Abstract
Background:
PD-L1 is expressed in tumor cells and immune cells and both have been associated with response to anti-PD-1 axis immunotherapy. Here, we examine the expression of PD-L1 to determine which cell type carries the predictive value of the test.
Methods:
We measured the expression of PD-L1 in multiple immune cells with two platforms and confocal microscopy on three retrospective Yale NSCLC cohorts (425 non-immunotherapy-treated cases and 62 pembrolizumab/nivolumab/atezolizumab-treated cases). The PD-L1 level was selectively measured in different immune cell subsets using two multiplexed QIF panels including CD56 for natural killer (NK) cells, CD68 for macrophages and CD8 for cytotoxic T cells.
Results:
PD-L1 was significantly higher in macrophages in both tumor and stromal compartment compared to other immune cells. Elevated PD-L1 in macrophages was correlated with high PD-L1 level in tumor as well as CD8 and CD68 level (P<0.0001). High PD-L1 expression in macrophages was correlated with better overall survival (OS; p=0.036 by cell count/ p=0.019 by molecular co-localization) while high PD-L1 expression in tumor cells was not.
Conclusion:
In nearly 500 NSCLC cases the predominant immune cell type that expresses PD-L1 is CD68+ macrophages. The level of PD-L1 in macrophages is significantly associated with the level of PD-L1 in tumor cells and infiltration by CD8+ T cells, suggesting a connection between high PD-L1 and “hot” tumors. In anti-PD-1 axis therapy treated patients, high levels of PD-L1 expression in macrophages is associated with longer overall survival and may be responsible for the predictive effect of the marker.
Keywords: PD1/PD-L1, immunotherapy, immune checkpoints, survival, macrophages, biomarkers, quantitative immunofluorescence
Introduction
Immune checkpoints are regulators of the immune system and they are crucial for maintaining the immune homeostasis by controlling the amplitude of immune responses1. In cancer, immune checkpoints are often expressed by tumor cells to suppress the antitumor immune responses against them as an adaptive escape mechanism2,3. Among the discovered immune checkpoints, the interaction between programmed death 1 receptor (PD-1) on tumor infiltrating lymphocytes (TILs) and its ligand programmed death ligand 1 (PD-L1) on tumor cells has been identified as a critical immunosuppressive mechanism in cancer4–6.
Several clinical studies have shown that antibodies targeting PD-1/PD-L1 pathway achieved durable clinical responses with unprecedented survival rate7,8. However, only a fraction of patients benefit from this therapy and some of the therapies are approved with a companion diagnostic test to identify patients who are more likely to respond. The only currently FDA approved companion diagnostic is the PD-L1 immunohistochemistry (IHC) test9. A number of IHC tests are approved with varied antibody clones, staining platforms, protocols and scoring system and the localization of PD-L1 detection. The first approved test (22c3) scored only PD-L1 expression on tumor cells while other assays, for example, the SP142 assay prescribed scoring of both tumor cells (TC) and immune cells (IC). More recently, in tumors other than NSCLC, the 22c3 test has evolved to a combined proportion score that includes both tumor cells and immune cells. The evolution toward inclusion of PD-L1 detection on immune cells in diagnostics reveals its importance to anti-PD-1 therapeutic efficacy as compared to PD-L1 expression by tumor cells. This evolution of diagnostic scoring coincides with recent work in mouse models that has shown the efficacy of anti-PD-1 axis blockade relies on the host expression of PD-L1 in the tumor microenvironment and draining lymph nodes rather than in tumor cells10–12.
In this study, we follow the mouse mechanistic studies to attempt to determine the cell type in which the PD-L1 expression is associated with the drug mechanism of action. We hypothesize that the immune cells scored in the tests are predominantly macrophages and that the expression of PD-L1 in these cells is the critical target for immune therapy and therefore the best target for future assessment.
