Abstract
Background:
Although many studies have established significant associations between short-term air pollution and the risk of getting cardiovascular diseases, there is a lack of evidence based on causal distributed lag modeling.
Methods:
Inverse probability weighting (ipw) propensity score models along with conditional logistic outcome regression models based on a case-crossover study design were applied to get the causal unconstrained distributed (lag0–lag5) as well as cumulative lag effect of short-term exposure to PM2.5/Ozone on hospital admissions of acute myocardial infarction (AMI), congestive heart failure (CHF) and ischemic stroke (IS) among New England Medicare participants during 2000–2012. Effect modification by gender, race, secondary diagnosis of Chronic Obstructive Pulmonary Diseases (COPD) and Diabetes (DM) was explored.
Results:
Each 10 μg/m3 increase in lag0–lag5 cumulative PM2.5 exposure was associated with an increase of 4.3% (95% confidence interval: 2.2%, 6.4%, percentage change) in AMI hospital admission rate, an increase of 3.9% (2.4%, 5.5%) in CHF rate and an increase of 2.6% (0.4%, 4.7%) in IS rate. A weakened lagging effect of PM2.5 from lag0 to lag5 could be observed. No cumulative short-term effect of ozone on CVD was found. People with secondary diagnosis of COPD, diabetes, female gender and black race are sensitive population.
Conclusions:
Based on our causal distributed lag modeling, we found that short-term exposure to an increased ambient PM2.5 level had the potential to induce higher risk of CVD hospitalization in a causal way. More attention should be paid to population of COPD, diabetes, female gender and black race.
Keywords: Causal Modeling, CVD, Distributed Lag, Ambient Air Pollution, Medicare
1. Introduction
Air pollution is a complex mixture of particulate and gaseous compounds and is usually measured in health studies as PM2.5 (fine particulate matter with diameter <= 2.5 μm) and PM10 (coarse particulate matter with diameter <= 10 μm). Cardiovascular disease (CVD), including both biomarkers of disease such as blood pressure and inflammatory markers, as well as acute events, such as myocardial infarctions (MI), strokes, and heart failure, have been associated with short-term exposures to air pollution (1–12). Ozone is another component of air pollution of interest in cardiovascular health studies. Strong heterogeneity exists in the findings from studies of the effect of ozone on cardiovascular diseases (13–16).
There is a dearth of information about the distributed lag between PM2.5/ozone exposure and acute CVD events in a two-pollutant model. Most studies have examined associations with exposure the day of the event, or the mean of two or three days prior, with only limited numbers of studies looking at large datasets. Because today’s pollution is correlated with yesterday’s pollution, most of those studies have used constrained distributed lags, such as polynomial distributed lags (17), spline distributed lags (18), or penalized spline (19) distributed lags to overcome collinearity, potentially at the risk of bias (17). Moreover, none of those studies used causal modeling techniques. Further, they have been conducted in cities, to ensure statistical power and the availability of monitors. This leaves questions about generalizability to smaller cities, towns, and rural areas that have been less studied.
Therefore, in this study, we aim to explore the causal short-term distributed lag effects of air pollution on CVD hospitalizations. Comprehensively, we assessed the effect of PM2.5 and ozone on the CVD hospital admission risk of elderly people across the New England region of US using Medicare. Both the health data and the exposure data covers more areas beyond big cities. We took a causal modeling path to get the causal distributed lag effect of PM2.5 / Ozone on our CVD outcomes, including acute myocardial infarction (AMI), congestive heart failure (CHF) and ischemic stroke (IS), as well as identified potentially susceptible subpopulations through effect modification analysis.
2. Methods
2.1. Study population
The study population was comprised of US New England region residents (states of Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont) who were Medicare beneficiaries more than 64 years old, and enrolled in the fee-for-service program. We included all emergency or urgent care hospital admissions due to AMI (n=156,717), CHF (n=204,774) and IS (n=170,663) between the years 2000 to 2012. Information on patient ID, emergency/urgent admission status, admission date, admission type, sex, age at admission, race, zip code of residence, eligibility for Medicaid, secondary diagnosis indicator of chronic obstructive pulmonary diseases (COPD) and secondary diagnosis indicator of diabetes was obtained from the Medpar (Medicare Provider Analysis and Review, which provides information for 100% Medicare beneficiaries using hospital inpatient services) files of the Center for Medicare and Medicaid Services.
2.2. Health outcomes
Our primary outcomes were AMI, CHF and IS. Admissions with a primary diagnosis of the International Classification and Disease, Ninth Revision (ICD 9) code of 410.xx (AMI), 428.xx (CHF) and 433.xx-435.xx (IS) were treated as cases. In addition, secondary diagnosis of 250.xx (Diabetes) and 490.xx-492.xx, 496.xx (COPD) were used to investigate the potential effect modification of having secondary diagnosis of diabetes or COPD.
2.3. Assessment of exposure
Daily ambient levels of PM2.5 (24 hours averaged, μg/m3) and ozone (8-hour maximum, ppb) from 2000 to 2012 were predicted at a spatial resolution of 1km from a machine learning algorithm that combined satellite remote censoring data, chemical transport models, land use, and meteorology, using a neural network (20, 21). Daily averaged values were constructed by averaging the exposure levels of all grid cells within each individual zip code. Air surface temperature (°C) as well as relative humidity (%) were obtained from the North American Regional Reanalysis data and daily mean values were generated for each 32 km × 32 km grid in the New England area. PM2.5, ozone, temperature and relative humidity were merged into the health outcome dataset for each day by zip code of individual residence.
