Abstract
Enterocytes, the absorptive cells of the small intestine, mediate the process of dietary fat absorption by secreting triacylglycerol (TAG) into circulation. When levels of dietary fat are high, TAG is stored in cytoplasmic lipid droplets (CLDs) and sequentially hydrolyzed for ultimate secretion. Mice with deficiency in acyl CoA: diacylglycerol acyltransferase 1 (Dgatl−/− mice) were previously reported to have a reduced rate of intestinal TAG secretion and abnormal TAG accumulation in enterocyte CLDs. This unique intestinal phenotype is critical to their resistance to diet-induced obesity; however, the underlying mechanism remains unclear. Emerging evidence shows that lysosomal TAG hydrolysis contributes to autophagy-mediated CLD mobilization, or lipophagy, and when disrupted results in CLD accumulation. In order to study how lipophagy contributes to the unique intestinal phenotype of Dgatl−/− mice, enterocytes from wild-type (WT) and Dgatl−/− mice were examined at 2 and 6 h after oral oil gavage. Through ultrastructural analysis we observed TAG present within autophagic vesicles (AVs) in mouse enterocytes, suggesting the role of lipophagy in intestinal CLD mobilization during dietary fat absorption. Furthermore, we found that Dgatl−/− mice had abnormal TAG accumulation within AVs and less acidic lysosomes compared to WT mice. Together these findings suggest that the delayed dietary fat absorption seen in Dgatl−/− mice is, in part, due to the dysregulated flux of autophagy-mediated CLD mobilization and impairment of lysosomal acidification in enterocytes. The present study highlights the critical role of lysosome in enterocyte CLD mobilization for proper dietary fat absorption.
Keywords: Diacylglycerol acyltransferase, enterocyte, cytoplasmic lipid droplet, dietary fat absorption, lysosome
1. Introduction
The absorption of dietary fat by the small intestine contributes to postprandial blood triacylglycerol (TAG) levels and dysregulation of this process increases the risk of developing obesity, diabetes, and cardiovascular disease [1–3]. Enterocytes mediate dietary fat absorption by taking up hydrolyzed products of TAG in the gut lumen, re-synthesizing TAG intracellularly and packaging TAG on chylomicrons for secretion into the blood [4, 5]. Accumulating studies have shown that enterocytes also package TAG on cytoplasmic lipid droplets (CLDs) upon pharmaceutical inhibition on chylomicron synthesis [6] and ingestion of high levels of dietary fat [7, 8]. The depletion of CLDs within enterocytes occurs upon removal of chylomicron synthesis blockade or happens naturally over time in response to high dietary fat. These observations suggest enterocyte CLD are a temporary storage pool of TAG and establish a consensus about the role of CLD in regulating the rate of TAG secretion. However, little is known about the process of enterocyte CLD mobilization during dietary fat absorption. A better understanding of enterocyte CLD mobilization is necessary to provide novel therapeutic strategies for managing postprandial hyperlipidemia and its related disease complications.
Either cytoplasmic or lysosomal TAG hydrolysis activities can contribute to CLD mobilization [9, 10], both of which are present in enterocytes. Cytoplasmic TAG hydrolysis is catalyzed by three cytoplasmic lipases (adipose triglyceride lipase, hormone sensitive lipase, and monoacylglycerol lipase). Mouse models deficient in cytoplasmic lipases have altered intestinal CLD metabolism, however none of them have an altered dietary fat absorption rate or develop severe fat malabsorption. These results suggest that cytoplasmic TAG hydrolysis exerts little effects on mobilizing stored TAG towards a secretory fate [9, 11–13]. Lysosomal TAG hydrolysis is catalyzed solely by lysosomal acid lipase (LAL). Lal−/− mice and humans with a homozygous mutation in LAL (Wolman disease) were reported to have massive accumulation of TAG and cholesterol esters in enterocytes as well as severe quantitative fat malabsorption as evidenced by steatorrhea [14, 15]. These observations strongly indicate that lysosomal TAG hydrolysis activity is critical for mobilizing CLDs towards a secretory fate in enterocytes.
Lysosomal TAG hydrolysis is part of a newly identified process called autophagy-mediated CLD mobilization, or lipophagy, in several cell types [16–18]. In the process of lipophagy, a double-membrane autophagosome engulfs a partial or whole CLD and then fuses with a lysosome, generating a single-membrane autolysosome. The fusion event allows LAL to get access to and hydrolyze the TAG and cholesterol esters present within the autolysosome, releasing fatty acids to other metabolic fates. The functional role of lipophagy in regulating the balance between TAG storage and secretion has been shown in the liver [19, 20]. Todate, there is only one study highlighting the potential role of lipophagy in regulating intestinal lipid metabolism [10]. In that study, they observed TAG within autophagic vesicles (AVs) in a human enterocyte cell line, Caco-2 cells, upon treatment of lipid micelles. They further demonstrated that inhibition of either autophagy initiation or LAL results in abnormal CLD accumulation and impaired TAG depletion in Caco-2 cells. Although this in vitro study indicates a significant impact of lipophagy on enterocyte CLD mobilization, whether this process is involved in regulating dietary fat absorption has not been examined in in vivo models.
Mice deficient in acyl CoA:diacylglycerol acyltransferase 1 (Dgatl−/− mice) have delayed dietary fat absorption, which are previously characterized to have greater CLD accumulation, a slower rate of TAG secretion and a blunted postprandial triglyceridemic response [21–24]. Such a unique intestinal phenotype is critical to their resistance to diet-induced hepatic steatosis and obesity with no quantitative fat malabsorption [21, 24, 25]; however, the underlying mechanism(s) remain unclear. Two DGAT enzymes, DGAT1 and DGAT2, catalyze the final, committed step of TAG synthesis, the acylation of diacylglycerol (DAG) with a fatty acyl-CoA [26]. Dgatl−/− mice are capable of synthesizing TAG for storage and secretion in enterocytes, likely due to the presence of DGAT2 [24–26]. Our recent study highlights the distinct functional roles of Dgat1 and Dgat2 in directing TAG to certain subcellular pools for CLD and chylomicron synthesis [23]. In the study, we employed transmission electron microscopy (TEM) to assess intracellular TAG distribution in CLDs versus chylomicrons in mice models with varying Dgat1 and Dgat2 levels, and observed abundant structures containing TAG resembling autophagosomes in Dgatl−/− mice. Given the emerging role of lipophagy in mediating CLD mobilization and the known phenotype of delayed dietary fat absorption in Dgatl−/− mice, we hypothesized that Dgatl−/− mice had dysregulated lipophagy in enterocytes resulting in slower TAG mobilization to the secretory fate.
To test this hypothesis, enterocytes from WT and Dgatl−/− mice were examined 2 and 6 h after an oral oil gavage, which represents early and late stages of dietary fat absorption, respectively. TAG present in CLDs and AVs, lysosomal pH, and associated mRNA levels of genes were assessed at these time points to understand how this process is disrupted in enterocytes of Dgatl−/− mice in response to dietary fat.
