Antiphospholipid syndrome (APS) is an autoimmune disorder associated with hypercoagulability. Despite clearly defined criteria (Miyakis et al, 2006), the laboratory component for diagnosing APS is highly complex, because no single assay identifies all antiphospholipid antibodies (Tripodi, 2009). Improved diagnostic accuracy for lupus anticoagulants (LA) has been particularly emphasized in recent guidelines (Pengo et al, 2009; Keeling et al, 2012; Moore, 2014). Demonstration of an antiphospholipid antibody by the LA assay may be the most critical component in APS diagnosis because these antibodies correlate highly with thrombotic complications (Devreese et al, 2011; de Groot & Urbanus, 2012). However, testing for LA is problematic due to several ‘diagnostic pitfalls’ such as interference by antithrombotic therapy and non-pathological phospholipid antibodies (Male et al, 2005; Tripodi, 2009). Hence, more work is needed to refine the approach to LA testing and interpretation.
Our current LA work-up includes simultaneous performance of a dilute Russell Viper Venom Test (dRVVT), a phospholipid-neutralization test (PNT) and a partial thromboplastin time (PTT). We noted recurring discrepant dRVVT/PNT results. In order to better understand this phenomenon, we undertook a study to: (i) determine a baseline discrepancy rate for dRVVT/PNT testing and (ii) correlate LA results with other laboratory assays to evaluate the significance of discrepancies.
We retrospectively reviewed all LA results since the introduction of the dRVVT (Life Diagnostics, Leiden, Netherlands) and PNT (Staclot; Diagnostic Stago, Parsippany, NJ, USA) combination at our institution (1 August, 2010 to 30 June, 2013). The PNT includes screening and confirmatory components in an integrated testing platform with addition of hexagonal phase phosphatidylethanolamine (HPE) as a LA neutralizing agent. The following were also collected: patient age/gender, results of simultaneously performed immunological APS studies (anticardiolipin and β−2-glycoprotein-I antibodies) and coagulation tests [PTT, prothrombin time (PT), mixing studies, heparin neutralization]. Our PTT reagent is calibrated to be of high sensitivity for LA detection. All assays were performed according to manufacturer’s instructions. The dRVVT screen/confirm ratio was positive for values >1·2, while a difference of >8 s was positive for the PNT. Discrepant LA results were defined as dRVVT+/PNT− or dRVVT−/PNT+ on the same patient sample using the above cut-off values. Heparin neutralization for a prolonged PTT was accomplished with polybrene, although polybrene neutralization was not performed with every LA evaluation. If the PTT remained prolonged after polybrene (>40 s), then a standard (1:1 pooled normal plasma) 37°C mixing study was undertaken. Full correction was defined as the PTT mix result within the normal range at both 0 and 60 min incubation. As applicable, data were analysed using the Fisher exact test, with significance at P < 0·05. The institutional review board approved this study (HIC 1404013833).
Among 4328 LA evaluations, 79·2% (3432/4328) were uniformly negative, while 14·3% (618/4328) were discrepant. Discrepancies were essentially equally distributed among Group A (i.e., dRVVT+/PNT−) and Group B (i.e., dRVVT−/PNT+). Figure 1 shows the data regarding the associated patient/coagulation testing characteristics for all groups, including discrepancies (Groups A and B) and consistent-positives (i.e., Group C, dRVVT+/PNT+). Overall, among patients with any LA discrepancy and additional coagulation testing (Groups A + B), 58·0% (123/212) had evidence of anticoagulation therapy. This was a significantly higher percentage than a matched patient cohort from Group C (70/196; 35·7%, P < 0·0001).
Fig 1.