Materials and Methods
Tissue Microarray and Patient Cohorts
Discovery Cohort
We used two retrospective collections of NSCLC from Yale University, cohort A (YTMA79; n=209) and cohort B (YTMA250; n=291), that consist of 500 patients from 1988–2003 (YTMA79) and 2004–2011 (YTMA250) as previously described13,14. Detailed clinicopathological characterization is summarized in Supplementary table 1. The cases that were finally included in the study were fewer (176 out of 209 in YTMA79; 249 out of 291 in YTMA250) due to the loss of tissue, missing data, or poor quality of the staining as seen in other TMA studies. All tissue was collected with the approval from the Yale Human Investigation committee protocol #9505008219. The Yale Human Investigation Committee approved the patient consent forms or in some cases a waiver of consent all in accordance with the ethical guidelines of the US Common Rule.
All tissue specimens from the two cohorts were obtained from formalin-fixed, paraffin embedded (FFPE) tissue and were prepared as a tissue microarray (TMA) with a previously described standard procedure15,16. FFPE cell line pellets were used as controls. For each TMAs, we used two different blocks containing different tumor areas of the same tumors. For analysis, spots from the same patients were averaged.
Immunotherapy Treated Cohort
We used tumor samples from patients with NSCLC, Cohort C (YTMA404; n=81) treated with anti-PD-1 axis therapy at Yale-New Haven Hospital between 2011 and 2018. As summarized in table 1, 62 out of 81 samples were collected before immunotherapy and treated with single agent therapy (pembrolizumab, nivolumab or atezolizumab). All tissue specimens were obtained from FFPE biopsy or resection tissue and prepared as TMA. Response status were determined using RECIST v1.1 criteria.
Table 1.
Immunotherapy Treated Cohort Patient Characteristics
| Characteristics | N (%) |
|---|---|
| Total | 81 |
| Gender | |
| Male | 43 (53.1) |
| Female | 38 (46.9) |
| Age | 42 (51.9) |
| < 70 yo | 39 (48.1) |
| >= 70 yo | |
| Smoking history | |
| Never smoker | 14 (17.3) |
| Current smoker | 18 (22.2) |
| Former smoker | 48 (59.3) |
| *Missing | 1 |
| Histology | |
| Adenocarcinoma | 61 (75.3) |
| Squamous-cell carcinoma | 15 (18.5) |
| Large-cell carcinoma | 4 (4.9) |
| Adeno-squamous carcinoma | 1 (1.2) |
| Stage | |
| III | 2 (2.5) |
| IV (M1a) | 21 (25.9) |
| IV (M1b) | 11 (13.6) |
| IV (M1c) | 47 (58) |
|
Actionable drivers Yes |
37 (45.7) |
|
|
| No | 44 (54.3) |
| Genotype-tailored therapies | |
| Yes | 13 (16) |
| No | 68 (84) |
| Site | |
|
|
| *Missing | 1 |
| N lines of therapy | |
| 0 | 16 (19.8) |
| 1 | 42 (51.9) |
| 2 | 19 (23.5) |
| 3 | 2 (2.5) |
| 4 | 1 (1.2) |
| *Missing | 1 |
| Radiotherapy prior to immunotherapy | |
| Yes | 28 (34.6) |
| No | 53 (65.4) |
| Moment of collection | |
| Pre immunotherapy | 73 (90.1) |
| Post immunotherapy | 8 (9.9) |
| Immunotherapy regimens | |
| Nivolumab | 57 (70.4) |
| Pembrolizumab | 5 (6.2) |
| Atezolizumab | 5 (6.2) |
| Others | 14 (17.2) |
Control Cohort
We used a control cohort YTMA337 which is a specially selected PD-L1 specific index array NSCLC tumors (n=30) and cell line pellets with variable levels of PD-L1 expression in two-fold redundancy. For standardization, we stained YTMA337 alongside with each experiment as quality control to show that the YTMA337 staining pattern is quantitatively consistent between experiments.
Antibody Validation
CD68 antibody (1:200; Mouse monoclonal, IgG3, clone PG-M1, DAKO) and CD8 antibody (1:250; Mouse monoclonal, IgG1, clone C8/144B, DAKO) were validated in our lab and previously published17,18. CD56 antibody (1:200; Mouse monoclonal, IgG1, clone 123C3, DAKO) was cross-validated with antibody clone 56C04 (1:100, Mouse monoclonal, ThermoFisher). Both CD56 antibodies were evaluated on the control cohort with immunofluorescence (IF). Comparison between two clones shows a high regression coefficients (R2) (>0.7). This, combined with vendor specificity validation assured to CD56 antibody validation. CD8 assays were validated in previous studies.
