Abstract
Tumor ablation by nanosecond pulsed electric fields (nsPEF) is an emerging therapeutic modality. We compared nsPEF cytotoxicity for human cell lines of cancerous (IMR-32, Hep G2, HT-1080, and HPAF-II) and non-cancerous origin (BJ and MRC-5) under strictly controlled and identical conditions. Adherent cells were uniformly treated by 300-ns PEF (0–2000 pulses, 1.8 kV/cm, 50 Hz) on indium tin oxide-covered glass coverslips, using the same media and serum. Cell survival plotted against the number of pulses displayed three distinct regions (initial resistivity, logarithmic survival decline, and residual resistivity) for all tested cell types, but with differences in LD50 spanning as much as nearly 80-fold. The non-cancerous cells were less sensitive than IMR-32 neuroblastoma cells but more vulnerable than the other cancers tested. The cytotoxic efficiency showed no apparent correlation with cell or nuclear size, cell morphology, metabolism level, or the extent of membrane disruption by nsPEF. Increasing pulse duration to 9 µs (0.75 kV/cm, 5 Hz) produced a different selectivity pattern, suggesting that manipulation of PEF parameters can, at least for certain cancers, overcome their resistance to nsPEF ablation. Identifying mechanisms and cell markers of differential nsPEF susceptibility will critically contribute to the proper choice and outcome of nsPEF ablation therapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-016-2434-4) contains supplementary material, which is available to authorized users.
Keywords: Nanosecond electric pulses, Microsecond electric pulses, Cytotoxicity, Cancer ablation, Electroporation
Introduction
Intense pulsed electric fields (PEF) can compromise the integrity of the cell plasma membrane, the process referred to as electroporation or electropermeabilization [1–5]. PEF of 0.1–10 ms duration are broadly employed to introduce otherwise impermeable molecules into cells, including the intracellular delivery of cytotoxic agents, such as bleomycin to kill tumors [6–8]. Ablation of cancerous tissue can also be achieved by more intense PEF treatments which cause irreversible electroporation (IRE) [9–11]. The major advantage of IRE over other ablative techniques, such as radiofrequency ablation or cryotherapy, is that it is able to preserve sensitive structures, such as adjacent major blood vessels and the extracellular matrix [12–15]. A recent review outlining the use of IRE in 16 independent clinical trials revealed encouraging results for the treatment of tumors located in close proximity to major vessels and bile ducts, including those in the liver, pancreas, lungs, and kidneys [16]. The authors identified IRE as being safe and effective in humans, with significant tumor regression and prolonged survival.
More recent research has extended PEF ablation protocols to nanosecond-duration pulses (nsPEF). Unlike IRE, nsPEF permeabilizes not only the plasma membrane, but also intracellular membranous structures, such as the mitochondria, nucleus, and endoplasmic reticulum [17–21]. The electropores formed by nsPEF are smaller (less than 1.5 nm in diameter) compared to IRE, and they have unique ion channel-like conductive properties [22]. Downstream effects of nsPEF include calcium mobilization [20, 23–26], cell swelling and blebbing [27, 28], disassembly of actin structures [29], activation of phosphoinositide signaling [30], as well as the induction of apoptotic or necrotic cell death or autophagy [31–36]. The role of Maxwell–Wagner polarization in membrane charging and electroporation gradually diminishes as pulses get shorter, and is replaced by other mechanisms, such as dielectric stacking. Certain phenomena, including a so-called “bipolar cancellation,” are unique to nanosecond-duration pulses (perhaps including shorter microsecond range), whereas the effect of longer micro- and millisecond pulses is exactly the opposite [37–39].
Different interaction mechanisms and bioeffects of nsPEF, as compared to traditional IRE, expand the options and offer new opportunities for tumor and tissue ablation. Killing cancer cells by nsPEF has been extensively explored in vitro [3, 31, 40–43], followed by successful tumor ablation trials in animals [44–47] and in humans [48, 49], without recurrence and with minimal side effects. In addition to inducing cell death, nsPEF inhibited cell proliferation and angiogenesis in tumors [47, 50] and triggered an immune response, preventing secondary tumor growth [44, 51]. Hence, the use of nsPEF to ablate tumors presents a promising therapeutic modality.
One of the most widely contemplated aspects of IRE and nsPEF in cancer therapy is their potential to selectively target tumors while sparing healthy tissues. Early studies observed profound differences in sensitivity of various tumor and normal cell lines when exposed to either 100- [52] or 10-µs [53] pulses, while the threshold for electropermeabilization was not different. More recent studies using nsPEF have reported differential sensitivity of various cell lines, with pulse durations ranging from 10 to 300 ns [40, 41, 48, 54–56]. We observed that selectivity for cell killing by PEF decreases as pulse duration increases [41]. It was also suggested that suspension cultures are more sensitive to nsPEF than adherent cultures [40, 54]. Yang et al. recently reported that a cancer cell line, basal cell carcinoma, was more sensitive to nsPEF than its paired normal cell line [55]. However, this study only compared two cell lines, and it is uncertain whether this phenomenon will extend to other types of cancers. Ivey et al. suggested that a finely tuned PEF could preferentially kill cancer cells in a heterogeneous model [57]. Cholesterol content could be a factor that determines cells’ susceptibility to nanoelectroporation [58].
While the data indicate a potential for nsPEF to selectively kill certain types of cells, the findings could be affected by media and protocol differences. Small differences in media composition and serum, including even batch-to-batch variation, are known to have a major impact on the survival of cells after electroporation [59]. Therefore, differential sensitivity to nsPEF of cell lines maintained in different media could in fact be a combined effect of nsPEF and media composition. Exposure of large quantities of adherent cells to nsPEF is not straightforward, and in previous studies, it required the detachment of cells from their substrate, to treat cells by nsPEF in suspension. This detachment and subsequent re-attachment can alter the cell’s physiology and survival, and the nsPEF response of cells in suspension may not be fully representative of the effect on adherent cells. At present, the selectivity of nsPEF and their ability to target cancer cells is an area which needs further investigation.
We recently introduced a new method of PEF exposure devoid of the stressful cell handling [60]. Adherent cells are grown on glass coverslips with an electroconductive but optically transparent indium tin oxide (ITO) layer. PEF exposures are accomplished simply by aseptically placing a coverslip with adherent cells into a standard electroporation cuvette and delivering PEF, thus eliminating the steps of cell detachment and re-attachment (Fig. 1a). The added benefits are the lack of electric field shielding by other cells, the ease of media change if needed, and reduced labor, enabling high-throughput protocols. In the present study, this method was employed to compare nsPEF susceptibility of different human cancer and non-cancer cells. Possible confounding impact of growth media was eliminated by selecting cell lines with the same growth medium requirement and procuring the medium and serum in the amounts sufficient for the entire set of experiments (to avoid batch-to-batch variations).
Fig. 1.
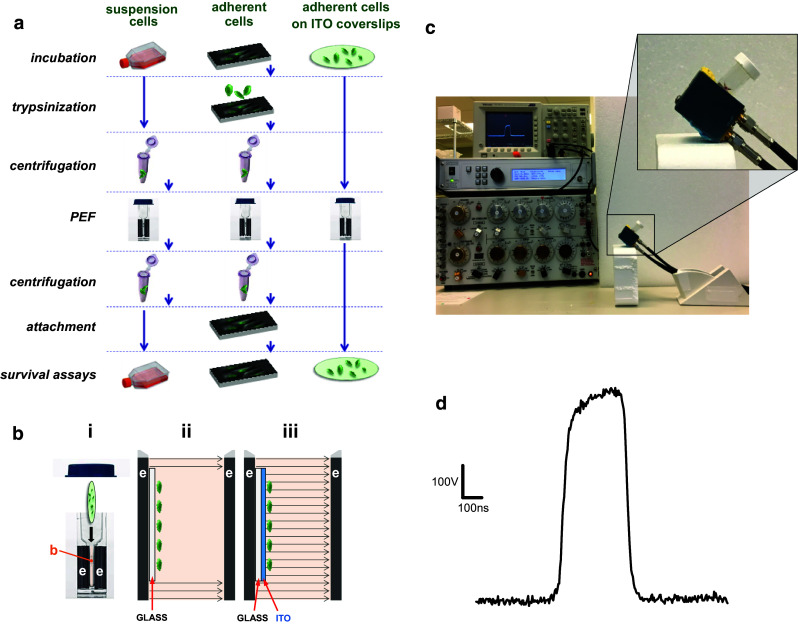
Streamlined pulsed electric field (PEF) treatment of adherent cells on coverslips with an electroconductive indium tin oxide (ITO) layer. a Standard sequence of procedures for PEF exposure in electroporation cuvettes for suspension-grown cells (left) and adherent cells (center). The use of ITO coverslips (right) eliminates multiple steps which may affect cell survival. b Schematic explaining PEF delivery to cells in an electroporation cuvette (i) with buffer (b) placed in a gap between two electrodes (e). Panels ii and iii show the enlarged view of this gap (not to scale) and the electric field lines when cells are attached to a “regular” glass coverslip (ii) or attached to an ITO-coated coverslip (iii). The regular glass coverslip shields cells from the electric field, whereas the ITO layer serves as an electrode and cancels shielding; see text for more details. c Exposure setup used in this study. The magnified area shows the electroporation cuvette tilted to keep the ITO coverslip resting flat on the bottom electrode. The ITO surface with cells is facing up into the medium. d Shape of a 300-ns pulse at 600 V (1.8 kV/cm)
Under these strict and uniform experimental conditions, we observed striking differences in susceptibility to nsPEF, with a nearly 80-fold difference between the most and the least sensitive cell lines. nsPEF was not selective against cancer cells, and its efficacy showed no apparent correlation with cell size, morphology, or tested physiological variables.
