Abstract
Background:
Birth weight is very important for long-term physical, mental, health, and brain development. Pesticide exposure is thought to interfere with fetal growth, among others, through disruption of the function of the insulin-like growth hormone-1 (IGF-1) hormone.
Objective:
To analyze the relationship between exposure to pesticides during pregnancy and low-birth weight (LBW) through the disruption of the IGF-1 hormone.
Methods:
In a case-control study, babies born with LBW (birth weight <2500 g) and those born later with normal birth weight (≥2500 g) at 2 hospitals in Brebes were chosen as cases and controls, respectively. Maternal pesticide exposure was measured by interview using a questionnaire. Umbilical serum IGF-I level was tested using the ELISA method.
Results:
There was a significant relationship between pesticide exposure during pregnancy and LBW (OR 6.8; 95% CI 2.0 to 22.9) and low umbilical serum IGF-1 levels (OR 3.6; 95% CI 1.2 to 11.1). There was a significant relationship between low umbilical serum IGF-1 levels and LBW (OR 8.9; 95% CI 2.4 to 32.1).
Conclusion:
There was a significant relationship between pesticide exposure during pregnancy and LBW through the umbilical serum IGF-1 reduction pathway.
Keywords: Pesticides, Fetal blood, Infant, low birth weight, Organophosphates, Insulinlike growth factor I
TAKE-HOME MESSAGE
Birth weight is known to have a close relationship with longterm brain development, baby's survival and the quality of physical and mental growth in the future.
Pesticide exposure could be a risk factor for the occurrence of growth disorders in children living in agricultural areas.
Environment toxic exposure during pregnancy can interfere with the synthesis and secretion of the insulin-like growth hormone-1, which increases the frequency of low-birth weight.
Introduction
Birth weight is one of the determinants of a baby’s survival; it determines the quality of physical and mental growth in the future. Birth weight is even known to have a close relationship with long-term brain development.1 Low-birth weight (LBW), ie, birth weight <2500 g,2 is an important health concern, particularly in developing countries.3 LBW is a major predictor of prenatal mortality and morbidity. Recent studies have found that LBW also increases the risk for noncommunicable diseases such as diabetes and cardiovascular disease, later in life.4,5
Optimal intrauterine growth is essential for fetal development and contributes to birth weight and long-term health. Fetal growth is influenced by interactions between genetic, nutritional, hormonal, and environmental factors.6 One environmental factor that needs attention is exposure to pesticides during pregnancy.7,8 Pregnant women in agricultural areas are at risk of exposure to pesticides due to their involvement in agricultural activities. Several types of pesticides are often used in agricultural areas; they are classified as endocrine disrupting chemicals (EDC), including organophosphate, chlorpyrifos, and malathion.9
Exposure to toxic substances from the environment can interfere with the synthesis and secretion of the insulin-like growth hormone-1 (IGF-1).10 IGF-1 is one of the most significant growth factors and hormones in fetal growth.11,12 It plays a significant role in fetal growth especially in advanced gestational ages.13 IGF-1 is a single-chain 7.5-kDa polypeptide that promotes growth before and after birth. During pregnancy, IGF-1 can affect fetal growth through effects on maternal metabolism and placenta, where IGF-1 plays a role in metabolism, mitogenesis, and differentiation of various types of cells including regulation and the development of trophoblast cells that make up the placenta.14,15
Many research studies on the association between pesticide exposure and LBW have so far been conducted. However, those that measure and analyze umbilical serum IGF-1 levels as an intermediate variable, are still very rare. We conducted this study to analyze the association between exposure to pesticides and LBW through interference with umbilical serum IGF-1 levels.
Materials and Methods
In a case-control study we studied mothers who gave birth in the period January–May 2018 in the Brebes Regional General Hospital and Bhakti Asih Hospital Brebes, Central Java Province. Mothers who did antenatal care in the study area, had a complete maternal and child health books, singleton pregnancy, and spontaneous delivery were included in the study. Mothers who had an emergency during labor, had a history of chronic diseases (diabetes mellitus, pulmonary tuberculosis, poor nutrition, etc ), and/or came from the outside Brebes Regency area were excluded.
