Abstract
Context.
Patients with decompensated cirrhosis have high rates of health care utilization at end of life (EOL). However, the impact of transplant candidacy on intensity of EOL care is currently unknown.
Objectives.
To assess the relationship between transplant candidacy and intensity of EOL care in the last year of life in an ambulatory cohort of patients with decompensated cirrhosis.
Methods.
We performed a retrospective analysis of 230 patients with decompensated cirrhosis who were evaluated for liver transplantation in a large health care system between 1/1/2010 and 12/31/2017 and died by 6/20/2018. We compared health care utilization in the last year of life and EOL care outcomes between transplant-listed (n = 133) and nonlisted (n = 97) patients. We examined predictors of palliative and hospice care utilization using multivariate logistic regression.
Results.
During the last year of life, patients had a median of three hospitalizations (IQR 2–5) and spent a median of 31 days (IQR 16–49) in the hospital. In all, 80% of patients died in the hospital, with 70% dying in the intensive care unit. The majority (70.0%) received a life-sustaining procedure (mechanical ventilation, renal replacement therapy, or cardiopulmonary resuscitation) during their terminal hospitalization, which did not differ between transplant-listed and nonlisted patients (74.4% vs. 63.9%, P = 0.09). Transplant-listed patients had lower odds of receiving specialty palliative care (odds ratio 0.43, P = 0.005). Patients with hepatocellular carcinoma had higher odds of receiving hospice care (odds ratio 2.03, P = 0.049).
Conclusion.
Patients with decompensated cirrhosis had intensive health care utilization during their last year of life regardless of transplant candidacy. Further work is needed to optimize their EOL care, particularly for patients who are ineligible for transplantation
Keywords: End-stage liver disease, liver transplantation, hospice, palliative care, advance care planning, code status
Introduction
Decompensated cirrhosis, a disease state in which patients with cirrhosis develop ascites, variceal bleeding, or hepatic encephalopathy, is a diagnosis for which liver transplantation remains the only curative therapy.1,2 Unfortunately, thousands of patients with decompensated cirrhosis annually are not eligible for liver transplantation because of significant comorbid disease, ongoing or recent substance use, inadequate social support, or advanced age. Among those patients listed for liver transplantation, more than 30% either die while awaiting transplantation or are removed from the waitlist.3 As such, decompensated cirrhosis is a terminal diagnosis for over 40,000 patients annually, irrespective of transplant candidacy.4
Patients with decompensated cirrhosis have high rates of health care utilization at end of life (EOL).5,6 Owing to their liver-related complications, patients with decompensated cirrhosis are frequently hospitalized during their last months of life, often receive invasive procedures, such as renal replacement therapy (RRT), mechanical ventilation, and cardiopulmonary resuscitation (CPR), and have high in-hospital mortality.5–10 Furthermore, these patients are rarely referred to palliative and hospice care services.10–13 However, factors contributing to intensive EOL care for patients with decompensated cirrhosis, particularly transplant candidacy, are unknown. Transplant candidacy has been associated with reduced rates of advance care planning and increased likelihood of receiving aggressive life-sustaining treatments near EOL in patients with other end-stage conditions.14,15 As such, patients with decompensated cirrhosis who are candidates for liver transplantation may represent a group that is particularly vulnerable to intensive medical care at EOL.
To date, studies evaluating differences in health care utilization and EOL care outcomes for patients with decompensated cirrhosis based on transplant candidacy are lacking. A more comprehensive under-standing of health care utilization and EOL care outcomes in this population is necessary to identify patients at higher risk for poor-quality EOL care. This study aims to address this gap in the literature by assessing the relationship between transplant candidacy and associated intensity of health care utilization and EOL care in the last year of life in a decedent cohort of patients who developed decompensated cirrhosis in the ambulatory setting. We sought to test our primary hypothesis that patients with decompensated cirrhosis on the transplant list will have more intensive patterns of EOL care compared with patients who are not listed.
Methods
Study Design and Data Collection
We performed a retrospective analysis of all adult (age ≥ 18 years) patients evaluated for liver transplantation between 1/1/2010 and 12/31/2017 in a network of nine acute care hospitals (Partners Health-care, Massachusetts), including one tertiary care medical center with a liver transplantation program (Massachusetts General Hospital, Boston, MA). We identified patients who had died by 6/30/2018, and within this decedent cohort, we identified all patients with decompensated cirrhosis. Decompensated cirrhosis was defined as clinical evidence of cirrhosis confirmed via imaging and/or liver biopsy with ascites, history of variceal bleeding, hepatic encephalopathy, and/or hepatocellular carcinoma (HCC).2 The presence and date of diagnosis of decompensated cirrhosis were determined through a review of medical records, including patient charts, laboratory values, radio-graphic studies, endoscopy reports, and pharmacy records.
