Abstract
Background and Purpose
Hydrogen sulfide (H2S)‐releasing agents are viewed as potential antihypertensive drugs. Recently, natural isothiocyanates emerged as original H2S‐donor agents. Among them, erucin, present in some edible cruciferous plants, shows suitable H2S‐releasing properties and features of “druggability.” The aim of this work was to investigate the erucin‐mediated release of H2S inside vascular cells, its vasorelaxing effects, and activity on BP of normo and hypertensive animals.
Experimental Approach
Intracellular H2S‐release and the hyperpolarizing effect of erucin were tested using fluorescent dye, in human aortic smooth muscle cells (HASMCs). Its direct vasorelaxing effect and ability to inhibit noradrenaline‐induced vasoconstriction were evaluated on endothelium‐intact or ‐denuded rat aortic rings. Its vasodilator properties were tested in coronary arteries using Langendorff‐perfused rat hearts. Finally, erucin's antihypertensive activity was evaluated in vivo in normotensive and spontaneously hypertensive rats (SHRs) by recording systolic BP using the tail‐cuff method.
Key Results
Erucin induced the release of H2S inside HASMCs. Moreover, erucin hyperpolarized the membrane of HASMCs membrane in a concentration‐dependent manner. It induced vasodilatation of rat aortic rings, in endothelium‐denuded vessels. This effect was further improved by the presence of endothelial NO. When pre‐incubated with rat aortic rings, erucin induced concentration‐dependent inhibition of noradrenaline‐induced vasoconstriction. Erucin did not affect basal coronary flow but restored the flow to normal in pre‐contracted coronary vessels. Finally, in vivo, erucin decreased systolic BP in SHRs by about 25%, and restored the BP to values observed in normotensive rats.
Conclusions and Implications
Erucin is an H2S donor endowed with vasorelaxing and antihypertensive effects.
Linked Articles
This article is part of a themed section on Hydrogen Sulfide in Biology & Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.4/issuetoc
What is already known
Hydrogen sulfide (H2S) donors are potential antihypertensive drugs.
Natural isothiocyanates are H2S donors.
What this study adds
The natural isothiocyanate erucin releases H2S inside vascular cells.
Erucin evokes vasorelaxing and antihypertensive effects mediated by H2S.
What is the clinical significance
Erucin may represent an attractive drug candidate for the treatment of hypertension.
Abbreviations
- AngII
angiotensin II
- BKCa
large‐conductance calcium‐activated potassium channels
- CF
coronary flow
- DADS
diallyldisulfide
- DiBac4(3)
bisoxonol dye bis(1,3‐dibutylbarbituric acid)
- Emax
maximal vasorelaxing efficacy
- HASMCs
human aortic smooth muscle cells
- L‐NAME
Nω‐nitro‐L‐arginine methyl ester
- NA
noradrenaline
- Psys
systolic blood pressure
- SHR
spontaneously hypertensive rat
- WSP‐1
Washington State Probe‐1
1. INTRODUCTION
Hypertension is still the most widespread chronic disease, it is the main cause of life‐threatening cardiovascular complications, such as stroke, coronary artery disease, and heart failure, and it is the principal risk factor for premature mortality worldwide. A further rise in prevalence of hypertension is expected, because of economic growth, lifestyle, and increased life expectancy (Kumar, 2013). Nowadays, this sneaky silent killer is estimated to be responsible for about 10 million deaths every year (Achelrod, Wenzel, & Frey, 2015; Noubiap et al., 2018). However, adequate control of correct levels of blood pressure is associated with an effective reduction in complications and mortality (Sundström et al., 2015; Xie et al., 2016). Consistently, many pharmacological targets useful for treating hypertension have been identified and a huge number of drugs of proven efficacy and safety is currently available. However, in spite of this wide and heterogeneous choice of pharmacological interventions, a significant number of hypertensive patients cannot achieve blood pressure control (Myat, Redwood, Qureshi, Spertus, & Williams, 2012; Pierdomenico et al., 2005; Sarafidis, Georgianos, & Bakris, 2013). In particular, the prevalence of true resistant hypertension (i.e., the resistance that occurs in fully adherent patients treated with well‐designed therapies and multiple antihypertensive drugs) is estimated to be about 10% (Noubiap et al., 2018). Therefore, the discovery of original pharmacological mechanisms and novel antihypertensive drug candidates is still a timely and compelling issue.
