Abstract
Purpose:
To characterize the longitudinal panretinal retinal vascular dynamics in diabetic macular edema (DME) and retinal vein occlusion (RVO) over a 12-month period while being treated with intravitreal aflibercept injections (IAI).
Design:
Prospective open-label study (clinicaltrials.org identifier, ).
Participants:
Thirty-one treatment-naïve eyes with foveal-involving retinal edema secondary to DME and RVO.
Methods:
Subjects received 2mg IAI q4 weeks for the first 6 months, followed by 2 mg q8 weeks. Ultra-widefield angiograms (UWFA, California Optos) and SD-OCT (Cirrus, Zeiss) scans were obtained and analyzed using a novel quantitative assessment platform. Visual acuity, central subfield thickness, and adverse events were also collected.
Main Outcome Measures:
The primary study endpoint was the mean change in panretinal leakage index at month 12 from baseline as measured by UWFA.
Results:
Baseline mean age was 67.1 years (range, 46-84). At month 12, visual acuity significantly improved by a mean of 18.4 ± 21.4 letters (P < 0.0001), and CST also significantly improved with a mean reduction of 301.3 ± 250.3 μm (P < 0.0001). Mean panretinal leakage index significantly improved, decreasing from 3.4% at baseline to 0.5% at month 6 (P < 0.0001) and 0.4% at month 12 (P < 0.0001). Panretinal ischemic index did not demonstrate any significant change but showed a nonsignificant increase from 5.5% at baseline to 6.1% at month 6 (P = 0.315) and 8.7% at month 12 (P = 0.193). Subjects diagnosed with DME showed a decrease in leakage index from 3.5 ± 2.7% at baseline to 1.6 ± 0.8% at month 12 (P = 0.018) and overall stability in ischemic index from 5.0 ± 4.1% at baseline to 4.7 ± 3.5% at month 12 (P = 0.689). Subjects with RVO presented a decrease in leakage index from 3.3 ± 1.1% at baseline to 0.02 ± 0.03% at 12 months (P < 0.0001) and a non-significant increase in ischemic index from 5.9 ± 4.5% at baseline to 12.6 ± 9.8% at month 12 (P = 0.172).
Conclusions:
Therapy with IAI resulted in a dramatic reduction in panretinal leakage index. Panretinal ischemic index did not improve and trended towards worsening.
Diabetes mellitus is a serious health problem worldwide and continues to increase in numbers and significance. It is expected that 552 million people will be affected globally with diabetes by 2030.1 Diabetic retinopathy (DR) represents a microvasculature complication of diabetes and is a leading cause of preventable blindness in the working-age population of developed countries.2,3 Diabetic macular edema (DME) is the most frequent cause of visual impairment among people with diabetes.3 In patients with DME, there is intraretinal fluid accumulation within the retina as a consequence of the inner blood-retinal barrier breakdown.2,3 Retinal vein occlusions (RVO) are the second most common retinal vascular disease after DR. Vision loss from RVO most commonly results from macular edema and/or macular ischemia.4
It is well known that vascular endothelial growth factor (VEGF) plays a major role for retinal vascular hyperpermeability in DME and RVO, and anti-VEGF agents have become first-line treatment for macula edema due to DR and RVO, improving macular thickening and vision.4-6 Multiple phase III studies have demonstrated clinical efficacy and safety of intravitreal aflibercept injection (IAI) for the management of DME and macula edema secondary to RVO.7-10 However, in clinical practice selected patients continue to have variable response to anti-VEGF therapy. No reliable methods exist to determine which patients will gain or lose vision following anti-VEGF injection, making such predictive biomarkers an important unmet need.
Although OCT is the primary imaging modality used to diagnose and monitor macular edema,11 OCT does not correlate well with visual function. Predictive imaging biomarkers are needed to better understand prognosis, facilitate optimal therapeutic choice, and enable more comprehensive patient education. Hence, emerging imaging modalities may provide additional opportunities for disease characterization. Studies have demonstrated that ultra-widefield angiography identifies significant panretinal vascular abnormalities in eyes with DME and RVO, including microaneurysms, leakage, and nonperfusions.12,13 Ultra-widefield angiography can image 3 times more peripheral retina than conventional angiography and shows 3.9 times more area of retinal nonperfusion.14 However, the assessment of angiographic phenotypes is limited primarily to subjective interpretation. Global assessments of angiographic features, including leakage and ischemia, may allow for better characterization of the disease burden.
There is limited understanding regarding longitudinal quantitative assessment of angiographic parameters. In this study, we utilize a previously described semi-automated analysis platform that provides a unique opportunity for evaluation of angiographic metrics and their potential role in disease activity and allow for the development of potential imaging biomarkers that have implications for therapeutic response or resistance.15 This report presents the overall study results of the PERMEATE study that prospectively evaluates quantitative ultra-widefield angiography during treatment with intravitreal aflibercept for macular edema secondary to DME or RVO.
Methods
Study Design
PERMEATE was a 12-month prospective open-label study for treatment-naïve eyes with foveal-involving retinal edema secondary to DME and RVO. The study protocol was approved by the Cleveland Clinic Investigational Review Board (IRB) and all study-related procedures were performed in accordance with the Declaration of Helsinki and US Code 21 of Federal Regulations. All patients provided informed consent. The study is registered with Clinicaltrials.gov (identifier, ).