2. Materials and Methods
2.1. Diet and Animals
All procedures were approved by the Purdue Animal Care and Use Committee. Whole body Dgatl-deficient (Dgatl−/−) mice were generated as previously described [21, 25]. Male Dgatl−/− mice, 4–6 months of age were used in this study. The mice were housed in a specific pathogen-free barrier facility with a 12 h light/dark cycle (6AM/6PM) and fed a low-fat, rodent chow diet (PicoLab 5053, Lab Diets, Richmond, IN). On the day of the experiment the mice were fasted for 4 h at the beginning of the light cycle and administered 200 μl of olive oil by oral gavage. No food was available after the gavage. The small intestine was then harvested from the mice 2 or 6 h after oil gavage. The excised small intestine was divided into five equal length segments and labeled S1–S5 (proximal to distal) in relation to the stomach, with S2–3 representing the jejunum.
2.2. Transmission electron microscopy (TEM)
Mice were anesthetized using inhaled isoflurane 2 and 6 h after a 200 μl oral olive oil gavage. We performed whole mouse perfusion fixation by cardiac infusion of 2% glutaraldehyde and 2% paraformaldehyde in 0.1M sodium cacodylate (pH 7.4). Small pieces of the jejunum were fixed in freshly prepared 2% glutaraldehyde in 0.1M sodium cacodylate (pH 7.4) for at least 2 h at 4°C. Pieces of tissue were stained with 1% osmium tetroxide in 0.1 M sodium cacodylate (pH 7.4) for 1 h at room temperature, dehydrated, and embedded in Embed 812 resin. Thick sections (0.5 μm) were stained with 1% toluidine blue and examined by light microscopy to confirm tissue orientation. Thin sections (80 nm) were cut on a Leica UC6 ultramicrotome and stained with 2% uranyl acetate and lead citrate. Images were acquired on a Tecnai T20 transmission electron microscope (FEI, Hillsboro, OR) equipped with a LaB6 source and operating at 100kV. The identification of TAG in CLDs and AVs was based on previously published studies [20, 23, 27–30]. To determine the % of enterocytes having CLDs or AVs, a total of 77 ± 25 (average ± STD) intact enterocytes from the middle area of villi per mouse per time point were examined by TEM (3 mice/group). To quantify the number and the amount of TAG stored in CLDs, 20 intact enterocytes that had TAG present in the subcellular pools for both secretion (ER lumen and Golgi) and storage (CLDs) [23] were selected for quantitative measurements (3 mice/group). The amount of TAG stored in CLDs within an individual cell was indicated by the sum of the areas of identified CLDs. To quantify the amount of TAG present within AVs, a total of 117 ± 22 (average ± STD) intact enterocytes from the middle area of villi per mouse per time point were examined by TEM (3 mice/group). The amount of TAG present within AVs of individual cells was estimated by the equation: (mean number of TAG-containing AVs in an individual cell) × (mean area of TAG inindividual TAG-containing AVs). The quantitative measurements were determined using ImageJ software (NIH, USA).
2.3. qPCR
Total RNA was extracted from jejunum mucosa with RNA STAT60 (Tel-Test, Friendswood, TX) and then DNase treated with a Turbo DNA-free Kit (Ambion, Austin, TX). cDNA was synthesized from 1μg DNase-treated RNA using the AffinityScript QPCR cDNA Synthesis Kit (Stratagene, La Jolla, CA). Total DNA was extracted from tissues using DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA). SYBR green qPCR was performed using the Mx3000P QPCR System (Stratagene) and Brilliant III SYBR Green Master Mix (Stratagene). Primers used for determination of relative mRNA levels were produced by Integrated DNA technologies (Coralville, IA) and validated for efficiency and correct product size of cDNA from mouse intestinal mucosa (see Table 1). The level of each gene was calculated with the comparative Ct method using WT mice as the reference group.
Table 1.
Primers used for q-PCR
| Gene | Primer Sequences |
|---|---|
| TFEB | F 5’-CCAGAAGCGAGAGCTCACAGAT-3’ |
| R 5’-TGTGATTGTCTTTCTTCTGCCG-3’ | |
| Wipi1 | F 5’-CGGCTACATGGGAAAGATG-3’ |
| R 5’-GTGGGTCCAAGTTGTAGATG-3’ | |
| Lamp1 | F 5’-CAAGATGCTCTCCCTCAATG-3’ |
| R 5’-CCAGGCTAGATGGTCTGATA-3’ | |
| Atp6v1b2 | F 5’-CACGCTGATGTGTCTAACC-3’ |
| R 5’-CCAGCCAATGTCCAAAGT-3’ | |
| Atp6v1a | F 5’-CTGCCCAGAGTGACAATAAG-3’ |
| R 5’-CGTGGAAGAGGGAAGAAATC-3’ | |
| Lal | F 5’-CTAGAATCTGCCAGCAAGCC-3’ |
| R 5’-AGTATTCACCGAATCCCTCG-3’ | |
| β-actin | F 5’-AGGCCCAGAGCAAGAGAGGTA-3’ |
| R 5’-GGGGTGTTGAAGGTCTCAAACA-3’ |
2.4. Fluorescence confocal microscopy
Small pieces of the jejunum were harvested from the mice, frozen in molds with optimal cutting temperature embedding media and stored at −80°C until processed for fluorescence confocal microscopy. The tissue sections (10–12 μm) were fixed with 2% paraformaldehyde, permeabilized with 0.1% saponin and stained with fluorescence labeling reagents or validated antibodies. The tissue sections were stained for TAG using 1 μg/ml boron dipyrromethene (BODIPY) (Life Technologies, Grand Island, NY, USA) and for nuclei using 300 nM DAPI (Life Technologies). The tissue sections were also probed with Lamp1 (1:200 dilution) (C-20; Santa Cruz Biotechnology, Dallas TX, USA), Plin3 (1:1000 dilution) [31], and a secondary AlexaFluor antibody (Life Technologies). The images were acquired using a Nikon A1R confocal microscope (Nikon Instruments Inc., Melville NY, USA) with a 60X oil objective.
To assess lysosome acidification, small pieces of jejunum explants were harvested from the mice, washed with cold PBS (pH 7.4) and incubated in 24-well plates containing Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 20 mM HEPES, 100 U/mL penicillin-streptomycin and 10% fetal bovine serum. Lysosomes in the tissue explants were stained by 2 μM LysoSensor™ Yellow/Blue DND-160 (Thermo Fisher Scientific) in DMEM at 37°C for 20 min. Horchst (Thermo Fisher Scientific) was used to stain the nuclei of live cells. Tissue explants were placed on glass slides with coverslips and imaged using an Olympus fv 10i confocal microscope with a 60X oil objective (Shinjuku, Tokyo, Japan). Emission signals of LysoSensor™ Yellow/Blue DND-160 were detected at 450 nm (yellow fluorescence) and 521 nm (blue fluorescence). The image processing, including quantification of fluorescence intensities and image overlay, were processed using ImageJ software (NIH) and NIS- Elements C acquisition.
