Abstract
Context:
Patients with implantable cardioverter defibrillators (ICD) are at risk for multiple physical and psychological symptoms. Identification of specific symptom profiles associated with poor outcomes may elucidate novel strategies to enhance symptom management.
Objectives:
The objectives were to determine common symptoms following initial ICD implant, identify classes of individuals with similar symptom profiles, describe patient characteristics associated with different symptom profiles, and determine if symptom profiles at hospital discharge predicted outcomes 3 and 12 months post-implant.
Methods:
This was a secondary data analysis of a randomized controlled trial that compared Patient+Partner versus Patient-Only interventions designed to help patients manage symptoms, prepare for ICD shocks, and resume daily activities. Symptoms were measured with the Patient Concerns Assessment. Latent class regression analysis was used to identify symptom classes at baseline, 3- and 12-month follow-up. Associations between patient characteristics, class membership, and outcomes were examined using chi-square, analysis of variance, and Poisson regression.
Results:
The study included 301 patients (74% male, mean age 64±11.9 years). Three classes were identified: Multi-Symptom (N=119, 40%), Tired-Rundown (N=130, 43%), and Mostly Asymptomatic (N=52, 17%). Patients in the Multi-Symptom class were younger (59.9 years, p < 0.001), and reported more anxiety (p < 0.001) and depression (p < 0.01) than the other classes. Membership in the Multi-Symptom class predicted lower quality of life and nearly double the rate of hospitalizations after 12 months (p = 0.02, IRR 1.9).
Conclusion:
Evaluation of symptom profiles after ICD implantation offers a promising strategy for identifying patients at risk for poor health outcomes.
Keywords: implantable cardioverter defibrillator, symptom profile, quality of life
Introduction
Implantable cardioverter defibrillators (ICDs) reduce mortality for patients with a history of, or at high-risk for, life-threatening ventricular arrhythmias (1). In the United States, approximately 60,000 ICDs are implanted each year (2). Individuals who receive an ICD are heterogeneous in terms of their clinical histories and the range of their conditions and comorbidities (3), which are likely to influence self-reported symptoms. Patients who receive ICDs also share common physical and psychological symptoms (4), including anxiety, fatigue, and sleep disturbances (5, 6). Device-related fears and behavioral avoidance are reported, including fears related to being in crowds or public spaces (7), water (e.g. showers, boats) (7), as well as engaging in sexual activity (8). Psychological symptoms are associated with poor outcomes including reductions in quality of life, heart failure hospitalizations, and death (9, 10). Although psychoeducational interventions have been developed to address the psychological needs of patients post-ICD implant (4), most intervention research has been conducted with the general ICD population, and not specifically focused on patients with the highest symptom burden, at greatest risk for psychological distress, or with poor physical outcomes (6). In addition, the content addressing physical symptoms in interventions tends to be general and limited, possibly curtailing improvement in important patient-centered outcomes influenced by both physical and psychological symptoms, such as quality of life and rehospitalizations.
Identification of distinct symptom profiles among ICD recipients has the potential to uncover common and concurrent physical and psychological symptoms, thereby elucidating novel strategies to enhance psychoeducational and symptom management interventions. The purpose of this secondary analysis was to: 1) determine the most common symptoms experienced following initial ICD implantation from the time of hospital discharge to 12 months post implant, 2) identify classes of individuals with similar symptom profiles, 3) describe demographic/health characteristics associated with different symptom profiles, and 4) determine if symptom profiles at time of hospital discharge predict patient outcomes at 3 and 12 months after ICD implant.
Methods
The Primary Study
This is a secondary analysis of a 2-group blocked randomized controlled trial that compared a Patient + Partner (P+P) versus a Patient-Only (P-Only) intervention conducted between 2009 and 2015 (N = 301 patient/partner dyads). A complete description of the original trial is available elsewhere (11), and the trial is registered at clinicaltrials.gov (NCT 01252615). In brief, participants were enrolled within 24 hours of discharge from the hospital from 17 hospitals in the Pacific Northwest and Southeast U.S. Inclusion criteria were as follows: 1) initial ICD implant for primary or secondary prevention of sudden cardiac arrest; 2) intimate partner (spouse, lover or life partner) living at the same residence; 3) English language proficient; and 4) telephone access for 1-year after ICD implant. The exclusion criteria were: 1) clinical comorbidities that impaired cognitive and/or physical functioning; 2) Orientation-Memory-Concentration Test (Short BLESSED) score > 6 (12, 13); 3) age < 21 years; 4) Alcohol Use Disorders Identification Test (AUDIT-C) score > 4 for alcohol abuse (14); and 5) Alcohol, Smoking and Substance Involvement Screening Test (ASSIST 2.0) score > 4 for daily non-medical use of opiates or hallucinogens (15). The University of Washington Institutional Review Board approved the trial, and all participants provided written informed consent.
All participants received usual care from their healthcare providers and were randomized to one of two intervention conditions, stratified by ICD indication (primary or secondary) and Charlson Comorbidity Index (≤ 2 or > 2) (16). Stratification was used to ensure study groups balanced by ICD indication and to control for the possibility that comorbidity profiles may influence intervention effectiveness. Elements for each intervention were derived from Social Cognitive Theory (17) and from the earlier study, “Experiences of Recovery Following Sudden Cardiac Arrest” (18, 19). Intervention goals included management of physical symptoms, resumption of activities of daily living and exercise, management of anxiety and depression, preparation for ICD shocks and management, gathering of ICD care information, and understanding when to activate the emergency medical system. Patients in both intervention conditions received the same intervention content, the P-Only intervention addressed patients, whereas the P+P intervention integrated the partner into the patient recovery process.
The Present Secondary Analysis Sample.
Sample
Patients with an initial ICD implant were randomized to either of the two intervention conditions, and received the same patient intervention content. The presence of symptoms associated with other non-cardiac health problems was controlled as a result of the exclusion criteria (e.g. cognitive and/or physical impairment and alcohol/substance abuse). Consequently, for this analysis, patient participants were combined into a single group (150 patients from P+P and 151 from P-Only, total N=301).
Measures.
Sociodemographic information was collected at baseline and included age, sex, race/ethnicity, education level, employment status, and income. Health history information collected at baseline included ICD indication, left ventricular ejection fraction (LVEF), Charlson Comorbidity Index, comorbidities, and body mass index (BMI).
Symptoms.