Materials and methods
Cell lines and culture conditions
Four cancer and two non-cancer human cell lines (Table 1) were chosen for comparison and obtained from American-Type Culture Collection (ATCC, Manassas, VA). All these cell lines required the same growth medium and were maintained at 37 °C, 5% CO2. The growth medium was the ATCC-formulated Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (certified OneShot format, FBS-OneShot, Life Technologies, Grand Island, NY), 100 IU/mL penicillin, and 0.1 µg/mL streptomycin (Corning Life Sciences, Tewksbury, MA). Both the media and FBS were purchased in amounts sufficient for the entire series of experiments to eliminate any variability in batches which might affect survival [59].
Table 1.
LD50 for 300-ns and 9-µs PEF for tested cell lines
| Cell line | 300 ns, 1.8 kV/cm, 50 Hz | 9 µs, 0.75 kV/cm, 5 Hz | ||||
|---|---|---|---|---|---|---|
| Number of pulses (LD50) ± 95% CIa | Absorbed dose (J/g)b | N c | Number of pulses (LD50) ± 95% CIa | Absorbed dose (J/g)b | N c | |
| Neuroblastoma (IMR-32) | 11.0 ± 4.3 | 0.16 | 193 | 1.6 ± 0.6 | 0.12 | 98 |
| Normal skin fibroblasts (BJ) | 22.5 ± 4.9 | 0.32 | 134 | – | – | – |
| Normal lung fibroblasts (MRC-5) | 45.7 ± 21.1 | 0.66 | 174 | – | – | – |
| Hepatocellular carcinoma (Hep G2) | 88.4 ± 18.8 | 1.27 | 139 | 2.0 ± 0.7 | 0.15 | 89 |
| Fibrosarcoma (HT-1080) | 132.7 ± 42.4 | 1.99 | 206 | – | – | – |
| Pancreatic adenocarcinoma (HPAF-II) | 841.3 ± 60.4 | 12.1 | 146 | 51.0 ± 20 | 3.8 | 75 |
a95% CI is a 95% confidence interval for the approximation of LD50 using a logarithmic fit. Non-overlapping confidence intervals indicate the significance of differences at p < 0.05 or better, see text for details
bAbsorbed dose, in J/g, corresponding to LD50 for the specified type of PEF exposure
cThe overall number of independent experiments conducted to generate the survival curve
PEF exposure in electroporation cuvettes
We employed a recently introduced technique for PEF exposure of adherent cells grown on glass coverslips with an electroconductive but optically transparent ITO layer, without cell detachment [60]. Approximately 6 h prior to PEF exposure, cells were harvested, diluted to approximately 0.2 × 106 cells/mL with fresh growth medium, and seeded on ITO coverslips (85 µL of cell suspension per coverslip). The goal was to achieve about 50% confluency by the time of PEF exposure, to make sure that the overnight growth of PEF- and sham-exposed samples was not restricted even if there was no cell death. We utilized 8 mm diameter, # 1.5 thickness glass coverslips covered with ITO to the sheet resistance of 8–12 Ohms/sq by Diamond Coatings Ltd. (Halesowen, UK). The ITO surface was coated with poly-l-lysine (Sigma-Aldrich, St. Louis, MO) to improve cell adherence.
ITO coverslips with cells attached were aseptically loaded into standard 1-mm gap electroporation cuvettes (BioSmith Biotech, San Diego, CA) filled with the exposure medium (EMEM supplemented with FBS-OneShot and 10 mM HEPES). The addition of HEPES was intended to maintain the constant pH 7.4, while the samples were at room temperature and outside the CO2 incubator. The antibiotics were omitted from the exposure medium (as well as from the growth medium for subsequent incubation), because their effect on electroporated cells is difficult to predict.
For a typical set of experiments, coverslips were loaded into 38 cuvettes (5 sham exposures and 33 nsPEF exposures, using different pulse numbers). The cuvette was kept tilted (Fig. 1c), to let the coverslip rest flat on the bottom electrode (anode), with the glass surface facing down, and the ITO surface with cells facing up into the buffer. Each coverslip was individually handled, exposed, and measured, and thus was regarded as a single experiment.
Trapezoidal 300-ns or rectangular 9-µs pulses from an AVTECH AVOZ-D2-B-ODA generator (AVTECH Electrosystems, Ottawa, Ontario, Canada) were delivered to the cuvette via a 50- to 10-Ohm transition module (AVOZ-D2-T, AVTECH Electrosystems) modified into a cuvette holder. Cells were exposed to different numbers of either 300-ns pulses (0–2000; 50 Hz, 1.8 kV/cm) or 9-µs pulses (0–200, 5 Hz, 0.75 kV/cm) at room temperature (22 ± 2 °C). The sequence of different exposures was varied between sets of experiments, and sham exposures were inserted randomly between nsPEF exposures. Pulse trains of pre-determined duration and repetition rate were triggered externally by a model S8800 simulator (Grass Instruments Co., Quincy, MA). The pulse shape and amplitude (Fig. 1d) were monitored using a 500 MHz, 5GS/s Tektronix TDS 3052C oscilloscope (Tektronix, Wilsonville, OR).
The maximal theoretically possible (adiabatic) heating (ΔT, °C) caused by PEF exposures was calculated as
| 1 |
where AD is the absorbed dose, J/g (Eq. 2, see below) and C is the specific heat of the medium [assumed at 4.2 J/(g °C)]. The temperature rise from a single 300-ns or 9-µs pulse was no more than 0.01 °C. For the most intense PEF treatment used (2000 pulses, 300 ns, 1.8 kV/cm, and 50 Hz), the maximal adiabatic heating was ~7 °C. Because the experiments were conducted at room temperature (24 °C), such heating was innocuous to cells which are cultured at 37 °C. Moreover, these calculations yield the “worst case scenario” heating, which in reality is offset by heat dissipation.
Once all exposures were completed, the coverslips were transferred to a 48-well cell culture plate containing the growth medium without antibiotics (300 µL per well) and incubated at 37 °C, 5% CO2 overnight.
Electric field simulation and measurements for PEF exposures on ITO coverslips
If cells grown on a “regular” glass coverslip are pulsed in a cuvette, the glass layer, which is a dielectric material, shields the cells from the electric field (Fig. 1b). However, this protection can be cancelled by covering the surface of the coverslip, on which the cells are grown, by a layer of a conductive material. This layer becomes an additional electrode, as the conductive electrolyte solution makes a conductive pathway from the underlying cuvette electrode to the layer. As a result, the cells growing attached to the surface of the ITO layer can be efficiently exposed to PEF.
Since the ITO layer is not directly connected to any of the electrodes of the PEF generator, the actual value of the electric field created within the electric chamber, especially near the surface of the ITO layer, where the cells are located, cannot be straightforwardly calculated. We employed a finite-element simulation for the electric field distribution within an electroporation cuvette in which a glass coverslip with the ITO layer was placed on one of the electrodes, and verified the simulation results by experimental measurements.
The simulation study, which was carried out with a COMSOL Multiphysics package version 4.4 (Burlington, USA) for 1-, 2-, and 4-mm gap cuvettes, has established that the electric field was uniform within a 3.5-mm radius from the center of the coverslip, with a gradual reduction further outward and an increase within 0.2-mm wide outer rim (see Supplementary Methods and Fig. S2 for details). This pattern of the electric field distribution was consistent with the uniform distribution of electropermeabilized cells over the surface of the coverslip [60]. For every 100 V applied across a 1-mm gap cuvette, the simulated electric field at the ITO surface was 0.28 kV/cm (Fig. S2). Taking into account that the actual cuvette gap often was larger than the nominal value (by 10–20%) and that the coverslip glass thickness could vary by 5–10%, the proportionality coefficient was rounded to 0.3 kV/cm. Hence, for the present study, we accepted that the application of 250 or 600 V across a 1-mm cuvette with an ITO coverslip produced the electric field of 0.75 or 1.8 kV/cm, respectively, at the coverslip surface.
The peak specific absorption rate (SARp; kW/g) and absorbed dose (AD; J/g) at the cell location were calculated as
| 2 |
| 3 |
where σ is the conductivity of the solution (14.8 mS/cm for the exposure medium), E is the local electric field at the coverslip surface (kV/cm), ρ is the density of the solution (~1 g/cm3), τ is the pulse duration (s), and N is the number of pulses [61].
The simulated electric field values were verified by direct measurements using a microelectrode glued to the ITO surface as a voltage probe and positioning the coverslip at different locations within a cuvette using a micromanipulator. The results of the measurements were remarkably close to the model predictions (Table S2).
