Abstract
Background:
Exposure to air pollution is associated with increased blood pressure (BP) in adults and children. Some evidence suggests that air pollution exposure during the prenatal period may contribute to adverse cardiorenal health later in life. Here we apply a distributed lag model (DLM) approach to identify critical windows that may underlie the association between prenatal particulate matter ≤ 2.5 μm in diameter (PM2.5) exposure and children’s BP at ages 4 to 6 years.
Methods:
Participants included 537 mother-child dyads enrolled in the Programming Research in Obesity, GRowth Environment, and Social Stress (PROGRESS) longitudinal birth cohort study based in Mexico City. Prenatal daily PM2.5 exposure was estimated using a validated satellite-based spatio-temporal model and BP was measured using the automated Spacelabs system with a sized cuff. We used distributed lag models (DLMs) to examine associations between daily PM2.5 exposure and systolic and diastolic BP (SBP and DBP), adjusting for child’s age, sex and BMI, as well as maternal education, preeclampsia and indoor smoking report during the second and third trimester, seasonality and average postnatal year 1 PM2.5 exposure.
Results:
We found that PM2.5 exposure between weeks 11 to 32 of gestation (days 80 to 226) was significantly associated with children’s increased SBP. Similarly, PM2.5 exposure between weeks 9 to 25 of gestation (days 63 to 176) was significantly associated with increased DBP. To place this into context, a constant 10 μg/m3 increase in PM2.5 sustained throughout this critical window would predict a cumulative increase of 2.6 mmHg (CI: 0.5, 4.6) in SBP and 0.88 mmHg (CI: 0.1, 1.6) in DBP at ages 4 to 6 years. In a stratified analysis by sex, this association persisted in boys but not in girls.
Conclusions:
Second and third trimester PM2.5 exposure may increase children’s BP in early life. Further work investigating PM2.5 exposure with BP trajectories later in childhood will be important to understanding cardiorenal trajectories that may predict adult disease. Our results underscore the importance of reducing air pollution exposure among susceptible populations, including pregnant women.
Keywords: particulate matter, blood pressure, distributive lag models, prenatal exposure, Bayesian distributed lag interaction models
Introduction
Air pollution exposure leads to millions of premature deaths worldwide, with cardiovascular diseases accounting for 60–80% of these deaths (Lelieveld et al., 2015; Lelieveld et al., 2019). The World Health Organization (WHO) recommends an annual mean level of particulate matter with aerodynamic diameter ≤2.5 microns (PM2.5) no more than 10 μg/m3 to protect human health, and notes that no level of PM2.5 has shown to be free of health impacts (Shi et al., 2016; WHO, 2018). In 2018, the estimated average PM2.5 level in Mexico City was 19.7 μg/m3, ranking it as the 30th highest (worst) capital city globally for estimated average PM2.5 level (AirVisual, 2018). Exposure to air pollution has been associated with increased blood pressure (BP) and hypertensive diseases in several populations (Bo et al., 2019; Curto et al., 2019; Sears et al., 2018). In Mexico, the location of our study, potential cardiovascular effects of air pollution are of critical economic and public health importance. In 2011, there were an estimated $5.7 billion (in U.S. dollars) in direct and indirect healthcare costs due to hypertension in Mexico (Arredondo and Aviles, 2014; Arredondo A. et al., 2013). According to the Mexican National Health Survey, in 2012 there were 22.4 million adult cases of hypertension, of which only (50%) had a diagnosis and only 25% had their hypertension controlled (Arredondo and Aviles, 2014; Campos-Nonato et al., 2013). A recent study of 1,452 children aged 6–14 years living in Mexico City reported prevalence of hypertension of 1.5% and 16.3% reported family history of hypertension (Vashi et al., 2016). Since childhood BP trajectories track with adult risk for high BP, early detection and intervention is imperative (Chen et al., 2008; Theodore et al., 2015).
Moreover, evidence suggests that early life exposures are associated with childhood BP. A recent cross-sectional study of 43,745 children and adolescents aged 7 to 18 years in China found that a 10 μg/m3 increase in PM2.5 exposure in the year before the measurement was associated with a 1.5 mmHg increase in systolic blood pressure (SBP) (Zhang et al., 2019). Further, recent evidence from both human and animal studies suggests that air pollution exposure during the prenatal period adversely contributes to adolescent cardiovascular health, and thus presents immediate and later life concerns. A study of 1293 mother-child dyads participating in the Boston Birth Cohort reported that maternal exposure to PM2.5 in the third trimester was associated with a 3.5 percentile increase in child SBP at ages 3 to 9 years (Zhang et al., 2018). In a study in mice, offspring born to dams exposed to PM2.5 in pregnancy showed evidence of cardiac dysfunction in adolescence compared to offspring born to dams exposed to filtered air in pregnancy (Tanwar et al., 2017). Although the prenatal period has been identified as a susceptible period of air pollution-related cardiovascular effects, no previous studies have identified specific windows of susceptibility during pregnancy.
