Abstract
Integrin β4 (ITGB4) has been shown to play an important role in the regulation of cancer stem cells (CSCs). Immune targeting of ITGB4 represents a novel approach to target this cell population with potential clinical benefit. We developed two immunologic strategies to target ITGB4: ITGB4 protein-pulsed dendritic cells (ITGB4-DC) for vaccination, and adoptive transfer of anti-CD3/anti-ITGB4 bispecific antibody (ITGB4 BiAb) armed tumor-draining lymph node T cells. Two immunocompetent mouse models were utilized to assess the efficacy of these immunotherapies in targeting both CSCs and bulk tumor populations: 4T1 mammary tumors and SCC7 head and neck squamous carcinoma cell line. Immunologic targeting of ITGB4 utilizing either ITGB4-DC or ITGB4 BiAb-T cells significantly inhibited local tumor growth and metastases in both the 4T1 and SCC7 tumor models. Furthermore, the efficacy of both of these ITGB4-targeted immunotherapies was significantly enhanced by the addition of anti-PD-L1. Both ITGB4-targeted immunotherapies induced endogenous T cell cytotoxicity directed at CSCs as well as non-CSCs which expressed ITGB4, and immune plasma-mediated killing of CSCs. As a result, ITGB4-targeted immunotherapy not only reduced the number of ITGB4high CSCs in residual 4T1 and SCC7 tumors but also their tumor-initiating capacity in secondary mouse implants. Additionally, treated mice demonstrated no apparent toxicity. The specificity of these treatments was demonstrated by the lack of effects observed using ITGB4 knockout 4T1 or ITGB4-negative CT26 colon carcinoma cells. Since ITGB4 is expressed by CSCs across a variety of tumor types, these results support immunologic targeting of ITGB4 as a promising therapeutic strategy.
Introduction
The development of cancer immunotherapy represents one of the most significant advances in oncology. Despite these successes, the benefits of immunotherapy are limited to a subset of patients and tumor types. Furthermore, the durability of these responses is often limited. There is increasing evidence that therapeutic resistance and tumor relapse may be mediated by a subset of tumor cells that display stem cell properties (1–3). These cancer stem cells (CSCs) lack expression of differentiation antigens and may display inherent resistant to a variety of immunotherapeutic approaches (2, 4). The ability of CSCs to escape recognition and elimination by the immune system may contribute to the limited clinical efficacy of current cancer immunotherapies. The targeting of shared CSC antigens represents an approach to overcome these limitations.
Integrins are heterodimeric transmembrane receptors that mediate interaction of cells with extracellular matrix components (5). Integrin β4 (ITGB4), which heterodimerizes exclusively with the α6 chain, functions as a receptor for the basement membrane protein laminin. ITGB4 expression is increased in a variety of malignancies including breast cancer cells (6, 7). ITGB4 is involved in and can enhance multiple signaling pathways, including ErbB2 (8, 9), PI3K (10, 11), FAK/AKT (12, 13), and c-Met (14, 15), to promote tumor progression (16). Exosome proteomics revealed the exosomal ITGB4 was associated with lung metastasis (17, 18). Furthermore, upregulation of ITGB4 is an adverse prognostic marker in pancreatic ductal adenocarcinoma (19) and breast cancer (20). Importantly, Integrin-β4 induces expansion of prostate tumor progenitors (21), and identifies cancer stem cell-enriched populations from breast cancer cells (22). It plays an important role in the metastasis and treatment resistance of these cells (23–25). We therefore hypothesized that immunologically targeting ITGB4 might improve the efficacy of immune checkpoint blockade by targeting the CSC population as well as bulk tumor cells.
In multiple tumor types, CSCs may be enriched by virtue of their increased expression of aldehyde dehydrogenase (ALDH) activity as accessed by the Aldefluor assay (26, 27). In mouse models of melanoma and head and neck (HN) cancer, we previously demonstrated the efficacy of a dendritic cell (DC) vaccine generated by pulsing these cells with a lysate of ALDHhigh CSCs (28, 29). This effect was mediated by cytotoxic CD8 T cells as well as antibodies that specifically targeted the CSC population. Furthermore, the therapeutic efficacy of ALDHhigh HN CSC-DC vaccine was significantly augmented by anti-PD-L1 administration (30). This immunotherapeutic augmentation was apparent in tumor models of advanced disease as well as those simulating the adjuvant setting (30). Although these studies demonstrated the feasibility of generating immune responses against the CSCs, the clinical application of this approach is limited by the need to obtain tumor tissue to isolate CSCs from patient. An alternate approach of targeting CSC shared antigens has the potential for providing an “off the shelf” reagent that can be utilized in patients whose tumors express the antigen. Since ITGB4 is express in CSCs across multiple tumor types (17, 18, 21, 22), it is well suited for such immunologic targeting.
T cell engaging bispecific antibodies (BiAb), which bring T effector cells in contact with tumor cells, represents another approach for immunologic targeting (31–33). We previously generated an anti-CD3/anti-CD133 bispecific antibody and bound it to cytokine-induced killer (CIK) cells as effector cells (BiAb-CIK) to target CD133high CSCs. CIK cells bound with anti-CD3/anti-CD133 bispecific antibodies effectively targeted CD133high CSCs both in vitro and in vivo (34).
In this study, we explored two approaches for immunologic targeting of ITGB4 utilizing breast and head & neck cancer models: ITGB4-DC vaccination and anti-CD3/anti-ITGB4 bispecific antibody armed T cells adoptive transfer. We also demonstrated that immunologic targeting of ITGB4 enhanced the efficacy of anti-PD-L1 checkpoint blockade in these models.
Materials and Methods
Mice
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan (Protocol number PRO00006536 & 8556). Six- to 8-week-old female Balb/c mice and C3H mice were purchased from The Jackson Laboratory and Charles River Laboratories.
Murine tumor cells
4T1 is a murine mammary carcinoma cell line, which is syngeneic to Balb/c mice (35). SCC7, a murine head and neck squamous carcinoma cell line syngeneic to C3H mice was described in our previous publications (28). The murine colon carcinoma CT26 cell line is also syngeneic to Balb/c mice (36). 4T1, SCC7 and CT26 cells were kindly provided by Dr. Michael S. Sabel, Dr. Jeffrey S Moyer and Dr. James Moon respectively. Luciferase-labelled 4T1 (4T1-Luc) cells were kindly provided by Dr. Max S. Wicha at the University of Michigan two years ago. 4T1, 4T1-Luc and SCC7 cells were utilized in this manuscript over approx. 2 years. Aliquots of these cells were frozen at the beginning of the experiments. The passage number of 4T1, 4T1-Luc and SCC7 cells is approximately 35. CT26 cells were used over the last 3 months, with a passage number of approximately 10. All cell lines were routinely tested for Mycoplasma contamination using MycoProbe™ Mycoplasma Detection Kit (R&D Systems, Inc., Minneapolis, MN) and any contaminated cell line was treated with Plasmocin™ treatment (InvivoGen, San Diego, CA), confirmed by negative detection of Mycoplasma before being used again. The most recent testing was 8 months ago. All cell lines were grown in complete medium (CM) consisting of RPMI1640 and supplements (30).
Knockout of ITGB4 gene in 4T1 cell line via CRISPR/Cas9
We generated ITGB4 knockout 4T1 (4T1-ITGB4KO) cells by using Integrin β4 CRISPR/Cas9 KO Plasmid (Santa Cruz Biotechnology, Dallas, TX). According to the manufacturer’s instructions, ~2.0 × 105 4T1 cells were seeded in a 6-well tissue culture plate in 3 ml of antibiotic-free standard growth medium per well 24 hours prior to transfection. At the 40–80% confluence, 4T1 cells were co-transfected with Integrin β4 CRISPR/Cas9 KO Plasmid and HDR Plasmid for 72 hours and then selected with media containing puromycin (6 μg/ml). Knockout of ITGB4 in 4T1 cells was confirmed by western blot and flow cytometry as described below.
Western Blotting
Total protein of CT26, 4T1 cells or 4T1-ITGB4KO cells were exacted by RIPA (Pierce Biotechnology, Waltham, MA) and concentrations determined by Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein lysates (30 μg) were resolved by 6% SDS–polyacrylamide gel electrophoresis (80 V: ~0.5 hours and 110 V: ~1.5 hours) and electrotransferred (320 mA, 2 hours) at 4°C onto Polyvinylidene difluoride membranes. Blots were blocked with 5% non-fat dry milk in Tris-buffered saline with 0.2% Tween-20 (TBST) for 1 h at room temperature and incubated overnight with primary murine ITGB4 antibody (Santa Cruz Biotechnology, Dallas, TX) (dilution: 1: 250) or β-actin antibody (Invitrogen, Waltham, MA) (dilution: 1:1000) at 4°C. After washing three times with TBST, the blots were incubated with peroxidase-conjugated secondary antibody (Invitrogen, Waltham, MA) (dilution: 1: 250) for 1 h at room temperature. Blots were washed three times and reactivity accessed by chemiluminescence (Thermo Fisher Scientific, Waltham, MA) using ChemiDoc™ Imaging System (Bio-Rad Laboratories, Hercules, CA).