2.4. Study design
We used a time stratified, bidirectional case-crossover design to examine the short-term effects of air pollutants (2-pollutant model, adjusting for pm2.5 and ozone simultaneously) on cardiovascular outcomes. Case days were when the subjects were admitted to the hospital. Control days were matched by day of week, month, and year with the case. Slowly varying or invariant covariates such as age, sex, race, smoking history, cholesterol, body mass index, and pre-existing medical conditions do not change day by day, and are controlled by matching for a case-crossover design. The institutional review board at Harvard University has approved this study.
2.5. Statistical analysis
We fit marginal structural models using weights based on propensity score models (usually referred to as inverse probability weighting method, ipw) following the methodology proposed by Cole at al. (22). A generalized ipw method is used. For each of the two exposures, pm2.5 and ozone, we assessed the distributed lag effect from day 0 (the current day of the event, lag0) to day 5 (5 days prior to the event, lag5). And for each lag of each exposure, we fitted a linear regression with the exposure lag of interest against all the other five lags of that pollutant and the six lags of the other pollutant along with linear and quadratic terms for temperature (lag 0 and 1), and linear terms for relative humidity (lag 0 and 1) to control for potential confounding by meteorological conditions with reference to Di et al’s lag choice (23). This may lead to the case that the future lags were actually controlled in models for previous lags of exposure. The plausibility for this modeling choice is based on the blockage of the backdoor pathway from previous lag (e.g. fine particle on day t-1, FPt-1) to CVD admission via weather/atmospheric condition (including cloudiness, wind speed, wind direction and other meteorological conditions apart from temperature and relative humidity) if we condition on future lags (eg. fine particle on day t, FPt), see example in Figure S1. The probability density of the residuals of this model is the probability density of receiving the exposure each person got, predicting from the variables included in the propensity score models. These probabilities are further stabilized by dividing by the marginal probability of the exposure the subject received. We used the inverse of these probabilities as the weights for each subject, for that lag. Hence the stabilized weights are SWA=f(A)/(f(A/L)) where A is exposure and L are covariates, and f is the Gaussian probability density. We did not transform exposures in our models since most of the observations had low exposure level and approximation to normal distribution could be applied to satisfy the assumption for linear models. Under the assumptions of no important omitted confounders and correct specification of the propensity score models, regressing outcome against exposure at a lag, using the weights specific to that lag, should provide the marginal effect of that particular lag of that exposure (e.g. pm25 lag0 or ozone lag5), independent of covariates. This was done for each of the six lags of each pollutant. We further truncated the weights to between 2.5th and 97.5th percentile (22). Positivity exclusion was also conducted with reference to Cole at al (22).
In addition to computing the marginal effect of exposure at each lag of each pollutant, we computed the cumulative overall effect of pm2.5 or ozone over the six lag days by taking an inverse variance weighted sum of the coefficients at each lag. Effect modification was examined for sex, race, secondary diagnosis of COPD and diabetes. P values for comparing every modifier’s two contrasting groups were reported based on two-sided two sample t test. All the analyses were conducted using R 3.5.0. Because of the potential confounding effects of NOx pollutants in the winter, supplementary analysis for the effect of ozone on CVD was specifically carried out with restriction to time window from April 1 to September 30. A series of sensitivity analyses on temperature adjustment (splines and more lags) were also conducted.
3. Results
3.1. Cohort and meteorological characteristics
There was a total of 156,717 individuals who were admitted for AMI, 204,774 for CHF and 170,663 for IS. Table 1 showed the cohort characteristics, pollution levels, and meteorological variables. Among all the cases, there were slightly more females. The majority of the study population was white and black. We also examined the eligibility for Medicaid insurance and found that about 20% of the population had dual insurance. For all case and control days, the average PM2.5 exposure level was around 10 μg/m3. The average all seasons ozone level was around 36 ppb. Summer time ozone was a little bit higher than all seasons ozone level. In addition, in terms of the density and spatial variation of CVD events, for AMI, we found that the average counts of cases per zip code is 119 and the average counts of zip codes involved per case day is 42, with 208 and 72 for CHF and 119 and 43 for IS, respectively. To convey a sense of the coverage of an US zip code, in Massachusetts, as of 2015, there is a total of 537 zip codes under use and for each one, it covers about 37.8 km2 and about 12,853 people (“Massachusetts”. United States Census Bureau. Retrieved June 10, 2015).
Table 1.
