Abstract
Objectives:
Fatigue is common among individuals with systemic lupus erythematosus (SLE) but causes are not well understood. We examined perceived stress and depressive symptoms as predictors of fatigue in SLE.
Methods:
Data from two years of the Lupus Outcomes Study (n=650), obtained through annual structured interviews, were used. Fatigue was measured with the SF-36 Vitality scale along with a variety of self-report measures of disease, depression, and stress. Multivariate linear regression models examined predictors of changes in fatigue. Model 1 tested the association of Time 1(T1) depression with Time 2(T2) fatigue; Model 2 added T1 perceived stress to Model 1; and final models added T1-T2 decrease in stress. All analyses controlled for T1 fatigue, age, sex, self-report of fibromyalgia, pain, and SLE duration, activity, and damage.
Results:
Mean (SD) age was 51(12) years, 92% were women, 68% were Caucasian. Mean (SD) SF-36 Fatigue score was 55(24). T1 depression significantly predicted T2 fatigue. When T1 stress was added, stress (β 1.7, 95% CI [1.1, 2.2], p <0.0001) significantly predicted T2 fatigue but depression was no longer significance. The addition of T1-T2 decrease in stress was associated with clinically meaningful decline in fatigue (β −11.8, 95% CI [15.6, −8.9], p <0.0001).
Conclusion:
While depressive symptoms initially predicted subsequent fatigue, the effects were mediated by stress. A decrease in stress, in addition, was associated with a clinically meaningful decrease in fatigue. These results suggest that perceived stress plays an important role in SLE fatigue and may be an important focus of interventions for fatigue.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystem disease with varied clinical manifestations and a complex disease course. Mortality in SLE has improved over the past 5 decades with the estimated 10-year survival among SLE patients increasing from 63% in the 1950s to 91% in the 2000s (1). With improved survival, the impact of disease and treatment on quality of life (QoL) has emerged as an important consideration in the management of SLE. Health related quality of life (HRQoL) of individuals with SLE is reported as significantly worse in comparison to patients with hypertension, congestive heart failure, myocardial infarction, depression, and adult onset diabetes mellitus, and this effect begins at an earlier age (2). The Outcome Measures in Rheumatology Clinical Trials (OMERACT) group highlighted the importance of measuring and reporting patients’ experience of SLE through self-reported HRQoL instruments and defined it as an independent core outcome in SLE, outside of disease activity and damage (3). Fatigue, for example, is one such primary patient-reported outcomes.
Fatigue in SLE remains an elusive symptom that is poorly understood by health care providers, yet widely prevalent. Fatigue may be defined as an overwhelming sense of tiredness, lack of energy and feeling of exhaustion (4) and is a primary determinant of HRQoL in SLE patients (5). A comprehensive review of fatigue in SLE trials identified that 67 to 90% of patients reported significant fatigue (6) (7), and half of these patients consider fatigue as their most disabling symptom (8). In a study evaluating perceived unmet needs that limit one’s ability to attain optimal health and QoL among 386 patients with SLE, “Tiredness” was recorded as a perceived area of unmet need by 81% of patients, and 54% of this group labeled the acuity of this unmet need to be at a moderate or high level (8). Using a disease-specific quality of life (QOL) tool, the LupusQoL, 322 patients with SLE marked fatigue as the domain with greatest impairment (9). In another study of 185 patients with SLE using the Lupus QoL-US, fatigue and physical health were the most affected domains (10).
The origin of fatigue in SLE is believed to be multifactorial and multidimensional. Identified factors associated with fatigue in SLE include stress (11,12); pain (7,12-15); poor sleep (13,16,17); depression (15,16,18-20); reduced levels of exercise (7,13); helplessness (7,13,20,21); abnormal illness behavior (7,13); and poor perceived social support (13,15). The association of fatigue with disease activity in SLE remains controversial with some studies showing a positive correlation (7,16-18,22) and others with no significant association (4,13,15). Fibromyalgia (FM) is intricately related with fatigue and if present may contribute to fatigue in SLE patients (14,17); however most studies on fatigue in SLE do not account for FM. Notably, one third of patients reporting fatigue in SLE fulfill the American College of Rheumatology (ACR) criteria for FM (16).
Psychological factors are recurrently identified as strong, independent predictors of fatigue. Previous analyses identify variability in the relationship between SLE disease, disease comorbidities, inflammatory processes, and psychological pathways. Inarguably, patients with SLE suffer from elevated psychological symptoms compared to the general population, and at the same time, the relationship between psychological factors and disease activity remains unclear. Patients with SLE commonly identify two prevalent psychological symptom clusters, stress and depression. Though similar, depression and stress are different, and variably influence patient outcomes. Stress, identified as negative perceptions of life events (threats to one’s health, threats to survival like insecurity in food, housing, or income, interpersonal abuse, or daily negative life events), results in neuroendocrine and physiological consequences and can be both acute or chronic (23). Alternatively, depression is commonly delineated by maladaptive cognitions and neuropsychiatric changes to both mood and behavior. Inflammatory SLE disease processes, and cognitive and emotional difficulties in coping with the disease are believed to explain both depression and stress. Though stress related to disease factors, personal factors and social relationships impacts fatigue, stress related to poverty states and work-place related stress also link to worsened disease activity (24). These same proposed mechanisms are believed to play a role in fatigue, yet they require clarity along the disease continuum.