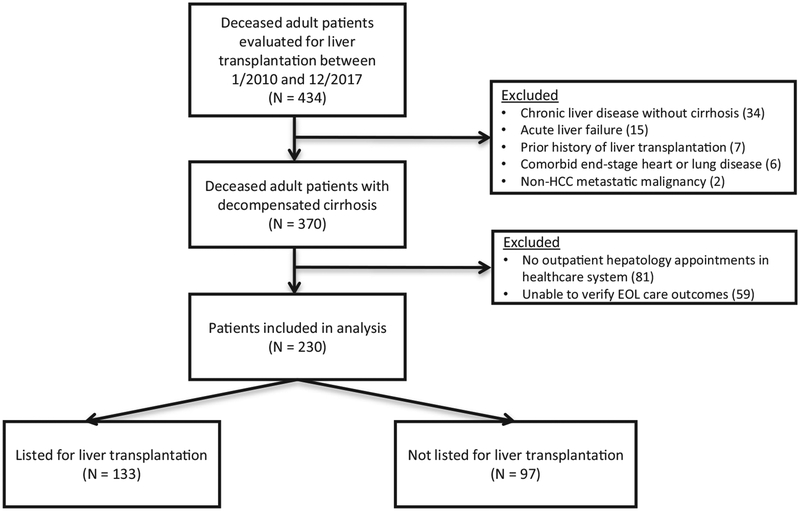
Because the aim of our study was to assess the impact of transplant candidacy on health care utilization and EOL care in an ambulatory cohort of patients who developed decompensated cirrhosis, we excluded patients who had any of the following: 1) no outpatient hepatology appointments in our health care system as we lacked access to these patients’ preclinical data before them developing decompensated cirrhosis and 2) no available records to verify their EOL outcomes, specifically place of death and/or cause of death. We additionally excluded patients who had a prior history of liver transplantation or a comorbid life-limiting condition such as end-stage heart or lung disease or non-HCC metastatic malignancy (Fig. 1). The Partners Institutional Review Board approved this study.
Fig. 1.
Flow diagram of patient selection. HCC = hepatocellular carcinoma; EOL = end of life.
We conducted a comprehensive chart review to obtain information regarding patients’ demographics, clinical data, liver transplantation evaluation, and the outcomes of health care utilization in the last year of life and EOL care. Demographic data included age at death, gender, race, and marital status. Clinical data included etiology of liver disease, presence of ascites, hepatic encephalopathy, or HCC, history of variceal bleeding, and Charlson Comorbidity Index score.16 Each patient in the cohort was evaluated at least once for liver transplantation. Our transplant-listed cohort included patients who were ever listed for liver transplantation, whereas our nonlisted cohort included patients who were evaluated for transplantation but were either deferred or declined for listing. We obtained their natural Model for End-Stage Liver Disease (MELD) score at the time of evaluation (for the nonlisted cohort) or listing (for the transplant-listed cohort).
We collected data on health care utilization in the last year of life, which included the frequency of hospitalizations, hospital length of stay (LOS), intensive care unit (ICU) admissions, ICU LOS, receipt of life-sustaining procedures, defined as CPR, mechanical ventilation, and/or RRT, and palliative care utilization, specifically referral to specialty palliative care in the inpatient and/or outpatient settings.17
We obtained data on EOL care, which included code status documentation, place of death, cause of death, hospice utilization, and LOS in hospice, through a review of the electronic medical record and, if needed, by search of the Social Security Death Index or online obituaries.
Statistical Analysis
We used descriptive statistics to summarize patient demographics, disease characteristics, and clinical outcomes of all study patients and stratified by status on the liver transplantation list (transplant-listed vs. nonlisted), using Chi-squared analysis for categorical variables and t-test or Wilcoxon rank sum test for continuous variables, where appropriate.
Sensitivity analyses were conducted comparing health care utilization and EOL care outcomes between transplant-listed and nonlisted patients after excluding patients who were deferred for transplantation after evaluation for the following two reasons: 1) required additional workup to determine transplant eligibility and 2) temporarily too well for transplantation.
To examine predictors of palliative care (either inpatient or outpatient referrals) and hospice care utilization in patients with decompensated cirrhosis, we used multivariate logistic regression models and tested the following a priori defined predictors: age, gender, race, marital status (married vs. not married), Charlson Comorbidity Index score, etiology of liver disease (alcohol-related, viral, or nonalcoholic steatohepatitis), presence of ascites, hepatic encephalopathy, or HCC, history of variceal bleeding, and status on the transplantation list (transplant-listed vs. nonlisted). In addition to age, variables significant in the univariate analyses with P-value < 0.15 were included in the multivariate logistic regression models.