CVD Hospital Admitted New England Medicare Participants Cohort Characteristics, Pollution Levels and Meteorological Variables (2000–2012)a (no color needed)
| Categories | AMI | CHF | IS |
|---|---|---|---|
| Population | |||
| Persons (No.) | 156717 | 204774 | 170663 |
| Case days (No.) | 230157 | 404996 | 232264 |
| Control days (No.) | 782169 | 1376263 | 789267 |
| Individual Covariates (among all cases) | |||
| Female sex N (%) | 120351 (52.3) | 234425 (57.9) | 136295 (58.7) |
| Race or ethnic group N (%) | |||
| Whites | 219240 (95.3) | 377420 (93.2) | 217093 (93.5) |
| Blacks | 5292 (2.3) | 16685 (4.1) | 8921 (3.8) |
| Other | 2174 (0.9) | 3578 (0.9) | 2366 (1.0) |
| Asians | 1067 (0.5) | 1968 (0.5) | 1323 (0.6) |
| Hispanics | 1649 (0.7) | 4048 (1.0) | 1799 (0.8) |
| Native Americans | 162 (0.1) | 297 (0.1) | 125 (0.1) |
| Unknown | 573 (0.2) | 1000 (0.2) | 637 (0.3) |
| Eligibility for Medicaid N (%) | 45558 (19.8) | 91257 (22.5) | 45029 (19.4) |
| Average age at admission (yr.) | 79.68 (8.13) | 81.45 (8.24) | 80.40 (7.86) |
| secondary diagnosis of COPD N (%) | 39754 (17.3) | 116606 (28.8) | 28100 (12.1) |
| secondary diagnosis of Diabetes N (%) | 64511 (28.0) | 133410 (32.9) | 60378 (26.0) |
| Pollution Levels & Meteorological Variables all days | |||
| PM2.5 (μg/m3) | 10.13 (6.48) | 10.08 (6.42) | 10.10 (6.47) |
| Ozone (ppb) | 36.44 (11.74) | 36.33 (11.73) | 36.72 (11.91) |
| Ozone (ppb)-warm seasons (4–9) | 42.93 (11.73) | 42.99 (11.56) | 43.14 (11.79) |
| Air surface temperature (°K) | 282.51 (9.83) | 282.64 (9.70) | 283.17 (9.74) |
| Relative humidity (%) | 78.63 (10.95) | 78.54 (11.04) | 78.62 (10.94) |
| case days | |||
| PM2.5 (μg/m3) | 10.14 (6.48) | 10.11 (6.46) | 10.17 (6.54) |
| Ozone (ppb) | 36.44 (11.74) | 36.28 (11.71) | 36.74 (11.93) |
| Ozone (ppb)-warm seasons (4–9) | 42.94 (11.73) | 42.94 (11.55) | 43.22 (11.79) |
| Air surface temperature (°K) | 282.47 (9.81) | 282.62 (9.67) | 283.13 (9.74) |
| Relative humidity (%) | 78.63 (10.95) | 78.59 (11.03) | 78.59 (10.95) |
| control days | |||
| PM2.5 (μg/m3) | 10.12 (6.49) | 10.07 (6.41) | 10.08 (6.45) |
| Ozone (ppb) | 36.44 (11.75) | 36.35 (11.73) | 36.72 (11.90) |
| Ozone (ppb)-warm seasons (4–9) | 42.93 (11.74) | 43.01 (11.56) | 43.12 (11.79) |
| Air surface temperature (°K) | 282.52 (9.84) | 282.65 (9.71) | 283.19 (9.74) |
| Relative humidity (%) | 78.63 (10.95) | 78.52 (11.04) | 78.63 (10.94) |
Abbreviations: AMI for acute myocardial infarction; CHF for congestive heart failure; IS for ischemic stroke.
Mean and standard deviation for continuous variables and number and percentages for categorical variables. Pollutants and Meteorological variables are at residence zipcode levels.
3.2. PM2.5 distributed lag effects
Overall, a weakened lagging effect of PM2.5 from lag0 to lag5 could be observed. Significant harmful effects were shown especially for PM2.5 exposure on lag0–lag2 days with respect to the risk of admissions for AMI and CHF. As to the effects of PM2.5 on IS, significantly increased risk was mainly observed for lag0 and lag1 days. Based on the cumulative effects summarizing all the lag days (lag0–5), each 10 μg/m3 increase in PM2.5 exposure was associated with an increase of 4.31% (95% confidence interval: 2.21%, 6.42%, percentage change) in the AMI hospital admission rate, an increase of 3.95% (2.37%, 5.53%) in the CHF rate and an increase of 2.56% (0.44%, 4.69%) in the IS rate. The results were based on daily pm2.5 exposures concerning all seasons (Figure 1, Table S1).
Figure 1. Distributed Lag Effects of PM2.5 and Ozone on Percentage Change of AMI, CHF and IS Hospital Admission Ratea.