Understanding predictors and correlates of fatigue in SLE is key to guiding the development of and providing effective and efficacious treatment. However, current literature is greatly limited by cross-sectional methodology, that does not permit for causal inferences or longitudinal understanding regarding the etiology of fatigue in SLE (19). Guided by our preliminary study using cross sectional data, suggesting a relationship between depression, stress and fatigue in SLE (19), we undertook this study using a larger SLE cohort to explore our hypothesis that perceived stress and depression would predict fatigue over time, as evaluated through a longitudinal study design data.
Materials and Methods
Participants
University of California San Francisco (UCSF) Lupus Outcomes study (LOS) data was used for this study. Participants in the LOS were recruited from both clinical and community sources. SLE diagnoses and fulfillment of the ACR classification criteria for SLE were confirmed by medical record review (25). Specifics regarding the LOS cohort have been previously published (26).
Data for the LOS were primarily obtained from annual structured telephone interviews. Interviews queried symptoms, medications and other treatments, patient-reported measures of lupus disease activity and damage, and a number of validated patient reported outcomes (PROs). Annual retention rates averaged 93%. These analyses use data from two consecutive interviews, Waves 5 and 6 (T1 and T2, hereafter). (These time points were chosen because the patient-reported measure of lupus damage [Brief Index of Lupus Damage-BILD] was added in Wave 5, which allowed us to control for disease damage in analyses.) The analysis cohort consisted of the 678 individuals with responses to both interviews. All study procedures were approved by the UCSF Committee on Human Research.
Measures
Demographic and health characteristics were collected through self-report variables including age, sex, body mass index (BMI), race, education (high school or less vs. greater than high school), income (below federal poverty vs. not), disease duration and self-reported history of a diagnosis of fibromyalgia.
Fatigue was measured using the vitality domain of the Medical Outcome Study (MOS) 36-item short-form health survey (SF-36), the most widely employed and validated generic self-reported tool in SLE studies (27). The vitality domain consists of 4 items pertaining to energy level and fatigue in the last 4 weeks. Scores range from 0-100, and were reversed so that higher scores on the scale meant higher levels of fatigue, i.e. feeling tired and worn out all of the time. Pain was assessed with the bodily pain subscale of MOS-SF-36, where scores range from 0-100, and higher score represents lower pain. The population standard deviation score is 10, where a 10-point changed is viewed as a clinically meaningful change.
Perceived stress was measured using the 4-item Perceived Stress (PS) scale, which assesses an individual’s general perceived level of stress, such as the sense of being in control and having the ability to handle personal problems in one’s life during the last month (28). Higher scores indicate higher levels of stress. Scores range from 0-20. A minimal clinically important difference MCID has not been defined for the PS scale and therefore we utilized 0.5 standard deviation change in score, which has been shown to approximate an MCID, equaling a 2-point difference in scores for the PS (29).
The 20-item Center for Epidemiologic Studies Depression scale (CESD) was used to evaluate current depressive symptom severity (30). Scores range from 0 – 60 with higher scores implying higher levels of depressive symptoms, where a score of ≥ 16 indicates high levels of depressive symptoms and highlights an elevated risk for clinical depression. The CES-D has been recommended by the ACR Ad Hoc Committee on Neuropsychiatric Lupus as the preferred measure of depressive symptom severity for patients with SLE, and is validated in SLE (31).
Current disease activity was assessed by a 0-10 self-reported rating of lupus activity over the past 3 months [0 (no activity) – 10 (high activity)]. This item was validated as part of the Systemic Lupus Activity Questionnaire (SLAQ) (32). The 0 – 10 rating item was used in analyses because the SLAQ includes items that query depressed mood and fatigue.
The Brief Index of Lupus Damage (BILD) (33) was used to estimate organ damage. The BILD is based on Systemic Lupus International Cooperating Clinics/American College of Rheumatology Damage Index (SDI) (34), and consists of 28 items capturing information on 26 SDI items including determinations of organ damage accumulation as cardiovascular disease and events, and diabetes. Scores range from 0 to 31 where higher scores indicate greater damage.
Statistical analysis
Descriptive statistics were calculated for the sample. Differences between individuals who completed both interviews (T1 and T2) and those who completed only the T1 interview were examined with t-tests or chi-square analyses. Pearson correlations were completed to identify primary predictors of the model. See supplement Table 1. Cross-sectional multivariate hierarchical linear regression analysis examined the association of CESD and perceived stress with fatigue at T1. These analyses controlled for age, sex, disease duration, self-reported disease activity and damage (SLAQ and BILD), self-reported fibromyalgia (Yes/No), SF-36 Pain score, and obesity (BMI ≥30).
Longitudinal analyses were constructed to identify predictors of changes in fatigue scores between T1 and T2 (SF-36 Vitality). The first model included the covariates above plus T1 CESD score and T1 fatigue. The second model added the T1 perceived stress score, and the third model added both the T1 perceived stress score and a numeric continuous variable representing any decline in perceived stress between T1 and T2. The final model (Model 4) replaced any decline in perceived stress with a binary variable denoting decline of ≥2 points in the perceived stress scale. This difference was estimated to be a meaningful decline (0.5 SD).
