Abstract
Objective
To report in vivo measurements of lumbar facet joint subchondral bone mineral density used in the description of facet joint loading patterns and to interrogate if low-back pain is associated with changes in subchondral bone mineral density.
Materials and Methods
In vivo measurements of lumbar facet joint subchondral bone mineral density (L1/2 to L5/S1) in Hounsfield units were performed on 89 volunteers (56 controls and 33 with low back pain) by computed tomography osteoabsorptiometry at subchondral regions between 1.5 mm and 2.5 mm below the joint surface. The facet surface was divided in five topographic zones: cranial, lateral, caudal, medial and central.
Results
1,780 facet joint surfaces were analyzed. Facets were denser (p<0.0001) both in superior facets and in low-back pain subjects (p<0.0001). For the entire cohort, the facet center zone subchondral bone mineral density was higher (p<0.0001) than that of the peripheral zones. The analyses indicate that subchondral bone mineral density is highest in patients with low back pain, the superior facets, and the center zone of the facets.
Conclusion
Subchondral bone mineral density is thought to reflect cumulative, long-term distribution of stress acting on a joint. This work shows that higher subchondral bone mineral density values in the center zone indicate predominant stress transmission through the center of the facet joints. Finally, the greater subchondral bone mineral density in patients with low back pain may reflect both increased load bearing by the facets secondary to disc degeneration and misdistribution of loading within the joint.
Keywords: Lumbar facet joint, subchondral bone density, low back pain
Introduction
Low back pain is the second most common cause of adult disability worldwide.[1] The lifetime prevalence of low back pain is 54–80% [2], and the yearly nationwide cost of low back pain is estimated at $100 billion [3]. Causes of low back pain include facet joint osteoarthritis, soft tissue sprain, disc degeneration, instability, nerve impingement, infection, and neoplasm. In particular, osteoarthritis of the facet joints, which are the load-bearing joints of the posterior spine [4–6], is thought to be the cause of low back pain in 15% to 45% of patients.[7, 8]
Any change in the long-term mechanical loading of a bone changes its microstructure [9–11]. In particular, loading on the subchondral surface of a joint typically increases the subchondral bone’s mineral density. This has been most commonly demonstrated for the joints of the upper and lower extremities. However, it has also been demonstrated in the joints of the spine. For example, Wagner et al. demonstrated that removal of posterior instrumentation in the lumbar spine increases the stress loading of the spine, consequently producing an increase in the facet joint subchondral bone density [12].
A primary pathologic change in osteoarthritis of a weight-bearing joint is degeneration and loss of articular cartilage. Studies have shown that articular cartilage degeneration is associated with increases in subchondral bone density, at least in part related to changes in the mechanical loading of subchondral bone [13–17]. Computed Tomography Osteoabsorbptiometry is a three-dimensional technique that enables volumetric measurement of bone mineral density [17–20]. This technique can be used to characterize bone mineral density in very specific areas, including the subchondral bone of joints throughout the body [19, 21].
Computed Tomography Osteoabsorbptiometry can be used to characterize bone mineral density in the subchondral bone of the facet joints of the lumbar spine [17–21]. This is useful in that it can help determine the distribution of loads within the facet joint [9–12] as well as for the study of pathologic processes in facet joint osteoarthritis [13–17].
Despite the importance of the facet joint as a cause of low back pain, research into both the loading patterns of the facet joint and the involvement of subchondral bone in osteoarthritis of the facet joint has been limited to date. In this context, the purposes of the present study are twofold: First, the present study tests the hypothesis that the presence of low back pain in patients is associated with the density of lumbar facet subchondral bone. Second, by using Computed Tomography Osteoabsorbptiometry to examine the distribution of subchondral bone density within the facet joint, the present study seeks to better elucidate the loading patterns of the facet joints of the lumbar spine.
