Abstract
A traceless approach to quinolin-4(1H)-one scaffolds through Rh(III)-catalyzed redox-neutral [3+3] cyclization of N-nitrosoanilines with cyclopropenones has been achieved. This protocol features short reaction time and atom-economical combination without extra additives, which can be further applied in the construction of privileged heterocyclic compounds in pharmaceutical chemistry.
Keywords: Rhodium(III), redox-neutral, [3+3] annulation, N-nitrosoaniline, cyclopropenones, quinolin-4(1H)-ones
1. Introduction
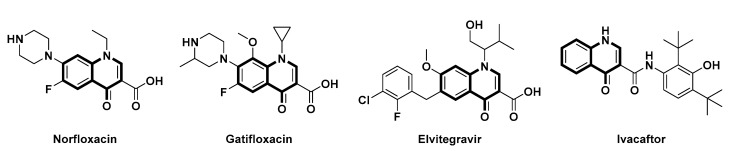
Quinolin-4(1H)-ones are ubiquitously present in numerous natural products and drugs, representing an important class of privileged structures in medicinal chemistry [1,2,3,4,5], such as antibiotics norfloxacin and gatifloxacin, a HIV integrase inhibitor, elvitegravir, and a modulator of ATP-binding cassette transporters, lvacaftor (Figure 1). Therefore, the development of highly efficient protocols both in transition-metal-catalyzed C-H activation and photocatalytic methods for the construction of such N-heterocyclic scaffolds is an extremely hot issue in modern organic chemistry [6,7,8,9,10,11,12]. However, the existing methodologies usually require elaborate-to-access starting materials, multiple steps, or harsh reaction conditions, failing to implement a wide range of applications. Given the importance of quinolin-4(1H)-ones with broad biological activities, there still remains the need to develop efficient, step- and atom-economic synthetic strategies.
Figure 1.
Representative drugs containing quinolin-4(1H)-ones.
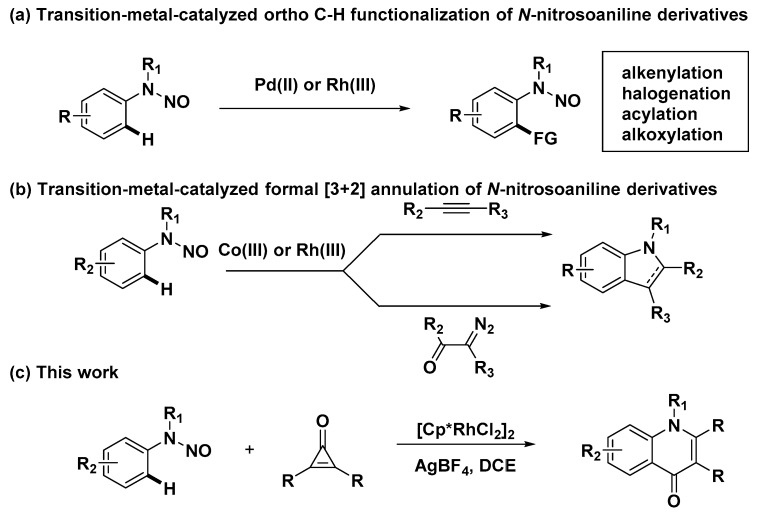
In the past decade, transition-metal-catalyzed redox-neutral C-H activation reactions have emerged as a robust and versatile methodology, avoiding stoichiometric amounts of external oxidants [13,14,15]. Recently, N-nitroso [16,17,18] as a novel directing group has aroused increasing attention and has been successfully employed in transition-metal (e.g., Pd, Rh, etc.) catalyzed C-H functionalization (Scheme 1a) [19,20,21,22]. In 2013, Zhu’s group reported the pioneering work of Rh(III)-catalyzed redox-neutral [3+2] annulation of N-nitrosoanilines with internal alkynes to form efficiently indole derivatives (Scheme 1b) [23,24,25,26,27,28]. Similarly, several formal [3+2] annulations between N-nitrosoanilines and diazo compounds [29] as well as propargyl alcohols [30] utilizing the N−nitroso group as an internal oxidant have been reported to prepare diversified indole scaffolds, in which the substrate involving the N−nitroso group seems to be an excellent synthon to build these intriguing privileged structures via a C-H bond activation and further annulation cascade. Therefore, in continuation of our recent efforts on transition-metal-catalyzed C-H annulations for the construction of heterocyclic scaffolds [31,32,33,34,35,36], we surprisingly found a new redox-neutral [3+3] annulation of N-nitrosoanilines with cyclopropenones [37,38,39] to generate a different substituted quinolin-4(1H)-one scaffold (Scheme 1c), which is a desirable privileged structure for further drug discovery. However, coincidentally, a similar work was reported by Cheng [40] after our work was finished and ready to submit. Compared with Cheng’s strategy, this method without extra additives also enables the efficient preparation of quinolin-4(1H)-ones in a much shorter time (2 h vs. 12 h), and has a good substrate scope.
Scheme 1.
Transition-metal-catalyzed C-H functionalization of N-nitrosoanilines.
2. Results and Discussions
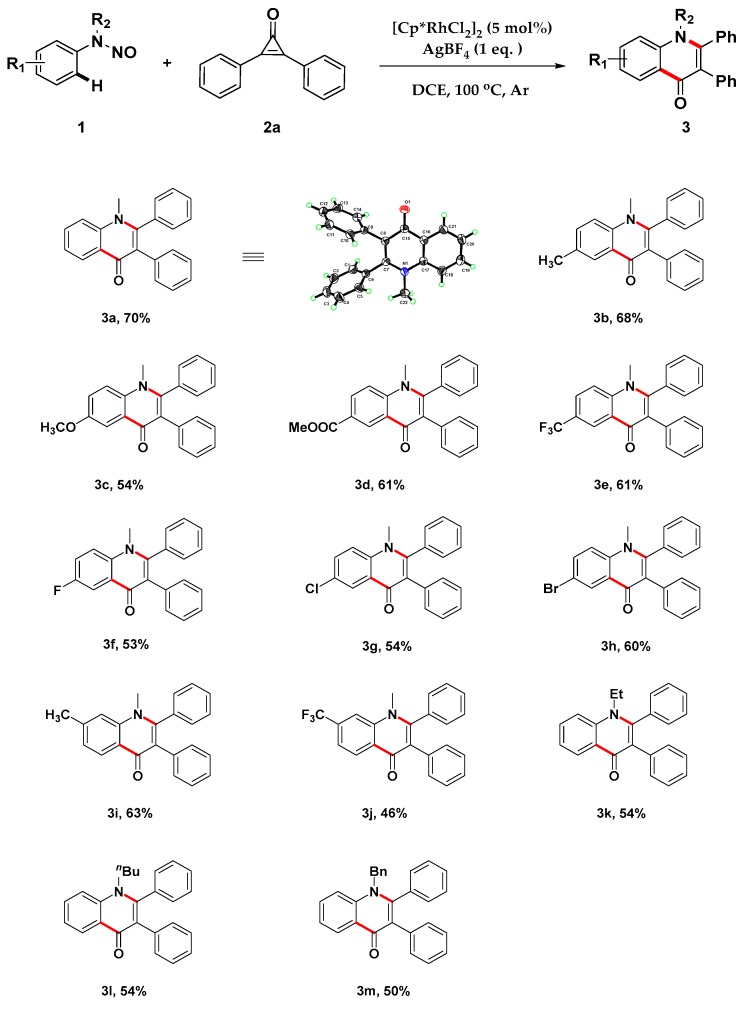
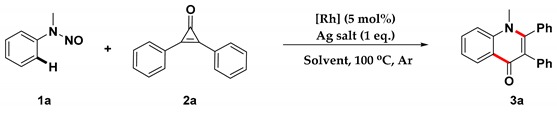
We initiated our studies by examining the reaction conditions of the coupling of N-nitrosoaniline, 1a, with diphenylcyclopropenone, 2a, in the presence of a Rh(III) catalyst. As shown in Table 1, three Rh(III) catalysts were firstly explored in dichloroethane (DCE), and the desired product, 3a, could only be afforded in 13% yield under the presence of [Cp*RhCl2]2, whereas the other two Rh(III) catalysts or Rh(III)-free were not effective (Table 1, entries 1–4). The structure of 3a was also unambiguously confirmed by an X-ray crystallographic analysis (see the Supplementary Material for details). However, further explorations demonstrated that a large amount of side product of dimerization of cyclopropenone [41,42] was generated simultaneously in this transformation, which resulted in a low yield of the desired product. Based on these results, we wondered whether lowering the concentration of cyclopropenone could inhibit the formation of the dimerization side product. To our delight, when the concentration was reduced from 0.1 M to 0.02 M, the yield of the desired product was increased dramatically, increasing the yield of 3a to 72% (entries 5,6). Inspired by the results, we further screened the silver salts and the results revealed that AgBF4 was still the most effective, while no desired product was formed in the absence of the silver additive (entries 7–9). Further explorations for reaction solvents displayed that DCE was the best choice for this transformation (entries 10,11). In addition, we attempted some complex additives with HOAc, CsF, or Zn(OAc)2, respectively, but they led to a slightly decreasing yield (entries 12–14). Similarly, reducing the reaction temperature to 80 °C or 60 °C was also detrimental to this transformation (entries 15,16).