Since the discovery of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9532) as an endogenous gasotransmitter, the potential utility of this small gaseous molecule in cardiovascular pharmacology has emerged (Bucci & Cirino, 2011; Martelli et al., 2012). Indeed, H2S acts as a vasorelaxing mediator and is a key regulator of vascular tone homeostasis (Gheibi, Jeddi, Kashfi, & Ghasemi, 2018). In vascular tissues, physiological levels of this gasotransmitter are mainly biosynthesized by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279#1444, although the contribution of the other two H2S‐generating enzymes http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279#1444 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279#1444 (MPST) cannot be excluded (Yang & Wang, 2015). An altered biosynthesis of H2S is presently known to account for “H2S‐rich” or “H2S‐poor” diseases, associated with an excess or deficiency of H2S respectively (Szabo & Papapetropoulos, 2017). In particular, H2S deficiency is likely to have a relevant significant role in hypertension (Ahmad et al., 2015; Meng, Ma, Xie, Ferro, & Ji, 2015). Indeed, an association between a deficiency in H2S and hypertension has been clearly demonstrated in rodents (Yan, Du, & Tang, 2004; Yang et al., 2008) and even in humans. In fact, reduced H2S concentrations have been detected in plasma of grade 2 and 3 hypertensive patients (Sun, Xi, Yang, Ma, & Tang, 2007), in patients with portal hypertension (Wang et al., 2014) and in maternal hypertension and preeclampsia (Wang et al., 2013). Conversely, the administration of exogenous H2S by suitable H2S donors can be considered as a useful innovative strategy for the pharmacological treatment of hypertension. Actually, different chemotypes of H2S donors exhibited vasorelaxing activity and blood pressure lowering effects in several scientific studies (Szabo & Papapetropoulos, 2017). The importance of most of these compounds has been to elucidate the molecular mechanism of action of H2S and understand the pathophysiological roles of H2S in the regulation of cardiovascular function. Nevertheless, for clinical applications an ideal H2S donor should combine well‐balanced H2S‐releasing properties (in particular, slow and long‐lasting release) with other essential pharmacological “druggability” features, such as good oral bioavailability, safety, known biotransformation, and safe metabolites.
In a recent paper, we first reported the H2S‐releasing properties of different natural isothiocyanates, which are phytochemical compounds typical of cruciferous plants (family of Brassicaceae; Citi et al., 2014). These properties were tested by “cell‐free” electrochemical approaches, but no pharmacological evaluation of their cardiovascular effects was carried out. Among the various isothiocyanates, erucin seems to be worthy of pharmacological investigation, because of its favourable characteristics. Indeed, erucin (Figure 1) showed slow and long‐lasting release of H2S. Moreover, erucin is present in edible plants, such as Eruca sativa Mill. (salad rocket), which are widely used in human nutrition and are not associated with any evidence of toxicity. Different pharmacokinetic studies on erucin have been carried out in animals and humans. These studies demonstrated that erucin is well absorbed after oral administration; they also described the metabolites of erucin derived from its biotransformation (Clarke et al., 2011; Platz et al., 2015).
Figure 1.
Molecular structure of erucin
The aim of this study was to evaluate the potential H2S‐releasing activity of erucin in vascular smooth muscle, investigate its typical H2S‐mediated effects in vascular smooth muscle cells and in isolated vessels, and finally, assess the in vivo effects of erucin in a suitable experimental model of hypertension.
2. METHODS
2.1. Cell cultures
Human aortic smooth muscle cells (HASMCs, Cat.no: C‐007‐5C) were purchased from Thermo Fisher (Waltham, MA, USA) and were cultured following the manufacturer's instructions. Briefly, HASMCs were cultured in Medium 231 (Thermo Fisher) supplemented with Smooth Muscle Growth Supplement (Thermo Fisher), 1% of 100 units·ml−1 penicillin and 100 mg·ml−1 streptomycin (Sigma‐Aldrich, St. Louis, MO, USA) in tissue culture flasks at 37°C in a humidified atmosphere of 5% CO2. Cells were split 1:2 twice a week and used until passage 16.
2.2. Intracellular H2S‐release measurement
HASMCs were cultured up to about 90% confluence and 24 hr before the experiment cells were seeded onto a 96‐well clear‐bottom black plate, pre‐coated with gelatin 1% (from porcine skin, Sigma‐Aldrich), at a density of 30 × 103 per well. After 24 hr, to allow cell attachment, the medium was replaced and cells were incubated for 30 min with a 100 μM solution of the fluorescent dye WSP‐1 (Washington State Probe‐1,3′‐methoxy‐3‐oxo‐3H‐spiro[isobenzofuran‐1,9′‐xanthen]‐6′‐yl‐2(pyridin‐2‐yldisulfanyl benzoate); Cayman Chemical, Ann Arbor, MI, USA). After the 30 min incubation of HASMCs with WSP‐1 (allowing cells to up‐load the dye), the supernatant was removed and replaced with different solutions of erucin (CREA, Bologna, Italy) dissolved in standard buffer (HEPES, 20 mM; NaCl, 120 mM; KCl, 2 mM; CaCl2·2H2O, 2 mM; MgCl2·6H2O, 1 mM; glucose, 5 mM; and pH 7.4, at room temperature) at three increasing concentrations (30, 100, and 300 μM). WSP‐1 is a specific and highly sensitive dye for H2S detection; indeed, when it reacts with H2S, it releases a fluorophore detectable with a spectrofluorometer at λ = 465–515 nm. The change in fluorescence (expressed as fluorescence index) was monitored every 5 min for 35 min, by means of an EnSpire (PerkinElmer, Waltham, MA, USA) spectrofluorometer and it was calculated as the difference between the fluorescence at time t, after the addition of erucin or vehicle (DMSO 1%, Sigma‐Aldrich), and the basal fluorescence. The AUC was also calculated. On the bases of previous experiments (Barresi et al., 2017), diallyldisulfide (DADS, Sigma‐Aldrich) 300 μM was used as slow H2S‐donor reference compound. Six different experiments (n = 6) were performed, each carried out in six replicates. The results are expressed as mean ± SEM.