Results
T1 sample characteristics
Characteristics of the cohort are shown in Table 1. Mean age was 51 years, 92% were female, 68% were white, 27% self-reported a diagnosis of fibromyalgia, and 26% had a body mass index (BMI) ≥30 consistent with obesity (Table 1). Mean duration of SLE was 17 ±8 years. Self-reported disease activity at T1 was 4.2 ± 2.7 (range 0 – 10), and T1 BILD score was 2.2 ± 2.0. At T1, almost three-quarters reported use of glucocorticoids and about one third reported use of an immunosuppressive medication. Participants who completed the T1 interview but not the T2 had significantly higher PS scores, but there were no other significant differences between the two groups (Table 1).
Table 1.
Sample characteristics and comparison of LOS participants who did and did not complete both T1 and T2 interviews
| Total sample (n = 726) (Cross- sectional analysis) |
In both T1 and T2 (n=678) (Longitudinal analysis) |
Dropped out, T1 only (n=48) |
P* | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 50.6 ± 12.6 | 50.6 ± 12.4 | 50.2 ± 15.7 | .80 |
| Female, % (n) | 92.2 (669) | 92.3 (626) | 89.6 (43) | .41 |
| Race | .01 | |||
| White, % (n) | 68.0 (494) | 69.0 (468) | 54.2 (26) | |
| African American | 6.3 (46) | 6.2 (42) | 8.3 (4) | |
| Asian | 8.5 (62) | 8.9 (60) | 4.2 (2) | |
| Other, mixed, or unknown | 17.1 (124) | 15.9 (108) | 33.3 (16) | |
| Hispanic ethnicity | 16.6 (120) | 16.5 (112) | 17.0 (8) | .99 |
| Income below poverty | 11.2 (80) | 10.7 (71) | 18.8 (9) | .10 |
| Education > high school | 85.5 (621) | 86.3 (585) | 75.0 (36) | .05 |
| Self-report Fibromyalgia, % (n) | 27.2 (194) | 27.0 (180) | 29.8 (14) | .73 |
| Obese (BMI ≥ 30), % (n) | 25.6 (183) | 26.0 (174) | 19.2 (9) | .39 |
| Pain (SF-36 Bodily Pain; lower → more pain) | 41.7 ± 11.2 | 41.8 ± 11.3 | 40.7 ± 11.0 | .53 |
| Perceived Stress Scale (PS-4; range 0-16) | 5.3 ± 3.6 | 5.2 ± 3.6 | 6.4 ± 4.1 | .04 |
| Center for Epidemiologic Studies Depression scale (CESD; range 0-60) | 14.1 ± 12.4 | 14.0 ± 12.2 | 16.1 ± 14.1 | .25 |
| SF-36 Fatigue (range 0-100) | 54.8 ± 23.9 | 54.5 ± 23.7 | 54.9± 24.0 | .92 |
| Lupus Characteristics | ||||
| Duration, years | 16.6 ± 8.4 | 16.7 ± 8.4 | 16.2 ± 8.2 | .71 |
| Self-reported SLE activity (range 0-10) | 4.2 ± 2.7 | 4.2 ± 2.7 | 4.8 ± 2.8 | .14 |
| BILD (Brief Index of Lupus Damage; range 0 – 18?) | 2.2 ± 2.0 | 2.2 ± 2.0 | 2.5 ± 2.2 | .36 |
| Current glucocorticoid use, % (n) | 40.4 (264) | 40.4 (274) | 39.6 (19) | .91 |
| Current immunosuppressive use | 36.4 (264) | 35.6 (241) | 47.9 (23) | .09 |
p-value from t-tests or chi-square analyses comparing individuals who were and were not included in the longitudinal analyses.