Table 1.
Optimization of Reaction Conditions a.
| Entry | [Rh] | [Ag] | Solvent | Yield (%) b |
|---|---|---|---|---|
| 1 | [Cp*RhCl2]2 | AgBF4 | DCE | 13 |
| 2 | Rh(PPh3)3Cl | AgBF4 | DCE | 0 |
| 3 | Rh(COD)2(BF4) | AgBF4 | DCE | 0 |
| 4 | / | AgBF4 | DCE | 0 |
| 5 c | [Cp*RhCl2]2 | AgBF4 | DCE | 49 |
| 6 d | [Cp*RhCl2]2 | AgBF4 | DCE | 72 (70) j |
| 7 d | [Cp*RhCl2]2 | AgSbF6 | DCE | 40 |
| 8 d | [Cp*RhCl2]2 | AgOTf | DCE | 23 |
| 9 d | [Cp*RhCl2]2 | / | DCE | 0 |
| 10 d | [Cp*RhCl2]2 | AgBF4 | THF | 69 |
| 11 d | [Cp*RhCl2]2 | AgBF4 | Acetone | 66 |
| 12 d,e | [Cp*RhCl2]2 | AgBF4 | DCE | 49 |
| 13 d,f | [Cp*RhCl2]2 | AgBF4 | DCE | 49 |
| 14 d,g | [Cp*RhCl2]2 | AgBF4 | DCE | 67 |
| 15 d,h | [Cp*RhCl2]2 | AgBF4 | DCE | 57 |
| 16 d,i | [Cp*RhCl2]2 | AgBF4 | DCE | 0 |
a Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), Ag salt (0.2 mmol), solvent (2 mL), sealed tube under argon, 2 h. b NMR yield using CH2Br2 as internal standard. c solvent (5 mL). d solvent (10 mL). e HOAc (20 mol%) was added. f CsF (20 mol%) was added. g Zn(OAc)2 (20 mol%) was added. h at 80 °C. i at 60 °C. j isolated yield. DCE: dichloroethane. THF: tetrahydrofuran.
With the optimized reaction conditions in hand, we firstly investigated the scope of N-nitrosoanilines, and the results indicated that this formal [3+3] annulation reaction could tolerate various substituents on both the aromatic ring (R1) and the nitrogen atom (R2) to generate diversified quinolin-4(1H)-one derivatives in moderate to good yields (Scheme 2). The introduction of electron-donating groups (CH3 and OCH3) or electron-withdrawing groups (COOMe and CF3) at the 4-position of aniline 1 was tolerant and had no influence on the yields (3b–3h). Likewise, halogen-substituted anilines were also compatible in this catalytic system, giving the target compounds 3f–3h. When meta-substituted anilines were employed, the C-H bond activation took place at the less sterically hindered position, irrespective of the electronic nature of the substituents, and both electron-donating and electron-withdrawing groups were converted smoothly into the desired products 3i and 3j. Additionally, different N-substituents were explored, and the results showed that the substrates bearing alkyl and benzylic substituents could afford the desired products in moderate yields (3k–3m).
Scheme 2.
Scope of N-nitrosoanilines. Reaction conditions: 1 (0.2 mmol), 2 (0.4 mmol), AgBF4 (0.2 mmol), DCE (10 mL), sealed tube under argon, 2 h. The percentage represents isolated yield.
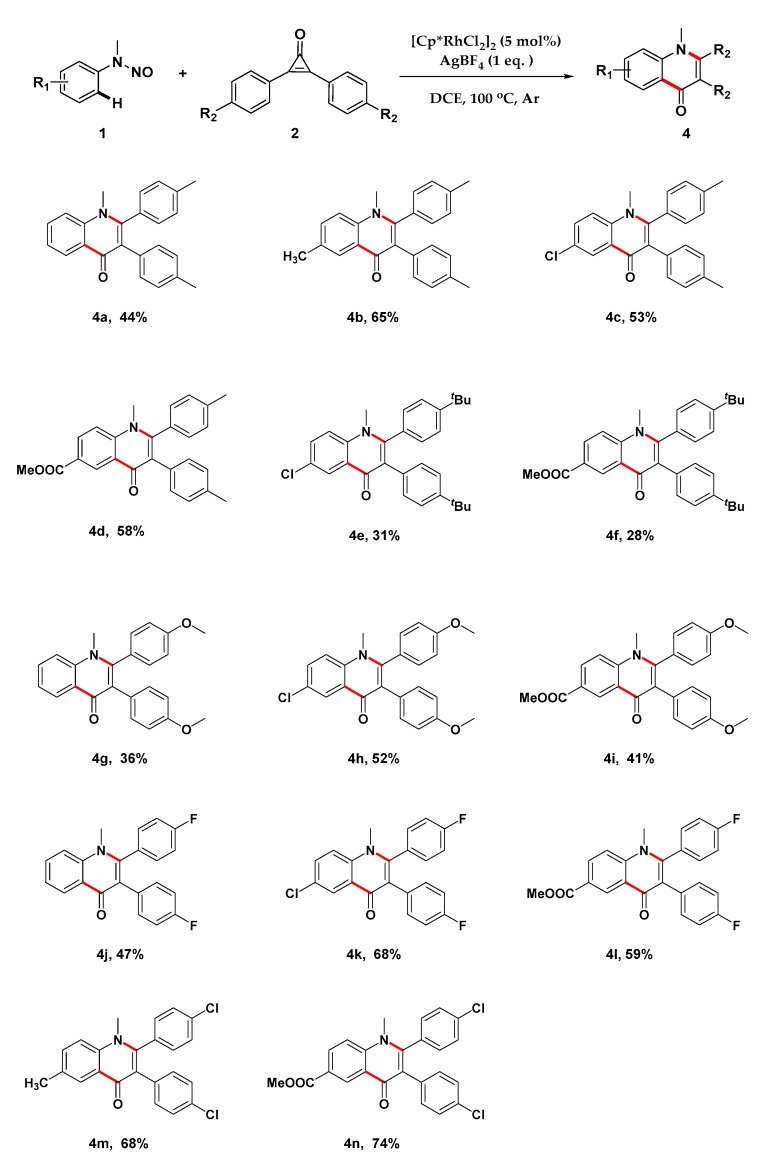
Next, the scope of cyclopropenones was further tested (Scheme 3), and the results demonstrated that different cyclopropenones could proceed smoothly to provide the corresponding products. The cyclopropenones bearing an electron-donating group at the para position of the phenyl group, such as methyl, tert-butyl, and methoxyl, were well tolerated under standard conditions, giving the desired products in moderate to good yields (4a–4i), regardless of whether electron-donating groups or electron-withdrawing groups were equipped into the N-aniline ring. Moreover, halogen-substituted phenyl groups could also be smoothly transformed into the corresponding products in moderate to good yields (4j–4n).
Scheme 3.
Scope of cyclopropenones. Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), AgBF4 (0.2 mmol), DCE (10 mL), sealed tube under argon, 2 h. The percentage represents isolated yield.
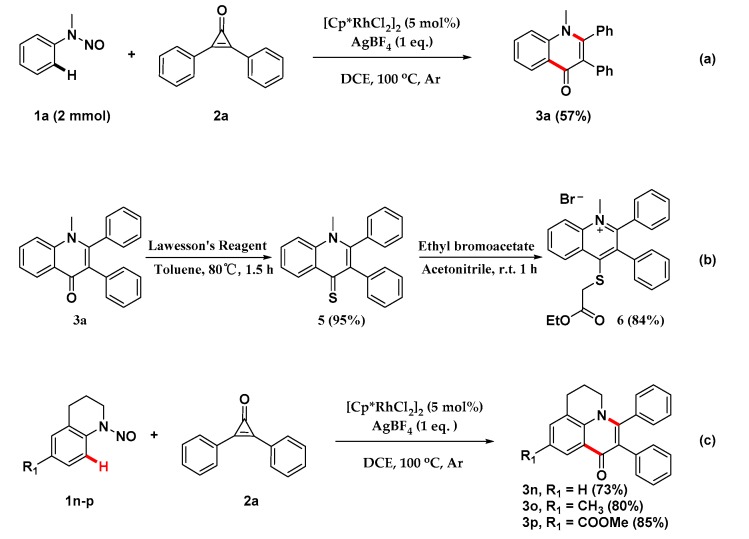
Intrigued by the privileged heterocyclic product derived from our strategy, we have further explored the gram-scale preparation of this transformation, its synthetic utility, and the late-stage functionalization for some important privileged scaffolds. As shown in Scheme 4, the redox-neutral [3+3] annulation could be carried out on a gram scale to produce 3a in a 57% yield (Scheme 4a). The synthetic utility of the obtained quinolin-4(1H)-one derivatives has been demonstrated by the following transformations into potentially bioactive molecules (Scheme 4b). Treatment of 3a with Lawesson’s reagent furnished thioketone 5 in a 95% yield, which could be further converted into thio-substituted product 6 in the presence of ethyl bromoacetate with a high yield. More interestingly, this strategy could also be used in the late-stage functionalization for tetrahydroquinoline privileged scaffolds to afford highly fused heterocyclic scaffolds, 3n–3p (Scheme 4c).
