Abstract
Introduction:
The 6-minute walk test (6MWT) is a well-established clinical assessment of functional endurance, validated as a measure of walking ability in spinal muscular atrophy (SMA). The current availability of disease-modifying therapies for SMA indicates a growing need for normative reference data to compare SMA patients with healthy controls.
Methods:
The literature was searched in two scientific databases. Studies were evaluated and selected based on adherence to American Thoracic Society guidelines for administering the 6MWT. Reference equations from the selected studies were applied to 6MWT data collected from SMA patients to calculate and compare % predicted values.
Results:
Three pediatric and six adult studies were selected for comparison. The % predicted values using the pediatric and adult equations ranged from 47.7 ± 18.2% to 67.6 ± 26.2% and 43.0 ± 17.9% to 59.5 ± 26.2%, respectively, and were significantly different (P < 0.001).
Discussion:
Results suggest significant variability between % predicted values derived from published reference equations in children and adults, despite adherence to 6MWT standardization.
Keywords: neuromuscular disease, normative reference, outcome measure, 6-minute walk test, spinal muscular atrophy
1 |. INTRODUCTION
The 6-minute walk test (6MWT) is a well-established clinical outcome measure used in many different adult and pediatric populations, and it has been validated for use in patients with spinal muscular atrophy (SMA).1 With the recent availability of disease modifying therapies for SMA,2 improvements in ambulatory function are anticipated in this patient population,3 and comparison with healthy controls becomes even more critical to assess treatment effects. However, it is unclear which published 6MWT normative reference values (norms) are best suited for this purpose.
The currently approved treatments for SMA provide optimal benefit if administered presymptomatically.4–6 As treated infants with SMA grow and develop, new phenotypes in SMA are emerging. Similarly, ambulatory SMA patients who receive treatment are demonstrating trajectories inconsistent with the natural history of the disease.7,8 Therefore, it is becoming apparent that healthy norms in 6MWT distance (6MWD) will be necessary in this population as people with SMA begin to close the gap with their healthy peers.
There are numerous healthy norms for the 6MWT available9–12 and it is unclear which of these would be most appropriate to compare with people with SMA. Moreover, reference equations from various normative data sets for the 6MWT in healthy people have not been compared with 6MWDs in people with SMA, including the normative reference equations most widely used in clinical populations.13–15 Therefore, the aims of this study were two-fold: first, to systematically review the literature of healthy normative data for the 6MWT in an effort to identify potential reference equations for use in SMA; and, second, to compare the walking distances from a 6MWT database of people with SMA using the selected normative reference equations in an effort to identify reference equations that may be suitable to assess treatment effects in people with SMA.
2 |. METHODS
2.1 |. Search strategies
PubMed and Scopus databases were searched using the key words search of “6 minute walk test” OR “6MWT” OR “six minute walk test” AND “healthy” AND “reference”. Articles searched were published between January 1960 and December 2017. Once duplicates and non-English studies were eliminated, titles and abstracts of the remaining articles were screened based on the following criteria. Studies were selected for inclusion if they evaluated healthy people (children and adults), and if the purpose of the study was to develop normative reference data (reference equation) for the 6MWT. Studies meeting these criteria were fully reviewed and selected for relevance. Reference lists of the selected studies also were examined for additional publications. Finally, the selected articles were entered into Google Scholar and the “related articles” link was explored for any additional studies.
2.2 |. Candidate study selection
An initial review was performed independently by two authors (A.G., K.C.) based on the following inclusion criteria: (1) the purpose of the study was to establish normative reference data for the 6MWT; (2) a reference equation was provided which included only variables commonly collected in a clinical setting such as height, weight, and age; (3) only the first test was used in developing the reference equation, if multiple tests were performed; and (4) the sample was a heterogeneous representative of a diverse, urban population (e.g., healthy participants recruited from a large university or medical center). The majority of studies did not report race and as such an indication of a heterogeneous sample was defined as samples that were collected in a large city at a large university or medical center that likely has a diverse representation of race/ethnicity. Exclusion criteria were studies with deviations from American Thoracic Society (ATS) protocols for administering the 6MWT (course length [<20 meters and >30 meters], course location, and/or lack of standardized instructions or encouragement), and single sex studies. Any disagreements from the initial review in applying the inclusion/exclusion criteria were settled by a blinded third party (J.M.). If a study met all inclusion and exclusion criteria, but a reference equation was not provided, the authors were contacted to provide the reference equation for inclusion in the analysis. A flow diagram of the systematic review is depicted in Supporting Information Figure S1, which is available online.
