Abstract
There is currently no effective treatment for Rett syndrome (RTT), a severe X-linked progressive neurodevelopmental disorder caused by mutations in the transcriptional regulator MECP2. Because MECP2 is subjected to X-inactivation, most affected individuals are female heterozygotes who display cellular mosaicism for normal and mutant MECP2. Males who are hemizygous for mutant MECP2 are more severely affected than heterozygous females and rarely survive. Mecp2 loss-of-function is less severe in mice, however, and male hemizygous null mice not only survive until adulthood, they have been the most commonly studied model system. Although heterozygous female mice better recapitulate human RTT, they have not been as thoroughly characterized. This is likely because of the added experimental challenges that they present, including delayed and more variable phenotypic progression and cellular mosaicism due to X-inactivation. In this review, we compare phenotypes of Mecp2 heterozygous female mice and male hemizygous null mouse models. Further, we discuss the complexities that arise from the many cell-type and tissue-type specific roles of MeCP2, as well as the combination of cell-autonomous and non-cell-autonomous disruptions that result from Mecp2 loss-of-function. This is of particular importance in the context of the female heterozygous brain, composed of a mixture of MeCP2+ and MeCP2− cells, the ratio of which can alter RTT phenotypes in the case of skewed X-inactivation. The goal of this review is to provide a clearer understanding of the pathophysiological differences between the mouse models, which is an essential consideration in the design of future pre-clinical studies.
1. Introduction
Rett syndrome (RTT) is a severe X-linked progressive neurodevelopmental disorder for which there is currently no effective treatment or cure. Males rarely survive past birth, and RTT impacts approximately 1:10,000 live female births (Chahrour and Zoghbi, 2007). Girls with RTT develop relatively normally for 6–18 months, after which they undergo a period of rapid regression, with loss of motor skills including purposeful hand movement, deceleration of head growth, and onset of repetitive, autistic behaviors (Hagberg, 1983; Rett, 1966). Social behavior and autonomic dysfunction, sleep problems, scoliosis, and seizures are also symptoms of this condition (Hagberg, 2005; Roze et al., 2007).
The discovery in 1999 that the majority of RTT cases are caused by mutations in the gene MECP2 (Amir et al., 1999), located on the X-chromosome, has led to an expansive field of research on the role of the transcriptional regulator MECP2 in brain function. Further, the monogenic nature of this disorder has led to the development of a number of mouse models of RTT. Mecp2 mutant mice exhibit a range of neurological abnormalities that recapitulate human RTT, and this model system has already provided crucial insight into the pathology of RTT. For example, selectively re-expressing Mecp2 in postmitotic neurons, either in the whole brain or neocortex and hippocampus, of adult mice has shown that RTT symptoms can be partially reversed (Giacometti et al., 2007; Guy et al., 2007; Luikenhuis et al., 2004), indicating that MeCP2 is necessary for both the development and maintenance of functional mature neurons (McGraw et al., 2011; Nguyen et al., 2012). These discoveries have uncovered the exciting potential of post-symptomatic reversal of RTT symptoms.
Although RTT is an X-linked disorder and human males rarely survive past birth, Mecp2 loss-of-function is less severe in mice and male hemizygous null mice (Mecp2−/y) not only survive until adulthood, they have been the most commonly studied model system to date and they have provided extensive insight into the molecular pathophysiology of Mecp2 loss-of-function. Heterozygous (Het; Mecp2+/−) female mice have not been as thoroughly characterized, likely because of the added experimental challenges that they present, including delayed and more variable phenotypic progression, and cellular mosaicism due to X-inactivation. However, they are a more clinically relevant RTT model and it is imperative that female heterozygotes are included in any studies of potential therapeutics (Katz et al., 2012). Further, because they have a more delayed phenotypic progression and longer lifespan than males, there is greater potential to investigate clinically relevant alterations in phenotypic development.
In this review, we discuss what is known about the phenotypes of Mecp2 Het female mice and the underlying pathophysiology, and we compare the female Het mice with the male hemizygous null mouse models. We discuss the lack of studies that compare the sexes and that employ the same phenotypic assessments in rescue or therapeutic intervention approaches. The goal of this review is to provide a clearer understanding of the pathophysiological differences between the mouse models, which is an essential consideration in the design of future pre-clinical studies.
2. MeCP2 is a transcriptional regulator with many functions
Although the genetic cause of RTT, mutations in the methyl CpG binding Protein 2 gene (MECP2; Mecp2 in mouse) was identified 20 years ago (Amir et al., 1999), the complex functions of the MeCP2 protein have hindered the elucidation of molecular disruptions underpinning RTT phenotypes. The many roles of MeCP2 have been comprehensively reviewed elsewhere (Bellini et al., 2014; Connolly and Zhou, 2019; Fasolino and Zhou, 2017; Guy et al., 2011; Horvath and Monteggia, 2018; Lyst and Bird, 2015); thus, we will only briefly summarize them here.
MeCP2 contains multiple domains, including the methyl-CpG-binding domain (MBD), transcriptional repression domain (TRD) and the nuclear repressor co-repressor (NCOR)-silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) interacting domain (NID), located within the TRD (Lyst et al., 2013; Nan et al., 1997, 1993). MeCP2 binds to DNA through its MBD domain and functions as a repressor of gene transcription by recruiting SIN3A, histone deacetylases and NCOR/SMRT co-repressors (Guo et al., 2014; Jones et al., 1998; Kokura et al., 2001; Lewis et al., 1992; Nan et al., 1998). In addition to interacting with methylated CpG dinucleotides, Mecp2 binds to methylated CpH (Guo et al., 2014). In fact, MeCP2 recruitment to methylated CpA plays a key role in the downregulation of long genes (Gabel et al., 2015). Additionally, because many of the dysregulated genes in Mecp2-mutant mice acquire high levels of methylated CpH as they mature, it has been proposed that the lack of MeCP2 binding to mCH as neurons mature could underpin the delayed onset of RTT symptoms (Chen et al., 2015).
Surprisingly, MeCP2 also acts as a transcriptional activator when it recruits cyclic AMP-responsive element-binding protein 1 (CREB1), with data suggesting that in the absence of Mecp2, a greater number of genes are downregulated than upregulated (Chahrour et al., 2008). Whether these transcriptional changes all represent direct targets of MeCP2 or secondary effects is not clear, however. It has been proposed that MeCP2 acts as a transcriptional repressor when it interacts with 5-methylcytosine (5mC) and as an activator when it binds to 5-hydroxymethylcytosine (5hmC) (Li et al., 2013; Mellén et al., 2012). However, more recent studies suggest that MeCP2 only has high affinity to 5hmCA and its affinity to 5hmCG is low, similar to unmethylated regions (Kinde et al., 2015; Mellén et al., 2017); therefore the low affinity of MeCP2 to 5hmCG would result in its diminished binding and consequently reduction in transcriptional repression by MeCP2 (Ip et al., 2018; Mellén et al., 2017).
Further, there is evidence that MeCP2 contributes to chromatin structural organization by displacing histone H1. Importantly, in the absence of Mecp2, there is an increase in histone deacetylation and histone H1, leading to alterations in chromatin structure (Nan et al., 1997; Skene et al., 2010). In addition, MeCP2 is involved in post-transcriptional regulation, evidenced by its enriched binding to alternatively spliced exons and by its interaction with Y box-binding protein 1 (YB-1), a protein that regulates RNA splicing events. Loss of MeCP2 leads to alterations in alternative splicing, in both mice and human cell lines (Maunakea et al., 2013; Young et al., 2005). MeCP2 also binds to DiGeorge syndrome critical region 8 (DGCR8), playing a role in miRNA processing by preventing the formation of the DGCR8-Drosha complex (Cheng et al., 2014).
With so many mechanisms of action and functions for MeCP2, it is not surprising that mutations in MECP2 lead to such a complex syndrome with several distinct symptoms. It should be noted that the majority of the mechanistic studies involving RTT model mice have been conducted in Mecp2-null mice, demonstrating their importance to RTT research. Even though the null model does not recapitulate the cellular mosaicism of human RTT, it has been invaluable for accelerating our understanding of MeCP2 and the underlying pathophysiology of RTT. However, due to the complete absence of MeCP2 in these mice, it is difficult to tease apart the direct effects of Mecp2 loss to the indirect outcomes of their severely compromised development, and further studies are needed to understand the mechanisms of action of MeCP2 in the context of the cellular mosaicism of the female Het brain.
3. Mecp2 Mutant Mouse Models
3.1. Female heterozygous mutant mice display milder phenotypes and delayed phenotypic progression
A number of Mecp2-mutant mouse models have been generated that recapitulate certain phenotypes of human RTT. Mecp2tm1.1Bird and Mecp2tm1.1Jae are the most common null allele models used, while Mecp2tm1Hzo (also known as Mecp2308) is one of the most common nonsense mutation models. The Mecp2tm1.1Bird model lacks exons 3 and 4, resulting in a complete loss of expression of Mecp2 mRNA and protein. Male null and female Het Mecp2tm1.1Bird mice display similar physical deficits, but with very distinct phenotypic progression timelines. These phenotypes include irregular gait and reduced mobility, breathing apneas, hindlimb clasping, reduced neuronal size and brain weight. Mecp2tm1.1Bird null males develop overt phenotypes as early as 3 weeks of age, while female Hets might not start showing obvious symptoms until 3 months. Further, female Het mice do not exhibit the rapid phenotypic progression seen in null males, which results in their early death at around 6 to 12 weeks of age (Guy et al., 2001).
