Abstract
Purpose of review:
Despite modern advances in medicine, non-healing wounds are the number one cause of non-traumatic, lower-limb amputation. Non-healing wounds are characterized by a healing process stalled between inflammation and tissue remodel/repair, a stage characterized by a shift in macrophage functional phenotype. Characterization of diversity in macrophage functional phenotype in wounds and metabolic contributions to macrophage polarization are discussed.
Recent findings:
Macrophage functional diversity in phenotype has recently evolved from duality (classically-activated, pro-inflammatory M1 and alternatively-activated, anti-inflammatory M2) to include an additional four alternately-activated sub-phenotypes (M2a, M2b, M2c, & M2d). Metabolic pathway utilization shifts characterize macrophage polarization with resulting metabolic and immune outcomes impacting host-pathogen interactions during wound healing.
Summary:
Recognition of the key role macrophage diversity plays in wound healing, along with better characterization of diverse macrophage phenotypes, will inform our understanding of pathogenicity in wound healing. Comprehensive profiling of the metabolism regulating macrophage polarization and host-pathogen interaction creates opportunity of discovery for innovative new diagnostics and therapeutics for treating non-healing wounds.
Keywords: macrophage, immunomodulation, metabolism, inflammation, wounds
INTRODUCTION
Over the past decade, significant progress has been made in the treatment of acute injuries. However, non-healing in chronic wounds such as diabetic foot ulcers (DFUs), venous insufficiency ulcers (VIUs), pressure ulcers (PUs), and unresolved hospital-acquired infections (HAIs) remains in an upward trajectory, despite introduction of innovative therapeutics (1). Such chronic wounds are defined as lasting greater than 30 days and are characterized by a failure to progress through the normal wound healing process (2). In chronic wounds, the healing process appears stalled at the resolution of inflammation and initiation of tissue re-organization and this transition from early to late stage inflammation is characterized by a shift in population from neutrophils to macrophages (3). Under conditions of metabolic dysregulation, a chronic inflammatory state of tissue-resident macrophages is found in insulin sensitive tissues such as adipose tissue (4), liver (5), and muscle (6); however, how metabolism contributes to macrophage functionality at the site of bacterial colonization in the wound has only recently started to be explored. Herein, the recently expanding role of macrophage functional phenotype and plasticity within the wound environment is discussed, including key findings that indicate this plasticity is achieved through metabolic immunomodulation. Viewed through the lens of infectious diseases, metabolism impact on macrophage functionality must play an essential role in pathogenesis, including the impact of metabolite exchange on host-pathogen interactions.
PLASTICITY IN MACROPHAGE FUNCTIONAL PHENOTYPE
While the earliest reports in macrophage literature focused primarily on two distinct phenotypes (termed classically- and alternatively-activated) (7), emerging research has identified several additional functional macrophage phenotypes that regulate immunological responses (8). The commonly employed M1-M2 nomenclature (pro-inflammatory vs anti-inflammatory, respectively) has expanded to include sub-phenotypes within the broader “alternately-activated” macrophage classification (M2) indicated by the addition of lower case letters (i.e. M2a, M2b, M2c, and M2d) (9). Emergent findings attempting to characterize and define each of these distinct macrophage functional phenotypes clearly suggest that macrophage plasticity is essential to all stages of wound healing, as outlined in Figure 1.
Figure 1: Macrophage phenotype functional diversity in the wound-healing environment.