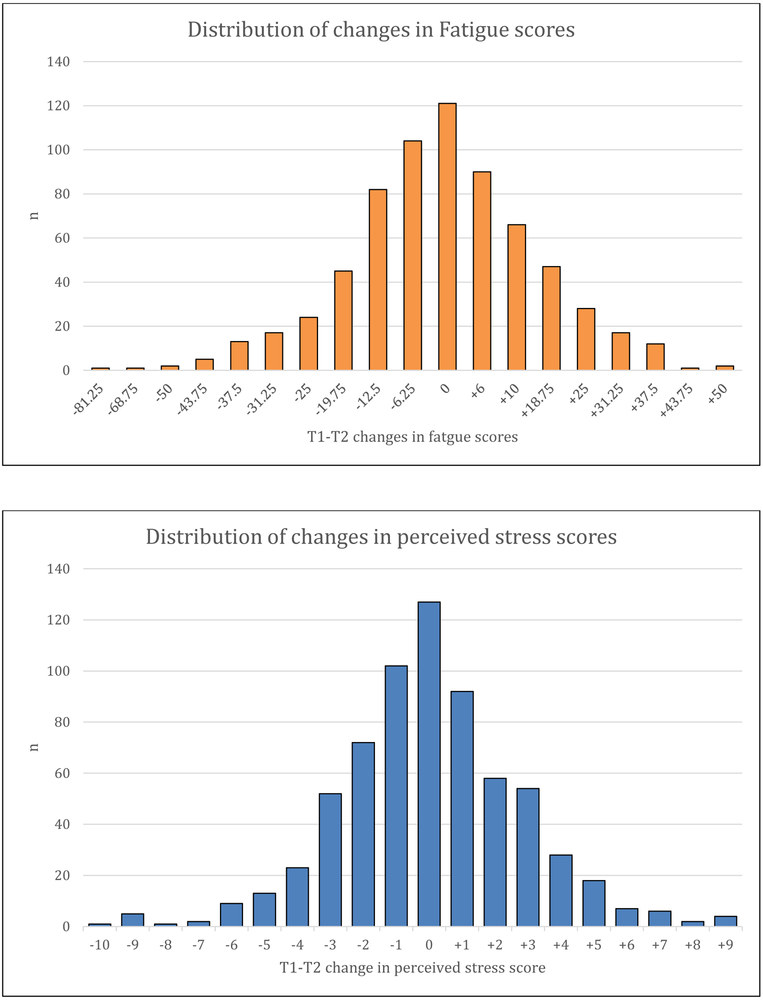
T1 fatigue was higher than the population mean of 50 (54.9 ± 24.0) (Table 1). Mean CESD scores were relatively high, at 14.0 ± 12.2. The mean perceived stress score was relatively low. The distributions of changes in fatigue and perceived stress scores are shown in Figure 1.
Figure 1.
Distribution of changes in fatigue scores and changes in perceived stress from T1-T2
Regression analyses
In cross-sectional multivariate regression analyses controlling for T1 age, sex, race, education (high school or less vs. greater than high school), income (below federal poverty vs. not), disease duration, self-reported disease activity (0 – 10 rating), BILD, pain, self-reported fibromyalgia, and obesity, both CESD and perceived stress were significantly and independently associated with fatigue (Table 2).
Table 2.
Results of cross-sectional and longitudinal multiple regression analyses
| Longitudinal | |||||
|---|---|---|---|---|---|
| Cross-sectional* | Model 1† | Model 2§ | Model 3¶ | Model 4** | |
| T1 depressive symptoms (CESD) | 0.70 (0.54, 0.86) | 0.17 (0.02, 0.31) | −0.01 (−0.19, 0.16) | −0.10 (−0.27, 0.07) | −0.08 (−0.25, 0.09) |
| p < 0.0001 | p = 0.02 | p = 0.87 | p = 0.24 | p = 0.38 | |
| T1 perceived stress | 0.84 (0.32, 1.36) | ---- | 0.91 (0.35, 1.46) | 1.73 (1.17, 2.29) | 1.73 (1.17, 2.29) |
| p = 0.002 | p = 0.001 | p < 0.0001 | p < 0.0001 | ||
| T1-T2 decrease in perceived stress | ---- | ---- | ---- | −10.77 (−13.28, −8.27) | ---- |
| p < 0.0001 | |||||
| T1-T2 decrease in perceived stress, ≥2 points | ---- | ---- | ---- | ---- | −12.06 (−14.93, −9.20) |
| p = <0.0001 | |||||
| Model R2 | 0.64 | 0.56 | 0.57 | 0.62 | 0.61 |
Tabled values are beta (95% confidence interval) and p-value from multiple regression analyses, with change in SF-36 Vitality score (fatigue) as the dependent variable.
Cross-sectional analysis controlled for T1 age, sex, race, education (high school or less vs. greater than high school), income (below federal poverty vs. not), disease duration, self-reported disease activity and damage, pain, self-reported fibromyalgia, and obesity.
Model 1 controlled for T1 age, sex, race, education, income, disease duration, self-reported disease activity and damage, pain, self-reported fibromyalgia, and obesity, plus T1 CESD and T1fatigue.
Model 2 added T1 perceived stress.
Model 3 added any T1 – T2 decrease in perceived stress
Model 4 added T1 – T2 decrease (≥2 points) in perceived stress score
In longitudinal analyses, after controlling for similar covariates plus T1 fatigue in Model 1, T1 CESD was found to be a significant predictor of T2 fatigue (b = 0.16, p < .05; Table 2) and accounted for 56% of the variance in T2 fatigue. When T1 perceived stress was added (Model 2), stress scores were found to be a significant predictor of T2 fatigue, such that greater stress was associated with greater fatigue. CESD was no longer significant in Model 2, signifying that perceived stress functioned as a mediator of the relationship between depression and fatigue over time.
Forty-one percent of participants had any T1-T2 decrease in perceived stress scores, and 26% had a decrease of ≥2 points. Figure 2 shows the mean change in fatigue scores according to decreases in stress. In Model 3, any decline in perceived stress from T1 to T2 was associated with a significant and meaningful decrease in fatigue (b = −11.75, approximately one-half standard deviation of the baseline mean value), where the beta coefficient was greater than an MCID of 10. T1 perceived stress remained a significant predictor of T2 fatigue, but again, T1 CESD did not contribute significantly to the model. Model 3 accounted for 61% of the variance in fatigue at T2. In the final model (4), decrease in stress was defined as a decrease of ≥2 points. Results in model 4 were similar to Model 3 (Table 2).