Susceptibility to prenatal air pollution exposure has also been shown to vary by fetal sex (Hsu et al., 2015; Lertxundi et al., 2019; Rosa et al., 2019). Only a few studies have examined sex as a potential effect modifier of the association between prenatal air pollution exposure and BP in childhood (van Rossem et al., 2015; Zhang et al., 2018; Zhang et al., 2019). These studies have reported mixed results, including no evidence of effect modification (van Rossem et al., 2015; Zhang et al., 2018), higher SBP in boys when compared to girls and higher diastolic blood pressure (DBP) in girls when compared to boys (Zhang et al., 2019).
In the present study, we sought to identify critical windows of exposure to PM2.5 associated with increased children’s BP at ages 4 to 6 years in the Programming Research in Obesity, GRowth Environment, and Social Stress (PROGRESS) longitudinal birth cohort study. We also examined potential effect modification by sex. Identifying potential critical windows of PM2.5 exposure during development and understanding the role of sex in these associations is important to our understanding of adverse life course cardiovascular events that may initiate even in utero. This work contributes to the literature examining the prenatal effects of air pollution, especially among susceptible populations such as pregnant women. It also supports the continued need for informed strategies for air pollution reduction.
Methods
Study population
Pregnant women who were receiving prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social –IMSS) were recruited into the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study between July 2007 and February 2011. Participants’ eligibility criteria at enrollment included: less than 20 weeks gestation, at least 18 years of age, had completed primary education, planned to stay in Mexico City for the next 3 years, had access to a telephone, had no medical history of heart or kidney disease, did not consume alcohol daily, and did not use any steroid or anti-epilepsy medications. Procedures were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai (#12–00751), and the Mexican National Institute of Public Health (project #560). After a detailed explanation of the project protocols, women provided written informed consent.
Prenatal PM2.5 levels
Daily residential exposure to PM2.5 was estimated during pregnancy using a previously described spatio-temporal model that incorporates Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements at a 1×1 km spatial resolution (Just et al., 2015). In brief, remote sensing data were calibrated with municipal ground level monitors of PM2.5, meteorological data and land use regression (LUR) variables (roadway density, temperature, relative humidity, planetary boundary layer and daily precipitation) to generate estimates of daily residential PM2.5 levels for each participant. Mixed effect models with spatial and temporal predictors and day-specific random effects were used to account for temporal variations in the PM2.5−AOD relationship. The model was fit with a seasonal smooth function of latitude and longitude and time-varying average incorporating local monitoring for days without AOD data. Model performance was assessed using monitor-level leave one out cross-validation; the model performed well with an R2 of 0.724. We refer the reader to (Just et al., 2015) for additional details of the spatio-temporal model methods and performance. The latitude and longitude of the maternal residence during pregnancy was captured by study personnel using hand-held GPS devices and cross-checked with mapping of major cross streets. In trimester-specific analyses, PM2.5 levels were averaged over clinically defined trimesters (in gestational weeks). In these trimester-specific analyses, we calculated the average PM2.5 over the 1st trimester defined as weeks 1–13 gestation, the 2nd trimester defined as weeks 14–27 gestation, and the 3rd trimester defined as 28 weeks’ gestation to delivery.
Blood pressure measurement
Children’s resting BP was measured using a Spacelabs Healthcare automated oscillometric device (Ambulatory BP 90207 monitor, WA, USA) as previously described (Sanders et al., 2018). After a rest period of 3–5 minutes, two BP measurements were taken using the automated Spacelabs system with a child-sized cuff. Advantages of the Spacelabs system over standard sphygmomanometer measurement include the elimination of observer bias and reduction of potential visit/procedural-induced anxiety or white coat hypertension (Gillman and Cook, 1995; Mattu et al., 2001). For secondary analyses, SBP and DBP percentiles, calculated based on the clinical guidelines published in 2017 (Flynn et al., 2017), were also considered as outcomes in linear models.