The ICD Patient Concerns Assessment (20) evaluates the frequency of 32 symptoms experienced by individuals with an ICD. Each symptom is rated on a Likert-type scale ranging from 0 (not at all) to 7 (every day).
Psychological distress.
The State-Trait Anxiety Inventory (STAI) is an assessment of trait and state anxiety used to measure psychological distress. Only the 20 state anxiety questions were used for this study: scores of 40 or more reflect clinically significant anxiety (21). The STAI is known to be sensitive to changes in anxiety in the sudden cardiac arrest population (22). The Patient Health Questionnaire (PHQ-9) is a brief scale used to measure depressed mood and provide an index of depression severity: scores of 10 or more represent moderate to severe depression. The PHQ-9 is highly correlated (r = 0.84) with professional mental health assessments of depression severity (23, 24).
Physical activity.
To evaluate physical activity, each participant wore a Cyma StepWatch for 5 out of 7 days at baseline, and at 3-, 6-, and 12-month follow-ups. Total steps/day during the active day were averaged over the 5-day period.
General health-related quality of life.
The Short Form Health Survey (SF-36) was used to assess mental and physical health based on derived composite scales of mental (MCS) and physical (PCS) health. Higher scores represent better functioning (25, 26).
Healthcare use.
Participants self-reported their hospital admissions at each follow-up study assessment. Discharge diagnosis and length of stay were verified with medical records by study personnel.
Procedures.
Measures of symptoms, psychological distress, actigraphy, and quality of life were assessed at baseline (at time of hospital discharge following initial ICD implant), and again at 1, 3, 6, and 12 months. Symptoms reported at baseline, 3, and 12 months are reported in this study. The total number of verified hospitalizations that occurred at 3 and 12 months are reported in this study. Sociodemographic and health information, collected via medical record review, was collected at baseline.
Analysis.
Descriptive statistics (proportions, means) were used to describe patient characteristics. The 10 most commonly experienced symptoms were identified using proportions (N, %) to determine the number of individuals that experienced each symptom and means (mean, SD) to describe the average number of days per week that participants reported experiencing the symptom. Symptoms were ranked from most common to least common based on the proportion of individuals who experienced each symptom.
Latent class regression analysis (Mplus version 7.31) was used to identify groupings of individuals with similar symptom profiles. This analysis was conducted using the 10 most commonly reported physical and psychological symptoms from the baseline Patient Concerns Assessment; symptoms were recoded as dichotomized values (0/1). Rather than including all 32 symptoms, we used the most common symptoms because our goal was to identify common symptom profiles. The underpinning theoretical rationale was that identification of common symptom profiles would be best achieved by including symptoms that were both prevalent in the population and frequently experienced at the individual level. The 10 most common symptoms occurred in > 35% of the sample and had an average frequency above or near one day per week (Table 2). This approach also averted statistical issues due to data sparseness (27). Guided by the Symptom Management Model (28), we selected 7 demographic and health history variables most likely to influence the symptom experience. Thus, included as covariates in the latent class regression model were: intervention condition, age, gender, income, ICD indication, Charlson Comorbidity Index, and LVEF. Covariates were added to the latent class model one at a time, and those that were statistically significant (p < 0.05) were retained in the final adjusted model. The optimal number of classes was determined by theoretical and clinical interpretability combined with comparisons of the Akaike information criterion (AIC), sample-size adjusted Bayesian information criterion (BICa), and the Vuong-Lo-Mendell-Rubin likelihood ratio test (VLMR) model fit indices (29). Probabilities by class for each symptom variable were defined as either high (≥ 0.5) or low (< 0.5).
Table 2.
Symptom Means and Percent Reporting at Baseline, 3 Months, and 12 Months
| Baseline N=301 | 3 Months N=290 | 12 Months N=280 | ||||
|---|---|---|---|---|---|---|
| Symptom | Mean | N (%) | Mean | N (%) | Mean | N (%) |
| Tiredness | 4.2 (±2.2) | 282 (94%) | 3.31 (±2.3) | 249 (86%) | 3.21 (±2.3) | 237 (85%) |
| Feeling rundown | 2.81 (±2.4) | 225 (75%) | 1.99 (±2.2) | 178 (61%) | 2.12 (±2.2) | 184 (66%) |
| Interrupted sleep | 2.97 (±2.6) | 222 (74%) | 2.35 (±2.3) | 195 (67%) | 2.58 (±2.4) | 196 (70%) |
| Reduced sexual activity | 3.36 (±3.0) | 182 (60%) | 2.51 (±2.8) | 147 (51%) | 2.66 (±2.9) | 151 (54%) |
| Shortness of breath | 1.98 (±2.3) | 180 (60%) | 1.75 (±2.1) | 165 (57%) | 1.72 (±2.2) | 151 (54%) |
| Difficulty falling asleep | 1.81 (±2.2) | 166 (55%) | 1.59 (±2.1) | 148 (51%) | 1.62 (±2.2) | 135 (48%) |
| Feeling anxious in general | 1.57 (±2.0) | 158 (53%) | 1.1 (±1.9) | 112 (39%) | 1.12 (±1.8) | 108 (39%) |
| Pains in lower back | 1.81 (±2.5) | 138 (46%) | 1.95 (±2.5) | 149 (51%) | 1.97 (±2.5) | 148 (53%) |
| Short-term memory loss | 0.95 (±1.6) | 113(38%) | 0.99 (±1.6) | 116 (40%) | 1.22 (±1.8) | 126 (45%) |
| Dizziness/near-fainting | 0.92 (±1.6) | 111 (37%) | 0.72 (±1.3) | 93 (33%) | 0.77 (±1.4) | 95 (34%) |