Cell viability assay
Approximately 20 h post-PEF exposure, Presto Blue reagent (Life Technologies, Grand Island, NY) was added to each well in a 1:10 dilution. After 1 h of incubation at 37 °C, 5% CO2, fluorescence was measured with a Synergy 2 microplate reader (BioTek, Winooski, VT) using excitation at 530 nm and detection at 590 nm. Background fluorescence was subtracted from each reading (“blank” samples containing ITO-coated coverslips with no cells), and readings were normalized to the mean value for sham-exposed parallel controls. The survival values were plotted against the number of pulses to generate the survival curve.
Cell imaging on ITO coverslips at 20 h following nsPEF or sham exposure
Following the “Cell viability assay”, selected coverslips were visually analyzed and imaged using the Olympus IX81/FV1000 setup (see “PEF delivery to individual cells and measurements of electroporative dye uptake”). The glass-bottomed chamber was filled with the exposure medium (see “Cell lines and culture conditions”) supplemented with 1 µg/mL Hoechst (exc./em. 405/430–470 nm) and 5 µg/mL propidium iodide (PI; exc./em. 543/560–660 nm). Hoechst labeled all nuclei on the coverslip, whereas PI labeled dead cells. Still images were taken to assess cell appearance, size, shape, nuclear size, and the presence and location of any survivors.
PEF delivery to individual cells and measurements of electroporative dye uptake
To quantify the extent of cell membrane permeabilization by PEF (see “Selective cytotoxicity of nsPEF does not correlate with the severity of electroporative damage of the cell membrane”), cells were grown on glass coverslips without an ITO layer. The experimental approach and dosimetry were similar to previously described [22, 62, 63]. Briefly, a coverslip with cells was placed in a glass-bottomed chamber mounted on an Olympus IX81 inverted microscope equipped with an FV1000 confocal laser scanning system. The chamber was filled with the exposure medium supplemented with 1 µM YO-PRO-1 (YP; exc./em. 488/505–525 nm) and 5 µg/mL PI. These membrane integrity markers are essentially non-fluorescent in the medium and do not penetrate into cells (or penetrate poorly) unless the membrane is compromised. Their penetration into cells is manifested by a profound increase in fluorescence and has been routinely used to quantify cell membrane permeabilization [62–64]. Nearly, rectangular 300-ns pulses were delivered to a selected cell or group of cells with a pair of tungsten rod electrodes (0.1 mm diameter, 0.25 mm gap). With a help of a robotic micromanipulator [MP-225, (Sutter, Novato, CA)], the electrodes were positioned precisely 30 µm above the coverslip surface, so that the selected cells were located in the center of the gap between the electrode tips. Electric pulses were triggered externally by a TTL pulse from the pClamp software via a Digidata 1322A output (Molecular Devices, Foster City, CA). The same software and Digidata output were used to synchronize nsPEF delivery and image acquisition. The electric field at the cell location was determined by 3D simulations with a finite-element Maxwell equation solver Amaze 3D (Field Precision, Albuquerque, NM). Images were taken every 10 s for 200 s, with nsPEF delivery triggered at 24 s into the experiment. Differential interference contrast (DIC) and fluorescent images were obtained with a 40×, 0.94 NA dry objective. Data were analyzed and quantified using MetaMorph Advanced v.7.7.0.0 (Molecular Devices).
Statistical analysis
Statistical analyses were performed using a two-tailed t test, where p < 0.05 was considered statistically significant. The region of logarithmic decline of the survival curve (see “Cell viability assay”) was fit with a logarithmic function to determine the lethal dose (number of pulses) that decreased cell survival to 50% (LD50). The fit and its 95% confidence interval were calculated using Grapher 11 (Golden Software, Golden, CO).
Results
Diverse susceptibility to 300-ns PEF across the different cell lines
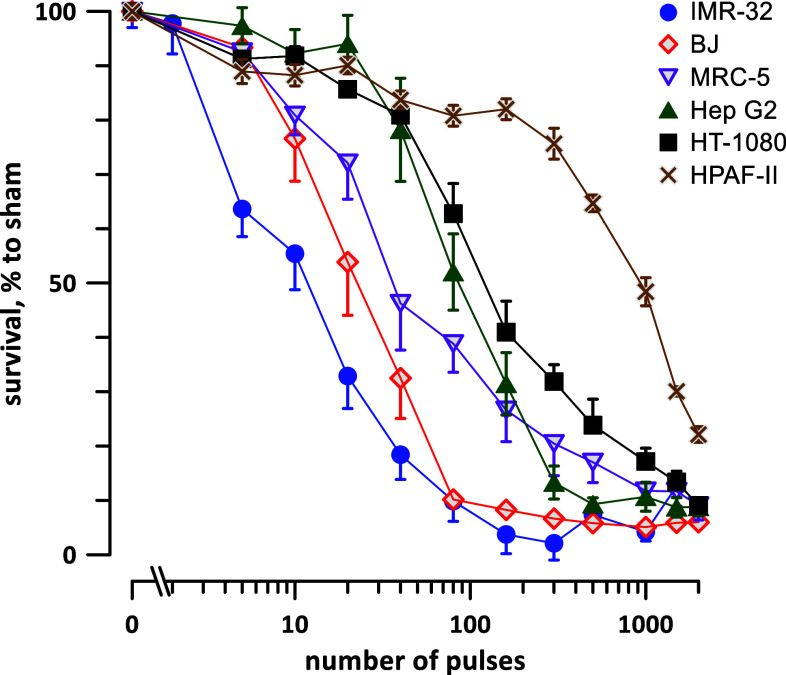
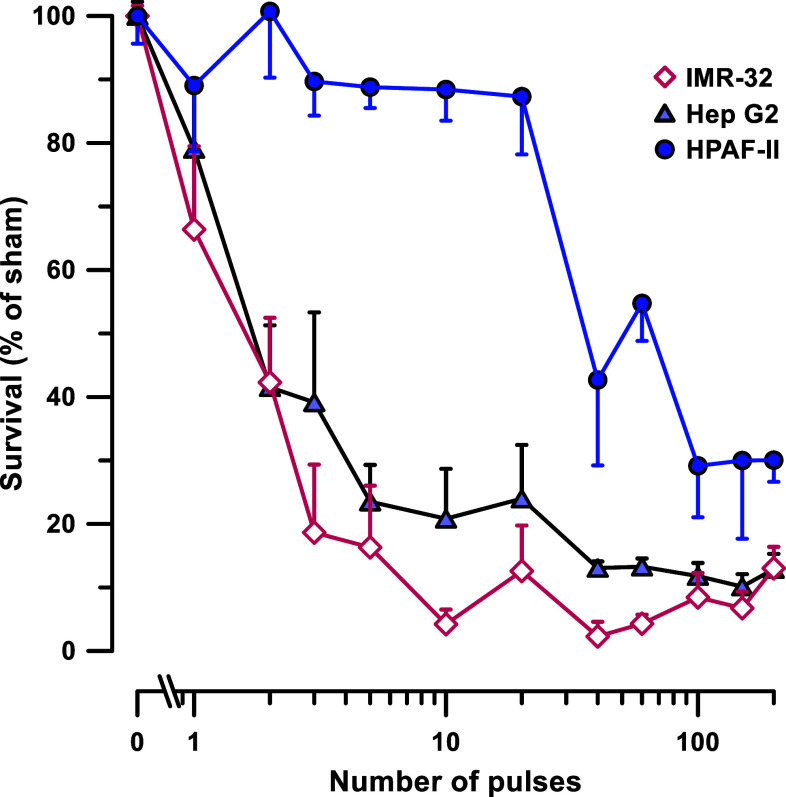
Four cancerous and two non-cancerous cells lines were treated by different numbers (from 0 to 2000) of 300 ns, 1.8 kV/cm, 50 Hz pulses. The 20-h survival was normalized to sham-exposed (0 pulses) parallel controls and plotted against the pulse number (Fig. 2). The plots displayed profoundly different susceptibility to nsPEF, along with some remarkable similarities in the curve shape.
Fig. 2.