For all analyses, an alpha of <0.05 was used to determine statistical significance. We conducted all analyses using STATA, version 15.1 (StataCorp., College Station, TX).
Results
Patient Characteristics
Fig. 1 depicts the study flow diagram. The cohort comprised 230 patients, 133 (58%) of whom were on the liver transplantation waiting list at the time of death, whereas 97 (42%) were not listed for transplantation. There were no significant differences in age, sex, race, marital status, cirrhosis etiology, presence of ascites, variceal bleeding, hepatic encephalopathy, or Charlson Comorbidity Index scores between both groups (Table 1). Patients on the liver transplantation waiting list were more likely to have HCC than patients who were not listed (32% vs. 19%, P = 0.03) and had a lower natural MELD score at the time of their evaluation for liver transplantation (18 vs. 23, P = 0.003). The median time between transplant evaluation and listing for the transplant-listed patients was 51 days (IQR 18–94.5 days).
Table 1.
Patient Characteristics
| Characteristic | All, N= 230 | Transplant Listed, n = 133 | Nonlisted, n = 97 |
|---|---|---|---|
| Age at death, mean (SD), yrs | 57.7 (8.7) | 58.1 (8.3) | 57.2 (9.1) |
| Male, n (%) | 148 (64.4) | 88 (66.2) | 60 (61.9) |
| Race, n (%) | |||
| White | 188 (81.7) | 109 (82.0) | 79 (81.4) |
| Black | 15 (6.5) | 10 (7.5) | 5 (5.2) |
| Asian | 8 (3.5) | 6 (4.5) | 2 (2.1) |
| Hispanic | 9 (3.9) | 4 (3.0) | 5 (5.1) |
| Other | 10 (4.3) | 4 (3.0) | 6 (6.2) |
| Marital status, n (%) | |||
| Single | 50 (21.7) | 26 (19.5) | 24 (24.7) |
| Married/life partner | 133 (57.8) | 86 (64.7) | 47 (48.5) |
| Divorced/legally separated | 26 (11.3) | 14 (10.5) | 12 (12.4) |
| Widowed | 10 (4.4) | 3 (2.3) | 7 (7.2) |
| Missing | 11 (4.8) | 4 (3.0) | 7 (7.2) |
| Cause of ESLD, n (%) | |||
| Alcohol | 58 (25.2) | 34 (25.6) | 24 (24.7) |
| NASH | 43 (18.7) | 24 (18.1) | 19 (19.6) |
| Hepatitis C | 58 (25.2) | 35 (26.3) | 23 (23.7) |
| Alcohol and hepatitis C | 30 (13.0) | 18 (13.5) | 12 (12.4) |
| Othera | 41 (17.8) | 22 (16.5) | 19 (19.6) |
| Clinical features, n (%) | |||
| Hepatocellular carcinoma | 60 (25.5) | 42 (31.6) | 18 (18.6) |
| Ascites | 208 (90.4) | 119 (89.5) | 89 (91.8) |
| Refractory ascites | 83 (36.1) | 44 (33.1) | 39 (40.2) |
| Variceal bleeding | 78 (33.4) | 50 (37.6) | 28 (28.9) |
| Hepatic encephalopathy | 191 (83.0) | 112 (84.2) | 79 (81.4) |
| MELD score (at time of transplant evaluation), median [IQR] | 20 [15–28] | 18 [14–24] | 23 [16–31] |
| Charlson Comorbidity Index score, median [IQR] | 5 [4–6] | 5 [4–6] | 5 [4–6] |
| Time from diagnosis of decompensated cirrhosis until death, median [IQR], days | 620.5 [291–1250] | 741.5 [350–1368] | 449.5 [184–1234.5] |
ESLD = end-stage liver disease; NASH = nonalcoholic steatohepatitis; MELD = Model for End-Stage Liver Disease.
“Other” includes hepatitis B, primary sclerosing cholangitis, primary biliary cholangitis, autoimmune hepatitis, hemochromatosis, and alpha-1 antitrypsin deficiency.
Overall, patients had a median survival of 620.5 days (IQR 291–1250 days) from the diagnosis of decompensated cirrhosis until death. Transplant-listed patients had a longer duration of time from their diagnosis of decompensated cirrhosis until death compared with those in the nonlisted cohort: 741.5 days (IQR 350–1368 days) vs. 449.5 days (IQR 184–1234.5 days) (P = 0.03). Patients who were listed spent a median of 196 days (IQR 57.5–495.5 days) on the waiting list before death. Among nonlisted patients, the median time from date of deferral or denial for transplant listing until death was 64 days (IQR 18.5–253.5 days). The reasons for transplant deferral or denial at the time of their evaluation were as follows: 39 (40.2%) were deemed too sick to undergo transplantation at time of evaluation, 15 (15.5%) required additional workup to determine transplant eligibility, 14 (14.4%) had active substance use disorder, nine (9.3%) had HCC beyond Milan criteria, eight (8.3%) had a medical contraindication to transplantation, four (4.1%) were temporarily too well, four (4.1%) declined transplantation, and four (4.1%) had psychosocial contraindications to transplantation.