2.5. Statistics
Values of quantitative data are reported as an average of biological replicates ± standard error of the mean (SEM). Statistical analyses of data were performed in SAS (SAS Institute, Inc., Cary, NC). A two-way ANOVA was used to determine the effects of the two main factors (genotype and time) and their interaction. When main factors or interaction resulted in significant differences, a Tukey post hoc test was used to identify differences between groups. In addition, a two-tailed student’s t test was used for two-group comparison. In all analyses, differences were considered statistically significant when P values were < 0.05.
3. Results
3.1. Greater TAG storage in CLDs at 2 but not 6 h after a dietary fat challenge, in enterocytes of Dgatl−/− compared to WT mice
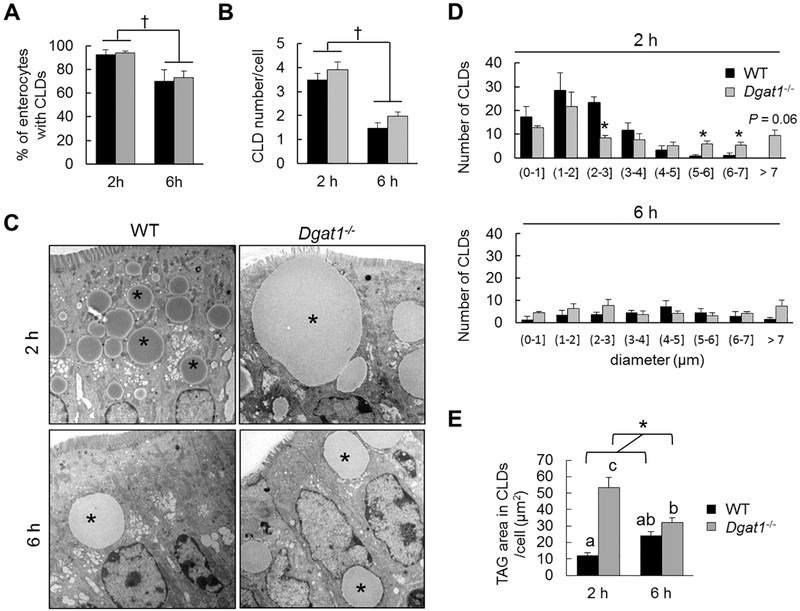
To determine the dynamic presence of CLDs in enterocytes of WT and Dgatl−/− mice, we administered an oral gavage of 200 μl olive oil and analyzed the presence of CLDs in enterocytes 2 and 6 h after oil gavage by TEM analysis. We selected 2 and 6 h after oil gavage as these are time points where CLDs are present, but reflect different balances between synthesis and mobilization of CLDs during dietary fat absorption [22]. No abnormal villus morphology was observed among the mice and the pattern of TAG distribution along the villi was similar between WT and Dgatl−/− mice at both time points (Supplementary figure 1 and 2). Quantifications of CLDs present in the middle region of villi were further analyzed. We found that the percent of enterocytes containing CLDs, as well as the number of CLDs per cell, were significantly less at 6 h than 2 h after an oil gavage in both WT and Dgatl−/− mice, with no differences observed between WT and Dgat1−/− mice (Figure 1A and B). However, at 2 h after an oil gavage, Dgatl−/− mice have an altered CLD size distribution, with fewer small CLDs and more large CLDs compared to WT mice (Figure 1C and D). This difference resulted in a significantly greater amount of TAG storage in CLDs per cell in Dgatl−/− compared to WT mice at 2 h after an oil gavage (Figure 1E). At 6 h after an oil gavage, this difference in CLD size distribution was no longer present (Figure 1C and D), resulting in similar levels of TAG storage in CLDs per cell in WT and Dgatl−/− mice at 6 h after an oil gavage (Figure 1E). Overall, we found greater TAG storage in CLDs per cell at 2 h, but not 6 h after a dietary fat challenge in enterocytes of Dgatl−/− compared to WT mice. This reflects an altered balance between CLD synthesis and mobilization in enterocytes of Dgatl−/− compared to WT mice only at early stage of dietary fat absorption.
Figure 1. Greater TAG storage in CLDs 2 h, but not 6 h after a dietary fat challenge in enterocytes from Dgatl−/− compared to WT mice.
(A) Percent of enterocytes containing CLDs in WT and Dgatl−/− mice after oil gavage. (B) Mean number of CLDs in individual enterocytes from WT and Dgatl−/− mice after oil gavage. (C) Representative electron micrographs of enterocytes from WT and Dgatl−/− mice after oil gavage. * = CLD. Scale bar = 2 μm. (D) The number of CLDs per size range out of 20 enterocytes per mouse in the indicated group. (E) The amount of TAG stored in CLDs of individual cells. For (A), (B) and (E), a two-way ANOVA with Tukey post hoc test was used for consideration of the two main factors (genotype and time) and their interaction (n = 3 mice/group). Significant effect of genotype (*) and time (f) are reported for P < 0.05. When the interaction resulted in significant differences, letters are used to indicate differences between groups at P < 0.05 (n=3 mice/ group). For (D), * denotes significant differences (P < 0.05) by two tailed student’s t test (n=3 mice/group).
3.2. Presence of larger AVs in enterocytes of Dgatl−/− compared to WT mice
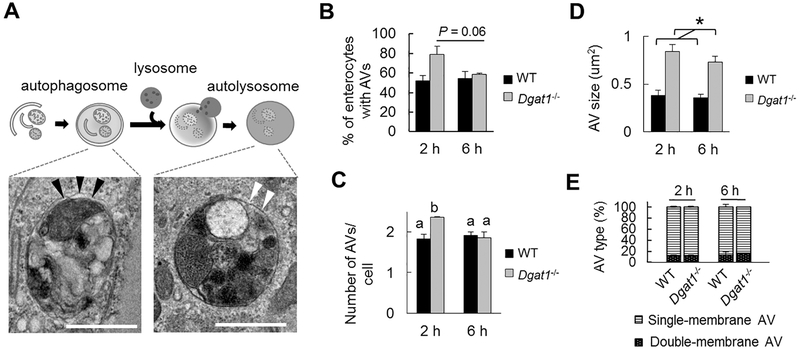
To assess whether the autophagy process is altered in enterocytes of Dgatl−/− compared to WT mice, we performed ultrastructural analysis on AVs identified by TEM at 2 and 6 h after an oil gavage. Based on previously published work describing and categorizing AVs [27, 28, 30, 32, 33], we were able to identify early-stage, double-membrane AVs (representing autophagosomes) as well as late-stage, single-membrane AVs (representing autolysosomes) in both WT and Dgatl−/− enterocytes (Figure 2A). The identified AVs varied in size (up to 2 μm in diameter) and contained mixed cellular materials. The percent of cells having AVs trended higher in Dgatl−/− compared to WT mice at 2 h, but was similar at 6 h after an oil gavage (Figure 2B). The enterocytes that contained AVs were further examined for quantitative measurements. More AVs per cell were present in Dgatl−/− compared to WT mice at 2 h after an oil gavage, but similar numbers of AVs per cell were present in WT and Dgatl−/− mice at 6 h after an oil gavage (Figure 2C). In addition, AVs present in enterocytes of Dgatl−/− mice were larger than those in WT mice (Figure 2D). Notably, the proportion of double-membrane and single-membrane AVs were similar among the mouse groups (Figure 2E), suggesting an intact initiation of autophagy and fusion events between autophagosomes and lysosomes in the absence of Dgat1. The accumulation of larger AVs in Dgatl−/− enterocytes indicates there is either a greater synthesis [34] or reduced turnover of AVs [35] in enterocytes of Dgatl−/− mice.