The Mecp2tm1.1Jae model, on the other hand, lacks only exon 3 and, although mice carrying this allele do not express the full MeCP2 protein, they do exhibit peptides of smaller size. Similar physical deficits are seen in Mecp2tm1.1Jae null males, which display overt symptoms by 5 weeks of age. Het females, on the other hand, are apparently asymptomatic for the first four months of life, later developing aberrant gait, body tremors, and reduced activity as seen with null males and the Mecp2tm1.1Bird model (Chen et al., 2001; Guy et al., 2001). The Mecp2tm1Hzo model produces a truncated protein, recapitulating a common mutation found in RTT. A premature stop codon was engineered after codon 308 of Mecp2; thus, the allele maintains the MBD and TRD domains and the nuclear localization signal (NLS), but lacks the C-terminal third of the sequence. Similar to the null allele models, the heterozygous female mice exhibit milder phenotypes with a delayed symptom onset relative to males. In female Hets, overt symptoms appear after 1 year of age, while males start showing symptoms after 4 months of age, with signs of mild tremor appearing at 6 weeks. (Shahbazian et al., 2002b).
Due to the fact that the testes of Mecp2-null mice remain internal and they are not able to breed, generating Mecp2−/− female mice is uncommon. However, Guy et al (2001) generated Mecp2−null females by crossing female mice heterozygous for Mecp2 and for a Cre transgene on the X chromosome to males carrying an allele flanking exons 3 and 4 of the Mecp2 gene. The resulting Mecp2-null female mice displayed similar phenotypic progression as Mecp2-null males (Guy et al., 2001), suggesting that the delayed and highly variable phenotypic progression seen in Het females is due to their cellular mosaicism rather than an overall sex difference. Additional studies on Mecp2−/− female mice would be necessary, however, to determine if there are sex-based differences in the RTT model mice that are independent of the cellular mosaicism inherent in the commonly employed Het model.
3.2. Phenotypes of both male and female Mecp2 mutant mice depend on genetic background
Importantly, genetic background greatly alters phenotypic presentation in Mecp2 mutant mice (Table 1). For example, Mecp2tm1.1Bird male mice on a C57BL/6 background undergo rapid weight loss before death, while males with the same allele on a 129 strain are significantly heavier than wildtype (Guy et al., 2001). Similarly, Mecp2tm1.1Jae male mice maintained on a mixed background (129, C57BL/6 and BALB/c) display increased body weight (Chen et al., 2001), as do heterozygous Mecp2tm1.1Bird females on an FVB.129F1 (FVB/N x 129S6/SvEv) background. The latter are overweight starting at 8 weeks of age, while heterozygous females on a 129.B6F1 (129S6/SvEv x C57BL/6) background only appear to be heavier at 52 weeks. Weight gain in mice is altered by the neuropeptide somatostatin in the hypothalamus, which increases as the expression level of MeCP2 rises. Remarkably, the negative correlation between Mecp2 expression and weight gain is only observed in females on the FVB.129F1 background, but not the 129.B6F1 strain, even though both strains show weight gain with the progression of RTT (Samaco et al., 2013).
Table 1.
Phenotypic variation is dependent on both Mecp2 mutant allele and genetic background.
| Phenotype | Male | Female | |||
|---|---|---|---|---|---|
| Allele | Background | Allele | Background | ||
| Weight | Loss | Mecp2tm1.1Bird | C57BL/6 | ||
| Gain | Mecp2tm1.1Bird | 129 | Mecp2tm1.1Bird | FVB.129F1 | |
| Mecp2tm1.1Jae | 129, C57BL/6, BALB/c | 129.B6F1 (delayed) | |||
| Social behavior | Increased | Mecp2tm1.1Bird | 129S1/SvImJ × B6/CBA | Mecp2tm1.1Bird | FVB.129F1 * |
| Mecp2tm1.1Jae | C57BL/6 | 129.B6F1 * | |||
| Mecp2tm1Hzo | C57BL/6 | ||||
| Decreased | Mecp2tm1Hzo | 129/SvEv | - | - | |
| Anxiety behavior | |||||
| Light/dark box | Increased | Mecp2tm1Hzo | 129SvEv × C57BL/6J | - | - |
| Mecp2LSL | 129S6SvEv × FVB | ||||
| Mecp2LSL | 129S6SvEvTac × FVB | ||||
| Decreased | - | - | Mecp2tm1.1Bird | FVB.129F1 | |
| 129.B6F1 | |||||
| Open field | Increased | Mecp2tm1Hzo | 129SvEv × C57BL/6J | Mecp2tm1.1Bird | C57BL/6 |
| Mecp2tm1Hzo | C57BL/6 | ||||
| Decreased | - | - | - | - | |
| Elevated Plus Maze | Increased | Mecp2tm1Hzo | 129SvEv × C57BL/6J | - | - |
| Mecp2tm1Hzo | C57BL/6 | ||||
| Decreased | Mecp2LSL | 129S6SvEv × FVB | Mecp2LSL | 129S6SvEv × FVB | |
| Mecp2LSL | 129S6SvEvTac × FVB | Mecp2tm1.1Bird | C57BL/6 | ||
| Mecp2tm1Tam | 129/C57BL6 | FVB.129F1 | |||
| Mecp2tm1.1Jae | C57BL/6 | 129.B6F1 | |||
| Mecp2tm1.1Jae | C57BL/6 | ||||
| Zero Maze | Increased | Mecp2tm1Hzo | C57BL/6 | - | - |
| Decreased | Mecp2tm1.1Jae | C57BL/6 | Mecp2tm1.1Jae | C57BL/6 | |
Increased time spent with novel mouse when compared to object, but decreased time spent with novel mouse when compared to wildtype littermate
Dash (-) indicates absence of phenotype or phenotype not investigated
Mecp2LSL: Mecp2 gene floxed by a stop codon
Although Mecp2tm1.1Bird mutant mice on a CD-1 background display similar overall phenotypes to those on a C57BL/6 background, considerations should be made to metabolic alterations, such as cholesterol levels, which appear to depend more on the genetic background than neurological phenotypes. Genetic background also modifies litter size and maternal care, with Mecp2 heterozygous mice on a CD-1 background having larger litters with greater survival than C57BL/6 dams (Gigli et al., 2016). Maternal care is particularly important for symptom onset and severity in Mecp2-mutant mice. This is highlighted by cross-fostering pups between Mecp2+/− and wildtype dams, which results in differences in the onset of adult phenotypes and easier detection of behavioral deficits of heterozygous female mice when compared to their wildtype littermates (Vogel Ciernia et al., 2017).
The sociability of Mecp2 mutant mice is also variable and dependent on the allele and strain studied. For males, the Mecp2tm1.1Jae and Mecp2tm1.1Bird alleles are associated with hypersociability (Kerr et al., 2008; Schaevitz et al., 2010); however, Mecp2tm1Hzo males only display increased sociability on a C57BL/6 background (Pearson et al., 2012), and not on the 129/SvEv background (Moretti et al., 2005). Mecp2tm1.1Bird female mice on either a FVB.129F1 or 129.B6F1 background spend more time investigating a novel mouse than a novel object, but less time investigating the novel mouse than their wildtype littermates, suggesting behavioral impairments in social approach (Samaco et al., 2013).
3.3. Mecp2 mutant male and female mice share a subset of phenotypes
Although phenotypic progression and severity are highly divergent between the two sexes, a number of neurological phenotypes are consistently comparable between male hemizygous null and female Het mice. This includes a reduction in the volume of cortical and subcortical regions, with only minor sex differences in the Mecp2tm1Hzo model, mainly in the inferior and superior colliculi of the brainstem (Allemang-Grand et al., 2017). Further, both Mecp2-null and Het females display reduced dendritic complexity, spine density and soma area of cortical neurons (Belichenko et al., 2009; Fukuda et al., 2005; Kishi and Macklis, 2004; Rietveld et al., 2015; Stuss et al., 2012; Tropea et al., 2009; Wang et al., 2013). Disrupted contextual fear learning (Pelka et al., 2006a; Samaco et al., 2013) and inability to complete hippocampal dependent tasks (Stearns et al., 2007) are also consistently observed in both male and female Mecp2 mutant mice.
Other robust phenotypes observed in mice of both sexes, as well as RTT patients, are respiratory (Mancini et al., 2018; Roux et al., 2007) and cardiac dysfunction (McCauley et al., 2011). A study using Sarizotan, a 5-HT1a and dopamine D2-like receptor agonist, showed reduction in apneas and breathing irregularities in both male and female mice, with only respiratory frequency increased in females and not altered in male mice (Abdala et al., 2014). This drug is currently in clinical trial to assess its efficacy in treating breathing abnormalities of RTT patients (). Reduction in apnea frequency was also observed in Mecp2 Het female mice exposed to metabotropic glutamate receptor allosteric modulators. Metabotropic glutamate receptor 7 (mGlu7) is downregulated in the cortex and hippocampus of male and female Mecp2 mutant mice, in addition to RTT patients, and its modulation also improves synaptic transmission between Schaffer collaterals and CA1 in the hippocampus, regardless of sex (Gogliotti et al., 2017).
Additionally, male mice generated with a conditional Mecp2 allele that exhibit a ~50% reduction in the expression of Mecp2 showed intact pain sensitivity, which is dependent on spinal cord reflex, but impaired pain recognition, which is dependent on the communication between the spinal cord and the brain (Samaco et al., 2008). The same phenotype is observed in female mice and RTT patients (Downs et al., 2010; Samaco et al., 2013). This indicates that either pain response is a common result of the loss of Mecp2 independent of sex, or that the similarity in phenotype is due to the roughly 50% reduction of MeCP2 in the brain.
Anxiety-like behavioral phenotypes are even more complex, with results dependent on the paradigm used to test the behavior. The elevated plus and zero mazes consistently show lower anxiety-like behavior in both male and female mutant mice (Meng et al., 2016; Pelka et al., 2006b; Samaco et al., 2013; Stearns et al., 2007; Ure et al., 2016; Vogel Ciernia et al., 2017), with the exception of the Mecp2tm1Hzo model (De Filippis et al., 2010; McGill et al., 2006). However, the open field test usually evidences increased anxiety-like behavior in both sexes (McGill et al., 2006; Shahbazian et al., 2002a; Vogel Ciernia et al., 2017), while the light/dark box paradigm shows variable results, indicating anxiogenic effect in some cases (McGill et al., 2006; Meng et al., 2016; Ure et al., 2016) and anxiolytic effect in others (Samaco et al., 2013).