Health Care Utilization During Last Year of Life
During their last year of life, patients had a median number of three hospitalizations (IQR 2–5) and one ICU admission (IQR 1–2), which did not vary based on status on the transplant list. Transplant-listed and nonlisted patients spent a similar number of total days in the hospital during their last year of life (28 days [IQR 16–51 days] vs. 33 days [IQR 14–45 days], P = 0.56). We found no difference in days in the hospital when limiting our analyses to the last 180 days (27 days [IQR 14.5–47 days] vs. 27 days [IQR 11.5–39.5 days], P = 0.48) and 90 days of life (22 days [IQR 10.5–39.5= days] vs. 22 days [IQR 10–37 days], P = 0.72).
End-of-Life Care Outcomes
The median LOS of patients’ last hospitalization before death was 13 days (IQR 5–23 days), which did not vary based on transplant candidacy (Table 2). Patients on the transplant list were more likely to be admitted to the ICU during their last hospitalization than nonlisted patients (79.7% vs.66.0%, P = 0.02). The majority of patients (161 of 230, 70.0%) received a life-sustaining procedure during their last hospitalization, defined as either mechanical ventilation (134, 58.3%), RRT (103, 44.8%), or CPR (15, 6.5%). The rates of receipt of life-sustaining procedures were similar for transplant-listed and nonlisted patients (74.4% vs.63.9%, P = 0.09).
Table 2.
Health Care Utilization and End-of-Life Care Outcomes
| Characteristic | Transplant Listed, n = 133 | Nonlisted n = 97 | P-value |
|---|---|---|---|
| LOS of last hospitalization before death, median [IQR], days | 14 [6.5, 24] | 10 [4, 21.5] | 0.18 |
| ICU admission during last hospitalization before death, n (%) | 106 (79.7) | 64 (66.0) | 0.02 |
| ICU LOS, median [IQR], days | 4 [1,11] | 2 [0, 9] | 0.07 |
| Intubation, n (%) | 81 (60.9) | 53 (54.6) | 0.34 |
| RRT, n (%) | 64 (48.1) | 39 (40.2) | 0.23 |
| CPR, n (%) | 10 (7.5) | 5 (5.2) | 0.59 |
| In-Hospital death, n (%) | 115 (86.5) | 76 (78.4) | 0.11 |
| Death in ICU, n (%) | 98 (73.7) | 60 (61.9) | 0.06 |
| Cause of death, n (%) | 0.30 | ||
| Infection | 63 (47.4) | 39 (40.2) | |
| Gastrointestinal bleeding | 18 (13.5) | 10 (10.3) | |
| Respiratory failure | 10 (7.5) | 9 (9.3) | |
| Renal failure | 8 (6.0) | 13 (13.4) | |
| HCC | 5 (3.8) | 7 (7.2) | |
| Other | 29 (21.8) | 19 (19.6) | |
| Outpatient palliative care referral, n (%) | 6 (4.5) | 6 (6.2) | 0.57 |
| Inpatient palliative care referral, n (%) | 33 (24.8) | 38 (39.2) | 0.02 |
| Hospice referral, n (%) | 29 (21.8) | 28 (28.9) | 0.22 |
LOS = length of stay; ICU = intensive care unit; RRT = renal replacement therapy; CPR = cardiopulmonary resuscitation; HCC = hepatocellular carcinoma.
The majority of patients died in the hospital (191 of 230, 83.0%), with most deaths occurring in the ICU (158, 68.7%). The most common causes of death were infection (102, 44.3%) and gastrointestinal bleeding (28, 12.2%). Both transplant-listed and nonlisted patients had similar rates of in-hospital mortality(86.5% vs. 78.4%, P = 0.11), and causes of death did not vary significantly between these two groups (Table 2).
Patients who died outside of the hospital had a median of eight days (IQR 4–28.5 days) from their last hospital discharge until death, which did not vary based on transplant candidacy. Only a minority of these patients (eight of 39, 20.5%) were admitted to the ICU during their last hospitalization, with similar rates of ICU admission between transplant-listed and nonlisted patients (16.7% vs. 23.8%, P = 0.70).