Figure 2. Accumulation of AVs and increased size of AVs in enterocytes of Dgatl−/− compared to WT mice after a dietary fat challenge.
(A) Representative electron micrographs of AVs in different stages of autophagy: an early-staged, double-membrane AV that contains undigested cellular components (left) and late-staged, single-membrane AVs that contain partially digested material (middle) or amorphous electron-dense material (right). Black arrows indicate doublemembrane structures. White arrows indicate single membrane structure of AVs. Scale bar = 1 μm. (B) Percent of enterocytes containing AVs in WT and Dgatl−/− mice after oil gavage. (C) Mean number of AVs in an individual cell and (D) mean size of the identified AVs in WT and Dgatl−/− mice. (E) Proportions of double-membrane and single-membrane AVs in WT and Dgatl−/− mice. A two-way ANOVA with Tukey post hoc test was used for consideration of the two main factors (genotype and time) and their interaction (n = 3 mice/group). Significant effect of genotype (*) and time (†) are reported for P < 0.05. When the interaction resulted in significant differences, letters are used to indicate differences between groups at P < 0.05.
3.3. Greater TAG accumulation in AVs in enterocytes of Dgatl−/− compared to WT mice
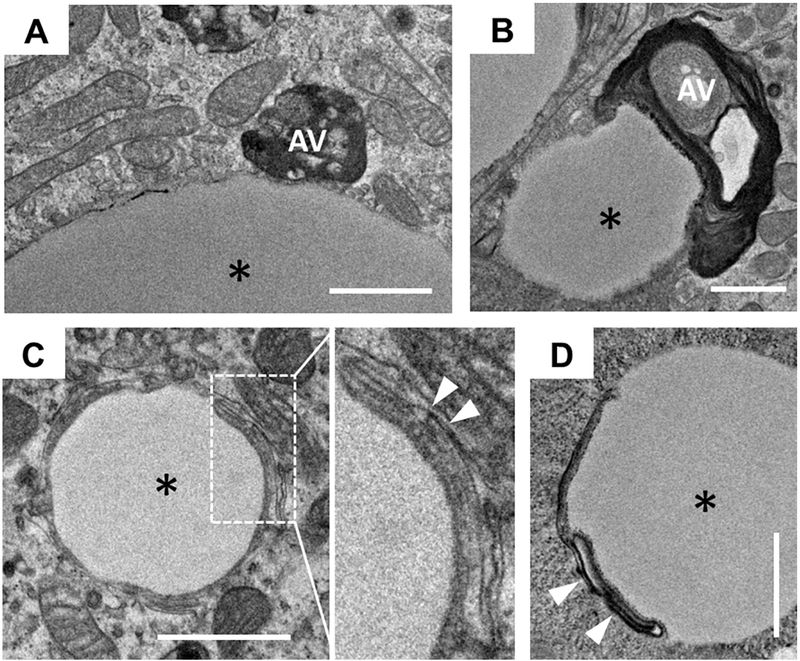
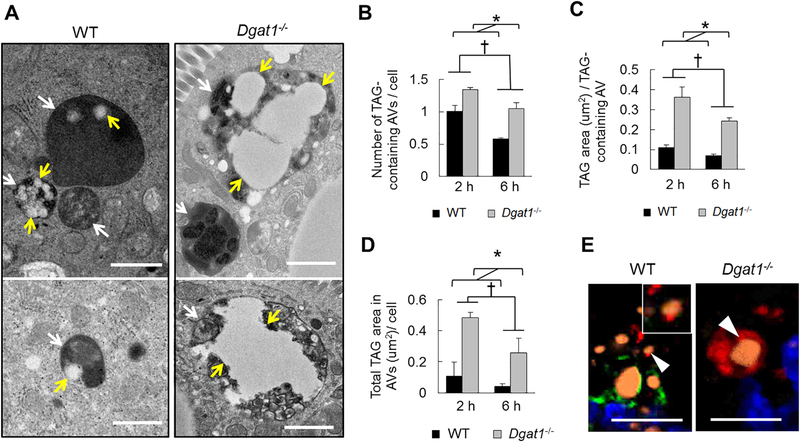
To determine whether the change in AVs may contribute to the altered TAG storage in Dgatl−/− mice, we assessed TAG content within AVs as an indication of lipophagy by TEM. Although it is unclear how AVs internalize TAG in enterocytes, we observed that some AVs have physical contact with CLDs and also observed perturbation of membrane structure on the surface of CLDs (Figure 3). In addition, both WT and Dgatl−/− mice have a subpopulation of the AVs containing TAG present in enterocytes after oil gavage (Figure 4A) and indeed these TAG-containing AVs are similar to those defined in previous ultrastructural analyses [10, 20, 32, 36, 37]. At 6 h after an oil gavage, the presence of TAG within AVs was reduced compared to 2 h after the gavage in enterocytes of both WT and Dgatl−/− mice (Figure 4B–D). However, enterocytes of Dgatl−/− mice had greater numbers of TAG-containing AVs per cell and a greater area of TAG per TAG-containing AV compared to WT mice at both time points (Figure 4B and C). Together, these differences contributed to a greater total area of TAG within AVs per cell in enterocytes of Dgatl−/− compared to WT mice (Figure 4D).
Figure 3. A subpopulation of CLDs has interconnections with AVs or is associated with membrane structures as observed by TEM.
(A and B) CLDs have physical contact with AVs. (C and D) Membrane structures are associated with the surface of CLDs. The images were taken from the enterocytes of Dgatl−/− mice 2 h after a dietary fat challenge. * = CLD; white arrows indicate membrane structures. Scale bar = 1 μm.
Figure 4. Greater TAG accumulation in AVs within enterocytes of Dgatl−/− compared to WT mice after a dietary fat challenge.