Taken together, these data suggest that a subset of RTT phenotypes are consistent between male and female Mecp2-mutant mice, and might respond similarly to therapeutic intervention. However, the preponderance of evidence indicates that male null and female Het mice have sex-specific responses to possible therapeutic interventions that must be considered.
4. MeCP2 has distinct functions in different circuits and cell types across the nervous system
The mammalian brain is comprised of a complex network of neuronal and glial subtypes, each with a distinct transcriptome and epigenome. MeCP2 is expressed in most (if not all) of these cells, but the molecular pathways regulated by MeCP2 are tissue- and cell-type specific and loss of MeCP2 function in defined CNS circuits results in distinct RTT phenotypes. To tease apart the contributions of different circuitries and brain regions to RTT phenotypes, cell type- and brain region-specific deletion and re-expression of Mecp2 have been employed. However, few studies have included both male and female mice in their experimental design, which is problematic when the severity of the phenotypes can be idiosyncratic to each sex, greatly impacting the potential for phenotypic rescue. Further, studies that have analyzed both males and female Mecp2 mutant mice have identified some distinct differences in phenotypes, highlighting the need to employ both sexes in such analyses.
4.1. Mecp2 mutant mice display sex-specific phenotypes in distinct neuronal subpopulations
An example of molecular phenotypic differences between males and females is found in the serotonergic system, which is more highly dysregulated in Mecp2-null mice than Het females (Vogelgesang et al., 2017). Behaviorally, loss of Mecp2 specifically in 5-hydroxytryptamine (5-HT) neurons leads to reduced depression-like and increased anxiety-like phenotypes in the novelty suppression feeding test in male Mecp2-null mice. In contrast, female Het mice display reduced anxiety-like behavior in the elevated plus maze test (Philippe et al., 2018). The authors suggest that the changes in behavior could be a result of increased expression of 5-HT1A autoreceptors due to the loss of MeCP2 enhancement of Deaf1-mediated repression in 5-HT neurons (Philippe et al., 2018).
Restoration of Mecp2 expression specifically in glutamatergic or inhibitory neurons also highlights the differences in male and female phenotypes, and possible limitations of preclinical studies that focus only on Mecp2-null mice. Female mice in which Mecp2 expression is maintained exclusively in glutamatergic neurons display more extensive phenotypic amelioration than male mice; for example, normalization of ataxia is only seen in Het females (Meng et al., 2016). In contrast, Het female mice expressing Mecp2 only in inhibitory neurons display less extensive phenotypic improvement than male null mice (Ure et al., 2016). The authors suggest that this phenotypic divergence is indicative of Mecp2 re-expression being less effective in inhibitory than glutamatergic neurons in Mecp2 mosaic Het brains (Meng et al., 2016; Ure et al., 2016).
Mecp2 mutant mice also display sex-specific differences relating to the cholinergic system. Mecp2 deficient mice express lower levels of choline acetyltransferase in a number of brain regions, such as basal forebrain and striatum (Ricceri et al., 2011; Zhou et al., 2017). With the injection of nicotine or nicotinic acetylcholine receptor (nAChR) agonist, male Mecp2-null mice displayed enhanced locomotion, contrary to the suppressive effect these agonists have on wildtype mice. Het female mice, on the other hand, show distinctive phenotypes after nicotine exposure, such as Straub tail, a dorsiflexion of the tail used to measure nicotine sensitivity in mice. Similar phenotypic heterogeneity was found in the expression levels of nAChR subtypes in the midbrain of RTT mice, with males having a significant reduction in α4 and α6 subtype mRNA levels, while females only showed differential expression of α6 (Leung et al., 2017). This could contribute to the sex difference seen in the behavioral response to nicotine exposure. Additionally, the loss of Mecp2 in the cholinergic system impairs memory recognition of Mecp2-null mice, a phenotype that can be rescued with the chronic administration of the acetylcholinesterase inhibitor donepezil (Ballinger et al., 2019).
The hippocampi of Mecp2-null and female heterozygous mice, on the other hand, share similar properties. Increased hippocampal activation (Calfa et al., 2011; Li et al., 2017), and a reduction in soma size of hippocampal neurons is observed in both males and females. The same is true for soma size of neurons in the locus coeruleus, which display smaller and hyperexcitable neurons in both male and female mutant mice. However, there are features in the locus coeruleus that distinguish males and females; the decrease in tyrosine hydroxylase levels between MeCP2- in the Het brain and wildtype females is greater than difference seen between Mecp2-null and wildtype male mice (Taneja et al., 2009). Neuronal phenotypes in the neocortex are also consistent between male nulls and female Hets. In both, cortical neurons demonstrate reduced dendritic complexity, spine density, and soma size (Belichenko et al., 2009; Fukuda et al., 2005; Kishi and Macklis, 2004; Rietveld et al., 2015; Stuss et al., 2012; Tropea et al., 2009; Wang et al., 2013), as well as spontaneous excitatory input (Asgarihafshejani et al., 2019). Physical and morphological phenotypes of dopaminergic neurons in the substantia nigra are comparable between males and females as well. Both sexes of Mecp2 mutant mice have reduced cell capacitance and dopamine current density, and increased resistance, although male mice show more severe reduction in capacitance (Gantz et al., 2011). Thus, some neuronal characteristics might provide a consistent measure of potential phenotypic rescue between mutant mice of both sexes, but distinct responses can be expected in other neuronal populations.
4.2. Mecp2 loss-of-function in glia disrupts their function and alters neuronal circuitry
Initially, RTT was thought to be predominantly caused by the loss of Mecp2 in neurons since the protein is expressed up to ten times higher in neuronal cells than other cell types in the brain (Chen et al., 2001; Skene et al., 2010). However, more recently, Mecp2 loss-of-function in glial cells has been shown to centrally contribute to RTT pathogenesis, although sex differences have yet to be characterized in these important cellular populations. Expressing Mecp2 exclusively in astrocytes improves several RTT phenotypes, including locomotion and anxiety-like behavior, in addition to prolonging lifespan (Lioy et al., 2011). Mecp2-null astrocytes grow slower in vitro and do not mediate immune response as effectively as wildtype astrocytes. Further, Mecp2-null astrocytes alter wildtype neuronal phenotypes and fail to provide essential support for neuronal health. The negative impact of Mecp2-null astrocytes might become more pronounced with age; hippocampal astrocytes of Het female mice display lower MeCP2 expression at 7 months than at 1 month of age. This Mecp2 deficiency appears to spread through gap junctions, highlighting the negative impact that MeCP2− cells have on MeCP2+ cells (Maezawa et al., 2009).
Microglia also contribute to circuit disruption in RTT, although their role in the pathogenesis of the disorder has been controversial. Wang et al (2015) found that restoration of MeCP2 expression in microglia or the introduction of wildtype microglia in the brain via bone marrow transplantation does not rescue RTT phenotypes in mice, as had been previously reported (Derecki et al., 2012; Wang et al., 2015). Nevertheless, microglia are a known physiological mediator of synaptic pruning by eliminating unnecessary synaptic connections in the retinogeniculate system of Mecp2-null mice at postnatal day 5 (Schafer et al., 2012) and between postnatal days 30 and 60 (Hong et al., 2014; Schafer et al., 2012). Interestingly, Mecp2-null and wildtype microglia behave similarly in early postnatal and juvenile timepoints; however, after postnatal day 56, microglia lacking Mecp2 excessively prune synaptic connections. This period coincides with late symptomatic stages of Mecp2-null mice, indicating that microglia could enable late stage circuit defects in males (Schafer et al., 2016). Because female heterozygous mice become highly symptomatic at a later timepoint than male mice, it would be interesting to see if the same microglia defects would be found in this population, at a later timepoint.
5. Rett Syndrome phenotypes are influenced by patterns of X-chromosome inactivation
Our ability to distinguish differences between male Mecp2-null and female Mecp2 Het mice is further compounded by phenotypic variability caused by skewed X-inactivation. X-chromosome inactivation (XCI) is an event that occurs very early in development, around the time of implantation, in both mice and humans. It allows for dosage compensation between females and males, who only possess one X-chromosome. The process randomly silences either the maternal or paternal X-chromosome in all somatic cells. The inactivated chromosome (Xi) expresses a non-coding RNA called XIST, which is upregulated prior to Xi undergoing extensive epigenetic modulation, such as DNA methylation and histone modification, responsible for its silencing (Clemson et al., 1996; Escamilla-Del-Arenal et al., 2011; Kohlmaier et al., 2004; Penny et al., 1996; Shahbazian et al., 2002a; Sharp et al., 2011).
Although XCI is a random process and it is expected that Het female mice are mosaic for Mecp2 expression, with roughly 50% of cells expressing the wildtype allele while the other half express the mutated allele, the transcript level of either allele can vary widely from 40 to 85% (Braunschweig et al., 2004; Samaco et al., 2013; Young and Zoghbi, 2004). Whilst the pattern of XCI is mostly skewed towards the wildtype allele in mice, it is interesting to note that MeCP2 protein levels in wildtype-expressing cells within the brain inversely correlates with the number of cells expressing the mutated Mecp2 allele (Braunschweig et al., 2004). Therefore, even with a favorable XCI ratio, overall MeCP2 protein level is decreased due to this non-cell-autonomous impact on the wildtype cells. Hence, it is problematic to base pre-clinical research only on male mice lacking any MeCP2 expression, since these animals will not show the impact the mutated allele could have on wildtype cells, which could greatly alter phenotypic presentation.