Sampling was carried out by a consecutive sampling method of all deliveries at the Brebes General Hospital and Bhakti Asih Hospital in the study period. Babies born with LBW were included in the case group; those born later with a birth weight of ≥2500 g were included in the control group. Data on gestational age at the time of labor was obtained from the medical birth registry from the hospital. Gestational age was categorized into preterm birth (<38 wks) and term (≥38 wks), because a normal pregnancy can range 38 to 42 weeks.16 The minimum sample size was calculated based on the equation proposed by Lemeshow, et al, for case-control studies.17
Structured questionnaires were used to collect the subjects’ characteristics and history of pesticide exposure. History of pesticide exposure was a composite variable of four sub-variables of exposure risks: (1) maternal involvement in agriculture before pregnancy, (2) maternal involvement in agriculture during pregnancy, (3) the maternal risk of exposure to pesticides in the home environment before pregnancy, and (4) the maternal risk of exposure to pesticides in the home environment during pregnancy. Subjects were considered “exposed to pesticides” if they had a minimum of two of the four exposure risks. Structured questionnaires were also used to obtain information about the characteristics of the subjects (age, type of occupation, level of education, maternal smoking habit, the presence of family members who have smoking habits, etc ).
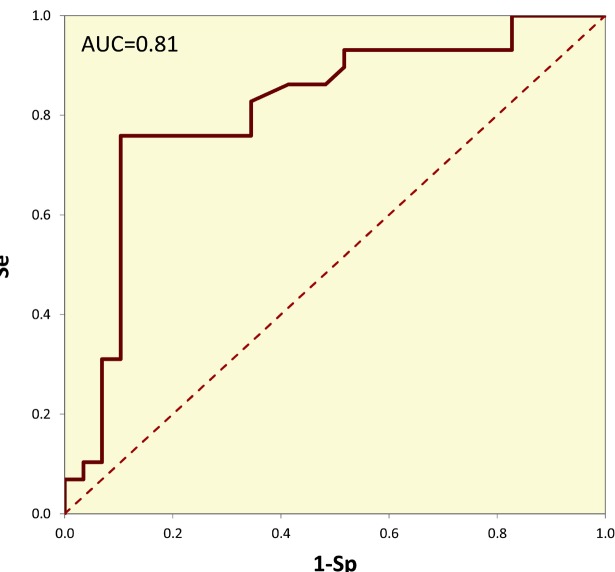
Blood sampling from the umbilical cord during labor was carried out by trained midwives. Plasma concentrations of IGF-1 were measured using microplate enzyme-linked immunosorbent assays (ELISA, Diagnostic Systems Laboratories, Webster, TX, USA) according to the manufacture instructions. Triplicate assays were performed for each sample. Absorbance was read using a microplate reader (DSL, Webster, TX, USA).18 The cut-off value for IGF-1 level for LBW was determined using ROC curve analysis by maximizing the Youden’s index,19,20 which revealed a cut-off value of 32.6 ng/dL, with a sensitivity and specificity of 0.862 and 0.586, respectively (Fig 1). An IGF-1 level <32.6 ng/dL was considered “low.”
Figure 1.
ROC curves for various umbilical serum IGF-1 cut-off values for LBW
Data were analyzed with SPSS® for Windows® ver 16.0. Student’s t test for independent samples and Mann-Whitney U test were used to compare the normally and non-normally distributed variables, respectively, between cases and controls. Spearman’s ρ was used to determine the correlation between serum IGF-1 level and the anthropometric data recorded (birth weight, head circumference, and birth length). χ2 test was used to determine the association between pesticide exposure, IGF-1 levels, and LBW. Binary logistic regression analysis was used to analyze the effect of pesticide exposure, IGF-1 levels, and gestational age on LBW.
Ethics
This study was approved by the Medical Research Ethics Committee of the Faculty of Medicine, Diponegoro University (688/EC/FK-RSDK/XII/2017).
Results
We studied 36 LBW newborns (cases) and 36 normal babies (controls). Of 36 LBW babies, seven were excluded: blood samples were lysed in four, one died because of eclampsia, and two refused to stay in the study, leaving 29 LBW babies in the case group. There were also seven babies who dropped out in the control group: blood samples were lysed in five and two refused to continue their participation in this study, leaving 29 babies in the control group.