Scheme 4.
Gram experiment and derivatization of coupled product. (a) Gram-scale experiment. (b) Derivatization of final product. (c) Late-stage functionalization for tetrahydroquinolines.
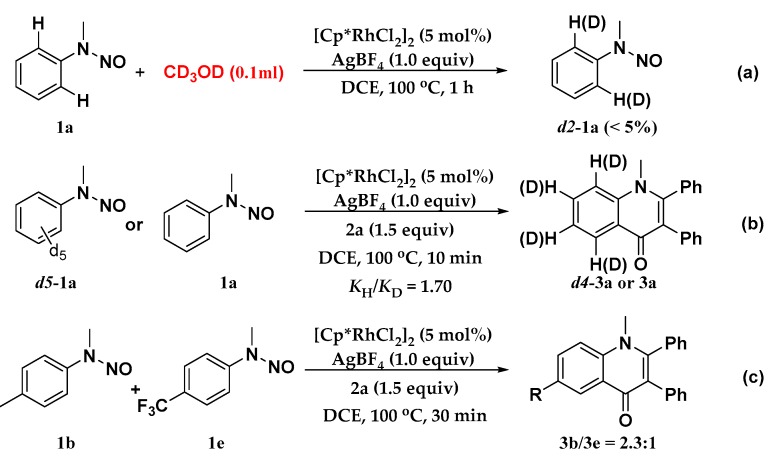
To understand the reaction mechanism, control experiments were carried out (Scheme 5). Firstly, the hydrogen–deuterium (H/D) exchange experiment was conducted to gain insight into the C-H cleavage step. No deuterated N-nitrosoaniline was observed after treating with CD3OD, indicating that rhodium-mediated C-H bond cleavage is irreversible (Scheme 5a). D5-1a and 1a were then subjected to the standard conditions, and the kinetic isotope effect (KIE) was measured. The value of kH/kD is 1.7, implying that the C-H bond cleavage was the rate-determining step in the transformation (Scheme 5b) [43]. Furthermore, to probe the electronic preference, an intermolecular competition experiment was carried out, and the result suggested that the electron-rich substrate, 1b, reacted at a higher rate (Scheme 5c).
Scheme 5.
Mechanistic studies. (a) Hydrogen–deuterium (H/D) exchange experiment. (b) KIE experiment. (c) Intermolecular competition experiment.
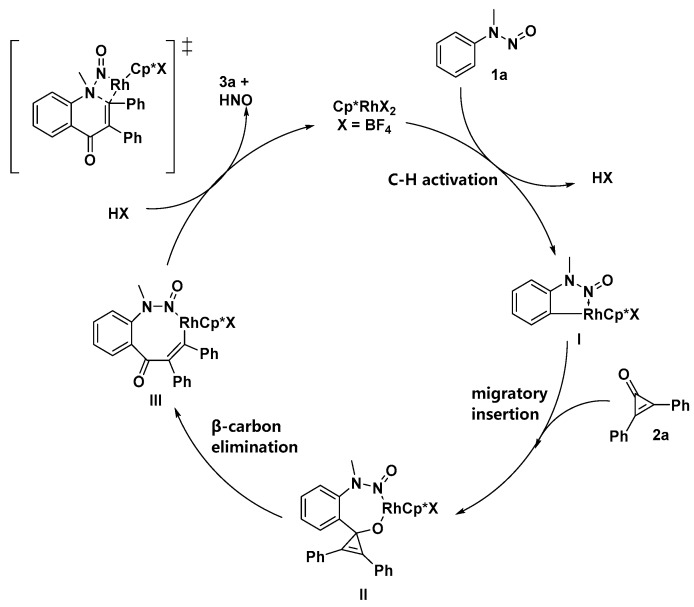
On the basis of these results and literature precedents [20,21], in order to gain insight into this reaction mechanism, the mechanism of the coupling of N-nitrosoaniline with cyclopropenone is proposed in Scheme 6. A cationic Rh(III) species can easily undergo ortho C-H insertion of N-nitrosoaniline 1a to afford intermediate I. Then, intermediate I can be saturated by cyclopropenone coordination and subsequently undergo migratory insertion of the Rh-C bond into the carbonyl group of cyclopropenone 2a to afford the alkoxide intermediate II, which is followed by β-carbon elimination to afford the Rh(III) alkenyl intermediate III. Finally, intermediate III undergoes a direct cyclization pathway to yield the six-membered ring product, 3a, releasing HNO with the regeneration of the Rh(III) catalyst.
Scheme 6.
Proposed mechanism.
3. Materials and Methods
3.1. General Information
Unless otherwise noted, the reagents (chemicals) were purchased from commercial sources and used without further purification. Water was deionized before being used. Analytical thin layer chromatography (TLC) was HSGF 254 (0.15–0.2 mm thickness). Compound spots were visualized by UV light (254 nm). Column chromatography was performed on silica gel FCP 300–400. NMR spectra were run on a 400 or 500 MHz instrument. Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). Low- and high-resolution mass spectra (LRMS and HRMS) were measured on a spectrometer. N-nitrosoanilines 1 and cyclopropenones 2 were prepared according to the previous literature [23,44,45,46].
3.2. General Procedures for Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones (3 and 4)
To a 35 mL Schlenk tube was sequentially added N-nitrosoanilines 1 (0.2 mmol), cyclopropenone 2 (0.4 mmol for product 3, 0.3 mmol for product 4), catalyst (5 mol%), Ag salt (0.2 mmol), and solvent (10 mL). The reaction was sealed under argon and stirred at 100 °C for 2 h. After the reaction was completed (detected by TLC), solvent was removed under reduced pressure, and the crude mixture was purified by flash column chromatography on silica gel with a PE/EA (4/1, v/v) solvent system to afford the final product, 3, and with a CH2Cl2/CH3OH (50/1, v/v) solvent system to afford the final product, 4.
3.3. General Procedures for 1-Methyl-2,3-diphenylquinoline-4(1H)-thione (5)
To a solution of 3a (50 mg, 0.16 mmol) in 20 mL toluene was added Lawesson’s reagent (64 mg, 0.16 mmol), and the reaction was stirred at 80 °C for 1.5 h. After the reaction was completed, the mixture was filtered, and the precipitate was washed with cold ethanol to afford the compound, 5, as a brown solid in a 95% yield.
3.4. General Procedures for 4-((2-Ethoxy-2-oxoethyl)thio)-1-methyl-2,3-diphenylquinolin-1-ium (6)
A 50 mL reaction flask was charged with acetonitrile and compound 5 (20 mg, 0.06 mmol), and ethyl bromoacetate (1.2 equiv) was added for 1 h at room temperature. After the reaction was completed, the solvent was removed, and the residue was purified by flash column chromatography on silica gel eluting with methanol to afford the compound, 6, in a 84% yield.
3.5. Analytical Characterization Data of Products
1-Methyl-2,3-diphenylquinolin-4(1H)-one (3a). Light yellow solid (70%). m.p. 218.3–218.8 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.59 (dd, J = 8.0, 1.7 Hz, 1H), 7.73 (ddd, J = 8.7, 7.0, 1.7 Hz, 1H), 7.57 (d, J = 8.6 Hz, 1H), 7.46–7.41 (m, 1H), 7.30–7.26 (m, 3H), 7.18–7.14 (m, 2H), 7.13–7.09 (m, 2H), 7.07–7.02 (m, 3H), 3.55 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.4, 152.2, 141.6, 135.9, 135.2, 132.4, 131.5, 129.7, 128.8, 128.4, 127.6, 127.6, 126.8, 126.2, 124.5, 123.7, 115.9, 37.8. IR (KBr, cm−1): 3056, 1616, 1531, 1482, 1321, 1068, 754. HRMS (Electrospray ionization ESI) m/z [M + H]+ calcd. for C22H18NO: 312.1383, found: 312.1384.
1,6-Dimethyl-2,3-diphenylquinolin-4(1H)-one (3b). Light yellow solid (68%). m.p. 187.5–187.8 °C; 1H NMR (400 MHz, Chloroform-d) δ 8.39–8.35 (m, 1H), 7.55 (dd, J = 8.7, 2.2 Hz, 1H), 7.48 (d, J = 8.8 Hz, 1H), 7.29–7.26 (m, 3H), 7.17–7.08 (m, 4H), 7.06–7.01 (m, 3H), 3.53 (s, 3H), 2.52 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 151.9, 139.7, 136.2, 135.3, 133.8, 133.6, 131.6, 129.8, 128.8, 128.4, 127.6, 126.9, 126.7, 126.2, 124.2, 115.8, 37.7, 21.1. HRMS (ESI) m/z [M + H]+ calcd. for C23H20NO: 326.1539, found: 326.1539.
6-Methoxy-1-methyl-2,3-diphenylquinolin-4(1H)-one (3c). Yellow solid (54%). m.p. 212.4–212.7 °C; 1H NMR (400 MHz, Chloroform-d) δ 8.00 (d, J = 3.1 Hz, 1H), 7.54 (d, J = 9.3 Hz, 1H), 7.37–7.35 (m, 1H), 7.29–7.28 (m, 3H), 7.18–7.08 (m, 4H), 7.07–7.01 (m, 3H), 3.95 (s, 3H), 3.56 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.9, 156.7, 151.8, 136.5, 136.4, 135.4, 131.8, 130.0, 129.1, 128.7, 128.1, 127.8, 126.5, 123.8, 123.3, 117.9, 106.7, 56.2, 38.2. HRMS (ESI) m/z [M + H]+ calcd. for C23H20NO2: 342.1489, found: 342.1495.