Flow Cytometry
Expression of surface proteins was evaluated by flow cytometry. Anti-ITGB4 antibody (Invitrogen, Waltham, MA) was used to define the ITGB4 expression. Other antibodies include anti-PD-L1 (BD Biosciences, La Jolla, CA), anti-IgG and isotype controls (both from BD Biosciences, San Jose, CA). Stained cells were fixed with 4% paraformaldehyde (Alfa Aesar, Wood Hill, MA), and detected using the BD LSRFortessa™ Flow Cytometer (BD Biosciences, Franklin Lakes, NJ) and analyzed using the FlowJo software (Treestar, San Carlos, CA).
ALDEFLUOR assay
The ALDEFLUOR Kit (StemCell Technologies, Durham, NC) was used to isolate ALDHhigh CSCs/ALDHlow non-CSCs from the 4T1 and SCC7 cells as described previously (28). We stained cells with 7-actinoaminomycin-D (7-AAD) to exclude dead and late apoptotic cells to sort CSCs with high viability. We used the top 10% of ALDHhigh cells and the lowest 10% ALDHlow cells for the subsequent experiments.
Murine integrin beta 4 (mITGB4) protein pulsed DC vaccine
mITGB4 proteins were synthesized by LifeTein company (Somerset County, NJ). Murine bone marrow derived DCs (BMDCs) were expanded with granulocyte macrophage colony-stimulating factor (GM-CSF) (GenScript, Piscataway, NJ) and 2-mercaptoethanol (Gibco™, Grand Island, NY) as previously described in our laboratory (30, 37). 1 × 106 BMDCs were loaded with 20 μg mITGB4 protein and co-cultured at 37 °C overnight to generate the mITGB4-pulsed DC vaccine (mITGB4-DC).
Murine anti-CD3/anti-ITGB4 BiAb armed tumor-draining lymph node (TDLN) T cells
Murine anti-ITGB4 monoclonal antibody (Invitrogen) and anti-CD3 antibody (BD Biosciences, San Jose, CA) were coupled by Dr. Lawrence G. Lum Lab (University of Virginia) to produce murine anti-CD3/anti-ITGB4 BiAbs (mITGB4 BiAb). In order to induce 4T1 TDLN, 1 × 106 4T1 or 4T1-Luc tumor cells in 0.1 ml PBS were injected subcutaneously (s.c.) into the lower flanks of immunocompetent Balb/c mice. To induce SCC7 TDLN, 1 × 106 SCC7 tumor cells in 0.1 ml PBS were injected (s.c.) into the lower flanks of immunocompetent C3H mice. We used the same method to induce CT26 TDLN in Balb/c mice. Nine days after cells inoculation of the tumor cells, the TDLNs were harvested, and single-cell suspensions were prepared mechanically (38). TDLN cells were activated with anti-CD3 mAb and anti-CD28 mAb immobilized 6-well tissue culture plates for 2 days and expanded in CM containing 200 ng/ml of human rIL-2 (Pepro Tech Inc., Rocky Hill, NJ) for 3 days to generate activated/expended TDLN T cells. 1 × 106 activated TDLN T cells were incubated with 50 ng of mITGB4 BiAb for 1 h at 4 °C to generate murine ITGB4 BiAb-armed TDLN T cells (TDLN T-mITGB4 BiAb) (39).
Tumor models and treatment protocols
Minimal tumor model treated with mITGB4-DC vaccine
4T1 cells were injected into the mammary fat pad of Balb/c mice. SCC7 cells were injected into the C3H mice subcutaneously. The tumor-bearing animals were then divided into different groups (n=5). Twenty-four hours (1 day) after tumor injection, PBS, mITGB4-DC vaccine, anti-PD-L1 (MedImmune LLC., Gaithersburg, MD) and the combination treatment of mITGB4-DC vaccine plus with anti-PD-L1 antibody were administered respectively. The vaccination was repeated on day 8. Anti-PD-L1 was administrated three times after each DC vaccine. Each vaccine contained 2 × 106 DCs per mouse administrated s.c., and anti-PD-L1 was administrated i.p. in minimal tumor models. For tumor monitoring, the long and short diameters of tumor masses were measured three times per week. The volumes were calculated as: tumor volume = (width2 × length)/2.
Established tumor model treated with mITGB4 BiAb-armed TDLN T cells
Fourteen day 4T1 or 4T1-Luc, or SCC7 tumor-bearing animals were divided into different groups (n=5): PBS, TDLN T cells, ITGB4 BiAb-armed TDLN T cells, anti-PD-L1, ITGB4 BiAb-armed TDLN T cells plus with anti-PD-L1 isotype control, and ITGB4 BiAb armed TDLN T cells plus anti-PD-L1, respectively. The mITGB4 BiAb-armed 4T1 TDLN T cells or SCC7 TDLN T cells were transferred on day 14 and day 21 into 4T1 and SCC7 tumor-bearing hosts, respectively. Each mouse was injected with 1 × 106 mITGB4 BiAb-armed TDLN T cells via tail vain. Anti-PD-L1 was administrated twice after each T cell transfer. Tumor growth was monitored in a same way as the tumor treated by the DC vaccine. In addition, luciferase imaging was performed using the PerkinElmer’s IVIS™ Spectrum imaging system (Shelton, CT).
Experimental lung metastasis model treated with mITGB4-DC vaccine or mITGB4 BiAb-armed TDLN T cells
An experimental lung metastasis model was utilized to investigate tumor metastasis (40–42). Balb/c mice were injected i.v with 4T1-Luc cells via tail vain, while C3H mice were injected i.v. with SCC7 cells, to produce experimental lung metastases. Mice were then divided into treatment and control groups. mITGB4-DC vaccine was administrated on day 1 and 8 respectively, with each vaccine being followed by anti-PD-L1 injection. mITGB4 BiAb-armed TDLN T cells were transferred on day 3 and 10 with each T cell transfer being followed by anti-PD-L1 injection. At the end of the experiments, lungs were harvested to enumerate metastases.
Purification and culture of host T cells
Spleens were harvested from animals subjected to various treatments at the end of the experiments. Splenic CD3+ T cells were selected by CD3 MicroBeads (Miltenyi Biotec, Auburn, CA), activated by anti-CD3 and anti-CD28 (both from BD Biosciences, San Jose, CA), and then expanded with IL-2 to generate cytotoxic T cells (CTLs) (28).
CTL or TDLN T cell cytotoxicity
CTL or TDLN T cell-mediated cytotoxicity was tested using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI) according to the manufacturer’s protocol. CTLs or TDLN T cells were co-cultured with target cells (e.g. unsorted/ALDHhigh/ALDHlow 4T1, SCC7 cells, 4T1-ITGB4KO or CT26 cells) for 6 hours, and then the supernatants were harvested to measure lactate dehydrogenase. The following formula was used to calculate cytotoxicity: % Cytotoxicity= (Experimental - Effector spontaneous - Target spontaneous)/ (Target maximum - Target spontaneous) × 100.
Binding of immune plasma to tumor cells
Plasma was collected from treated hosts at the end of the experiments. IgG levels were quantified using ELISA. Unsorted/ALDHhigh/ALDHlow 4T1 or SCC7 cells were incubated with the plasma containing equal quantities of IgG for 60 minutes on ice. Cells were washed twice and incubated with anti-mouse IgG (eBioscience, San Diego, CA) for 30 minutes on ice. Cells were then washed twice and their binding to plasma IgG was detected by flow cytometry.
Complement-dependent cytotoxicity (CDC)
Cell lysis mediated by antibodies in immune plasma was assessed by incubation of 105 viable ALDHhigh/ALDHlow 4T1 or SCC7 cells with equal quantities of IgG on ice for 1 hour, followed by culturing in the presence of rabbit complement (Calbiochem, La Jolla, CA) in a 37 °C water bath for another 1 hour. Viable cells were then counted after trypan blue (Gibco, Carlsbad, CA) staining to calculate cell lysis: % of viable cells = viable cells after plasma/complement incubation/105. Lower percentage of viable cells indicates more cell lysis.