| Ringing in ears | 1.53 (±2.6) | 106 (35%) | 1.51 (±2.5) | 102 (35%) | 1.59 (±2.6) | 104 (37%) |
| Constipation | 0.88 (±1.5) | 105 (35%) | 0.75 (±1.5) | 76 (26%) | 0.77 (±1.6) | 76 (27%) |
| Insomnia | 1.21 (±2.1) | 103 (34%) | 1.06 (±1.9) | 101 (35%) | 1.08 (±1.9) | 96 (34%) |
| Difficulty walking | 1.16 (±2.0) | 103 (34%) | 1.11 (±2.0) | 96 (33%) | 1.18 (±2.0) | 99 (35%) |
| Headaches | 0.73 (±1.4) | 93 (31%) | 0.71 (±1.4) | 93 (32%) | 0.80 (±1.4) | 102 (36%) |
| Palpitations/flip-flopping | 0.94 (±1.8) | 90 (30%) | 0.81 (±1.7) | 67 (27%) | 0.84 (±1.7) | 86 (31%) |
| Diarrhea | 0.77 (±1.5) | 91 (30%) | 0.70 (±1.3) | 91 (31%) | 0.69 (±1.4) | 78 (28%) |
| Heartburn | 0.69 (±1.5) | 81 (27%) | 0.77 (±1.4) | 91 (31%) | 0.71 (±1.3) | 89 (32%) |
| Fast pulse | 0.68 (±1.5) | 75 (25%) | 0.73 (±1.4) | 92 (32%) | 0.75 (±1.3) | 100 (36%) |
| Nausea | 0.59 (±1.3) | 73 (24%) | 0.44 (±1.2) | 51 (18%) | 0.62 (±1.4) | 62 (22%) |
| Abdominal pain | 0.49 (±1.1) | 68 (23%) | 0.47 (±1.1) | 61 (21%) | 0.53 (±1.3) | 63 (23%) |
| Long-term memory loss | 0.63 (±1.5) | 69 (23%) | 0.74 (±1.5) | 82 (30%) | 0.76 (±1.5) | 88 (31%) |
| Blurred vision | 0.56 (±1.3) | 65 (22%) | 0.53 (±1.3) | 65 (23%) | 0.61 (±1.3) | 75 (27%) |
| Angina/chest pain | 0.49 (±1.2) | 64 (21%) | 0.47 (±1.3) | 57 (20%) | 0.45 (±1.2) | 51 (18%) |
| Confusion | 0.49 (±1.2) | 61 (20%) | 0.59 (±1.3) | 70 (24%) | 0.61 (±1.2) | 75 (27%) |
| Sensitivity to light | 0.57 (±1.4) | 61 (20%) | 0.70 (±1.6) | 66 (23%) | 0.69 (±1.4) | 80 (29%) |
| Tremors/shaking of hands | 0.55 (±1.4) | 56 (19%) | 0.56 (±1.4) | 55 (19%) | 0.61 (±1.5) | 56 (20%) |
| Skin rash | 0.48 (±1.4) | 44 (15%) | 0.35 (±1.2) | 36 (12%) | 0.51 (±1.4) | 52 (19%) |
| Metallic taste in mouth | 0.28 (±1.0) | 32 (11%) | 0.24 (±0.8) | 31 (11%) | 0.23 (±0.9) | 25 (9%) |
| Halo vision | 0.2 (±0.8) | 28 (9%) | 0.23 (±0.9) | 28 (10%) | 0.34 (±1.0) | 40 (14%) |
| Vomiting | 0.12 (±0.5) | 20 (7%) | 0.16 (±0.8) | 18 (6%) | 0.18 (±0.8) | 21 (8%) |
| Fainting/passing out | 0.03 (±0.3) | 6 (2%) | 0.06 (±0.5) | 8 (3%) | 0.12 (±0.6) | 15 (5%) |
| Total Symptom Count | 10.9 (±6.0) | 10.4 (±6.8) | 10.9 (±7.4) | |||
Values are mean (± standard deviation) or N (%). Mean scores reflect the average number of days per week study participants reported feeling the symptom using scored response options, ranging from 0 = not at all to 7 = every day. N (%) reflects the number/percentage of participants that reported experiencing the symptom between 1 and 7 days per week. Total Symptom Count represents the mean number of symptoms experienced between 1 and 7 days per week out of the 32 symptoms on the Patient Concerns Assessment.
Health and demographic characteristics of the participants assigned to each of the three classes were compared using chi-square for dichotomous variables, and Kruskal-Wallis one-way analysis of variance for continuous and ordinal variables (SPSS V19). Consonant with the exploratory purpose of this work, unadjusted p-values < 0.05 were considered statistically significant (30). Chi-square was used to determine whether latent class membership differed by study intervention condition.
The influence of latent class membership on patient outcomes was examined. The ability of baseline latent class membership to predict mental health status (MCS) and physical health status (PCS) at 3 and at 12 months after ICD implant was examined using univariate analysis of variance. Poisson regression was used to determine if baseline latent class membership predicted frequency of hospitalization at 3 and 12 months post ICD implant. Using an approach similar to the latent class regression analysis, the same 7 demographic and health history variables were included as covariates in the outcome analyses (intervention condition, age, gender, income, ICD indication, Charlson Comorbidity Index, and LVEF). Covariates that were statistically significant (p < 0.05) when added to the model one at a time were retained in the final adjusted models.
Results
Sample Characteristics
Participants were primarily male (74%), ranging in age from 26 to 93 years, with a mean age of 64 (±11.9) years. The sample included more participants with an ICD for primary (60%) versus secondary (40%) prevention of sudden cardiac arrest. Primary prevention ICDs are implanted in individuals who are at risk for, but have not experienced, sustained ventricular arrhythmias or sudden cardiac arrest (e.g. individuals with ischemic heart disease or nonischemic cardiomyopathy with LVEF ≤ 35%). Additional sample characteristics are provided in Table 1.
Table 1.
Demographic and Health Characteristics
| Characteristic (N=301) | |
|---|---|
| Age, years | 64.1 (±11.9) |
| Female | 79 (26%) |
| Hispanic/Latino | 6 (2%) |
| Secondary | 121 (40%) |
| Comorbidity (CCI) | 2.3 (±1.5) |
| BMI | 29.6 (±6.2) |
| Left Ventricular Ejection Fraction | 34.1 (±14.3) |
| Atrial or Ventricular Arrhythmia | 208 (69%) |
| COPD | 56 (19%) |
| Thyroid Disease | 40 (13%) |
| Diabetes Mellitus | 85 (28%) |
| Renal Disease | 12 (4%) |
| Anxiety (STAI ≥ 40) | 67 (22%) |
| Depressive Disorder (PHQ-9 ≥ 10) | 50 (17%) |
| Total Steps/Day | 6,895 (±3,671) |
| Physical Health Status (SF-36 PCS) | 36.2 (±10.2) |
| Mental Health Status (SF-36 MCS) | 51.7 (±10.9) |
| Hospitalizations, 3 months | 0.22 (± 0.6) |
| Hospitalizations, 12 months | 0.56 (±1.1) |
Baseline values provided unless otherwise specified. Values are mean (± standard deviation) or N (%). ICD: implantable cardioverter defibrillator. CCI: Charlson Comorbidity Index. BMI: Body Mass Index. COPD: Chronic Obstructive Pulmonary Disease. SF-36: Short Form Health Survey. MCS: mental health composite scale. PCS: physical health composite scale.