Study Population
Thirty-one subjects were recruited at the Cole Eye Institute. Inclusion criteria were as follows: ≥ 18 years of age, foveal-involving retinal edema secondary to DME or RVO based on spectral-domain (SD) OCT, Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) of 20/25 to HM (i.e., 0 to 83 ETDRS letters) in the study eye. Exclusion criteria included previous therapy with any investigational agent to treat DME or RVO; previous intravitreal anti-VEGF or steroid therapy; systemic anti-VEGF therapy 3 months prior to first dose; any history of laser photocoagulation (focal or panretinal); significant media opacities; aphakia; other causes of macular edema; uncontrolled glaucoma; prior filtration surgery; full-thickness penetrating keratoplasty; history of uveitis; any ocular or periocular infection within 2 weeks of screening; therapeutic radiation in the region of the study eye; allergy to fluorescein sodium; pregnancy; or currently breast-feeding.
Treatment
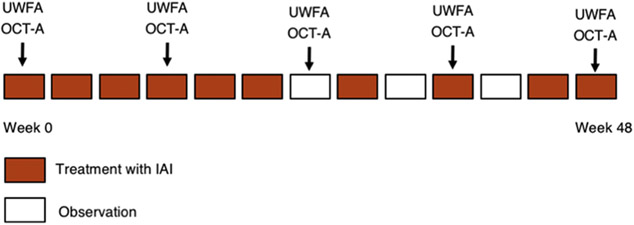
Subjects received 2mg IAI (0.05 mL) monthly (i.e., every 4 weeks +/− 1 week; “q4w”) for the first 6 months, followed by 2 mg IAI bimonthly (i.e., every 8 weeks +/− 1 week; “q8w”). Subjects were also treated at the exit visit on week 48 (Figure 1). Following the transition to q8w dosing, if rescue criteria were met the subject could return to q4w dosing at investigator’s decision. Rescue criteria included loss of 10 or more ETDRS letters from best previous measurement and/or increase in 75 microns of retinal thickness on SD-OCT as compared to the best previous visit.
Figure 1:
Study Design. Additional treatment with intravitreal IAI injection at observation visits if criteria met. OCT-A is optional at month 0, 3, 6, 9 and 12.
Image Acquisition
Evaluations occurred monthly for the duration of the study. Each patient underwent standardized ETDRS manifest refraction, slit lamp and fundus examination, tonometry, and SD-OCT (Cirrus HD-OCT; Zeiss, Oberkochen, Germany). Ultra-widefield fluorescein angiography (UWFA) and SD-OCT angiography (OCT-A) were performed at baseline, month 3, month 6, month 9, and month 12 (Figure 1). Angiographic imaging was performed with the California system (Optos, Dunfermline, Scotland, United Kingdom). All SD-OCT files were deidentified and exported as .dcm files and UWFA images were exported as .tiff files.
Study Endpoint
The primary study endpoint was the mean change in panretinal leakage index at month 12 from baseline as measured by UWFA. Secondary endpoints included the mean change in total panretinal leakage index at month 6, the change in ischemic index at month 6 and 12, the mean absolute change from baseline central subfield thickness (CST) at month 6 and 12, the mean change from baseline in BCVA score at month 6 and 12, the percentage of subjects that were anatomically dry by SD-OCT at month 6 and 12, the percentage of participants who gained or lost 15 ETDRS letters or more of vision at month 6 and 12, the percentage of subjects that are 20/40 or better at month 6 and 12, and the percentage of subjects that are 20/200 or worse at month 6 and 12. Missing values were assigned using the last observation carried forward (LOCF) method for all subjects who continued the study for at least 3 months. A sub-analysis was also performed based on underlying diagnosis (e.g., DME vs RVO). Ocular and non-ocular adverse event reports were generated by study coordinators through nondirective questioning, laboratory testing, or eye examinations and were recorded in patient’s source documents.
UWFA Image Analysis and SD-OCT Analysis
For ultra-widefield angiographic analysis, images were corrected for warping by processing them through a previously described dewarping transformation software to create the projected retinal image.16 A semi-automated quantitative angiographic assessment tool was utilized to evaluate quantitative angiographic dynamics, as previously described.15 Optimal early and late images were selected for image analysis based on image timing, image quality, and absence of artifacts. Early and late images were utilized for leakage assessment and the early image was utilized for ischemia assessment. The images were analyzed with the automated software platform and any segmentation errors manually corrected by trained image analysts. The same analyst evaluated all images from the same subject throughout the course of the study to reduce variability within a given subject’s image interpretations. A secondary confirmatory review was performed by the project lead (NF) to facilitate consistency and annotation accuracy for each metric. Leakage was defined as a region of increasing hyperfluorescence in size and intensity in the late phase FA compared with early phase (Figure 2). Nonperfusion/ischemia was defined as an area of hypofluorescence with loss of visible vascular flow (Figure 2). Threshold confidence levels were utilized for all readers. The region of interest (ROI) was determined by the image analyst based on the areas of the image that were of sufficient quality for analysis. This area was selected as the denominator for the index calculations. Leakage and ischemic indices were defined as the percentage area of abnormality of interest (e.g., leakage, ischemia) over the total analyzable retina area (i.e., ROI), as previously described.17,18
Figure 2:
Ultra-widefield fluorescein angiograms from two representative PERMEATE subjects. A-C, Late-phase UWFA image of a patient diagnosed with CRVO. UWFA with overlay of the leakage in red (B), and binary mask containing the segmented leakage (C). D-F, Early phase UWFA of a patient diagnosed with PDR. UWFA with ischemia overlay in green (E), and binary mask containing the segmented ischemia (F).