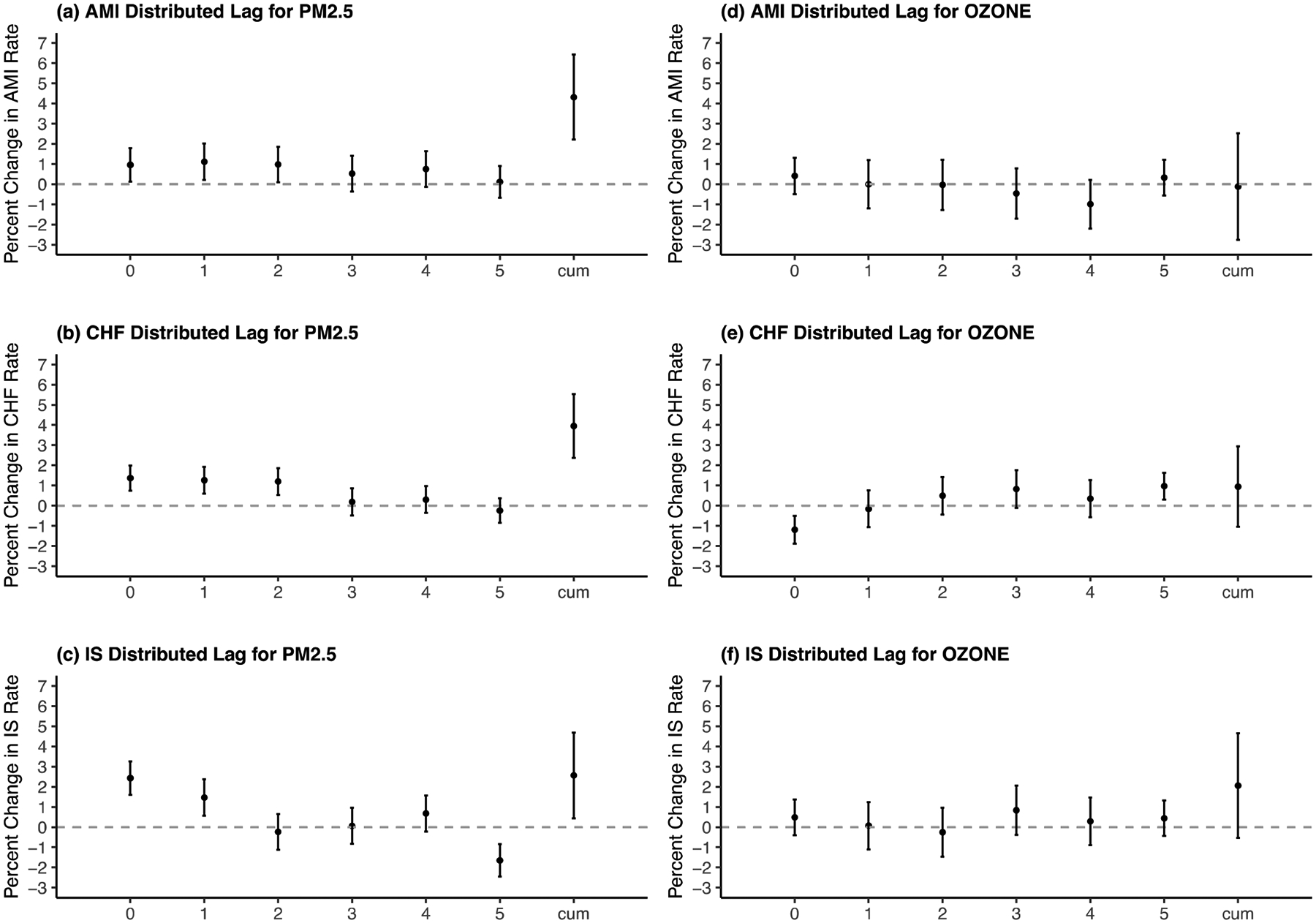
Abbreviations: AMI for acute myocardial infarction; CHF for congestive heart failure; IS for ischemic stroke.
a X axis is for different lag days and cumulative lag0–5; Y axis is for percentage change in the outcome hospital admission rate (%) per 10-unit increase of pm2.5 (μg/m3) or ozone (ppb), CVD Hospital Admitted New England Medicare Participants 2000–2012.
3.3. Ozone distributed lag effects
No clear pattern of distributed lag effects of ozone could be seen. In addition, no significant cumulative effects were observed. With respect to most of the lag days of ozone exposure, no significantly protective or harmful effects could be observed. Results were based on all-season ozone level (Figure 1, Table S1). In the sub-analysis of ozone during warm seasons, results were similar. (Table S3)
3.4. Modification analysis
We examined the effect of four potential modifiers, including secondary diagnosis of COPD, secondary diagnosis of diabetes, sex and race, in our analysis. The detailed results were presented in Figure 2 and Table S2. Having secondary diagnosis of COPD seemed to modify the association between PM2.5 and hospital admissions of AMI, CHF and IS. People with COPD had a significantly higher risk of AMI and CHF hospital admission with percentage increase of AMI: 7.60% (1.63%, 13.57%), CHF: 6.05% (2.10%, 10.00%) compared to AMI: 3.62% (1.30%, 5.94%) and CHF: 3.08% (1.20%, 4.96%) among those without COPD, for every 10 μg/m3 increase in cumulative PM2.5 (P=0.031, P=0.022, respectively). However, for IS, people without COPD had a higher risk instead. People with COPD also had a significantly higher risk of getting IS admission for each 10 ppb increase in ozone level (P=0.043). Diabetic elderly people turned out to have a higher risk of getting AMI admission for each 10 μg/m3 increase in PM2.5, but not for the other two events or the ozone exposure, compared to non-diabetic ones (P=0.010). Females had a higher chance of getting CHF hospitalizations associated with 10 μg/m3 increase in PM2.5 than males (P=0.040). One of the biggest findings is that among black population, we examined a 26.36% (12.70%, 40.02%) admission rate increase of AMI for 10 μg/m3 increase in PM2.5 compared to only 4.46% (2.30%, 6.62%) increase among white population (P=0.001). Similar strong modification by race was also detected in the association between ozone and IS admission with much higher risk among black population approaching about 15% IS admission rate increase for 10 ppb increase in ozone.