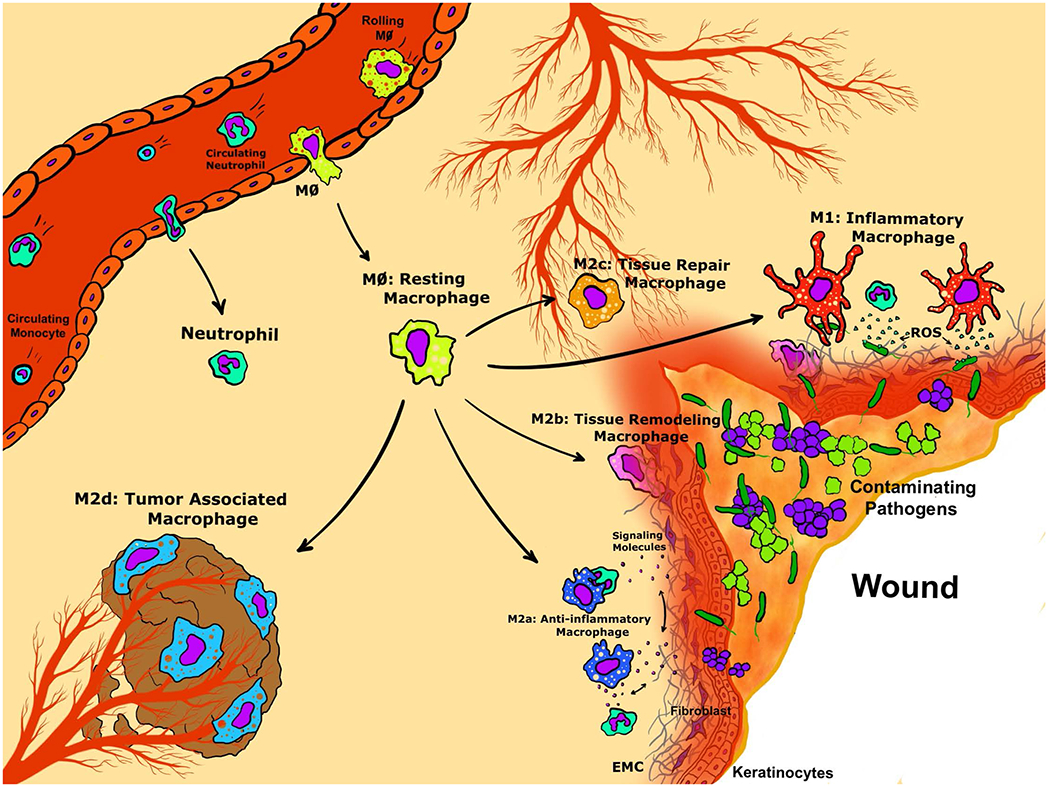
Graphical representation of macrophage functional phenotypes follow normal wound healing progression from post-injury recruitment of circulating innate immune cells to localized macrophage differentiation (M0: Resting Macrophage, yellow cells). Activation of macrophage polarization may result in one of five distinct functional phenotypes as follows: M1 Inflammatory Macrophages (red cells), M2a Anti-Inflammatory Macrophages (dark blue cells), M2b Tissue Remodeling Macrophages (purple cells), M2c Tissue Repair Macrophages (orange cells), or M2d Tumor Associated Macrophages (light blue cells).
Source: Original art by co-author Tyler Lawton.
Upon signaling of tissue damage and bacterial colonization, resting M0 macrophages (yellow cells, Figure 1) are activated from tissue-resident macrophages, recruited from circulating macrophages, or differentiated from circulating monocytes that traffic to the site of injury. While in a homeostatic metabolic state, the M0 macrophages represent the initiating step toward macrophage activation into the following variety of functional phenotypes. Most well characterized, the M1 or classically activated macrophage (red cells, Figure 1) initiates the pro-inflammatory immune responses to pathogen colonization (10, 11). Recent advances describing M1 polarization suggest activation of circulating macrophages to this phenotype (M0 to M1) results in development of systemic inflammatory response syndrome in humans (12)*, demonstrating the lethality of inappropriate M1 sustained activation. As the inflammation abates, these alarmins are believed to facilitate the onset of anti-inflammatory or wound healing responses characterized by the presence of alternatively activated macrophages or the M2 phenotypes.