2.3 |. Application of SMA clinical data to the selected studies
A retrospective chart review of 6MWT data from children and adults with SMA collected between August 2008 and December 2017 at the Columbia University Irving Medical Center (CUIMC) Neuromuscular Clinic was performed (Institutional Review Board [IRB] AAAE8252). These tests were performed by trained physiotherapists as part of a regular clinical care visit. A 25-meter course was used and the test was administered according to a standardized protocol that adheres to the ATS guidelines for administering the test16 and is suitable for SMA.17 For individuals who performed multiple tests, only the first test was included for analysis to ensure consistent comparison across the sample. Only ambulatory individuals with SMA Type 3 were included. No participants received any investigational or approved disease-modifying therapies for SMA at the time of the assessment. All participants or their parents gave written informed consent, and the study conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee, CUIMC IRB.
2.4 |. Statistical analysis
Data analyses were performed using SPSS (IBM Corp. Released 2016. SPSS Statistics for Windows, Version 24.0; Armonk, NY). The percentage of the predicted distance walked (% predicted) was calculated by comparing the observed 6MWD of people with SMA to the predicted 6MWD derived from the selected reference equations [% predicted distance = (observed 6MWD / predicted 6MWD) * 100]. The selected pediatric healthy reference equations were used for the pediatric SMA sample (age range, 4-18 years), and the selected adult healthy reference equations were used for the adult SMA sample (age range, 19-49 years). The means of the % predicted percentages calculated from the selected pediatric and adult reference equations were calculated and compared. Means and standard deviations (SDs) were calculated for continuous data, and frequencies were calculated for categorical data. After checking for normality using the Shapiro-Wilk test, repeated measures ANOVA was used to compare the means of the calculated % predicted values between the included studies, derived from the adult and pediatric SMA data. Tests of between-study effects were used for comparisons. Pairwise comparisons among the three pediatric and six adult equations were performed post hoc, adjusting for multiple comparisons. Observed 6MWDs from the pediatric and adult SMA clinical samples were also individually examined and compared with predicted distances from the selected pediatric and adult normative reference equations. While a meta-analysis to understand the variation in normative data may have been informative, it was not possible, as we did not use or have access to the raw normative data for creating the reference equations (three equations for pediatric, six equations for adult); as such we applied each of the equations to applicable patients in our clinical data set. Statistical significance was set a priori at α < 0.05.
3 |. RESULTS
3.1 |. Systematic review
The literature search in PubMed and Scopus databases resulted in 148 and 173 articles, respectively. After removing duplicates and non-English studies, and performing a title and abstract review for relevance, the total number of articles to be considered for review was 47 (19 pediatric) (Supporting Information Table S1). Once strict inclusion and exclusion criteria were applied, three pediatric18–20 and six adult19,21–25 studies were included for analysis (Table 1). The most widely used normative datasets in neuromuscular disorders including SMA did not meet strict inclusion criteria and were not considered candidate studies for analyses.13–15
TABLE 1.