Figure 2. Stratified Results for the Cumulative Effects (lag0–5) of PM2.5 and Ozone on Percentage Change of AMI, CHF and IS Hospital Admission Ratea.
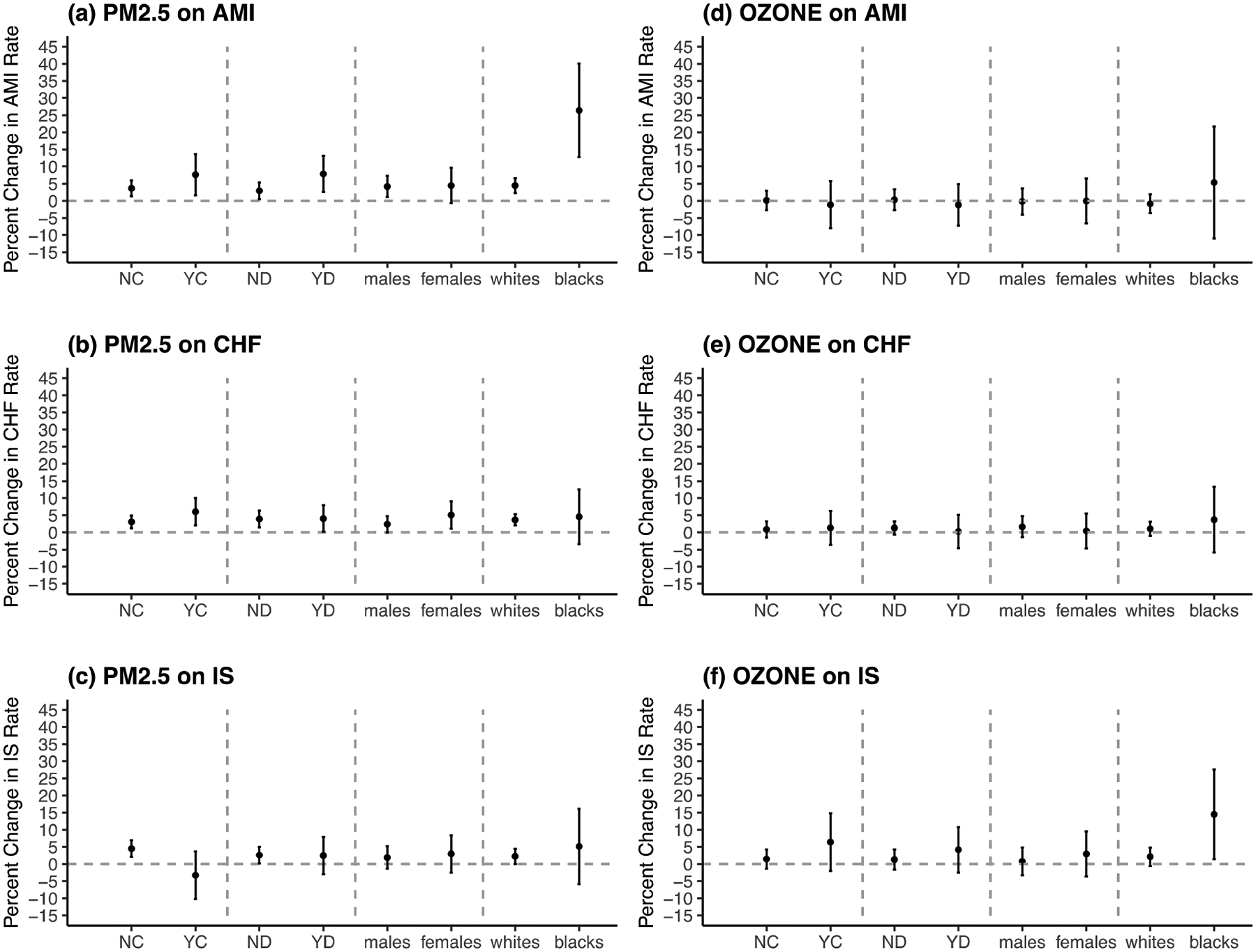
Abbreviations: AMI for acute myocardial infarction; CHF for congestive heart failure; IS for ischemic stroke.
a X axis is for different subgroups including people without secondary diagnosis of COPD (NC), people with secondary diagnosis of COPD (YC), people without secondary diagnosis of diabetes (ND), people with secondary diagnosis of diabetes (YD), males, females, whites and blacks; Y axis is for percentage change in the outcome hospital admission rate (%) per 10-unit increase in cumulative pm2.5 (μg/m3) or ozone (ppb), CVD Hospital Admitted New England Medicare Participants 2000–2012.
3.5. Sensitivity analysis
Sensitivity analysis on temperature adjustment (Appendix, Table S4) does not show significant departure from the effect estimates reported in our main analysis.