Highly selective sensitivity of different cell lines to 300-ns PEF. The plots display cell survival 20 h after exposure to different numbers of 300-ns pulses (1.8 kV/cm, 50 Hz). The survival was normalized to the sham-exposed parallel control, mean ± SEM, 9–18 independent experiments per data point. For clarity, most error bars are plotted in one direction only and significant differences are not marked, see text and Table 1. Note the log scale for abscissa
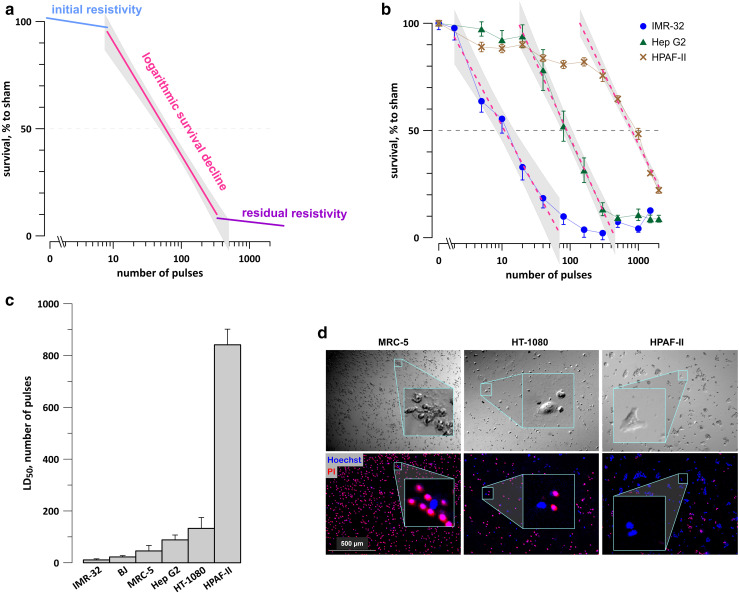
For each tested cell line, the survival curve could be described as having three distinct regions: it started with an initial resistivity shoulder (when cell survival remained unchanged or just modestly decreased with increasing the pulse number), continued with a logarithmic survival decline, and ended with a residual resistivity shoulder (Fig. 3a). Fitting the second region with a logarithmic function (Fig. 3b) has enabled the quantification of nsPEF susceptibility, by determining LD50 as the number of pulses which reduced survival to 50%. The 95% confidence interval for LD50 was determined by calculation of a 95% confidence interval “corridor” range for the best fit (shown by shaded areas in Fig. 3b) and measuring its intercepts with the 50% survival level. When these intercepts were at different distances from the mean LD50 value, we conservatively used the larger of the two intervals.
Fig. 3.
Analysis of survival curves after 300-ns PEF exposure. a Survival curves shown in Fig. 2 share a similar shape which is indicative of three distinct regions: the initial resistivity shoulder, logarithmic survival decline, and the residual resistivity. For quantitation of PEF sensitivity, the logarithmic decline region can be fit with a log function. b Representative log fits (dashed lines) using the survival data for IMR-32, Hep G2, and HPAF-II cells. Shaded areas define the 95% confidence intervals for each fit. LD50 was determined as the best fit value at 50% survival; the confidence interval for LD50 is the width of the shaded area at 50% survival level. When the lower and upper limits of the confidence interval were at a different distance from the mean, the larger of the two values was used. c LD50 for tested cell lines, measured as outlined in a and b. The error bars are the confidence intervals; the lack of error bar overlap indicates the significance of differences at p < 0.05 or better, see Table 1 for numerical data. d Imaging confirms the presence of survivors even after the maximum tested PEF exposure (2000 pulses, 300 ns, 1.8 kV/cm, 50 Hz). Representative DIC (top) and fluorescence images (bottom) from the center of ITO coverslips at 22–24 h after PEF exposure in indicated cell lines. Dead cells were stained with PI (red), and live cells were stained with Hoechst (blue). Insets show magnifications of selected areas
The quantification of the survival curves has revealed striking differences in nsPEF susceptibility, nearly 80-fold between the most sensitive IMR-32 neuroblastoma cells and the most resistant HPAF-II adenocarcinoma cells (Fig. 3c; Table 1). Each of the remaining cell lines, including both non-cancerous (BJ and MRC-5) and cancerous cell types (Hep G2 and HT-1080), had intermediate sensitivities to nsPEF. Thus, under strictly uniform experimental protocols, using the same media and serum, and avoiding cell detachment for nsPEF exposure, we established that nsPEF was highly selective for cell killing, but was not particularly selective against cancer cells.
The residual resistivity was yet another unexpected characteristic of the survival curves. While it was reported earlier that exposures of suspension cells in electroporation cuvettes do not result in 100% cell killing [40, 41], it was explained by possible non-uniform electric field exposure at the edges of the cuvette and in the suspension meniscus, as well as by possible shielding of some cells by other cells. However, shielding is not expected to occur on ITO coverslips, where cells are in a monolayer. The electric field, however, was somewhat reduced at the periphery of the coverslip (Fig. S2), which could help cells at the peripheral rim to escape the lethal exposure. Another possibility was that the Presto Blue assay detected some residual metabolic activity from actually dead cells. To test if the residual resistivity was real or an experimental artifact, the coverslips were analyzed by fluorescence microscopy. We were able to find isolated live cells in the center of coverslips even after the most intense treatments (Fig. 3d). The nuclei of all cells were stained with a cell-permeable Hoechst (blue fluorescence), but in dead cells, it was quenched by red fluorescence of PI (red). The live cells could be identified by both DIC transillumination and blue fluorescence. Thus, the residual resistivity was not an artifact but reflected an unusual level of nsPEF tolerance in a limited sub-population of cells. Understanding the mechanism underlying this tolerance, as well as methods to overcome it, is essential for efficient tumor ablation by nsPEF and remains an area for future investigation.
nsPEF susceptibility does not correlate with cell or nuclear size or with other morphological features
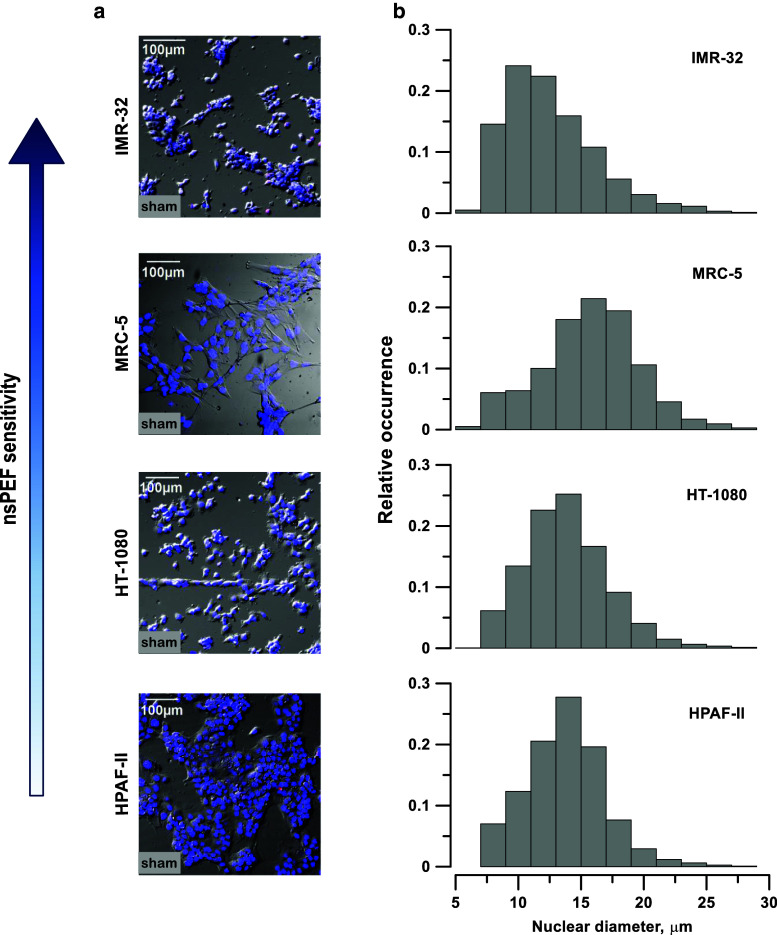
As a first step to reveal why tested cells respond to nsPEF so differently, we checked if any cytological characteristics, such as shape, size, nuclear size, or anything else in cell appearance, might correlate with nsPEF susceptibility. A number of previous studies, in particular those relying on the electric circuit modeling, suggested that larger cells and/or cells with larger nuclei might be more vulnerable [1, 57, 65], but it was not necessarily true in experimental studies [52]. However, we were unable to identify any morphological characteristics which would seem to correlate with nsPEF susceptibility. The nucleus size could be measured accurately by labeling it with Hoechst dye and using specialized modules of the MetaMorph software; however, it was not possible to measure the exact cell size in cells that formed tight clusters (such as HPAF-II or IMR-32). As far as we could judge by a visual inspection, the most sensitive cells, IMR-32, were among the smallest (Fig. 4a) and also had small nuclei (Fig. 4b). The largest cells with large nuclei (MRC-5) had an intermediate sensitivity to nsPEF. HT-1080 cells had the same size nuclei as HPAF-II, but were six-fold more sensitive.
Fig. 4.
nsPEF sensitivity has no apparent correlation with cell shape, size, or growth pattern (a) or with nucleus size (b). a Representative images of monolayers of IMR-32, MRC-5, HT-1080, and HPAF-II cells on ITO coverslips in control samples (no PEF exposure). Cell nuclei were labeled with Hoechst dye (blue), and fluorescence was laid over DIC images. Cells most sensitive to nsPEF are at the top and the least sensitive at the bottom. b Bar plots showing the frequency distributions of the nuclear diameters in the same cell lines; 2000–3000 cells were measured per group. MRC-5 cells, which have an intermediate sensitivity to nsPEF, had the largest diameter nuclei
One might also expect that a smaller cell height above the coverslip surface could result in smaller induced membrane potential and diminished electroporation. However, the MRC-5 fibroblasts, which were clearly the thinnest above the coverslip, had an intermediate sensitivity. Contrary to the expectation, the most resistant cells (HPAF-II) formed a relatively “tall” monolayer. It might be packaging into tight clusters that reduced the loss of vital solutes into the medium and reduced the vulnerability of HPAF-II. However, HT-1080 was the second least vulnerable and did not form such clusters, whereas IMR-32, the most vulnerable, packed into tight clusters.
