Advances in our understanding of tumour biology have inspired new treatments for cancer. One approach is to deliver drugs using nanoscale vehicles that can overcome the biological barriers in the body that otherwise prevent drugs from reaching the tumour1. These nanovehicles can capitalize on the enhanced permeability and retention (EPR) effect to passively accumulate in tumours. First described in 1986, the EPR effect posits that macromolecules and nanovehicles (usually between 10 and 125 nm in diameter) are small enough to escape from the leaky blood vessels found in tumours, but they remain in the tumour because of poor lymphatic drainage2. Perhaps the best-known antitumour therapy that capitalizes on EPR is Doxil (which involves the use of liposomes loaded with the drug doxorubicin)3. However, the efficacies of Doxil and other nanomedicines have been variable because the EPR effect can be smaller than expected for some tumours; additionally, irregularities in the size and connectivity of tumour blood vessels increase the interstitial fluid pressure inside tumours and impair drug delivery4.
Writing in Nature Nanotechnology, Rakesh Jain of Harvard Medical School and colleagues from Massachusetts Institute of Technology and the University of Cyprus5 show experimentally and mathematically that nanoparticle transport into tumours can be improved by making the pores of leaky tumour blood vessels smaller. This process, known as vascular normalization6, reduces the interstitial fluid pressure inside the tumours and encourages convective penetration of molecules (Fig. 1).
Figure 1 |.
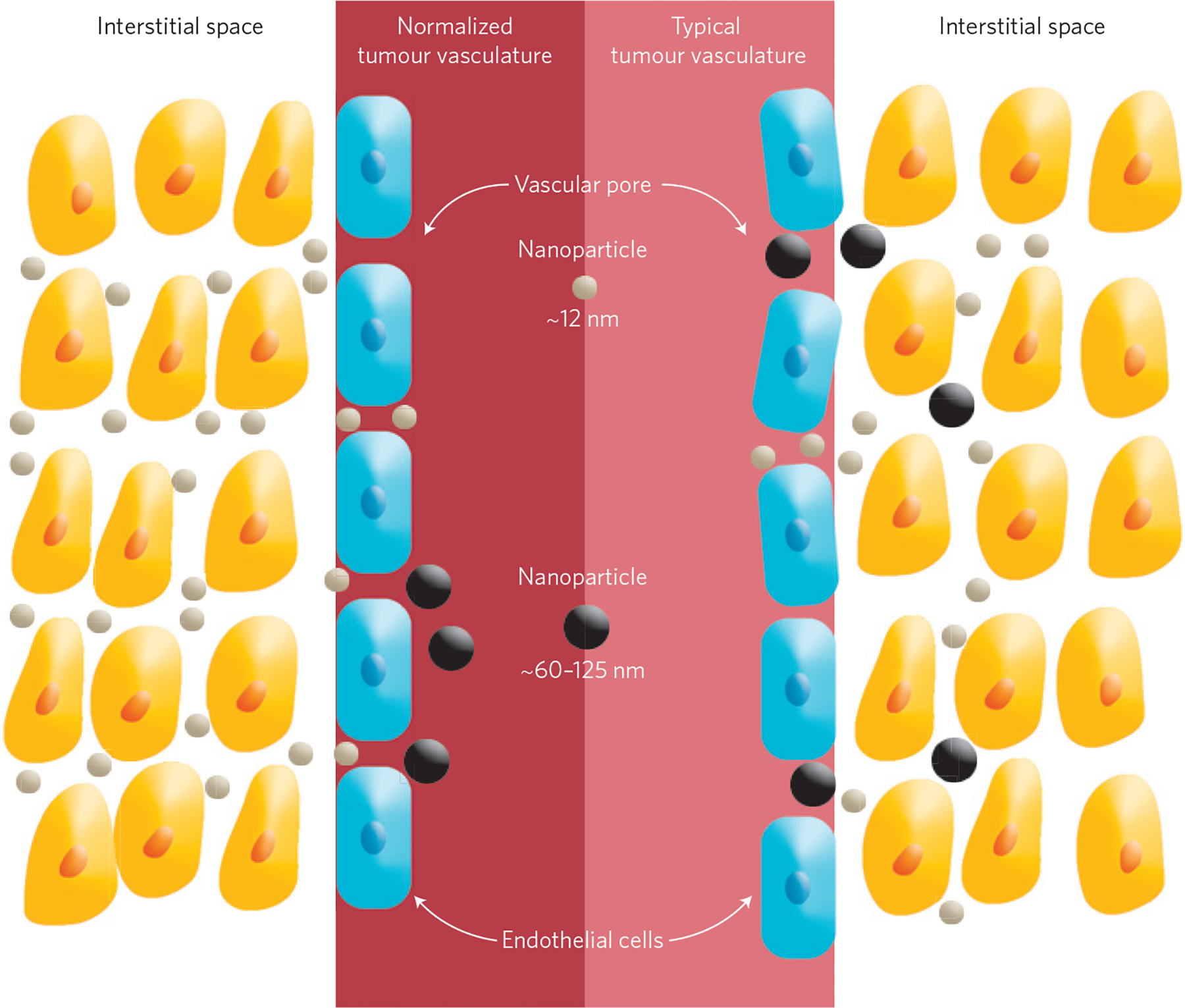
Size-dependent transport of nanotherapeutics. The blood vessels in a typical tumour contain pores of various sizes, which allow nanoparticles of different sizes (black and grey) to enter the tumour (right). However, these pores also cause the interstitial fluid pressure to increase, which limits convective transport of nanoparticles into the tumour. Decreasing the size of these pores (a process called vascular normalization) increases convection and the interstitial penetration of small (~12 nm) nanotherapeutics (left). However, for larger nanoparticles (~60–125 nm) this increase in convection is overridden by an increase in steric and hydrodynamic hindrances.