Tumorigenicity of residual ALDHhigh CSCs or whole residual tumor cells
Treated primary tumors were harvested at the end of experiments and processed into single-cell suspensions, using 1 × collagenase/hyaluronidase solution (Stemcell Technologies, Vancouver, BC, Canada). Residual ALDHhigh CSCs were isolated from the cell suspension. We ensured that viable ALDHhigh CSCs or whole residual tumor cells were injected by staining these cells with Trypan Blue before cell counts and injection. Tumor growth was then monitored (28). Similarly, whole residual tumor cells were injected into the syngeneic mice to compare the tumorigenicity of the residual cells after different treatments.
Statistical analysis
All data are presented as mean ± standard error of mean (SEM). Differences between groups were analyzed by one-way ANOVA or two-way ANOVA. The software GraphPad Prism 7 & 8 (GraphPad Software Inc., La Jolla, CA) was used to perform the statistical data analysis. Significant differences among data groups were assigned when p < 0.05.
Results
Expression of ITGB4 and PD-L1 on 4T1 and SCC7 cells
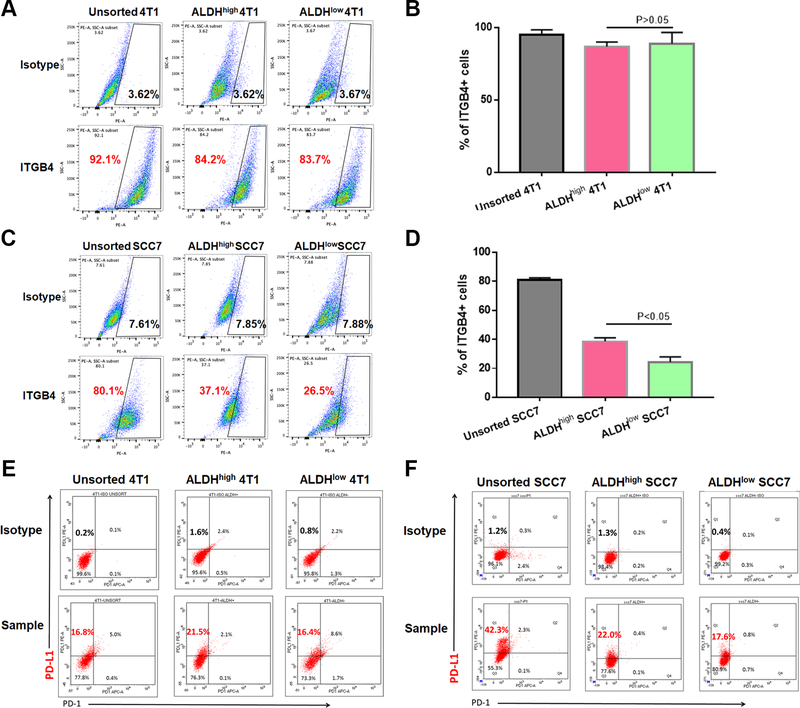
We analyzed ITGB4 expression in 4T1 murine breast cancer and SCC7 murine squamous cancer cell lines by flow cytometry. We compared the expression of ITGB4 on bulk cells as well as CSCs, which have been previously defined at ALDHhigh cells (30, 37). We found that 4T1 cells highly expressed ITGB4 in bulk unsorted cells (92.1%), in isolated ALDHhigh cells (84.2%) as well as in ALDHlow cells (83.7%) (Fig. 1A, B). In contrast in SCC7 cells, the expression of ITGB4 on ALDHhigh cells (37.1%) was significantly (p<0.05) greater than that on ALDHlow cells (26.5%) (Fig. 1C, D). These results allow us to determine the effects of ITGB4 targeting on both bulk and CSC populations.
Figure 1.
Expression of ITGB4 and PD-L1 on 4T1 and SCC7 unsorted cells, ALDHhigh cells and ALDHlow cells. A: 4T1 cells highly expressed ITGB4 in unsorted, ALDHhigh and ALDHlow cell populations. B: Statistical data showed no difference in the expression of ITGB4 in ALDHhigh and ALDHlow 4T1 sub-populations (p>0.05). C: SCC7 cells also expressed high level of ITGB4 on unsorted cells, and its expression was significantly higher on ALDHhigh CSCs than on ALDHlow non-CSCs. D: Statistically, ITGB4 cell-surface abundance on ALDHhigh SCC7 cells was approximately 1.5-fold higher than that on ALDHlow SCC7 cells (p<0.05). E-F: PD-L1 expression on the 4T1 (E) and SCC7 (F) cells. Flow cytometry performed at least twice, and one presesentitive datum is shown for each experiment.
We also accessed the expression of the immune checkpoint molecule PD-L1 on these cell populations. The percentage of PD-L1-expressing unsorted, ALDHhigh and ALDHlow 4T1 cells was 21.8 (16.8 + 5.0) %, 23.6 (21.5 +2.1) % and 25 (16.4 +8.6) % respectively (Fig. 1E). For SCC7 cells, 44.6 (42.3 +2.3) % of unsorted cells, 22.4 (22.0 + 0.4) % of ALDHhigh cells, and 18.4 (17.6 + 0.8) % of ALDHlow cells expressed PD-L1 (Fig. 1F). These data provide the rationale to determine whether the addition of anti-PD-L1 checkpoint blockade can augment the effects of ITGB4 targeting.
mITGB4-DC vaccination inhibited both local tumor growth and lung metastases in both 4T1 and SCC7 models
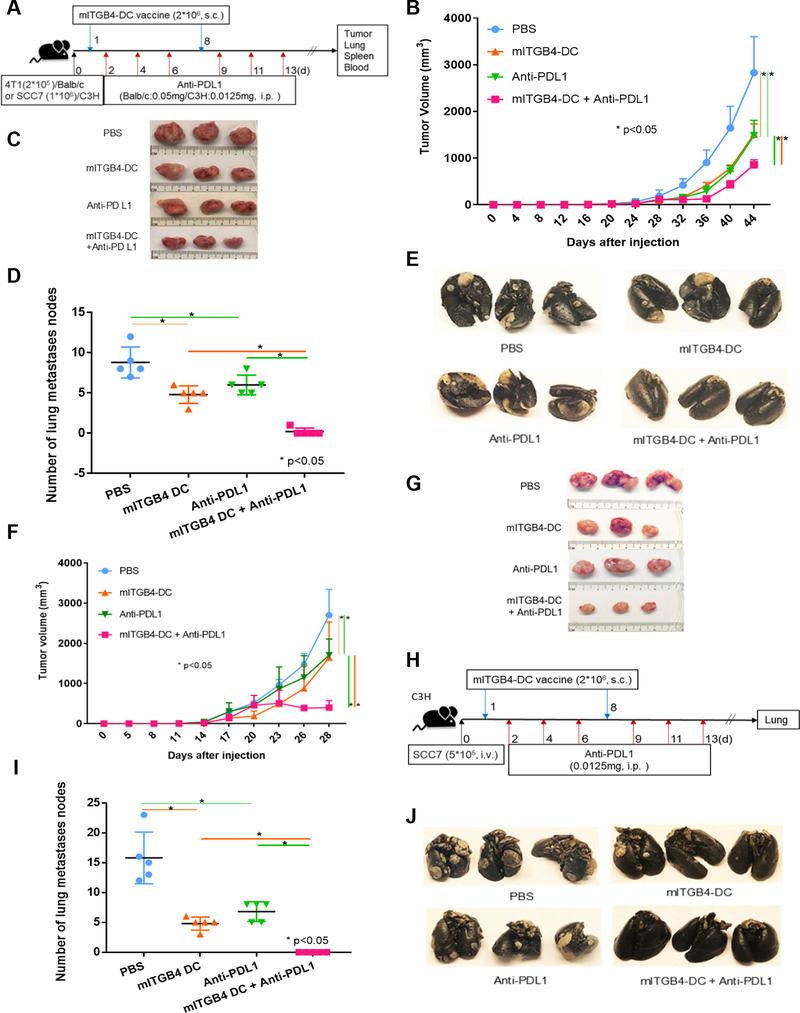
We have previously shown that ALDHhigh CSC lysate-pulsed DC vaccine conferred significant antitumor immunity in both tumor protection (28) and therapeutic models (29). To determine whether ITGB4 presented by DC can effectively target ITGB4-expressing tumors, we prepared a mITGB4-DC vaccine and treated mice with minimal disease burden in 4T1 or SCC7 tumor-bearing mice. In these therapeutic experiments, some groups were also treated with anti-PD-L1 mAb (Fig. 2A). In the 4T1 model, mITGB4-DC vaccination significantly inhibited local tumor growth (Fig. 2B, C) as well as the development of spontaneous lung metastases (Fig. 2D, E). In addition, the therapeutic effectiveness was significantly (p<0.05) enhanced by anti-PD-L1 co-administration (Fig. 2B–E). In the SCC7 model, mITGB4-DC vaccination alone or in combination with anti-PD-L1 inhibited local tumor growth (Fig. 2F, G).