4. Discussion
In summary, we found that short-term exposure to increased PM2.5 level induces higher risk of CVD hospitalizations among elderly people in New England area. We could also observe a pattern of weakened effect of PM2.5 on AMI, CHF and IS from lag0 to lag5. We did not see evidence of acute effects of ozone on the cardiovascular outcomes and no clear pattern of distributed lag effects of ozone could be concluded based on this study. People with secondary diagnosis of COPD, diabetes and of black race were more susceptible to PM2.5 in terms of the risk of AMI hospitalizations while with respect to CHF, females and people with COPD were more sensitive. In addition, people with COPD and blacks had higher risk of getting ischemic stroke induced by increased level of ozone.
The underlying mechanism linking exposure to pm2.5 and CVD has not been fully investigated. However, a few theories have been proposed: first, cardiovascular events including heart attack and heart failure could be the results of white cells accumulated in the artery forming clots, due to the immune response in human body induced by the exhalation of particles (24). In addition, oxidative stress and inflammation induced by the inhalation of PM have the potential to lead to central nervous system pathology because of the penetration of the inflammatory compounds into the blood-brain barrier or direct entry of PM through the olfactory bulb, especially for triggering stroke (5, 25). There is also growing evidence indicating that genetic susceptibility is likely to play a role in response to air pollution. Genetic differences may determine who will have worse health damage from short-term or protracted exposure to air pollution (26).
The main findings of this study are supported by many other studies (but in a non-causal modeling way) (16, 27–31). A quantitative meta-analysis comprising 20 related studies stated that the current literature evidence for short-term pm2.5-related ischemic stroke was especially strong (27). An increased risk of mortality, especially mortality due to all CVD events, AMI and stroke, was found for a 10 μg/m3 increase in 2-day averaged PM2.5 level based on a US national time series study on short-term effects of PM2.5 and PM10 using 112 cities (28). A significantly positive association was also observed between heart disease mortality and short-term PM2.5 at lag0 day in Japan (31). However, there were also other studies that concluded no significant associations (32–35). This inconsistency in research findings could be due to the major difference in study design, dataset used, study population, analytical method as well as temporal and spatial properties. Further larger nationwide or worldwide original studies or systematic reviews concerning the causal effect of short-term exposure to particulate matter pollution on CVD risk are needed to resolve this discrepancy. In addition, we did find a small portion of effect trends that are difficult to interpret. For example, COPD population were at a lower risk of getting IS for exposure to pm2.5 adjusting for ozone, temperature and rh. This is unusual with what most of the past studies found with their data. However, one possible reason is that they did a single pollutant model instead of two-pollutant model here. Our effect modification results reported were adjusting for ozone. COPD patients were shown in our study to be at a higher risk of getting IS when exposed to higher levels of ozone while ozone and pm2.5 levels are commonly in a negative correlation with each other (36, 37). These might suggest that more research is needed to devote efforts in warranting and exploring the modification effect of COPD status on ischemic stroke hospital admission risk when simultaneously adjusting for more atmospheric co-pollutants that are correlated with each other. We also detected significant protective effect of PM2.5 lag5 day exposure on IS risk as well as ozone lag0 day exposure on CHF, which could possibly be due to the residual confounding effects by co-pollutants or short-term daily variation of smoking pattern.
Comparing the conventional approach reported by the past studies with our analyses, our study improves the adjustment and produces effect estimates that are more than association. The past studies did many analyses using the conventional conditional logistic association regression putting different lags (most commonly up to lag2 days of exposure) or moving average of multiple lags into the same outcome regression model. These kinds of results are actually conditional on levels of all the other lags, co-exposure, meteorological variables and thus has limited generalizability. Casual modeling methods, including ipw, analyze observational data in a way that approximates conducting a randomized experiment to make exposure independent of all potential confounders, rather than to control for the confounders in the outcome regression. Under specified assumptions, they yield causal estimates of the effects of exposure (38). Often, they provide marginal estimates of the effects of exposure, that is, ones that are not conditional on the distribution of covariates and are therefore more generalizable. Therefore, in this case, after we used the weights generated from the propensity score models predicting the exposure lag of interest out of the other lags, important co-pollutant exposure and related meteorological variables that change in a short time and later created a pseudo randomized population based on weighting, in the final step of the marginal outcome regression model, we could obtain the causal estimate.
We understand that one may have questions about what kind of assumptions we need to make for such a causal model and how they hold in this setting. According to Hernan and Robins (38), they are: 1) exchangeability; it assumes no unmeasured confounding but the real case is that we do not have the resources to get all the potential unmeasured confounders, thus this condition is assumed here. However, this assumption can be tested through sensitivity analysis by including more covariates concerned, Cole S.R. and Hernan, M.A, when developing the ipw causal inference method, suggested the readers instead to assume that the most important confounders were identified using expert knowledge and well-adjusted in the propensity score models (22). We actually tested this assumption using the data we have via sensitivity analysis of temperature adjustment by including more lags of temperature and spline adjustments. Results from the sensitivity analysis on temperature did not have significant deviation from our main analysis estimates. We tend to believe that the most important and available confounders have been adjusted in such a case-cross over study design. Residual confounding is more likely to result from co-pollutants that we do not have the information on (and actually we already tried to control the ozone and pm2.5 at the same time as co-pollutants to each other). But this is the best we could do using the exposure data we have right now. 2) positivity; guaranteed in our analysis through positivity exclusion. It means that the condition that there are both exposed and unexposed individuals at every level of the confounders. 3) Consistency; assumed here (one cannot prove this to be true, and this needs to be assumed). It is often understood as “The observed outcome is exactly the same as the potential outcome the person will have under the exposure assigned” (39).