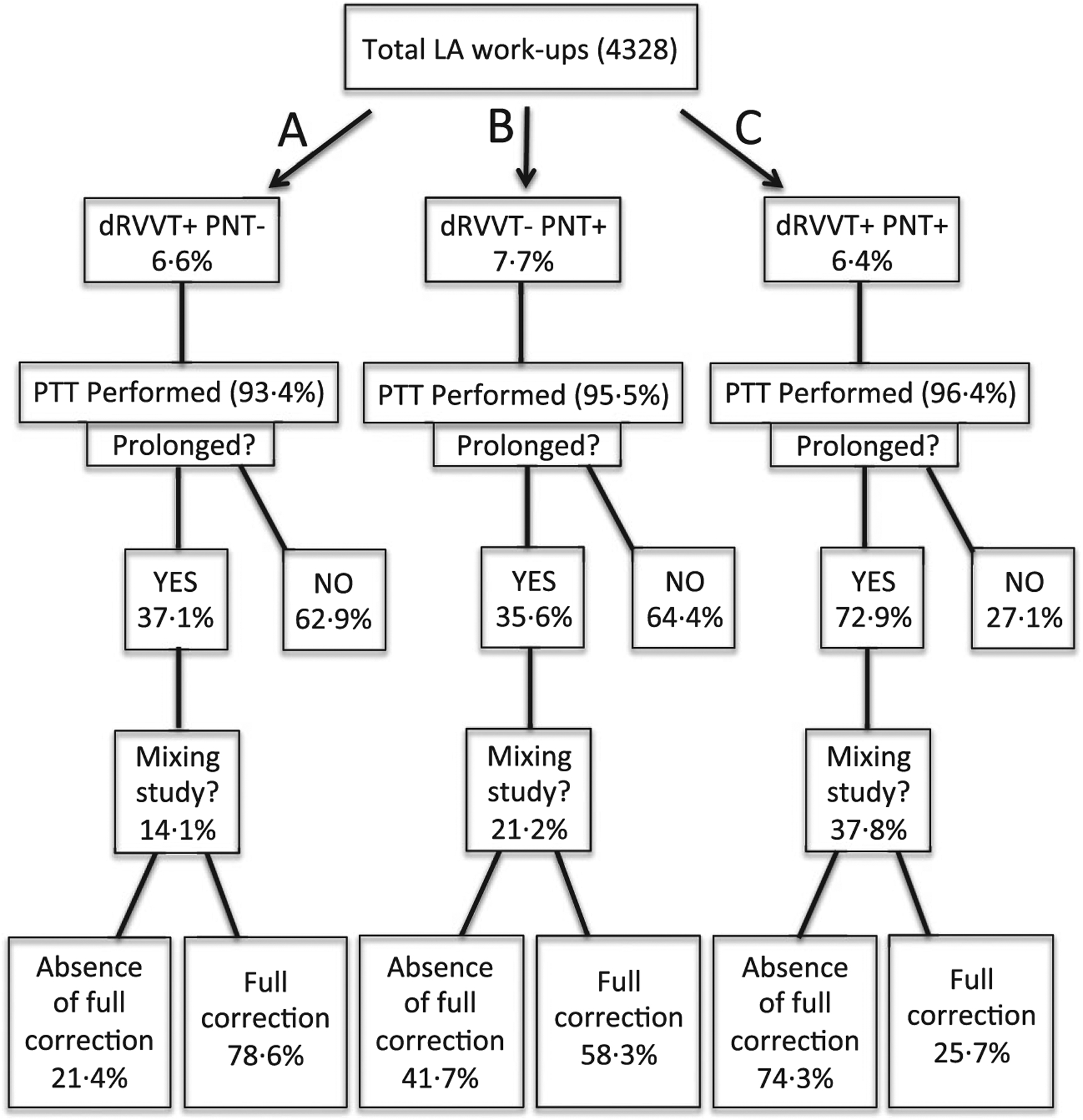
Flow chart depicting LA and coagulation testing in discrepant (A, B) and consistently positive (C) groups. In total, 4328 dRVVT/PNT combination assays were evaluated. The study population included patients ranging in age from 4 months to 93 years with a female:male ratio of 1·7:1. PTT assays were performed in all three groups at a high rate. PT was not as frequently performed, but was prolonged in many of those tested: A, 51·2% (64/125); B, 22·1% (30/136); and C, 29·9% (38/127) (data not shown). PTT testing with the addition of polybrene showed patients with unfractionated heparin anticoagulation: A, 10·1% (10/99); B, 31·9% (36/113); and C, 11·7% (23/196), (data not shown). LA, lupus anticoagulant; dRVVT, dilute Russell Viper Venom Test; PNT, phospholipid-neutralization test; PTT, partial thromboplastin time; PT, prothrombin time.