Figure 2.
mITGB4-DC vaccine in ITGB4-targeted immunotherapy. A: Protocol of the 4T1 and SCC7 therapeutic minimal tumor models. Treatment groups were as follows: PBS, mITGB4-DC vaccine, anti-PD-L1, and the combination of mITGB4-DC vaccine and anti-PD-L1(n=5). In 4T1 model, the administration of mITGB4-DC vaccine inhibited both local tumor growth (B, C) and spontaneous lung metastases (D, E); co-administration of anti-PD-L1 mAb significantly enhanced the therapeutic effectiveness (B-E). Pictures of local tumors (C) and spontaneous lung metastases (E) from treated animals bearing 4T1 tumors are shown. F, G: mITGB4-DC vaccine and/or anti-PD-L1 inhibited the local tumor growth in SCC7 therapeutic minimal tumor. H: SCC7 experimental lung metastasis model using mITGB4-DC vaccine. I, J: mITGB4-DC vaccine and/or anti-PD-L1 significantly inhibited lung metastases in SCC7 experimental lung metastasis model. The experiment of 4T1 or SCC7 therapeutic minimal models was repeated four times.
Unlike 4T1, SCC7 does not spontaneously metastasize when implanted subcutaneously. In order to determine the effect of mITGB4-DC vaccine on tumor metastases in this model, we experimentally induced lung metastases by i.v. injection (41–43). SCC7 cells were injected via tail vein into syngeneic C3H mice, followed by mITGB4-DC vaccination with or without anti-PD-L1 administration (Fig. 2H). As shown in Fig. 2I, J, the mITGB4-DC vaccine significantly inhibited lung metastases, and this inhibition was significantly enhanced by anti-PD-L1 administration.
mITGB4 BiAb-armed TDLN T cell transfer inhibited local tumor growth and lung metastases
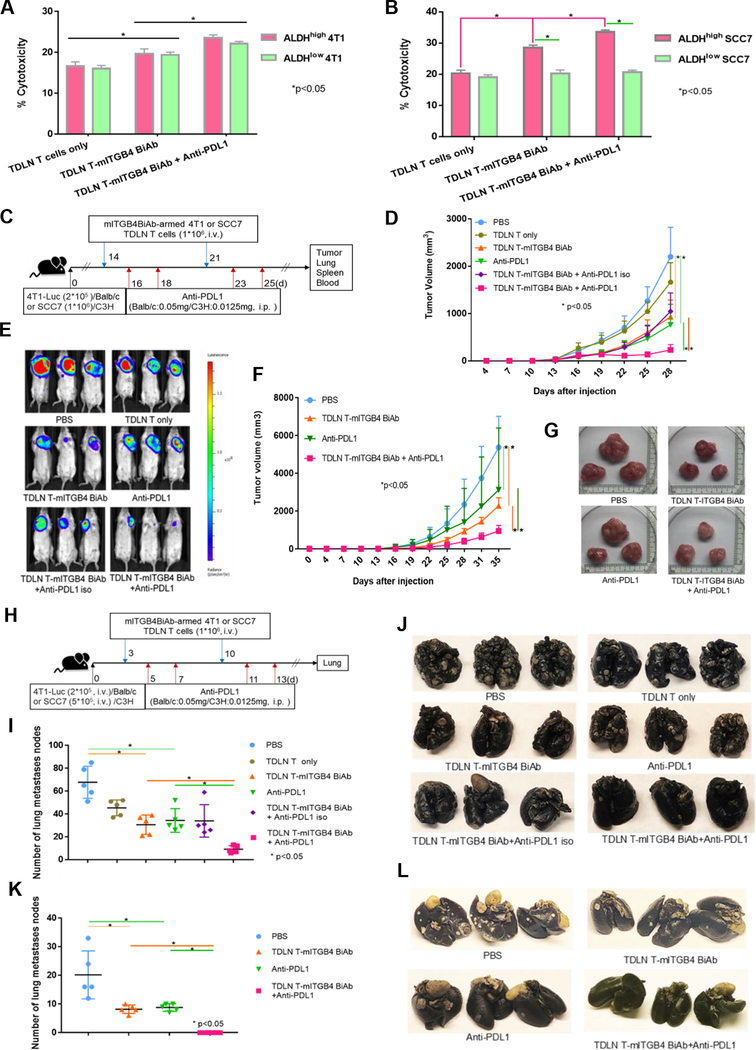
To confirm that ITGB4 can serve as an effective target for cancer immunotherapy, we evaluated a different immunological strategy to target ITGB4. To this end, we generated murine anti-CD3/anti-ITGB4 bispecific antibodies (mITGB4 BiAb) and tested the direct cytotoxicity of activated mITGB4 BiAb-armed TDLN T cells on ALDHhigh and ALDHlow 4T1 or SCC7 cells in vitro. mITGB4 BiAb-armed TDLN T cells killed ALDHhigh and ALDHlow 4T1 cells similarly, higher than non-armed TDLN T cells, and the killing could be enhanced by the addition of anti-PD-L1 in culture (Fig. 3A). However, mITGB4 BiAb-armed TDLN T cells killed more ALDHhigh SCC7 cells than ALDHlow SCC7 cells, and the killing was significantly enhanced by anti-PD-L1 as well (Fig. 3B).
Figure 3.
ITGB4-targeted immunotherapy using mITGB4 BiAb-armed TDLN T cells via adoptive transfer. A, B: In vitro CTL activity of TDLN T cells co-cultured with 4T1 (A) and SCC7 (B) ALDHhigh vs. ALDHlow populations. C: Experimental protocol of the therapeutic 4T1 and SCC7 established (day 14) tumor models by adoptive transfer of mITGB4 BiAb-armed T cells. Treatment groups included: PBS, TDLN T cells, TDLN T-mITGB4 BiAb, anti-PD-L1, TDLN T-mITGB4 BiAb + anti-PD-L1 isotype, and TDLN T-mITGB4 BiAb + anti-PD-L1(n=5). D: TDLN T-mITGB4 BiAb significantly inhibited tumor growth in therapeutic 4T1 model, which was enhanced by anti-PD-L1. E: Luminescence imaged by IVIS at the end of experiments to display tumor size in treated mice of the therapeutic 4T1 model. F, G: In therapeutic SCC7 model, TDLN T-mITGB4 BiAb inhibited tumor growth, and co-injection and anti-PD-L1 significantly boosted the therapeutic effectiveness. H: Experimental lung metastasis protocol. I-L: In both 4T1 and SCC7 experimental lung metastasis models, TDLN T-mITGB4 BiAb significantly suppressed the metastases. Statistics (I) and representative photos (J) of 4T1 tumors from treated Balb/c mice are shown. Statistics (K) and representative photos (L) of SCC7 tumors from treated C3H mice are shown. The therapeutic experiments on 4T1 and SCC7 local tumors were repeated three times.
Based on these in vitro data, we proceeded to test mITGB4 BiAb-armed TDLN T cell transfer in mouse models bearing established (14 day) tumors (Fig. 3C). Local tumor growth was significantly reduced after mITGB4 BiAb-armed TDLN T cell transfer in the 4T1 (Fig. 3D, E) and the SCC7 (Fig. 3F, G) models. Combining anti-PD-L1 therapy resulted in significant reduction in local tumor burden compared to mITGB4 BiAb-T cells transfer alone (Fig. 3D–G).
We then tested experimental lung metastases of 4T1 and SCC7 tumors treated with mITGB4 BiAb-armed TDLN T cells. 4T1-Luc cells and SCC7 cells were injected via tail vein into syngeneic Balb/c mice and C3H mice respectively, followed by treatments with mITGB4 BiAb-TDLN T cells and/or anti-PD-L1 (Fig. 3H). Lung metastases were significantly reduced by mITGB4 BiAb-TDLN T cell transfer and this therapeutic effectiveness was significantly augmented by combining with anti-PD-L1 mAb in both 4T1 (Fig. 3I, J) and SCC7 (Fig. 3K, L) models.