Covariates
Covariates were selected a priori based on previous literature (Aris et al., 2019) and included child’s sex, age, and body mass index (BMI) i.e. weight divided by height squared (kg/m2) at BP measurement, average first postnatal year PM2.5, seasonality of PM2.5 exposure, maternal educational attainment at enrollment (categorized as <high school, some high school or high school graduate, >high school), preeclampsia (yes/no), and prenatal tobacco smoke exposure. Prenatal exposure to environmental tobacco smoke was defined as report of any smoker in the home during the second or third trimester of pregnancy. Seasonality of PM2.5 exposure was defined as sine and cosine of time of year (Stolwijk et al., 1999; van Rossem et al., 2015). In linear models, where no time series data were used, season of conception was used to account for seasonality and was defined according to weather patterns in Mexico City as dry cold (January-February; November-December), dry warm (March-April) and rainy (May-October).
Statistical Analyses
In this study, 602 PROGRESS participants had BP measured at ages 4 to 6. Of these, 2 were missing PM2.5 data. Children born preterm (<37 weeks gestation) and post term (>42 weeks gestation) were excluded from these analyses (final n=537). Ultrasounds are not routinely performed as standard of care in Mexico, therefore gestational age was based on last menstrual period (LMP) and by a standardized physical examination to determine gestational age at birth (Capurro et al., 1978). Physical exam gestational age was used instead of the gestational age determined by LMP If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP. A sensitivity analysis was performed including these individuals. We fitted distributive lag models (DLMs) to estimate the time-varying association between BP at ages 4 to 6 years and estimated daily PM2.5 level from 60 days prior to conception to 60 days post birth (390 days). This method incorporates data from all time points simultaneously and assumes that the association between the outcome and exposure at a given time point, controlling for exposure at all other time points, varies smoothly as a function of time. For SBP a DLM that modeled a smooth function using B-splines with 2 degrees of freedom was fit (Gasparrini, 2011; Gasparrini et al., 2010), DBP was modeled using B-splines with 1 degree of freedom, both were chosen due to their parsimony and best AIC value; additional smoothing did not significantly improve the model. A sensitive window was identified when the pointwise 95% confidence bands did not contain zero. DLMs were implemented using the dlnm package version 2.3.9 (Gasparrini, 2011) in R Version 3.5.1 (Boston, MA) and other analyses were performed in SPSS version 24 (Chicago, IL). Traditional linear regression models with adjustment for the same covariates were also run to compare DLM results to clinically defined trimesters and average PM2.5 over pregnancy. In order to avoid bias in estimates (Wilson et al., 2017b), average exposure from all three trimesters were included in a single model.
Exploratory Bayesian distributed lag interaction models (BDLIM) were employed to determine effect modification by child sex using weekly average PM2.5. In brief, BDLIM estimates the cumulative effect of PM2.5 exposure over the entire pregnancy for each sex-specific subgroup, accounting for identified sensitive windows and within-window effects (Wilson et al., 2017a). In these models, each stratum can have either the same or different sensitive windows or the same or different within-window effects (Wilson et al., 2017a). The model quantifies the likelihood of each pattern of heterogeneity and estimates the association between exposure and outcome under the effect modification pattern that is best supported by the data. In addition to sensitive windows, BDLIM estimates the cumulative effect of PM2.5 exposure over pregnancy for each stratum accounting for sensitive windows and within-window effects.
Results
Participants’ demographic characteristics are shown in Table 1. The majority of mothers included in this study had 12 or fewer years of schooling (76.2%) and more than a third reported exposure to a smoker in the home during pregnancy. The average child’s SBP and DBP levels were 86/53, within the normal range for 4- to 6-year-olds (Baker-Smith et al., 2018); only three children (1%) had BPs above the 90th percentile for either SBP or DBP.
Table 1.
Demographics for mother-child dyads participating in the PROGRESS study at age 4 to 6 years.
| Characteristic | N=537 |
|---|---|
| n (%) | |
| Child sex | |
| Female | 267 (49.7) |
| Male | 270 (50.3) |
| Prenatal environmental tobacco smoke (ETS) exposure (yes) | 189 (35.2) |
| Maternal education at enrollment | |
| < High school | 210 (39.1) |
| Some high school or high school graduate | 199 (37.1) |
| > High school | 128 (23.8) |
| Maternal preeclampsia | 21 (3.9) |
| Mean ± std | |
| Maternal age at enrollment (years) | 27.7 ± 5.59 |
| Gestational age (weeks) | 38.7 ± 1.14 |
| Child age at BP measurement (years) | 4.80 ± 0.56 |
| Child BMI (kg/m2) | 15.7 ± 1.66 |
| Average prenatal PM2.5 (μg/m3) | 22.6 ± 2.62 |
| Child BP at 4–6 years (mm Hg) | |
| SBP | 86.1 ± 7.08 |
| DBP | 52.5 ± 5.84 |
In the linear models of trimester averaged PM2.5, we observed significant associations for 2nd trimester PM2.5 as well as average PM2.5 over pregnancy with children’s SBP and DBP (Table 2). Specifically, for each 10 μg/m3 increase in 2nd trimester PM2.5 we found a higher 2.1 mmHg SBP (95%CI: 0.4, 3.8) and 1.6 mmHg DBP (95%CI: 0.2, 3.1). Similarly, per each 10 μg/m3 increase in average PM2.5 over pregnancy we found a higher 2.2 mmHg DBP (95%CI: 0.3, 4.2) in childhood. A similar trend (2.0 mmHg increase per 10 μg/m3 average PM2.5 (95%CI: − 0.2, 4.3)) was observed for SBP although not statistically significant. We observed null relationships for the 1st and 3rd trimester averages with both SBP and DBP. We saw similar patterns but attenuated associations in sensitivity analyses including children born pre- and post-term (Supplemental Table S1) and when we used BP percentiles as outcomes (Supplemental Table S2).