6-Methoxycarbonyl-1-methyl-2,3-diphenylquinoline-4(1H)-one (3d). Light yellow solid (61%). m.p. 236.7–237.2 °C; 1H NMR (600 MHz, DMSO-d6) δ 8.86 (d, J = 2.3 Hz, 1H), 8.27 (dd, J = 9.0, 2.2 Hz, 1H), 7.92 (d, J = 9.1 Hz, 1H), 7.23–7.06 (m, 4H), 6.94–6.91 (m, 2H), 6.88–6.85 (m, 2H), 3.92 (s, 3H), 3.47 (s, 3H), 2.27 (s, 3H), 2.19 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 166.7, 152.6, 144.2, 135.3, 134.8, 132.8, 131.3, 130.1, 129.6, 129.1, 128.6, 127.7, 126.5, 126.2, 125.6, 125.3, 116.2, 52.4, 38.1. HRMS (ESI) m/z [M + H]+ calcd. for C24H20NO3: 370.1438, found: 370.1430.
6-(Trifluoromethyl)-1-methyl-2,3-diphenyl-quinolin-4(1H)-one (3e). Light yellow solid (61%). m.p. 226.7–227.0 °C; 1H NMR (400 MHz, Chloroform-d) δ 8.86 (d, J = 2.2 Hz, 1H), 7.95–7.90 (m, 1H), 7.67 (d, J = 9.0 Hz, 1H), 7.33–7.27 (m, 3H), 7.18–7.06 (m, 5H), 7.06–6.98 (m, 2H), 3.57 (s, 3H). 13C NMR (125 MHz, Chloroform-d) δ 175.9, 152.9, 143.3, 135.2, 134.6, 131.3, 129.6, 129.2, 128.6, 128.5 (q, J = 3.0 Hz) 127.7, 126.6, 126.3, 125.9, 125.6 (q, J = 3.0 Hz), 125.1, 123.3 (q, J = 271.8 Hz), 116.9, 38.0. 19F NMR (471 MHz, Chloroform-d) δ -61.9. HRMS (ESI) m/z [M + H]+ calcd. for C23H17F3NO: 380.1257, found 380.1251.
6-Fluoro-1-methyl-2,3-diphenylquinolin-4(1H)-one (3f). White solid (53%). m.p. 213.5–213.8 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.22 (dd, J = 8.9, 3.1 Hz, 1H), 7.58 (dd, J = 9.3, 4.1 Hz, 1H), 7.46 (ddd, J = 9.3, 7.5, 3.1 Hz, 1H), 7.30–7.27 (m, 3H), 7.17–7.09 (m, 4H), 7.08–7.00 (m, 3H), 3.56 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.6, 159.4 (d, J = 243.9 Hz), 152.3, 138.1, 135.7, 134.9, 131.4, 129.7, 129.0, 128.5, 128.3 (d, J = 6.8 Hz), 127.7, 126.4, 123.9, 120.9 (d, J = 24.8 Hz), 118.2 (d, J = 7.6 Hz), 112.1 (d, J = 22.3 Hz), 38.1. 19F NMR (471 MHz, Chloroform-d) δ -118.0. HRMS (ESI) m/z [M + H]+ calcd. for C22H17FNO: 330.1289, found: 330.1288.
6-Chloro-1-methyl-2,3-diphenylquinolin-4(1H)-one (3g). Light yellow solid (54%). m.p. 248.4–248.9 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.53 (d, J = 2.5 Hz, 1H), 7.66 (dd, J = 9.1, 2.6 Hz, 1H), 7.52 (d, J = 9.1 Hz, 1H), 7.30–7.27 (m, 3H), 7.18–7.09 (m, 4H), 7.09–6.98 (m, 3H), 3.54 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.3, 152.4, 140.0, 135.5, 134.9, 132.6, 131.4, 130.0, 129.7, 129.0, 128.5, 127.8, 127.7, 126.9, 126.5, 124.8, 117.7, 38.0. HRMS (ESI) m/z [M + H]+ calcd. for C22H17ClNO: 346.0993, found: 346.0996.
6-Bromo-1-methyl-2,3-diphenylquinolin-4(1H)-one (3h). Light yellow solid (60%). m.p. 243.1–244.2 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.69 (d, J = 2.5 Hz, 1H), 7.79 (dd, J = 9.0, 2.5 Hz, 1H), 7.45 (d, J = 9.1 Hz, 1H), 7.30–7.28 (m, 3H), 7.16–7.09 (m, 4H), 7.08–6.99 (m, 3H), 3.53 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.2, 152.4, 140.4, 135.5, 135.3, 134.8, 131.4, 130.1, 129.7, 129.0, 128.5, 128.2, 127.7, 126.5, 125.0, 117.9, 117.6, 37.9. HRMS (ESI) m/z [M + H]+ calcd. for C22H17Br NO: 390.0488, found: 390.0482.
1,7-Dimethyl-2,3-diphenylquinolin-4(1H)-one (3i). Light yellow solid (63%). m.p. 286.1–287.6 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.46 (d, J = 8.2 Hz, 1H), 7.35 (s, 1H), 7.30–7.26 (m, 4H), 7.16–7.14 (m, 2H), 7.12–7.08 (m, 2H), 7.06–7.01 (m, 3H), 3.52 (s, 3H), 2.56 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 151.9, 143.1, 141.8, 136.0, 135.3, 131.6, 129.8, 128.8, 128.4, 127.5, 127.4, 126.2, 125.4, 124.8, 124.3, 115.6, 37.7, 22.5. HRMS (ESI) m/z [M + H]+ calcd. for C23H20NO: 326.1539, found: 326.1546.
1-Methyl-2,3-diphenyl-7-(trifluoromethyl)quinolin-4(1H)-one (3j). Light yellow solid (46%). m.p. 221.4–222.7 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.69 (d, J = 8.3 Hz, 1H), 7.84 (s, 1H), 7.65 (dd, J = 8.4, 1.4 Hz, 1H), 7.32–7.28 (m, 3H), 7.18–7.11 (m, 4H), 7.09–7.00 (m, 3H), 3.59 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.8, 153.1, 141.2, 135.3, 134.7, 134.0 (q, J = 32.5 Hz), 131.3, 129.6, 129.2, 129.0, 128.6, 127.7, 126.6, 125.6, 124.0 (q, J = 262.5 Hz), 119.7 (q, J = 3.2 Hz), 113.6 (q, J = 4.2 Hz), 38.0. 19F NMR (471 MHz, Chloroform-d) δ -62.6. HRMS (ESI) m/z [M + H]+ calcd. for C23H17F3NO: 380.1257, found: 380.1252.
1-Ethyl-2,3-diphenylquinolin-4(1H)-one (3k). White solid (54%). m.p. 247.4–247.9 °C; 1H NMR (400 MHz, Chloroform-d) δ 8.61 (d, J = 8.0 Hz, 1H), 7.75–7.68 (m, 1H), 7.60 (d, J = 8.7 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.29–7.26 (m, 3H), 7.22–7.17 (m, 2H), 7.13–7.07 (m, 2H), 7.06–7.00 (m, 3H), 4.08 (q, J = 7.0 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H); 13C NMR (150 MHz, Chloroform-d) δ 176.5, 152.2, 140.3, 136.2, 135.2, 132.6, 131.6, 129.6, 129.0, 128.6, 128.2, 127.8, 127.4, 126.5, 125.0, 123.8, 116.3, 43.9, 14.7. HRMS (ESI) m/z [M + H]+ calcd. for C23H20NO: 326.1539, found: 326.1532.
1-Butyl-2,3-diphenylquinolin-4(1H)-one (3l). White solid (54%). m.p. 119.1–120.4 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.61 (dd, J = 8.1, 1.7 Hz, 1H), 7.71 (ddd, J = 8.7, 7.0, 1.7 Hz, 1H), 7.56 (d, J = 8.7 Hz, 1H), 7.44–7.40 (m, 1H), 7.30–7.27 (m, 3H), 7.19–7.16 (m, 2H), 7.10 (dd, J = 8.3, 6.5 Hz, 2H), 7.06–7.00 (m, 3H), 3.99–3.92 (m, 2H), 1.70–1.68 (m, 2H), 1.20–1.13 (m, 2H), 0.77 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.2, 152.1, 140.3, 136.0, 135.0, 132.3, 131.5, 129.5, 128.8, 128.3, 127.9, 127.6, 127.1, 126.3, 124.7, 123.6, 116.2, 48.7, 30.9, 19.9, 13.5. HRMS (ESI) m/z [M + H]+ calcd. for C25H24NO: 354.1852, found: 354.1857.