Both mITGB4-DC vaccination and mITGB4 BiAb-armed TDLN T cell transfer specifically targeted ITGB4
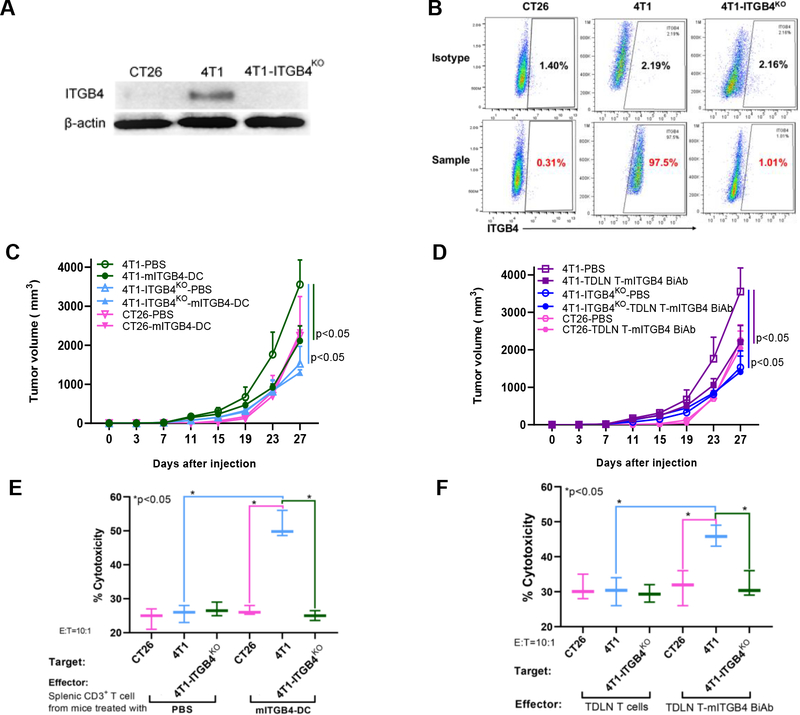
To determine the specificity of ITGB4 targeting we generated ITGB4 knockout 4T1 (4T1-ITGB4KO) cells and in addition assessed treatment effects in CT26 murine colon carcinoma cells, which do not express ITGB4. Lack of ITGB4 expression in these cells was confirmed by western blot (Fig. 4A) and flow cytometry (Fig. 4B). We treated 4T1, 4T1-ITGB4KO or CT26 tumor-bearing mice with mITGB4-DC vaccine in minimal tumor model (similar to Fig. 2A) or with TDLN T-mITGB4 BiAb in established tumor model (similar to Fig. 3C). Compared to PBS group, mITGB4-DC vaccine (Fig. 4C) or TDLN T-mITGB4 BiAb (Fig. 4D) significantly (p<0.05) inhibited the tumor growth of 4T1 cells. However, mITGB4-DC vaccine (Fig. 4C) or TDLN T-mITGB4 BiAb (Fig. 4D) did not affect growth of 4T1-ITGB4KO cells or CT26 cells. Interestingly, knockout of ITGB4 significantly (p<0.05) reduced the tumor growth of 4T1 cells even without treatment (Fig. 4C, D). This suggests that ITGB4 plays an important biological role in these cells.
Figure 4.
Both mITGB4-DC vaccination and mITGB4 BiAb-armed TDLN T cell transfer specifically target ITGB4. A, B: Confirmation of ITGB4 knockout in 4T1-ITGB4KO cells and lack of expression of ITGB4 in CT26 cells via western blot (A) and flow cytometry (B). C, D: ITGB4-targeted cancer immunotherapies specifically target ITGB4 in vivo. mITGB4-DC (C) and TDLN T-mITGB4 BiAb (D) significantly inhibited the tumor growth of 4T1 cells, but not 4T1-ITGB4KO or ITGB4-negative CT26 tumors. Experimental groups included 4T1, 4T1-ITGB4KO or CT26 tumor-bearing mice treated with PBS vs. mITGB4-DC vaccine in minimal tumor model (C) or treated with PBS vs. TDLN T-mITGB4 BiAb in established tumor model (D). E: CD3+ T cells isolated from the spleens of Balb/c mice treated with mITGB4-DC vaccine killed ITGB4-expressing 4T1 cells specifically in vitro. F: TDLN T-mITGB4 BiAb mediated significant greater cytotoxicity to ITGB4-expressing 4T1 cells than to 4T1-ITGB4KO cells or ITGB4-negative CT26 cells in vitro.
To confirm the specific targeting of ITGB4 by mITGB4-DC vaccine or TDLN T-mITGB4 BiAb, we performed in vitro killing experiments using splenic CD3+ T cells from animals subjected to vaccination or the mITGB4 BiAb-armed TDLN T cells. CD3+ T cells were isolated from the spleens of Balb/c mice treated with PBS or mITGB4-DC vaccine respectively. After activation and expansion as described in Materials and Methods, these T cells were co-cultured with 4T1, 4T1-ITGB4KO or CT26 cells for 6 hours respectively. Splenic CD3+ T cell-mediated cytotoxicity was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI). We found (Fig. 4E) that while splenic CD3+ T cell from PBS-treated mice killed 4T1, 4T1-ITGB4KO and CT26 cells equally, splenic CD3+ T cell from mITGB4-DC vaccine-treated mice killed 4T1 cells significantly (p<0.05) more than splenic CD3+ T cell from PBS-treated mice, and splenic CD3+ T cell from mITGB4-DC vaccine-treated mice killed 4T1 cells significantly (p<0.05) more than 4T1-ITGB4KO and CT26 cells. These data thus confirm that mITGB4-DC vaccine induced T cell targeting of ITGB4, and this targeting is ITGB4 specific (Fig. 4E).
In separate experiments, non-armed TDLN T cells and mITGB4 BiAb-armed TDLN T cells were co-cultured with 4T1, 4T1-ITGB4KO or CT26 cells and killing assays were performed by harvesting the co-cultured supernatants to measure lactate dehydrogenase. We found (Fig. 4F) that while non-armed TDLN T cells killed 4T1, 4T1-ITGB4KO and CT26 cells equally, armed TDLN T-mITGB4 BiAb killed 4T1 significantly (p<0.05) more than non-armed TDLN T cells, and TDLN T-mITGB4 BiAb cells killed 4T1 cells significantly (p<0.05) more than their killing of 4T1-ITGB4KO and CT26 cells. These data thus further confirm that TDLN T-mITGB4 BiAb cells target ITGB4-expressing tumor cells, and this targeting is ITGB4 specific (Fig. 4F).
Together, our data demonstrated that both mITGB4-DC vaccine and mITGB4 BiAb-armed T cell adoptive transfer specifically target ITGB4-expressing tumor cells in vitro and in vivo.
ITGB4-targeted immunotherapies conferred host immunity against ALDHhigh CSCs and ALDHlow non-CSCs
To understand the mechanism(s) underlying therapeutic efficacy, we harvested host spleens and immune plasma at the end of the therapeutic experiments from 4T1 and SCC7 models respectively, and evaluated host T and B cell responses.
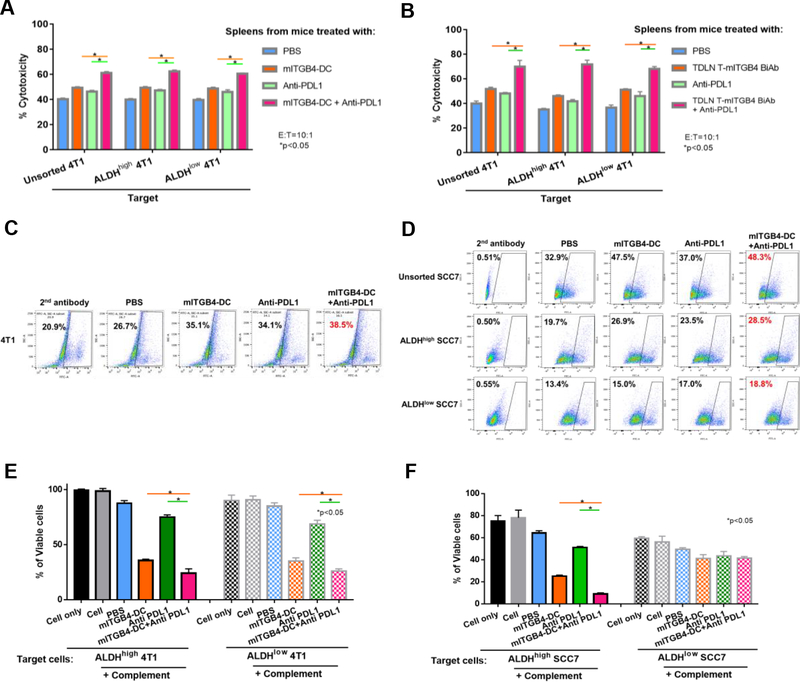
Splenic CD3+ T cells isolated from the 4T1-bearing mice (from Fig. 2B) treated with mITGB4-DC vaccine + anti-PD-L1 mAb killed unsorted, ALDHhigh, and ALDHlow 4T1 cells in vitro, significantly (p<0.05) higher than CD3+ T cells isolated from mice subjected to each mono-therapy or PBS control (Fig. 5A). Of note, similar killing was observed in ALDHhigh 4T1 cells and ALDHlow 4T1 cells. In addition, splenic CD3+ T cells isolated from the 4T1-bearing mice (from Fig. 3D) treated with mITGB4 BiAb-armed TDLN T cells + anti-PD-L1 mAb killed unsorted, ALDHhigh, and ALDHlow 4T1 cells in vitro, significantly (p<0.05) higher than CD3+ T cells isolated from the mice subjected to each mono-therapy and PBS control (Fig. 5B). Again, such killing was comparable against ALDHhigh 4T1 cells vs. ALDHlow 4T1 cells.