Healthy normative 6MWT reference studies selected for comparison
| Study | Sample | Standardization | Outcomes | ||||
|---|---|---|---|---|---|---|---|
| First author | Country | Age range (y) | N Total (M/F) | Course length (m) | Course location reported | Reference values provided | Predictor variables |
| Pediatric | |||||||
| Chen18 | Taiwan | 7-17 | 762 (382/380) | 30 | Hallways at schools, validated at hospital | Normal reference centile charts by height for 6MWD for males and females | Age, gender, height |
| McKay19 | Australia | 3-19 | 300 (150/150) | 25 | Laboratory (hallway) | Normal reference percentiles by age groups and gender for 6MWD | Age, gender, height, weight |
| Oliveira20 | Brazil | 6-13 | 161 (77/84) | 22 | Corridors at schools | Normal reference equation for 6MWD | Age, gender, height, weight |
| Adult | |||||||
| Ajiboye21 | Nigeria | 21-67 | 422 (224/198) | 30 | Straight, flat surface of medical gymnasium | Normal reference equation for 6MWD | Age, gender, height, weight |
| Chetta22 | Italy | 20-50 | 102 (48/54) | 30 | Undisturbed, indoor, level, hospital corridor | Normal reference equation for 6MWD | Age, gender, height, |
| Fernandes23 | West India | 25-75 | 169 (80/89) | 30 | Undisturbed, straight corridor | Normal reference equation for 6MWD | Age, gender |
| McKay19 | Australia | 20-59 | 400 (200/200) | 25 | Laboratory (hallway) | Normal reference percentiles by age groups and gender for 6MWD | Age, gender, height, weight |
| Palaniappan24 | India | 25-80 | 125 (58/67) | 30 | Indoor hospital corridor | Normal reference equation for 6MWD | Age, gender, height, weight |
| Shrestha25 | Nepal | 18-81 | 250 (166/76) | 30 | Long, flat, straight corridor in an outpatient center | Normal reference equation for 6MWD | Age, gender, weight |
3.2 |. SMA clinical data
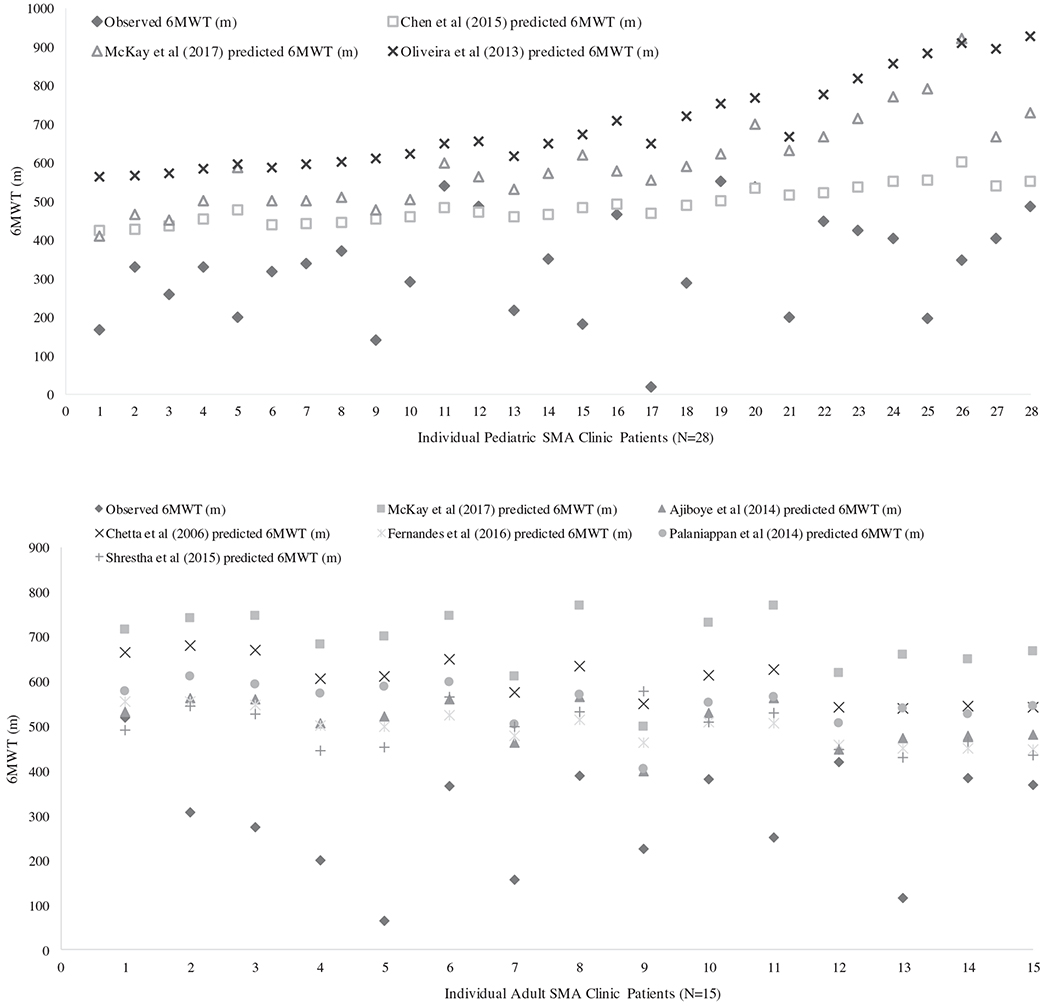