Almost all (211 of 230, 91.7%) patients were admitted with full code status during their terminal hospitalization, with similar rates seen in both transplant-listed and nonlisted patients (94.7% vs.87.6%, P = 0.09). Almost all (216 of 230, 93.9%) patients were transitioned to do not resuscitate/do not intubate (DNR/DNI) or comfort measures only (CMO) during their terminal hospitalization. The majority (80.1%) of code status transitions from full code to DNR/DNI or CMO occurred within 72 hours of death, with over half (55.5%) of code status transitions taking place on the day of death. Among those patients who were transitioned from full code to DNR/DNI or CMO only, 64.3% of code status discussions and transitions were coordinated by members of the ICU teams. These findings did not vary based on transplant candidacy.
Sensitivity Analysis
In a sensitivity analysis excluding nonlisted patients who required additional workup to determine transplant eligibility or were temporarily too well for transplantation at the time of evaluation, there remained no difference in the number of hospitalizations and ICU admissions in the last year of life, hospital LOS in the last 90 days of life, terminal hospitalization LOS, rates of receipt of life-sustaining procedures, and rate of in-hospital death between listed and nonlisted cohorts (P > 0.05 for all comparisons, results not shown).
Palliative Care and Hospice Care Utilization
Within the entire cohort, 78 patients (33.9%) were referred to specialty palliative care, with almost all (91.0%) of these consultations occurring in the inpatient setting. Patients who were only referred to palliative care in the inpatient setting were seen by the service in a median of 10 days (IQR 3–28 day) before death. Multivariate logistic regression modeling (Table 3) demonstrated that transplant-listed patients had significantly lower odds of being referred to specialty palliative care (odds ratio 0.43, 95% CI0.24–0.78, P = 0.005).
Table 3.
Univariate and Multivariate Analyses of Predictors of Palliative Care Referral
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| Odds Ratio (95% GI) | P-value | Odds Ratio (95% CI) | P-value | |
| Age, yrs | 1.02 (0.98–1.05) | 0.30 | 1.01 (0.97–1.05) | 0.63 |
| Female gender | 0.86 (0.50–1.59) | 0.71 | ||
| White race | 1.02 (0.50–2.08) | 0.96 | ||
| Married | 0.70 (0.40–1.25) | 0.23 | ||
| Charlson Comorbidity Index score | 1.15 (0.98–1.34) | 0.09 | 1.12 (0.95–1.32) | 0.17 |
| Alcohol | 1.10 (0.63–1.91) | 0.74 | ||
| Viral hepatitis | 1.38 (0.79–2.4) | 0.26 | ||
| NASH | 0.77 (0.40–1.5) | 0.45 | ||
| HCC | 1.64 (0.89–3.03) | 0.112 | 1.60 (0.82–3.19) | 0.16 |
| Ascites | 1.77 (0.62–5.03) | 0.28 | ||
| Variceal bleed | 1.13 (0.63–2.01) | 0.67 | ||
| Hepatic encephalopathy | 0.84 (0.41–1.74) | 0.64 | ||
| Transplant—listed | 0.50 (0.29–0.88) | 0.02 | 0.43 (0.24–0.78) | 0.005 |
NASH = nonalcoholic steatohepatitis; HCC = hepatocellular carcinoma.
In total, 57 patients (24.8%) were referred to hospice, with a median LOS in hospice of six days (IQR 3–17 days) before death. Among those referred to hospice, 23 patients (40.4%) died at home. In addition, the majority (36 of 39, 92.3%) of patients who died outside of the hospital had been referred to hospice before death. Multivariate logistic regression modeling (Table 4) demonstrated that patients with HCC had significantly higher odds of receiving hospice care (odds ratio 2.03, 95% CI 1.00–4.12, P = 0.049). Liver transplant candidacy was not found to be associated with referral to hospice.
Table 4.
Univariate and Multivariate Analyses of Predictors of Hospice Care Referral
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | |
| Age, yrs | 1.03 (0.99–1.07) | 0.10 | 1.01 (0.97–1.05) | 0.59 |
| Female gender | 0.78 (0.41–1.49) | 0.46 | ||
| White race | 0.77 (0.36–1.63) | 0.50 | ||
| Married | 0.75 (0.40–1.41) | 0.38 | ||
| Charlson Comorbidity Index score | 1.15 (0.98–1.36) | 0.08 | 1.10 (0.93–1.31) | 0.27 |
| Alcohol | 0.94 (0.51–1.73) | 0.85 | ||
| Viral hepatitis | 2.04 (1.11–3.74) | 0.02 | 1.5 (0.81–2.95) | 0.19 |
| NASH | 0.84 (0.41–1.74) | 0.64 | ||
| HCC | 2.81 (1.47–5.34) | 0.002 | 2.03 (1.00–4.12) | 0.049 |
| Ascites | 1.16 (0.69–2.36) | 0.44 | ||
| Variceal bleed | 0.85 (0.46–1.60) | 0.61 | ||
| Hepatic encephalopathy | 0.50 (0.24–1.06) | 0.07 | 0.57 (0.26–1.25) | 0.16 |
| Transplant listed | 0.69 (0.37–1.26) | 0.23 | ||
NASH = nonalcoholic steatohepatitis; HCC = hepatocellular carcinoma.