Figure 5.
ITGB4-targeted immunotherapies elicited ITGB4-specific host immune responses against CSCs as well as non-CSCs. A, B: Splenetic T cells harvested from mice treated with ITGB4-DC vaccine (A) or mITGB4 BiAb-armed TDLN T cells (B) mediated significant cytotoxicity against 4T1 cells. Data were replicated in a second experiment for both ITGB4-DC vaccine and mITGB4 BiAb-armed TDLN T cell adoptive transfer experiments. C-F: ITGB4-targeted immunotherapy conferred significant host anti-ITGB4 humoral immunity in both 4T1 and SCC7 models. Immune plasma was collected from treated mice and IgG was quantified using ELISA. C: Plasma harvested from treated mice-bearing 4T1 tumor as indicated bound to 4T1 cells. D: Plasma IgG induced by ITGB4-DC vaccine bound to unsorted, ALDHhigh, and ALDHlow SCC7 cells to different extend. E: Cytotoxic effects of immune plasma via CDC in 4T1 model. Plasma harvested from animals-bearing 4T1 tumor subjected to treatment was incubated with ALDHhigh 4T1 or ALDHlow 4T1 cells for 1 hour followed by culturing in the presence of rabbit complement for another 1 hour. Lower percentage of viable cells at the end of incubation indicates more cell lysis. F: Similar CDC assay as in E was tested in the SCC7 model. Data were repeated twice.
We also accessed the induction of humoral anti-CSC immunity by ITGB4-DC based immunotherapy. To this end, we collected immune plasma from the peripheral blood of 4T1 (from Fig. 2B) or SCC7 (from Fig. 2F)-bearing mice subjected to treatments. We first accessed the specificity of ITGB4-DC vaccine-primed antibody by binding assays of the plasma to tumor cells. Immune plasma from Balb/c mice that received the mono-treatment of ITGB4-DC vaccine or anti-PD-L1 bound to 4T1 cells (35.1% and 34.1% respectively) more effectively than the immune plasma collected from PBS-treated (26.7%) (Fig. 5C). The binding activity was enhanced by the ITGB4-DC vaccine + anti-PD-L1 mAb treatment (38.5%) (Fig. 5C). In the SCC7 model, immune plasma harvested from ITGB4-DC vaccinated mice bound to unsorted, ALDHhigh and ALDHlow SCC7 cells more than the immune plasma harvested from anti-PD-L1 treated mice or from PBS-treated controls, respectively, and the combination treatment did not show additive binding effect (Fig. 5D). Notably, equal amounts of IgG in the immune plasma collected from the ITGB4-DC vaccine + anti-PD-L1-treated C3H mice bound to unsorted SCC7 (48.9%) more than its binding to ALDHhigh and ALDHlow SCC7. However, the binding to ALDHhigh SCC7 CSCs (28.5%) was higher than that to ALDHlow SCC7 non-CSCs (18.8%) (Fig. 5D).
To examine the consequence of the binding of immune plasma to tumor cells, we utilized antibody and CDC assays in both 4T1 and SCC7 models. ITGB4-DC vaccine-primed immune plasma killed both ALDHhigh and ALDHlow 4T1 cells significantly, and the ITGB4-DC vaccine plus anti-PD-L1-primed immune plasma killed both ALDHhigh and ALDHlow 4T1 cells significantly higher than the immune plasma collected from all the other groups (Fig. 5E). In the SCC7 model, ITGB4-DC vaccine-primed immune plasma killed ALDHhigh SCC7 cells more than ALDHlow SCC7 cells, and the ITGB4-DC vaccine plus anti-PD-L1-primed immune plasma killed ALDHhigh significantly more than the immune plasma collected from all the other groups against ALDHhigh cells (Fig. 5F). Together, these experiments demonstrated that ITGB4-targeted immunotherapies induced host immunity against ALDHhigh CSCs as well as non-CSCs.
ITGB4-targeted immunotherapy reduced the proportion of ALDHhigh and ITGB4high cells in residual tumors and reduced the tumor initiating capacity of these cells
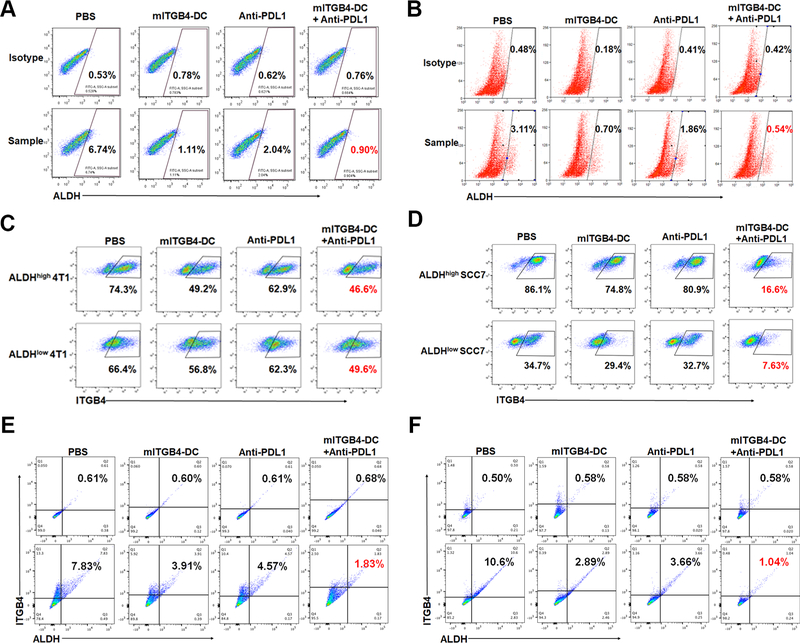
The experiments described above suggested that ITGB4 immunotherapy could target ALDHhigh CSCs that express this protein. To confirm this, we determined the proportion of ALDHhigh cells and ITGB4high cells in residual tumors post treatment. After treatment with ITGB4-DC vaccine, the ALDHhigh populations was reduced in both 4T1 (from 6.74% to 1.11%) (Fig. 6A) and SCC7 (from 3.11% to 0.70%) (Fig. 6B). This number was further decreased by co-treatment with anti-PD-L1 to 0.90% for 4T1 (Fig. 6A) and to 0.54% for SCC7 (Fig. 6B). In parallel, the frequencies of ITGB4-expressing ALDHhigh and ALDHlow 4T1 cells (Fig. 6C) and ALDHhigh and ALDHlow SCC7 cells (Fig. 6D) in the mITGB4-DC vaccine plus anti-PD-L1 treated tumors were lower than those in mono-therapy groups and PBS controls.
Figure 6.
ITGB4-targeted immunotherapy reduced the number of ALDHhigh/ITGB4high CSCs. Flow cytometric proportions of ALDHhigh 4T1 (A) and SCC7 (B) cells in residual tumor subjected to treatment as indicated. Frequencies of ITGB4-expressing ALDHhigh and ALDHlow 4T1 (C) and SCC7 (D) cells from residual tumors treated as indicated. The co-expression of ALDH and ITGB4 in residual 4T1 tumors (E) and SCC7 tumors (F). The number of ALDHhigh/ITGB4high residual 4T1 (E) and SCC7 (F) cells were significantly reduced, particularly after combined treatment of DC-vaccine plus anti-PD-L1. Results of flow cytometry were representative of two separate experiments performed.
We examined the change of ALDHhighITGB4high cells in the treated residual tumors by double staining, and found that the percentage of ALDHhighITGB4high cells was markedly reduced from 7.83% and 10.6% in the control (PBS) group of 4T1 tumor (Fig. 6E) and SCC7 tumor (Fig. 6F) to 1.83% and 1.04% respectively after combination treatment with mITGB4-DC vaccine plus anti-PD-L1 administration.