Participant characteristics for the pediatric and adult SMA clinical sample are provided in Table 2. The Shapiro–Wilk test for normality indicated the observed 6MWDs were normally distributed for both pediatric and adult samples (W = 0.973, P = 0.659, W = 0.967, P = 0.814, respectively). Figure 1 shows observed 6MWDs in each SMA individual in the clinical sample compared with the predicted 6MWD calculated from the reference equations reported or obtained from the selected healthy pediatric and adult studies in the systematic review, respectively.
TABLE 2.
CUIMC SMA clinic participant characteristics
| Participant characteristic | Pediatric SMA sample (N = 28) | Adult SMA sample (N = 15) |
|---|---|---|
| Age (years) | 9.7 ± 4.9 | 36.6 ± 10.7 |
| Sex (% male) | 68% | 47% |
| Height (cm) | 134.2 ± 26.1 | 166. 5 ± 11.2 |
| Weight (kg) | 38.7 ± 22.1 | 71.1 ± 16.9 |
| BMI (kg/m2) | 19.7 ± 5.3 | 25.8 ± 7.2 |
| 6MWT (m) | 332.2 ± 135.3 | 294.9 ± 125.2 |
Note: Results are presented as mean ± SD or % of sample. SMA sample comprised of people with SMA Type 3.
FIGURE 1.
A, Pediatric observed 6MWD in clinical data set compared with predicted 6MWD, for three selected studies. Predicted distances calculated using reference equations from the selected pediatric studies and observed 6MWT for individual participants (N = 28). B, Adult observed 6MWD in clinical data set compared with predicted 6MWT distance, for six selected studies. Predicted distances calculated using reference equations from the selected adult studies and observed 6MWT for individual participants (N = 15)
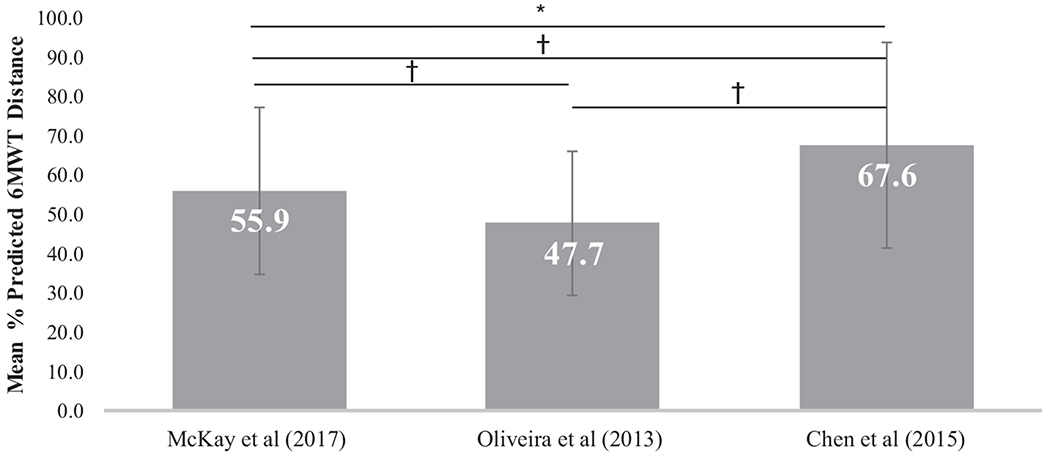
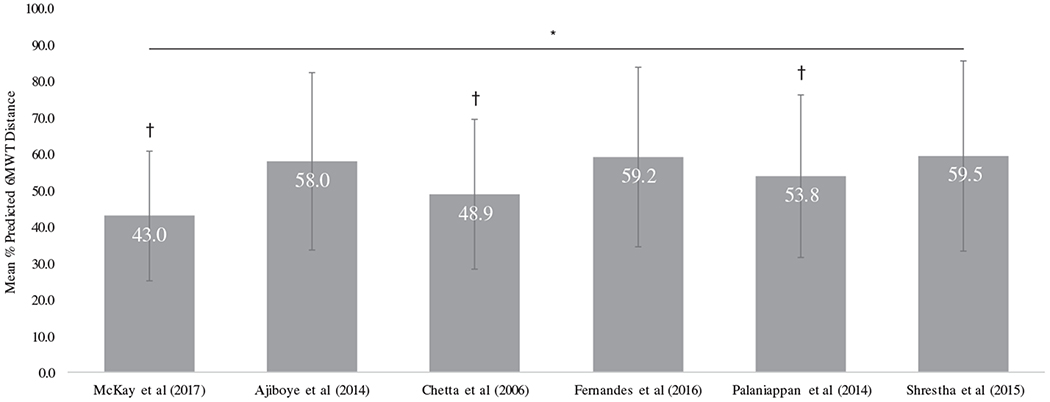
When comparing the mean % predicted values, there were significant differences across the three pediatric normative reference equations when the SMA clinical data was applied (Figure 2). Pairwise comparisons among the three pediatric equations, adjusting for multiple comparisons, revealed significant differences between all three studies. In adults with SMA, there also were significant differences between the % predicted values from the clinical data using the six selected equations (Figure 3). Pairwise comparisons among the six adult equations, adjusting for multiple comparisons, revealed three studies (McKay,19 Chetta,22 and Palaniappan2) were significantly different from all other studies. Three studies (Ajiboye,21 Fernandes,23 and Shrestha25) were not significantly different from each other (P > .05). All three of these studies had lower predicted distances which resulted in higher % predicted distance values when the clinical data was applied.