Previously observed in fungal, parasite, and helminth infections, the M2a phenotype is known to antagonize pro-inflammatory responses (dark blue cells, Figure 1) and is observed in the tissue environment promoting wound resolution (13). Distinct to the M2a phenotype, the mannose receptor (CD206) is thought to play a role in the elimination of pro-inflammatory proteins in wounds, but recent work using partially depleted CD206-M2a macrophages suggests these cells may also play a role in adipose tissue browning and insulin sensitivity (14)**. A hybrid between the well-described M1 and M2a phenotypes, the M2b macrophages (purple cells, Figure 1) secrete both pro- and anti-inflammatory cytokines/chemokines, and are thought to modulate the breadth and depth of an inflammatory response, blunting the immune response to infection, specifically in cases of infection post-burning (15), without complete suppression of the inflammatory response as initiated at wounding.
Resolution of inflammation and transition to tissue formation is thought to be mediated by the M2c phenotype (orange cells, Figure 1). M2c polarized macrophages demonstrate strong phagocytic activity targeted at uptake of apoptotic neutrophils and are thought to appropriately control wound repair response and limit collagen deposition in scar tissue (16). Recent findings by E. B. Lurier, et al. (2017) demonstrated that the M2c phenotype upregulated several genes and cell-markers instrumental in normal wound healing through regulation of clot formation and angiogenesis, phagocytosis of wound debris, and the deposition of ECM components (17). While M2a and M2c functional phenotype seem to overlap, temporal activation of these two phenotypes occurs separately in the process of normal wound healing with M2c gene expression peaking around six hours post-injury and M2a gene expression peaking around 25 days post-injury (17, 18).
Finally, tumor-associated macrophages (TAMs) or M2d macrophages (light blue cells, Figure 1), have been observed within the tumor mass microenvironment, as implicated by the commonly used nomenclature. This phenotype is associated with potent immunosuppressive functions and angiogenesis promotion contributing to the survival of the tumor mass **(19) and is thought to arise via tumor-secreted factors (20). While typically observed associated with a tumor mass, M2d macrophages have recently emerged as an important functional phenotype in the chronic wound environment, possibly through the suppression of T-cell immunity **(21). Chronically inflamed wounds are oxygen restricted, leading to miRNA epigenetic modification of hypoxia-related genes, which drives the phenotypic shift from M1 to M2d and contributes to M2d functionality *(22, 23). Much debate remains about the identification and functional of wound-associated macrophage subpopulations, including contribution to healing in wounds (reviewed in Wermuth and Jimenez (24)); however, metabolic immunomodulation of macrophage plasticity in infectious disease is one of the most innovative area of research and has revived interest in these overlooked immune cells in pathogenesis.
METABOLISM AND MACROPHAGE FUNCTIONAL PHENOTYPE
Recent characterization of metabolic immunomodulation has demonstrated the important role metabolism plays in the polarization of macrophages (Reviewed in O’Neill, et al. (25) and outlined in Figure 2). Despite recent demonstrations of macrophage diversity, the majority of current research favors comparison of polarization to M1 (classically-activated macrophages) relative to M2 (alternately-activated macrophages) without the additional complexity of comparisons within the M2 subgroup functional phenotypes (i.e. M2a, M2b, M2c, M2d). While basic macrophage homeostasis is maintained in resting and activated (M1/M2) macrophages through persistence of glycolysis metabolism (gray text box, pathway (i), Figure 2), the M1/M2 activation results in specific energy investment in pathways supportive of the inflammatory function of acute healing (red text boxes, Figure 2) or supportive of inflammatory resolution and tissue repair/regeneration (yellow text boxes, Figure 2), as shown for the proposed model of immunomodulation of macrophages via metabolic pathways (red & yellow text boxes labeled (ii)-(xi), Figure 2).
Figure 2: Metabolic immunomodulation of macrophage functional phenotype and host-pathogen interactions.