(A) Representative electron micrographs of AVs containing TAG from WT and Dgatl−/− mice. White arrows indicate AVs and yellow arrows indicate TAG present within AVs in enterocytes. Scale bar = 1 μm. (B) Mean number of TAG-containing AVs within an individual cell and (C) mean area of TAG per TAG-containing AV were determined in the indicated groups. (D) The amount of TAG present within AVs of individual cells was estimated by the equation: (mean number of TAG-containing AVs in an individual cell) × (mean area of TAG in individual TAG-containing AVs). For (B), (C) and (D) a two-way ANOVA with Tukey post hoc test was used for consideration of the two main factors (genotype and time) and their interaction (n = 3 mice/group). Significant effect of genotype (*) and time (†) are reported for P < 0.05. (E) Confocal fluorescence micrographs of jejunum tissue stained with BODIPY (orange; TAG), Lamp1 (red; lysosome marker), Plin3 (green; a CLD associated protein) and DAPI (blue; nuclei). White arrows indicate TAG associated with LAMP1. Scale bar = 10 μm.
To determine what contributes to accumulation of TAG within AVs in enterocytes of Dgatl−/− compared to WT mice, we assessed the type of AVs containing TAG in WT and Dgatl−/− mice. Among the TAG-containing AVs, the proportions of double-membrane and singlemembrane AVs were similar between WT and Dgatl−/− mice (data not shown). In addition, by using fluorescence confocal microscopy we were able to identify TAG (stained by BODIPY, orange) associated with lysosomes (Lamp1 positive, red) in enterocytes of both WT and Dgatl−/− mice (Figure 4E). These observations collectively suggest that Dgatl-deficiency does not impair the ability of TAG to be targeted by autophagosomes and delivered to autolysosomes.
3.4. Transient dysregulation of lysosome acidification in enterocytes of Dgatl−/− compared to WT mice
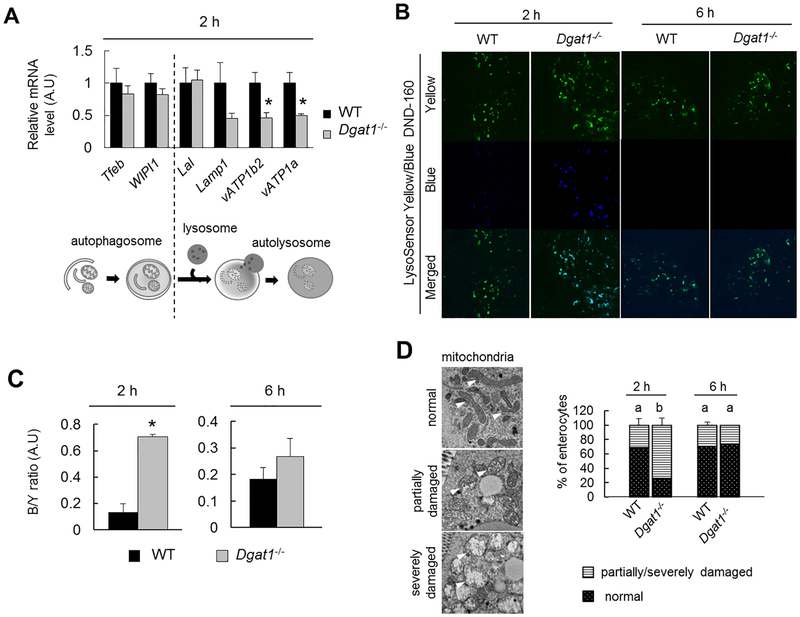
To assess which stage of lipophagy is altered by Dgatl-deficeincy, mRNA levels of autophagy- and lysosome-related genes (Tfeb, Wipil, Lal, Lampl, vATPla, and vATPlb2) in mucosa from the jejunum of WT and Dgatl−/− mice 2 h after an oil gavage were determined. We found that levels of Tfeb, Wipil, Lal and Lampl were similar between WT and Dgatl−/− mice (Figure 5A). However, mRNA levels of vacuolar ATPase subunits (vATPla and vATPlb2), which mediate lysosome acidification, were significantly lower in enterocytes of Dgatl−/− mice compared to WT mice (Figure 5A). Interestingly, the decrease in mRNA levels of vATPase subunits were only present at 2h, but not 6h, after oil gavage in enterocytes of Dgatl−/− mice compared with WT mice (qPCR data at 6h data point not shown).
Figure 5. Dysregulation of lysosome function in enterocytes of Dgatl−/− compared to WT mice after a dietary fat challenge.
(A) qPCR analysis of relative mRNA levels of autophagy or lysosome-related genes in enterocytes from Dgatl−/− compared to WT mice 2 h after oil gavage. (n=3–4 mice/group). (B) Representative confocal fluorescence micrographs of jejunum tissue stained with LysoSensor Yellow/Blue dye. (C) Blue/yellow fluorescence intensity determined from (B). The fluorescent signal intensity was determined by the average of three representative images from each mouse (n= 5 mice/group). (D) Representative electron micrographs (left panel) and percent (right panel) of enterocytes with indicated mitochondrial morphology For (D) and, a two-way ANOVA with Tukey post hoc test was used for consideration of the two main factors (genotype and time) and their interaction (n = 3 mice/group). Significant effect of genotype (*) and time (†) are reported for P < 0.05. When the interaction resulted in significant differences, letters are used to indicate differences between groups at P < 0.05. For (A) and (C), * denotes a significant difference between mouse models by two-tailed student’s t test (p < 0.05).
To determine whether the lower mRNA levels of vATPase subunits were consistent with reduced lysosome acidification in enterocytes of Dgatl−/− compared to WT mice, lysosomes were stained with pH-sensitive LysoSensor™ Yellow/Blue DND-160 in fresh intestinal tissues from mice. Yellow fluorescence is predominantly generated in acidic lysosomes, whereas blue fluorescence is intensified when lysosome pH is increased. We found a significantly greater blue/yellow fluorescence ratio in enterocytes of Dgatl−/− compared to WT mice at 2 h after oil gavage (Figure 5B and C), suggesting reduced lysosome acidification in enterocytes of Dgatl−/− mice. In addition, consistent with the qPCR data (Figure 5A), the lysosome dysfunction as indicated by higher fluorescent B/Y ratios was only present at 2 h, but not 6 h after an oil gavage, in enterocytes of Dgatl−/− mice (Figure 5B and C).
Lysosome function is required for the appropriate turnover of organelles, and studies have shown an accumulation of morphologically damaged, dysfunctional mitochondria in models of lysosome dysfunction [38, 39]. We observed mitochondria with damaged cristae and swelling morphology present more frequently at 2 h, but not 6 h,after oil gavage in enterocytes of Dgatl−/− compared to WT mice (Figure 5D), indicating a transient impairment of mitochondrial turnover and further supporting impaired lysosome acidification in Dgatl−/− mice
Taken together, these observations of lysosome dysregulation were consistent with the finding of abnormal CLD accumulation only at 2 h after an oil gavage in Dgatl−/− compared to WT mice (Figure 1E). A compensatory effect for Dgatl-deficiency may take place between 2 and 6 h after oil gavage, allowing lysosomes to restore function and the ability to mobilize TAG stored in CLDs at later time points.