Quantitative Immunofluorescence (QIF)
TMA slides were baked at 60°C for 1 hour and then soaked in xylene twice for 20 minutes each. Rehydration was performed with two 1-minute washes in 100% ethanol followed by 1-minute wash in 70% ethanol and 5-minute rinse under streaming tap water. Antigen retrieval was then performed with pH8 EDTA buffer for 20 minutes at 97°C in the Lab Vision PT module (Thermo Scientific). 30-minute incubation in 2.5% hydrogen peroxide in methanol was performed to block endogenous peroxidases and subsequently unspecific antigens were blocked using a 0.3% BSA in TBST for 30 minutes. For the PD-L1/CD68/CD8 multiplex panel, a cocktail of primary antibodies including PD-L1 (1:800; Rabbit monoclonal, clone SP142, Spring), CD68 and CD8 were incubated in 0.3%BSA in TBST overnight at 4 °C. Horseradish peroxidases (HRPs) conjugated secondary antibodies specific to each primary antibody isotype were used sequentially (anti-mouse IgG3, 1:1000, Abcam; anti-rabbit EnVision, DAKO; anti-mouse IgG1, 1:100, eBioscience). Note that the primary antibody used for PD-L1 here is the SP142 antibody, not the SP142 assay. In other works, the antibody has been shown to be equivalent to other cytoplasmic domain PD-L1 antibodies19, even though the assay has proven to be of lower sensitivity20,21. Tyramide-bound fluorophores were added after each secondary antibody to bind to the HRPs. Specifically, biotinylated tyramide (1:50, PerkinElmer) and Alexa750-streptavidin (1:100, Life Technologies) were used for CD68, Cyanine 5 (Cy5) tyramide (1:50, PerkinElmer) was used for PD-L1, and Cy3 tyramide plus (1:100, PerkinElmer) was used for CD8. Epithelial tumor cells were detected with 1-hour incubation of anti-rabbit pan-cytokeratin(CK) (1:100, DAKO) and following 1-hour incubation of goat-anti-rabbit Alexa488 (1:100; eBioscience) at RT. Finally, nucleus was stained with 4,6-diamidino-2-phenylin-dole (DAPI) (1:1000, Life Technologies) for 10 mins at RT and mounted with Prolong Gold mounting reagent (Life Technologies). For the PD-L1/CD68/CD56 multiplex panel, a cocktail of primary antibodies including PD-L1, CD68 and CD56 were used and the rest of the protocol is the same as previously described.
Quantitative immunofluorescence was quantified using two platforms: image collection was done on either the PM2000 (HistoRx, Branford, CT) or Vectra Polaris (Perkin Elmer, Hopkinton, MA) automated fluorescence microscopy platforms. The resultant images were analyzed and quantified using either the AQUAnalysis ™ software (Navigate Biopharma, Carlsbad CA) or the InForm software (Perkin Elmer, Hopkinton MA) on all tumor spots as previously described13. Briefly, fluorescent images of DAPI, FITC (CK), Cy5 (PD-L1) Cy3 (CD56/CD8) and Cy7 (CD68) for each core were collected on either platform except for the Vectra Polaris, multispectral images were captured in five channels on all tumor spots. With the trainable feature-recognition inForm software, specific cells and tissue types will be identified. The cell count for phenotype of interest (PD-L1 and CD68/CD8/CD56 double positive phenotype) was calculated in both tumor and stromal tissue for final analysis. All acquired tumor spots were evaluated visually and spots with less than 2% of tumor or staining artifacts were excluded from the final analysis. An AQUA score of 500AU was used to define PD-L1 positivity in order to reproduce a 1% TPS threshold and was determined by visualization of immunofluorescence staining of PD-L1.