Central subfield thickness was measured based on the standard Cirrus software segmentation. The segmentation was reviewed for accuracy and corrected for any segmentation errors. Masked qualitative grading of SD-OCT images was performed to identify anatomic changes in intraretinal and subretinal fluid.
Statistical Analysis
Demographic and baseline characteristics were summarized descriptively. Continuous variables were summarized with mean, range, median, and standard deviation. Categorical variables were summarized with frequency and percentage. Values are reported as mean ± standard deviation (SD) through the manuscript unless otherwise specified. A P value < 0.05 was considered statistically significant. Paired t-tests were used to compare continuous variables, and Fisher’s exact tests and chi-square tests were used to compare categorical data points. Data analyses were performed using IBM® SPSS® Statistics for Mac version 24 (SPSS, Inc, Chicago, Illinois, USA).
Results
Clinical Demographics
The PERMEATE study included 31 eyes. Two subjects did not continue the study past month 3 due to elective study withdrawal (1) and death (1). Thereby, 29 subjects were included in this efficacy analysis. All subjects are included in the safety analysis. The mean age of the participants was 67.1 years (range, 46-84). Eighteen subjects were male (62.1%) and 11 (37.9%) were female. Fourteen patients (48.3%) had DME as a primary diagnosis. Diabetic retinopathy severity in these subjects were as follows: mild non-proliferative diabetic retinopathy (NPDR; 1/14, 7.1%), moderate NPDR (6/14, 42.9%), severe NPDR (4/14, 28.6%), and proliferative diabetic retinopathy (PDR; 3/14, 21.4%). Fifteen subjects (51.7%) had RVO as a primary diagnosis. The specific type of RVO included central RVO (CRVO) in 66.7% (10/15) and branch RVO (BRVO) in 33.3% (5/15). Twenty-three (79.3%) subjects had diagnosis of hypertension at baseline. Mean blood pressure across all subjects was 147.6/83.4 mmHg at baseline and 145.9/88.9 at month 12 (P > 0.05). Patients’ baseline demographic and clinical characteristics are shown in Table 1.
Table 1:
Baseline demographic and clinical data of patients enrolled in the PERMEATE Study.
| BASELINE CHARACTERISTICS | TOTAL (N = 29) |
|---|---|
| Mean age, years (SD) | 67.1 (9.7) |
| Male, n (%) | 18 (62.1) |
| Female, n (%) | 11 (37.9) |
| Race, n (%) | |
| Caucasian | 23 (79.3) |
| African-American | 6 (20.7) |
| Primary Diagnosis, n (%) | |
| DME | 14 (48.3) |
| Mild NPDR | 1 (7.1) |
| Moderate NPDR | 6 (42.9) |
| Severe NPDR | 4 (28.6) |
| PDR | 3 (21.4) |
| RVO | 15 (51.7) |
| CRVO | 10 (66.7) |
| BRVO | 5 (33.3) |
| Right study eye, n (%) | 17 (58.6) |
| Lens Status, n (%) | |
| Phakic | 22 (75.9) |
| Pseudophakic | 7 (24.1) |
| Glaucoma, n (%) | 9 (31) |
| Hypertension, n (%) | 23 (79.3) |
| Mean systolic pressure, mmHg | 147.6 |
| Mean diastolic pressure, mmHg | 83.4 |
DME: diabetic macular edema; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy; RVO: retinal vein occlusion; CRVO: central retinal vein occlusion; BRVO: branch retinal vein occlusion.
Quantitative Ultra-widefield Angiography Outcomes
At baseline, mean panretinal leakage index was 3.4 ± 3.8% (range, 0.1%-17.4%). Overall, mean panretinal leakage index significantly decreased from 3.4% at baseline to 0.5% at month 6 (−86.2%, P < 0.0001) and 0.4% at month 12 (−88.7%, P < 0.0001; Figures 3 and 4). Mean baseline panretinal ischemic index was 5.5 ± 8% (range, 0.1%-31.6%) at the baseline visit. Although not significant, mean ischemic index increased from 5.5% at baseline to 6.1% at month 6 (+12.3%, P = 0.315) and 8.7% at month 12 (+59.2%, P = 0.193; Figures 3 and 4).
Figure 3:
UWFA images from two PERMEATE subjects. The left column is the baseline visit, middle column shows month-6, and the right column is month-12. A-C, Late-phase UWFA images of a subject diagnosed with CRVO. D-F, Leakage overlay in red. G-I, Early-phase angiograms of a subject with PDR and a close-up view of the inferonasal quadrant presenting an area of ischemia which remains the same over the three time points. J-L, Ischemia overlay in green.