Jain and co-workers normalized the tumour blood vessels by treating mice with the vascular endothelial growth factor receptor-2-blocking antibody, and used near-infrared multiphoton microscopy to monitor the penetration of nanoparticles (ranging from 12 to 125 nm) into tumours. The experimental data was compared with mathematical models to predict how vascular normalization affects the distribution of the pore sizes of the blood vessels, and how this can be used to optimize the delivery of nanomedicines.
Nanoparticles that are 12 nm in diameter entered normalized tumours more efficiently than larger (60 nm and 125 nm) nanoparticles (Fig. 1), indicating that normalization can improve the delivery of the smallest nanoparticles. This difference can be explained by the fact that normalization decreases the size of the pores of the blood vessels. This means that larger nanoparticles, whose diameter comes close to the size of the average pore of a normalized blood vessel, experience steric and hydrodynamic hindrances that impede transport.
The model was further validated in mice: the commercially available nanomedicine Abraxane (10 nm in diameter) showed better antitumour efficacy in mice that underwent vascular normalization than in animals that were not normalized. In contrast, Doxil (a 100-nm-diameter commercially available nanomedicine) showed the same efficacy with or without tumour normalization. This trend of improved transport in tumours using nanomedicines of smaller sizes has been shown in the studies that first defined the EPR effect2. However, Jain and co-workers showed that decreasing the pore size of blood vessels in tumours can impact the delivery of nanoparticles. Ultimately, this study brings attention to the unrealistic expectations that we have developed for nanomedicines: not all nanoscale systems can effectively penetrate tumours.
There are some important caveats to consider: first, predictive models are useful but because tumours are heterogeneous, have high interstitial pressure and an acidic microenvironment, and are poorly oxygenated, they do not always obey the rules that govern normal tissue physiology. Furthermore, within a tumour, nanoparticles are likely to exhibit dynamic behaviours that depend on how they navigate through the network of blood vessels and how they interact with cells and components of the tissue. For example, nanoparticles can bind to positively charged collagen fibres and/or be removed by inflammatory cells. Moreover, after systemic administration nanoparticles often adsorb proteins and aggregate7. Because surface charge and other physicochemical properties can directly impact the effective size and biological interactions of nanotherapeutics, these parameters should be considered when modelling nanoparticle transport.
Second, although Jain and co-workers showed that tumours preconditioned with vascular normalization can improve the efficacy of established anticancer nanoparticles, it is worth noting that Abraxane and Doxil differ dramatically in properties other than size. Doxil is a cationic liposome containing polyethylene glycol on its surface, whereas Abraxane has a human serum albumin protein backbone. Despite this, the present results suggest that the impact of these other differences on transport may be small compared with the effect of size.
Finally, vascular normalization may seem counterintuitive as a therapeutic strategy because it improves blood flow to tumours and lowers interstitial fluid pressures, which could promote undesirable tumour growth. In fact, the main aim of anti-angiogenic therapies that destroy the blood vessels in tumours is to prevent the delivery of oxygen and nutrients to tumours. However, it is now clear that destruction of the vessels can impede the delivery of drugs and nanomedicines. This means that for successful delivery, vascular normalization requires very careful timing and precise dosing6. Although such precision can be difficult to achieve, the improved transport attained for small nanoparticles may be worth the efforts to precondition tumours for treatment.
It is encouraging that the principles of hindered transport through nanoscale pores in tumour vessels can be used as the basis for designing new methods to treat the biggest killer in the developed world. Jain and co-workers present compelling evidence that only the smallest (12 nm) nanotherapeutics will have a transport advantage in normalized tumours. This might be taken as extremely bad news because 12 nm is about the size of a common antibody, and is much smaller than most nanosystems being studied. However, the researchers showed that if we learn to modulate the average size and the distribution of pore sizes in the blood vessels of tumours, new therapies are possible. This work has profound implications on the impact of manipulating tumours to be more amenable to anticancer nanotherapeutics.
Contributor Information
Christopher J. Cheng, Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06511, USA;
W. Mark Saltzman, Department of Biomedical Engineering, Yale University, New Haven, Connecticut 06511, USA..
References
- 1.Ruoslahti E, Bhatia SN & Sailor MJ J. Cell Biol 188, 759–768 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumura Y & Maeda H Cancer Res. 46, 6387–6392 (1986). [PubMed] [Google Scholar]
- 3.Duggan ST & Keating GM Drugs 71, 2531–2558 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Nakamura H & Maeda H Adv. Drug Del. Rev. 63, 136–151 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Chauhan V et al. Nature Nanotech. 7, 383–388 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK Science 307, 58–62 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Alexis F, Pridgen E, Molnar LK & Farokhzad OC Mol. Pharm 5, 505–515 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