The second major advantage is that in such a marginal structured way, we could bypass the high collinearity issue between different lags or between different correlated exposures (such as pm2.5 and ozone here) and get the marginal independent lag effects of exposure on the outcome, and by inverse variance weighting the estimates for each lag, compute the cumulative effect over the full lagging period. There is very few studies that did a detailed analysis for each lag’s effect from lag0 day exposure to lag5 day exposure, partially because they were faced with the high collinearity issue and if they put all the lags together into the outcome regression models, that kind of models would be highly unstable and the estimates generated would be very noisy. Although some of the previous studies tried to combine the information from more lags altogether by using the moving average levels, yet by doing that, they could not identify which lag of exposure is more influential than the others and the distributed lag effects pattern like we showed in our Figure 1. Besides, constrained distributed lag model is theoretically hard to incorporate into the causal modeling approaches.
Although the conventional unconstrained distributed lag model tends to produce more unstable effect estimates compared with the constrained one which allows coefficients to follow a flexible curve (either spline or polynomial) more simulating the plausible biological mechanisms, in order to explore the short-term causal effects of air pollution on hospitalization of CVDs for this cohort, we need to combine distributed lag models with the causal modeling method we chose to use which is ipw (22) here. Due to the natural feature of the ipw modeling, we have to put only one lag of exposure at a time to run a series of marginal structured models after we created a pseudo population that approximate a randomized population using the weights generated from the propensity score models. Therefore, the final effect estimates of each lag of exposure is unconstrained with more robust estimates resulting from the weighting process instead of from the collinearity between different lags in the same outcome regression model.
Apart from the two major advantages we mentioned above, this is also a relatively large-sample study, providing enough power for the analysis, as well as better research evidence supporting the causal harmful effect of short-term exposure of particulate matter on CVD at least among New England elderly population. By applying ipw, we could explore marginal distributed lag effects with no parameter constraints and we identified the potential causal effects of each lag independent of other lags with relevant confounders controlled.
This study also comes along with a few limitations. In this analysis, no sub-analysis was conducted, looking at the effect estimates with restriction to only low-level individual daily exposure for all case and control days. Current literature has demonstrated that low-level air pollution below the US EPA short-term national ambient air quality standard (NAAQS) of 35 μg/m3 for particulate matter (https://www.epa.gov/criteria-air-pollutants/naaqs-table), yielded a higher risk increase in health outcomes compared to same unit increase of high-level air pollution above the standard, including all-cause mortality (23, 36). Compared with the conventional distributed lag modeling approach, our approach excluded certain subjects that violated the positivity assumption required by causal modeling, which is likely to reduce the analysis power. However, this should not be a big problem if the study has a large sample size, which is the case for our study. There would always be residual confounding from other pollutants, such as NOx, CO and SOx, emitted by the traffic especially in the winter months, which would bias the results for the ozone models a little bit for winter months. However, in our sub-analysis of the ozone effect during the warm seasons, which is from April to September, similar pattern and insignificant association could be observed compared to the all-year ozone results.
In conclusion, inverse probability weighted distributed lag modeling is a way to choose when investigating short-term causal effects of environmental exposures on health outcomes and it gives unconstrained, less conditional effect estimates that are less influenced by highly correlated covariates, compared to the conventional approach. Short-term exposure to an increased ambient fine particles level has a strong potential to induce higher risk of getting CVD hospitalizations among New England elderly residents. People with secondary diagnosis of COPD, diabetes, female gender and black race are sensitive population.
Supplementary Material
Key Messages.
Our application of causal distributed lag modeling showed harmful effects of short-term pm2.5 exposure on CVD hospitalizations in a causal way among elderly population.
Each 10 μg/m3 increase in lag0–lag5 cumulative PM2.5 exposure on average brings an increase of 4.3% in AMI hospital admission rate, an increase of 3.9% in CHF rate and an increase of 2.6% in IS rate among New England Medicare participants.
Overall, the effect of PM2.5 on CVD hospital admission risk declined from lag0 to lag5 days. Significant harmful effects were shown especially for lag0–lag2 days with respect to the risk of hospital admissions for AMI and CHF. And lag0–lag1 days for IS hospitalization.
People with secondary diagnosis of COPD, diabetes, female gender and black race are sensitive population.
Acknowledgements:
Many thanks to Professor Francesca Dominici and Dr. Yun Wang at Harvard T.H. Chan School of Public Health for providing access to the Medicare data. Thank Professor Miguel Hernan at Harvard T.H. Chan School of Public Health for his help in causal modeling.