FIGURE 2.
Comparison of mean % predicted 6MWD across the selected pediatric normative studies. Mean % predicted 6MWD calculated using the clinical data set applied separately across the three selected pediatric equations. Error bars indicate SDs. *P < 0.001 for the repeated measures ANOVA. †P < .001 for the pairwise comparisons
FIGURE 3.
Comparison of mean % predicted 6MWD across the selected adult normative studies. Mean % predicted 6MWD calculated using the clinical data set applied separately across the six selected adult equations. Error bars indicate SDs. *P < 0.001 for the repeated measures ANOVA. †P < .01 for the pairwise comparisons
4 |. DISCUSSION
We found significant variability in the calculated % predicted 6MWDs of pediatric and adult SMA samples across the selected healthy normative data sets. It remains unclear which reference equation should be used when evaluating the performance of SMA patients. All studies included in this systematic review adhered to ATS guidelines for administering the 6MWT and were representative of a diverse population commonly seen in specialty clinics for people with SMA, suggesting that this variability likely cannot be attributed to deviations in test standardization.
The protocol for the 6MWT, as set forth by the ATS, includes specific guidelines for course length, location, and standardized instructions/encouragement.16 Procedures established for SMA patients adhere to these guidelines, and the data collected in our clinical sample adhered to these.17 Understandably, the methodology and administration of the 6MWT can impact total distance covered.26 Therefore, we excluded any studies that deviated from the ATS guidelines. Our strict inclusion and exclusion criteria allowed us to observe differences in the reference equations that could not be attributed to testing standard bias.