We next evaluated immunological APS results in discrepant versus consistent-positive LA cases (Fig 2). In order to eliminate artifactual results, the primary criterion for inclusion in this analysis was prolongation of the PTT, which could not be attributed to heparin or factor deficiency. This analysis included 295 test samples (from 248 patients) from Groups A, B and C. When multiple LA evaluations were performed on a single patient, the patient was characterized as having ≥1 LA discrepancy if at least one of the LA work-ups demonstrated a simultaneous dRVVT/PNT discrepant result (Fig 2B, D).
Fig 2.
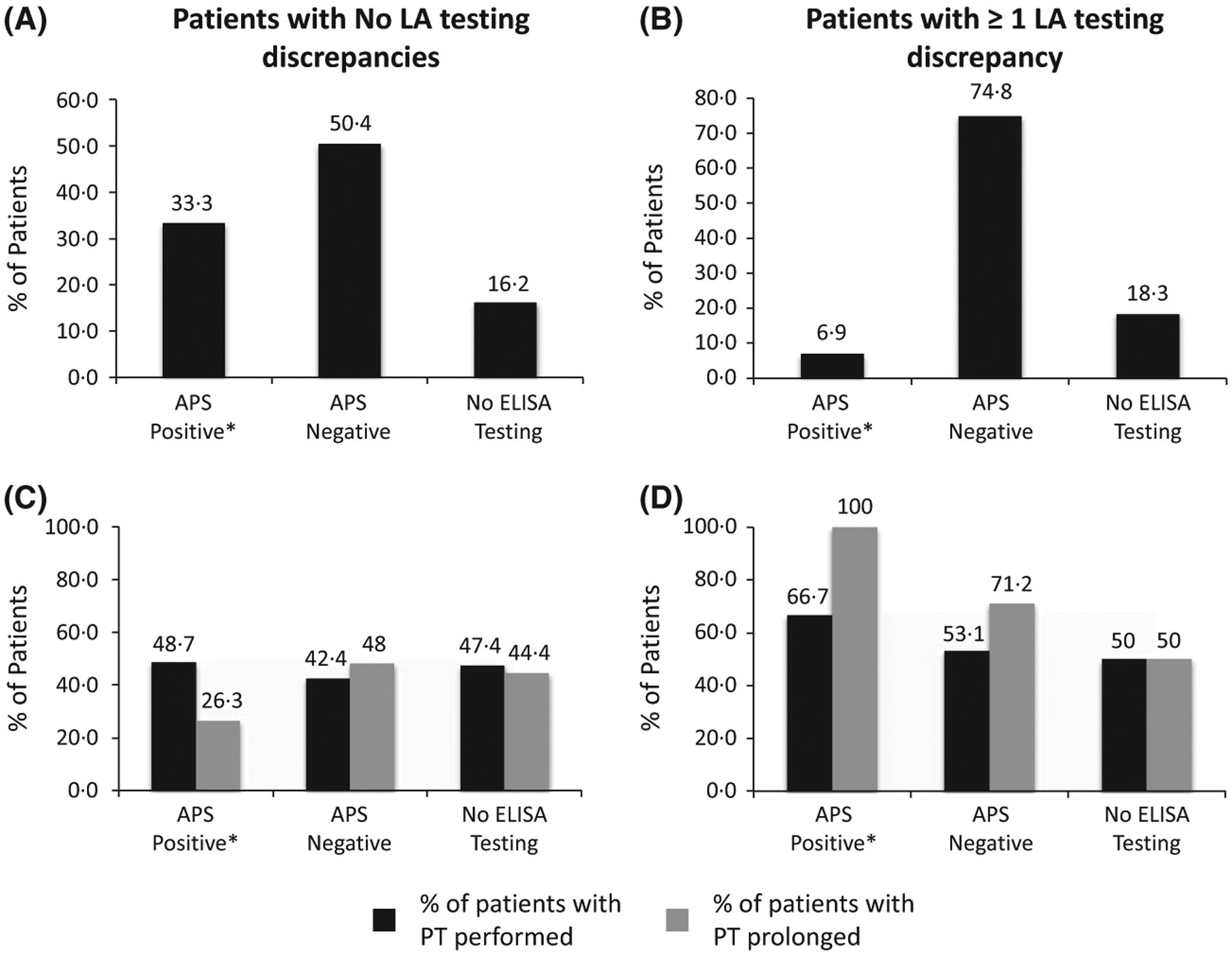
Comparison between consistently positive and discrepant LA results utilizing immunological APS test results in patients (n = 248) with PTT prolongation (not due to heparin or factor deficiency, as noted in the text). Plots A and C represent patients with consistently positive LA results: (A) 33·3% (39/117) APS-positive, 50·4% (59/117) APS-negative, and 16·2% (19/117) with no ELISA testing. (C) PT was performed in 48·7% (19/39) APS-positive, 42·4% (25/59) APS-negative and 47·4% (9/19) with no ELISA testing. Of those tested, 26·7% (5/19), 48% (12/25) and 44·4% (4/9) of the PTs were prolonged, respectively. Plots B and D represent patients with discrepant LA results: (B) 6·9% (9/131) APS-positive, 74·8% (98/131) APS-negative and 18·3% (24/131) with no ELISA testing. (D) PT was performed in 