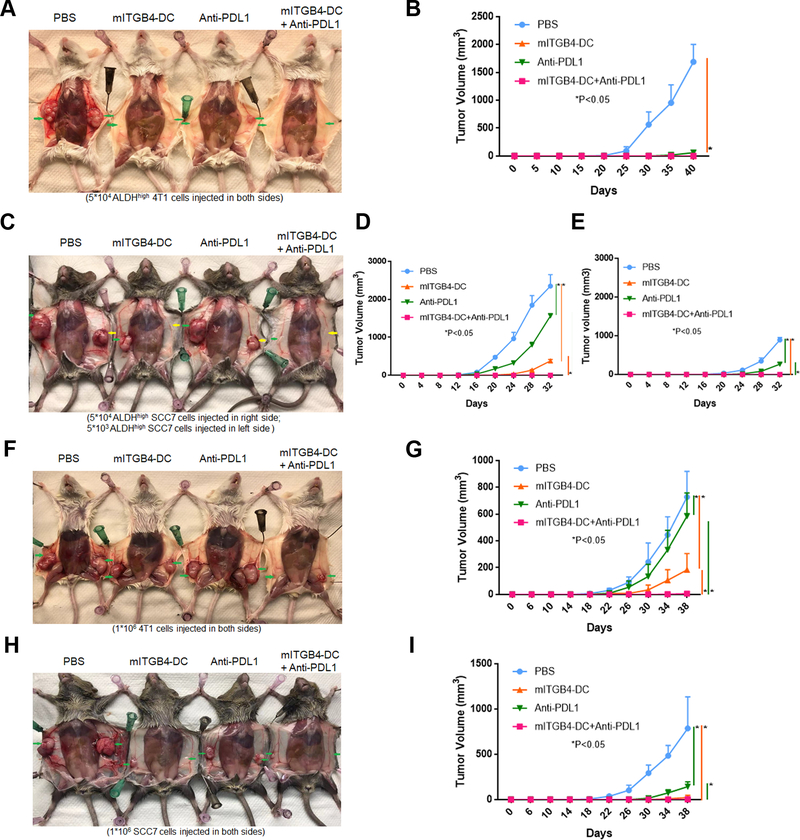
To assess if the immunotherapies also reduced the tumor initiating capacity of 4T1 and SCC7 CSCs, we isolated ALDHhigh 4T1 (Fig. 6A) and SCC7 (Fig. 6B) cells from the residual tumors subjected to mITGB4-DC vaccine and/or anti-PD-L1 treatment and injected them into normal syngeneic Balb/c and C3H mice respectively to test their tumorigenicity. We found that while 50,000 ALDHhigh 4T1 cells from PBS treated control mice formed large tumors in 40 days, equal number of ALDHhigh 4T1 cells from mITGB4-DC vaccination alone or combination treatment with mITGB4-DC vaccination + anti-PD-L1 failed to form tumors within the same period of time (Fig. 7A, B). In the SCC7 model, 50,000 ALDHhigh SCC7 cells from mITGB4-DC vaccine +/− anti-PD-L1 treated mice grew significantly slower than that of other groups (Fig. 7C, D). Similar results were observed at lower numbers (5,000) of ALDHhigh cells injected (Fig. 7C, E).
Figure 7.
ITGB4-targeted immunotherapy reduced the tumorigenicity of CSCs and residual tumors. A-E: Tumor growth of residual ALDHhigh CSCs in syngeneic mice was significantly polarized in the 4T1 model (A, B) and in the SCC7 model (C-E). A: 5 × 104 residual ALDHhigh 4T1 cells was injected in both sides of Balb/c mice. C: 5 × 104 residual ALDHhigh SCC7 cells were injected in right side of C3H mice (Green arrows), while 5 × 103 residual ALDHhigh SCC7 cells were injected to the left side (Yellow arrows). B, D, E: Tumor growth curves resulted from injected different doses of ALDHhigh tumor cells from treated residual tumors: 5 × 104 residual ALDHhigh 4T1 cells injected to Balb/c mice (B), 5 × 104 residual ALDHhigh SCC7 cells injected to C3H mice (D), and 5 × 103 residual ALDHhigh SCC7 cells injected to C3H mice (E). F-I tested the tumorigenicity of the whole residual tumor cells. F, H: Photos of representative tumors grown from injected whole residual tumor cells subjected to treatment as indicated. 1 × 106 residual 4T1 (F) or SCC7 (H) cells were injected in both sides of syngeneic Balb/c and C3H mice respectively. Tumor growth curves from whole residual 4T1 (G) and SCC7 (I) are shown.
To confirm that integrin β4-targeted cancer immunotherapy has the ability to reduce the tumorigenicity of the residual tumor cells, we also tested the tumor initiating capacity of the whole residual 4T1 and SCC7 cells. When 1 × 106 residual 4T1 or SCC7 cells from treated mice were injected into both sides of syngeneic Balb/c and C3H mice respectively, equal number of the residual tumor cells from combined mITGB4-DC vaccine + anti-PD-L1 treated mice formed significantly smaller tumors than that from PBS or mono-therapy treated mice (Fig. 7F, G for 4T1; Fig. 7H, I for SCC7). Together, these data show that ITGB4-targeted immunotherapy reduced both the number of ALDHhigh and ITGB4high cancer stem cells in residual 4T1 and SCC7 tumors and their ability to form tumors, as well as suppressing the tumorigenicity of the whole residual tumors subjected to ITGB4-targeted immunotherapy.
Lack of systemic toxicity form ITGB4-targeted therapies
To address the potential toxicity as well as off target effects of engaging ITGB4, we performed a group of experiments, including: (1) Histopathological analysis of internal organs and glands from treated mice; (2) Hematologic analysis, and (3) Access of Insulin-like growth factor-1 (IGF-1) levels following treatment. As shown in Supplemental data Fig. S1 and Tab. S1, these results as well as lack of weight loss in treated animals vs. controls demonstrate the safety of this approach.
Discussion
In this study, we investigated the immunological targeting of ITGB4 utilizing two different approaches: mouse ITGB4 protein as antigen to pulse DCs or coating TDLN T cells with mouse anti-CD3/anti-ITGB4 BiAb to prepare mITGB4 BiAb-T cells for targeted adoptive transfer. Data obtained from applying two distinct immunological methods confirmed that ITGB4 can serve as an effective target for cancer immunotherapies.
4T1 cells highly expressed ITGB4 in unsorted, ALDHhigh, and ALDHlow cells. The 4T1 mammary carcinoma is highly tumorigenic and invasive and can spontaneously metastasize from the primary tumor in the mammary gland to multiple distant sites including lymph nodes, blood, liver, lung, brain, and bone (43). In the 4T1 minimal tumor model, mITGB4-DC vaccine inhibited both local tumor growth and spontaneous pulmonary metastases. In addition, mITGB4 BiAb armed T cell adoptive transfer suppressed tumor growth of established macroscopic 4T1 tumor. In an experimental lung metastasis model, BiAb-armed T cell adoptive transfer also inhibited lung metastases. Together, these data indicate that both mITGB4-DC vaccine and mITGB4 BiAb-armed T cell adoptive transfer can suppress local tumor growth and metastasis of a tumor with high expression of ITGB4 on both ALDHhigh CSCs and bulk tumor cells.
In contrast, SCC7 cells expressed ITGB4 on the ALDHhigh cells significantly more than that on the ALDHlow cells. mITGB4-DC vaccine and mITGB4 BiAb-armed T cell adoptive transfer effectively suppressed the local tumor growth as well as the experimental metastasis of SCC7, suggesting that successful immunological targeting of ITGB4 is able to target ITGB4-expressing CSCs that mediate tumor metastasis, as well as ITGB4-expressing bulk tumor cells.
By using 4T1-ITGB4KO cells and CT26 cells lacking ITGB4 expression as negative controls, our data confirmed the specificity of ITGB4 targeting. Interestingly, knockout of ITGB4 significantly reduced the tumor growth of 4T1 cells. This suggests that ITGB4 plays an important biological role and supports a recent report that knockout of ITGB4 reduced the migration and invasion of HCT116 colorectal cancer cells (44). Together, these data strongly support the conclusion that ITGB4 represents an attractive target for cancer immunotherapy.
We also examined the ability of ITGB4 immunotherapy to augment the efficacy of anti-PD-L1 immune checkpoint blockade since PD-L1 was expressed in our tumor cell models. Furthermore, Wang et al reported that PD-L1 can promote the growth and metastasis of tumor by activating the ITGB4/SNAI1/SIRT3 signaling pathway, suggesting another potential mechanism of synergy between these pathways (45). Our data indicate that anti-PD-L1 antibody significantly augmented the therapeutic efficacy of ITGB4-DC vaccine and ITGB4 BiAb-armed T cell adoptive transfer in both 4T1 and SCC7 models.
We found in this study that ITGB4-targeted immunotherapies could elicit ITGB4 specific cellular immune responses. CTLs generated from the spleens of treated 4T1-bearing mice specifically killed ITGB4-expressing 4T1 tumor cells. In addition, ITGB4-DC vaccine conferred significant host anti-ITGB4 humoral immunity. ITGB4-DC vaccine-primed immune plasma specifically bound to 4T1 and SCC7 cells resulting in significant killing of ITGB4-expressing 4T1 and SCC7 cells. Toxicity remains a major concern for any kind of immunotherapy. ITGB4 is expressed in the skin and its expression correlated with epidermal lysis bullosa (46). However, in this study we did not detect systemic toxicity associated with ITGB4 immunotherapy in our mouse models.