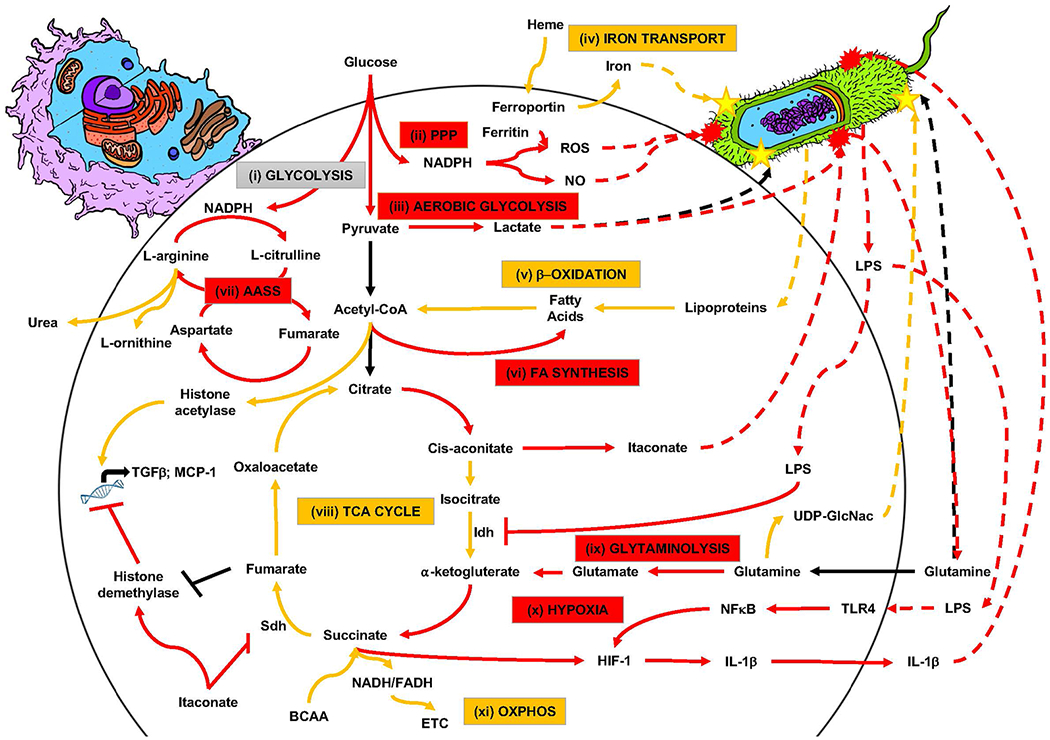
Current schematic model of metabolic immunomodulation of macrophage functional phenotype presented as classically activated M1 (red boxes and lines) and alternately activated M2 (yellow boxes and lines), based on currently published research. Grey “glycolysis” textbox indicative of homeostatic metabolism in M0/M1/M2. Colored textboxes indicate energetic investment in metabolic pathways during polarization (M1/M2). Solid lines indicate metabolic pathways of interest to polarization (M1/M2; simplified for overall view). Dashed lines indicate pathogen-derived metabolites or pathogen-directed metabolites of host-pathogen metabolic interaction. Yellow stars on pathogen indicate effects of benefit to survival of the pathogen and red explosive shapes on pathogen indicate effects of harm to pathogen survival.
Source: Original art by co-author Tyler Lawton; pathway model based on referenced research in body of review article.
As far back as 1958, metabolic pathway contribution to immune function has been described through the Warburg Effect wherein immune activation is coupled to metabolic shift from oxidative phosphorylation (pathway xi, Figure 2) to aerobic glycolysis (pathway (iii), Figure 2) (26); however, recent metabolic and transcriptional profiling indicates that this shift to aerobic glycolysis is specific to M1 polarization and oxidative phosphorylation is favored by M2 polarization (27, 28). Significant metabolic pathway shift during M1 activation to the pentose phosphate pathway (PPP) activates the anti-microbial NADPH oxidase and generates reactive oxygen species (ROS) (pathway (ii), Figure 2) (29). The TCA cycle (pathway (viii), Figure 2) plays a key role in macrophage polarization, as an intact cycle is associated with the M2 phenotype and an uncoupling of the TCA cycle at both citrate and succinate is associated with the M1 phenotype (28, 29). Finally, selective amino acid uptake and metabolism plays a significant role in macrophage polarization. For example, the glutaminolysis pathway (pathway (ix), Figure 2) and the aspartate-arginosuccinate shunt pathway (pathway (vii), Figure 2) play an important role in maintaining cellular redox homeostasis during ROS and NO production, respectively, associated with M1 anti-microbial functionality (30, 31). In contrast, arginine flux through the arginase pathway is associated with M2 polarization, immune tolerance, and wound healing (32).