Description of Symptoms
The 10 most common symptoms immediately following an initial ICD implant were tiredness, feeling rundown, interrupted sleep, reduced sexual activity, shortness of breath, difficulty falling asleep, feeling anxious, pains in the lower back, short-term memory loss, and dizziness/near-fainting (Table 2). Tiredness was the most common symptom reported at baseline, 3 and 12 months, affecting 94% (N=282) of participants at baseline and 85% (N=237) of participants after 12 months of follow-up. At baseline, participants reported feeling tired an average of 4.2 days per week, with a reduction to 3.2 days per week by 12 months.
The top 10 symptoms did not differ by ICD indication. The most common symptoms for both primary and secondary prevention participants remained tiredness, feeling rundown, and interrupted sleep. Compared to the full study sample, a single symptom differed at baseline in the primary prevention group, with ringing of the ears replacing dizziness/near-fainting. Similarly, a single symptom differed at baseline in the secondary prevention group, with insomnia replacing dizziness/near-fainting.
Symptom Classes
The unadjusted baseline model fit indices indicated that a 3-class model was the optimal latent class solution. Sociodemographic and health variables considered likely to influence symptoms were tested in an adjusted model, and included age, gender, ICD indication, Charlson Comorbidity Index, income, and LVEF. When added to the model one at a time, two covariates were statistically significant, patient age (p < 0.001) and Charlson Comorbidity Index (p = 0.04), and were thus retained in the final adjusted model. The fit indices for the final adjusted model indicated that the 3- or 4-class solution could be considered appropriate (Table 3). To determine the optimal solution, the 3-class and 4-class symptom profiles were compared for interpretability. Based on this review, and relevant model fit indices (BICa and VLMR), the 3-class model was determined to be the optimal solution.
Table 3.
Summary of Latent Class Analysis Fit Indices for 2- through 4-Class Solutions
| Model | AIC† | BICa† | VLMR |
|---|---|---|---|
| 2-class | 3334.46 | 3346.78 | −1821.20* |
| 3-class | 3287.95 | 3307.24 | −1644.23* |
| 4-class | 3284.41 | 3310.66 | −1607.98ns |
Lower numbers indicate a better model fit;
p < 0.01; NS = non-significant
The 3-class solution was deemed the most appropriate based on the lowest BICa and the VLMR results. The statistical significance of the VLMR for the 3-class solution indicates that the 3-class solution is a better fit than the 2-class solution. Although the AIC indicates the 4-class solution may be best, AIC is more likely to indicate an overfit model. Further, the VLMR for the 4-class solution is non-significant, indicating the 3-class solution is a better fit than the 4-class solution.
Symptoms by Class Membership.
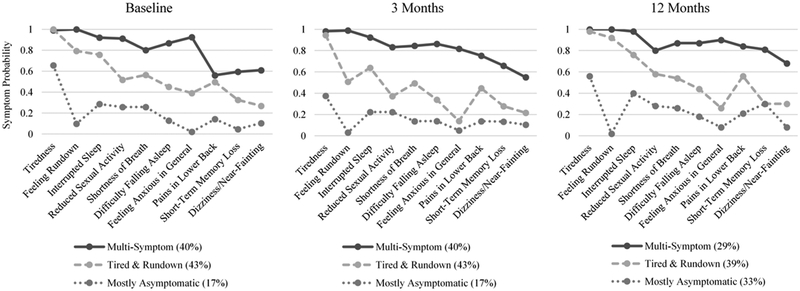
The three classes of participants were labeled: 1) Multi-Symptom (N=119, 40%), 2) Tired-Rundown (N=130, 43%), and 3) Mostly Asymptomatic (N=52, 17%). The Multi-Symptom class consisted of participants who had a high probability for every symptom. Participants in the Tired-Rundown class had a high probability of tiredness, feeling rundown, interrupted sleep, reduced sexual activity, and shortness of breath; of these, the first 3 symptoms had 75% or greater probability and were thus the most representative of the class. Individuals in the Mostly Asymptomatic class had a high probability of reporting a single symptom, tiredness. The Mostly Asymptomatic class was the smallest group, with only 17% of participants (N=52) assigned to this class at baseline (Figure 1).
Figure 1.
Symptom probabilities by class at baseline, three months, and 12 months.
The 3-class solution at 3 and 12 months showed minimal variation in terms of symptom probabilities compared to the baseline solution (Figure 1). By 12-months post ICD implant, the proportion of participants in the Mostly Asymptomatic class was increased to 33% (N=92) compared to 17% at baseline and 3 months.
Characteristics by Class Membership.
Participants in the Multi-Symptom class at baseline had a mean age of 59.9 (±11.8) compared to 66.52 (±12.1) for the Tired-Rundown class and 67.8 (±8.3) for the Mostly Asymptomatic class (p < 0.001). Differences in the Charlson Comorbidity Index were evident although not statistically significant (p = 0.14) at baseline. Anxiety (p < 0.001) and depression (p < 0.01) were more common in the Multi-Symptom class than in the Tired-Rundown or Mostly Asymptomatic classes. Diabetes was most common in the Mostly-Asymptomatic class (p = 0.04). Participants in the Multi-Symptom and Tired-Rundown classes took fewer steps per day than those in the Mostly Asymptomatic class (p < 0.01). Additional baseline demographic and heath history comparisons are reported in Table. 4
Table 4.