Figure 2.
Changes in fatigue among individuals with and without a decrease in stress, and similar with a ≥ 2 point decrease in stress
Discussion
Individuals with SLE commonly report significant impairments in HRQoL, at levels greater than those with other chronic illnesses, where fatigue is consistently identified as a primary cause of decreased function and HRQoL (2,3,35-37). Recently, a Swedish cross-sectional study showed that in addition to disease activity and corticosteroids, fatigue drives increased health care costs for those with SLE (38). Understanding factors affecting that affect fatigue in SLE is key to patient-centered management for this poorly understood, under-diagnosed, and under-treated phenomena and its widespread impact.
The objectives of these analyses were to assess possible predictors of fatigue using a longitudinal model; we adjusted for previously identified predictors and comorbidities including age, socioeconomic status (race, education, and income), gender, pain, obesity, comorbidities like fibromyalgia, disease duration, activity and damage. Depression and perceived stress predicted fatigue one year later in individuals with SLE. Specifically, though depression initially predicted fatigue over time, this relationship was mediated by perceived stress and led to clinically meaningful changes in fatigue over time.
Loss of energy or exhaustion can characterize fatigue and can also be regarded as a depressive symptoms. In a cross-sectional study of 148 female participants [50 diagnosed with SLE, 45 with major depressive disorder (MDD), and 53 age-matched controls], fatigue was reported in 90% of women with SLE and 77.8% of patients with MDD in contrast to 39.6% in the control group (39). High prevalence of fatigue seen in patients with clinically diagnosed depression points towards a relationship between the two. Depressed mood has a strong positive association with fatigue in SLE (15,16,19,40,41). Anxiety and depressive symptoms are highly prevalent among patients with SLE and are thought to represent central nervous system involvement, immune dysfunction manifestation, and cognitive processes related to the emotional burden of the disease (39). Several studies attempted to elucidate the relationship between depression and fatigue in SLE, although many are limited by their cross-sectional design. Da Costa et al in their study of 139 women with SLE showed that both components of fatigue, i.e. physical and mental, were associated with depression, where in depressed mood was a stronger determinant of mental fatigue (17). In one previous longitudinal analysis, the data failed to demonstrate the contribution of baseline depression to fatigue at follow up (18). To understand predictors of depressed mood that may better explain fatigue, Azizoddin et al examined 124 lupus patients and identified that the relationships between socioeconomic status and depressed mood and anxiety were mediated by stress, social support, and low self-esteem, above and beyond disease factors (42). Such findings illuminate the strength of psychological factors and their impact on depressive symptomatology and consequently fatigue, as seen in this study. Overall, our results add to the body of literature and consolidate the association between depression and fatigue.
When adding perceived stress to the longitudinal model analysis, we demonstrated that effects of depression on fatigue were mediated through perceived stress. These methodologies and results re-iterate the role and need for biopsychosocial models of care that address fatigue for SLE patients. The 4-item Perceived Stress scale, which was used in our study, is a reliable measure of global perceptions of stress, where social support and perceived health status are major predictors (28). Stress, unlike fatigue and depression, is not a compilation of symptoms but is rather a subjective measure of receptivity and interpretation of life events. Though depression is marked by neurophysiological changes and is diagnostically complex, stress can result from perceptual deficits. Stress also results in cascading psychophysiological consequences through increased and irregular Hypothalamic-pituitary-adrenal (HPA) cortex activity, and resulting inflammatory and oxidative processes (43,44). Stress in lupus, therefore, relates not only to lupus disease processes and disease burden, but also results from personal factors (relationships, self-esteem, helplessness, and poverty status) that exist in one’s life (24,43,44). As an example, Omdal et al showed that stressors including anxiety, difficulty coping, and social dysfunction were associated with fatigue independent of depression and hopelessness in a sample SLE patients (41). Our study is in line with the above findings and provides substantial evidence to the etiology of SLE fatigue. These findings validate SLE patients’ experience of increased levels of perceived stress, whether they be due to disease burden, poverty and sociological factors, or helplessness, with an increased likelihood to be depressed, and in turn experience higher levels of fatigue. Notably, the effects of perceived stress and depression on fatigue are independent of other disease variables, including disease activity and damage. It may be that chronic stress or recurrent acute stress episodes lead to the development of depression overtime, thereby triggering and maintaining resulting fatigue.