Table 2. .
Association of trimester and average over pregnancy PM2.5 levels with blood pressure outcomes at age 4–6 years
| SBP β (95% CI) | p-value | DBP β (95% CI) | p-value | |
|---|---|---|---|---|
| Model 1 | ||||
| 1st trimester PM2.5 | −0.4 (−2.0, 1.2) | 0.60 | 0.3 (−1.1, 1.7) | 0.70 |
| 2nd trimester PM2.5 | 2.1 (0.4, 3.8) | 0.01 | 1.6 (0.2, 3.1) | 0.03 |
| 3rd trimester PM2.5 | 0.3 (−1.3, 1.9) | 0.70 | 0.1 (−1.2, 1.5) | 0.84 |
| Model 2 | ||||
| Average pregnancy PM2.5 | 2.0 (−0.2, 4.3) | 0.07 | 2.2 (0.3, 4.2) | 0.03 |
Estimates shown for a 10 μg/m3 increase in PM2.5. Models adjusted for child’s age, sex and BMI, maternal education and preeclampsia, prenatal ETS exposure, season of conception, other trimester PM2.5 concentrations (Model 1) and postnatal year 1 average PM2.5.
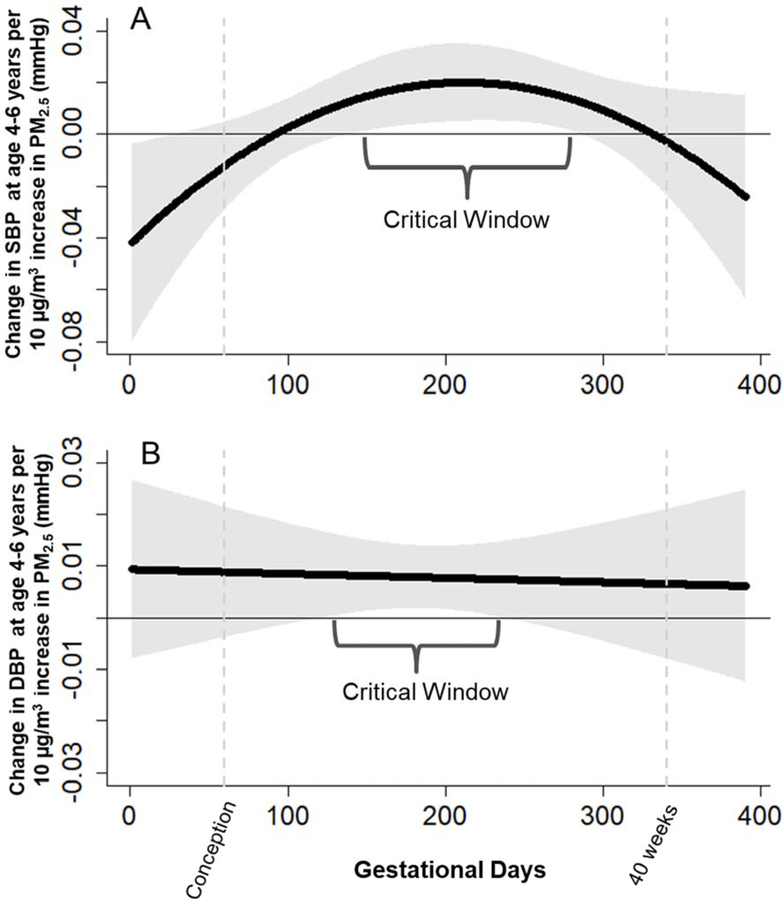
Next, we applied a DLM approach adjusting for maternal education, preeclampsia, seasonality of PM2.5 exposure, and prenatal tobacco smoke exposure, as well as child sex, age, and BMI at BP visit (Figure 1). We found significant associations between prenatal PM2.5 exposures in mid to late pregnancy and both SBP (Figure 1a) and DBP (Figure 1b) among children. Specifically, we identified a critical window of PM2.5 exposure between weeks 11 to 32 of gestation (days 80 to 226) and higher children’s SBP. We also identified a significant association between PM2.5 exposure between weeks 9 to 25 (days 63 to 176) higher DBP. To place this into context, a constant 10 μg/m3 increase in PM2.5 sustained throughout this critical window would predict a cumulative increase of 2.6 mmHg (CI: 0.5, 4.6) in SBP and 0.88 mmHg (CI: 0.1, 1.6) in DBP at ages 4 to 6 years. We did not observe significant associations between average first postnatal year PM2.5 and BP in any of our models.