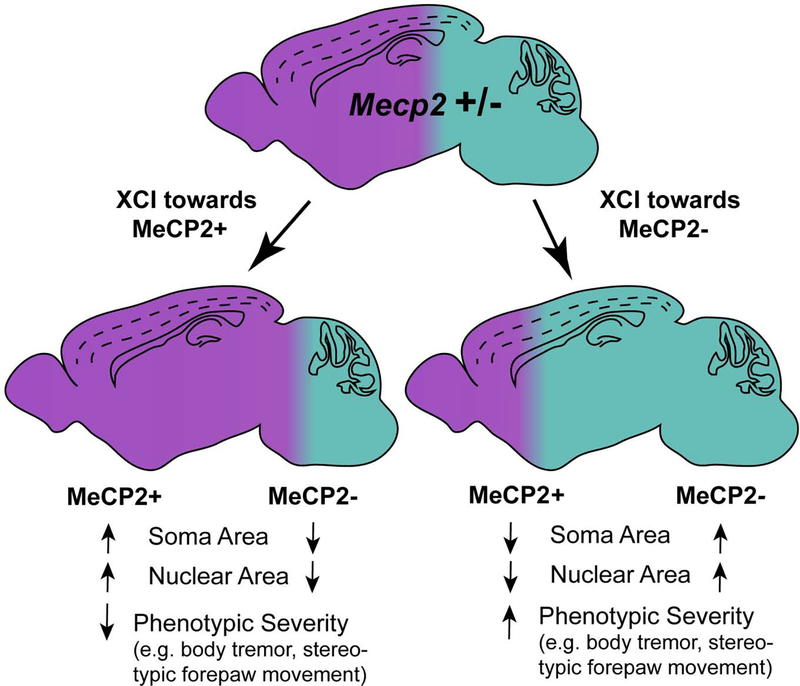
In mice, multiple phenotypes are influenced by the XCI ratio, with body tremor and stereotypic forepaw movements being highly susceptible to changes in the ratio (Young and Zoghbi, 2004). However, how mouse behavior is influenced by levels of MeCP2 is dependent on the region of the brain; protein expression in the cortex inversely correlates with overall phenotype severity, while MeCP2 levels in the cerebellum, hippocampus and spinal cord are not significantly correlated with overall phenotype severity. Specific behaviors, on the other hand, are correlated with MeCP2 expression levels in these regions. For example, open field activity increases as Het mice display higher MeCP2 expression in the hippocampus, but no correlation is found in the cortex, cerebellum or spinal cord. The same is true for anxiety-like behavior (Wither et al., 2013). This disparity is not only restricted to physical or behavioral phenotypes as it has been shown that neuronal morphology is also impacted by the ratio of cells expressing wildtype or mutant Mecp2; the nuclear area of cells expressing MeCP2 rises with the increase in the number of cells expressing the wildtype Mecp2 allele, while the nuclear area of the cells expressing the null allele decreases (Rietveld et al., 2015). This shows that neurons expressing the mutant Mecp2 allele will have a more severe phenotype when surrounded by skewed XCI favoring the wildtype allele (Fig. 1), perhaps indicating the inability of the null neurons to compete with the wildtype expressing cells. In addition, the soma size of MeCP2+ neurons is also reduced in mosaic brains when compared to neurons in wildtype brains, demonstrating a non-cell autonomous effect impacted by variable XCI (Rietveld et al., 2015; Wither et al., 2013).
Figure 1: Skewed X-Chromosome Inactivation alters RTT phenotypes in Mecp2 heterozygous female brain.
The female Het brain is a mosaic of cells expressing the wildtype allele (MeCP2+) and those expressing the mutant or null allele (MeCP2−). Although a 1:1 ratio of MeCP2+ and MeCP2− cells is the norm (top), skewed X-chromosome inactivation (XCI) can lead to an increase in the relative percentage of either the MeCP2+ cells (bottom left) or the MeCP2− cells (bottom right). This change in the overall cellular environment alters specific phenotypes of both MeCP2+ and MeCP2− neurons, through non-cell-autonomous mechanisms. For both MeCP2+ and MeCP2− neurons, the direction of phenotypic change (arrows) is depicted relative to the same cell type under balanced (1:1) XCI. Magenta indicates expression of wildtype allele and cyan indicates expression of null allele.
In contrast to Mecp2 mutant mouse models of the disorder, only a few cases of skewed XCI pattern have been documented in RTT patients, with balanced XCI predominantly found in the population (LaSalle, J. M., Goldstine, J., Balmer, D., Greco, 2001; Shahbazian et al., 2002a). However, the small number of variable XCI cases seen in patients could be explained by undiagnosed cases in which a skewed ratio towards wildtype MECP2 expression could be neurologically protective, preventing or lessening RTT symptoms (Amir et al., 2000; Knudsen et al., 2006; Young and Zoghbi, 2004; Zhang et al., 2012). The paternal X-chromosome is the most commonly inactivated in instances where there is skewed XCI in humans (Nielsen et al., 2001); because de novo mutations in MECP2 usually have a paternal origin (Girard et al., 2001; Trappe et al., 2001; X. Zhang et al., 2012), this could enhance the protective effect of skewed XCI.
In humans (and in mice), different mutations in MECP2 underpin phenotypic variability. For example, missense mutations are more likely to result in scoliosis while truncating mutations frequently cause breathing abnormalities (Amir et al., 2000). Despite this phenotypic variability, the major determinant of RTT phenotypes is still the XCI ratio, which will most likely determine if the patient will or will not meet diagnostic criteria for RTT (Amir et al., 2000; Wan et al., 1999). This further highlights the importance of employing female mice, which are also subject to XCI, in RTT research.
6. MeCP2 exerts both cell autonomous and non-cell-autonomous control
The impact that skewed XCI ratios have not only on cells expressing the mutated allele of Mecp2, but also on the cells expressing the wildtype allele reinforces that Mecp2 mutant phenotypes result from both cell-autonomous and non-cell-autonomous disruptions. For example, Mecp2-null neocortical projection neurons exhibit reduced dendritic arborization even when transplanted into the cortex of wildtype mice; the reduction is not worsened when Mecp2-null neurons are transplanted in Mecp2-null cortices, indicating that the loss of Mecp2 is the main contributor to this phenotype. In contrast, wildtype neurons transplanted into the Mecp2-null cortex demonstrate reduced soma area, similar to null-neurons, indicating a non-cell-autonomous impact on soma size (Kishi and Macklis, 2010).
6.1. MeCP2+ and MeCP2− cells in the Het brain are distinct from wildtype and null
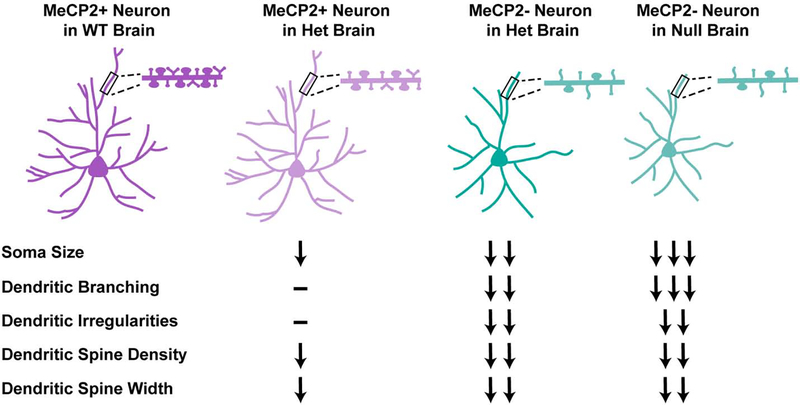
Similar observations of both cell autonomous and non-cell-autonomous effects of Mecp2 loss-of-function can be made when comparing wildtype (MeCP2+) with null (MeCP2−) layer V-VI pyramidal neurons in the motor cortex of Het female mice (Fig. 2). In addition to altered dendritic spine density and dendritic width in MeCP2− neurons, disruptions were seen between MeCP2+ cells in Het female brain and wildtype littermates. However, the number of dendritic spines on MeCP2+ neurons was reduced when compared to MeCP2− neurons, which was similar to the number of spines found in the brains of Mecp2-null male mice, indicating the influence of both cell autonomous and non-cell-autonomous mechanisms in this phenotype. In contrast, the number of dendrites with irregularities, such as narrowing and swelling, appears to be influenced only by cell autonomous processes, since MeCP2+ cells are not different from wildtype neurons, while MeCP2− and Mecp2-null neurons show a significant increase in frequency of those dendrites when compared to wildtype controls (Belichenko et al., 2009). Soma and nuclear size of MeCP2+ cortical neurons in a Het brain are also significantly reduced relative to those in a wildtype brain, although they are not quite as small as MeCP2− neurons. Overall dendritic length and branching of MeCP2+ neurons is not different from wildtype, on the other hand, but is significantly reduced in MeCP2− neurons (Rietveld et al., 2015). Further, electrophysiological parameters of cortical neurons are under the influence of cell autonomous (reduction in excitatory tone) and non-cell-autonomous (increased IPSCs frequency) mechanisms as well (Asgarihafshejani et al., 2019). Thus, RTT neuronal morphology phenotypes are comprised of both cell-autonomous and non-cell-autonomous disruptions.
Figure 2: Neurons display both cell-autonomous and cell-non-autonomous disruptions in the female heterozygous brain.
Mecp2-mutant neurons in the brains of both female Hets and male nulls display aberrant size and morphology phenotypes. MeCP2− neurons in a Het brain display distinct morphologies from MeCP2+ neurons; however, they are not as severely disrupted as MeCP2− neurons in a null brain. In addition, MeCP2+ neurons in a Het brain are distinct from MeCP2+ neurons in a wildtype (WT) brain, for example, displaying decreased soma size and dendritic spine density. Other phenotypes, such as dendritic branching and morphology, are dependent only on MeCP2 expression within that neuron. Thus, specific phenotypes respond differently to the cellular environment, indicating that they are controlled by distinct cell-autonomous and cell-non-autonomous mechanisms. Arrows denote phenotypic change relative to MeCP2+ in a WT brain, dash indicates no change.