Figure 4:
Percentage of mean leakage index values (Top) and mean ischemic index values (Bottom) from baseline to month 12. Black lines show overall results; orange lines show results for subjects diagnosed with DME; blue lines show results for patients diagnosed with RVO. *P < 0.05, **P < 0.01, and ***P < 0.0001 versus baseline.
When evaluating eyes with DME, mean panretinal leakage index showed a reduction of 78.1%. Mean leakage index was 3.5 ± 2.7% at baseline and improved to 1.6 ± 0.8% at month 12 (P = 0.018). Additionally, mean panretinal ischemic index showed a slight improvement of 5.8% from baseline (5.0 ± 4.1%) to month 12 (4.7 ± 3.5%, P = 0.689).
In subjects presenting with RVO, mean leakage index significantly decreased from 3.3 ± 1.1% at baseline to 0.02 ± 0.03% at 12 months (−99.3%, P < 0.0001). Mean ischemic index demonstrated a non-significant increase from 5.9 ± 4.5% at baseline to 12.6 ± 9.8% (+114.9%, P = 0.172) at month 12.
Functional Outcomes
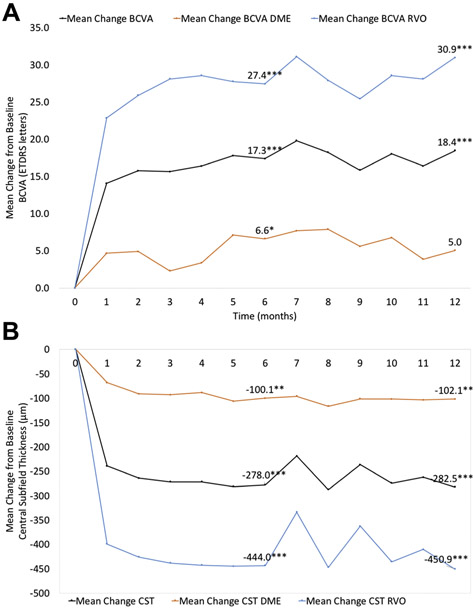
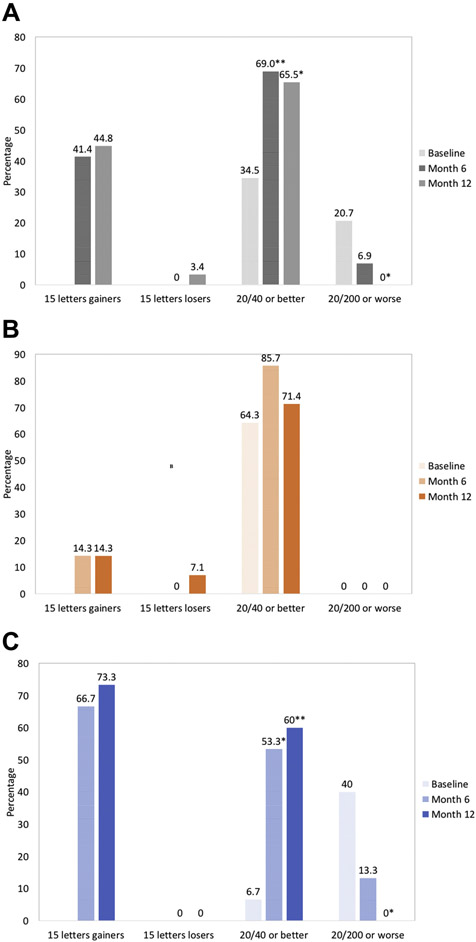
The mean BCVA was 20/80 (54.3 ± 23.3 ETDRS letters) at baseline. Mean BCVA significantly improved by 17.3 ± 19.1 letters (P < 0.0001) and 18.4 ± 21.4 letters (P < 0.0001) at month 6 and month 12, respectively (Figure 5). At month 6, 41.4% (n = 12) of eyes gained 3 lines or more of vision (≥ 15 letters) and no eye lost 3 lines (≥ 15 letters) from baseline. After month 12, 44.8% (n = 13) of participants gained ≥ 15 letters, and 3.4% (n = 1) lost ≥ 15 letters. The percentage of eyes that had a BCVA ≥ 20/40 was significantly greater in both month 6 and month 12 compared with baseline. Sixty-nine percent (n = 20, P < 0.009) and 65.5% (n = 19, P = 018) of eyes were 20/40 or better at month 6 and month 12, respectively, while 34.5% (n = 10) of eyes had a BCVA ≥ 20/40 at baseline. In addition, fewer eyes had ≤ 20/200 BCVA at months 6 and 12, although not significant at month 6. At baseline, 20.7% of eyes (n = 6) presented BCVA of 20/200 or worse versus 6.9% (n = 2, P = 0.253) and 0% (n = 0, P = 0.023) of eyes at month 6 and month 12, respectively (Figure 6A).
Figure 5:
Graphs showing the functional and anatomical outcomes of PERMEATE subjects. (Top) Mean change in best-corrected visual acuity (BCVA) from baseline to month 12. (Bottom) Mean change in central (OCT) subfield thickness (CST) at each study visit. Black lines represent overall results; orange lines are values for patients with DME; blue lines represent patients with RVO. *P < 0.05, **P < 0.01, and ***P < 0.0001 versus baseline.