Materials and Methods
Internal Review Board approval for the following procedures was obtained. Inclusion criteria for the low back pain group were recurrent pain of the low back with at least two episodes of at least six weeks brought on by modest physical exertion. Subjects with the following conditions were excluded from both groups: claustrophobia or contraindication to magnetic resonance or computed tomographic imaging, previous spinal surgery, presence of lumbosacral transitional vertebrae, scoliosis, and congenital malformation of vertebrae or facet joints. In total, 89 volunteers provided informed consent to participate in the study. Of these, 33 were recruited as subjects with low back pain and 56 were recruited as subjects without low back pain; 47 were male and 42 were female; the mean age (± standard deviation; range) was 36.5 years (± 12.9; 20–59).
Creation of Facet Joint Surface Models
Each subject underwent lumbar computed tomography (CT) scans in a clinical unit (Volume Zoom, Siemens, Malvern, PA) at 1.0 mm slice thickness and a pixel size of approximately 0.5 mm in the supine position including spinal levels from L1 to S1. The CT image data in Digital Imaging and Communications (DICOM) format were used to prepare three-dimensional (3D) joint surface models of each facet joint including subchondral bone density information underneath each facet joint surface. Said facet joint surface models were created by manual tracing the facet joint surface contours on each axial CT slice using a tablet digitizer (Wacom Intuos 3, Wacom, Saitama, Japan) as previously described by Otsuka et al.[22] During tracing, care was taken trace only the joint surface excluding osteophytes.
Measurement of Subchondral Bone Density
Subchondral bone density underneath the facet joint surface was automatically recorded during tracing of the joint surface in an increment of 0.5 mm down to a depth of 4.5 mm. The subchondral bone density at each location was determined by linear interpolation of Hounsfield Units (HU) at four adjacent pixels in each CT slice. The method was implemented using a custom-written C++ routine (Microsoft Visual C++ 2003 under Microsoft Foundation Class programming environment). The subchondral bone layers located at these three depths: a) 1.5 mm, b) 2.0 mm and c) 2.5 mm were used for the following analyses considering a partial volume effect at the joint surface pixels and typical subchondral bone plate thickness of the human lumbar spine [23] (Figure 1). The subchondral bone density at each location was determined as an average of the bone density at these three layers. This analysis method covered the entire surface of the facet joint, but to provide meaningful anatomical information, the facet joint surfaces were further divided into five topographic zones (center, superior, inferior, medial, and lateral) as in Simon et al. [24]. The resulting mean value for each topographic zone is the final value reported here.
Fig. 1.
Distributions of subchondral bone density distribution of the facet joint at layers of 1.5 mm, 2.0 mm and 2.5 mm underneath the joint surface
Statistical Analysis
The eighty-nine subjects yielded 1,780 facet articular processes for analysis (10 superior articular processes and 10 inferior articular processes per patient). The subchondral bone density data in each superior or inferior articular process were averaged between left and right sides. The subchondral bone density was tested for association with facet zones, lumbar levels, patient age, and subject gender using ANOVA with post hoc Fisher pairwise comparisons. The results were presented as mean ± standard deviation. The level of significance was set at α=0.05 (p<0.05). All analyses were carried out in Statview 5.0, (SAS Institute, Cary, NC).
Results
The mean subchondral bone density of entire surface of the facet joint surface was greater for superior than inferior facets (1061.8 HU [±212.4] versus 1044.1 HU [±204.2], p<0.0001). When facets were subdivided into anatomic zones, the sequence of highest to lowest densities for the superior facets was: center > medial > cranial > lateral > caudal (Figure 2). The sequence of highest to lowest densities for the inferior facets was: center > cranial > lateral > medial > caudal. For both superior and inferior facets, the mean values of subchondral bone density for each of the five zones were statistically significantly different from each other on pairwise testing, with the exception of the pairwise test between the inferior facet center and cranial zones (Figure 2).
Fig. 2.