Within the limits of this study, we were unable to single out specific morphological characteristics responsible for nsPEF tolerance, and focused on cell physiological traits instead.
nsPEF susceptibility is not related to differences in cell metabolism
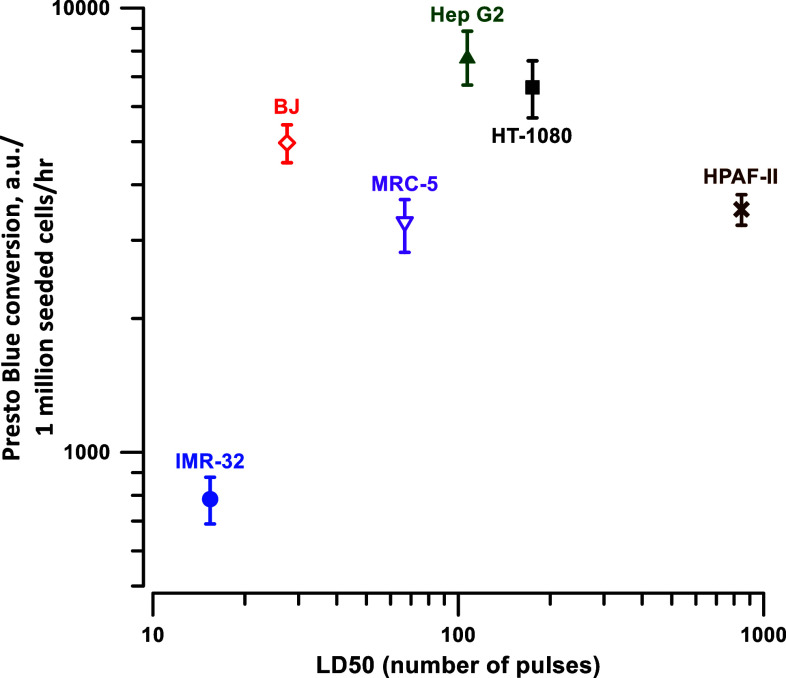
We noticed that control IMR-32 cells converted Presto blue relatively slowly compared to other cell lines. Since it was the most vulnerable cell line, we hypothesized that the low basal level of metabolism could be responsible for the reduced ability of cells to repair damages and survive after an nsPEF. This idea was tested by plotting the Presto blue conversion rate (as measured in control cells and normalized to the number of cells seeded) against the LD50 value in the respective cell line (Fig. 5). With the exception of IMR-32 cells, the rate of Presto Blue conversion did not appear to correlate with the sensitivity to nsPEF.
Fig. 5.
Metabolic rate in different cell lines does not correlate with their sensitivity to nsPEF. Cell metabolic rate was calculated in control samples (not exposed to nsPEF) as a rate of Presto Blue conversion to resorufin normalized to cell seeding density, and plotted against the respective LD50 values. Mean ± SEM for 4–6 separate sets of experiments
Selective cytotoxicity of nsPEF does not correlate with the severity of electroporative damage of the cell membrane
Earlier studies on cells [66, 67], lipid vesicles [68], and planar lipid bilayers [69] showed that electroporation was dependent on the chemical composition of the plasma membrane, such as the cholesterol content. Recent experimental [58] and molecular dynamics [70] studies confirmed that cholesterol content could affect the degree of electroporation by nsPEF as well. Thus, it was logical to hypothesize that unknown differences in the plasma membrane composition could result in more severe permeabilization and, consequently, reduced survival in certain cell lines.
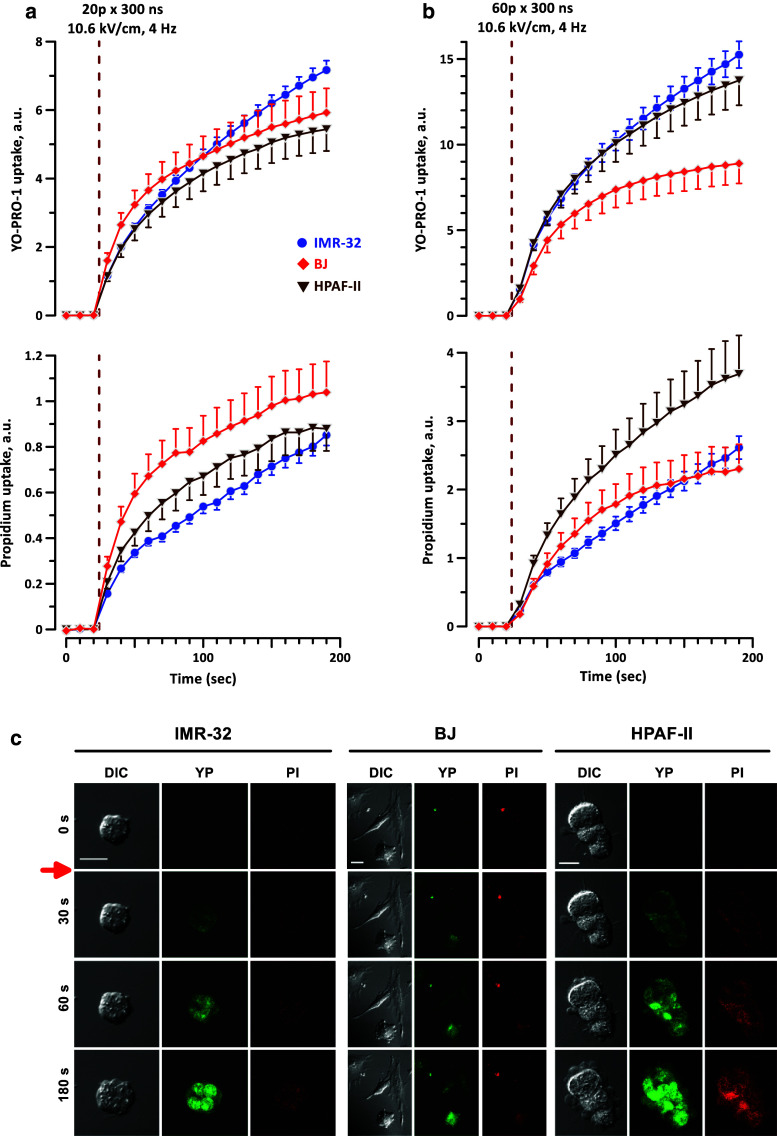
The extent of membrane permeabilization was tested by time lapse imaging of PI and YP uptake in cells grown and exposed to nsPEF on “regular” (non-ITO) coverslips, using the same approaches as we employed before [27, 62–64, 71]. The efficiency of electroporation of individual cells between a pair of tungsten rod electrodes is much lower than on ITO coverslips [34], thus we had to use a higher electric field, 10.6 kV/cm. The YP cation is known to enter the cell through smaller electropores than the propidium cation, so comparing the uptake of the two dyes could potentially help to reveal differences in the size of pores after nsPEF treatments [63].
Trains of 20 or 60, 300-ns PEF, 4 Hz, were tested in three cell lines selected for their diverse sensitivity to killing by nsPEF: IMR-32 (the most sensitive), BJ (intermediate sensitivity), and HPAF-II (the least sensitive). The experiments established a similar degree of membrane permeabilization to both dyes in all three tested cell lines (Fig. 6); under some conditions, the dye uptake was even higher in the least sensitive cells, HPAF-II. Interestingly, clustering of HPAF-II cells rendered no protection from the permeabilization by nsPEF, and they appeared more prone to blebbing than the other two cell lines (Fig. 6c).
Fig. 6.
nsPEF permeabilizes cell membrane similarly in nsPEF-sensitive and nsPEF-resistant cell lines. Electroporative entry of marker dyes YO-PRO-1 (YP) and propidium iodide (PI) was monitored by time lapse fluorescence imaging in cell lines with high, intermediate, and low sensitivity to killing by nsPEF (IMR-32, BJ, and HPAF-II, respectively). A train of either 20 (a) or 60 (b) 300-ns pulses (10.6 kV/cm, 4 Hz) was delivered starting at 24 s into the experiment (vertical dashed line). Mean ± SEM for 14–34 cells in each group. The observed differences in dye uptake are not indicative of a stronger membrane permeabilization in more sensitive cell lines. Control cells showed no appreciable dye uptake within 200 s of the experiment (data not shown). c Representative images of morphological changes and dye uptake for exposures quantified in a. Red arrow indicates when the pulses were delivered. Scale bars are 20 µm. Note the enhanced blebbing in HPAF-II cells
These results revealed little to no difference in the degree of electroporation by nsPEF between the different cell lines, and any differences observed did not correlate with the relative sensitivity to nsPEF.
Compared to the other two tested cell lines, the PI uptake in IMR-32 cells exposed to either 20 or 60 p became linear after 40 or 20 s, respectively. This linear growth in dye uptake indicates that the pores formed by nsPEF did not recover. Instead, it suggests that the pores remained open and did not shrink or reseal, which can ultimately lead to membrane rupture and cell death. Therefore, the enhanced sensitivity of IMR-32 cells to nsPEF may be due, at least in part, to reduced recovery of cells following exposure. However, this does not fully account for the selectivity by nsPEF, since there was similar recovery between the remaining two cell lines, BJ and HPAF-II, which still had very different sensitivities to nsPEF. Thus, there is likely some other factor contributing to the high degree of selectivity by nsPEF, and remains an area for future investigation.