Soma size and synaptic connectivity have been shown to be influenced by cell-autonomous and non-cell-autonomous mechanisms in the hippocampus as well. In the absence of MeCP2, there is a decline in Brain-derived neurotrophic factor (BDNF) synthesis and release, which results in a reduction of soma area, nuclear size, and dendritic length of hippocampal neurons, in addition to diminished glutamatergic synaptic outputs in vitro. The same morphological and presynaptic defects are observed in wildtype neurons in which the BDNF pathway is blocked (Sampathkumar et al., 2016). Importantly, increasing the expression of BDNF in vitro, either by normalizing its production in the Mecp2-null neurons or by exogenous application to cell culture medium, rescued both the synaptic and morphological defects of Mecp2-null hippocampal neurons. Overexpressing BDNF in wildtype neurons co-cultured with Mecp2-null neurons did not rescues their phenotypes, however, indicating that BDNF regulates dendritic complexity in a cell-autonomous and autocrine manner (Sampathkumar et al., 2016). The BDNF pathway could play a similar role in this phenotype within cortical neurons, which also express lower levels of BDNF (Chang et al., 2006).
Mecp2 loss-of-function also leads to both cell-autonomous and non-cell-autonomous disruptions in gene expression. In the brain of Het female mice, MeCP2− and MeCP2+ cortical pyramidal neurons display distinct patterns of gene expression, demonstrating cell autonomous disruptions in the regulation of gene expression by the loss of MeCP2 (Johnson et al., 2017). However, a large of number of genes are also differentially expressed in MeCP2+ neurons from Het female mice when compared to wildtype neurons from a control mouse brain. Importantly, these non-cell-autonomous disruptions in transcriptional regulation are more prevalent in mice carrying the R106W human mutation, which results in severe RTT phenotype, than in those with the T158M mutation, which has a less severe phenotype, suggesting that disease progression drives these secondary changes in gene expression. Interestingly, the non-cell-autonomous differentially expressed genes are predominantly associated with cell-to-cell signaling and protein phosphorylation while the cell-autonomous differentially expressed genes are associated with transcriptional regulation (Johnson et al., 2017). Another study found that the genes that are differentially expressed between MeCP2+ neurons in wildtype and Het mouse brains are associated with neuronal activity-dependent gene expression and neurotrophin signaling. In agreement with the previous study, the non-cell-autonomous differentially expressed genes do not seem to be directly regulated by MeCP2, mostly likely resulting from indirect effects of the mosaic RTT brain environment (Renthal et al., 2018).
6.2. MeCP2+ and MeCP2− glia alter neuronal morphology and function via non-cell-autonomous mechanisms
Wildtype hippocampal neurons co-cultured with astrocytes derived from Mecp2-null mice or with astrocytic conditioned media (ACM) from Mecp2-null and wildtype astrocytes, mimicking Mecp2 mosaicism in heterozygous brains, fail to thrive, displaying a reduction in neuronal processes and neuronal density (Ballas et al., 2009). Wildtype mouse hippocampal neurons co-cultured with astrocytes differentiated from induced pluripotent stem cell (iPSC) from RTT patients carrying 3 distinct MECP2 mutations similarly show reduced neuronal outgrowth, when compared to wildtype neurons cultured on isogenic control iPSC-derived astrocytes (Williams et al., 2014). Additionally, culturing Mecp2-null hippocampal neurons with ACM derived from wildtype astrocytes ameliorates disrupted neuronal growth (Ballas et al., 2009), providing further evidence of the non-cell-autonomous influence of glia in RTT pathology. Loss of Mecp2 specifically in astrocytes also impairs synaptic transmission. Proper neuronal synaptic response to astrocyte stimulation only occurs in the presence of an astrocyte that expresses Mecp2; an MeCP2+ neuron within a Het brain will demonstrate impaired synaptic transmission if coupled with an MeCP2− astrocyte (Rakela et al., 2018).
These findings that Mecp2 loss-of-function exerts both cell-autonomous and non-cell-autonomous effects highlights the complexity of RTT. Further, it reinforces the need to study the molecular underpinnings of RTT and to investigate potential therapeutics within female Het mice. MeCP2+ and MeCP2− neurons likely respond differently to any intervention, and could modify the response of neighboring cells accordingly. Although male hemizygous null mice remove this variability and allow for the study of underlying mechanisms in a more straightforward context, they do not recapitulate the complexity of the human disorder.
7. Translation of Pre-Clinical Studies performed in mice to human RTT
Although behavioral and physical deficits of Mecp2 mutant mice are dependent on the allele, genetic background, and sex of the animal being studied, the most confounding aspect of RTT research is, perhaps, the fact that phenotypic rescue experiments in mice that show very promising results are not recapitulated in clinical trials. One such example is the drug desipramine, an antidepressant that inhibits norepinephrine reuptake, which was evaluated for breathing disorders in RTT patients. Preclinical experiments showed robust rescue in the number of apneas and tyrosine hydroxylase-expressing neurons in the brainstem, and increased lifespan with treatment of Mecp2-null mice with desipramine (Roux et al., 2007; Zanella et al., 2008). However, the clinical trial was unsuccessful, showing no improvement in the patients treated with desipramine when compared to the placebo group (Mancini et al., 2018). An underlying cause of such dichotomy could be that the pre-clinical research focused only on male mice.
Many of the insulin-like growth factor 1 (IGF1) preclinical studies were also done in male mice. Recombinant human IGF1 (rhIGF1) extends Mecp2-null lifespan, and improves apnea, bradycardia and locomotion in these animals (Castro et al., 2014; Mellios et al., 2014; Tropea et al., 2009). Females were only used to study visual plasticity due to the severe phenotypes of male mice in adulthood; in this study, rhIGF1 treatment curbed the enhanced critical period of heterozygous females (Castro et al., 2014). Although, a few studies with human female RTT patients found IGF1 treatment to be well tolerated and to improve some features of the disorder (Glaze et al., 2017; Khwaja et al., 2014; Pini et al., 2012), another clinical trial did not see significant improvement in patients, with some parameters worsening (O’Leary et al., 2018).
It will be interesting to observe the outcome of other clinical trials currently underway that were also based on preclinical studies focused on Mecp2-null mice, and whether the expanding use of female Het mouse models will improve our understanding of therapeutic targets. Any therapeutic approach will need to consider the immense challenges posed by the cell-type specific transcriptional targets and functions of MeCP2 and the unique stoichiometry of its expression between neurons and glia, in addition to the cellular mosaicism of the Het brain.
8. Summary
It would be a gross understatement to say that RTT syndrome is a complex disorder. MeCP2 has multiple functions, and the pathways that it regulates are cell-type and tissue-type specific. It is the combination of Mecp2 loss-of-function across these distinct cell types and circuits that leads to the amalgam of symptoms that characterize RTT. Teasing these apart and identifying the disruptions that are paramount for targeting in therapeutic intervention is a monumental task. In so doing, however, it is imperative to recognize the cellular mosaicism of the female Het brain as a critical factor in the pathophysiology of RTT. Therapeutic strategies for Mecp2 re-expression, such as via adeno-associated virus (AAV) Mecp2 transgenes, must consider the cellular mosaicism of the female Het brain in designing a targeting strategy. For example, exogenously expressing Mecp2 within the MeCP2+ cells as well as the MeCP2− cells would be highly detrimental as MeCP2 overexpression leads to severe neurological disruptions (Collins et al., 2004). Similarly, driving expression of Mecp2 at the same levels in glia as in neurons would likely lead to an over-expression phenotype as neurons express MeCP2 at a 10-fold higher level than other cell types in the brain. Further, Mecp2 loss-of-function causes both cell-autonomous and non-cell-autonomous disruptions in the brain. Thus, we must consider not only the phenotypes and molecular underpinnings of MeCP2− neurons and glia, but also the impact of these cells on MeCP2+ cells. MeCP2+ cells in a Het brain are distinct from MeCP2+ cells in a wildtype brain, and MeCP2− cells in a Het brain are distinct from MeCP2− cells in a male hemizygous null brain. These variations contribute to the distinct phenotypes observed in MeCP2 mutant female mice in comparison to male null mice, as well as their increased variability in phenotypic progression.
Highlights.
Rett syndrome is caused by mutations in the X-linked gene MECP2
Male Mecp2 hemizygous null mice have been the predominantly studied model system
Female heterozygotes are a mosaic of MeCP2+ and MeCP2− cells
Mecp2 loss-of-function causes both cell-autonomous and non-cell-autonomous disruptions
Male and female Mecp2 mutant mice display sex-specific phenotypic differences
Acknowledgments
Funding: This work was supported by the National Institutes of Health [Grant number 1R01NS106285], and the International Rett Syndrome Foundation [Grant number 30534].