Mean values for subchondral bone density in HU segregated by facet location (superior or inferior) and subdivided by anatomical zone (caudal, central, cranial, lateral or medial) following the system used in Simon et al. [24]. Error bars span one SD. ** shows significant differences at the p < 0.0001 level, and * at p < 0.05
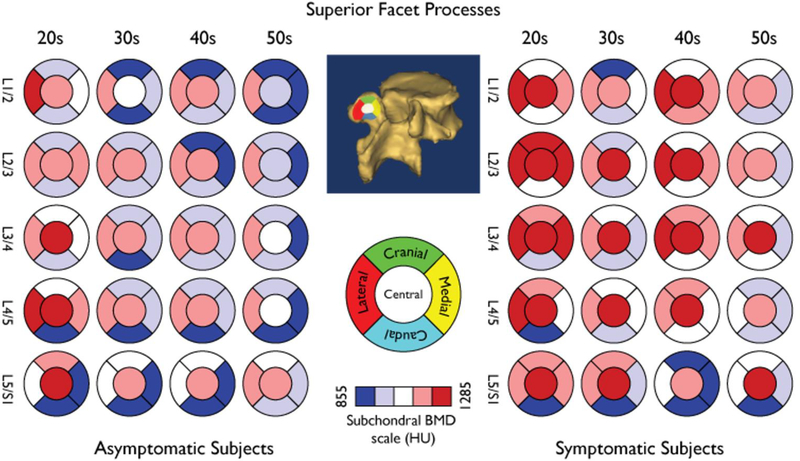
The general distribution for subchondral bone density in the superior facets is shown in Figure 3 and that of the inferior facets in Figure 4, respectively. Subchondral bone density decreased along with each descending lumbar level (Table 1). This pattern was replicated in subgroup analyses stratified by anatomic position and presence of low back pain (subgroup analyses not shown).
Fig. 3.
False color map showing the distribution of subchondral BMD by age group and spinal levels in the Superior Facet Processes. The anatomical facet zoning scheme is set at the facet surface centroid
Fig. 4.
False color map showing the distribution of subchondral BMD by age group and spinal levels in the Inferior Facet Processes. The anatomical facet zoning scheme is set at the facet surface centroid. In the superior processes, the medial and lateral regions are mirror images of the corresponding inferior process zones
Table 1.
Comparison of subchondral BMD between different spinal levels. Asterisks denote significant differences.
| Level Comparison | p-values |
|---|---|
| L1L2 vs. L2L3 | 0.0241* |
| L1L2 vs. L3L4 | 0.0007* |
| L1L2 vs. L4L5 | <0.0001* |
| L1L2 vs. L5S1 | <0.0001* |
| L2L3 vs. L3L4 | 0.2564 |
| L2L3 vs. L4L5 | 0.0004* |
| L2L3 vs. L5S1 | <0.0001* |
| L3L4 vs. L4L5 | 0.0246* |
| L3L4 vs. L5S1 | 0.0009* |
| L4L5 vs. L5S1 | 0.2856 |
A comparison between asymptomatic and symptomatic subjects showed that subchondral bone density was greater in subjects with than without low back pain (1074.3 HU [±201.4] versus 1040.3 HU [±211.6], p<0.0001). Correspondingly, among the low back pain subjects, subchondral bone density was greater in the superior facets (p ≤ 0.032). However, solely among asymptomatic subjects, subchondral bone density did not differ between the superior and inferior facets (p = 0.65).
Female subjects had greater subchondral bone density than males (1062.4 HU [±209.4] vs. 1044.46 HU [±207.4], p < 0.0001). Nonetheless, within the low back pain group, subchondral bone density was lower in females than in males (1051.5 HU [±208.7] versus 1091.1 HU [±194.2], p<0.0001). In contrast, for the asymptomatic subjects, subchondral bone density was greater in females than in males (1067.8 HU [±209.6] versus 1012.8 HU [±210.2], p<0.0001).
Finally, younger age was associated with higher subchondral bone (Table 2). The youngest group (subjects in their 20s) had the densest facets, and there was a significant drop in density for the next-densest age group (subjects in their 30s; 1096.1 HU [±200.6] versus 1030.5 HU [±218.3], p<0.0001). Although there were statistically significant differences between the subjects in their 30s and 40s (p = 0.0036), the subchondral bone density values displayed an asymptotic behavior with increasing age, and neither the 40s versus 50s nor the 30s versus 50s subchondral bone density tests were statistically significant (p≥0.05 for each; Table 2).