The studied groups were well matched for their basic characteristics, with the exception of the gestational age and anthropometric data—birth weight, birth length, and head circumference (Table 1). The anthropometric measurements, ie, birth weight, birth length, and head circumference, were significantly different between the case and control groups (Table 2).
| Table 1: Characteristics of the studied mothers. Values are either median (IQR) or n (%). | |||
| Characteristics | Case (n=29) | Control (n=29) | p value |
| Age (yrs) | 31.0 (11.0) | 29.0 (11.0) | 0.999* |
| Weight (kg) | 60.0 (12.0) | 60.0 (11.0) | 0.861* |
| Height (cm) | 155.0 (7.0) | 154.0 (8.0) | 0.318† |
| Gestational age (wks) | 36.0 (3.5) | 38.0 (2.5) | 0.001† |
| Hemoglobin levels (g/dL) | 11.0 (0.4) | 11.0 (0.5) | 0.415† |
| Gravida | |||
| Primigravida | 10 (35%) | 9 (31.0) | 1.000‡ |
| Multigravida | 19 (66%) | 20 (69.0) | |
| Parity | |||
| Primipara | 9 (31%) | 9 (31.0) | 0.523‡ |
| Multipara | 20 (69%) | 20 (69.0) | |
| Maternal Education | |||
| Elementary school | 20 (69%) | 21 (72.4) | 0.577‡ |
| Junior high school | 6 (21%) | 7 (24.1) | |
| High school | 29 (100%) | 1 (3.4) | |
| Maternal Occupation | |||
| Private employees | 1 (3%) | 4 (13.8) | 0.438‡ |
| Traders | 3 (10%) | 5 (17.2) | |
| Farmer/owner | 1 (3%) | 2 (7%) | |
| Farm workers | 13 (45%) | 8 (28%) | |
| Housewife | 10 (35%) | 10 (35%) | |
| Others | 1 (3%) | 0 (0%) | |
| Husband’s education | |||
| Elementary school | 20 (69%) | 21 (72%) | 0.340‡ |
| Junior high school | 5 (17%) | 7 (24%) | |
| High school | 4 (14%) | 1 (3%) | |
| Husband’s occupation | |||
| Private | 4 (14%) | 6 (21%) | 0.900‡ |
| Traders | 9 (31%) | 8 (28%) | |
| Farmer/owner | 0 (0%) | 1 (3%) | |
| Farm workers | 14 (48%) | 12 (41%) | |
| Fisherman | 1 (3%) | 1 (3%) | |
| Others | 1 (3%) | 1 (3%) | |
| Maternal’s smoking habit | |||
| Yes | 0 (0%) | 0 (0%) | 1.000‡ |
| No | 29 (100%) | 29 (100%) | |
| Family members smoking habits | |||
| Yes | 25 (86%) | 24 (83%) | 1.000‡ |
| No | 4 (14%) | 5 (17%) | |
| *Independent-sample Student’s t test, †Mann-Whitney U test, ‡χ2 test | |||
| Table 2: The median (IQR) of infants’ anthropometric parameters between cases and controls | |||
| Anthropometrics | Case (n=29) | Control (n=29) | p value |
| Birth weight (g) | 2100 (445) | 3100 (750) | <0.001 |
| Birth length (cm) | 44 (3) | 48.0 (4) | <0.001 |
| Head circumference (cm) | 30 (2) | 33.0 (3) | <0.001 |
Maternal involvement in agricultural activities before pregnancy and the risk of pesticide exposure in the home environment during pregnancy were proved as risk factors for LBW (OR of 4.9 and 6.2, respectively) (Table 3).