Figure 1.
Associations between daily prenatal PM2.5 and a) SBP and b) DBP at 4–6 years. Models adjusted for child’s age, sex and BMI, maternal education and preeclampsia, prenatal ETS exposure, seasonality and postnatal year 1 average PM2.5.
In BDLIM analysis, we found that the association with SBP persisted only in boys and not in girls (Supplemental Figure S1) and found no sex-modification with DBP (data not shown). The BDLIM analysis suggested that effect modification by sex was attributable to both different windows and the difference in the magnitude of the within-window association (the normalized posterior density was 0.93 for the SBP model, interpreted as the probability that this was the best-fitting pattern of effect modification). We saw a significant window at 17–29 weeks of gestation in which increased PM2.5 was associated with higher SBP only in boys. We note that the direction of the association among girls was the same over this time period but did not reach statistical significance and we observed a greater magnitude of effect in boys. We also observed putative windows indicating sex-specific associations with lower BP from the postnatal period of weeks 41–45 in boys, and the preconception period of 1–10 weeks in girls.
Discussion
While the potential benefits of air pollution reduction to mortality among adults are widely studied, a growing body of evidence suggests children’s exposure to PM2.5 has substantial acute and latent effects on developing respiratory, renal, and cardiovascular physiology. These effects can directly impact child health and may also track into adult life with even greater societal importance. Herein, we assessed children’s vulnerability during the prenatal period and our findings suggest that prenatal exposure to PM2.5 during a specific window in mid to late pregnancy (between weeks 17 to 35 of gestation) is associated with higher SBP and DBP in childhood. Indeed, we observed a critical window of PM2.5 exposure between weeks 11 to 32 of gestation associated with children’s increased SBP, as well as between weeks 9 to 25 associated with increased DBP.
Our findings contribute to a limited but growing body of literature examining in utero air pollution exposure and children’s BP. Similar results were observed in a Boston Birth Cohort study that reported an association between third trimester PM2.5 exposure with increased offspring SBP percentile and risk for elevated BP (with an exposure distribution generally below that in our Mexican cohort). Specifically, each 5 μg/m3 increment in PM2.5 during the third trimester was associated with a 3.5 percentile increase in children ages 3–9 (Zhang et al., 2018). In the Child Health Study in California, the majority of trimester levels of PM2.5, PM10, NO2 and O3 were not associated with SBP and DBP measured at 11 years of age, except for third-trimester exposure to NO2, which was significantly associated with a 2.33 mmHg increase in SBP per 21 ppb increase in NO2 (Breton et al., 2016). In addition, two studies assessed newborn BP and reported similar findings. A study of 1131 mother-infant pairs participating in the Project Viva birth cohort in Boston, MA reported that higher mean PM2.5 exposure in the third trimester was marginally associated with increased newborn SBP (an IQR increase in PM2.5 was associated with a 0.5 mmHg higher SBP) (van Rossem et al., 2015). A study of 427 term infants in the Belgian ENVIRONAGE cohort reported that a 5 μg/m3 increase in prenatal PM2.5 was associated with a 2.4 mmHg higher SBP and 1.8 mmHg higher DBP at birth (Madhloum et al., 2019), with the last 4–5 weeks of pregnancy being particularly important. To our knowledge, no studies have assessed whether newborn BP tracks with adult trajectory for high BP or hypertensive diseases. The differences in observed effect sizes can be due to a variety of factors that vary by study, including geographic location and relative level of ambient PM2.5, chemical composition and size distribution of PM2.5, local temperature and weather patterns, age of children at time of BP assessment, and methodological exclusions based on known risk factors.