Other considerations are necessary to develop an optimal reference equation for people with SMA. For example, one approach may be to aim to closely match the demographics of the healthy control cohort to the patient population, or consider which predictive variables may be most important for evaluating 6MWT performance in a larger sample of patients with SMA. Weight was included in the majority of reference equations included in our analysis. However, in SMA due to decreased muscle mass, weight may not adequately reflect the impact of body composition on function. Other anthropometric measures, such as waist circumference, may make comparisons of SMA patients to their healthy peers more meaningful. Furthermore, shorter course lengths may disproportionately impact individuals with SMA who have more difficulty with turns at the end of the 6MWT course and may reduce the overall walking distance achieved.
It is important to point out that three healthy normative reference equations, commonly used in various neuromuscular studies,27–33 fail to meet our inclusion criteria. Currently, the published norms for the 6MWT most widely used are the Geiger norms13 for pediatric populations, and the Enright14 or Gibbons15 norms for adult populations. Surprisingly perhaps, these studies posed various methodological and population concerns that forced us to exclude them from this analysis. For example, Geiger and colleagues collected normative data from a homogenous population from Austria with body mass index (BMI) that was, on average, very low. Additionally, several testing aspects of the study by Geiger et al. did not adhere to ATS protocols, such as an outdoor course, the use of an incentive device, and the development of a reference equation using the greatest distance of three recorded trials. In adults, similar methodological and sample concerns were identified. In fact, Enright and Sherrill caution others when applying their regression equation to non-Caucasians and to participants under age 40 years.14 While Gibbons and colleagues followed 6MWT protocols similar to ATS guidelines, they used the best recorded score out of four tests to model their equation, a criterion that would disadvantage adults with SMA who perform only one test.15 These equations may in fact be suitable in the setting in which they were obtained and in certain clinic settings, but their exclusion from our analysis suggests that the methods and sample used in developing healthy norms need to more precisely match the protocols and clinical population where they are being applied, such as an SMA clinic.
Recently, the first disease-modifying treatment was approved for SMA2,34 and other therapies are currently in clinical trials.35 As new phenotypes in SMA are emerging following treatment,4–6 there is a growing need to compare patients with SMA with healthy controls in an attempt to assess treatment effects. This will be particularly important as treated patients with SMA begin to close the performance gap in ambulatory function and endurance relative to their unaffected peers. Unfortunately, based on this systematic review, the optimal reference equations for calculating % predicted 6MWD in this patient population is not known. Until an appropriate reference equation is determined, we recommend using the raw 6MWD and percentage fatigue36 when analyzing the 6MWT performance of patients with SMA. By so doing, one can evaluate an individual over time to assess relative performance and to capture a possible therapeutic trajectory.
This study is not without limitations. The systematic review was limited to articles written in English. The clinical SMA data being used for comparison was reviewed retrospectively; therefore, only commonly collected clinical variables could be used in the reference equations considered for inclusion. The sample sizes for the SMA clinical data were also small (pediatric N = 28; adult N = 15), which may have contributed to the significant variability in % predicted values. Additionally, race/ethnicity was not available for comparison between our clinical SMA data and the normative reference equations. A prospective study may make it possible to include a more extensive list of variables such as heart rate, blood pressure, and other anthropometric measures (e.g., waist circumference) to determine the most relevant information for predicting 6MWT performance. Importantly, caution will be necessary in selecting variables in new equations that could be affected by the disease. A prospective study with these considerations is a direction to be considered for future research.
In conclusion, the most representative healthy normative reference equation for estimating the % predicted 6MWT performance in patients with SMA is unclear. Our results show significant variability between % predicted values derived from existing published reference equations in children and adults, despite strict adherence to 6MWT standardization criteria. The variability in currently available equations emphasizes the need for further examination.
Supplementary Material
ACKNOWLEDGEMNT
We thank and acknowledge all patients and families who receive clinical care at the CUIMC Neuromuscular Clinic, and all clinical staff involved in collecting 6MWT data. We also acknowledge Dr. Michael McDermott for his review of the statistical methods, and Dr. Jennifer Baldwin for her involvement in the collection of the 1000 norms data. Material from this manuscript was contained in a presentation at the Cure SMA Annual Meeting in Dallas, Texas, June 15, 2018.