Patient Demographic and Health Characteristics by Baseline Class Membership at Baseline
| Baseline Variable | Multi-Symptom (N=119, 40%) | Tired & Rundown (N=130, 43%) | Mostly Asymptomatic (N=52, 17%) | p-value |
|---|---|---|---|---|
| Patient-Only Intervention | 58 (49%) | 67 (52%) | 26 (50%) | 0.91 |
| Age | 59.9 (±11.8) | 66.5 (±12.1) | 67.8 (±8.3) | <0.001 |
| Male | 87 (73%) | 96 (74%) | 39 (75%) | 0.97 |
| Primary Prevention ICD | 73 (61%) | 73 (56%) | 34 (65%) | 0.47 |
| Comorbidity (CCI) | 2.4 (±1.5) | 2.4 (±1.5) | 1.9 (±1.3) | 0.14 |
| BMI | 29.9 (±6.7) | 29.4 (±6.0) | 29.4 (±5.3) | 0.69 |
| LVEF | 33.4 (±14.5) | 34.7 (±14.4) | 34.1 (±14.0) | 0.68 |
| Atrial or Ventricular Arrhythmia | 79 (66%) | 91 (70%) | 38 (73%) | 0.66 |
| COPD | 25 (21%) | 27 (21%) | 4 (8%) | 0.08 |
| Thyroid Disease | 17 (14%) | 14 (11%) | 9 (17%) | 0.46 |
| Diabetes Mellitus | 32 (27%) | 31 (24%) | 22 (42%) | 0.04 |
| Renal Disease | 3 (3%) | 9 (7%) | 0 (0%) | 0.06 |
| Anxiety (STAI ≥ 40) | 45 (38%) | 19 (15%) | 3 (6%) | <0.001 |
| Depression (PHQ-9 ≥ 10) | 38 (32%) | 10 (8%) | 2 (4%) | <0.001 |
| Total Steps/Day | 6494 (±3281) | 6542 (±3442) | 8677 (±4514) | <0.01 |
Values are mean (± standard deviation) or N (%). ICD: implantable cardioverter defibrillator. CCI: Charlson Comorbidity Index. BMI: Body Mass Index. LVEF: Left Ventricular Ejection Fraction. COPD: Chronic Obstructive Pulmonary Disease. STAI: State-Trait Anxiety Inventory. PHQ-9: Patient Health Questionnaire.
The observed differences in age, Charlson Comorbidity Index, total steps per day, anxiety, and depression persisted through 12 months. Charlson Comorbidity Index scores were higher for participants in the Multi-Symptom and Tired-Rundown classes compared to the Mostly Asymptomatic class at 3 months (p < 0.01) and 12 months (p = 0.02). We did not find statistically significant differences in class membership by intervention condition at any time point.
Health Outcomes
Compared to the Mostly Asymptomatic class, which served as the reference class in the predictive models, membership in the Multi-Symptom class at baseline predicted significantly poorer mental (MCS) and physical (PCS) health status (p < 0.001, Table 5) at 3 and 12 months post ICD implant. Membership in the Tired-Rundown class at baseline predicted poorer physical health (p < 0.001) at 3 and 12 months. Membership in the Multi-Symptom class at baseline did not influence hospitalizations at 3 months post ICD implant, but resulted in nearly double the rate of hospitalizations at the 12-month follow-up (p = 0.02, incident rate ratio 1.9). Membership in the Tired-Rundown class at baseline did not have a statistically significant impact on hospitalizations at the 3- or 12-month follow-up. ICD indication (primary vs. secondary), Charlson Comorbidity Index, age, and income remained significant predictors of quality of life and hospitalizations in the fully adjusted models (Table 5). Specifically, primary prevention ICD indication was associated lower mental health status at 12 month follow up and fewer hospitalizations at 3 month follow up. Higher baseline comorbidity status was associated with lower physical health status at both time points, and more frequent hospitalizations at 12 month follow up. Older age was associated higher mental health status at both time points, and lower income levels predicted lower physical health status at both time points.
Table 5.
Baseline Symptom Class Prediction of Quality of Life at Hospitalizations at 3 and 12 Months Post ICD Implant
| 3 Months Post Implant | 12 Months Post Implant | |||||||
|---|---|---|---|---|---|---|---|---|
| QOL: Mental Health Status (SF-36 MCS)* | ||||||||
| B | SE | t | p-value | B | SE | t | p-value | |
| Multi-Symptom Class | −6.35 | 1.57 | −4.04 | < 0.001 | −7.09 | 1.56 | −4.55 | < 0.001 |
| Tired-Rundown Class | −2.12 | 1.5 | −1.41 | 0.16 | −2.12 | 1.48 | −1.43 | 0.15 |
| Income: < $10,000 | −4.45 | 3.55 | −1.25 | 0.21 | ||||
| Income: $10,000–29,000 | −2.59 | 1.67 | −1.55 | 0.12 | ||||
| Income: $30,000–49,000 | −1.8 | 1.56 | −1.15 | 0.25 | ||||
| Income: $50,000–69,000 | 0.14 | 1.7 | 0.08 | 0.94 | ||||
| Income: $70,000–89,000 | −2.15 | 1.78 | −1.21 | 0.23 | ||||
| Primary Prevention ICD | −2.62 | 1.19 | −2.2 | 0.03 | ||||
| Age | 0.11 | 0.05 | 2.36 | 0.02 | 0.14 | 0.05 | 2.99 | <0.01 |
| LVEF | 0.05 | 0.04 | 1.17 | 0.25 | ||||
| QOL: Physical Health Status (SF-36 PCS)* | ||||||||
| B | SE | t | p-value | B | SE | t | p-value | |
| Multi-Symptom Class | −11.5 | 1.73 | −6.66 | < 0.001 | −10.35 | 1.77 | −5.85 | < 0.001 |
| Tired-Rundown Class | −7.3 | 1.65 | −4.42 | < 0.001 | −6.48 | 1.68 | −3.86 | < 0.001 |
| Income: < $10,000 | −7.85 | 3.91 | −2.01 | 0.05 | −6.63 | 4.02 | −1.65 | 0.1 |
| Income: $10,000–29,000 | −5.61 | 1.88 | −2.99 | <0.01 | −6.85 | 1.94 | −3.53 | < 0.001 |
| Income: $30,000–49,000 | −4.95 | 1.69 | −2.92 | <0.01 | −5.81 | 1.78 | −3.26 | <0.01 |
| Income: $50,000–69,000 | −2.61 | 1.84 | −1.42 | 0.16 | −5.06 | 1.92 | −2.64 | <0.01 |
| Income: $70,000–89,000 | −2.24 | 1.91 | −1.17 | 0.24 | −3.59 | 2.01 | −1.78 | 0.08 |
| Primary Prevention ICD | −0.55 | 1.29 | −0.43 | 0.67 | −2.03 | 1.34 | −1.52 | 0.13 |
| Comorbidity (CCI) | −2.03 | 0.41 | −4.93 | < 0.001 | −2.39 | 0.44 | −5.43 | < 0.001 |
| Age | −0.06 | 0.05 | −1.22 | 0.22 | −0.9 | 0.05 | −1.75 | 0.08 |
| LVEF | 0.03 | 0.05 | 0.68 | 0.5 | 0.01 | 0.05 | 0.1 | 0.92 |
| Hospitalizations** | ||||||||
| Baseline Variable | B | SE | Wald χ2 | p-value | B | SE | Wald χ2 | p-value |
| Multi-Symptom Class | 0.33 | 0.41 | 0.65 | 0.42 | 0.63 | 0.27 | 5.44 | 0.02 |
| Tired-Rundown Class | 0.13 | 0.41 | 0.09 | 0.76 | 0.11 | 0.28 | 0.14 | 0.71 |
| Income: $10,000–29,000 | 0.81 | 0.41 | 3.9 | 0.05 | 0.55 | 0.28 | 3.72 | 0.05 |
| Income: $30,000–49,000 | 0.88 | 0.39 | 5.17 | 0.02 | 0.46 | 0.27 | 2.99 | 0.08 |
| Income: $50,000–69,000 | −0.42 | 0.6 | 0.49 | 0.48 | 0.04 | 0.32 | 0.01 | 0.91 |
| Income: $70,000–89,000 | 0.13 | 0.52 | 0.06 | 0.81 | 0.27 | 0.32 | 0.73 | 0.39 |
| Primary Prevention ICD | −0.57 | 0.27 | 4.47 | 0.03 | ||||
| Comorbidity (CCI) | 0.18 | 0.05 | 10.61 | 0.001 | ||||
QOL: Quality of Life. ICD: implantable cardioverter defibrillator. CCI: Charlson Comorbidity Index. LVEF: Left Ventricular Ejection Fraction.