The potential mechanisms underlying the observed associations are unclear, but likely include a number of adverse pathways. Cardiovascular effects of air pollution include inflammation, oxidative stress and mitochondrial abnormalities (Bourdrel et al., 2017; Calderon-Garciduenas et al., 2019). Postnatally, the particle size of PM can affect adverse health outcome severity, where smaller particle diameters including ultrafine PM0.1 (diameter < 0.1 μm) and fine PM2.5 (diameter < 2.5 μm) are associated with worse outcomes than coarse PM10 (diameter < 10 μm) (Lee et al., 2014) as these particles can penetrate the lung and enter the bloodstream. During the prenatal period, fine particulate matter components circulating in the bloodstream may enter the uteroplacental vascular system (Liu et al., 2016). Recently, the ENVIRONAGE birth cohort in Belgium reported that ambient black carbon particles can reach and accumulate in the fetal side of the placenta (Bove et al., 2019). Several mechanisms through which particulate air pollution exposures lead to adverse perinatal/childhood outcomes are proposed, including placentally-mediated inflammation, thrombosis, and oxidative stress resulting in impaired vascularization decreased transplacental function or placental insufficiency (Familari et al., 2019; Liu et al., 2016; Yue et al., 2019). Moreover, our group and others have shown complex layers of genetic and epigenetic regulation in placental tissue and cord blood including global and site-specific methylation changes (Breton et al., 2016; Maghbooli et al., 2018) and shorter telomere length (Rosa et al., 2019) may play a role. More studies are needed to understand the complex etiology of in utero PM2.5 on the developing cardiovascular, renal, and respiratory systems which could all contribute to altered childhood BP.
A limited number of studies have examined potential effect modification by sex of these associations. In the prospective Boston Birth Cohort study, Zhang et al. did not observe evidence of a sex by PM2.5 interaction but PM2.5 was associated with higher SBP in boys when compared to girls (Zhang et al., 2018). In a cross-sectional analysis of PM2.5 and PM10 exposure and BP in Chinese children and adolescents, there was a suggestive interaction between PM10 and sex, with boys having higher SBP than girls while for DBP, PM2.5 and PM10 were associated with higher measures in girls when compared to boys (Zhang et al., 2019). Van Rossem and colleagues reported no evidence of effect modification by sex in their study of trimester-averaged air pollution exposure and newborn BP (van Rossem et al., 2015). Current data support male sex as an important risk factor for primary hypertension in children (Theodore et al., 2015; Wang et al., 2006). Furthermore, boys have been previously shown to be more susceptible to in utero exposure to particulate matter including higher risk of adverse fetal outcomes (Lakshmanan et al., 2015), differences in body composition (Chiu et al., 2017) and measures of oxidative stress (Song et al., 2019). Additional work is needed to fully elucidate these sex-specific effects.
Our study had many strengths, including a sample size of 537 mother-child dyads with carefully collected outcome and covariate data collected longitudinally over several years. We leveraged highly spatially- and temporally-resolved ambient air pollution data reflecting each individual participant’s residential exposure during the entire pregnancy. We excluded mothers and children with known clinical risk factors for high BP including preterm birth and adjusted for maternal preeclampsia (Aris et al., 2019). We used data-driven statistical methods to identify sensitive windows to air pollution exposure and the observed associations remained significant after adjustment for a number of important potential confounders and covariates. We also acknowledge some limitations. Air pollution exposure in microenvironments outside of or within the home might lead to personal exposure levels that differed from our residential ambient exposure estimates, although such exposure misclassification is likely to be nondifferential and would drive effect estimates towards the null. Air pollution is a complex mixture of contaminants including polycyclic aromatic hydrocarbons and lead, which in addition to PM2.5, have been linked to changes in blood pressure (Holme et al., 2019; Trasande et al., 2015). In this study were unable to disentagle the effects of potentially relevant mixture components. As with any observational study, we cannot rule out residual confounding due to unmeasured factors related to residential location that may also influence BP in childhood. Nevertheless, our findings were limited to specific time periods within pregnancy, suggesting any unmeasured confounders would likely need to co-vary with PM2.5 and time-invariant characteristics would be less likely to explain these associations (Weisskopf et al., 2015). Our BP outcome relied on an average measure collected at a single time point. Ongoing studies will examine children’s BP and cardiometabolic trajectories through adolescence as persistent elevation in BP is an established risk factor for adult cardiovascular and renal disorders. Finally, the generalizability of our findings may be limited due the composition of our cohort which consisted of low-income Mexican families with substantial PM2.5 exposure.