Funding
This study was supported by the National Institutes of Health (NIH) grant [R01 ES024332-01A1], [ES-000002], Health Effects Institute (HEI) grant [4953-RFA14-3/16-4]. This publication was made possible also by U.S. Environmental Protection Agency (EPA) grant [RD-835872-01]. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Approval
This study got approval from the Institutional Review Board at Harvard University before conducting the data analysis.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chuang H-C, Ho K-F, Lin L-Y, Chang T-Y, Hong G-B, Ma C-M, et al. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environ Int. 2017;106:91–6. [DOI] [PubMed] [Google Scholar]
- 2.Bind MA, Peters A, Koutrakis P, Coull B, Vokonas P, Schwartz J. Quantile Regression Analysis of the Distributional Effects of Air Pollution on Blood Pressure, Heart Rate Variability, Blood Lipids, and Biomarkers of Inflammation in Elderly American Men: The Normative Aging Study. Environmental health perspectives. 2016;124(8):1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, et al. Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2017;37(9):1793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai L, Bind MA, Koutrakis P, Coull BA, Sparrow D, Vokonas PS, et al. Fine particles, genetic pathways, and markers of inflammation and endothelial dysfunction: Analysis on particulate species and sources. J Expo Sci Environ Epidemiol. 2016;26(4):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yitshak Sade M, Novack V, Ifergane G, Horev A, Kloog I. Air Pollution and Ischemic Stroke Among Young Adults. Stroke. 2015;46(12):3348–53. [DOI] [PubMed] [Google Scholar]
- 6.Araujo JA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual Atmos Health. 2010;4(1):79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, et al. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. 2006;114(7):1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, et al. Air Pollution and Myocardial Infarction in Rome: A Case-Crossover Analysis. Epidemiology. 2003:in press. [DOI] [PubMed] [Google Scholar]
- 11.Albert MA, Ridker PM. The role of C-reactive protein in cardiovascular disease risk. Curr Cardiol Rep. 1999;1(2):99–104. [DOI] [PubMed] [Google Scholar]
- 12.Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172(3):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occupational and environmental medicine. 2007;64(7):439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koken PJ, Piver WT, Ye F, Elixhauser A, Olsen LM, Portier CJ. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in Denver. Environmental health perspectives. 2003;111(10):1312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Annals of neurology. 2008;64(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. Jama. 2012;307(7):713–21. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz J The distributed lag between air pollution and daily deaths. Epidemiology (Cambridge, Mass). 2000;11(3):320–6. [DOI] [PubMed] [Google Scholar]
- 18.Zanobetti A, Wand MP, Schwartz J, Ryan LM. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1(3):279–92. [DOI] [PubMed] [Google Scholar]
- 19.Zanobetti A, Schwartz J. Mortality displacement in the association of ozone with mortality: an analysis of 48 cities in the United States. Am J Respir Crit Care Med. 2008;177(2):184–9. [DOI] [PubMed] [Google Scholar]
- 20.Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. Assessing PM2.5 Exposures with High Spatiotemporal Resolution across the Continental United States. Environmental science & technology. 2016;50(9):4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. Journal of the Air & Waste Management Association (1995). 2017;67(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168(6):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. Association of Short term Exposure to Air Pollution With Mortality in Older Adults. Jama. 2017;318(24):2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steenhof M, Janssen NA, Strak M, Hoek G, Gosens I, Mudway IS, et al. Air pollution exposure affects circulating white blood cell counts in healthy subjects: the role of particle composition, oxidative potential and gaseous pollutants - the RAPTES project. Inhalation toxicology. 2014;26(3):141–65. [DOI] [PubMed] [Google Scholar]
- 25.von Bornstadt D, Kunz A, Endres M. Impact of particulate matter exposition on the risk of ischemic stroke: epidemiologic evidence and putative mechanisms. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(2):215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanobetti A, Baccarelli A, Schwartz J. Gene-air pollution interaction and cardiovascular disease: a review. Progress in cardiovascular diseases. 2011;53(5):344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin HH, Fann N, Burnett RT, Cohen A, Hubbell BJ. Outdoor fine particles and nonfatal strokes: systematic review and meta-analysis. Epidemiology (Cambridge, Mass). 2014;25(6):835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environmental health perspectives. 2009;117(6):898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo R, Michikawa T, Ueda K, Ago T, Nitta H, Kitazono T, et al. Short-Term Exposure to Fine Particulate Matter and Risk of Ischemic Stroke. Stroke. 2016;47(12):3032–4. [DOI] [PubMed] [Google Scholar]
- 30.Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet (London, England). 2013;382(9897):1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda K, Nitta H, Ono M. Effects of fine particulate matter on daily mortality for specific heart diseases in Japan. Circulation journal : official journal of the Japanese Circulation Society. 2009;73(7):1248–54. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Kindzierski W, Kaul P. Air Pollution and Acute Myocardial Infarction Hospital Admission in Alberta, Canada: A Three-Step Procedure Case-Crossover Study. PloS one. 2015;10(7):e0132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart (British Cardiac Society). 2014;100(14):1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing JJ, Adar SD, Sanchez BN, Morgenstern LB, Smith MA, Lisabeth LD. Ethnic differences in ambient air pollution and risk of acute ischemic stroke. Environmental research. 2015;143(Pt A):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology (Cambridge, Mass). 2011;22(3):422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. The New England journal of medicine. 2017;376(26):2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia M, Zhao T, Cheng X, Gong S, Zhang X, Tang L, et al. Inverse relations of PM2. 5 and O3 in air compound pollution between cold and hot seasons over an urban area of east China. Atmosphere. 2017;8(3). [Google Scholar]
- 38.Hernan MA, Robins JM. Causal inference. CRC: Boca Raton, FL; 2010. [Google Scholar]
- 39.Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology (Cambridge, Mass). 2009;20(1):3–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