DNA-tagged Quantitative Immunofluorescence
We stained one slide from the same NSCLC TMA cohort C block with the Ultimapper® kit (Ultivue Inc). the slide was deparaffinized in an oven at 60°C (oven company) for 20 minutes followed by immediate immersion in xylene (twice) for 20 minutes each. The slide was rehydrated using graded alcohols (100%, 100%, 70% ethanol) followed by tap water. Antigen retrieval was done using EDTA (company) at pH9 in a PT Module pressure boiler with pre-heating the solution to 85°C then 20 mins at 97°C followed by cooling at 75°C, then the module tank was then kept in under running tap water for 10 minutes. The slide was then washed in PBS thrice by submersion. Blocking was done using the blocking solution in the Ultimapper kit® at Room temperature (RT: 20–25°C) for 15 minutes in a humid chamber. The antibody solution was made (mixing of single strand DNA-tagged antibodies and antibody diluent) and intubated for 60 minutes at RT in a humid chamber. Amplification of the DNA-strand was done with Pre-amplification solution for 25 minutes at RT in humid chamber followed by Amplification solution (Amplification enzyme and Amplification buffer) at 30°C in a sealed humid chamber (Slide Moat, Boekel Scientific). This was followed by nuclear staining (Hoescht) and amplification of Fluorescent tagged DNA probes for 25 minutes at RT. The slides where then cover slipped and scanned using the PM2000 machines and AQUA® Scores were generated for each marker and studied for the impact in to overall survival.
Confocal Microscopy
For high resolution images, the TMA slides were examined using a Leica SP5 Confocal Microscope at 100× magnification in the fluorescent mode. Image acquisition and analysis were performed on the LAS AS software (Leica). The excitation and emission wavelengths used to detect were at 405nm, 561nm, 633nm and a multi-line Argon.
Statistical Analysis
Reproducibility of the assays is measured with the control cohort with Pearson’s correlation coefficient (R). An AQUA score of 500AU was used to stratify PD-L1 SP142 staining scores into positive or negative groups of patients for analysis. This threshold was determined with visual inspection of all NSCLC tumor cohorts and the control array. AQUA scores and inForm cell counts between responders (PR/CR) and non-responders (SD/PD) were compared with fisher’s exact test two-sided. Survival analysis was carried out using Kaplan-Meier curves and significance was determined with the log-rank test. Joinpoint 4.6.0.0 was used to identify the the marker score which is an apparent change in trend that is statistically significant without referring to the outcome data22. The univariate analyses were performed with GraphPad Prism 7, and multivariate analyses were performed using JMP11. All p values were descriptive and based on two-sided tests and were not adjusted for multiple comparisons.
Results
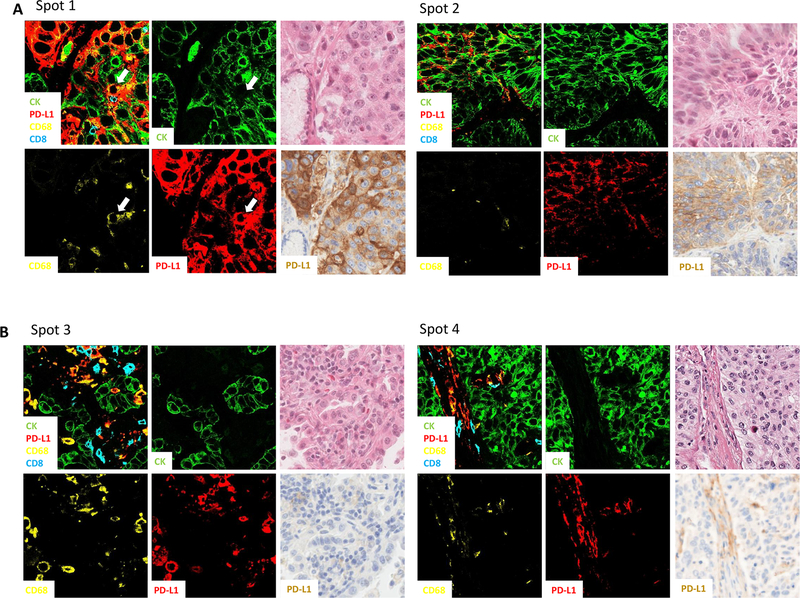
Among the 425 NSCLC cases in discovery cohorts A&B, CD68+, CK- cells, defined as macrophages were present in 391/425 (92%) cases, while CD8+, CK- T cells and CD56+, CK- NK cells were present in 388/425 (91.3%) and 90/209 (43.1%) respectively. A visually estimated threshold of 500 arbitrary units of fluorescence (au) of AQUA score was used to determine the patients into PD-L1 positive (126/425, 29.6%) or negative group (299/425, 70.4%). Then inForm was used to assess PD-L1 expression on both tumor tissue compartment and stromal tissue compartment where tumor was defined by a machine learning algorithm that defines CK positive regions as tumor tissue compartment. A similar training approach was used for the stromal compartment definition using DAPI and absence of CK. Finally, a third compartment was defined/trained where there is no tissue. PD-L1 cellular localization was then determined within the PD-L1 positive group (determined by AQUA, n=126) by the cell