Table 2.
Subchondral Bone Density in Hounsfield Units (HU) by age group.
| Age Group | Mean ± SD (HU) | Significant Differences |
|---|---|---|
| 20s | 1096.2 ± 200.6 | ** vs. 30s, 40s and 50s |
| 30s | 1030.5 ± 218.3 | * vs. 40s and ** vs. 20s |
| 40s | 1048.0 ± 196.4 | * vs. 30s and ** vs. 20s |
| 50s | 1038.6 ± 201.3 | ** vs. 20s |
** p<0.0001 and * p = 0.0036. All other comparisons were not significant. A peak is observed in the youngest group followed by a largely stable period from the 30s onwards.
Taken together, the analyses indicate that subchondral bone density was highest in subjects with low back pain, female subjects, younger subjects, the superior facets, the center zone of the facets, and the upper lumbar levels. Correspondingly, subchondral bone density was lowest in subjects without low back pain, male subjects, older subjects, the inferior facets, the caudal zone of the facets, and lower lumbar levels.
Discussion
The present study also demonstrates that the overall facet joint subchondral bone density is greater for patients with than without low back pain. Given that back pain is a multi-factorial condition, it can often be difficult to narrow the differential diagnosis for patients. As described, subchondral bone density is known to be associated with loading distribution in non-spine [9–11] and facet joints [12]. Hence, one possible explanation for the present study’s finding that subchondral bone density is greater in patients with than without low back pain is that in patients with facet osteoarthritis (and hence, highly likely to have low back pain), the mechanical arrangement of the facets is altered, such that at least certain regions experience increased loading [4, 6, 25]. These regions may then respond with an increase in subchondral bone density. Whether said subchondral bone density increases play any role in the development of the pain syndrome is an area for future research
The present study demonstrates distinct patterns in the distribution of subchondral bone density in lumbar facet joints. Specifically, the study demonstrates that the center of both the superior and inferior facet joints have the greatest subchondral bone density. Correspondingly, the caudal zones of both the superior and inferior facet joints have the lowest subchondral bone density. Furthermore, the present study demonstrates that the distribution of subchondral bone density is different in the superior and inferior facet joints. Specifically, while both have the greatest density in the center zone, the superior facet joints have the second-greatest density in the medial zone, while the inferior facets have the second-greatest density in the cranial zone. The literature to date does not present a complete picture of the biomechanics of the facet joint. The areas of greatest subchondral bone density in the present study are representative of the areas of greatest loading on the joint surface. Hence, the present study makes an important contribution to our understanding of the facet joints by indicating that loading stresses are concentrated in the center zone of both facets, the medial zone of the superior facet, and the cranial zone of the inferior facet.
In the present study, the subchondral bone density of the superior facets was higher than that of inferior facets. In most joints, the concave component has denser subchondral bone, while the convex component has less dense subchondral bone [26, 27]. In the lumbar spine, the superior facet is concave, while the inferior facet is convex. Hence, the results of the present study with respect to the facet joints fit the pattern observed by others with respect to other joints in the body.
Unexpectedly, subchondral bone density was overall greater in females than males. Otsuka et al. reported that in subjects both with and without low back pain, the mean facet surface area in males was larger than in females by 12% [22]. While loading in the facet joint is expected to be higher in males, smaller facet joint surface area in females may increase higher stress and, in turn, increase subchondral bone density. Similar results were found when the data were analyzed by spinal level. The facet joint subchondral bone density was greatest at the L1/2 level and decreased with each successive lower spinal level. Since the facet surface areas are smaller at the upper-level vertebrae [22], it is also possible that higher stresses at the facet joints in upper vertebral levels due to a smaller facet joint area may be implicated as a cause for denser facet joints in the upper levels. Future studies on stress distribution at the facet joint in different genders and spinal levels and its relation with subchondral bone density will be required to test this hypothesis. It is worth acknowledging that the sample size and demographics of this study may be a limitation when considering conclusions about the influence of age and gender on subchondral bone density.