Discussion
In this study, we examined health care utilization and EOL care outcomes in a decedent cohort of patients with decompensated cirrhosis receiving longitudinal ambulatory care within a large health care system who were evaluated for liver transplantation. Patients in our study cohort had a median survival of approximately two years from the time of diagnosis of decompensated cirrhosis until death, which is consistent with the natural history of end-stage liver disease as previously reported.2 Both transplant-listed and nonlisted patients experienced frequent hospital admissions in the last year of life, and almost one-quarter of their last 90 days of life were spent in the hospital, with a substantial majority requiring ICU level care during their terminal hospitalizations. Nearly all patients were full code in the days before their death and had similar rates of receipt of invasive life-sustaining therapies, irrespective of transplant candidacy. The majority of patients died in the hospital, with most dying in the ICU. Only a minority of patients received palliative or hospice care services, with patients on the transplant list having significantly lower odds of being referred to palliative care and patients with HCC having significantly higher odds of being referred to hospice.
Contrary to our primary hypothesis, patients with decompensated cirrhosis who were deferred or declined for listing for liver transplantation received similarly intensive medical care at EOL as those who were listed for transplantation, despite their terminal diagnosis. Specifically, we found similar rates of receipt of CPR, mechanical ventilation, and RRT near the EOL for both transplant-listed and nonlisted patients. These results persisted after performing a sensitivity analysis removing patients who were deferred for transplant listing due to a need for additional medical workup to determine suitability for transplantation and those who were temporarily too well at the time of evaluation. These findings are particularly concerning because patients not listed for transplant represent a high-risk population with a very poor prognosis and no likelihood of cure. These intensive patterns of health care utilization in patients with decompensated cirrhosis may in part be due to nature of the illness itself, as patients frequently can have unpredictable and severe life-threatening complications, such as variceal hemorrhage, sepsis, and worsening encephalopathy. Nonetheless, there is a critical need to optimize EOL care for patients with decompensated cirrhosis who are transplant-ineligible given their poor prognosis.
The intensity of EOL care for patients who are not candidates for liver transplantation may also reflect delayed advance care planning conversations. In our study, 88% of nonlisted patients were full code at the time of their terminal hospitalization, despite having relative or absolute contraindications to transplantation, and the majority of code status transitions to DNR/DNI or CMO only occurred in the ICU setting within 72 hours of death. These findings have been similarly noted in prior studies, in which rates of code status documentation for patients with cirrhosis range from 14% to 28%.10,18,19 Decisions about EOL care options are an integral part of advance care planning, and studies have shown that patients with life-limiting illnesses who discuss their EOL care preferences with their clinicians are less likely to opt for intensive medical interventions at the EOL.20–25 Our findings have identified an important gap in care delivery to transplant-ineligible patients and highlight a need to develop interventions to improve advance care planning for this population that is particularly vulnerable to receiving intensive EOL care.
Despite their substantial morbidity, patients with decompensated cirrhosis were rarely referred to palliative care services. Only 34% of patients in the entire cohort received a palliative care consultation, with over 90% of these visits occurring in the inpatient setting during the terminal hospitalization. Transplant listing was associated with lower odds of referral to palliative care in our cohort, a finding that has been noted in previous observational studies.6,26 While rates of palliative care referrals for patients with decompensated cirrhosis have been increasing annually, early palliative care remains underutilized for this population.6,11 For patients with decompensated cirrhosis who are on the transplant list, misperceptions equating palliative care with “EOL care,” delivered only when curative therapy is no longer an option, may be an important barrier to referral.26 This high-lights a missed opportunity to improve care delivery to patients with decompensated cirrhosis throughout the course of their illness trajectory and at EOL, as palliative care referrals have been associated with a reduction in health care utilization and costs for hospitalized patients with liver disease.6,11,27