There exists a strong need to include the assessment of stress in usual care with SLE, especially in those with unremitting and severe fatigue. At the same time, identification of stress is only meaningful if paired with appropriate and effective interventions targeted towards stress reduction (45-48). Previous psychological interventions show improvements in fatigue and HRQoL factors. In a randomized-controlled trial with 92 patients with SLE who received either biofeedback-assisted cognitive behavioral therapy or usual care, those in this intervention experienced significant improvements in both psychological function and in pain (49). Another targeted, 6-week stress intervention, Balancing Lupus Experiences with Stress Strategies (BLESS), for African Americans with SLE, resulted in significant reductions in fatigue, depression, activity engagement, and lupus self-efficacy (46). A more recent study that explored the effects of a peer support group for patients with lupus, resulted in similar improvements in anxiety and depression, and even positive changes to Th1/Th2 cytokine balances, “indicating a possible underlying mechanism of action” (47). Though formalized, targeted stress interventions are likely most potent and can result in broad improvements in patients QoL, they can be difficult to implement and may require mental health clinicians for implementation. As an alternative, psychoeducational interventions and community resources can be easier to implement and can also result in improvements in stress and fatigue (50). To better understand and treat lupus related fatigue, there lies a strong need for longitudinal research that assesses the relationships between multifactorial disease factors and psychosocial symptomatology, while evaluating clinical effectiveness and long-term outcomes of such programs. At the same time, concurrent to controlling disease activity and damage, health care providers need to be cognizant of the role stress plays towards health outcomes in SLE patients, so they inquire and address it appropriately. Interventions may include active listening, education, referral to appropriate resources, services and interdisciplinary health care providers to address stress in SLE patients. In the same respect, it is important to connect individuals with sociological needs including subsidized housing, Medicaid entitlement, supplemental nutrition assistance programs and the like as complementary strategies to target underlying stress and resulting fatigue.
Limitations and strengths
Firstly, our measure of stress included perceptions of stress rather than the presence of specific stressors; however, the PS-4 is widely used and has consistently been shown to be a valid representation of perceived stress. Secondly, study findings are generalizable to similar individual cohorts. The study cohort was primarily Caucasian and as lupus is more common ethnic minorities, findings should be generalized accordingly. Although about one quarter of our subjects were minorities, we included only English-speaking individuals, another limitation with the pre-existent dataset. Minority patients, some of whom may be non-English-speaking, often have more severe disease and may experience higher levels of social stressors which may not be captured well. However, this study paves the way for futures studies with ethnically heterogeneous groups of patients with SLE. Thirdly, fibromyalgia was measured through self-report and though this may be less valid, this is an improvement to most studies in the literature that do not include presence of FM. As these analyses were undertaken with pre-existent data, we did not have access to more granular data on FM. Fourth, previous analyses have identified anxiety, poor sleep, reduced functionality, helplessness, and poor social support as correlated with fatigue. Unfortunately, all previously identified variables were not available in the LOS dataset. Previously identified primary correlates of fatigue however, were included in our model (19). Even still, given the strong overlap with anxiety and stress, including them both in future analyses would allow for a comparison of the unique role of clinical anxiety and perceived stress individually. Lastly, LOS participants may not represent the full spectrum of disease in SLE; those with severe disease or elevated stress symptomatology are especially likely to be under-represented because they may have been unable to respond to interviews or were more likely to drop out, as seen in this cohort, therefore limiting generalizability.
In spite of these limitations, the study had a number of strengths. The longitudinal design of the study extended previous cross-sectional findings (19). Our prospective model was tested in a large multi-ethnic population that included validated measures of both depressive symptoms and perceived stress. We also adjusted for potential confounders including disease activity, damage and fibromyalgia, which are known to have potential associations with fatigue in SLE patients.
Conclusion
Depression and stress are primary factors that explain the development of fatigue overtime in patients with SLE, irrespective of their concurrent FM diagnosis, disease activity, and damage. Stress is the primary driver for fatigue and should be targeted through comprehensive evaluation and management. If clinicians are to succeed in helping patients improve fatigue across the disease spectrum, they must include evaluation of stress and pursue evidence-based interventions targeting stress that mobilize patients’ engagement in self-management, concurrent with ongoing management of their lupus.
Supplementary Material
Significance and innovations:
Depression and stress were identified as primary predictors of fatigue at one year follow up in a sample of 650 individuals with SLE
Specifically, though depression was significantly related to fatigue, stress mediated the relationship between depression and fatigue over time. Furthermore, decline in stress resulted in clinically meaningful improvements in fatigue over time.
For individuals with SLE suffering from fatigue, stress should be a routinely assessed and targeted through comprehensive, evidence-based treatments that mobilize self-management and their reaction to stress.