4. Discussion
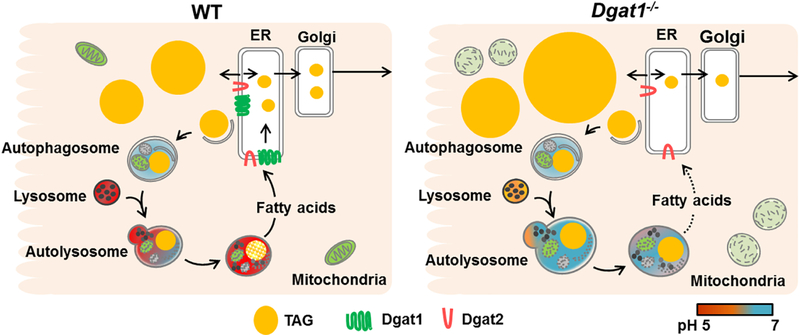
The intestinal phenotype of delayed dietary fat absorption in Dgatl−/− mice is well documented in the literature but its underpinning remains unclear. In response to an acute dietary fat challenge, Dgatl−/− mice have abnormal CLD accumulation within enterocytes and a slower rate of TAG secretion into blood [21, 23]. In addition, while abnormal TAG accumulation in CLDs of Dgatl−/− mice was observed in the fed state under a chronic high fat dietary intervention, this TAG accumulation disappears after an overnight fast and they do not develop overt fat malabsorption [24, 25]. This suggests that the TAG in enterocyte CLDs is mobilized for secretion at a slower rate, contributing to their resistance to diet-induced obesity [25]. However, the possible mechanism(s) has not been explored. Here we provide evidence that the unique intestinal phenotype of Dgatl−/− mice is, in part, due to the disruption of autophagy-mediated CLD mobilization. Specifically, we observed greater TAG accumulation in both CLDs and AVs in enterocytes of Dgatl−/− compared to WT mice after an acute fat challenge. Furthermore, we demonstrated that the lysosomes of Dgatl−/− mice are less acidic compared to WT mice during the early stage of dietary fat absorption. This suggests that Dgatl−/− mice have impaired lysosome acidification, which is essential for optimal LAL enzyme activity to complete autophagy-mediated CLD mobilization. Based on the findings of this study, we propose that the reduced lysosome acidification in enterocytes of Dgatl−/− mice delays fatty acid supply for TAG resynthesis and secretion, ultimately resulting in a slow rate of intestinal TAG secretion that contributes to their lean phenotype (Figure 6). Overall, this work highlights the potential of targeting lysosome function for managing postprandial hyperlipidemia and related diseases.
Figure 6. A proposed role of Dgat1-deficiency in delaying dietary fat absorption through dysregulated lysosome function.
Under normal conditions, TAG is either incorporated onto chylomicrons for secretion or into CLDs for temporary storage in response to a high dietary fat challenge. The mobilization of TAG from the storage pool to a secretory fate is contributed by lipophagy, where CLDs are engulfed by autophagosomes and delivered to lysosomes for TAG hydrolysis. The TAG is hydrolyzed by Lal, which favors acidic environment for optimal enzyme activity, and redirected to secretory fate. In the absence of Dgat1, lysosome acidification is impaired, resulting in large, non-acidic autolysosomes and TAG accumulation within autophagic vesicles and CLDs. This disruption of lipophagy limits the amount of fatty acids liberated from CLDs and therefore decreases the rate of intestinal TAG secretion into the circulation.
The present study supports a role for lysosomes in regulating autophagic flux. Impaired autophagy and reduced AV turnover have been observed in models with defective lysosome function or lysosomal storage disorders across multiple tissue types [18, 35, 38, 40]. These models are characterized by AV accumulation due to one or multiple defects in the autophagy process, including impaired autophagosome-lysosome fusion, reduced activities of lysosome hydrolases, and reduced lysosome acidification. In this study, we found that the AV accumulation in enterocytes of Dgatl−/− mice was likely due to reduced lysosome acidification, as suggested by reduced mRNA levels of subunits of vATPase and a higher lysosome pH compared to WT mice (Figure 4). vATPase is known to tightly regulate lysosome function by maintaining an acidic environment favored for diverse lysosome enzymes [41–44]. vATPase-deficiency in Drosophila results in accumulation of giant, non-functional autolysosomes but has little effect on autophagosome-lysosome fusion [42]. Consistently, in enterocytes of Dgatl−/− mice where levels of vATPase subunits are reduced, we observed accumulation of larger AVs and non-acidic lysosomes without impaired autophagosome-lysosome fusion. Taken together, our findings in Dgatl−/− mice suggest that lysosome acidification is an important contributor to proper lysosome function and autophagic flux in enterocytes.
LAL is critical to complete the lipophagy process by mediating the hydrolysis of TAG and cholesterol esters within lysosomes. Although we found similar mRNA levels of Lal in enterocytes of Dgatl−/− and WT mice after an oil gavage, several observations made in the present study suggest a reduction of Lal activity in enterocytes of Dgatl−/− mice. First, Dgatl−/− mice have an abnormal accumulation of TAG in AVs, indicatinginefficient lysosomal TAG hydrolysis. This is consistent with a study showing TAG accumulation within autolysosome-like structures (LAMP1 positive) of Caco-2 cells treated with an LAL inhibitor [10]. Second, the lysosomes present in Dgatl−/− enterocytes are less acidic compared to WT mice. Dysregulated lysosomal acidification can greatly decrease Lal enzyme activity given that the pH range of optimized LAL enzyme activity is 3.5 – 4.5 [45, 46]. Thus, targeting lysosome acidification could be a potential approach to effectively regulate enterocyte CLD mobilization during dietary fat absorption.
The exact mechanism through which Dgat1-deficiency leads to dysregulation of lysosomal acidification still remains unknown. Several reports demonstrated that high intracellular levels of free fatty acid and DAG, which is substrate of Dgat1, can cause lysosome dysfunction in several tissue types [35, 47–49]. We speculated that Dgat1-deficiency dysregulates lysosome function in enterocytes by altering cellular levels of DAG and/or FFA. However, our preliminary data from a targeted lipidomics analysis showed no significant differences in cellular levels of DAG and FFA between WT and Dgatl−/− mice at either 2 or 6 h after oil gavage (data not shown). In addition, studies have reported that the abnormal CLD accumulation is associated with a reduced lysosome acidification in the liver of obese KKAy mice [47, 50]. These observations indicate that the abnormal accumulation of CLD could be not only the outcome of lysosome dysregulation but also part of the mechanism contributing to such dysregulation. Although this speculation needs to be closely examined, it is supported by our observations in enterocytes of Dgatl−/− mice, where abnormal CLD accumulation and lysosome dysregulation are present only at the early stage of dietary fat absorption and both are eventually resolved. One potential explanation for this transient dysregulation is that the activity of cytoplasmic TAG hydrolysis remains intact and therefore the lysosome dysregulation is eventually alleviated by the slow flux of TAG from storage to secretion in enterocytes. Although the exact “recovery mechanism” remains unclear at this point, our findings in Dgatl−/− mice are consistent with their overall healthy phenotype and absence of overt fat malabsorption[25]. While the data from the present study is mostly observational, the approach of TEM analysis enables a direct visualization of disruption of autophagy-mediated CLD mobilization when Dgat1 is absent. Such dynamic process which has multiple cellular machineries/organells involved, indeed, warrants mechanistic studies in the future.