The subchondral bone density in the second decade was substantially higher than in the other groups, but the subchondral bone density then displayed an asymptotic behavior with increasing age. In general, bone mass reaches a peak in the third decade of life, and then gradually declines. It is possible that the “running average” value of the facet joint subchondral bone density in the present study over the 30s, 40s, and 50s subgroups represents the result of a combination of competing forces. The pull to increase subchondral bone density due to increasing load misdistributions and the increasing load due to the degenerating disc [28], versus the pull to decrease subchondral bone density as part of the natural decrease in bone density with age. Further studies are required to shed light on the contributions of these two factors.
Another limitation of the current study is lack of information on menopausal state and bone density of the vertebral body which are relevant to evaluate general osteoporotic status. Since association between the bone mineral density and muscle mass and quality has been reported[29] additional measurements on the paravertebral muscles, such as cross-sectional area and density of the muscle, should be also considered in the future study to better understand the relationship between the mechanical loading and facet joint subchondral bone density.
In conclusion, the present study demonstrates differences in subchondral bone density between subjects with and without LBP. Likely, the observed increases in subchondral bone density in subjects with LBP is related to increased load bearing due to degeneration of the lumbar discs and/or abnormal distribution of the load within the joint due to degeneration of the joint’s articular cartilage. The present study also demonstrated variation in facet joint subchondral bone density that helps clarify the joints’ loading patterns and biomechanical properties.
Acknowledgements
This study was supported by the NIH:
National Institute on Arthritis and Musculoskeletal and Skin Diseases Grant (5P01 AR048152-10, PI: GBJ Andersson).
National Center for Complementary and Integrative Health (NCCIH) grant 1R01-AT006692-01A1, PI: N Inoue)
The authors wish to thank Dr. Daniel Bohl for his careful help with editing this manuscript. The Manuscript submitted does not contain information about medical device(s)/drug(s).
Funding This study was funded by the NIH - NIAMS and NCCIH institutes.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Compliance with Ethical Standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boswell MV, Trescot AM, Datta S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain physician. 2007; 10(1):7–111. [PubMed] [Google Scholar]
- 3.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. The Journal of bone and joint surgery American volume. 2006; 88 Suppl 2:21–24. [DOI] [PubMed] [Google Scholar]
- 4.Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. The Journal of bone and joint surgery British volume. 1980; 62(3):358–362. [DOI] [PubMed] [Google Scholar]
- 5.Jaumard NV, Welch WC, Winkelstein BA. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. Journal of biomechanical engineering. 2011; 133(7):071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984; 9(6):557–565. [DOI] [PubMed] [Google Scholar]
- 7.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of a randomized, double-blind controlled trial: Clinical Trial NCT00355914. Pain physician. 2008; 11(2):121–132. [PubMed] [Google Scholar]
- 8.Varlotta GP, Lefkowitz TR, Schweitzer M, et al. The lumbar facet joint: a review of current knowledge: part 1: anatomy, biomechanics, and grading. Skeletal radiology. 2011; 40(1):13–23. [DOI] [PubMed] [Google Scholar]
- 9.Carlson KJ, Patel BA. Habitual use of the primate forelimb is reflected in the material properties of subchondral bone in the distal radius. Journal of anatomy. 2006; 208(6):659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewire P, Simkin PA. Subchondral plate thickness reflects tensile stress in the primate acetabulum. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1996; 14(5):838–841. [DOI] [PubMed] [Google Scholar]