In both approaches of ITGB4-targeted immunotherapy we observed a significantly reduced number of ITGB4-expressing 4T1 cells and SCC7 cells. In parallel, immunotherapeutic targeting of ITGB4 significantly reduced the ALDHhigh 4T1 cells and ALDHhigh SCC7 CSCs in the residual tumors subjected to immunotherapy. Importantly, the residual ALDHhigh 4T1 cells and SCC7 CSCs demonstrated significantly reduced tumorigenicity. In addition, significantly reduced tumorigenicity of whole residual tumors subjected to ITGB4-targeted immunotherapy confirmed that ITGB4-targeted immunotherapy could reduce both the number and the tumorigenicity of the residual 4T1 and SCC7 tumors. Since ITGB4 is expressed in CSCs across a number of tumor types, immunologic targeting of this protein has significant therapeutic potential.
Supplementary Material
Acknowledgments
This work was partially supported by 1R56DE024385-01 (Li Q), Medimmune Inc. (Wicha MS & Li Q), Guangzhou Improve Medical Instruments Co., Ltd. (Li Q), Gillson Longenbaugh Foundation (Chang AE & Li Q), R01 CA 140314 (Lum LG), R01 CA 182526 (Lum LG), and the University of Michigan MICHR Grant UL1TR000433 (Li Q), the NCI research grant 1R-35CA 197585 (Wicha MS), as well as the NCI research grant P30CA046592 (Moore T).
Footnotes
Conflict of Interest: Elaine M. Hurt is a full-time employee of MedImmune. Lawrence G. Lum is co-founder of Transtarget, Inc and Member of SAB for Rapa Therapeutics. No other potential conflicts of interests were disclosed.
References
- 1.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature Reviews Clinical Oncology 2017;14(10):611–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Reports 2017;50(3):117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 2014;28(12):1101–7, 1110. [PubMed] [Google Scholar]
- 4.Feng D, Wang N, Hu J, Li W. Surface markers of hepatocellular cancer stem cells and their clinical potential. Neoplasma 2014;61(5):505–13. [DOI] [PubMed] [Google Scholar]
- 5.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110(6):673–87. [DOI] [PubMed] [Google Scholar]
- 6.Kajiji S, Tamura RN, Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. Embo Journal 1989;8(3):673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews Cancer 2010;10(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006;126(3):489–502. [DOI] [PubMed] [Google Scholar]
- 9.Muthuswamy SK. ErbB2 makes beta 4 integrin an accomplice in tumorigenesis. Cell 2006;126(3):443–5. [DOI] [PubMed] [Google Scholar]
- 10.Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol 2008;226(2):178–91. [DOI] [PubMed] [Google Scholar]
- 11.Muranen T, Iwanicki MP, Curry NL, Hwang J, DuBois CD, Coloff JL, et al. Starved epithelial cells uptake extracellular matrix for survival. Nature Communications 2017;8:13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JG, Ahn JH, Jin KT, Ho LJ, Choi JH. Mutant p53 promotes ovarian cancer cell adhesion to mesothelial cells via integrin beta4 and Akt signals. Sci Rep 2015;5:12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng C, Zhang ZG, Chen WX, Luo HP, Song J, Dong W, et al. An integrin beta4-EGFR unit promotes hepatocellular carcinoma lung metastases by enhancing anchorage independence through activation of FAK-AKT pathway. Cancer Letters 2016;376(1):188–96. [DOI] [PubMed] [Google Scholar]
- 14.Ephstein Y, Singleton PA, Chen W, Wang L, Salgia R, Kanteti P, et al. Critical role of S1PR1 and integrin beta4 in HGF/c-Met-mediated increases in vascular integrity. Journal of Biological Chemistry 2013;288(4):2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai YL, Lai IR, Peng YJ, Ding ST, Shen TL. Activation of focal adhesion kinase through an interaction with beta4 integrin contributes to tumorigenicity of colon cancer. Febs Letters 2016;590(12):1826–37. [DOI] [PubMed] [Google Scholar]
- 16.Cooper J, Giancotti FG. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019;35(3):347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic MM, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527(7578):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami K, Fujita Y, Kato T, Mizutani K, Kameyama K, Tsumoto H, et al. Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. International Journal of Oncology 2015;47(1):384–90. [DOI] [PubMed] [Google Scholar]
- 19.Masugi Y, Yamazaki K, Emoto K, Effendi K, Tsujikawa H, Kitago M, et al. Upregulation of integrin beta4 promotes epithelial-mesenchymal transition and is a novel prognostic marker in pancreatic ductal adenocarcinoma. Laboratory Investigation 2015;95(3):308–19. [DOI] [PubMed] [Google Scholar]
- 20.Brendle A, Lei H, Brandt A, Johansson R, Enquist K, Henriksson R, et al. Polymorphisms in predicted microRNA-binding sites in integrin genes and breast cancer: ITGB4 as prognostic marker. Carcinogenesis 2008;29(7):1394–9. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka T, Otero J, Chen Y, Kim YM, Koutcher JA, Satagopan J, et al. beta4 Integrin signaling induces expansion of prostate tumor progenitors. Journal of Clinical Investigation 2013;123(2):682–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, et al. Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci U S A 2017;114(12):E2337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci U S A 2019;116(15):7353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. Cancer stem cells: The root of tumor recurrence and metastases. Seminars in Cancer Biology 2017;44:10–24. [DOI] [PubMed] [Google Scholar]
- 25.Chang JC. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore) 2016;95(1 Suppl 1):S20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1(5):555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince M, Zhou L, Moyer JS, Tao H, Lu L, Owen J, et al. Evaluation of the immunogenicity of ALDH(high) human head and neck squamous cell carcinoma cancer stem cells in vitro. Oral Oncology 2016;59:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Research 2012;72(7):1853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. OncoImmunology 2015;4(3):e990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE, et al. Therapeutic Efficacy of Cancer Stem Cell Vaccines in the Adjuvant Setting. Cancer Research 2016;76(16):4661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lum LG, Thakur A. Targeting T cells with bispecific antibodies for cancer therapy. Biodrugs 2011;25(6):365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur A, Rathore R, Kondadasula SV, Uberti JP, Ratanatharathorn V, Lum LG. Immune T cells can transfer and boost anti-breast cancer immunity. OncoImmunology 2018;7(12):e1500672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lum LG, Thakur A, Al-Kadhimi Z, Colvin GA, Cummings FJ, Legare RD, et al. Targeted T-cell Therapy in Stage IV Breast Cancer: A Phase I Clinical Trial. Clinical Cancer Research 2015;21(10):2305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, et al. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clinical Immunology 2013;149(1):156–68. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clinical Cancer Research 2011;17(15):4987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brattain MG, Strobel-Stevens J, Fine D, Webb M, Sarrif AM. Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Research 1980;40(7):2142–6. [PubMed] [Google Scholar]
- 37.Li Q, Lu L, Tao H, Xue C, Teitz-Tennenbaum S, Owen JH, et al. Generation of a novel dendritic-cell vaccine using melanoma and squamous cancer stem cells. J Vis Exp 2014(83):e50561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4–1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Research 2003;63(10):2546–52. [PubMed] [Google Scholar]
- 39.Thakur A, Lum LG, Mittal S. Bispecific Antibody Armed T Cells to Target Cancer Cells. Methods Mol Biol 2018;1722:117–26. [DOI] [PubMed] [Google Scholar]
- 40.Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Research 2011;71(9):3364–76. [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Zhang JJ, Huang XY. Mouse models for tumor metastasis. Methods Mol Biol 2012;928:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price JE. Spontaneous and experimental metastasis models: nude mice. Methods Mol Biol 2014;1070:223–33. [DOI] [PubMed] [Google Scholar]
- 43.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol 2001;Chapter 20:20–2. [DOI] [PubMed] [Google Scholar]
- 44.Choi S, Marco M, Chen C, Pelossof R, O’Rourke K, Smith J, et al. KRAS mutation is associated with upregulation of integrin beta-4 expression leading to tumor invasion in colorectal cancer. Journal of Clinical Oncology; 2019;37;576-. [Google Scholar]
- 45.Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, et al. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene 2018;37(30):4164–80. [DOI] [PubMed] [Google Scholar]
- 46.Nakano A, Pulkkinen L, Murrell D, Rico J, Lucky AW, Garzon M, et al. Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the beta 4 integrin gene (ITGB4) and genotype/phenotype correlations. Pediatric Research 2001;49(5):618–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.