Effects of increasing the pulse duration to 9 µs
In a previous study, we showed that increasing the pulse duration into the microsecond range increased the cell killing efficiency but eliminated the selectivity between two suspension cell lines [41]. Therefore, we tested if this observation holds true for adherent cells used in the present study. Three cell lines with high, intermediate, and low susceptibility to 300-ns PEF (IMR-32, Hep G2, and HPAF-II, respectively) were exposed to trains from 0 to 200 of 9-µs pulses (5 Hz, 0.75 kV/cm). The experiment protocol and data analyses were the same as with 300-ns pulses.
We found that IMR-32 and Hep G2 cells had essentially the same sensitivity to 9-µs PEF, while HPAF-II was about 25-fold more resistant (Fig. 7; Table 1). Thus, shifting from 300- to 9-µs PEF has eliminated the selectivity between IMR-32 and Hep G2, reduced the selectivity between IMR-32 and HPAF-II, but increased the selectivity between Hep G2 and HPAF-II. However, it is possible that the difference between IMR-32 and Hep G2 was not revealed, simply because the LD50 was already reached with just 1–2 pulses.
Fig. 7.
Cell survival after exposure to 9-µs pulses (0.75 kV/cm, 5 Hz). Survival was measured in three indicated cell lines, using the same protocols, as described in Fig. 2. Mean ± SEM, N = 4–9 independent experiments per data point, see text, Fig. 2, and Table 1 for more details
Consistent with the previous observations [41], the equivalent absorbed dose (J/g) to reach 50% lethality was threefold (HPAF-II) or ninefold (Hep G2) lower with 9-µs PEF (Table 1) compared to 300-ns PEF. The dose reduction was negligible for IMR-32; however, this result is not reliable, again because of high efficiency of even a single pulse and possibly inaccurate LD50 calculation for IMR-32.
Increasing the pulse duration to 9 µs did not eliminate the residual resistivity in any of the three cell lines, with survival leveling off at around 10–20% (IMR-32 and Hep G2) or as high as 30% (HPAF-II).
Discussion
The principal result of this study is the demonstration of profound, up to 80-fold differences in nsPEF sensitivity between several human cell lines. The experimental procedures, protocols, and media were identical for all tested cell lines. Moreover, we adopted a novel experimental approach using ITO coverslips, which eliminated stressful cell handling and possible confounding impact of detachment and re-attachment of cells. We conclude that the established differences in nsPEF sensitivity are indeed a result of physiological differences in studied cells. However, it remains to be understood which specific characteristics determined the nsPEF sensitivity. To the extent it was studied, nsPEF sensitivity showed little or no relevance to cell shape or size, growth pattern, nucleus size, metabolic rate, or the degree of electroporative damage to cell membrane. This is consistent with the previous studies which could not correlate PEF sensitivity to cell size or other morphological features [40, 52].
Increasing the pulse duration from 300 ns to 9 µs reduced the energy deposition needed to reach LD50, consistent with previous observations in suspension cells [41]. The selectivity for cell killing was altered in a complex way, by eliminating some differences which were present with 300-ns pulses, but emphasizing other differences. Such altered sensitivity pattern could potentially be reflecting the difference in cell death mechanisms triggered by PEF of different durations.
nsPEF selectivity showed no preference for killing based on whether the cells were cancerous or not, as the normal cell lines BJ and MRC-5 had an intermediate sensitivity. This is in contrast to a study by Yang et al. [55] which reported heightened sensitivity to 30-ns PEF in a cancerous cell line, basal cell carcinoma, compared to normal cells. However, this study only compared two cell lines, and its conclusions cannot be readily extrapolated to other cancerous and non-cancerous cells.
Our findings highlight the potential for using PEF to specifically target certain types of tumors. In contrast, other cancers are highly resistant to PEF and may require tuning of PEF exposure conditions for more efficient ablation, such as increasing the pulse duration, increasing EF amplitude, or increasing the extracellular Ca2+ concentration during PEF exposures [60, 72].
Irrespective of the different sensitivity to nsPEF of the different cell lines, the survival curves had a common shape with three distinct phases: initial resistivity, logarithmic survival decline, and residual resistivity. In this last phase, cell survival leveled off to ~5–10%, even at the highest doses, indicating the presence of a small population of cells that remained highly resistant to nsPEF. Although some residual resistivity was observed in the previous studies [40, 41, 54], it could be an experimental artifact due to shielding of cells in the suspension, or due to a reduced electric field in the meniscus of the suspension. These explanations do not hold for ITO coverslip exposures, which, therefore, provided evidence for the highly diverse nsPEF sensitivity not only between cell lines, but also between individual cells within each cell line. The presence of these resistant cells could have a major impact on the successful ablation of tumors, thus stressing the need to identify the physiological characteristics responsible for nsPEF tolerance.
It has been previously shown that nsPEF can permeabilize and damage the mitochondrial membrane [17, 18, 33], leading to ATP depletion and ROS formation [73, 74]. Different degrees of nsPEF-induced ROS formation or ATP depletion could potentially underlie the susceptibility to nsPEF-induced cell death. Indeed, nsPEF-resistant U937 cells had less ROS formation and better survival following nsPEF exposure than nsPEF-sensitive Jurkat cells [74], and oxidation-damaged areas of the cell membrane were more sensitive to electroporation [75]. Therefore, damage to mitochondria by nsPEF may result in varying levels of ATP loss, ROS formation, and/or induction of either apoptosis or necrosis, which could contribute to the cells’ sensitivity to exposure.
Another factor possibly related to nsPEF selectivity is the composition of the plasma membrane, particularly the phospholipid profile and cholesterol content. The depletion of plasma membrane cholesterol increased nsPEF sensitivity [58]. Interestingly, it has been reported that various solid tumors and malignancies have an accumulation of cholesterol and dysregulated cholesterol metabolism [76]. Similarly, the phospholipid profile in several cancer cell lines has been shown to be altered compared to normal cell lines, correlating directly with the malignancy of the cancer [77–81]. The phospholipids that were increased in cancer cell lines were those that have a tendency to accumulate in lipid rafts, which are rich in cholesterol. When lipid rafts are dysregulated in cancer, they promote cell transformation, tumor progression, and metastasis [81]. The accumulation of cholesterol, as well as alterations in the phospholipid profile, has been correlated with increased drug and hormone resistance [76, 78] and could potentially be responsible for the nsPEF tolerance as well.
Understanding the nsPEF susceptibility and residual resistivity mechanisms is critical for the development of individualized cancer ablation protocols and for the accurate prognosis of the treatment outcome.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a Grant from Pulse Biosciences Inc., by R01GM088303 from the National Institute of General Medical Sciences and by an AFOSR MURI grant FA9950-15-1-0517 on Nanoelectropulse-Induced Electromechanical Signaling and Control of Biological Systems, administered through Old Dominion University (to AGP).