1-Benzyl-2,3-diphenylquinolin-4(1H)-one (3m). Light yellow solid (50%). m.p. 74.3–75.6 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.60 (dd, J = 8.0, 1.7 Hz, 1H), 7.55 (ddd, J = 8.7, 7.0, 1.7 Hz, 1H), 7.41–7.35 (m, 2H), 7.32–7.26 (m, 3H), 7.19–7.02 (m, 10H), 7.01–6.98 (m, 2H), 5.25 (s, 2H); 13C NMR (125 MHz, Chloroform-d) δ 176.6, 152.6, 140.9, 136.6, 135.9, 134.7, 132.4, 131.5, 129.4, 129.1, 128.9, 128.2, 127.7, 127.6, 127.5 127.0, 126.3, 125.7, 124.9, 123.8, 117.2, 52.7. HRMS (ESI) m/z [M + H]+ calcd. for C28H22NO: 388.1696, found: 388.1698.
2,3-Diphenyl-6,7-dihydro-1H,5H-pyrido [3,2,1-ij]quinolin-1-one (3n). Yellow solid (73%). m.p. 280.2–281.4 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.42 (dd, J = 8.1, 1.6 Hz, 1H), 7.45 (dd, J = 7.1, 1.5 Hz, 1H), 7.33–7.24 (m, 4H), 7.18–7.13 (m, 2H), 7.13–7.07 (m, 2H), 7.06–7.00 (m, 3H), 3.82–3.75 (m, 2H), 3.08–3.04 (m, 2H), 2.12–2.05 (m, 2H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 151.3, 138.2, 136.0, 134.9, 131.5, 131.5, 129.5, 128.7, 128.4, 127.5, 126.9, 126.8, 126.2, 125.5, 124.1, 123.2, 50.2, 28.0, 22.1. IR (KBr, cm−1): 3045, 1616, 1544, 1484, 1438, 1307, 1039, 703. HRMS (ESI) m/z [M + H]+ calcd. for C24H20NO: 338.1539, found: 338.1536.
9-Methyl-2,3-diphenyl-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinolin-1-one (3o). Yellow solid (80%). m.p. 251.2–252.3 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.22–8.17 (m, 1H), 7.30–7.26 (m, 2H), 7.26–7.23 (m, 2H), 7.16–7.07 (m, 4H), 7.05–7.00 (m, 3H), 3.81–3.70 (m, 2H), 3.81–3.70 (m, 2H), 2.46 (s, 3H), 2.11–2.03 (m, 2H); 13C NMR (125 MHz, Chloroform-d) δ 176.1, 150.9, 136.3, 136.2, 134.9, 133.0, 133.0, 131.6, 129.5, 128.6, 128.4, 127.5, 126.8, 126.7, 126.1, 124.7, 123.8, 50.1, 27.9, 22.2, 21.1. IR (KBr, cm−1): 3056, 1625, 1536, 1490, 1317, 1274, 1079, 711. HRMS (ESI) m/z [M + H]+ calcd. for C25H22NO: 352.1696, found: 352.1697.
9-Methoxycarbonyl-2,3-diphenyl-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinolin-1-one (3p). Yellow solid (85%). m.p. 249.1–250.7 °C; 1H NMR (400 MHz, Chloroform-d) δ9.06–9.03 (m, 1H), 8.09–8.06 (m, 1H), 7.30–7.26 (m, 3H), 7.18–7.08 (m, 4H), 7.08–6.99 (m, 3H), 3.95 (s, 3H), 3.82–3.75 (m, 2H), 3.12–3.06 (m, 2H), 2.14–2.05 (m, 2H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 166.9, 151.8, 141.0, 135.4, 134.4, 131.5, 131.3, 129.4, 128.9, 128.6, 128.1, 127.6, 127.3, 126.5, 126.2, 125.2, 124.6, 52.3, 50.4, 27.9, 21.8. IR (KBr, cm−1): 3057, 1714, 1627, 1498, 1442, 1301, 1215, 1072, 701. HRMS (ESI) m/z [M + H]+ calcd. for C26H22NO3: 396.1594, found: 396.1593.
1-Methyl-2,3-di-p-tolylquinolin-4(1H)-one (4a). White solid (44%). m.p. 186.3–186.7 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.59 (dd, J = 8.0, 1.7 Hz, 1H), 7.74–7.67 (m, 1H), 7.56 (d, J = 8.6 Hz, 1H), 7.42 (ddd, J = 8.0, 7.0, 0.9 Hz, 1H), 7.09 (d, J = 7.8 Hz, 2H), 7.04 (d, J = 8.1 Hz, 2H), 6.94–6.91 (m, 4H), 3.53 (s, 3H), 2.32 (s, 3H), 2.22 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.7, 152.6, 141.9, 138.9, 135.8, 133.1, 132.6, 132.5, 131.5, 129.8, 129.4, 128.6, 127.9, 126.9, 124.7, 123.9, 116.1, 38.0, 21.7, 21.6. HRMS (ESI) m/z [M + H]+ calcd. for C24H22NO: 340.1696, found: 340.1694.
1,6-Dimethyl-2,3-di-p-tolylquinolin-4(1H)-one (4b). White solid (65%). m.p. 233.7–233.9 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.39–8.33 (m, 1H), 7.52 (dd, J = 8.8, 2.1 Hz, 1H), 7.45 (d, J = 8.7 Hz, 1H), 7.08 (d, J = 7.9 Hz, 2H), 7.02 (d, J = 8.1 Hz, 2H), 6.92–6.90 (m, 4H), 3.50 (s, 3H), 2.50 (s, 3H), 2.31 (s, 3H), 2.22 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.1, 151.7, 139.4, 138.3, 135.2, 133.4, 133.1, 132.8, 132.2, 131.1, 129.4, 128.8, 128.1, 126.7, 126.3, 123.9, 115.5, 37.4, 21.2, 21.1, 20.8. HRMS (ESI) m/z [M + H]+ calcd. for C25H24NO: 354.1852, found: 354.1850.
6-Chloro-1-methyl-2,3-di-p-tolylquinolin-4(1H)-one (4c). Light yellow solid (53%). m.p. 240.2–241.7 °C; 1H NMR (600 MHz, DMSO-d6) δ 8.19 (d, J = 2.5 Hz, 1H), 7.87 (d, J = 9.1 Hz, 1H), 7.83 (dd, J = 9.1, 2.6 Hz, 1H), 7.18–7.12 (m, 4H), 6.93–6.89 (m, 2H), 6.87–6.83 (m, 2H), 3.45 (s, 3H), 2.26 (s, 3H), 2.18 (s, 3H); 13C NMR (150 MHz, Chloroform-d) δ 175.7, 152.7, 140.2, 139.1, 136.0, 132.7, 132.6, 132.2, 131.3, 129.9, 129.7, 129.4, 128.6, 127.9, 126.9, 124.9, 118.0, 38.1, 21.7, 21.6. HRMS (ESI) m/z [M + H]+ calcd. for C24H21ClNO: 374.1306, found: 374.1305.
6-Methoxycarbonyl-1-methyl-2,3-di-p-tolylquinolin-4(1H)-one (4d). White solid (58%). m.p. 238.1–239.4 °C; 1H NMR (600 MHz, DMSO-d6) δ 8.86 (d, J = 2.3 Hz, 1H), 8.27 (dd, J = 9.0, 2.2 Hz, 1H), 7.92 (d, J = 9.1 Hz, 1H), 7.23–7.06 (m, 4H), 6.94–6.91 (m, 2H), 6.88–6.85 (m, 2H), 3.92 (s, 3H), 3.47 (s, 3H), 2.27 (s, 3H), 2.19 (s, 3H); 13C NMR (150 MHz, Chloroform-d) δ 176.7, 167.0, 152.8, 144.4, 139.1, 136.1, 132.8, 132.5, 132.2, 131.3, 130.3, 129.7, 129.5, 128.7, 126.2, 125.7, 125.3, 116.4, 52.6, 38.2, 21.7, 21.6. HRMS (ESI) m/z [M + H]+ calcd. for C26H24NO3: 398.1751, found: 398.1744.
2,3-Bis(4-(tert-butyl)phenyl)-6-chloro-1-methylquinolin-4(1H)-one (4e). White solid (31%). m.p. 296.0–297.6 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.56 (d, J = 2.5 Hz, 1H), 7.66 (dd, J = 9.1, 2.6 Hz, 1H), 7.54 (d, J = 9.0 Hz, 1H), 7.28 (d, J = 6.2 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 8.1 Hz, 2H), 6.91 (d, J = 8.0 Hz, 2H), 3.62 (s, 3H), 1.27 (s, 9H), 1.22 (s, 9H); 13C NMR (150 MHz, Chloroform-d) δ 175.5, 153.1, 152.2, 148.9, 140.1, 132.8, 132.6, 132.1, 131.1, 130.0, 129.7, 128.0, 127.0, 125.3, 125.1, 124.6, 117.9, 38.3, 35.0, 34.6, 31.6, 31.5. HRMS (ESI) m/z [M + H]+ calcd. for C30H33ClNO: 458.2245, found: 458.2248.