Only a minority of patients in our study received hospice care before death, and hospice enrollment occurred relatively late in the disease course. Our findings revealed that only 25% of decedents were referred to hospice before death, and among those referred, the median hospice LOS was only six days. Notably, 40% of patients who were referred to hospice in our cohort died at home. Hospice services are likely underutilized for many patients with decompensated cirrhosis because of the unpredictable illness trajectory, which often hinders the clinician’s ability to accurately prognosticate and determine hospice candidacy.28–30 Of note, we found that HCC was a significant predictor of hospice care referral for patients with decompensated cirrhosis, likely due to the predictable terminal nature of the cancer diagnosis. Importantly, we also found that transplant candidacy was not a predictor of hospice referral, a concerning finding given that patients not listed for transplantation had a median of only 64 days of survival after the date they were deferred or declined for liver transplantation. Timely hospice referral has been associated with improved patient and caregiver satisfaction with EOL care, and in patients with HCC in particular, hospice care has been associated with lower rates of acute care use at EOL.31–36 Therefore, additional studies are needed to better identify patients with decompensated cirrhosis who may benefit from hospice referral to optimize the quality of their EOL care.37–39
Our study has limitations. First, this study was conducted at a single health care system and liver transplant program; thus, our sample size may limit the generalizability of our findings. Importantly, local practice patterns may limit the generalizability of outcomes related to palliative care or hospice referral. As such, larger multicenter studies are needed to assess national trends in health care utilization and EOL care outcomes in this patient population. Second, given the retrospective study design, there is a risk for selection bias and unmeasured confounding. However, baseline characteristics between the transplant-listed and nonlisted groups were relatively well matched, and we performed a multivariate analysis to adjust for demographic and clinical factors that could be associated with palliative care or hospice utilization. Third, we excluded patients who had no outpatient hepatology appointments in our health care system or incomplete EOL care outcomes and hospitalizations that occurred outside of our health care system may not have been fully captured in our medical records; thus, our observed rates of health care utilization may be underestimates. Fourth, we focused our study on patients with decompensated cirrhosis who were evaluated for liver transplantation. Studies assessing the EOL care outcomes of patients with decompensated cirrhosis who have absolute contraindications to transplantation are warranted.
In conclusion, our study demonstrates that patients with decompensated cirrhosis, irrespective of transplant candidacy, spend a substantial portion of their last 90 days of life in the hospital, have high rates of health care utilization at the EOL with low rates of palliative and hospice care utilization, and often die in the hospital setting. Notably, most patients with decompensated cirrhosis who were not listed for liver transplantation were full code on their terminal admission and received intensive life-sustaining therapies such as RRT, mechanical ventilation, and CPR at EOL. These findings underscore the need to develop interventions to enhance early advance care planning for patients with decompensated cirrhosis, particularly those who are not transplant candidates, to improve the quality of their EOL care.
Disclosures and Acknowledgments
This work was supported by the National Institutes of Health (T32DK007191 to N. N. U. and K24DK078772 to R. T. C.). Dr. El-Jawahri is a scholar in clinical research of the Leukemia & Lymphoma Society.
The authors have no financial disclosures or conflicts of interest to declare related to the topic of this manuscript.
References
- 1.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 annual data report: liver. Am J Transpl 2019;19(Suppl 2): 184–283. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: Final data for 2016. Natl Vital Stat Rep 2018;67: 1–76. [PubMed] [Google Scholar]
- 5.Kelly EM, James PD, Murthy S, et al. Health care utilization and costs for patients with end-stage liver disease are significantly higher at the end of life compared to those of other decedents. Clin Gastroenterol Hepatol 2019;17: 2339–2346.e1. [DOI] [PubMed] [Google Scholar]
- 6.Patel AA, Walling AM, Ricks-Oddie J, May FP, Saab S, Wenger N. Palliative care and health care utilization for patients with end-stage liver disease at the end of life. Clin Gastroenterol Hepatol 2017;15:1612–1619.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CL, Hammill BG, Qualls LG, Curtis LH, Muir AJ. Significant morbidity and mortality among hospitalized end-stage liver disease patients in medicare. J Pain Symptom Manage 2016;52:412–419.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology 2011;54:1864–1872. [DOI] [PubMed] [Google Scholar]
- 9.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012;107:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poonja Z, Brisebois A, van Zanten SV, Tandon P, Meeberg G, Karvellas CJ. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014;12:692–698. [DOI] [PubMed] [Google Scholar]
- 11.Rush B, Walley KR, Celi LA, Rajoriya N, Brahmania M. Palliative care access for hospitalized patients with end stage liver disease across the United States. Hepatology 2017;66: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 12.Altaii H, Al-Kindi SG, Yaqoob Z, Al-Khazaraji A, Romero-Marrero C. Place of death and hospice utilization among patients who die from cirrhosis in the United States. Clin Gastroenterol Hepatol 2018;16:142–143. [DOI] [PubMed] [Google Scholar]