Abbreviations
- IRE
Irreversible electroporation
- ITO
Indium tin oxide
- µsPEF
Microsecond pulsed electric field
- nsPEF
Nanosecond pulsed electric field
- PEF
Pulsed electric field
- PI
Propidium iodide
- YP
YO-PRO-1
References
- 1.Neumann E, Sowers AE, Jordan CA. Electroporation and electrofusion in cell biology. New York: Plenum Press; 1989. [Google Scholar]
- 2.Teissie J, Eynard N, Gabriel B, Rols MP. Electropermeabilization of cell membranes. Adv Drug Deliv Rev. 1999;35(1):3–19. doi: 10.1016/S0169-409X(98)00060-X. [DOI] [PubMed] [Google Scholar]
- 3.Breton M, Mir LM. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnetics. 2012;33(2):106–123. doi: 10.1002/bem.20692. [DOI] [PubMed] [Google Scholar]
- 4.Pakhomov AG, Miklavcic D, Markov MS, editors. Advanced electroporation techniques in biology in medicine. Boca Raton: CRC Press; 2010. [Google Scholar]
- 5.Zimmermann U, Friedrich U, Mussauer H, Gessner P, Hämel K, Sukhorukov V. Electromanipulation of mammalian cells: fundamentals and application. IEEE Trans Plasma Sci. 2000;28(1):72–82. doi: 10.1109/27.842868. [DOI] [Google Scholar]
- 6.Belehradek J, Jr, Orlowski S, Poddevin B, Paoletti C, Mir LM. Electrochemotherapy of spontaneous mammary tumours in mice. Eur J Cancer. 1991;27(1):73–76. doi: 10.1016/0277-5379(91)90065-L. [DOI] [PubMed] [Google Scholar]
- 7.Mir LM, Orlowski S, Belehradek J, Jr, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27(1):68–72. doi: 10.1016/0277-5379(91)90064-K. [DOI] [PubMed] [Google Scholar]
- 8.Miklavcic D, Mali B, Kos B, Heller R, Sersa G. Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 2014;13(1):29. doi: 10.1186/1475-925X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4(6):699–705. doi: 10.1177/153303460500400615. [DOI] [PubMed] [Google Scholar]
- 10.Neal RE, 2nd, Davalos RV. The feasibility of irreversible electroporation for the treatment of breast cancer and other heterogeneous systems. Ann Biomed Eng. 2009;37(12):2615–2625. doi: 10.1007/s10439-009-9796-9. [DOI] [PubMed] [Google Scholar]
- 11.Rubinsky B. Irreversible electroporation. Series in biomedical engineering. Berlin: Springer; 2010. [Google Scholar]
- 12.Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53(7):1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 13.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6(4):295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- 14.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 15.Bower M, Sherwood L, Li Y, Martin R. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol. 2011;104(1):22–28. doi: 10.1002/jso.21899. [DOI] [PubMed] [Google Scholar]
- 16.Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, Meijer S, van Kuijk C, van den Tol PM, Meijerink MR. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25(7):997–1011. doi: 10.1016/j.jvir.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Batista Napotnik T, Wu YH, Gundersen MA, Miklavcic D, Vernier PT. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics. 2012;33(3):257–264. doi: 10.1002/bem.20707. [DOI] [PubMed] [Google Scholar]
- 18.Beebe SJ, Chen YJ, Sain NM, Schoenbach KH, Xiao S. Transient features in nanosecond pulsed electric fields differentially modulate mitochondria and viability. PLoS One. 2012;7(12):e51349. doi: 10.1371/journal.pone.0051349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenov I, Xiao S, Pakhomov AG. Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim Biophys Acta. 2013;1828(3):981–989. doi: 10.1016/j.bbamem.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenov I, Xiao S, Pakhomova ON, Pakhomov AG. Recruitment of the intracellular Ca2+ by ultrashort electric stimuli: the impact of pulse duration. Cell Calcium. 2013;54(3):145–150. doi: 10.1016/j.ceca.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson GL, Roth CC, Kuipers MA, Tolstykh GP, Beier HT, Ibey BL. Permeabilization of the nuclear envelope following nanosecond pulsed electric field exposure. Biochem Biophys Res Commun. 2016;470(1):35–40. doi: 10.1016/j.bbrc.2015.12.092. [DOI] [PubMed] [Google Scholar]
- 22.Pakhomov AG, Bowman AM, Ibey BL, Andre FM, Pakhomova ON, Schoenbach KH. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem Biophys Res Commun. 2009;385(2):181–186. doi: 10.1016/j.bbrc.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernier PT, Sun Y, Chen MT, Gundersen MA, Craviso GL. Nanosecond electric pulse-induced calcium entry into chromaffin cells. Bioelectrochemistry. 2008;73(1):1–4. doi: 10.1016/j.bioelechem.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Craviso GL, Choe S, Chatterjee P, Chatterjee I, Vernier PT. Nanosecond electric pulses: a novel stimulus for triggering Ca2+ influx into chromaffin cells via voltage-gated Ca2+ channels. Cell Mol Neurobiol. 2010;30(8):1259–1265. doi: 10.1007/s10571-010-9573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wegner LH, Frey W, Schonwalder S. A critical evaluation of whole cell patch clamp studies on electroporation using the voltage sensitive dye ANNINE-6. Bioelectrochemistry. 2013;92:42–46. doi: 10.1016/j.bioelechem.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Craviso GL, Choe S, Chatterjee I, Vernier PT. Modulation of intracellular Ca(2+) levels in chromaffin cells by nanoelectropulses. Bioelectrochemistry. 2012;87:244–252. doi: 10.1016/j.bioelechem.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Nesin OM, Pakhomova ON, Xiao S, Pakhomov AG. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim Biophys Acta. 2011;3:792–801. doi: 10.1016/j.bbamem.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rassokhin MA, Pakhomov AG. Electric field exposure triggers and guides formation of pseudopod-like blebs in U937 monocytes. J Membr Biol. 2012;245(9):521–529. doi: 10.1007/s00232-012-9433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pakhomov AG, Xiao S, Pakhomova ON, Semenov I, Kuipers MA, Ibey BL. Disassembly of actin structures by nanosecond pulsed electric field is a downstream effect of cell swelling. Bioelectrochemistry. 2014;100:88–95. doi: 10.1016/j.bioelechem.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolstykh GP, Beier HT, Roth CC, Thompson GL, Payne JA, Kuipers MA, Ibey BL. Activation of intracellular phosphoinositide signaling after a single 600 nanosecond electric pulse. Bioelectrochemistry. 2013;94:23–29. doi: 10.1016/j.bioelechem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Beebe SJ, Fox PM, Rec LJ, Willis EL, Schoenbach KH. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003;17(11):1493–1495. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 32.Beebe SJ, Blackmore PF, White J, Joshi RP, Schoenbach KH. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol Meas. 2004;25(4):1077–1093. doi: 10.1088/0967-3334/25/4/023. [DOI] [PubMed] [Google Scholar]
- 33.Ren W, Sain NM, Beebe SJ. Nanosecond pulsed electric fields (nsPEFs) activate intrinsic caspase-dependent and caspase-independent cell death in Jurkat cells. Biochem Biophys Res Commun. 2012;421(4):808–812. doi: 10.1016/j.bbrc.2012.04.094. [DOI] [PubMed] [Google Scholar]
- 34.Pakhomova ON, Gregory BW, Semenov I, Pakhomov AG. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS One. 2013;8(7):e70278. doi: 10.1371/journal.pone.0070278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullery JC, Tarango M, Roth CC, Ibey BL. Activation of autophagy in response to nanosecond pulsed electric field exposure. Biochem Biophys Res Commun. 2015;458(2):411–417. doi: 10.1016/j.bbrc.2015.01.131. [DOI] [PubMed] [Google Scholar]
- 36.Morotomi-Yano K, Akiyama H, Yano K. Different involvement of extracellular calcium in two modes of cell death induced by nanosecond pulsed electric fields. Arch Biochem Biophys. 2014;555–556:47–54. doi: 10.1016/j.abb.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Ibey BL, Ullery JC, Pakhomova ON, Roth CC, Semenov I, Beier HT, Tarango M, Xiao S, Schoenbach KH, Pakhomov AG. Bipolar nanosecond electric pulses are less efficient at electropermeabilization and killing cells than monopolar pulses. Biochem Biophys Res Commun. 2014;443(2):568–573. doi: 10.1016/j.bbrc.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pakhomov AG, Semenov I, Xiao S, Pakhomova ON, Gregory B, Schoenbach KH, Ullery JC, Beier HT, Rajulapati SR, Ibey BL. Cancellation of cellular responses to nanoelectroporation by reversing the stimulus polarity. Cell Mol Life Sci. 2014;71(22):4431–4441. doi: 10.1007/s00018-014-1626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gianulis EC, Lee J, Jiang C, Xiao S, Ibey BL, Pakhomov AG. Electroporation of mammalian cells by nanosecond electric field oscillations and its inhibition by the electric field reversal. Sci Rep. 2015;5:13818. doi: 10.1038/srep13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibey BL, Roth CC, Pakhomov AG, Bernhard JA, Wilmink GJ, Pakhomova ON. Dose-dependent thresholds of 10-ns electric pulse induced plasma membrane disruption and cytotoxicity in multiple cell lines. PLoS One. 2011;6(1):e15642. doi: 10.1371/journal.pone.0015642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibey BL, Pakhomov AG, Gregory BW, Khorokhorina VA, Roth CC, Rassokhin MA, Bernhard JA, Wilmink GJ, Pakhomova ON. Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochim Biophys Acta. 2010;1800(11):1210–1219. doi: 10.1016/j.bbagen.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenbach KS, Hargrave B, Joshi RP, Kolb J, Osgood C, Nuccitelli R, Pakhomov AG, Swanson J, Stacey M, White JA, Xiao S, Zhang J, Beebe SJ, Blackmore PF, Buescher ES. Bioelectric effects of nanosecond pulses. IEEE Trans Dielectr Electr Insul. 2007;14(5):1088–1109. doi: 10.1109/TDEI.2007.4339468. [DOI] [Google Scholar]