2,3-Bis(4-(tert-butyl)phenyl)-6-methoxycarbonyl-1-methylquinolin-4(1H)-one (4f). White solid (28%). m.p. 273.8–274.5 °C; 1H NMR (500 MHz, Chloroform-d) δ 9.23 (d, J = 2.2 Hz, 1H), 8.35 (dd, J = 9.0, 2.2 Hz, 1H), 7.61 (d, J = 9.0 Hz, 1H), 7.28 (d, J = 2.4 Hz, 2H), 7.12–7.08 (m, 2H), 7.06–7.03 (m, 2H), 6.93–6.89 (m, 2H), 3.99 (s, 3H), 3.64 (s, 3H), 1.27 (s, 9H), 1.21 (s, 9H); 13C NMR (150 MHz, Chloroform-d) δ 176.5, 167.0, 153.2, 152.3, 149.0, 144.4, 132.8, 132.6, 132.1, 131.1, 130.3, 129.7, 126.4, 125.9, 125.4, 125.3, 124.6, 116.4, 52.6, 38.4, 35.0, 34.6, 31.6, 31.5. HRMS (ESI) m/z [M + H]+ calcd. for C32H36NO3: 482.269, found: 482.2686.
2,3-Bis(4-methoxyphenyl)-1-methylquinolin-4(1H)-one (4g). White solid (36%). m.p. 192.7–193.2 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.57 (d, J = 8.1 Hz, 1H), 7.71 (t, J = 8.0 Hz, 1H), 7.55 (d, J = 8.7 Hz, 1H), 7.42 (t, J = 7.5 Hz, 1H), 7.06 (d, J = 8.1 Hz, 2H), 6.96 (d, J = 8.2 Hz, 2H), 6.81 (d, J = 8.1 Hz, 2H), 6.68 (d, J = 8.1 Hz, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 3.55 (s, 3H);13C NMR (125 MHz, Chloroform-d) δ 176.9, 159.9, 158.1, 152.4, 141.9, 132.8, 132.5, 131.3, 128.7, 127.9, 127.8, 127.0, 124.5, 123.8, 116.2, 114.2, 113.5, 55.6, 55.5, 38.1. HRMS (ESI) m/z [M + H]+ calcd. for C24H22NO3: 372.1594, found: 372.1596.
6-Chloro-2,3-bis(4-methoxyphenyl)-1-methylquinolin-4(1H)-one (4h). White solid (52%). m.p. 241.5–242.1 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.51 (d, J = 2.5 Hz, 1H), 7.63 (dd, J = 9.1, 2.6 Hz, 1H), 7.50 (d, J = 9.1 Hz, 1H), 7.05 (d, J = 8.6 Hz, 2H), 6.94 (d, J = 8.6 Hz, 2H), 6.81 (d, J = 8.3 Hz, 2H), 6.68 (d, J = 8.3 Hz, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 3.53 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.8, 160.1, 158.2, 152.7, 140.3, 132.7, 132.7, 131.3, 130.0, 128.3, 128.0, 127.5, 127.0, 124.8, 118.0, 114.2, 113.6, 55.6, 55.5, 38.2. HRMS (ESI) m/z [M + H]+ calcd. for C24H21ClNO3: 406.1204, found: 406.1203.
6-Methoxycarbonyl-2,3-bis(4-methoxyphenyl)-1-methylquinolin-4(1H)-one (4i). White solid (41%). m.p. 242.3–243.7 °C; 1H NMR (500 MHz, Chloroform-d) δ 9.19 (d, J = 2.2 Hz, 1H), 8.32 (dd, J = 9.0, 2.2 Hz, 1H), 7.58 (d, J = 9.0 Hz, 1H), 7.10–7.03 (m, 2H), 6.98–6.92 (m, 2H), 6.85–6.78 (m, 2H), 6.73–6.66 (m, 2H), 3.96 (s, 3H), 3.79 (s, 3H), 3.73 (s, 3H), 3.55 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.8, 167.0, 160.1, 158.2, 152.8, 144.5, 132.9, 132.7, 131.3, 130.4, 128.1, 127.5, 126.3, 125.6, 125.4, 116.5, 114.3, 113.6, 55.6, 55.5, 52.6, 38.3. HRMS (ESI) m/z [M + H]+ calcd. for C26H24NO5: 430.1649, found: 430.1648.
2,3-Bis(4-fluorophenyl)-1-methylquinolin-4(1H)-one (4j). White solid (47%). m.p. 239.2–241.8 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.56 (dd, J = 8.1, 1.7 Hz, 1H), 7.74 (ddd, J = 8.6, 6.9, 1.6 Hz, 1H), 7.57 (d, J = 8.6 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.17–7.10 (m, 2H), 7.05–6.93 (m, 4H), 6.83 (t, J = 8.8 Hz, 2H), 3.55 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.4, 162.7 (d, J = 248.9 Hz), 161.5 (d, J = 243.6 Hz), 151.3, 141.6, 133.1 (d, J = 8.0 Hz), 132.6, 131.7 (d, J = 8.2 Hz), 131.1 (d, J = 3.7 Hz), 129.0 (d, J = 4.6 Hz), 127.6, 126.7, 124.0, 123.7, 116.0, 115.9 (d, J = 13.2 Hz), 114.8 (d, J = 21.2 Hz), 37.8. 19F NMR (471 MHz, Chloroform-d) δ −111.0, −116.2. HRMS (ESI) m/z [M + H]+ calcd. for C22H16F2NO: 348.1194, found: 348.1192.
6-Chloro-2,3-bis(4-fluorophenyl)-1-methylquinolin-4(1H)-one (4k). White solid (68%). m.p. 293.3–294.2 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.51 (d, J = 2.6 Hz, 1H), 7.67 (dd, J = 9.1, 2.6 Hz, 1H), 7.52 (d, J = 9.1 Hz, 1H), 7.17–7.10 (m, 2H), 7.06–6.93 (m, 4H), 6.84 (t, J = 8.7 Hz, 2H), 3.54 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 175.2, 162.8 (d, J = 249.3 Hz), 161.5 (d, J = 244.1 Hz), 151.5, 140.0, 133.0 (d, J = 8.0 Hz), 132.8, 131.6 (d, J = 8.2 Hz), 131.3 (d, J = 3.5 Hz), 130.8 (d, J = 3.7 Hz), 130.3, 126.8, 124.1, 117.8, 116.0 (d, J = 21.7 Hz), 114.9 (d, J = 213 Hz), 114.8, 38.0. 19F NMR (471 MHz, Chloroform-d) δ -110.6, -115.8. HRMS (ESI) m/z [M + H]+ calcd. for C22H15ClF2NO: 382.0805, found: 382.0795.
6-Methoxycarbonyl-2,3-bis(4-fluorophenyl)-1-methylquinolin-4(1H)-one (4l). White solid (59%). m.p. 213.5–214.2 °C; 1H NMR (500 MHz, Chloroform-d) δ 9.19 (d, J = 2.1 Hz, 1H), 8.35 (dd, J = 9.0, 2.2 Hz, 1H), 7.60 (d, J = 9.0 Hz, 1H), 7.17–7.12 (m, 2H), 7.06–6.94 (m, 4H), 6.84 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H), 3.57 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.5, 166.8, 163.1 (d, J = 249.3 Hz), 161.0 (d, J = 244.3 Hz), 152.0, 144.4, 133.3 (d, J = 9.4 Hz), 133.1, 131.9 (d, J = 8.3 Hz), 131.3 (d, J = 3.2 Hz), 130.9 (d, J = 3.6 Hz), 130.3, 126.3, 125.9, 125.1, 116.5 (d, J = 14.1 Hz), 116.2, 115.2 (d, J = 21.3 Hz), 52.7, 38.4. 19F NMR (471 MHz, Chloroform-d) δ −110.5, −115.7. HRMS (ESI) m/z [M + H]+ calcd. for C24H18F2NO3: 406.1249, found: 406.1247.
2,3-Bis(4-chlorophenyl)-1,6-dimethylquinolin-4(1H)-one (4m). White solid (68%). m.p. 226.1–227.3 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.35–8.32 (m, 1H), 7.56 (dd, J = 8.7, 2.3 Hz, 1H), 7.47 (d, J = 8.8 Hz, 1H), 7.32–7.28 (m, 2H), 7.14–7.07 (m, 4H), 6.99–6.92 (m, 2H), 3.52 (s, 3H), 2.51 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 150.9, 139.9, 135.5, 134.6, 134.4, 134.3, 133.6, 133.1, 132.6, 131.3, 129.3, 128.3, 127.1, 126.8, 123.2, 116.1, 38.0, 21.3. HRMS (ESI) m/z [M + H]+ calcd. for C23H18Cl2NO: 394.0760, found: 394.0758.
2,3-Bis(4-chlorophenyl)-6-methoxycarbonyl-1-methylquinolin-4(1H)-one (4n). White solid (74%). m.p. 278.4–279.1 °C; 1H NMR (500 MHz, Chloroform-d) δ 9.17 (d, J = 2.1 Hz, 1H), 8.35 (dd, J = 9.0, 2.2 Hz, 1H), 7.59 (d, J = 9.0 Hz, 1H), 7.36–7.29 (m, 2H), 7.16–7.07 (m, 4H), 6.99–6.91 (m, 2H), 3.97 (s, 3H), 3.55 (s, 3H); 13C NMR (125 MHz, Chloroform-d) δ 176.3, 166.8, 151.6, 144.4, 135.9, 133.8, 133.3, 133.1, 133.0, 132.9, 131.2, 130.3, 129.5, 128.4, 126.3, 125.9, 124.6, 116.5, 52.7, 38.4. HRMS (ESI) m/z [M + H]+ calcd. for C24H18Cl2NO3: 438.0658, found: 438.0661.