In summary, we demonstrated that Dgatl−/− mice have alterations in the process of autophagy-mediated CLD mobilization in enterocytes, likely due to the impairments of AV turnover and lysosome acidification during early stage of dietary fat absorption. We propose that this lysosome dysregulation results in a slower rate of intestinal TAG secretion that is critical to their resistance to diet-induced obesity (Figure 6). The present study highlights the important role of lysosome function in regulating autophagy-mediated CLD mobilization for proper dietary fat absorption. Most importantly, our findings suggest lysosome function as a potential therapeutic target for managing postprandial hyperlipidemia.
Supplementary Material
Highlights.
Cytoplasmic lipid droplets and autophagic vesicles are present in enterocytes in response to dietary fat consumption.
Deficiency of an enzyme in triacylglycerol synthesis, diacylglycerol acyltransferase 1, results in more and larger cytoplasmic lipid droplets in enterocytes, larger autophagic vesicles containing larger lipid droplets in enterocytes, and disrupted lysosome acidification in response to dietary fat consumption.
Lipophagy is a regulatable process within enterocytes involved in dietary fat absorption.
5. Acknowledgement
We acknowledge the Multi-Scale Imaging Center, Purdue University, particularly Christopher Gilpin and Laurie Mueller for helping with the TEM imaging. We thank Dr. Chun-Ju Chang in the Department of Basic Medical Sciences at Purdue University for kindly providing the confocal microscopy. We thank Tzu-Ching Wu for the assistance of fluorescent image analysis. We thank Alyssa Zembroski for feedback on the manuscript. This work was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute, funded in part by Award Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Abbreviations
- AV
autophagic vesicle
- BODIPY
boron dipyrromethene
- CLD
Cytoplasmic lipid droplet
- DAG
diacylglycerol
- DGAT
acyl CoA: diacylglycerol acyltransferase
- FFA
free fatty acid
- TAG
Triacylglycerol
- Lal
lysosomal acid lipase
- Lamp1
Lysosomal-associated membrane protein 1
- TEM
transmission electron microscopy
- Tfeb
Transcription Factor EB
- vATP
Vacuolar-type H+ -ATPase
- Wipi1
WD repeat domain phosphoinositide-interacting protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Nikolopoulou A, Kadoglou NP, Obesity and metabolic syndrome as related to cardiovascular disease, Expert Rev. Cardiovasc. Ther, 10 (2012) 933–939. [DOI] [PubMed] [Google Scholar]
- [2].Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J, Origins and evolution of the Western diet: health implications for the 21st century, Am. J. Clin. Nutr, 81 (2005) 341–354. [DOI] [PubMed] [Google Scholar]
- [3].Arca M, Alterations of intestinal lipoprotein metabolism in diabetes mellitus and metabolic syndrome, Atheroscler Suppl, 17 (2015) 12–16. [DOI] [PubMed] [Google Scholar]
- [4].Mansbach CM, Siddiqi SA, The biogenesis of chylomicrons, Annu. Rev. Physiol, 72 (2010) 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yen CL, Nelson DW, Yen MI, Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism, J. Lipid Res, 56 (2015) 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Halpern J, Tso P, Mansbach CM 2nd, Mechanism of lipid mobilization by the small intestine after transport blockade, J. Clin. Invest, 82 (1988) 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu J, Lee B, Buhman KK, Cheng JX, A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging, J. Lipid Res, 50 (2009) 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Uchida A, Whitsitt MC, Eustaquio T, Slipchenko MN, Leary JF, Cheng JX, Buhman KK, Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice, Front Physiol, 3 (2012) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Obrowsky S, Chandak PG, Patankar JV, Povoden S, Schlager S, Kershaw EE, Bogner-Strauss JG, Hoefler G, Levak-Frank S, Kratky D, Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARalpha signaling, J. Lipid Res, 54 (2013) 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Khaldoun SA, Emond-Boisjoly MA, Chateau D, Carriere V, Lacasa M, Rousset M, Demignot S, Morel E, Autophagosomes contribute to intracellular lipid distribution in enterocytes, Mol. Biol. Cell, 25 (2014) 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grober J, Lucas S, Sorhede-Winzell M, Zaghini I, Mairal A, Contreras JA, Besnard P, Holm C, Langin D, Hormone-sensitive lipase is a cholesterol esterase of the intestinal mucosa, J. Biol. Chem, 278 (2003) 6510–6515. [DOI] [PubMed] [Google Scholar]
- [12].Douglass JD, Zhou YX, Wu A, Zadrogra JA, Gajda AM, Lackey AI, Lang W, Chevalier KM, Sutton SW, Zhang SP, Flores CM, Connelly MA, Storch J, Global deletion of MGL in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity, J. Lipid Res, 56 (2015) 1153–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chon SH, Douglass JD, Zhou YX, Malik N, Dixon JL, Brinker A, Quadro L, Storch J, Over-expression of monoacylglycerol lipase (MGL) in small intestine alters endocannabinoid levels and whole body energy balance, resulting in obesity, PLoS One, 7 (2012) e43962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J, Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span, J. Lipid Res, 42 (2001) 489–500. [PubMed] [Google Scholar]
- [15].Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS, Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease, J. Biol. Chem, 250 (1975) 8487–8495. [PubMed] [Google Scholar]
- [16].Singh R, Cuervo AM, Lipophagy: connecting autophagy and lipid metabolism, Int J Cell Biol, 2012 (2012) 282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cingolani F, Czaja MJ, Regulation and Functions of Autophagic Lipolysis, Trends Endocrinol. Metab, 27 (2016) 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ward C, Martinez-Lopez N, Otten EG, Carroll B, Maetzel D, Singh R, Sarkar S, Korolchuk VI, Autophagy, lipophagy and lysosomal lipid storage disorders, Biochim. Biophys. Acta, 1861 (2016) 269–284. [DOI] [PubMed] [Google Scholar]
- [19].Skop V, Cahova M, Papackova Z, Palenickova E, Dankova H, Baranowski M, Zabielski P, Zdychova J, Zidkova J, Kazdova L, Autophagy-lysosomal pathway is involved in lipid degradation in rat liver, Physiol. Res, 61 (2012) 287–297. [DOI] [PubMed] [Google Scholar]