General Linear Model, Univariate Analysis of Variance.
Poisson regression.
Discussion
This study addresses a notable gap in knowledge related to symptom profiles during the first year post-ICD implant and how symptoms change over time in this patient population. The most common symptoms experienced over the first year following an initial ICD implant were identified, and 3 classes of individuals experiencing similar symptom profiles were distinguished. Further, two symptom profiles present at the time of ICD implant, characterized by multiple physical and psychological symptoms and by fatigue, predicted long-term health outcomes. Thus, we demonstrated that assessment of physical and psychological symptoms at the time of ICD implantation is an appropriate approach for identification of patients at risk for poor quality of life and rehospitalization during the first year following ICD implant.
To our knowledge, this is the first study to demonstrate that specific physical and psychological symptom profiles predict health outcomes following an initial ICD implant for primary or secondary prevention of sudden cardiac arrest. Other research has demonstrated associations between distinct symptom profiles and health outcomes—such as functional status, hospitalizations, and event-free survival—in heart failure, atrial fibrillation, and acute coronary syndrome populations (31, 32). Similar to our findings, Herr (33) reported that for adults with heart failure, a cluster of physical and psychological symptoms resulted in acceleration of functional limitations compared to those with a cluster of physical symptoms alone. In our study, the Multi-Symptom class, characterized by both physical and psychological symptoms and comorbidities, predicted poorer health outcomes than the Tired-Rundown class, characterized primarily by physical symptoms. Neither ICD indication nor LVEF were significant predictors of latent class membership. However, receiving an ICD for primary prevention was associated with fewer hospitalizations during the first 3 months following an initial ICD implant, and lower mental health-related quality of life at 12 months. These findings indicate that symptom profiles and health history characteristics are important indictors of health outcomes in the first year following an initial ICD implant.
Results of the current study revealed that individuals who experience multiple symptoms are younger and more likely to report comorbid anxiety and/or depression. The impact of high symptom burden among younger patients has been observed in other serious or life-threatening illnesses (34, 35). Moreover, the number and severity of symptoms in patients with cardiovascular disease is known to be associated with both anxiety and depression (36, 37). The prevalence of psychological distress after receiving an ICD is high, with a prevalence rate of approximately 20% for anxiety and/or depressive disorders a year or more after implantation (5). The high prevalence of psychological distress after an ICD may explain why individuals in the Multi-Symptom class reported poorer mental health-related quality of life at 3 and 12 months. Additionally, prior research shows that younger patients receiving an ICD have greater risk for psychological distress and reduced quality of life (38, 39). The higher symptom burden and lower mental health-related quality of life reported by the younger ICD recipients could be explained by the ‘response shift’ phenomenon, whereby conceptualization of good health changes with age (40). Thus, younger patients may perceive their health status as worse based on pre-conceived expectations about their health. Psychological symptoms specific to the ICD itself are also common, including behavioral avoidance, anxiety related to physical and sexual activity, symptoms of post-traumatic stress related to prior shocks or sudden cardiac arrest, and fear of cardiac arrest (4, 7, 41–43).
Participants with diabetes were more likely to classified with the Mostly-Asymptomatic profile. Of note, studies reveal that patients with diabetes are at risk for asymptomatic or ‘silent’ ischemic cardiac events (44). That is, diabetic patients are at risk for developing cardiac autonomic neuropathy that can result in dysfunction of the cardiac autonomic afferent nervous system (45). This is a potential explanation for the observed differences in perceived symptoms by diabetic patients in this study.
The results of this study contribute to the development of symptom science by expanding earlier descriptive work related to symptoms in the ICD patient population. More than a decade ago, Dougherty (46) similarly reported that tiredness, interrupted sleep, feeling dependent on others, shortness of breath, and reduced sexual activity were the most common symptoms immediately following ICD implantation for secondary prevention. In that study, which tested an 8-week educational intervention for participants receiving an initial ICD for secondary prevention, the most common symptoms 12 months post-ICD implant remained relatively unchanged. Berg (6) conducted an RCT of a psychoeducational intervention following an initial ICD implant for primary or secondary prevention; the intervention focused on sleep education and counseling. The investigators found high levels of poor sleep quality (67%) and sleepiness (40%) at the time of initial ICD implant. No significant differences were observed between usual care and intervention conditions in the Berg (6) study, and a limited number of participants exhibited improvement in sleep quality (57%) or sleepiness (33%) 6 months post implant. Taken together, these studies suggest that the management of physical symptoms has not been optimized during ICD implant recovery and demonstrates that interventional research is warranted to target management of the high burden of both physical and psychological symptoms in this patient population.