Figure 6:
Additional key secondary endpoints. Bar graph showing functional outcomes after 6 months and 12 months of treatment for the full analysis set (A), patients diagnosed with DME (B), and patients diagnosed with RVO (C). Proportion of eyes that gained and lost ≥ 15 letters from baseline; proportion of eyes that had a best-corrected visual acuity (BCVA) ≥ 20/40 and ≤20/200. *P < 0.05, **P < 0.01, and ***P < 0.0001 versus baseline.
Eyes with DME showed a mean change from baseline BCVA of 6.6 ± 5.9 letters at month 6 (P = 0.049) and 5.0 ± 7.4 letters at month 12 (P = 0.207, Figure 5). At months 6 and 12, 14.3% (n = 2) of subjects gained 15 letters or more of vision. No subject lost 15 letters from baseline at month 6 and 7.1% (n =1) of subjects lost 15 letters at month 12. At baseline, 64.3% (n = 9) of eyes had BCVA ≥ 20/40 versus 85.7% (n = 12, P = 0.385) and 71.4% (n = 10, P = 0.686) of eyes at month 6 and month 12, respectively. No eye had BCVA ≤ 20/200 at baseline, month 6, or month 12 (Figure 6B).
Subjects with RVO had a statistically significant improvement of 27.4 ± 10 letters at 6 months (P < 0.0001) and 30.9 ± 9.9 letters at 12 months (P < 0.0001, Figure 5). At month 6, 66.7% (n = 10) of eyes gained 15 letters or more of vision. After 12 months,73.3% (n = 11) of eyes gained ≥ 15 letters. No eye lost greater or equal to 15 letters of vision at months 6 or 12. At baseline, 6.7% (n = 1) of eyes presented BCVA ≥ 20/40 compared with 53.3% (n = 8, P = 0.014) and 60.0% (n = 9, P = 0.005) of eyes at month 6 and month 12, respectively. Forty percent (n = 6) of eyes had BCVA ≤ 20/200 at baseline, while 13.3% (n = 2, P = 0.215) of eyes had BCVA ≥ 20/200 at month 6 and no eye (P = 0.017) presented BCVA ≥ 20/200 at month 12 (Figure 6C).
Mean baseline CST and macular volume across all participants were 541.2 ± 242.3 μm and 13.7 ± 4.3 mm2, respectively. Overall, mean CST demonstrated a significant reduction of 278 ± 251.3 μm (P < 0.0001) at month 6 and 301.3 ± 250.3 μm (P < 0.0001) at month 12 (Figure 5). Overall, mean macular volume also improved significantly at month 6 (−3.9 ± 4.0 mm2; P < 0.0001) and month 12 (−4.4 ± 4.1 mm2; P < 0.0001). At baseline, 100% of eyes presented with intraretinal or subretinal fluid or both on SD-OCT. After 6 months and 12 months of treatment, 55.2% (n = 16, P < 0.0001) and 62.1% (n = 18, P < 0.0001) were anatomically ‘dry’ by SD-OCT, respectively.
Mean CST value presented by DME subjects was 281.1 ± 46.2 μm at month 6 (−100.1 ± 63.9 μm from baseline, P = 0.009) and 279.1 ± 43.0 μm at month 12 (−102.1 ± 61.1 μm from baseline, P = 0.006, Figure 5). Mean macula volume was 9.8 ± 0.6 mm3 at month 6 (−1.0 ± 0.4 mm3 from baseline, P < 0.001) and 9.7 ± 0.5 mm3 at month 12 (−1.1 ± 0.5 mm3 from baseline, P < 0.001). At months 6 and 12, 26.7% (n = 4, P = 0.098) of subjects were anatomically ‘dry’ by SD-OCT.
The mean CST improvement from baseline (690.5 ± 125.0 μm) showed by RVO subjects was 444.0 ± 119.6 μm at month 6 (246.5 ± 15.6 μm, P < 0.0001) and 450.9 ± 118.9 μm at month 12 (239.6 ± 22.3 μm, P < 0.0001, Figure 5). Mean macula volume was 16.4 ± 2.2 mm3 at baseline and improved to 9.7 ± 0.3 mm3 at month 6 (−6.7 ± 2.0 mm3, P < 0.0001) and 9.5 ± 0.4 mm3 at month 12 (−6.9 ± 2.0 mm3, P < 0.0001). At months 6 and 12, 80.0% (n = 12, P < 0.0001) and 93.3% (n = 14, P < 0.0001) of subjects were anatomically ‘dry’ by SD-OCT.
Safety and Adverse Events
There were no ocular serious adverse events (SAEs), including no cases of endophthalmitis. One patient suffered a stroke, and there were 2 deaths during the study. One patient died of multiple organ failure due to elderly age (93 years) 10 months after enrollment and 1 patient died from an unknown cause thought to be related to uncontrolled diabetes 3 months after enrollment.