count of the following phenotype: PD-L1/CK double positive, PD-L1/CD68 double positive, PD-L1/CD56 double positive and PD-L1/CD8 double positive in both tumor and stromal compartments. Figure 1A illustrates a series of case examples. Case 1, where tumor PD-L1 was predominantly localized with CK, also showing some expression in tumor infiltrating macrophages. Case 2 shows PD-L1 localization with CK in the tumor compartment and with CD68 in stromal compartment. Case 3 shows PD-L1 was only localized with CD68 in both tumor and stromal. Finally, case 4 shows PD-L1 only expressed by CK in the tumor compartment. Then these data were averaged across 126 cases of PD-L1 positive NSCLC and shown in summary pie charts in Figure 1B. PD-L1 localization was significantly higher in CD68+ macrophages compared to CD56+ NK cells and CD8+ T cells: in the tumor compartment, PD-L1 was predominantly co-localized with CK (53%) while 41% of PD-L1 was found to be colocalized with CD68, 4% with CD8 and 2% with CD56; in the stromal compartment, the majority of PD-L1 positive cells were CD68 positive (80%), while only 5% from CD8 and 3% from CD56.
Figure 1.
Quantification of PD-L1 localization in tumor and stroma. Four representative cases to demonstrate typical PD-L1 localization patterns with cell counts and confocal images of regions of the counted case (A). Summary pie charts of PD-L1 localization calculated by double positive phenotype cell counts (PD-L1/CK, PD-L1/CD68, PD-L1/CD8, PD-L1/CD56) on the average of 457 NSCLC cases was presented as percentages in both tumor and stromal compartment (B).
To better illustrate the compartmentalization of PD-L1 expression, Figure 2 shows high magnification confocal images of 4 examples, both combined and separated by the channel along with the same region stained with H&E after capturing the fluorescent images and the chromogenic IHC image from a serial section of the same tissue region. Note that is hard to visualize the colocalization in either the CD8 or CD56 compartments since they represent a relatively small percentage of the total PD-L1. Since the PD-L1 expression in this study was mainly found in CK and CD68+ cells, and PD-L1 in CD56 and CD8 was relatively low, the remainder of this work focuses on the expression of PD-L1 by tumor cell and CD68+ macrophage only. Correlation between PD-L1 level in CD68+ macrophages and PD-L1 level in tumor, CD8 and CD68 levels was identified, indicating a connection between high PD-L1 level in macrophages and “hot” tumors (Supplementary Figure 1).
Figure 2.
Colocalization of phenotypic markers and PD-L1. Example confocal images of PD-L1 colocalization with tumor cells (A) and with macrophages (B). In Fig. 2A, white arrow is a tumor infiltrating macrophage with CD68 and PD-L1 double staining, but without cytokeratin staining.
Next, we assessed the association with outcome for PD-L1 expression in each compartment. The potential correlation between the prognostic value of PD-L1 level in macrophages and patients’ outcome was evaluated in a merged analysis of Yale cohorts A and B. No significant association between PD-L1 level in macrophages and major clinicopathological variables was found. PD-L1 expression in CD68+ macrophages was defined as cases where PD-L1 expression level was above a visual threshold of 3000 au of AQUA score (156/425, 36.7%). The remainder of the case were PD-L1 negative in macrophage group (269/425, 63.3%). High PD-L1 expression in macrophages was significantly associated with high PD-L1 level in tumor as well as CD8 level and CD68 level (P <0.0001) (Figure 3A). With the median cut-off, or any discovered cut-point, we found PD-L1 level in CD68 was not associated with patient’s survival in patients treated with standard of care therapy received by the patients in these retrospective cohorts gathered prior to the availability of immune therapy (Figure 3B, Supplementary Figure 2, 3).