Improvements in quality of life and reduction of hospitalizations in the year following ICD implantation may be accomplished by targeting interventions to individuals with high physical and psychological symptom burden at the time of ICD implant (4), and designing interventions that address physical and psychological symptoms concurrently. Across time, characteristics associated with the Multi-Symptom class included higher rates of depression and anxiety, younger age, and higher Charlson Comorbidity Index. In addition, age and Charlson Comorbidity Index were statistically significant covariates in predicting mental and physical health-related quality of life and hospitalizations. Each of these characteristics could be systematically evaluated prior to hospital discharge to identify individuals at the greatest risk for sustained physical and psychological symptom burden and poor health outcomes. Interventions to reduce hospitalizations and target symptom management and quality of life could be tailored for these individuals. For example, cognitive behavioral therapy (CBT) is effective for the treatment of both insomnia and depression (47), indicating the potential to improve both physical (i.e. tiredness, feeling rundown, interrupted sleep) and psychological symptoms following ICD implant. Better managed symptoms are likely to improve quality of life and reduce hospitalizations. Similarly, combining CBT and exercise is a promising approach for improving symptom management following ICD implant, while concurrently improving physical function and quality of life (48, 49).
The study design is an important limitation to consider. This work was a secondary analysis of a block randomized controlled trial that compared two interventions without a usual care control group. All patients received an intervention, with components of the intervention focused on symptom management. However, all patients received the same intervention components; the difference between interventions was whether or not the partner also participated. Thus, we examined for the effect of partner involvement in the intervention by including intervention condition as a covariate in the latent class and regression analyses. Finally, the sample was 91% Caucasian and 74% male, which limits the overall generalizability of the findings. The relatively low number minority participants in the study may be partially explained by the general demographics of the Pacific Northwest, where the most participants were recruited. However, it is possible that the low number of minorities and women in this sample reflects health disparities that exist in the general patient population that receives ICD’s in the United States (50).
Conclusion
Three unique symptom profiles of patients with an initial ICD implant were derived, labeled specifically as Multi-Symptom, Tired-Rundown, and Mostly Asymptomatic classes. Individuals in the Multi-Symptom class compared to the other classes were younger and reported more anxiety and depression. Membership in the Multi-Symptom class was predictive of lower quality of life and more frequent hospitalizations for up to a year following ICD implant. Given the dramatic increase in the prevalence of ICDs, further research is warranted to determine if the use of symptom profiles provides an efficacious and cost-effective option for targeted interventions and personalized symptom management following ICD implantation.
Key Message.
Three classes of individuals were identified based on symptom profiles, including one class marked by multiple physical and psychological symptoms. Individuals with the multi-symptom profile at the time of ICD implant were younger, had more depression and anxiety, and experienced lower quality of life and increased hospitalizations 12 months following ICD implant.
Funding:
The clinical trial was funded by the National Institutes of Health, National Heart Lung and Blood Institute (R01 HL 086580).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
There are no conflicts of interest to disclose for the study authors (MS, ET, CD).
References
- 1.Bardy G, Lee K, Mark D, Poole J, Packer D, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352(3):225–237.. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm 2017;15(10):e190–e252. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar SB, Dougherty CM, Sears SF, Carroll DL, Goldstein NE, Mark DB, et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: A scientific statement from the American Heart Association. Circulation 2012;126(17):2146–2172. [DOI] [PubMed] [Google Scholar]
- 5.Magyar-Russell G, Thombs BD, Cai JX, Baveja T, Kuhl EA, Singh PP, et al. The prevalence of anxiety and depression in adults with implantable cardioverter defibrillators: A systematic review. J Psychosom Res 2011;71(4):223–231. [DOI] [PubMed] [Google Scholar]
- 6.Berg SK Higgins M, Reilly CM, Langberg JJ, Dunbar SB. Sleep quality and sleepiness in persons with implantable cardioverter defibrillators: Outcome from a clinical randomized longitudinal trial. Pacing Clin Electrophysiol 2012;35(4):431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemon J, Edelman S, Kirkness A. Avoidance behaviors in patients with implantable cardioverter defibrillators. Heart Lung 2004;33(3):176–182. [DOI] [PubMed] [Google Scholar]
- 8.Steinke EE, Gill-Hopple K, Valdez D, Wooster M. Sexual concerns and educational needs after an implantable cardioverter defibrillator. Heart Lung 2005;34(5):299–308. [DOI] [PubMed] [Google Scholar]
- 9.Thylén I, Dekker RL, Jaarsma T, Strömberg A, Moser DK. Characteristics associated with anxiety, depressive symptoms, and quality-of-life in a large cohort of implantable cardioverter defibrillator recipients. J Psychosom Res 2014;77(2):122–127. [DOI] [PubMed] [Google Scholar]
- 10.Shalaby AA, Brumberg GE, Pointer L, Bekelman DB, Rumsfeld JS, Yang Y, et al. Depression and outcome among veterans with implantable cardioverter defibrillators with or without cardiac resynchronization therapy capability. Pacing Clin Electrophysiol 2104;37(8):994–1001. [DOI] [PubMed] [Google Scholar]
- 11.Dougherty CM, Thompson EA, Kudenchuk PJ. Development and testing of an intervention to improve outcomes for partners following receipt of an implantable cardioverter defibrillator in the patient. ANS Adv Nurs Sci 2012;35(4):359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for alzheimer’s disease (CERAD). part I. clinical and neuropsychological assessment of alzheimer’s disease. Neurology 1989;39(9):1159–1165. [DOI] [PubMed] [Google Scholar]
- 13.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry 1983;140(6):734–739. [DOI] [PubMed] [Google Scholar]