| Table 3: The relationship between pesticide exposure and LBW. Values are n (%). | |||
| Variables | Cases (n=29) | Controls (n=29) | OR (95% CI) |
| Maternal involvement in agricultural activities before pregnancy | |||
| Yes | 21 (72) | 10 (35) | 4.9 (1.6 to 15.2) |
| No | 8 (28) | 19 (66) | 1 |
| Maternal involvement in agricultural activities during pregnancy | |||
| Yes | 13 (45) | 7 (24) | 2.5 (0.8 to 7.8) |
| No | 16 (53) | 22 (76) | 1 |
| The risk of pesticide exposure in the home environment before pregnancy | |||
| Yes | 18 (62) | 11 (38) | 2.6 (0.9 to 7.7) |
| No | 11 (38) | 18 (62) | 1 |
| The risk of pesticide exposure in the home environment during pregnancy | |||
| Yes | 18 (62) | 6 (21) | 6.2 (1.9 to 20.2) |
| No | 11 (38) | 23 (79) | 1 |
| History of maternal pesticide exposure (composite variable)* | |||
| Yes | 17 (59) | 5 (17) | 6.8 (2.0 to 22.9) |
| No | 12 (41) | 24 (83) | 1 |
| *Subjects were considered to be exposed to pesticides if they had a minimum of two out of four exposure risks. | |||
Those with a history of pesticide exposure had lower median IGF-1 levels than those without a history of exposure (p=0.003; 29.7 [IQR 17.4] and 52.7 [40.1] ng/dL, respectively). Meanwhile, mothers with a history of pesticide exposure had a 3.6-fold increase in the risk of having lower IGF-1 levels than those without a history of pesticide exposure (Table 4).
| Table 4: The association between pesticide exposure and IGF-1 levels. Values are n (%). | |||
| Maternal ex posure to pesticide | Umbilical serum IGF-1 levels | OR (95% CI) | |
| Low (<32.6 ng/dL) | High (≥32.6 ng/dL) | ||
| Yes (n=22) | 12 (55) | 10 (46) | 3.6 (1.2 to 11.1) |
| No (n=36) | 9 (25) | 27 (75) | 1 |
The median IGF-1 level in the case group was significantly (p<0.001) lower than the control group (30.6 [IQR 16.9] and 57.1 [35.9] ng/dL, respectively). Mothers with low IGF-1 levels were at significant risk of giving birth to babies with LBW compared with mothers with high IGF-1 levels (Table 5).
| Table 5: The association between umbilical serum IGF-1 levels and LBW. Values are n (%). | |||
| Umbilical serum IGF-1 levels | Cases (n=29) | Controls (n=29) | OR (95% CI) |
| Low (<32.6 ng/dL) | 17 (59) | 4 (14) | 8.9 (2.4 to 32.1) |
| High (≥32.6 ng/dL) | 12 (41) | 25 (86) | |
There were two independent predictors of LBW—pesticide exposure (adj OR 4.1; 95% CI 1.1 to 16.2) and low IGF-1 level (adj OR 7.2; 95% CI 1.8 to 29.2); gestational age <38 weeks was not an independent risk factor (adj OR 2.7; 95% CI 0.7 to 9.8).
There were significant (p<0.001) correlations (0.445≤ ρ ≤0.596) between umbilical serum IGF-1 level and all anthropometric parameters measured in the babies.
Discussion
We found an association between exposure to pesticides in pregnant women and giving birth to LBW babies. A study in France concludes that exposure to pesticides in pregnant women has influence on fetal development in utero.21 Involvement of pregnant women in agricultural activities puts them at risk of exposure to pesticides, for example, when farmers spray plants with pesticides in the fields while pregnant women are looking for pests or pulling grass from plants in the same area. Our previous study conducted in the same location, shows that pesticide exposure is a risk factor for developing goiter,22 hypothyroidism,23 and stunting in children24. The pesticides used in the study region were classified as EDCs. These chemicals can interfere with the synthesis, secretion, transport, metabolism, binding action, and elimination of hormones in the body that function to maintain balance (homeostasis), reproductive, and growth processes.25 One of the hormones that can be disrupted by exposure to pesticides is IGF-1, a hormone that is needed in the process of the growth process. IGF-1 is a mitogenic hormone peptide with a single 70-amino acid chain; its structure is similar to proinsulin, which stimulates systemic body growth in various species. IGF-1 is produced in almost all organs, but the liver is the main source of circulating IGF-1.26 It is believed that the mechanism of the observed growth disturbance in the fetal and childhood periods is mainly due to the presence of these chemicals in the environment.