Funding information
This work was supported by the SMA Foundation, the Program in Physical Therapy at Columbia University Irving Medical Center, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development 1K01HD084690.
CONFLICT OF INTEREST
Ashley Goodwin, Kayla Cornett, and Marnee McKay have no conflicts of interest to disclose. Joshua Burns: None pertaining to this study. General: Joshua Burns’ research and clinical activities are funded by the Australian Department of Health (Medical Research Future Fund), US National Institutes of Health, Charcot-Marie Tooth Association of Australia, Charcot-Marie Tooth Association (USA), Diabetes Australia, Elizabeth Lottie May Rosenthal Bone Bequest, Perpetual Limited, Humpty Dumpty Foundation. Consultancies: Acceleron Pharma, Pharnext. Carol Ewing Garber has no conflicts of interest to disclose. Darryl De Vivo: None pertaining to this study. General: Darryl De Vivo has received personal compensation for activities with AveXis, Biogen, Roche, IONIS, Sarepta, Cytokinetics Pharmaceuticals, PTC, Mallinckrodt, Santhera, Scholar Rock and the SMA Foundation and has received research support from NIH, DOD, SMA Foundation, Glut1 Disease Foundation and Hope for Children Research Foundation. Jacqueline Montes: None pertaining to this study. General: Jacqueline Montes has served as a member of advisory boards for Biogen, Cytokinetics, Roche, and Scholar Rock; consultant to Biogen and Scholar Rock; and received research support from the Muscular Dystrophy Association and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health.
Abbreviations:
- 6MWD
6-minute walk test distance
- 6MWT
6-minute walk test
- ANOVA
analysis of variance
- ATS
American Thoracic Society
- BMI
body mass index
- CUIMC
Columbia University Irving Medical Center
- IRB
Institutional Review Board
- norm
normative reference value
- SD
standard deviation
- SMA
spinal muscular atrophy
Footnotes
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Dunaway Young S, Montes J, et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. 2016;54(5):836–842. [DOI] [PubMed] [Google Scholar]
- 2.Corey DR. Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat Neurosci. 2017;20(4):497. [DOI] [PubMed] [Google Scholar]
- 3.Montes J, Young SD, Mazzone E, et al. Ambulatory function and fatigue in nusinersen-treated children with spinal muscular atrophy. (P2.322). Neurology. 2018;90(15 Suppl):P2.322. [Google Scholar]
- 4.De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29:842–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraszewski JN, Kay DM, Stevens CF, et al. Pilot study of population-based newborn screening for spinal muscular atrophy in New York state. Genet Med. 2018;20(6):608–613. [DOI] [PubMed] [Google Scholar]
- 6.Servais L “The Times They Are a-Changin’.” In reply to El-Zaidy et al., AVXS-101 (Onasemnogene Abeparvovec) for SMA1: comparative study with a prospective natural history cohort. J Neuromuscul Dis. 2019;6(3):319–320. [DOI] [PubMed] [Google Scholar]
- 7.Montes J, Dunaway Young S, et al. Nusinersen improves walking distance and reduces fatigue in later-onset spinal muscular atrophy. Muscle Nerve. 2019;60(4):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darras BT, Chiriboga CA, Iannaccone ST, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology. 2019;92(21):e2492–e2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacau LA, de Santana-Filho VJ, Maynard LG, Neto GM, Fernandes M, Carvalho VO. Reference values for the 6-minute walk test in healthy children and adolescents: a systematic review. Braz J Cardiovasc Surg. 2016;31(5):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dourado VZ. Equações de referência para o teste de caminhada de seis minutos em indivíduos saudáveis. Arq Bras Cardiol. 2011;96:e128–e138. [PubMed] [Google Scholar]
- 11.Mylius CF, Paap D, Takken T. Reference value for the 6-minute walk test in children and adolescents: a systematic review. Exp Rev Respir Med. 2016;10(12):1335–1352. [DOI] [PubMed] [Google Scholar]
- 12.Salbach NM, O’Brien KK, Brooks D, et al. Reference values for standardized tests of walking speed and distance: a systematic review. Gait Posture. 2015;41(2):341–360. [DOI] [PubMed] [Google Scholar]