- 13.Kathpalia P, Smith A, Lai JC. Underutilization of palliative care services in the liver transplant population. World J Transplant 2016;6:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joffe S, Mello MM, Cook EF, Lee SJ. Advance care planning in patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transpl 2007;13:65–73. [DOI] [PubMed] [Google Scholar]
- 15.Walling AM, Asch SM, Lorenz KA, Wenger NS. Impact of consideration of transplantation on end-of-life care for patients during a terminal hospitalization. Transplantation 2013;95:641–646. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40: 373–383. [DOI] [PubMed] [Google Scholar]
- 17.Barnato AE, Farrell MH, Chang CC, Lave JR, Roberts MS, Angus DC. Development and validation of hospital “end-of-life” treatment intensity measures. Med Care 2009;47:1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plunkett A, Mortimore M, Good P. Palliative care in cirrhosis with decompensation. Intern Med J 2019;49: 904–908. [DOI] [PubMed] [Google Scholar]
- 19.Wachter RM, Luce JM, Hearst N, Lo B. Decisions about resuscitation: inequities among patients with different diseases but similar prognoses. Ann Intern Med 1989;111: 525–532. [DOI] [PubMed] [Google Scholar]
- 20.Weeks WB, Kofoed LL, Wallace AE, Welch HG. Advance directives and the cost of terminal hospitalization. Arch Intern Med 1994;154:2077–2083. [PubMed] [Google Scholar]
- 21.Chambers CV, Diamond JJ, Perkel RL, Lasch LA. Relationship of advance directives to hospital charges in a Medicare population. Arch Intern Med 1994;154:541–547. [PubMed] [Google Scholar]
- 22.Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 2014;28: 1000–1025. [DOI] [PubMed] [Google Scholar]
- 23.Zheng NT, Mukamel DB, Caprio T, Cai S, Temkin-Greener H. Racial disparities in in-hospital death and hospice use among nursing home residents at the end of life. Med Care 2011;49:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu CY, Johantgen M. Factors associated with treatment restriction orders and hospice in older nursing home residents. J Clin Nurs 2011;20:377–387. [DOI] [PubMed] [Google Scholar]
- 25.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ufere NN, Donlan J, Waldman L, et al. Physicians’ perspectives on palliative care for patients with end-stage liver disease: a national Survey study. Liver Transpl 2019;25: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rush B, Fruhstofer C, Walley KR, Celi LA, Brahmania M. Palliative medicine and hospital readmissions in end-stage liver disease. BMJ 2019. 10.1136/bmjspcare-2018-001635. [DOI] [PubMed] [Google Scholar]
- 28.Freeborne N, Lynn J, Desbiens NA. Insights about dying from the SUPPORT project. The study to understand prognoses and preferences for outcomes and risks of treatments. J Am Geriatr Soc 2000;48(5 Suppl):S199–S205. [PubMed] [Google Scholar]
- 29.Fox E, Landrum-McNiff K, Zhong Z, et al. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645. [DOI] [PubMed] [Google Scholar]
- 30.Kimbell B, Boyd K, Kendall M, Iredale J, Murray SA. Managing uncertainty in advanced liver disease: a qualitative, multiperspective, serial interview study. BMJ Open 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA 2004;291:88–93. [DOI] [PubMed] [Google Scholar]
- 32.Bainbridge D, Giruparajah M, Zou H, Seow H. The care experiences of patients who die in residential hospice: a qualitative analysis of the last three months of life from the views of bereaved caregivers. Palliat Support Care 2018;16:421–431. [DOI] [PubMed] [Google Scholar]
- 33.Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA 2016;315:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanoff HK, Chang Y, Reimers M, Lund JL. Hospice utilization and its effect on acute care needs at the end of life in medicare beneficiaries with hepatocellular carcinoma. J Oncol Pract 2017;13:e197–e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang S-J, Chang H-T, Hwang IH, Wu C-Y, Yang W-H, Li C-P. Hospice offers more palliative care but costs less than usual care for terminal geriatric hepatocellular carcinoma patients: a nationwide study. J Palliat Med 2013;16: 780–785. [DOI] [PubMed] [Google Scholar]
- 36.Mudumbi SK, Bourgeois CE, Hoppman NA, et al. Palliative care and hospice interventions in decompensated cirrhosis and hepatocellular carcinoma: a rapid review of literature. J Palliat Med 2018;21:1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medici V, Rossaro L, Wegelin JA, et al. The utility of the model for end-stage liver disease score: a reliable guide for liver transplant candidacy and, for select patients, simultaneous hospice referral. Liver Transpl 2008;14:1100–1106. [DOI] [PubMed] [Google Scholar]
- 38.Antaki F, Lukowski A. The model for end-stage liver disease (MELD) predicts survival of liver cirrhosis patients after discharge to hospice. J Clin Gastroenterol 2007;41:412–415. [DOI] [PubMed] [Google Scholar]
- 39.Fukui N, Golabi P, Otgonsuren M, Mishra A, Venkatesan C, Younossi ZM. Demographics, resource utilization, and outcomes of elderly patients with chronic liver disease receiving hospice care in the United States. Am J Gastroenterol 2017;112:1700–1708. [DOI] [PubMed] [Google Scholar]