Our study showed that pregnant women with a history of pesticide exposure had lower umbilical serum IGF-1 levels than those not exposed to pesticides. Umbilical serum IGF-1 levels in the case group also proved to be much lower than that in the control group. These results were consistent with studies in Sudan27 and Norway28. Our previous study also proved that IGF-1 levels in stunting children were lower than in normal children.24 Analysis of all subjects (n=59) showed a significant correlation between umbilical serum IGF-1 levels and infants’ anthropometric parameters (birth weight, length of birth and head circumference). There is a growing body of evidence linking IGF-1 with neonatal birth weight.29 This association is manifested by any increase in neonatal IGF-1 associated with an increase in birth weight29 and any decrease in IGF-1 associated with a decrease in birth weight.30 In the fetus, IGF-1 and insulin, which promotes IGF-1 production,31 rather than growth hormone is the main driver of growth.32 IGF-1-dependent growth is mediated by the glucose-insulin axis, which allows a rapid response to nutritional fluctuations,33 and concentrations of IGF-1 normally rise throughout mid-late gestation to support the accelerated growth that normally occurs in the third trimester in utero.33,34
As a result of its estrogenic/anti-androgenic, it is possible that pesticides can also directly influence the growth hormone IGF-1 system.35 Studies in animals and humans have shown that environmental pollution, such as benzopyrene, dioxin, dibenzofurans, and hexachlorobenzene can alter the normal synthesis or secretion of IGF-1.36 Exposure to pesticides, especially organochlorine groups, can also interfere with IGF-1 function.10,37 Pesticide exposure could be a risk factor for the occurrence of growth disorders in children living in agricultural areas.24 Research in Spain proved that the average IGF-1 level in the female in whom DDT metabolites were detected in urine was lower than that in those in whom the metabolites were not detected.10 Based on logistic regression analysis, it was shown that pesticide exposure and low umbilical serum IGF-1 levels (<32.6 ng/dL) were independent risk factors for LBW. During pregnancy, levels of growth factors such as IGF-1 and IGF-2, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), factor growth fibroblasts (FGF-2 and FGF-4), and transforming growth factor (TGF-β) increase in the maternal circulation and continue to be elevated during pregnancy. This suggests that the hormone has an important role in the growth of the developing fetus.38 Research by Karamizadeh in Iran shows a positive and significant correlation between maternal IGF-1 levels and infant birth weight.39 IGF-1 plays a role in growth through its role as a mitogen and stimulator of cell proliferation and plays an important role in the process of mild repair/regeneration.40 Exposure to toxic materials that occur during the growth period (fetal, infancy and childhood periods) is more likely to have a more negative impact than exposure during adulthood.41
The limitation in this study was that IGF-1 level was only measured once. It just described the condition at that moment, so the temporal relationship and causality could not be examined. It is necessary to consider serial measurements of serum umbilical IGF-1 in future studies—at the beginning of the first, second and third trimester—so an explanation of the role of IGF-1 as an intervening/intermediate variable of fetal growth disturbance due to pesticide exposure can be better studied.
In conclusion, there is a significant association between exposure to pesticides during pregnancy and giving birth to LBW babies through affecting the umbilical serum IGF-1 pathway.
Conflicts of Interest:
None declared.
Financial Support:
This research was funded by Doctoral Grant from DP2M DIKTI with grant number 032/K6/KM/SP2H/PPM/2018, February 19, 2018.
Cite this article as: Widyawati SA, Suhartono S, Mexitalia M, Soejoenoes A. The relationship between pesticide exposure and umbilical serum IGF-1 levels and low-birth weight: A case-control study in brebes, Indonesia. Int J Occup Environ Med 2020;11:15-23. doi: 10.15171/ijoem.2020.1809
References
- 1.Walhovd KB, Fjell AM, Brown TT. et al. Long-term influence of normal variation in neonatal characteristics on human brain development. PNAS. 2012;109:20089–94. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Guidelines on Optimal Feeding of Low Birthweight Infants in Low-and Middle-Income Countries. 2011. [PubMed]
- 3. World Health Organization. WHA Global Nutrition Targets 2025: Low Birth Weight Policy Brief. 2014.