Acknowledgments
Funding: NIH/NIAMS P60 AR053308
Footnotes
Disclosures: None for these authors
References
- 1.Mak A, Cheung MWL, Chiew HJ, Liu Y, Ho RC man. Global Trend of Survival and Damage of Systemic Lupus Erythematosus: Meta-Analysis and Meta-Regression of Observational Studies from the 1950s to 2000s. Semin Arthritis Rheum 2012;41:830–839. [DOI] [PubMed] [Google Scholar]
- 2.Jolly M How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–1708. [PubMed] [Google Scholar]
- 3.Strand V, Gladmna D, Isenberg D, Petri M, Smolen J, Tugwell P. Endpoints: consensus recommendations from OMERACT IV. Lupus2 2000;9:322–327. [DOI] [PubMed] [Google Scholar]
- 4.Omdal R, Mellgren SI, Koldingsnes W, Jacobsen EA, Husby G. Fatigue in patients with systemic lupus erythematosus: Lack of associations to serum cytokines, antiphospholipid antibodies, or other disease characteristics. J Rheumatol 2002;29:482–486. [PubMed] [Google Scholar]
- 5.Jolly M, Pickard AS, Block JA, Kumar RB, Mikolaitis RA, Wilke CT, et al. Disease-specific patient reported outcome tools for systemic lupus erythematosus. Semin Arthritis Rheum 2012;42:56–65. Available at: 10.1016/j.semarthrit.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Cleanthous S, Tyagi M, Isenberg DA, Newman SP. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus 2012;21:465–476. [DOI] [PubMed] [Google Scholar]
- 7.Zonana-Nacach A, Roseman JM, McGwin GJ, Friedman AW, Baethge BA, Reveille JD. Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. Lupus 2000;9:101–109. [DOI] [PubMed] [Google Scholar]
- 8.Moses N, Wiggers J, Nicholas C, Cockburn J. Prevalence and correlates of perceived unmet needs of people with systemic lupus erythematosus. Patient Educ Couns 2005;57:30–38. [DOI] [PubMed] [Google Scholar]
- 9.McElhone K, Castelino M, Abbott J, Bruce IN, Ahmad Y, Shelmerdine J, et al. The LupusQoL and associations with demographics and clinical measurements in patients with systemic lupus erythematosus. J Rheumatol 2010;37:2273–2279. [DOI] [PubMed] [Google Scholar]
- 10.Jolly M, Pickard AS, Wilke C, Mikolaitis RA, Teh LS, McElhone K, et al. Lupus-specific health outcome measure for US patients: The LupusQoL-US version. Ann Rheum Dis 2010;69:29–33. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoni D, Cicognani E. Positive and problematic support, stress and quality of life in patients with systemic lupus erythematosus. Anxiety, Stress Coping 2016;29:542–551. [DOI] [PubMed] [Google Scholar]
- 12.Somers TJ, Kurakula PC, Criscione-Schreiber L, Keefe FJ, Clowse MEB. Self-efficacy and pain catastrophizing in systemic lupus erythematosus: relationship to pain, stiffness, fatigue, and psychological distress. Arthritis Care Res (Hoboken) 2012;64:1334–40. [DOI] [PubMed] [Google Scholar]
- 13.Wallace DJ. Additional components to measuring fatigue in patients with systemic lupus erythematosus: Comment on the article by the Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Arthritis Care Res 2008;59:1049. [DOI] [PubMed] [Google Scholar]
- 14.Burgos PI, Alarcón GS, McGwin G, Crews KQ, Reveille JD, Vilá LM. Disease activity and damage are not associated with increased levels of fatigue in systemic lupus erythematosus patients from a multiethnic cohort: LXVII. Arthritis Care Res 2009;61:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jump RL, Robinson ME, Armstrong AE, Barnes E V., Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: Contributions of disease activity, pain, depression, and perceived social support. J Rheumatol 2005;32:1699–1705. [PubMed] [Google Scholar]
- 16.Tench CM, McCurdie I, White PD, D’Cruz D. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology 2000;39:1249–1254. Available at: https://academic.oup.com/rheumatology/article-lookup/doi/10.1093/rheumatology/39.11.1249. [DOI] [PubMed] [Google Scholar]
- 17.Da Costa D, Dritsa M, Bernatsky S, Pineau C, Ménard HA, Dasgupta K, et al. Dimensions of fatigue in systemic lupus erythematosus: Relationship to disease status and behavioral and psychosocial factors. J Rheumatol 2006;33:1282–1288. [PubMed] [Google Scholar]
- 18.Tayer W, Nicassio P, Weisman M, Schuman C, Daly J. Disease status predicts fatigue in systemic lupus erythematosus. J Rheumatol 2001;28:1999–2007. [PubMed] [Google Scholar]
- 19.Azizoddin DR, Gandhi N, Weinberg S, Sengupta M, Nicassio PM, Jolly M. Fatigue in Systemic Lupus: The Role of Disease Activity and its Correlates. Lupus, 28(2), pp. 163–173. DOI: 10.1177/0961203318817826. [DOI] [PubMed] [Google Scholar]
- 20.Moldovan I, Cooray D, Carr F, Katsaros E, Torralba K, Shinada S, et al. Pain and depression predict self-reported fatigue/energy in lupus. Lupus 2013;22:684–689. [DOI] [PubMed] [Google Scholar]
- 21.Mills SD, Azizoddin D, Gholizadeh S, Racaza GZ, Nicassio PM. The mediational role of helplessness in psychological outcomes in systemic lupus erythematosus. Lupus 2018;27:1185–1189. [DOI] [PubMed] [Google Scholar]
- 22.Hart SL, Hoyt M a., Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst 2012;104:990–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res 2006;60:113–124. [DOI] [PubMed] [Google Scholar]
- 24.Yelin E, Trupin L, Bunde J, Yazdany J. Poverty, Neighborhoods, Persistent Stress, and SLE Outcomes:A Qualitative Study of the Patients’ Perspective. Arthritis Care Res (Hoboken) 2018;056476 Available at: http://doi.wiley.com/10.1002/acr.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan EM, Cohen AS, Fries JF, Masi AT, Mcshane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 26.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Care Res 2007;57:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware JE. SF-36 health survey update. use Psychol Test Treat Plan outcomes Assess 2004;3:693–718. [Google Scholar]