- 11.Muller-Gerbl M The subchondral bone plate. Advances in anatomy, embryology, and cell biology. 1998; 141:Iii-xi, 1–134. [DOI] [PubMed] [Google Scholar]
- 12.Wagner S, Weckbach A, Muller-Gerbl M. The influence of posterior instrumentation on adjacent and transfixed facet joints in patients with thoracolumbar spinal injuries: a morphological in vivo study using computerized tomography osteoabsorptiometry. Spine. 2005; 30(7):E169–178. [DOI] [PubMed] [Google Scholar]
- 13.Bland JH. The reversibility of osteoarthritis: a review. The American journal of medicine. 1983; 74(6a):16–26. [DOI] [PubMed] [Google Scholar]
- 14.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nature reviews Rheumatology. 2012; 8(11):665–673. [DOI] [PubMed] [Google Scholar]
- 15.Grynpas MD, Alpert B, Katz I, Lieberman I, Pritzker KP. Subchondral bone in osteoarthritis. Calcified tissue international. 1991; 49(1):20–26. [DOI] [PubMed] [Google Scholar]
- 16.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clinical orthopaedics and related research. 1986(213):34–40. [PubMed] [Google Scholar]
- 17.Ebel CM, Prodinger PM, Muhlhofer H, Muller-Gerbl M, Linsenmaier U, Putz R. Morphological adaptation of the tarso-metatarsal joints onto load transmission in the foot. Surgical and radiologic anatomy : SRA. 2010; 32(2):107–113. [DOI] [PubMed] [Google Scholar]
- 18.Kraljevic M, Zumstein V, Wirz D, Hugli R, Muller-Gerbl M. Mineralisation and mechanical strength of the glenoid cavity subchondral bone plate. International orthopaedics. 2011; 35(12):1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller-Gerbl M, Putz R, Hodapp N, Schulte E, Wimmer B. Computed tomography-osteoabsorptiometry for assessing the density distribution of subchondral bone as a measure of long-term mechanical adaptation in individual joints. Skeletal radiology. 1989; 18(7):507–512. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Gerbl M, Putz R, Kenn R. Demonstration of subchondral bone density patterns by three-dimensional CT osteoabsorptiometry as a noninvasive method for in vivo assessment of individual long-term stresses in joints. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992; 7 Suppl 2:S411–418. [DOI] [PubMed] [Google Scholar]
- 21.Zumstein V, Kraljevic M, Huegli R, Muller-Gerbl M. Mineralisation patterns in the subchondral bone plate of the humeral head. Surgical and radiologic anatomy : SRA. 2011; 33(9):775–779. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka Y, An HS, Ochia RS, Andersson GB, Espinoza Orias AA, Inoue N. In vivo measurement of lumbar facet joint area in asymptomatic and chronic low back pain subjects. Spine. 2010; 35(8):924–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan CY, Espinoza Orias AA, Shott S, et al. In vivo measurement of the subchondral bone thickness of lumbar facet joint using magnetic resonance imaging. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011; 19(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon P, Espinoza Orias AA, Andersson GB, An HS, Inoue N. In vivo topographic analysis of lumbar facet joint space width distribution in healthy and symptomatic subjects. Spine. 2012; 37(12):1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams MA, Hutton WC. The mechanical function of the lumbar apophyseal joints. Spine. 1983; 8(3):327–330. [DOI] [PubMed] [Google Scholar]
- 26.Simkin PA, Graney DO, Fiechtner JJ. Roman arches, human joints, and disease: differences between convex and concave sides of joints. Arthritis and rheumatism. 1980; 23(11):1308–1311. [DOI] [PubMed] [Google Scholar]
- 27.Simkin PA, Heston TF, Downey DJ, Benedict RS, Choi HS. Subchondral architecture in bones of the canine shoulder. Journal of anatomy. 1991; 175:213–227. [PMC free article] [PubMed] [Google Scholar]
- 28.Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990; 15(2):111–113. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Guo J, Duanmu Y, et al. Quantitative analysis of modified functional muscle-bone unit and back muscle density in patients with lumbar vertebral fracture in Chinese elderly men: a case-control study. Aging clinical and experimental research. 2019; 31(5):637–644. [DOI] [PubMed] [Google Scholar]