We note that while our models predicted a cumulative increase of 2.6 mmHg SBP and 0.9 mmHg DBP with a constant 10 μg/m3 increase in PM2.5 sustained throughout the identified critical windows, the effect magnitudes are not clinically significant. Only 3 cases of suspected high BP were identified at age 4 to 6 years; ongoing follow-up at age 8–11 will inform whether harmful trajectories persist throughout childhood. Taken together, our work supports the continued need for informed strategies for air pollution reduction especially among susceptible populations. More studies on environmentally-associated BP trajectories assessed in childhood are prudent to examine how sequelae from in utero to adulthood may shape lifelong cardiovascular and renal health outcomes.
Conclusions
Our findings suggest that in utero air pollution exposure affects children’s BP several years following birth, indicating there might be a biological programming effect. Specifically, we found that second and third trimester PM2.5 exposure was associated with higher children’s BP at ages 4 to 6 years old. The critical windows we identified for both SBP and DBP had a large degree of overlap, however, additional research is needed to understand these processes and examine whether a common biologial mechanism may exist. Further work investigating PM2.5 exposure with longitudinal BP trajectories later in childhood will also be important to understanding cardiorenal trajectories that may predict adult disease. Our results underscore the importance of reducing air pollution exposure among susceptible populations.
Supplementary Material
Highlights.
Examined sensitive windows of prenatal PM2.5 exposure on BP in childhood.
Sensitive window identified at gestational weeks 11–32 for SBP and 9–25 for DBP.
PM2.5 exposure during windows was associated with elevated SBP and DBP.
Findings suggest sex-specific associations.
PM2.5 more strongly associated with higher SBP in boys compared to girls
Acknowledgements:
This work was supported by NIEHS grants R00ES027496 (Rosa MJ, PI) and R00ES027508 (Sanders AP, PI). The PROGRESS project has been supported by grants R01ES014930, R01ES013744, R24ES028522 (Wright RO, PI), R01ES021357 (Baccarelli A and Wright RO, MPI) and R00ES023450 (Just AC, PI). This study was supported by the National Institute of Public Health/Ministry of Health of Mexico, and the National Institute of Perinatology. We thank the ABC (American British Cowdray Medical Center) in Mexico for providing some of the needed research facilities.
Abbreviations:
- BDLIM
Bayesian distributed lag model
- BP
Blood pressure
- CI
confidence interval
- DBP
Diastolic blood pressure
- DLM
Distributed lag model
- PM2.5
Particulate matter less than or equal 2.5 microns in diameter
- SBP
Systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AirVisual I, 2018. World Air Quality Report Region & City PM2.5 Ranking. 2018. [Google Scholar]
- Aris IM, et al. , 2019. Early Life Predictors of Systolic Blood Pressure Trajectories from Infancy to Adolescence: Findings from Project Viva. Am J Epidemiol. [DOI] [PMC free article] [PubMed]
- Arredondo A, Aviles R, 2014. Hypertension and its effects on the economy of the health system for patients and society: suggestions for developing countries. Am J Hypertens. 27, 635–6. [DOI] [PubMed] [Google Scholar]
- Arredondo A, et al. , Costos de enfermedades crónicas en México Informe Técnico de Memoria Metodológica. National Institute of Public Health, Cuernavaca, México, 2013, pp. 23–27. [Google Scholar]
- Baker-Smith CM, et al. , 2018. Diagnosis, Evaluation, and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 142. [DOI] [PubMed] [Google Scholar]
- Bo Y, et al. , 2019. Dynamic Changes in Long-Term Exposure to Ambient Particulate Matter and Incidence of Hypertension in Adults. Hypertension. 74, 669–677. [DOI] [PubMed] [Google Scholar]
- Bourdrel T, et al. , 2017. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 110, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove H, et al. , 2019. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 10, 3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, et al. , 2016. Prenatal Air Pollution Exposures, DNA Methyl Transferase Genotypes, and Associations with Newborn LINE1 and Alu Methylation and Childhood Blood Pressure and Carotid Intima-Media Thickness in the Children’s Health Study. Environ Health Perspect. 124, 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2019. Air Pollution, Combustion and Friction Derived Nanoparticles, and Alzheimer’s Disease in Urban Children and Young Adults. J Alzheimers Dis. [DOI] [PubMed]
- Campos-Nonato I, et al. , 2013. [Hypertension: prevalence, early diagnosis, control and trends in Mexican adults]. Salud Publica Mex. 55 Suppl 2, S144–50. [PubMed] [Google Scholar]
- Capurro H, et al. , 1978. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 93, 120–2. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. , 2008. Impacts of measurement protocols on blood pressure tracking from childhood into adulthood: a metaregression analysis. Hypertension. 51, 642–9. [DOI] [PubMed] [Google Scholar]
- Chiu YM, et al. , 2017. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ Res. 158, 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto A, et al. , 2019. Ambient Particulate Air Pollution and Blood Pressure in Peri-urban India. Epidemiology. 30, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familari M, et al. , 2019. Exposure of trophoblast cells to fine particulate matter air pollution leads to growth inhibition, inflammation and ER stress. PLoS One. 14, e0218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JT, et al. , 2017. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 140. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, 2011. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. Journal of Statistical Software. 43, 1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, et al. , 2010. Distributed lag non-linear models. Statistics in Medicine. 29, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Cook NR, 1995. Blood pressure measurement in childhood epidemiological studies. Circulation. 92, 1049–57. [DOI] [PubMed] [Google Scholar]