- 4.Risnes K, Vatten L, Baker J. et al. Birthweight and mortality in adulthood : a systematic review and meta-analysis. Int J Epidemiol. 2011;40:647–61. doi: 10.1093/ije/dyq267. [DOI] [PubMed] [Google Scholar]
- 5.Larroque B, Bertrais S, Czernichow P, Leger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics. 2001;108:111–5. doi: 10.1542/peds.108.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Grissa O, Yessoufou A, Mrisak I. et al. Growth factor concentrations and their placental mRNA expression are modulated in gestational diabetes mellitus : possible interactions with macrosomia. Pregnancy and Childbirth. 2010;10:1–10. doi: 10.1186/1471-2393-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabrowski S, Hanke W, Polanska K. et al. Pesticide exposure and birthweight: An epidemiological study in Central Poland. Int J Occup Med Environ Health. 2003;16:31–9. [PubMed] [Google Scholar]
- 8. National Pesticide Information Center. Pesticides and Pregnancy. Published 2018. Available from http://npic.orst.edu/health/preg.html (Accessed September 20, 2019).
- 9. Allsop M, Huxdorff C, Johnston P, et al. Pesticides and Our Health. A Growing Concern. United Kingdom: Greenpeace Recearch Laboratories; 2015.
- 10.Boada L, Lara P, Álvarez-León E. et al. Serum levels of insulin-like growth factor-I in relation to organochlorine pesticides exposure. Growth Horm IGF Res. 2007;17:506–11. doi: 10.1016/j.ghir.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Hellström A, Ley D, Hansen-pupp I. et al. Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. Am J Perinatol. 2016;33:1067–71. doi: 10.1055/s-0036-1586109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrogiannis G, Sifakis S, Patsouris E, Konstantinidou A. Insulin-like growth factors in embryonic and fetal growth and skeletal development ( Review ) Mol Med Rep. 2014;10:579–84. doi: 10.3892/mmr.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gluckman P, Pinal C. Fetus and infant regulation of fetal growth by the somatotrophic axis. J Nutr. 2003;133:1741S–6S. doi: 10.1093/jn/133.5.1741S. [DOI] [PubMed] [Google Scholar]
- 14.Sferruzzi-Perri A, Owens J, Pringle K, Roberts C. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol. 2011;1:7–20. doi: 10.1113/jphysiol.2010.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagey F, Joice K, Indriani I. IGF-1 levels related to incidence of macrosomia. Maj Obstet Ginekol. 2013;21:121–4. [Google Scholar]
- 16. The American College of Obstetricians and Gynecologists. Definition of Term Pregnancy. 2013.
- 17. Lemeshow S, Hosmer Jr D, Klar J, Lwanga S. Adequacy of Sample Size in Health Studies. New York: John Wiley & Sons Ltd, 1993.
- 18.Berrigan D, Potischman N, Dodd K. et al. Serum levels of insulin-like growth factor-I and insulin-like growth factor-I binding protein-3: Quality control for studies of stored serum. Cancer Epidemiol Biomarkers Prev. 2007;16:1017–22. doi: 10.1158/1055-9965.EPI-07-0044. [DOI] [PubMed] [Google Scholar]
- 19.Florkowski C. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. Clin Biochem Rev. 2008;29:S83–S87. [PMC free article] [PubMed] [Google Scholar]
- 20.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Medica. 2016;26:297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petit C, Chevrier C, Durand G. et al. Impact on fetal growth of prenatal exposure to pesticides due to agricultural activities : a prospective cohort study in Brittany, France. Environ Heal. 2010;9:12p. doi: 10.1186/1476-069X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kartini A, Suhartono S, Pangestuti D. et al. Goiter and hypothyroidism among elementary school children in lowland agricultural area, Brebes District Indonesia. Indian J Public Heal Res Dev. 2018;9:120–5. [Google Scholar]