1-Methyl-2,3-diphenylquinoline-4(1H)-thione (5). Brown solid (95%). 1H NMR (400 MHz, DMSO-d6) δ 9.02 (dd, J = 8.3, 1.5 Hz, 1H), 8.00 (d, J = 8.7 Hz, 1H), 7.89 (ddd, J = 8.6, 7.0, 1.6 Hz, 1H), 7.63–7.58 (m, 1H), 7.33–7.23 (m, 5H), 7.11–7.06 (m, 2H), 7.02–6.98 (m, 1H), 6.96–6.91 (m, 2H), 3.61 (s, 3H). 13C NMR (125 MHz, DMSO-d6) δ 140.8, 137.3, 136.9, 135.2, 133.8, 132.9, 131.5, 130.7, 129.6, 129.0, 128.5, 127.6, 126.1, 126.0, 118.7, 39.2. HRMS (ESI) m/z [M + H]+ calcd. for C22H17NS: 327.1082, found: 327.1072.
4-((2-Ethoxy-2-oxoethyl)thio)-1-methyl-2,3-diphenylquinolin-1-ium bromide (6). White solid (84%). 1H NMR (400 MHz, DMSO-d6) δ 8.97 (d, J = 8.5 Hz, 1H), 8.70 (d, J = 9.0 Hz, 1H), 8.38 (t, J = 8.0 Hz, 1H), 8.20 (t, J = 7.8 Hz, 1H), 7.40 (s, 5H), 7.27 (d, J = 5.6 Hz, 3H), 7.17–7.10 (m, 2H), 4.25 (s, 3H), 3.95 (q, J = 7.1 Hz, 2H), 3.54 (s, 2H), 1.05 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, DMSO-d6) δ 168.0, 158.3, 155.2, 140.3, 138.3, 136.1, 135.7, 132.9, 131.0, 130.6, 130.5, 129.6, 129.1, 128.8, 128.6, 128.5, 121.1, 61.8, 43.7, 37.2, 14.2. HRMS (ESI) m/z [M + H]+ calcd. for C26H24NO2S+: 414.1522, found: 414.1512.
4. Conclusions
In summary, we have developed a traceless approach to quinolin-4(1H)-one derivatives through rhodium(III)-catalyzed C−H annulation of N-nitrosoanilines with cyclopropenones. This reaction system provides a straightforward and atom-economical route for constructing the six-membered quinolin-4(1H)-one scaffolds, which may find important synthetic applications in the construction of heterocyclic compounds in pharmaceutical chemistry.
Acknowledgments
We gratefully acknowledge the financial support received from the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. We would like to thank all the reviewers for their great comments.
Supplementary Materials
The following are available online: Figures S1 and S2, Control Experiments for the Mechanistic Studies; Figure S3, X-ray Crystallographic Data; Figure S4, Copies of the 1H-NMR, 13C-NMR, and 19F-NMRspectra.
Author Contributions
Conceptualization, L.L.; experiments and analyses, L.L., J.L., W.D., and F.G.; writing—original draft preparation, L.L.; writing—review and editing, H.L., K.C., and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No.21672232, 21977106, 81620108027), National S & T Major Projects (2018ZX09711002), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12040217).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Carta D., Ferlin M.G. An Overview on 2-arylquinolin-4(1H)-ones and Related Structures as Tubulin Polymerisation Inhibitors. Curr. Top. Med. Chem. 2014;14:2322–2345. doi: 10.2174/1568026614666141127120421. [DOI] [PubMed] [Google Scholar]
- 2.Hadida S., Van Goor F., Zhou J., Arumugam V., McCartney J., Hazlewood A., Decker C., Negulescu P., Grootenhuis P.D. Discovery of N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (VX-770, ivacaftor), a potent and orally bioavailable CFTR potentiator. J. Med. Chem. 2014;57:9776–9795. doi: 10.1021/jm5012808. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez J.P., Gogliotti R.D., Domagala J.M., Gracheck S.J., Huband M.D., Sesnie J.A., Cohen M.A., Shapiro M.A. The synthesis, structure-activity, and structure-side effect relationships of a series of 8-alkoxy- and 5-amino-8-alkoxyquinolone antibacterial agents. J. Med. Chem. 1995;38:4478–4487. doi: 10.1021/jm00022a013. [DOI] [PubMed] [Google Scholar]
- 4.Sato M., Motomura T., Aramaki H., Matsuda T., Yamashita M., Ito Y., Kawakami H., Matsuzaki Y., Watanabe W., Yamataka K., et al. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J. Med. Chem. 2006;49:1506–1508. doi: 10.1021/jm0600139. [DOI] [PubMed] [Google Scholar]
- 5.Wentland M.P., Bailey D.M., Cornett J.B., Dobson R.A., Powles R.G., Wagner R.B. Novel amino-substituted 3-quinolinecarboxylic acid antibacterial agents: Synthesis and structure-activity relationships. J. Med. Chem. 1984;27:1103–1108. doi: 10.1021/jm00375a003. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Z.P., Li H.Q., Shi L., Lv P.C., Song Z.C., Zhu H.L. Synthesis, antiproliferative activity, and structure-activity relationships of 3-aryl-1H-quinolin-4-ones. ChemMedChem. 2008;3:1077–1082. doi: 10.1002/cmdc.200800057. [DOI] [PubMed] [Google Scholar]
- 7.Soural M., Hradil P., Krupkova S., Hlavac J. An Interesting Synthetic Pathway to Some Quinolin-4(1H)ones: Phenacylanthranilates Rearrangement-Limits and Scopes. Mini-Rev. Org. Chem. 2012;9:426–432. doi: 10.2174/157019312804699483. [DOI] [Google Scholar]
- 8.Coffman K.C., Palazzo T.A., Hartley T.P., Fettinger J.C., Tantillo D.J., Kurth M.J. Heterocycle-heterocycle strategies: (2-nitrophenyl)isoxazole precursors to 4-aminoquinolines, 1H-indoles, and quinolin-4(1H)-ones. Org. Lett. 2013;15:2062–2065. doi: 10.1021/ol400787y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang S., Park S., Kim K.S., Song C., Lee Y. Copper-Catalyzed Aza-Michael Addition of 2-Aminobenzoate to beta-Substituted α,β-Unsaturated Ketones: One-Pot Synthesis of 3-Carbonyl-2-Substituted Quinolin-4(1H)-ones. J. Org. Chem. 2018;83:2694–2705. doi: 10.1021/acs.joc.7b03162. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.R., Hu X.Q., Lu L.Q., Xiao W.J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016;49:1911–1923. doi: 10.1021/acs.accounts.6b00254. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B., Studer A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015;44:3505–3521. doi: 10.1039/C5CS00083A. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L., Lokman Hossain M., Xiao T. Synthesis of N-Containing Heterocyclic Compounds Using Visible-light Photoredox Catalysis. Chem. Rec. 2016;16:319–334. doi: 10.1002/tcr.201500228. [DOI] [PubMed] [Google Scholar]
- 13.Akram M.O., Banerjee S., Saswade S.S., Bedi V., Patil N.T. Oxidant-free oxidative gold catalysis: The new paradigm in cross-coupling reactions. Chem. Commun. 2018;54:11069–11083. doi: 10.1039/C8CC05601C. [DOI] [PubMed] [Google Scholar]
- 14.Cui X., Mo J., Wang L., Liu Y. Transition-Metal-Catalyzed Direct C–H Functionalization under External-Oxidant-Free Conditions. Synthesis. 2015;47:439–459. doi: 10.1055/s-0034-1379890. [DOI] [Google Scholar]
- 15.Xu P., Li W., Xie J., Zhu C. Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond. Acc. Chem. Res. 2018;51:484–495. doi: 10.1021/acs.accounts.7b00565. [DOI] [PubMed] [Google Scholar]
- 16.Li D.D., Cao Y.X., Wang G.W. Palladium-catalyzed ortho-acyloxylation of N-nitrosoanilines via direct sp2 C-H bond activation. Org. Biomol. Chem. 2015;13:6958–6964. doi: 10.1039/C5OB00691K. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Zeng H., Zhang W., Song C., Yang F., Liu Y., Zhu J. Rh(III)-catalyzed N-nitroso-directed C-H olefination polymerization. Polymer. 2019;172:152–159. doi: 10.1016/j.polymer.2019.03.063. [DOI] [Google Scholar]
- 18.Peng Q., Hu J., Huo J., Yuan H., Xu L., Pan X. Cp*Rh(iii) catalyzed ortho-halogenation of N-nitrosoanilines by solvent-controlled regioselective C-H functionalization. Org. Biomol. Chem. 2018;16:4471–4481. doi: 10.1039/C8OB00601F. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Wang G.-W. Solvent-free rhodium(III)-catalyzed synthesis of 2-aminoanilides via C−H amidation of N -nitrosoanilines under ball-milling conditions. Tetrahedron. 2018;74:4188–4196. doi: 10.1016/j.tet.2018.06.003. [DOI] [Google Scholar]