- Holme JA, et al. , 2019. Potential role of polycyclic aromatic hydrocarbons as mediators of cardiovascular effects from combustion particles. Environmental Health. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HHL, et al. , 2015. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children Identifying Sensitive Windows and Sex Differences. American Journal of Respiratory and Critical Care Medicine. 192, 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AC, et al. , 2015. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environ Sci Technol. 49, 8576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, et al. , 2015. Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environmental Research. 137, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, et al. , 2014. Air pollution exposure and cardiovascular disease. Toxicol Res. 30, 71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, et al. , 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 525, 367–71. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, et al. , 2019. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 40, 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertxundi A, et al. , 2019. Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ Res. 174, 114–121. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. , 2016. Effect of Fine Particulate Matter (PM2.5) on Rat Placenta Pathology and Perinatal Outcomes. Med Sci Monit. 22, 3274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhloum N, et al. , 2019. Neonatal blood pressure in association with prenatal air pollution exposure, traffic, and land use indicators: An ENVIRONAGE birth cohort study. Environment International. 130. [DOI] [PubMed] [Google Scholar]
- Maghbooli Z, et al. , 2018. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS One. 13, e0199772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattu GS, et al. , 2001. Comparison of the oscillometric blood pressure monitor (BPM-100(Beta) ) with the auscultatory mercury sphygmomanometer. Blood Press Monit. 6, 153–9. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, et al. , 2019. Association between prenatal particulate air pollution exposure and telomere length in cord blood: Effect modification by fetal sex. Environ Res. 172, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, et al. , 2018. Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environ Int. 120, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CG, et al. , 2018. The association of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy in the HOME study cohort. Environ Int. 121, 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, et al. , 2016. Low-Concentration PM2.5 and Mortality: Estimating Acute and Chronic Effects in a Population-Based Study. Environ Health Perspect. 124, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LL, et al. , 2019. Effects of maternal exposure to ambient air pollution on newborn telomere length. Environment International. 128, 254–260. [DOI] [PubMed] [Google Scholar]
- Stolwijk AM, et al. , 1999. Studying seasonality by using sine and cosine functions in regression analysis. Journal of Epidemiology and Community Health. 53, 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar V, et al. , 2017. PM2.5 exposure in utero contributes to neonatal cardiac dysfunction in mice. Environ Pollut. 230, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore RF, et al. , 2015. Childhood to Early-Midlife Systolic Blood Pressure Trajectories: Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension. 66, 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, et al. , 2015. Polycyclic aromatic hydrocarbons, brachial artery distensibility and blood pressure among children residing near an oil refinery. Environmental Research. 136, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossem L, et al. , 2015. Prenatal Air Pollution Exposure and Newborn Blood Pressure. Environmental Health Perspectives. 123, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashi N, et al. , 2016. Genetic markers of inflammation may not contribute to metabolic traits in Mexican children. PeerJ. 4, e2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. , 2006. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 114, 2780–7. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, et al. , 2015. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Curr Environ Health Rep. 2, 430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, Ambient (outdoor) air quality and health. Vol. 2019 World Health Organization, 2018. [Google Scholar]
- Wilson A, et al. , 2017a. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children’s health. Biostatistics. 18, 537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, et al. , 2017b. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am J Epidemiol. 186, 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H, et al. , 2019. Gestational exposure to PM2.5 impairs vascularization of the placenta. Sci Total Environ. 665, 153–161. [DOI] [PubMed] [Google Scholar]
- Zhang MY, et al. , 2018. Maternal Exposure to Ambient Particulate Matter <= 2.5 mu m During Pregnancy and the Risk for High Blood Pressure in Childhood. Hypertension. 72, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. , 2019. Exposure to ambient particulate matter air pollution, blood pressure and hypertension in children and adolescents: A national cross-sectional study in China. Environ Int. 128, 103–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.