66·7% (6/9) APS-positive, 53·1% (52/98) APS-negative and 50% (12/24) with no ELISA testing. Of those tested, 100% (6/6), 71·2% (37/52) and 50% (6/12) of the PTs were prolonged, respectively. Note: Immunological APS studies [anticardiolipin (aCL) and β−2-glycoprotein-I (β2GPI), both IgG and IgM antibodies] were measured by ELISA (QUANTA LiteTM; Inova Diagnostics Inc., San Diego, CA, USA). Positivity for APS (manufacturer’s recommendations) was modified from the Sapporo criteria (Miyakis et al, 2006) and defined as an aCL antibody of IgG and/or IgM isotype with titres >40 IgG antiphospholipid units (GPL) or IgM antiphospholipid units (MPL). The β2GPI antibody titres of IgG and/or IgM isotypes were defined by titres >20 GPL or MPL. APS, antiphospholipid syndrome; ELISA, enzyme-linked immunosorbent assay; LA, lupus anticoagulant; PTT, partial thromboplastin time; PT, prothrombin time.
There was a five-fold higher rate of positive APS results in patients with consistently positive LA findings as compared to patients with discrepant LA results (Fig 2A, B, first columns). The majority of discrepant cases were APS-negative by immunological testing (11:1 negative/positive ratio). A separate analysis of PT values was also done (Fig 2C, D). All LA-discrepant patients, APS-positive patients (Fig 2D, first paired columns) showed PT prolongation (mean PT = 25·1 s), compared with only one-quarter of LA-consistent, APS-positive patients (Fig 2C, mean PT = 20·6 s).