Discussion
In our literature review, the PERMEATE study represents the first prospective study to evaluate quantitative panretinal vascular dynamics following IAI therapy for DME and RVO. The study demonstrates that IAI q4 weeks for the first 6 months followed by q8 weeks significantly reduces panretinal leakage index at months 6 and 12. Participants exhibited an average decrease in panretinal leakage index from 3.4% at baseline to 0.4% at month 12. Subjects with DME as primary diagnosis showed a reduction of 78.1% in leakage index at month 12, while subjects with RVO presented a reduction of 99.3% at month 12. Conversely, overall panretinal ischemic index showed a nonsignificant worsening during the course of the study. In addition, similar to other phase III studies and clinical trials, this treatment-naïve cohort demonstrates dramatic and significant improvements in visual acuity and anatomy in patients with macular edema due to DME or RVO treated with IAI.7-10
Both diabetic eye disease and retinal venous occlusive disease typically involve the macula and the retinal periphery. Silva and associates and Fan and associates reported that mid and far periphery ischemia in DR eyes identified by UWFA accounted for 86.3% and 67.4% of the total ischemic area, respectively.19,20 Therefore, the use of UWFA for panretinal assessment provides a unique opportunity for evaluating potential imaging biomarkers for treatment response, disease burden, and prognosis.
While several investigators have described panretinal ischemic index, there is limited information on panretinal leakage index. Sim et al performed a retrospective analysis of UWFA images from 47 patients with DR and reported a median peripheral leakage index of 17.7%.17 This high value may be partially explained by the fact that in Sim’s study 74.5% of patients were graded with PDR. Quantitative reduction of leakage index in patients with DME has been pioneered reported by Allingham and associates. In their retrospective study of 29 subjects, they used a semi-automated segmentation algorithm and found that total leakage decreased by a mean of 40.1% after treatment with at least 3 consecutive anti-VEGF injections.21 After 12 months, the PERMEATE study showed a decrease of 88% in overall panretinal leakage index and a reduction of 78.1% when analyzing only subjects with DME. The methodology in this study diverges from ours due to the prospective approach of our study and the assessment of global retinal features by UWF images. The panretinal analysis may explain our higher values for leakage index. There is hardly any data in prospective trials evaluating UWFA leakage dynamics in these retinal vascular pathologies.
The mean ischemic index in this study was 5.5% at baseline and 8.7% after treatment. Subjects with DME presented a mean ischemic index of 5.0% at baseline which slightly reduced to 4.7% at month 12. The average ischemic index in other studies involving diabetic retinopathy have been generally higher than described in this report. Fan and associates published a case series of 40 eyes with treatment naive DME, and found an ischemic index of 31%.20 In a retrospective study of 68 eyes from 37 diabetic patients, Silva et al described similar results. They reported that eyes without predominantly peripheral lesions (PPL) showed a nonperfusion index of 25% and eyes with PPL had an index of 43%. Patel and associates found an even higher ischemic index of 47% among patients with recalcitrant DME.22 But it should be noted that in Patel’s study nearly 50% of the eyes had PDR, including values of ischemic index of 99%.
Studies involving RVO patients also presented higher ischemic index values comparing to this study. Our study found an ischemic index increase from 5.9% at baseline to 12.6% at month 12 among the patients with RVO. Tsui et al, in the first report about ischemic index in eyes with CRVO, described a mean ischemic index of 25%.18 This result was confirmed by Kwon et al analysis of the WAVE cohort, which reported an ischemic index of 30.5% at baseline and 23.5% at the 1-year follow-up in patients with RVO and persistent ME.23 The study that presented more similar results to ours was Singer’s.13 The authors included eyes with RVO and recurrent ME and found a mean ischemic index of 14.8%. One possible explanation of the overall lower ischemic index found in this study is that the reading center protocol for ischemia utilized highly specific thresholds for nonperfusion. As a result of this specific approach, many areas of the far periphery that may have been ischemic were excluded due to lack of definitive evidence of ischemia given the image quality. In addition, the segmentation techniques may be different. In previous studies, the segmentation has included “regions” as ischemia while we segmented well-defined focal areas without including the vessels. Lastly, some studies did not address the issue of warping due to retinal concavity.13,18,22
The results from this study would suggest that ischemia remains stable after 12 months of IAI treatment in patients with DME, despite improvements in functional and anatomic parameters. Campochiaro et al retrospective analysis of RISE and RIDE trials reflects the same finding.24 They reported that the percentage of subjects with no posterior retinal nonperfusion did not change over 2 years in the groups treated with ranibizumab. It is important to note that two subtypes of nonperfused retinal tissue were described with different features, as published by Fang et al.25 They analyzed 40 eyes with treatment naïve DME from the DAVE study and found that nonperfusion with leakage was positively correlated with central macular thickness while nonperfusion without leakage was negatively correlated. This finding supports the hypothesis that nonperfusion represents both injured tissue that is ischemic but still have viable tissue actively producing anti-VEGF and inflammatory cytokines as well as infarcted tissue which may no longer be producing cytokines. This may account for different results regarding nonperfusion in DME eyes, such as a previous result of the DAVE trial which failed to find a correlation between the severity of DME and nonperfusion.20
On the other hand, in patients diagnosed with RVO, nonperfusion trended toward worsening at 12 months after IAI treatment. Campochiaro et al showed that retinal nonperfusion reduced at 6 months in patients with RVO treated with ranibizumab.26 Further, Singer et al described improved ischemic index (10.3 vs. 14.8%) in eyes with RVO after resolution of macular edema and treatment with intravitreal ranibizumab and dexamethasone implant.13 Both of these studies speculate that anti-VEGF treatment could contribute to reperfusion. The WAVE cohort also found a reduction in the ischemic index for the entire retina after 1 year (23.5 vs. 30.5%) in patients with RVO with recalcitrant ME following multiple anti-VEGF injections.23 The authors also reported that the severity of macula edema appears to be associated with the ischemic index in the perimacular region (i.e., the closest area to the fovea). A secondary analysis of the WAVE trial reported that overall macular nonperfusion remained stabled and a minority of eyes showed progression over 1 year in patients diagnosed with RVO.27 In addition, Mir et al reported that monthly ranibizumab injections promoted improvement of nonperfusion in patients with BRVO but no significant reduction was observed in patients with CRVO, suggesting that the VEGF suppression was not sufficient in CRVO.28 There are three explanations that may account for our slightly different result. In the first months after the RVO episode, UWFA images usually show diffuse areas of blockage from retinal hemorrhages which were not analyzed as ischemia in this study. In addition, this study included severe RVO cases, mostly CRVO, which presented a high baseline mean CST value (690.5μm) and a low baseline mean BCVA (41.0 letters). Finally, our sample size of RVO subjects was not adequate to be conclusive.