Colonizing bacterial interaction macrophages in the wound also mediates metabolic activation and subsequent M1/M2 polarization (33). For example, bacterially-derived lipopolysaccharides (LPS) directly inhibit the isocitrate dehydrogenase (Idh), contributing to TCA cycle decoupling, and indirectly lead to macrophage production of the metabolite itaconate, a known antimicrobial metabolite utilized by M1 polarized macrophages (34, 35). In contrast, bacterially-derived lipoproteins can feed into β-oxidation metabolism (pathway (v), Figure 2), providing a key resource for M2 polarization (29, 36). M2 polarization also favors production of uridine diphosphate N-acetyl glucosamine (UDP-GlcNac, Figure 2) for protein glycosylation of the M2 mannose receptor (CD206) (28), but which can also be co-opted by colonizing bacteria for structural support of the biofilm matrix.
CONCLUSION
Recent observations of macrophage diversity and phenotypic plasticity demonstrate the broad impact of these cells throughout the body (37); however, temporal progression of wound-associated macrophage function from inflammation to resolution to tissue repair/remodeling indicates this plasticity plays a key role in the normal wound-healing process and dysregulation of this progression contributes to pathogenic non-healing. Further characterization of macrophage diversity and description of functional phenotype will provide essential leads for novel diagnostic and therapeutic approaches to wound healing (38). Finally, the recent surge in immunometabolism research has contributed significantly to our understanding of the mechanisms of macrophage polarization (39–41); however, how the metabolic interactome between wound-associated macrophages and wound-colonizing bacteria contributes to healing and non-healing remains an area of active discovery.
Key Points:
Chronic wounds are characterized by the healing process stalling at the transition between inflammation and tissue repair/remodeling.
Macrophage functional diversity and plasticity in the changing environment makes these innate immune cells essential mediators of wound progression through the normal wound healing process.
Metabolic immunomodulation of macrophage functional phenotype is key to innate immune response to wounding and host-pathogen metabolic interaction may be a key determinate of whether a wound resolves or stalls in the healing process.
Acknowledgements:
Financial support and sponsorship:
C.B.A., T.L., and M.C.B.A. are supported by NIH-NIAID grant R03AI135998 (PI-Ammons) and a subproject to NIH-NIGMS grant P20GM109007 (PD-Stevens, PL-Ammons).
Footnotes
Conflicts of interest:
None
References and recommended reading:
- 1.Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34(3):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(1):37–44. [DOI] [PubMed] [Google Scholar]
- 3.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341(22):1661–9. [DOI] [PubMed] [Google Scholar]
- 4.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59(2):347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. [DOI] [PubMed] [Google Scholar]
- 7.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudan B, Wacker MA, Wilson ME, Graff JW. A Systematic Approach to Identify Markers of Distinctly Activated Human Macrophages. Front Immunol. 2015;6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–12. [DOI] [PubMed] [Google Scholar]
- 11.Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27(4):237–48. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan A, Sun Y, Sukumaran P, Quenum Zangbede FO, Jondle CN, Sharma A, et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience. 2018;8:85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This original report demonstrates for the first time the role of regulated calcium flux in macrophage polarization to classically-activated, pro-inflammatory macrophages.
- 13.Roszer T Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi Y, Nawaz A, Kado T, Bilal M, Kuwano T, Yamamoto S, et al. Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci Rep. 2018;8(1):14567. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this original report, the co-authors present molecular interaction between environment of the host, metabolic health and fat deposition, and contribution of alternately-activated M2 macrophage functional phenotype in mediating cell-cell interactions.