Figure 3.
High PD-L1 expression in macrophages was correlated with high CD8 level, high CD68 level and high PD-L1 expression by tumor cells in 457 cases of NSCLC (A). Error bars represent mean with 95% confidence interval. PD-L1 expression in macrophages is not prognostic in this cohort (B).
The potential predictive value of PD-L1 in macrophages was evaluated in Yale cohort C, the immunotherapy treated cohort. The joinpoint method is independent of outcome22, so it was used to define a cohort stratification threshold. With the normalized cell count of total PD-L1/CK double positive phenotype and PD-L1/CD68 double positive phenotype, a joinpoint analysis for natural population breaks identified a significant breakpoint at the 25th percentile of the PD-L1/CK cell count (n=15, total n=59, p=0.00222) and the 21st percentile of the PD-L1/CD68 cell count within total cell count (n=12, total n=61, p=0.00222) (Supplementary Figure 5). With these joinpoints, no predictive value was found for high cell count for the PD-L1/CK phenotype (Figure 4A) while high cell count of PD-L1/CD68 phenotype was associated with better OS in patients treated with single therapy (pembrolizumab/nivolumab/atezolizumab) (P=0.036) (Figure 4B). Multivariate analysis indicated that the predictive value of high cell count of PD-L1/CD68 phenotype towards OS was independent of age, sex, stage smoking history and CD8 level (Supplementary Table 3). No significance was found between the PD-L1 level in CD68+ macrophages and response to immunotherapy or progression free survival (PFS) in this small cohort.
Figure 4.
PD-L1 level in macrophages predicts patients’ overall survival to anti-PD-1 axis blockade therapy using two different QIF methods. Using InForm to count cells, double positive PD-L1&CK cell (count n=15) was not associated with overall survival (A). Double positive PD-L1/CD68 cells (count n=12) was significantly associated with overall survival of NSCLC patients treated with single drug immunotherapy (B). Using AQUA assessment of PD-L1 in the tumor(C) or stromal (D) compartments was not associated with better outcome on monotherapy, while PD-=L1 in the CD68 compartment was statistically significantly associate with better overall survival(E).
To confirm this observation, a second independent assay method was used using DNA-Based Quantitative Immunofluorescence on the same Yale cohort C. This method uses different antibodies and no secondary antibodies, but rather a direct labeling of primary antibodies with oligonucleotide codes. These oligonucleotides provide specificity and can be differentially amplified and assessed by multiplex QIF using either the AQUA method, inForm method, or other QIF methods. The antibodies in the panel detect PD-L1, CD68, CD8 and CK. An AQUA based analysis was done to measure PD-L1 expression in the stroma, tumor and CD68 defined compartments. When assessed in tertiles, only PD-L1 in the CD68 compartment shows significance (p=0.019) (Figure 4C, D and E).
Besides PD-L1 expression in CD68+ cells, the level of CD8 was also found to be associated with response to immunotherapy (Supplementary Figure 6). When assessed in quartiles, high level of CD8 is found to be significantly associated with better OS (p=0.0045).
Discussion
Mechanistic studies have examined the function of PD-L1 expression in macrophages in mouse models and demonstrated that PD-L1 expression in host cells influenced PD-L1 blockade therapy10–12, but few studies have examined the expression of PD-L1 in human tumors, beyond their general characterization as “immune cells”. In this study, we evaluated the PD-L1 co-localization in 3 immune cell subtypes in 457 NSCLC patients’ tissue and show that the majority of PD-L1 expression is in CD68+ macrophages, as confirmed by two methods of QIF and confocal microscopy. Furthermore, expression of PD-L1 in macrophages was correlated with better overall survival in patients treated with immunotherapy.