Hence, due to confounding factors that may interfere with ischemia accurate analysis and uncertainties regarding the mechanism of nonperfusion, leakage index may more accurately represent inflammatory/VEGF burden and be a better metric to evaluate overall disease activity and treatment response. We are currently examining regional panretinal vascular metrics and their association with ME, treatment rebound, and VA outcomes.
Accurately assessing quantitative data from UWFA can be challenging. Even though we corrected the inherent peripheral retinal distortion of UWF images and removed the dynamic aspect of dye using UWFA, some limitations must be considered. First, we used a single angiogram image and not montage, therefore eyelashes, peripheral blurring, and motion artifacts may affect the size of analyzable area. Second, variations in image quality and illumination are frequent and may interfere the analysis consistency. Further, the small sample size of treatment group, the heterogeneous patient group (mixing patients diagnosed with DME and RVO in one study), lack of a control group, and the potential bias of readers not being masked limit the impact of our study. Additional research is needed with a larger dataset and longer follow-up to validate this preliminary analysis.
This study provides a unique assessment of longitudinal angiographic quantitative metrics in eyes with retinal vascular disease treated with IAI. This report study demonstrated that IAI injections effectively reduced panretinal leakage index to almost zero in a large number of patients. However, a small percentage of patients still presented with leakage at the final visit. In addition, this report supports the assertion that anti-VEGF therapy may not have significant modulatory impact on underlying ischemic areas. Ongoing research include quantitative differentiation of leakage sub-types (e.g., perivascular, focal, diffuse), correlation of DRSS changes with quantitative UWFA outcomes, zonal assessment of quantitative metrics, and evaluation of these metrics as a potential indicator for treatment. Similar to the presence of fluid on OCT in DME or neovascular age-related macular degeneration, the availability of an objective metric for assessing need for treatment in retinal vascular disease, particularly diabetic retinopathy, could be a major advancement for pharmacotherapeutic era of managing diabetic eye disease. Ongoing studies, such as the DRCR Protocol AA and the PRIME diabetic retinopathy study () will help better to define the role of widefield imaging and quantitative metrics in progression risk and treatment decision-making. Overall, the PERMEATE study demonstrates the feasibility of utilizing quantitative UWFA to evaluate longitudinal retinal vascular metrics and demonstrates dramatic improvement in panretinal leakage index without evidence of improvement in ischemic index.
Acknowledgments
Financial Support: Regeneron VGFTe-DME-1431 (JPE); NIH/NEI K23-EY022947 (JPE); Research to Prevent Blindness (Cole Eye Institutional Grant); Statement related to financial support: Regeneron provided input on study design but the authors had full control of all data and manuscript drafting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: JPE and RPS are consultants for Alcon, Allegro and Genentech/Roche. JPE is also a consultant for Thrombogenics, Novartis and Aerpio. JPE and RPS receive research support from Regeneron and Genentech. SKS receives research support from Allergan. No other specific conflicts of interest exist related to this study for any of the other authors.
Financial Disclosures:
NF: None; SKS: Leica (P), Bausch and Lomb (C), Santen (C), Regeneron (R), Allergan (R), Gilead (R); RPS: Zeiss (C), Genentech (C, R), Regeneron (C, R), Shire (C), Optos (C), Apellis (R), Alcon (R); AB: Genentech (C); SS: Eyepoint (C); AVR: Zeiss (C), Alcon (C), Allergan (C); KT: None; JR: None; MH: None; JPE: Zeiss (C), Leica (C, P), Aerpio (C, R), Novartis (C, R), Thrombogenics (C, R), Alcon (C, R), Genentech (C), Roche (C), Allergan (C), Alimera Sciences (C), Santen (C), Boerhinger Ingelheim (R), Regeneron (R).