Our analysis of LA testing indicated that a substantial percentage of the cases were associated with discrepant results, most likely representing false positives. The single largest contributing factor to LA discrepancies appeared to be testing done on patients undergoing active anticoagulation. Almost 80% of total LA evaluations were negative, indicating that LA testing was being ordered in many patients with a low clinical suspicion for disease. Efforts to improve accuracy in LA identification could culminate in less secondary testing and guide appropriate antithrombotic therapy (Jennings et al, 2002).
Lack of definitive knowledge of anticoagulation status was a limitation of our study. Although the dRVVT/PNT assays contain additives to neutralize heparin, complete neutralization may be difficult to achieve. Another limitation was that few patients had repeat testing over the study period. Therefore, patients were defined as APS-positive if their immunological testing was positive at a single time (Miyakis et al, 2006). Nonetheless, and despite these limitations, we believe that our results are helpful in identifying causes for discrepancies in LA evaluation at single time points.
In summary, a considerable LA discrepancy rate appears to overwhelmingly represent false-positive results, attributable primarily to anticoagulation. Given that patient anticoagulation status may be unknown to reference laboratories at the time of LA testing, and that clinicians may be unaware of the effect of anticoagulants on APS assays, we propose the following LA evaluation algorithm: [i] include PT testing with all LA evaluations; and [ii] cancel LA testing when both PT and PTT are prolonged; [iii] in the event that only the PT or PTT is prolonged, then heparin neutralization and mixing studies should be done, and subsequent LA testing should only be performed on those plasmas that do not demonstrate correction. While this approach could theoretically miss rare, very weakly reactive LAs (primarily due to lack of sensitivity of our PT reagent), it would eliminate most discrepancies.
Footnotes
Conflicts of interest
The authors (JBC, RT, HMR, and CAT) have no relevant, competing financial conflicts of interest.
References
- Devreese K, Peerlinck K & Hoylaerts MF (2011) Diagnostic test combinations associated with thrombosis in lupus anticoagulant positive patients. Thrombosis and Haemostasis, 105, 736–738. [DOI] [PubMed] [Google Scholar]
- de Groot PG & Urbanus RT (2012) The significance of autoantibodies against beta2-glycoprotein I. Blood, 120, 266–274. [DOI] [PubMed] [Google Scholar]
- Jennings I, Greaves M, Mackie IJ, Kitchen S, Woods TA & Preston FE; on behalf of the UK National External Quality Assessment Scheme for Blood Coagulation (2002) Lupus anticoagulant testing: improvements in performance in a UK NEQAS proficiency testing exercise after dissemination of national guidelines on laboratory methods. British Journal of Haematology, 119, 364–369. [DOI] [PubMed] [Google Scholar]
- Keeling D, Mackie I, Moore GW, Greer IA & Greaves M; for the British Committee for Standards in Haematology (2012) Guidelines on the investigation and management of antiphospholipid syndrome. British Journal of Haematology, 157, 47–58. [DOI] [PubMed] [Google Scholar]
- Male C, Foulon D, Hoogendoorn H, Vegh P, Silverman E, David M & Mitchell L (2005) Predictive value of persistent versus transient antiphospholipid antibody subtypes for the risk of thrombotic events in pediatric patients with systemic lupus erythematosus. Blood, 106, 4152–4158. [DOI] [PubMed] [Google Scholar]
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG & Krilis SA (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). Journal of Thrombosis and Haemostasis, 4, 295–306. [DOI] [PubMed] [Google Scholar]
- Moore GW (2014) Recent guidelines and recommendations for laboratory detection of lupus anticoagulants. Seminars in Thrombosis & Hemostasis, 40, 163–171. [DOI] [PubMed] [Google Scholar]
- Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M & De Groot PG; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis (2009) Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Journal of Thrombosis and Haemostasis,7, 1737–1740. [DOI] [PubMed] [Google Scholar]
- Tripodi A (2009) Testing for lupus anticoagulants: all that a clinician should know. Lupus, 18, 291–298. [DOI] [PubMed] [Google Scholar]