- 20.Liu B., Fan Y., Gao Y., Sun C., Xu C., Zhu J. Rhodium(III)-catalyzed N-nitroso-directed C-H olefination of arenes. High-yield, versatile coupling under mild conditions. J. Am. Chem. Soc. 2013;135:468–473. doi: 10.1021/ja3099245. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y., Feng L.J., Lu X., Kwong F.Y., Luo H.B. Palladium-catalyzed oxidative C-H bond acylation of N-nitrosoanilines with toluene derivatives: A traceless approach to synthesize N-alkyl-2-aminobenzophenones. Chem. Commun. 2014;50:15352–15354. doi: 10.1039/C4CC07440H. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y., Sun L., Chen Y., Zhou Q., Huang J.W., Miao H., Luo H.B. Palladium-Catalyzed Decarboxylative Acylation of N-Nitrosoanilines with alpha-Oxocarboxylic Acids. J. Org. Chem. 2016;81:1244–1250. doi: 10.1021/acs.joc.5b02535. [DOI] [PubMed] [Google Scholar]
- 23.Hu X., Chen X., Shao Y., Xie H., Deng Y., Ke Z., Jiang H., Zeng W. Co(III)-Catalyzed Coupling-Cyclization of Aryl C–H Bonds with α-Diazoketones Involving Wolff Rearrangement. ACS Catal. 2018;8:1308–1312. doi: 10.1021/acscatal.7b03668. [DOI] [Google Scholar]
- 24.Liang Y., Jiao N. Cationic Cobalt(III) Catalyzed Indole Synthesis: The Regioselective Intermolecular Cyclization of N-Nitrosoanilines and Alkynes. Angew. Chem. Int. Ed. 2016;55:4035–4039. doi: 10.1002/anie.201511002. [DOI] [PubMed] [Google Scholar]
- 25.Liu B., Song C., Sun C., Zhou S., Zhu J. Rhodium(III)-catalyzed indole synthesis using N-N bond as an internal oxidant. J. Am. Chem. Soc. 2013;135:16625–16631. doi: 10.1021/ja408541c. [DOI] [PubMed] [Google Scholar]
- 26.Yu S., Li X. Rhodium(III)-catalyzed C-C coupling of arenes with 2-vinyloxiranes: Synthesis of allylic alcohols. Org. Lett. 2014;16:1200–1203. doi: 10.1021/ol5000764. [DOI] [PubMed] [Google Scholar]
- 27.Zhao D., Shi Z., Glorius F. Indole synthesis by rhodium(III)-catalyzed hydrazine-directed C-H activation: Redox-neutral and traceless by N-N bond cleavage. Angew. Chem. Int. Ed. 2013;52:12426–12429. doi: 10.1002/anie.201306098. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S., Wang J., Zhang F., Song C., Zhu J. A Versatile, Traceless C-H Activation-Based Approach for the Synthesis of Heterocycles. Org. Lett. 2016;18:2427–2430. doi: 10.1021/acs.orglett.6b00949. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Wang M., Chen K., Zha S., Song C., Zhu J. C-H Activation-Based Traceless Synthesis via Electrophilic Removal of a Directing Group. Rhodium(III)-Catalyzed Entry into Indoles from N-Nitroso and alpha-Diazo-beta-keto Compounds. Org. Lett. 2016;18:1178–1181. doi: 10.1021/acs.orglett.6b00310. [DOI] [PubMed] [Google Scholar]
- 30.Song X., Gao C., Li B., Zhang X., Fan X. Regioselective Synthesis of 2-Alkenylindoles and 2-Alkenylindole-3-carboxylates through the Cascade Reactions of N-Nitrosoanilines with Propargyl Alcohols. J. Org. Chem. 2018;83:8509–8521. doi: 10.1021/acs.joc.8b01098. [DOI] [PubMed] [Google Scholar]
- 31.Fang F., Zhang C., Zhou C., Li Y., Zhou Y., Liu H. Rh(III)-Catalyzed C-H Activation of Benzoylacetonitriles and Tandem Cyclization with Diazo Compounds to Substituted Benzo[ de]chromenes. Org. Lett. 2018;20:1720–1724. doi: 10.1021/acs.orglett.8b00103. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Li J., Wu X., Zhou Y., Liu H. Rh(III)-Catalyzed C-H Cyclization of Arylnitrones with Diazo Compounds: Access to 3-Carboxylate Substituted N-Hydroxyindoles. J. Org. Chem. 2017;82:8984–8994. doi: 10.1021/acs.joc.7b01393. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Zhou J., Fang F., Xu B., Liu H., Zhou Y. Rhodium(III)-Catalyzed C-H Activation of alpha-Iminonitriles or alpha-Imino Esters and Cyclization with Acrylates to 2 H-Isoindoles. J. Org. Chem. 2018;83:11736–11746. doi: 10.1021/acs.joc.8b01664. [DOI] [PubMed] [Google Scholar]
- 34.Wu X., Wang B., Zhou S., Zhou Y., Liu H. Ruthenium-Catalyzed Redox-Neutral [4 + 1] Annulation of Benzamides and Propargyl Alcohols via C–H Bond Activation. ACS Catal. 2017;7:2494–2499. doi: 10.1021/acscatal.7b00031. [DOI] [Google Scholar]
- 35.Yang Q., Wu C., Zhou J., He G., Liu H., Zhou Y. Highly selective C–H bond activation of N-arylbenzimidamide and divergent couplings with diazophosphonate compounds: A catalyst-controlled selective synthetic strategy for 3-phosphorylindoles and 4-phosphorylisoquinolines. Org. Chem. Front. 2019;6:393–398. doi: 10.1039/C8QO01148F. [DOI] [Google Scholar]
- 36.Zhou J., Li J., Li Y., Wu C., He G., Yang Q., Zhou Y., Liu H. Direct Synthesis of 3-Acylindoles through Rhodium(III)-Catalyzed Annulation of N-Phenylamidines with alpha-Cl Ketones. Org. Lett. 2018;20:7645–7649. doi: 10.1021/acs.orglett.8b03383. [DOI] [PubMed] [Google Scholar]
- 37.Kondo T., Taniguchi R., Kimura Y. Ruthenium- and Rhodium-Catalyzed Ring-Opening Coupling Reactions of Cyclopropenones with Alkenes or Alkynes. Synlett. 2018;29:717–722. doi: 10.1055/s-0037-1609339. [DOI] [Google Scholar]
- 38.Kong L., Zhou X., Xu Y., Li X. Rhodium(III)-Catalyzed Acylation of C(sp3)-H Bonds with Cyclopropenones. Org. Lett. 2017;19:3644–3647. doi: 10.1021/acs.orglett.7b01650. [DOI] [PubMed] [Google Scholar]
- 39.Xie F., Yu S., Qi Z., Li X. Nitrone Directing Groups in Rhodium(III)-Catalyzed C-H Activation of Arenes: 1,3-Dipoles versus Traceless Directing Groups. Angew. Chem. Int. Ed. 2016;55:15351–15355. doi: 10.1002/anie.201609658. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y., Tian Y., Su K., Wang P., Guo X., Chen B. Rhodium(iii)-catalyzed [3 + 3] annulation reactions of N-nitrosoanilines and cyclopropenones: An approach to functionalized 4-quinolones. Org. Chem. Front. 2019;6:3973. doi: 10.1039/C9QO01250H. [DOI] [Google Scholar]
- 41.Mochida S., Hirano K., Satoh T., Miura M. Synthesis of Functionalized α-Pyrone and Butenolide Derivatives by Rhodium-Catalyzed Oxidative Coupling of Substituted Acrylic Acids with Alkynes and Alkenes. J. Org. Chem. 2009;74:6295–6298. doi: 10.1021/jo901077r. [DOI] [PubMed] [Google Scholar]
- 42.Tamura Y., Bayomi S.M., Tsunekawa M., Ikeda M. Nucleophilic Reactions of N-Unsubstituted Sulfoximines with Activated Acetylenes, Olefins, and Diphenylcyclopropenone. Chem. Pharm. Bull. 1979;27:2137–2142. doi: 10.1248/cpb.27.2137. [DOI] [Google Scholar]
- 43.Simmons E.M., Hartwig J.F. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 44.Cai S., Zeng J., Li Y., Liu X.-W. Polysubstituted pyrrole derivatives via 1,2-alkenyl migration of novel γ-amino-α,β-unsaturated aldehydes and α-diazocarbonyls. RSC Adv. 2014;4:7275–7278. doi: 10.1039/c3ra47020b. [DOI] [Google Scholar]
- 45.Li X., Han C., Yao H., Lin A. Organocatalyzed [3 + 2] Annulation of Cyclopropenones and beta-Ketoesters: An Approach to Substituted Butenolides with a Quaternary Center. Org. Lett. 2017;19:778–781. doi: 10.1021/acs.orglett.6b03737. [DOI] [PubMed] [Google Scholar]
- 46.Vanos C.M., Lambert T.H. Development of a catalytic platform for nucleophilic substitution: Cyclopropenone-catalyzed chlorodehydration of alcohols. Angew. Chem. Int. Ed. 2011;50:12222–12226. doi: 10.1002/anie.201104638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.