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5hmC
5-hydroxymethylcytosine
- 5mC
5-methylcytosine
- AAV
adeno-associated virus
- ACM
Astrocytic conditional media
- BDNF
Brain-derived neurotrophic factor
- CREB1
Cyclic AMP-responsive element-binding protein 1
- DGCR8
Di George syndrome critical region 8
- Het
Heterozygous
- IGF1
Insulin-like growth factor 1
- IPSC
Inhibitory postsynaptic current
- iPSC
Induced pluripotent stem cell
- MBD
Methyl-CpG-binding domain
- MECP2
Methyl CpG binding protein 2
- mGlu7
Metabotropic glutamate receptor 7
- nAChR
Nicotinic acetylcholine receptor
- NCOR
Nuclear repressor co-repressor
- NID
NCOR-SMRT interacting domain
- NLS
Nuclear localization signal
- rhIGF1
Recombinant human insulin-like growth factor 1
- RTT
Rett syndrome
- SMRT
Silencing mediator of retinoic acid and thyroid hormone receptor
- TRD
Transcriptional repression domain
- XCI
X-chromosome inactivation
- Xi
Inactivated X-chromosome
- YB-1
Y box-binding protein
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala AP, Lioy DT, Garg SK, Knopp SJ, Paton JFR, Bissonnette JM, 2014. Effect of sarizotan, a 5-HT1a and D2-like receptor agonist, on respiration in three mouse models of rett syndrome. Am. J. Respir. Cell Mol. Biol 50, 1031–1039. 10.1165/rcmb.2013-0372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allemang-Grand R, Ellegood J, Spencer Noakes L, Ruston J, Justice M, Nieman BJ, Lerch JP, 2017. Neuroanatomy in mouse models of Rett syndrome is related to the severity of Mecp2 mutation and behavioral phenotypes. Mol. Autism 8, 1–13. 10.1186/s13229-017-0138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van Den Veyver IB, Schultz R, Malicki DM, Tran CQ, Dahle EJ, Philippi A, Timar L, Percy AK, Motil KJ, Lichtarge O, Smith EOB, Glaze DG, Zoghbi HY, 2000. Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann. Neurol 47, 670–679. [DOI] [PubMed] [Google Scholar]
- Amir RE, van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY, 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet 23, 185–188. [DOI] [PubMed] [Google Scholar]
- Asgarihafshejani A, Nashmi R, Delaney KR, 2019. Cell-Genotype Specific Effects of Mecp2 Mutation on Spontaneous and Nicotinic Acetylcholine Receptor-Evoked Currents in Medial Prefrontal Cortical Pyramidal Neurons in Female Rett Model Mice. Neuroscience 414, 141–153. 10.1016/j.neuroscience.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G, 2009. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat. Neurosci 12, 311–317. 10.1038/nn.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger EC, Schaaf CP, Patel AJ, de Maio A, Tao H, Talmage DA, Zoghbi HY, Role LW, 2019. Mecp2 deletion from cholinergic neurons selectively impairs recognition memory and disrupts cholinergic modulation of the perirhinal cortex. eNeuro 6, 1–13. 10.1523/ENEURO.0134-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Mobley WC, 2009. Evidence for both neuronal cell autonomous and nonautonomous effects of methyl-CpG-binding protein 2 in the cerebral cortex of female mice with Mecp2 mutation. Neurobiol. Dis 34, 71–77. 10.1016/j.nbd.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Bellini E, Pavesi G, Barbiero I, Bergo A, Chandola C, Nawaz MS, Rusconi L, Stefanelli G, Strollo M, Valente MM, Kilstrup-Nielsen C, Landsberger N, 2014. MeCP2 post-translational modifications: A mechanism to control its involvement in synaptic plasticity and homeostasis. Front. Cell. Neurosci 8, 1–15. 10.3389/fncel.2014.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Simcox T, Samaco RC, LaSalle JM, 2004. X-chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum. Mol. Genet 13, 1275–1286. 10.1093/hmg/ddh142 [DOI] [PubMed] [Google Scholar]
- Calfa G, Hablitz JJ, Pozzo-Miller L, 2011. Network hyperexcitability in hippocampal slices from Mecp2 mutant mice revealed by voltage-sensitive dye imaging. J. Neurophysiol 105, 1768–1784. 10.1152/jn.00800.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Garcia RI, Kwok S, Banerjee A, Petravicz J, Woodson J, 2014. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome. PNAS 111, 9941–9946. 10.1073/pnas.1311685111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY, 2008. MeCP2, a Key Contributor to Neurological Disease, Activates and Represses Transcription. Science (80-.). 320, 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY, 2007. The Story of Rett Syndrome: From Clinic to Neurobiology. Neuron 56, 422–437. 10.1016/j.neuron.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R, 2006. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49, 341–348. 10.1016/j.neuron.2005.12.027 [DOI] [PubMed] [Google Scholar]
- Chen L, Chen K, Lavery LA, Baker SA, Shaw CA, Li W, Zoghbi HY, 2015. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc. Natl. Acad. Sci. U. S. A 112, 5509–5514. 10.1073/pnas.1505909112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R, 2001. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet 27, 327–331. 10.1038/85906 LK - http://limo.libis.be/resolver?&sid=EMBASE&issn=10614036&id=doi:10.1038%2F85906&atitle=Deficiency+of+methyl-CpG+binding+protein-2+in+CNS+neurons+results+in+a+Rettlike+phenotype+in+mice&stitle=Nat.+Genet.&title=Nature+Genetics&volume=27&issue=3&spage=327&epage=331&aulast=Chen&aufirst=Richard+Z.&auinit=R.Z.&aufull=Chen+R.Z.&coden=NGENE&isbn=&pages=327-331&date=2001&auinit1=R&auinitm=Z [DOI] [PubMed] [Google Scholar]
- Cheng TL, Wang Z, Liao Q, Zhu Y, Zhou WH, Xu W, Qiu Z, 2014. MeCP2 Suppresses Nuclear MicroRNA Processing and Dendritic Growth by Regulating the DGCR8/Drosha Complex. Dev. Cell 28, 547–560. 10.1016/j.devcel.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Clemson CM, McNeil JA, Willard HF, Lawrence JB, 1996. XIST RNA paints the inactive X chromosome at interphase: Evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132, 259–275. 10.1083/jcb.132.3.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, Sweatt JD, Zoghbi HY, 2004. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet 13, 2679–2689. 10.1093/hmg/ddh282 [DOI] [PubMed] [Google Scholar]
- Connolly DR, Zhou Z, 2019. Genomic insights into MeCP2 function: A role for the maintenance of chromatin architecture. Curr. Opin. Neurobiol 59, 174–179. 10.1016/j.conb.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Ricceri L, Laviola G, 2010. Early postnatal behavioral changes in the Mecp2–308 truncation mouse model of Rett syndrome. Genes, Brain Behav 9, 213–223. 10.1111/j.1601-183X.2009.00551.x [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SBG, Guyenet PG, Kipnis J, 2012. Wild type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105–109. 10.1038/nature10907.Wild [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J, Géranton SM, Bebbington A, Jacoby P, Bahi-Buisson N, Ravine D, Leonard H, 2010. Linking MECP2 and pain sensitivity: The example of Rett syndrome. Am. J. Med. Genet. Part A 152, 1197–1205. 10.1002/ajmg.a.33314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla-Del-Arenal M, Da Rocha ST, Heard E, 2011. Evolutionary diversity and developmental regulation of X-chromosome inactivation. Hum. Genet 130, 307–327. 10.1007/s00439-011-1029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolino M, Zhou Z, 2017. The crucial role of DNA methylation and MeCP2 in neuronal function. Genes (Basel). 8 10.3390/genes8050141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto YI, 2005. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J. Neuropathol. Exp. Neurol 64, 537–544. 10.1093/jnen/64.6.537 [DOI] [PubMed] [Google Scholar]
- Gabel HW, Kinde B, Stroud H, Gilbert CS, Harmin DA, Kastan NR, Hemberg M, Ebert DH, Greenberg ME, 2015. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature 522, 89–93. 10.1038/nature14319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Ford CP, Neve KA, Williams JT, 2011. Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. J. Neurosci 31, 12629–12637. 10.1523/JNEUROSCI.0684-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R, 2007. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. PNAS 104, 1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli CC, Scaramuzza L, Gandaglia A, Bellini E, Gabaglio M, Parolaro D, Kilstrup-Nielsen C, Landsberger N, Bedogni F, 2016. MeCP2 related studies benefit from the use of CD1 as genetic background. PLoS One 11, 1–14. 10.1371/journal.pone.0153473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M, Couvert P, Carrié A, Tardieu M, Chelly J, Beldjord C, Bienvenu T, 2001. Parental origin of de novo MECP2 mutations in Rett syndrome. Eur. J. Hum. Genet. 9, 231–236. 10.1038/sj.ejhg.5200618 [DOI] [PubMed] [Google Scholar]
- Glaze DG, Neul JL, Percy A, Feyma T, Beisang A, Yaroshinsky A, Stoms G, Zuchero D, Horrigan J, Glass L, Jones NE, 2017. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Rett Syndrome. Pediatr. Neurol 76, 37–46. 10.1016/j.pediatrneurol.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Fisher NM, Adams J, Zamorano R, Walker AG, Blobaum AL, Engers DW, Hopkins CR, Daniels JS, Jones CK, Lindsley CW, Xiang Z, Conn PJ, Niswender CM, 2017. MGlu7 potentiation rescues cognitive, social, and respiratory phenotypes in a mouse model of Rett syndrome. Sci. Transl. Med 9, 1–12. 10.1126/scitranslmed.aai7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Shin JH, Shin J, Li H, Xie B, Zhong C, Hu S, Le T, Fan G, Zhu H, Chang Q, Gao Y, Ming GL, Song H, 2014. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat. Neurosci 17, 215–222. 10.1038/nn.3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A, 2011. The Role of MeCP2 in the Brain. Annu. Rev. Cell Dev. Biol 27, 631–652. 10.1146/annurev-cellbio-092910-154121 [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A, 2007. Reversal of Neurological Defects in a Mouse Model of Rett Syndrome. Science (80-. ). 315, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A, 2001. A mouse Mecp2-null mutation causes neurological symptoms that mimic rett syndrome. Nat. Genet 27, 322–326. 10.1038/85899 [DOI] [PubMed] [Google Scholar]
- Hagberg B, 2005. Rett Syndrome : Long-Term Clinical Follow-Up. J. Child Neurol 722–727. [DOI] [PubMed] [Google Scholar]