PD-L1 has been found to be expressed not just by macrophages, cytotoxic T cells and NK cells, but rather broadly expressed by hematopoietic and non-hematopoietic cells, including B cells, dendritic cells, Treg cells, etc23–25. In addition, early stage tumor associated macrophages have been found to express both M1 and M2 markers thus the traditional M1/M2 associations with aggressiveness, or lack thereof, are not found26,27. While early stage TAMs are associated with increased probability of recurrence and they have no effect on T cells26,28. It has been suggested that PD-L1 expressed by TAMs in early-stage lung cancer does not inhibit effector T cell function26.
With our current panels, we were able to demonstrate that the majority of PD-L1 expression by non-neoplastic cells were from CD68+ cells that are likely be classified as macrophages. Garris et all have recently shown that the efficacy of anti-PD-1 immunotherapy requires intratumoral dendritic cells29. One possible explanation is that dendritic cells can express PD-L1 and bind to PD-1 thus resulting in downstream immune inhibition. We have been unable to assess the percentage of PD-L1 in dendritic cells due to the absence of a definitive, single antibody dendritic cell marker. These findings suggest further translational studies on the role PD-L1 in other types of immune cells and its predictive value to immunotherapy with treated cohort.
There are several limitations to this study. Perhaps the greatest limitation is that this work is entirely performed on TMAs that may over or underrepresent the biomarker expression in whole tissue section because of tumor and microenvironmental heterogeneity. However, study has shown good concordance between TMAs and corresponding whole tissue section and between two TMA cores from two paraffin blocks of the same tumor in breast cases30. Moreover, previous work in our lab assessed the PD-L1 QIF score in TMA cases and compared it with the corresponding WTS cases. We found a comparable PD-L1 detection between TMA and WTS in NSCLC cohorts31. The advantage of large numbers of cases accessible by TMA is favored here. A second limitation is the relatively small size of the immunotherapy treated cohort and the heterogeneity of treatment of this cohort. Access to tissue from treated patients is limited and similarly long term follow up is just now becoming available for treatment of patients with these medications after their FDA approval and acceptance as standard of care. As such this work should be considered as preliminary and should be validated by larger prospective studies
Another concern is that the tissue used in this study was archival tissue, not collected specifically for companion diagnostic testing. The issue of antigen aging has recently been addressed by Herbst and colleagues and suggests that this concern does appear to affect the predictive value of the PD-L1 test32. Finally, CD68 may not stain all tumor-associated macrophages and CD56 may also capture NKT cells. As such, this data must be considered hypothesis generating, or discovery data and the observation that PD-L1 expression in macrophages as the key predictive factor requires validation in external cohorts, or potentially future clinical trials.
In summary, we find PD-L1 expression is particularly frequent in macrophages as compared to other immune cell types in NSCLC patients. Elevated level of PD-L1 in macrophages could be evaluated in future studies as potentially contributory to the therapeutic efficacy of anti-PD-1 blockade therapy
Supplementary Material
Translational relevance.
Many studies have shown that “immune cells”, without specific characterization, are a critical component of the companion diagnostic tests used for PD-1 axis immunotherapy. The exact type of immune cells that express PD-L1 is unclear. By quantitatively measuring PD-L1 in CD68+ macrophages, CD56+ NK cells and CD8+ cytotoxic T cells we show that the vast majority of PD-L1 expression colocalizes with CD68, inferring that the key immune cell is a macrophage. It is more challenging to prove that the macrophage carries the predictive power of the test. However, in a small pilot scale, retrospective cohort, we show significant association with overall survival attributable to PD-L1 in the macrophage compartment, and not in the tumor cell compartment. This work raises the possibility that the companion diagnostic test for PD-1 axis therapies may be improved by assessing PD-L1 expression only when it is colocalized with CD68.
Acknowledgments
This work was primarily supported by funds from Yale SPORE in Lung Cancer (RSH, KAS, SNG and DLR) (P50-CA196530) and also funds from the Yale Cancer Center (P30CA016359) and funds from Navigate BioPharma (Novartis subsidiary) to DLR and KAS, The funding sources had no role in study design; collection, analysis, and interpretation of data; preparation of the manuscript or the decision to submit for publication.
Financial Support: This work was supported by funds from Navigate BioPharma (Novartis subsidiary), Yale SPORE in Lung Cancer and Yale Cancer Center to D.L. Rimm. The funding sources had no role in study design; collection, analysis, and interpretation of data; preparation of the manuscript or the decision to submit for publication.