- 13.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150(4):395–399. e392. [DOI] [PubMed] [Google Scholar]
- 14.Enright PL, Sherrill DL. Reference equations for the 6-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5):1384–1387. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21(2):87–93. [DOI] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the 6-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 17.Montes J, Blumenschine M, Dunaway S, et al. Weakness and fatigue in diverse neuromuscular diseases. J Child Neurol. 2013;28(10):1277–1283. [DOI] [PubMed] [Google Scholar]
- 18.Chen CA, Chang CH, Lin MT, et al. Six-minute walking test: normal reference values for Taiwanese children and adolescents. Acta Cardiol Sin. 2015;31(3):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay MJ, Baldwin JN, Ferreira P, et al. Reference values for developing responsive functional outcome measures across the lifespan. Neurology. 2017;88(16):1512–1519. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira AC, Rodrigues CC, Rolim DS, et al. Six-minute walk test in healthy children: is the leg length important? Pediatr Pulmonol. 2013; 48(9):921–926. [DOI] [PubMed] [Google Scholar]
- 21.Ajiboye OA, Anigbogu CN, Ajuluchukwu JN, Jaja SI. Prediction equations for 6-minute walk distance in apparently healthy Nigerians. Hong Kong Physiother J. 2014;32(2):65–72. [Google Scholar]
- 22.Chetta A, Zanini A, Pisi G, et al. Reference values for the 6-min walk test in healthy subjects 20-50 years old. Respir Med. 2006;100(9): 1573–1578. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes L, Mesquita AM, Vadala R, Dias A. Reference equation for 6 minute walk test in healthy western India population. J Clin Diagn Res. 2016;10(5):CC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palaniappan Ramanathan R, Chandrasekaran B. Reference equations for 6-min walk test in healthy Indian subjects (25-80 years). Lung India. 2014;31(1):35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha SK, Srivastava B. Six minute walk distance and reference equations in normal healthy subjects of Nepal. Kathmandu Univ Med j (KUMJ). 2015;13(50):97–101. [DOI] [PubMed] [Google Scholar]
- 26.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428. [DOI] [PubMed] [Google Scholar]
- 27.Argov Z, Bronstein F, Esposito A, et al. Characterization of strength and function in ambulatory adults with GNE myopathy. J Clin Neuromuscul Dis. 2017;19(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014;50(4):477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henricson E, Abresch R, Han JJ, Nicorici A. Percent-predicted 6-minute walk distance in duchenne muscular dystrophy to account for maturational influences. PLoS Curr. 2012;4:RRN1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtebekk ME, Berntsen S, Rasmussen M, Jahnsen RB. Physical activity and motor function in children and adolescents with neuromuscular disorders. Pediatr Phys Ther. 2013;25(4):415–420. [DOI] [PubMed] [Google Scholar]
- 31.Montes J, Glanzman AM, Mazzone ES, et al. Spinal muscular atrophy functional composite score: a functional measure in spinal muscular atrophy. Muscle Nerve. 2015;52(6):942–947. [DOI] [PubMed] [Google Scholar]
- 32.Salci Y, Karanfil E, Balkan AF, et al. Functional exercise capacity evaluated by timed walk tests in myasthenia gravis. Muscle Nerve. 2019;59(2):208–212. [DOI] [PubMed] [Google Scholar]
- 33.Wokke JHJ, Wokke JHJ, Escolar DM, Pestronk A, Jaffe KM. Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve. 38(4):1236–1245. [DOI] [PubMed] [Google Scholar]
- 34.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017–3026. [DOI] [PubMed] [Google Scholar]
- 35.Scoto M, Finkel R, Mercuri E, Muntoni F. Therapeutic approaches for spinal muscular atrophy (SMA). Gene Ther. 2017;24(9):514–519. [DOI] [PubMed] [Google Scholar]
- 36.Montes J, McDermott M, Martens W, et al. Six-minute walk test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010;74(10):833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.