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011. ;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029 [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 3.Tan GS, Cheung N, Simó R, Cheung GCM, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5(2):143–155. doi: 10.1016/S2213-8587(16)30052-3 [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro PA. Anti-vascular endothelial growth factor treatment for retinal vein occlusions. Ophthalmologica. 2012;227(SUPPL. 1):30–35. doi: 10.1159/000337157 [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Moon BG, Cho AR, Yoon YH. Optical Coherence Tomography Angiography of DME and Its Association with Anti-VEGF Treatment Response. Ophthalmology. 2016;123(11):2368–2375. doi: 10.1016/j.ophtha.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 6.Diabetic T, Clinical R. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korobelnik JF, Do DV., Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 copernicus study. Am J Ophthalmol. 2013;155(3):429–437. doi: 10.1016/j.ajo.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 9.Ogura Y, Roider J, Korobelnik JF, et al. Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am J Ophthalmol. 2014;158(5):1032–1038. doi: 10.1016/j.ajo.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 10.Clark WL, Boyer DS, Heier JS, et al. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion 52-Week Results of the VIBRANT Study. Ophthalmology. 2016;123(2):330–336. doi: 10.1016/j.ophtha.2015.09.035 [DOI] [PubMed] [Google Scholar]
- 11.Virgili G, Menchini F, Casazza G, et al. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;2015(1):1–57. doi: 10.1002/14651858.CD008081.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694–698. doi: 10.1136/bjophthalmol-2011-300774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M, Tan CS, Bell D, Sadda SR. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34(9):1736–1742. doi: 10.1097/IAE.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 14.Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra–Wide-Field Angiography Improves the Detection and Classification of Diabetic Retinopathy. Retina. 2012;32(4):785–791. doi: 10.1097/IAE.0b013e3182278b64 [DOI] [PubMed] [Google Scholar]
- 15.Ehlers JP, Wang K, Vasanji A, Hu M, Srivastava SK. Automated quantitative characterisation of retinal vascular leakage and microaneurysms in ultra-widefield fluorescein angiography. Br J Ophthalmol. 2017;101(6):696–699. doi: 10.1136/bjophthalmol-2016-310047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croft DE, van Hemert J, Wykoff CC, et al. Precise Montaging and Metric Quantification of Retinal Surface Area From Ultra-Widefield Fundus Photography and Fluorescein Angiography. Ophthalmic Surgery, Lasers Imaging Retin. 2014;45(4):312–317. doi: 10.3928/23258160-20140709-07 [DOI] [PubMed] [Google Scholar]
- 17.Sim DA, Keane PA, Rajendram R, et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014;158(1). doi: 10.1016/j.ajo.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Tsui I, Kaines A, Havunjian MA, et al. Ischemic index and neovascularization in central retinal vein occlusion. Retina. 2011;31(1): 105–110. doi: 10.1097/IAE.0b013e3181e36c6d [DOI] [PubMed] [Google Scholar]
- 19.Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122(12):2465–2472. doi: 10.1016/j.ophtha.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 20.Fan W, Wang K, Ghasemi Falavarjani K, et al. Distribution of Nonperfusion Area on Ultra-widefield Fluorescein Angiography in Eyes With Diabetic Macular Edema: DAVE Study. Am J Ophthalmol. 2017;180:110–116. doi: 10.1016/j.ajo.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 21.Allingham MJ, Mukherjee D, Lally EB, et al. A Quantitative Approach to Predict Differential Effects of Anti-VEGF Treatment on Diffuse and Focal Leakage in Patients with Diabetic Macular Edema: A Pilot Study. Transl Vis Sci Technol. 2017;6(2):7. doi: 10.1167/tvst.6.2.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel RD, Messner LV., Teitelbaum B, Michel KA, Hariprasad SM. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155(6):1038–1044.e2. doi: 10.1016/j.ajo.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 23.Kwon S, Wykoff CC, Brown DM, van Hemert J, Fan W, Sadda SR. Changes in retinal ischaemic index correlate with recalcitrant macular oedema in retinal vein occlusion: WAVE study. Br J Ophthalmol. 2018:bjophthalmol-2017–311475. doi: 10.1136/bjophthalmol-2017-311475 [DOI] [PubMed] [Google Scholar]
- 24.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121(9):1783–1789. doi: 10.1016/j.ophtha.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 25.Fang M, Fan W, Shi Y, et al. Classification of Regions of Nonperfusion on Ultra-widefield Fluorescein Angiography in patients with Diabetic Macular Edema. Am J Ophthalmol. 2019. doi: 10.1016/j.ajo.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 26.Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4):795–802. doi: 10.1016/j.ophtha.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 27.Ou WC, Lampen SIR, Wykoff CC. Longitudinal Quantification of Retinal Nonperfusion in the Macula of Eyes With Retinal Vein Occlusion Receiving Anti-VEGF Therapy: Secondary Analysis of the WAVE Randomized Trial. Ophthalmic Surg Lasers Imaging Retina. 2018;49(4):258–264. doi: 10.3928/23258160-20180329-08 [DOI] [PubMed] [Google Scholar]
- 28.Mir TA, Kherani S, Hafiz G, et al. Changes in Retinal Nonperfusion Associated with Suppression of Vascular Endothelial Growth Factor in Retinal Vein Occlusion. Ophthalmology. 2016;123(3):625–634.e1. doi: 10.1016/j.ophtha.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]