergillus. Nat. Prod. Res. 2014;28:776–781. doi: 10.1080/14786419.2014.880911. [DOI] [PubMed] [Google Scholar]
- 59.Nong X.H., Zhang X.Y., Xu X.Y., Qi S.H. Antifouling compounds from the marine-derived fungus Aspergillus terreus SCSGAF0162. Nat. Prod. Commun. 2015;10:1033–1034. doi: 10.1177/1934578X1501000659. [DOI] [PubMed] [Google Scholar]
- 60.Prompanya C., Fernandes C., Cravo S., Pinto M.M.M., Dethoup T., Silva A.M.S., Kijjoa A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. Mar. Drugs. 2015;13:1432–1450. doi: 10.3390/md13031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou X.M., Zhang Y.H., Hai Y., Zheng J.Y., Gu Y.C., Wang C.Y., Shao C.L. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar. Drugs. 2017;15:363. doi: 10.3390/md15110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X.F., Fang W., Tan S.Y., Lin X.P., Xun T.R., Yang B.J., Liu S.W., Liu Y.H. Aspernigrins with anti-HIV-1 activities from the marine-derived fungus Aspergillus niger SCSIO Jcsw6F30. Bioorg. Med. Chem. Lett. 2016;26:361–365. doi: 10.1016/j.bmcl.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Chen M., Shao C.L., Meng H., She Z.G., Wang C.Y. Anti-respiratory syncytial virus prenylated dihydroquinolone derivatives from the gorgonian-derived fungus Aspergillus sp. XS-20090B15. J. Nat. Prod. 2014;77:2720–2724. doi: 10.1021/np500650t. [DOI] [PubMed] [Google Scholar]
- 64.Wu Z.H., Liu D., Xu Y., Chen J.L., Lin W.H. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin. J. Nat. Med. 2018;16:219–224. doi: 10.1016/S1875-5364(18)30050-5. [DOI] [PubMed] [Google Scholar]
- 65.Zhuravleva O.I., Kirichuk N.N., Denisenko V.A., Dmitrenok P.S., Pivkin M.V., Afiyatullov S.S. New kipukasin from marine isolate of the fungus Aspergillus flavus. Chem. Nat. Compd. 2016;52:266–268. doi: 10.1007/s10600-016-1610-y. [DOI] [Google Scholar]
- 66.Chen M., Fu X.M., Kong C.J., Wang C.Y. Nucleoside derivatives from the marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2014;28:895–900. doi: 10.1080/14786419.2014.891114. [DOI] [PubMed] [Google Scholar]
- 67.Peng X.P., Wang Y., Zhu T.H., Zhu W.M. Pyrazinone derivatives from the coral-derived Aspergillus ochraceus LCJ11-102 under high iodide salt. Arch. Pharm. Res. 2018;41:184–191. doi: 10.1007/s12272-017-0928-8. [DOI] [PubMed] [Google Scholar]
- 68.Zheng C.J., Wu L.Y., Li X.B., Song X.M., Niu Z.G., Song X.P., Chen G.Y., Wang C.Y. Structure and absolute configuration of Aspergilumamide A, a novel lumazine peptide from the mangrove-derived fungus Aspergillus sp. Helv. Chim. Acta. 2015;98:368–373. doi: 10.1002/hlca.201400197. [DOI] [Google Scholar]
- 69.You M., Liao L., Hong S.H., Park W., Kwon D.I., Lee J., Noh M., Oh D.C., Oh K.B., Shin J. Lumazine peptides from the marine-derived fungus Aspergillus terreus. Mar. Drugs. 2015;13:1290–1303. doi: 10.3390/md13031290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W.T., Luo D., Huang J.N., Wang L.L., Zhang F.G., Xi T., Liao J.M., Lu Y.Y. Antibacterial constituents from Antarctic fungus, Aspergillus sydowii SP-1. Nat. Prod. Res. 2018;32:662–667. doi: 10.1080/14786419.2017.1335730. [DOI] [PubMed] [Google Scholar]
- 71.Ding C.H., Wu X.D., Auckloo B.N., Chen C.T., Ye Y., Wang K.W., Wu B. An unusual stress metabolite from a hydrothermal vent fungus Aspergillus sp. WU 243 induced by cobalt. Molecules. 2016;21:105. doi: 10.3390/molecules21010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strobel G., Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bugni T.S., Ireland C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004;21:143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 74.Rateb M.E., Ebel R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011;28:290–344. doi: 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- 75.Zhang P., Li X., Wang B.G. Secondary metabolites from the marine algal-derived endophytic fungi: Chemical diversity and biological activity. Planta Med. 2016;82:832–842. doi: 10.1055/s-0042-103496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.