Footnotes
Possible Conflict of Interest
Kurt Schalper has served as a consultant, advisor or served on a Scientific Advisory Board for Celgene, Moderna Therapeutics Shattuck Labs, Astra Zeneca, Pierre-Fabre and Abbvie. He has received research funding from Genoptix/Navigate (Novartis), Vasculox/Tioma, Tesaro, Moderna Therapeutics, Tesaro Pharmaceuticals, Surface Oncology, Pierre-Fabre Research Institute, Merck and Bristol-Myers Squibb.
David Rimm has served as a consultant, advisor or served on a Scientific Advisory Board for Amgen, Astra Zeneca, Agendia, Biocept, BMS, Cell Signaling Technology, Cepheid, Daiichi Sankyo, GSK, Merck, NanoString, Perkin Elmer, PAIGE, and Ultivue. He has received research funding from Astra Zeneca, Cepheid, Nanostring, Navigate/Novartis, NextCure, Lilly, Ultivue, and Perkin Elmer
Jon Zugazagoitia has received consulting honoraria from Guardant Health.
References
- 1.Sharpe AH, Wherry EJ, Ahmed R, et al. : The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8:239–45, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A: Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov 5:915–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, et al. : Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. : Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–34, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W, Wolchok JD, Chen L: PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 8:328rv4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gettinger SN, Horn L, Gandhi L, et al. : Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 33:2004–12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Soria JC, Kowanetz M, et al. : Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udall M, Rizzo M, Kenny J, et al. : PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagnostic Pathology 13:12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang H, Liang Y, Anders RA, et al. : PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 128:580–588, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H, Wei S, Hurt EM, et al. : Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 128:805–815, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau J, Cheung J, Navarro A, et al. : Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 8:14572, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schalper KA, Brown J, Carvajal-Hausdorf D, et al. : Objective Measurement and Clinical Significance of TILs in Non-Small Cell Lung Cancer. Jnci-Journal of the National Cancer Institute 107, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altan M, Pelekanou V, Schalper KA, et al. : B7-H3 Expression in NSCLC and Its Association with B7-H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clinical Cancer Research 23:5202–5209, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe A, Dolled-Filhart M, Camp RL, et al. : Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. Journal of the National Cancer Institute 97:1808–1815, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Camp RL, Chung GG, Rimm DL: Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med 8:1323–7, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Brown JR, Wimberly H, Lannin DR, et al. : Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res 20:5995–6005, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelekanou V, Villarroel-Espindola F, Schalper KA, et al. : CD68, CD163, and matrix metalloproteinase 9 (MMP-9) co-localization in breast tumor microenvironment predicts survival differently in ER-positive and -negative cancers. Breast Cancer Res 20:154, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaule P, Smithy JW, Toki M, et al. : A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao MS, Kerr KM, Kockx M, et al. : PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 13:1302–1311, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Morilla S, McGuire J, Gaule P, et al. : Quantitative assessment of PD-L1 as an analyte in immunohistochemistry diagnostic assays using a standardized cell line tissue microarray. Lab Invest, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Fay MP, Feuer EJ, et al. : Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335–51, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Dorfman DM, Brown JA, Shahsafaei A, et al. : Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 30:802–10, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curiel TJ, Wei S, Dong H, et al. : Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–7, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Francisco LM, Sage PT, Sharpe AH: The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singhal S, Stadanlick J, Annunziata MJ, et al. : Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavin Y, Kobayashi S, Leader A, et al. : Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 169:750–765 e17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mony JT, Schuchert MJ: Prognostic Implications of Heterogeneity in Intra-tumoral Immune Composition for Recurrence in Early Stage Lung Cancer. Front Immunol 9:2298, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garris CS, Arlauckas SP, Kohler RH, et al. : Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity 49:1148–1161 e7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyndi M, Sorensen FB, Knudsen H, et al. : Tissue microarrays compared with whole sections and biochemical analyses. A subgroup analysis of DBCG 82 b&c. Acta Oncol 47:591–9, 2008 [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin J, Han G, Schalper KA, et al. : Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2:46–54, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbst RS, Baas P, Perez-Gracia JL, et al. : Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol 30:281–289, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.