- 23.Suhartono S, Kartini A, Subagio H. et al. Pesticide exposure and thyroid function in elementary school children living in an agricultural area, Brebes District, Indonesia. Int J Occup Environ Med. 2018;9:137–44. doi: 10.15171/ijoem.2018.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kartini A, Subagio H, Hadisaputro S. et al. Pesticide exposure and stunting among children in agricultural areas. Int J Occup Environ Med. 2019;10:17–29. doi: 10.15171/ijoem.2019.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamanti-kandarakis E, Bourguignon J, Giudice L. et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellstrom A, Ley D, Hansen-pupp I. et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016;105:576–86. doi: 10.1111/apa.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elzein A, Ali A, Hamdan H. et al. Materno-foetal leptin and insulin-like growth factor in low birth weight neonates. J Obstet Gynaecol (Lahore) 2016;36:31–3. doi: 10.3109/01443615.2015.1030607. [DOI] [PubMed] [Google Scholar]
- 28.Asvold BO, Eskild A, Jenum P, Vatten LJ. Maternal concentrations of Insulin-like Growth Factor I and Insulin-like Growth Factor Binding Protein 1 during pregnancy and birth weight of offspring. Am J Epidemiol. 2011;174:129–37. doi: 10.1093/aje/kwr067. [DOI] [PubMed] [Google Scholar]
- 29.Vidal AC, Murtha AP, Murphy SK. et al. Maternal BMI , IGF-I levels , and birth weight in African American and White infants. Int J Pediatr. 2013:191472. doi: 10.1155/2013/191472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akman I, Arioglu P, Koroglu O. et al. Maternal zinc and cord blood zinc, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 levels in small-for-gestational-age newborns. Clin Exp Obs Gynecol. 2006;33:238–40. [PubMed] [Google Scholar]
- 31.LeRoith D, Yakar S. Mechanisms of Disease : metabolic effects of growth hormone and insulin-like growth factor 1. Endocrinol Metab. 2007;3:302–10. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 32.Murray P, Clayton P. Endocrine Control of Growth. Am J Med Genet. 2013;163:76–85. doi: 10.1002/ajmg.c.31357. [DOI] [PubMed] [Google Scholar]
- 33.Kajantie E, Dunkel L, Rutanen E. et al. IGF-I, IGF Binding Protein (IGFBP)-3, Phosphoisoforms of IGFBP-1, and Postnatal Growth in Very Low Birth Weight Infants. J Clin Endocrinol Metab. 2002;87:2171–9. doi: 10.1210/jcem.87.5.8457. [DOI] [PubMed] [Google Scholar]
- 34.Chiesa C, Osborn J, Haass C. et al. Insulin Concentrations at Birth: Is There a Relationship with Fetal Growth and Neonatal Anthropometry? Pediatr Clin Chem. 2008;5:550–8. doi: 10.1373/clinchem.2007.095299. [DOI] [PubMed] [Google Scholar]
- 35.Gore A, Crews D, Doan L. et al. Introduction to Endocrine Disrupting Chemicals (EDCs)-A Guide for Public Interest Organizations and Policy-Makers. Endocrine Society. 2014 [Google Scholar]
- 36.Holloway A, Petrik J, Younglai E. Influence of dichlorodiphenylchloroethylene on vascular endothelial growth factor and insulin-like growth factor in human and rat ovarian cells. Reprod Toxicol. 2007;24:359–64. doi: 10.1016/j.reprotox.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Zumbado M, Luzardo O, Lara P. et al. Growth Hormone & IGF Research Insulin-like growth factor-I ( IGF-I ) serum concentrations in healthy children and adolescents : Relationship to level of contamination by DDT-derivative pesticides. Growth Horm IGF Res. 2010;20:63–7. doi: 10.1016/j.ghir.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Romanowicz L, Galewska Z. Extracellular matrix remodeling of the umbilical cord in pre-eclampsia as a risk factor for fetal hypertension. J Pregnancy. 2011:542695. doi: 10.1155/2011/542695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karamizadeh Z, Saki S, Kashef S, Saki F. Comparison of the umbilical cord and maternal serum levels of IGF-1, leptin and cortisol in appropriate for gestational age and small for gestational age neonates. Int J Endocrinol Metab. 2008;2:89–94. [Google Scholar]
- 40.Guntur A, Rosen C. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013;2:1–6. doi: 10.1038/bonekey.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeker J. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166:E1–7. doi: 10.1001/archpediatrics.2012.241. [DOI] [PubMed] [Google Scholar]