- [20].Shanmugam M, McBrayer SK, Qian J, Raikoff K, Avram MJ, Singhal S, Gandhi V, Schumacker PT, Krett NL, Rosen ST, Targeting glucose consumption and autophagy in myeloma with the novel nucleoside analogue 8-aminoadenosine, J Biol Chem, 284 (2009) 26816–26830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee B, Fast AM, Zhu J, Cheng JX, Buhman KK, Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1−/− mice, J. Lipid Res, 51 (2010) 1770–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uchida A, Slipchenko MN, Eustaquio T, Leary JF, Cheng JX, Buhman KK, Intestinal acyl-CoA:diacylglycerol acyltransferase 2 overexpression enhances postprandial triglyceridemic response and exacerbates high fat diet-induced hepatic triacylglycerol storage, Biochim. Biophys. Acta, 1831 (2013) 1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hung YH, Carreiro AL, Buhman KK, Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat, Biochim Biophys Acta, 1862 (2017) 600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF Jr., Burri BJ, Hamilton RL, Abumrad NA, Farese RV Jr., DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis, J. Biol. Chem, 277 (2002) 25474–25479. [DOI] [PubMed] [Google Scholar]
- [25].Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr., Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat, Nat. Genet, 25 (2000) 87–90. [DOI] [PubMed] [Google Scholar]
- [26].Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr., Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis, J. Lipid Res, 49 (2008) 22832301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mizushima N, Yoshimori T, Levine B, Methods in mammalian autophagy research, Cell, 140 (2010) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eskelinen EL, To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells, Autophagy, 4 (2008) 257–260. [DOI] [PubMed] [Google Scholar]
- [29].Zhang H, Chang JT, Guo B, Hansen M, Jia K, Kovacs AL, Kumsta C, Lapierre LR, Legouis R, Lin L, Lu Q, Melendez A, O’Rourke EJ, Sato K, Sato M, Wang X, Wu F, Guidelines for monitoring autophagy in Caenorhabditis elegans, Autophagy, 11 (2015) 9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Goeritzer M, Vujic N, Schlager S, Chandak PG, Korbelius M, Gottschalk B, Leopold C, Obrowsky S, Rainer S, Doddapattar P, Aflaki E, Wegscheider M, Sachdev V, Graier WF, Kolb D, Radovic B, Kratky D, Active autophagy but not lipophagy in macrophages with defective lipolysis, Biochim Biophys Acta, 1851 (2015) 1304–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].D’Aquila T, Sirohi D, Grabowski JM, Hedrick VE, Paul LN, Greenberg AS, Kuhn RJ, Buhman KK, Characterization of the proteome of cytoplasmic lipid droplets in mouse enterocytes after a dietary fat challenge, PLoS One, 10 (2015) e0126823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rost-Roszkowska MM, Chajec L, Vilimova J, Tajovsky K, Kszuk-Jendrysik M, Does autophagy in the midgut epithelium of centipedes depend on the day/night cycle?, Micron, 68 (2015) 130–139. [DOI] [PubMed] [Google Scholar]
- [33].Eskelinen EL, Fine structure of the autophagosome, Methods Mol Biol, 445 (2008) 11–28. [DOI] [PubMed] [Google Scholar]
- [34].Yang L, Li P, Fu S, Calay ES, Hotamisligil GS, Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance, Cell Metab, 11 (2010) 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jaishy B, Zhang Q, Chung HS, Riehle C, Soto J, Jenkins S, Abel P, Cowart LA, Van Eyk JE, Abel ED, Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity, J. Lipid Res, 56 (2015) 546–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kanai M, Soji T, Herbert DC, Biogenesis and function of lipolysosomes in developing chick hepatocytes, Microsc Res Tech, 39 (1997) 444–452. [DOI] [PubMed] [Google Scholar]
- [37].Iancu TC, Manov I, Shaoul R, Haimi M, Lerner A, What’s in a name?-”Lipolysosome”: ultrastructural features of a lipid-containing organelle, Ultrastruct Pathol, 37 (2013) 293–303. [DOI] [PubMed] [Google Scholar]
- [38].Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A, A block of autophagy in lysosomal storage disorders, Hum. Mol. Genet, 17 (2008) 119–129. [DOI] [PubMed] [Google Scholar]
- [39].Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y, Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease), Am. J. Pathol, 167 (2005) 1713–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sarkar S, Carroll B, Buganim Y, Maetzel D, Ng AH, Cassady JP, Cohen MA, Chakraborty S, Wang H, Spooner E, Ploegh H, Gsponer J, Korolchuk VI, Jaenisch R, Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease, Cell Rep, 5 (2013)1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Colacurcio DJ, Nixon RA, Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease, Ageing Res Rev, 32 (2016) 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mauvezin C, Nagy P, Juhasz G, Neufeld TP, Autophagosome-lysosome fusion is independent of V-ATPase-mediated acidification, Nat Commun, 6 (2015) 7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mangieri LR, Mader BJ, Thomas CE, Taylor CA, Luker AM, Tse TE, Huisingh C, Shacka JJ, ATP6V0C knockdown in neuroblastoma cells alters autophagy-lysosome pathway function and metabolism of proteins that accumulate in neurodegenerative disease, PLoS One, 9 (2014)e93257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sobota JA, Back N, Eipper BA, Mains RE, Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins, Journal of cell science, 122 (2009) 3542–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takano T, Black WJ, Peters TJ, de Duve C, Assay, kinetics, and lysosomal localization of an acid cholesteryl esterase in rabbit aortic smooth muscle cells, J Biol Chem, 249 (1974) 6732–6737. [PubMed] [Google Scholar]
- [46].Haley NJ, Fowler S, de Duve C, Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells, J. Lipid Res, 21 (1980) 961–969. [PubMed] [Google Scholar]
- [47].Nakadera E, Yamashina S, Izumi K, Inami Y, Sato T, Fukushima H, Kon K, Ikejima K, Ueno T, Watanabe S, Inhibition of mTOR improves the impairment of acidification in autophagic vesicles caused by hepatic steatosis, Biochem. Biophys. Res. Commun, 469 (2016) 1104–1110. [DOI] [PubMed] [Google Scholar]
- [48].Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ, Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway, Hepatology, 40 (2004) 185–194. [DOI] [PubMed] [Google Scholar]
- [49].Jaishy B, Abel ED, Lipids, lysosomes, and autophagy, J. Lipid Res, 57 (2016) 1619–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Inami Y, Yamashina S, Izumi K, Ueno T, Tanida I, Ikejima K, Watanabe S, Hepatic steatosis inhibits autophagic proteolysis via impairment of autophagosomal acidification and cathepsin expression, Biochem Biophys Res Commun, 412 (2011) 618–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.