- 15.Nishiguchi T, Ito I, Lee JO, Suzuki S, Suzuki F, Kobayashi M. Macrophage polarization and MRSA infection in burned mice. Immunol Cell Biol. 2017;95(2):198–206. [DOI] [PubMed] [Google Scholar]
- 16.Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermohlen O, et al. Accelerated wound closure in mice deficient for interleukin-10. Am J Pathol. 2007;170(1):188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurier EB, Dalton D, Dampier W, Raman P, Nassiri S, Ferraro NM, et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology. 2017;222(7):847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel VA, McClellan EA, Scheuermann RH. Response of human skin to esthetic scarification. Burns. 2014;40(7):1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura R, Tanaka T, Yamamoto Y, Akasaki Y, Sasaki H. Dual role of macrophage in tumor immunity. Immunotherapy. 2018;10(10):899–909. [DOI] [PubMed] [Google Scholar]; **Excellent recent review of polarization into functional phenotype of TAMs and the associated interaction of polarized macrophages with the tumor in both beneficial and detrimental pathogenesis.
- 20.Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–12. [DOI] [PubMed] [Google Scholar]
- 21.Lai YS, Wahyuningtyas R, Aui SP, Chang KT. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J Cell Mol Med. 2019;23(2):1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Original report demonstrating autocrine growth factor signaling in TAMs resulting in suppression of tumorogenic T cells through cell to cell signaling.
- 22.Shukla S, Levine C, Sripathi RP, Elson G, Lutz CS, Leibovich SJ. The Kat in the HAT: The Histone Acetyl Transferase Kat6b (MYST4) Is Downregulated in Murine Macrophages in Response to LPS. Mediators Inflamm. 2018;2018:7852742. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This original report demostrates the next level of metabolic immunomodulation by connecting metabolic polarization in macrophages through chromatin remodeling and epigenitic regulatory mechanisms.
- 23.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17(12):774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wermuth PJ, Jimenez SA. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med. 2015;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nature reviews Immunology. 2016;16(9):553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warburg O, Gawehn K, Geissler AW. [Metabolism of leukocytes]. Z Naturforsch B. 1958;13B(8):515–6. [PubMed] [Google Scholar]
- 27.Assmann N, Finlay DK. Metabolic regulation of immune responses: therapeutic opportunities. J Clin Invest. 2016;126(6):2031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30. [DOI] [PubMed] [Google Scholar]
- 29.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy C, Newsholme P. Importance of glutamine metabolism in murine macrophages and human monocytes to L-arginine biosynthesis and rates of nitrite or urea production. Clinical science. 1998;95(4):397–407. [PubMed] [Google Scholar]
- 31.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–76. [DOI] [PubMed] [Google Scholar]
- 32.Albina JE, Mills CD, Barbul A, Thirkill CE, Henry WL Jr., Mastrofrancesco B, et al. Arginine metabolism in wounds. Am J Physiol. 1988;254(4 Pt 1):E459–67. [DOI] [PubMed] [Google Scholar]
- 33.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181(6):3733–9. [DOI] [PubMed] [Google Scholar]
- 34.Meiser J, Kramer L, Sapcariu SC, Battello N, Ghelfi J, D’Herouel AF, et al. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. J Biol Chem. 2016;291(8):3932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naujoks J, Tabeling C, Dill BD, Hoffmann C, Brown AS, Kunze M, et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog. 2016;12(2):e1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–55. [DOI] [PubMed] [Google Scholar]
- 37.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. [DOI] [PubMed] [Google Scholar]
- 38.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017;122:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artyomov MN, Sergushichev A, Schilling JD. Integrating immunometabolism and macrophage diversity. Semin Immunol. 2016;28(5):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. [DOI] [PubMed] [Google Scholar]
- 41.Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen. 2016;24(4):613–29. [DOI] [PubMed] [Google Scholar]