- Hagberg B, 1983. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. ann neurol 14, 471–479. [DOI] [PubMed] [Google Scholar]
- Hong YK, Park SH, Litvina EY, Morales J, Sanes JR, Chen C, 2014. Refinement of the Retinogeniculate Synapse by Bouton Clustering. Neuron 84, 332–339. 10.1016/j.neuron.2014.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath PM, Monteggia LM, 2018. MeCP2 as an Activator of Gene Expression. Trends Neurosci 41, 72–74. 10.1016/j.tins.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JPK, Mellios N, Sur M, 2018. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci 19, 368–382. 10.1038/s41583-018-0006-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Zhao YT, Fasolino M, Lamonica JM, Kim YJ, Georgakilas G, Wood KH, Bu D, Cui Y, Goffin D, Vahedi G, Kim TH, Zhou Z, 2017. Biotin tagging of MeCP2 in mice reveals contextual insights into the Rett syndrome transcriptome. Nat. Med 23, 1203–1214. 10.1038/nm.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJC, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP, 1998. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet 19, 187–191. 10.1038/561 [DOI] [PubMed] [Google Scholar]
- Katz DM, Berger-Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo-Miller L, Blue ME, Christian D, Crawley JN, Giustetto M, Guy J, Howell CJ, Kron M, Nelson SB, Samaco RC, Schaevitz LR, Hillaire-Clarke CS, Young JL, Zoghbi HY, Mamounas LA, 2012. Preclinical research in Rett syndrome: Setting the foundation for translational success. DMM Dis. Model. Mech 5, 733–745. 10.1242/dmm.011007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Alvarez-saavedra M, Sáez MA, Saona A, Young JI, 2008. Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Hum. Mol. Genet 17, 1707–1717. 10.1093/hmg/ddn061 [DOI] [PubMed] [Google Scholar]
- Khwaja OS, Ho E, Barnes KV, O’Leary HM, Pereira LM, Finkelstein Y, Nelson CA, Vogel-Farley V, DeGregorio G, Holm IA, Khatwa U, Kapur K, Alexander ME, Finnegan DM, Cantwell NG, Walco AC, Rappaport L, Gregas M, Fichorova RN, Shannon MW, Sur M, Kaufmann WE, 2014. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A 111, 4596–4601. 10.1073/pnas.1311141111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinde B, Gabel HW, Gilbert CS, Griffith EC, Greenberg ME, 2015. Reading the unique DNA methylation landscape of the brain: Non-CpG methylation, hydroxymethylation, and MeCP2. Proc. Natl. Acad. Sci. U. S. A 112, 6800–6806. 10.1073/pnas.1411269112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD, 2010. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp. Neurol 222, 51–58. 10.1016/j.expneurol.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD, 2004. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell. Neurosci 27, 306–321. 10.1016/j.mcn.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Knudsen GPS, Neilson TCS, Pedersen J, Kerr A, Schwartz M, Hulten M, Bailey MES, Ørstavik KH, 2006. Increased skewing of X chromosome inactivation in Rett syndrome patients and their mothers. Eur. J. Hum. Genet 14, 1189–1194. 10.1038/sj.ejhg.5201682 [DOI] [PubMed] [Google Scholar]
- Kohlmaier A, Savarese F, Lachner M, Martens J, Jenuwein T, Wutz A, 2004. A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol. 2 10.1371/journal.pbio.0020171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokura K, Kaul SC, Wadhwa R, Nomura T, Khan MM, Shinagawa T, Yasukawa T, Colmenares C, Ishii S, 2001. The Ski Protein Family Is Required for MeCP2-mediated Transcriptional Repression. J. Biol. Chem 276, 34115–34121. 10.1074/jbc.M105747200 [DOI] [PubMed] [Google Scholar]
- LaSalle JM, Goldstine J, Balmer D, Greco CM, 2001. Quantitative localization of heterogeneous methyl-CpG-binding protein 2 (MeCP2) expression phenotypes in normal and Rett syndrome brain by laser scanning cytometry. Hum. Mol. Genet. 10, 1729–1740. 10.1093/hmg/10.17.1729 [DOI] [PubMed] [Google Scholar]
- Leung J, McPhee DM, Renda A, Penty N, Farhoomand F, Nashmi R, Delaney KR, 2017. MeCP2-deficient mice have reduced α4 and α6 nicotinic receptor mRNA and altered behavioral response to nicotinic agonists. Behav. Brain Res. 330, 118–126. 10.1016/j.bbr.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A, 1992. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to Methylated DNA. Cell 69, 905–914. 10.1016/0092-8674(92)90610-O [DOI] [PubMed] [Google Scholar]
- Li W, Bellot-Saez A, Phillips ML, Yang T, Longo FM, Pozzo-Miller L, 2017. A small-molecule TrkB ligand restores hippocampal synaptic plasticity and object location memory in Rett syndrome mice. DMM Dis. Model. Mech 10, 837–845. 10.1242/dmm.029959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Muffat J, Cheng AW, Orlando DA, Lovén J, Kwok SM, Feldman DA, Bateup HS, Gao Q, Hockemeyer D, Mitalipova M, Lewis CA, Vander Heiden MG, Sur M, Young RA, Jaenisch R, 2013. Global transcriptional and translational repression in human-embryonic- stem-cell-derived rett syndrome neurons. Cell Stem Cell 13, 446–458. 10.1016/j.stem.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G, 2011. A role for glia in the progression of Rett-syndrome. Nature 475, 497–500. 10.1038/nature10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikenhuis S, Giacometti E, Beard CF, Jaenisch R, 2004. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl. Acad. Sci 101, 6033–6038. 10.1073/pnas.0401626101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyst MJ, Bird A, 2015. Rett syndrome: A complex disorder with simple roots. Nat. Rev. Genet 16, 261–274. 10.1038/nrg3897 [DOI] [PubMed] [Google Scholar]
- Lyst MJ, Ekiert R, Ebert DH, Merusi C, Nowak J, Selfridge J, Guy J, Kastan NR, Robinson ND, De Lima Alves F, Rappsilber J, Greenberg ME, Bird A, 2013. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci 16, 898–902. 10.1038/nn.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW, 2009. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J. Neurosci 29, 5051–5061. 10.1523/JNEUROSCI.0324-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini J, Dubus JC, Jouve E, Roux JC, Franco P, Lagrue E, Castelnau P, Cances C, Chaix Y, Rougeot-Jung C, Cornu C, Desportes V, Vallée L, Bahi-Buisson N, Truillet R, Attolini L, Villard L, Blin O, Micallef J, 2018. Effect of desipramine on patients with breathing disorders in RETT syndrome. Ann. Clin. Transl. Neurol 5, 118–127. 10.1002/acn3.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunakea AK, Chepelev I, Cui K, Zhao K, 2013. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res 23, 1256–1269. 10.1038/cr.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang TW, Ward CS, Skinner S, Percy AK, Glaze DG, Wehrens XHT, Neul JL, 2011. Rett syndrome: Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: Implication for therapy in Rett syndrome. Sci. Transl. Med 3 10.1126/scitranslmed.3002982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY, 2006. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A 103, 18267–18272. 10.1073/pnas.0608702103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw CM, Samaco RC, Zoghbi HY, 2011. Adult neural function requires MeCP2. Science (80-. ). 333, 186 10.1126/science.1206593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellén M, Ayata P, Dewell S, Kriaucionis S, Heintz N, 2012. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell 151, 1417–1430. 10.1016/j.cell.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellén M, Ayata P, Heintz N, 2017. 5-Hydroxymethylcytosine accumulation in postmitotic neurons results in functional demethylation of expressed genes. Proc. Natl. Acad. Sci. U. S. A 114, E7812–E7821. 10.1073/pnas.1708044114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Woodson J, Garcia RI, Crawford B, Sharma J, Sheridan SD, Haggarty SJ, Sur M, 2014. β2-Adrenergic receptor agonist ameliorates phenotypes and corrects microRNA-mediated IGF1 deficits in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A 111, 9947–9952. 10.1073/pnas.1309426111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang W, Lu H, He LJ, Chen W, Chao ES, Fiorotto ML, Tang B, Herrera JA, Seymour ML, Neul JL, Pereira FA, Tang J, Xue M, Zoghbi HY, 2016. Manipulations of MeCP2 in glutamatergic neurons highlight their contributions to rett and other neurological disorders. Elife 5, 1–21. 10.7554/eLife.14199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY, 2005. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet 14, 205–220. 10.1093/hmg/ddi016 [DOI] [PubMed] [Google Scholar]
- Nan X, Campoy FJ, Bird A, 1997. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481. 10.1016/S0092-8674(00)81887-5 [DOI] [PubMed] [Google Scholar]
- Nan X, Meehan RR, Bird A, 1993. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res 21, 4886–4892. 10.1093/nar/21.21.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng H, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A, 1998. Transcriptional repressionby the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389. [DOI] [PubMed] [Google Scholar]
- Nguyen MVC, Du F, Felice CA, Shan X, Nigam A, Mandel G, Robinson JK, Ballas N, 2012. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J. Neurosci 32, 10021–10034. 10.1523/JNEUROSCI.1316-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Henriksen KF, Hansen C, Silahtaroglu A, Schwartz M, Tommerup N, 2001. MECP2 mutations in Danish patients with Rett syndrome: High frequency of mutations but no consistent correlations with clinical severity or with the X chromosome inactivation pattern. Eur. J. Hum. Genet 9, 178–184. 10.1038/sj.ejhg.5200600 [DOI] [PubMed] [Google Scholar]
- O’Leary HM, Kaufmann WE, Barnes KV, Rakesh K, Kapur K, Tarquinio DC, Cantwell NG, Roche KJ, Rose SA, Walco AC, Bruck NM, Bazin GA, Holm IA, Alexander ME, Swanson LC, Baczewski LM, Mayor Torres JM, Nelson CA, Sahin M, 2018. Placebo-controlled crossover assessment of mecasermin for the treatment of Rett syndrome. Ann. Clin. Transl. Neurol 5, 323–332. 10.1002/acn3.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Defensor EB, Pobbe RLH, Yamamoto LHL, Bolivar VJ, Blanchard DC, Blanchard RJ, 2012. Mecp2 truncation in male mice promotes affiliative social behavior. Behav. Genet 42, 299–312. 10.1007/s10519-011-9501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PPL, 2006a. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain 129, 887–898. 10.1093/brain/awl022 [DOI] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PPL, 2006b. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain 129, 887–898. 10.1093/brain/awl022 [DOI] [PubMed] [Google Scholar]
- Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N, 1996. Requirement for Xist in X chromosome inactivation. Nature 379, 131–137. 10.1038/379131a0 [DOI] [PubMed] [Google Scholar]
- Philippe TJ, Vahid-Ansari F, Donaldson ZR, Le François B, Zahrai A, Turcotte-Cardin V, Daigle M, James J, Hen R, Merali Z, Albert PR, 2018. Loss of MeCP2 in adult 5-HT neurons induces 5-HT1A autoreceptors, with opposite sex-dependent anxiety and depression phenotypes. Sci. Rep 8, 1–13. 10.1038/s41598-018-24167-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini G, Scusa MF, Congiu L, Benincasa A, Morescalchi P, Bottiglioni I, Di Marco P, Borelli P, Bonuccelli U, Della-Chiesa A, Prina-Mello A, Tropea D, 2012. IGF1 as a Potential Treatment for Rett Syndrome: Safety Assessment in Six Rett Patients. Autism Res. Treat 2012, 1–14. 10.1155/2012/679801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakela B, Brehm P, Mandel G, 2018. Astrocytic modulation of excitatory synaptic signaling in a mouse model of Rett syndrome. Elife 7, 1–23. 10.7554/eLife.31629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Boxer LD, Hrvatin S, Li E, Silberfeld A, Nagy MA, Griffith EC, Vierbuchen T, Greenberg ME, 2018. Characterization of human mosaic Rett syndrome brain tissue by single-nucleus RNA sequencing. Nat. Neurosci 21, 1670–1679. 10.1038/s41593-018-0270-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rett A, 1966. [On a unusual brain atrophy syndrome in hyperammonemia in childhood]. Wien. Med. Wochenschr 116, 723–726 (in German). [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Fuso A, Laviola G, 2011. Cholinergic hypofunction in MeCP2–308 mice: Beneficial neurobehavioural effects of neonatal choline supplementation. Behav. Brain Res 221, 623–629. 10.1016/j.bbr.2011.03.051 [DOI] [PubMed] [Google Scholar]
- Rietveld L, Stuss DP, McPhee D, Delaney KR, 2015. Genotype-specific effects of Mecp2 loss-of-function on morphology of Layer V pyramidal neurons in heterozygous female Rett syndrome model mice. Front. Cell. Neurosci 9, 1–17. 10.3389/fncel.2015.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux JC, Dura E, Moncla A, Mancini J, Villard L, 2007. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur. J. Neurosci. 25, 1915–1922. 10.1111/j.1460-9568.2007.05466.x [DOI] [PubMed] [Google Scholar]
- Roze E, Cochen V, Sangla S, Bienvenu T, Roubergue A, Leu-Semenescu S, Vidaihet M, 2007. Rett syndrome: An overlooked diagnosis in women with stereotypic hand movements, psychomotor retardation, Parkinsonism, and dystonia? Mov. Disord. 22, 387–389. 10.1002/mds.21276 [DOI] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL, 2008. A partial loss of function allele of Methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 17, 1718–1727. 10.1093/hmg/ddn062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mcgraw CM, Ward CS, Sun Y, Neul JL, Zoghbi HY, 2013. Female Mecp2+/− mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet. 22, 96–109. 10.1093/hmg/dds406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar C, Wu YJ, Vadhvani M, Trimbuch T, Eickholt B, Rosenmund C, 2016. Loss of MeCP2 disrupts cell autonomous and autocrine BDNF signaling in mouse glutamatergic neurons. Elife 5, 1–23. 10.7554/eLife.19374.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz LR, Moriuchi JM, Nag N, Mellot TJ, Berger-Sweeney J, 2010. Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol. Behav. 100, 255–263. 10.1016/j.physbeh.2009.12.025 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Heller CT, Gunner G, Heller M, Gordon C, Hammond T, Wolf Y, Jung S, Stevens B, 2016. Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. Elife 5, 1–19. 10.7554/eLife.15224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, 2012. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Sun Y, Zoghbi HY, 2002a. Balanced X chromosome inactivation patterns in the Rett syndrome brain. Am. J. Med. Genet. 111, 164–168. 10.1002/ajmg.10557 [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Young JI, Yuva-Paylor LA, Spencer CM, Antalffy BA, Noebels JL, Armstrong DL, Paylor R, Zoghbi HY, 2002b. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35, 243–254. 10.1016/S0896-6273(02)00768-7 [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Stathaki E, Migliavacca E, Brahmachary M, Montgomery SB, Dupre Y, Antonarakis SE, 2011. DNA methylation profiles of human active and inactive X chromosomes. Genome Res. 21, 1592–1600. 10.1101/gr.112680.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr ARW, James KD, Turner DJ, Andrews R, Bird AP, 2010. Neuronal MeCP2 Is Expressed at Near Histone-Octamer Levels and Globally Alters the Chromatin State. Mol. Cell 37, 457–468. 10.1016/j.molcel.2010.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns NA, Schaevitz LR, Bowling H, Nag N, Berger UV, Berger-Sweeney J, 2007. Behavioral and anatomical abnormalities in Mecp2 mutant mice: A model for Rett syndrome. Neuroscience 146, 907–921. 10.1016/j.neuroscience.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Stuss DP, Boyd JD, Levin DB, Delaney KR, 2012. MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS One 7, 1–11. 10.1371/journal.pone.0031896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB, 2009. Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. J. Neurosci 29, 12187–12195. 10.1523/JNEUROSCI.3156-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe R, Laccone F, Cobilanschi J, Meins M, Huppke P, Hanefeld F, Engel W, 2001. MECP2 Mutations in Sporadic Cases of Rett Syndrome Are Almost Exclusively of Paternal Origin. Am. J. Hum. Genet. 68, 1093–1101. 10.1086/320109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Dong DF, Flannery R, Jaenisch R, Sur M, 2009. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 106, 2029–2034. 10.1073/pnas.0812394106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ure K, Lu H, Wang W, Ito-Ishida A, Wu Z, He LJ, Sztainberg Y, Chen W, Tang J, Zoghbi HY, 2016. Restoration of Mecp2 expression in GABAergic neurons is sufficient to rescue multiple disease features in a mouse model of Rett syndrome. Elife 5, 1–21. 10.7554/eLife.14198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Ciernia A, Pride MC, Durbin-Johnson B, Noronha A, Chang A, Yasui DH, Crawley JN, Lasalle JM, 2017. Early motor phenotype detection in a female mouse model of Rett syndrome is improved by cross-fostering. Hum. Mol. Genet. 0, 1–16. 10.1093/hmg/ddx087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelgesang S, Niebert S, Renner U, Möbius W, Hülsmann S, Manzke T, Niebert M, 2017. Analysis of the serotonergic system in a mouse model of Rett syndrome reveals unusual upregulation of serotonin receptor 5b. Front. Mol. Neurosci 10, 1–15. 10.3389/fnmol.2017.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Lee SSJ, Zhang X, Houwink-Manville I, Song HR, Amir RE, Budden S, Naidu SB, Pereira JLP, Lo IFM, Zoghbi HY, Schanen NC, Francke U, 1999. Rett syndrome and beyond: Recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am. J. Hum. Genet. 65, 1520–1529. 10.1086/302690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I-TJ, Reyes A-RS, Zhou Z, 2013. Neuronal morphology in MeCP2 mouse models is intrinsically variable and depends on age, cell type, and Mecp2 mutation. Neurobiol. Dis. 58, 3–12. 10.1016/j.nbd.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wegener JE, Huang T-W, Sripathy S, DeJesus-Cortes H, PinXu6 ST, Knobbe W, Leko V, Britt J, Starwalt R, McDaniel LS, Ward C, Parra D, Newcomb B,L,U, Nourigat C, Flowers DA, Sean Cullen, Jorstad NL, Yang Y, Glaskova L, Vigneau S, Kozlitina J, Yetman MJ, Jankowsky JL, Reichardt SD, Reichardt HM, Gartner J, Bartolomei MS, Fang M, Loeb K, Keene CD, Bernstein I, Goodell M, Brat DJ, Huppke P, Neul JL, Bedalov A, Pieper AA, 2015. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521 10.1038/nature10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Zhong X, Mohamed A, Li R, Liu Y, Dong Q, Ananiev GE, Choongmok JC, Lin BR, Lu J, Chiao C, Cherney R, Li H, Zhang SC, Chang Q, 2014. Mutant astrocytes differentiated from Rett syndrome patients-specific iPSCs have adverse effects on wildtype neurons. Hum. Mol. Genet. 23, 2968–2980. 10.1093/hmg/ddu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wither RG, Lang M, Zhang L, Eubanks JH, 2013. Regional MeCP2 expression levels in the female MeCP2-deficient mouse brain correlate with specific behavioral impairments. Exp. Neurol. 239, 49–59. 10.1016/j.expneurol.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, Kang D, Richman R, Johnson JM, Berget S, Zoghbi HY, 2005. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2 102, 17551–17558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JI, Zoghbi HY, 2004. X-Chromosome Inactivation Patterns Are Unbalanced and Affect the Phenotypic Outcome in a Mouse Model of Rett Syndrome. Am. J. Hum. Genet. 74, 511–520. 10.1086/382228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Mebarek S, Lajard AM, Picard N, Dutschmann M, Hilaire G, 2008. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir. Physiol. Neurobiol. 160, 116–121. 10.1016/j.resp.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang X, Wang J, Li J, Wu Q, Wen Y, Zhao Y, Zhang X, Yao H, Wu X, Yu S, Wei L, Bao X, 2018. Genomic mosaicism in the pathogenesis and inheritance of a Rett syndrome cohort. Genet. Med. 21, 2–10. 10.1038/s41436-018-0348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao X, Zhang Jingjing, Zhao Y, Cao G, Pan H, Zhang Jie, Wei L, Wu X, 2012. Molecular characteristics of Chinese patients with Rett syndrome. Eur. J. Med. Genet. 55, 677–681. 10.1016/j.ejmg.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Zhou H, Wu W, Zhang Y, He H, Yuan Z, Zhu Z, Zhao Z, 2017. Selective preservation of cholinergic MeCP2 rescues specific Rett-syndrome-like phenotypes in MeCP2stop mice. Behav. Brain Res. 322, 51–59. 10.1016/j.bbr.2017.01.023 [DOI] [PubMed] [Google Scholar]