- 43.Muratori C, Pakhomov AG, Xiao S, Pakhomova ON. Electrosensitization assists cell ablation by nanosecond pulsed electric field in 3D cultures. Sci Rep. 2016;6:23225. doi: 10.1038/srep23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuccitelli R, Berridge JC, Mallon Z, Kreis M, Athos B, Nuccitelli P. Nanoelectroablation of murine tumors triggers a CD8-dependent inhibition of secondary tumor growth. PLoS One. 2015;10(7):e0134364. doi: 10.1371/journal.pone.0134364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuccitelli R, Huynh J, Lui K, Wood R, Kreis M, Athos B, Nuccitelli P. Nanoelectroablation of human pancreatic carcinoma in a murine xenograft model without recurrence. Int J Cancer. 2013;132(8):1933–1939. doi: 10.1002/ijc.27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuccitelli R, Tran K, Athos B, Kreis M, Nuccitelli P, Chang KS, Epstein EH, Jr, Tang JY. Nanoelectroablation therapy for murine basal cell carcinoma. Biochem Biophys Res Commun. 2012;424(3):446–450. doi: 10.1016/j.bbrc.2012.06.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuccitelli R, Chen X, Pakhomov AG, Baldwin WH, Sheikh S, Pomicter JL, Ren W, Osgood C, Swanson RJ, Kolb JF, Beebe SJ, Schoenbach KH. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int J Cancer. 2009;125(2):438–445. doi: 10.1002/ijc.24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garon EB, Sawcer D, Vernier PT, Tang T, Sun Y, Marcu L, Gundersen MA, Koeffler HP. In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. Int J Cancer. 2007;121(3):675–682. doi: 10.1002/ijc.22723. [DOI] [PubMed] [Google Scholar]
- 49.Nuccitelli R, Wood R, Kreis M, Athos B, Huynh J, Lui K, Nuccitelli P, Epstein EH., Jr First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: proof of method. Exp Dermatol. 2014;23(2):135–137. doi: 10.1111/exd.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren Z, Chen X, Cui G, Yin S, Chen L, Jiang J, Hu Z, Xie H, Zheng S, Zhou L. Nanosecond pulsed electric field inhibits cancer growth followed by alteration in expressions of NF-kappaB and Wnt/beta-catenin signaling molecules. PLoS One. 2013;8(9):e74322. doi: 10.1371/journal.pone.0074322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuccitelli R, Tran K, Lui K, Huynh J, Athos B, Kreis M, Nuccitelli P, De Fabo EC. Non-thermal nanoelectroablation of UV-induced murine melanomas stimulates an immune response. Pigment Cell Melanoma Res. 2012;25(5):618–629. doi: 10.1111/j.1755-148X.2012.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cemazar M, Jarm T, Miklavcic D, Lebar AM, Ihan A, Kopitar NA, Sersa G. Effect of electric-field intensity on electropermeabilization and electrosensitivity of various tumor-cell lines in vitro. Electro- Magnetobiol. 1998;17(2):263–272. doi: 10.3109/15368379809022571. [DOI] [Google Scholar]
- 53.O’Hare MJ, Ormerod MG, Imrie PR, Peacock JH, Asche W. Electropermeabilization and electrosensitivity of different types of mammalian cells. In: Neumann E, Sowers AE, Jordan CA, editors. Electroporation and electrofusion in cell biology. Boston: Springer; 1989. pp. 319–330. [Google Scholar]
- 54.Stacey M, Stickley J, Fox P, Statler V, Schoenbach K, Beebe SJ, Buescher S. Differential effects in cells exposed to ultra-short, high intensity electric fields: cell survival, DNA damage, and cell cycle analysis. Mutat Res. 2003;542(1–2):65–75. doi: 10.1016/j.mrgentox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Yang W, Wu YH, Yin D, Koeffler HP, Sawcer DE, Vernier PT, Gundersen MA. Differential sensitivities of malignant and normal skin cells to nanosecond pulsed electric fields. Technol Cancer Res Treat. 2011;10(3):281–286. doi: 10.7785/tcrt.2012.500204. [DOI] [PubMed] [Google Scholar]
- 56.Yin D, Yang WG, Weissberg J, Goff CB, Chen W, Kuwayama Y, Leiter A, Xing H, Meixel A, Gaut D, Kirkbir F, Sawcer D, Vernier PT, Said JW, Gundersen MA, Koeffler HP. Cutaneous papilloma and squamous cell carcinoma therapy utilizing nanosecond pulsed electric fields (nsPEF) PLoS One. 2012;7(8):e43891. doi: 10.1371/journal.pone.0043891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivey JW, Latouche EL, Sano MB, Rossmeisl JH, Davalos RV, Verbridge SS. Targeted cellular ablation based on the morphology of malignant cells. Sci Rep. 2015;5:17157. doi: 10.1038/srep17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantu JC, Tarango M, Beier HT, Ibey BL. The biological response of cells to nanosecond pulsed electric fields is dependent on plasma membrane cholesterol. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbamem.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Sukhorukov VL, Reuss R, Zimmermann D, Held C, Muller KJ, Kiesel M, Gessner P, Steinbach A, Schenk WA, Bamberg E, Zimmermann U. Surviving high-intensity field pulses: strategies for improving robustness and performance of electrotransfection and electrofusion. J Membr Biol. 2005;206(3):187–201. doi: 10.1007/s00232-005-0791-2. [DOI] [PubMed] [Google Scholar]
- 60.Pakhomova ON, Gregory B, Semenov I, Pakhomov AG. Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim Biophys Acta. 2014;1838(10):2547–2554. doi: 10.1016/j.bbamem.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibey BL, Xiao S, Schoenbach KH, Murphy MR, Pakhomov AG. Plasma membrane permeabilization by 60- and 600-ns electric pulses is determined by the absorbed dose. Bioelectromagnetics. 2009;30:92–99. doi: 10.1002/bem.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gianulis EC, Pakhomov AG. Gadolinium modifies the cell membrane to inhibit permeabilization by nanosecond electric pulses. Arch Biochem Biophys. 2015 doi: 10.1016/j.abb.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pakhomov AG, Gianulis E, Vernier PT, Semenov I, Xiao S, Pakhomova ON. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim Biophys Acta. 2015;1848(4):958–966. doi: 10.1016/j.bbamem.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pakhomova ON, Gregory BW, Pakhomov AG. Facilitation of electroporative drug uptake and cell killing by electrosensitization. J Cell Mol Med. 2013;17(1):154–159. doi: 10.1111/j.1582-4934.2012.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agarwal A, Zudans I, Weber EA, Olofsson J, Orwar O, Weber SG. Effect of cell size and shape on single-cell electroporation. Anal Chem. 2007;79(10):3589–3596. doi: 10.1021/ac062049e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goncharenko MS, Katkov II. Effect of cholesterol on the stability of human erythrocyte membranes to electric breakdown. Biofizika. 1985;30(3):441–445. [PubMed] [Google Scholar]
- 67.Raffy S, Teissie J. Control of lipid membrane stability by cholesterol content. Biophys J. 1999;76(4):2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Needham D, Hochmuth RM. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys J. 1989;55(5):1001–1009. doi: 10.1016/S0006-3495(89)82898-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koronkiewicz S, Kalinowski S. Influence of cholesterol on electroporation of bilayer lipid membranes: chronopotentiometric studies. Biochim Biophys Acta. 2004;1661(2):196–203. doi: 10.1016/j.bbamem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Casciola M, Bonhenry D, Liberti M, Apollonio F, Tarek M. A molecular dynamic study of cholesterol rich lipid membranes: comparison of electroporation protocols. Bioelectrochemistry. 2014;100:11–17. doi: 10.1016/j.bioelechem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Pakhomova ON, Gregory BW, Khorokhorina VA, Bowman AM, Xiao S, Pakhomov AG. Electroporation-induced electrosensitization. PLoS One. 2011;6(2):e17100. doi: 10.1371/journal.pone.0017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansen EL, Sozer EB, Romeo S, Frandsen SK, Vernier PT, Gehl J. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS One. 2015;10(4):e0122973. doi: 10.1371/journal.pone.0122973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. doi: 10.1158/0008-5472.CAN-11-3782. [DOI] [PubMed] [Google Scholar]
- 74.Pakhomova ON, Khorokhorina VA, Bowman AM, Rodaite-Riseviciene R, Saulis G, Xiao S, Pakhomov AG. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch Biochem Biophys. 2012;527(1):55–64. doi: 10.1016/j.abb.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vernier PT, Levine ZA, Wu YH, Joubert V, Ziegler MJ, Mir LM, Tieleman DP. Electroporating fields target oxidatively damaged areas in the cell membrane. PLoS One. 2009;4(11):e7966. doi: 10.1371/journal.pone.0007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168(4):1107–1118. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merchant TE, Kasimos JN, Vroom T, de Bree E, Iwata JL, de Graaf PW, Glonek T. Malignant breast tumor phospholipid profiles using (31)P magnetic resonance. Cancer Lett. 2002;176(2):159–167. doi: 10.1016/S0304-3835(01)00780-7. [DOI] [PubMed] [Google Scholar]
- 78.Sterin M, Cohen JS, Ringel I. Hormone sensitivity is reflected in the phospholipid profiles of breast cancer cell lines. Breast Cancer Res Treat. 2004;87(1):1–11. doi: 10.1023/B:BREA.0000041572.07837.ec. [DOI] [PubMed] [Google Scholar]
- 79.Doria ML, Cotrim Z, Macedo B, Simoes C, Domingues P, Helguero L, Domingues MR. Lipidomic approach to identify patterns in phospholipid profiles and define class differences in mammary epithelial and breast cancer cells. Breast Cancer Res Treat. 2012;133(2):635–648. doi: 10.1007/s10549-011-1823-5. [DOI] [PubMed] [Google Scholar]
- 80.Doria ML, Cotrim CZ, Simoes C, Macedo B, Domingues P, Domingues MR, Helguero LA. Lipidomic analysis of phospholipids from human mammary epithelial and breast cancer cell lines. J Cell Physiol. 2013;228(2):457–468. doi: 10.1002/jcp.24152. [DOI] [PubMed] [Google Scholar]
- 81.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785(2):182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.