- 28.Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4). J Health Psychol 2013;18:1617–1628. [DOI] [PubMed] [Google Scholar]
- 29.Norman G, Sloan J, Wyrwich K. Interpretation of changes in health-related quality of life. The remarkable universality of half a standard deviation. Med Care 2003;41. [DOI] [PubMed] [Google Scholar]
- 30.Radloff L The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1:385–400. [Google Scholar]
- 31.Liang MH, Corzillius M, Bae SC, Lew RA, Fortin PR, Gordon C, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 32.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus 2003;12:280–286. [DOI] [PubMed] [Google Scholar]
- 33.Drenkard C, Yazdany J, Trupin L, Katz PP, Dunlop-Thomas C, Bao G, et al. Validity of a self-administered version of the brief index of lupus damage in a predominantly African American systemic lupus erythematosus cohort. Arthritis Care Res 2014;66:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gladman DD, Urowitz MB, Goldsmith CH, Fortin PR, Ginzler EM, Gordon C, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997;40:809–13. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9153540. [DOI] [PubMed] [Google Scholar]
- 35.Choi ST, Kang JI, Park IH, Lee YW, Song JS, Park YB, et al. Subscale analysis of quality of life in patients with systemic lupus erythematosus: Association with Depression, fatigue, disease activity and damage. Clin Exp Rheumatol 2012;30:665–672. [PubMed] [Google Scholar]
- 36.Williams EM, Lorig K, Glover S, Kamen D, Back S, Merchant A, et al. Intervention to Improve Quality of life for African-American lupus patients (IQAN): Study protocol for a randomized controlled trial of a unique a la carte intervention approach to self-management of lupus in African Americans. BMC Health Serv Res 2016;16:1–13. Available at: 10.1186/s12913-016-1580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarrete-Navarrete N, Peralta-Ramírez MI, Sabio JM, Martínez-Egea I, Santos-Ruiz A, Jiménez-Alonso J. Quality-of-life predictor factors in patients with SLE and their modification after cognitive behavioural therapy. Lupus 2010;19:1632–1639. [DOI] [PubMed] [Google Scholar]
- 38.Bexelius C, Wachtmeister K, Skare P, Jönsson L, Van Vollenhoven R. Drivers of cost and health-related quality of life in patients with systemic lupus erythematosus (SLE): A Swedish nationwide study based on patient reports. Lupus 2013;22:793–801. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca R, Bernardes M, Terroso G, De Sousa M, Figueiredo-Braga M Silent burdens in disease: Fatigue and depression in SLE. Autoimmune Dis 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yilmaz-Oner S, Ilhan B, Can M, Alibaz-Oner F, Polat-Korkmaz O, Ozen G, et al. Fatigue in systemic lupus erythematosus. Z Rheumatol 2016. Available at: http://link.springer.com/10.1007/s00393-016-0185-0. [DOI] [PubMed] [Google Scholar]
- 41.Omdal R, Waterloo K, Koldingsnes W, Husby G, Mellgren SI. Fatigue in patients with systemic lupus erythematosus: The psychosocial aspects. J Rheumatol 2003;30:283–287. [PubMed] [Google Scholar]
- 42.Azizoddin DR, Zamora-Racaza G, Ormseth SR, Sumner LA, Cost C, Ayeroff JR, et al. Psychological Factors that Link Socioeconomic Status to Depression/Anxiety in Patients with Systemic Lupus Erythematosus. J Clin Psychol Med Settings 2017;24:302–315. [DOI] [PubMed] [Google Scholar]
- 43.Morris G, Berk M, Galecki P, Walder K, Maes M. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Mol Neurobiol 2016;53:1195–1219. [DOI] [PubMed] [Google Scholar]
- 44.Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 2015;9:28 Available at: http://www.ncbi.nlm.nih.gov/pubmed/25698933. Accessed February 24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Riordan R, Doran M, Connolly D. Fatigue and Activity Management Education for Individuals with Systemic Lupus Erythematosus. Occup Ther Int 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams EM, Kamen D, Penfield M and Oates JC An Intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: The balancing lupus experiences with stress strategies (BLESS) study. Health (Irvine Calif) 2014;6:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams EM, Hyer JM, Viswanathan R, Faith TD, Egede L, Oates JC, et al. Cytokine balance and behavioral intervention; findings from the Peer Approaches to Lupus Self-Management (PALS) project. Hum Immunol 2017;78:574–581. Available at: 10.1016/j.humimm.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horesh D, Glick I, Taub R, Agmon-Levin N, Shoenfeld Y. Mindfulness-based group therapy for systemic lupus erythematosus: A first exploration of a promising mind-body intervention. Complement Ther Clin Pract 2017;26:73–75. Available at: 10.1016/j.ctcp.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: A randomized controlled trial. Arthritis Care Res (Hoboken) 2004;51:625–634. Available at: http://doi.wiley.com/10.1002/art.20533. [DOI] [PubMed] [Google Scholar]
- 50.Karlson EW, Liang MH, Eaton H, Huang J, Fitzgerald L, Rogers MP, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum 2004;50:1832–1841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.