- 14.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 15.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008;103(6):1039–1047. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A Social cognitive theory: An agentic perspective. Ann Rev Psychol 2001;52:1–26. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty CM, Benoliel JQ, Bellin C. Domains of nursing intervention after sudden cardiac arrest and automatic internal cardioverter defibrillator implantation. Heart Lung 2000;29(2):79–86. [PubMed] [Google Scholar]
- 19.Dougherty CM, Pyper GP, Benoliel JQ. Domains of concern of intimate partners of sudden cardiac arrest survivors after ICD implantation. J Cardiovasc Nurs 2004;19(1):21–31. [DOI] [PubMed] [Google Scholar]
- 20.Dunbar SB, Jenkins LS, Hawthorne M, Kimble LP, Dudley WN, Slemmons M, et al. Factors associated with outcomes 3 months after implantable cardioverter defibrillator insertion. Heart Lung 1999;28(5):303–315. [DOI] [PubMed] [Google Scholar]
- 21.CS R G RL. Manual for the state-trait anxiety inventory (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists; 1970. [Google Scholar]
- 22.Bloome LJ. Psychology and cardiology: Collaboration in coronary treatment and prevention. Professional Psychology 1979;10(4):485–490. [Google Scholar]
- 23.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the patient health questionnaire (PHQ): A diagnostic meta-analysis. J Gen Intern Med 2007;22(11):1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC. Diagnostic accuracy of the mood module of the patient health questionnaire: A systematic review. Gen Hosp Psychiatry 2007;29(5):388–395. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE. Standards for validating health measures: Definition and content. Journal of Chronic Diseases 1987;40(6):473–480. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30(6):473–483. [PubMed] [Google Scholar]
- 27.Wurpts I, Geiser C. Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Frontiers in Psychology 2014;5:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs 2001;33(5):668–76. [DOI] [PubMed] [Google Scholar]
- 29.Collins LM, Lanza ST. Latent class and latent transition analysis Hoboken, New Jersey: Wiley; 2010. [Google Scholar]
- 30.Rubin M Do p values lose their meaning in exploratory analyses? it depends how you define the familywise error rate. Review of General Psychology 2017;21(3):269–275. [Google Scholar]
- 31.DeVon HA, Vuckovic K, Ryan CJ, Barnason S, Zerwic JJ, Pozehl B, et al. Systematic review of symptom clusters in cardiovascular disease. European Journal of Cardiovascular Nursing 2017;16(1):6–17. [DOI] [PubMed] [Google Scholar]
- 32.Streur MM, Ratcliffe SJ, Callans DJ, Shoemaker MB, Riegel BJ. Atrial fibrillation symptom profiles associated with healthcare utilization: A latent class regression analysis. Pacing Clin Electrophysiol 2018;epub ahead of print: doi: 10.1111/pace.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herr JK, Salyer J, Flattery M, Goodloe L, Lyon DE, Kabban CS, et al. Heart failure symptom clusters and functional status - a cross-sectional study. J Adv Nurs 2015;71(6):1274–1287. [DOI] [PubMed] [Google Scholar]
- 34.Miaskowski C, Dunn L, Ritchie C, Paul S, Cooper B, Aouizerat B, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. Journal of Pain and Symptom Management 2015;50(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Moser D, Griffith K, Harring J, Johantgen M. Exploring symptom clusters in people with heart failure. Clinical Nursing Research 2019;28(2):165–181. [DOI] [PubMed] [Google Scholar]
- 36.Bekelman D, Havranek E, Becker D, Kutner J, Peterson P, Wittstein I, et al. Symptoms, depression, and quality of life in patients with heart failure. Journal of Cardiac Failure 2007;13(8):643–648. [DOI] [PubMed] [Google Scholar]
- 37.Sears S, Serber E, Alvarez L, Schwartzman D, Hoyt R, Ujhelyi M. Understanding atrial symptom reports: objective versus subjective predictors. Pacing and Clinical Electrophysiology 2005;28(8):801–807. [DOI] [PubMed] [Google Scholar]
- 38.Sears SF, Lewis TS, Kuhl EA, Conti JB. Predictors of quality of life in patients with implantable cardioverter defibrillators. Psychosomatics 2005;46(5):451–457. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez LD, Kuhl EA, Shea JB, Kirkness A, Lemon J, Whalley D, et al. Age-specific differences in women with implantable cardioverter defibrillators: An international multi center study. Pacing Clin Electrophysiol 2008;31(12):1528–1534. [DOI] [PubMed] [Google Scholar]
- 40.Galenkamp H, Huisman M, Braam A, Deeg D. Estimates of prospective change in self-rated health in older people were biased owing to potential recalibration response shift. Journal of clinical epidemiology 2012;65(9):978–988. [DOI] [PubMed] [Google Scholar]
- 41.Steinke EE. Sexual concerns of patients and partners after an implantable cardioverter defibrillator. Dimensions of Critical Care 2003;22(2):89–96. [DOI] [PubMed] [Google Scholar]
- 42.Irvine J, Firestone J, Ong L. A randomized controlled trial of cognitive behavior therapy tailored to psychological adaptation to an implantable cardioverter defibrillator. Psychosom Med 2011;73(3):226–233. [DOI] [PubMed] [Google Scholar]
- 43.Habibović M, Denollet J, Pedersen SS. Posttraumatic stress and anxiety in patients with an implantable cardioverter defibrillator: Trajectories and vulnerability factors. Pacing Clin Electrophysiol 2017;40(7):817–823. [DOI] [PubMed] [Google Scholar]
- 44.Wackers F, Young L, Inzucchi S, Chyun D, Davey J, Barrett E, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care 2004;27(8):1954–1961. [DOI] [PubMed] [Google Scholar]
- 45.Pop-Busui R Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010;33(2):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dougherty CM, Thompson EA, Lewis FM. Long-term outcomes of a telephone intervention after an ICD. Pacing Clin Electrophysiol 2005;28(11):1157–1167. [DOI] [PubMed] [Google Scholar]
- 47.Ashworth DK, Sletten TL, Junge M, Simpson K, Clarke D, Cunnington D, et al. A randomized controlled trial of cognitive behavioral therapy for insomnia: An effective treatment for comorbid insomnia and depression. Journal of Counseling Psychology 2015;62(2):115–123. [DOI] [PubMed] [Google Scholar]
- 48.Donta ST, Clauw DJ, Engel CC, Guarino P, Peduzzi P, Williams DA, et al. Cognitive behavioral therapy and aerobic exercise for gulf war veterans’ illnesses: A randomized controlled trial. JAMA 2003;289(11):1396–1404. [DOI] [PubMed] [Google Scholar]
- 49.Alswyan AH, Liberato ACS, Dougherty CM. A systematic review of exercise training in patients with cardiac implantable devices. J Cardiopulm Rehabil 2018;38(2):70–84. [DOI] [PubMed] [Google Scholar]
- 50.Hess PL, Hernandez AF, Bhatt DL, Helkamp AS, Yancy CW, Schwamm LH, et al. Sex and Race/Ethnicity Differences in Implantable Cardioverter-Defibrillator Counseling and Use Among Patients Hospitalized With Heart Failure: Findings from the Get With The Guidelines-Heart Failure Program. Circulation 2016;134(7):517–526. [DOI] [PubMed] [Google Scholar]