2.3. Evaluation of HASMC membrane hyperpolarization
HASMCs were seeded onto a 96‐well black plate, clear‐bottom pre‐coated with gelatin 1% (Sigma‐Aldrich), at density of 72 × 103 cells per well. After 24 hr, the medium was replaced, and cells were incubated for 1 hr with the bisoxonol dye bis(1,3‐dibutylbarbituric acid) (DiBac4(3), 2.5 μM, Sigma‐Aldrich) dissolved in a standard buffer. This membrane potential‐sensitive dye DiBac4(3) allowed us a non‐electrophysiological measurement of changes in cell membrane potential as already described (Martelli et al., 2014). An increase in fluorescence, due to an inward flow of the dye, is a consequence of membrane depolarization; conversely, a decrease in fluorescence, consequent to an outward flow of the dye, reflects membrane hyperpolarization. The spectrofluorometric measurement was set at excitation and emission wavelengths of 488 and 520 nm respectively (Multiwells reader, Enspire, PerkinElmer). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4272 (10 μM, Sigma‐Aldrich), a well‐known activator of BKCa potassium channels, was used as a reference hyperpolarizing drug. After the basal fluorescence had been measured, cells were treated with different concentrations of erucin (30, 100, and 300 μM), NS1619, or vehicle (DMSO 1%), and changes in fluorescence were recorded for 35 min. The relative reduction in fluorescence, consequent to membrane hyperpolarization, was recorded every 2.5 min and was expressed as (Ft − F0)/F0, where F0 represents the basal fluorescence and Ft is the fluorescence at time t after the administration of the test compounds. Moreover, the AUC was also calculated. The results were expressed as a % of the AUC induced by NS1619 10 μM. Six different experiments (n = 6) were performed, each carried out in six replicates.
2.4. Animal procedures and ethical statements
All the procedures involving animals were carried out following the guidelines of the European Community Council Directive 86‐609 and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki, EU Directive 2010/63/EU for animal experiments). The experiments were authorized by the Ethical Committee of the University of Pisa (Protocol number 0037321/2013) and by the Italian Ministry of Health (authorization number 751/2018‐PR). All the animals were housed in humidity‐ and temperature‐controlled rooms (22°C and 50%, respectively) with 12‐hr light/dark cycles, water, and food availability ad libitum. Three animals were housed in each cage. All efforts to reduce and minimize the number of animals and their suffering were carried out. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. A randomization of animals between groups and treatments was carried out. The investigators responsible for data analysis were blind to which animals represent which treatments and controls.
2.5. Functional assays on aortic rings
Adult male normotensive Wistar rats (300–350 g, Envigo, Huntingdon, UK, RGD Cat# 2312511, RRID:RGD_2312511) were given an overdose of sodium thiopental (100 mg·kg−1 i.p., Pentothal MSD, Kenilworth, NJ, USA), killed by cervical dislocation and bled. Thoracic aorta was rapidly excised and freed of extraneous tissues. When required for the experimental procedures, the endothelial layer was removed by gently rubbing the intimal surface of the aortae with a hypodermic needle. The aorta was cut in 5‐mm‐wide rings which were suspended, under a preload of 2 g, in organ baths, containing 20 ml of Tyrode solution (NaCl, 136.8 mM; KCl, 2.95 mM; CaCl2·2H2O, 1.80 mM; MgSO4·7H2O, 1.05 mM; NaH2PO4·H2O, 0.41 mM; NaHCO3, 11.9 mM; and glucose, 5.5 mM), thermostated at 37°C, and continuously gassed with Clioxicarb, a mixture of O2 (95%) and CO2 (5%). An isometric transducer (Grass FTO3, Harvard Apparatus, Holliston, MA, USA), connected to a preamplifier (Buxco Electronics, Troy, NY, USA) and with software for data acquisition (BIOPAC Systems Inc., MP 100, Goleta, CA, USA), was used to record changes in tension. After an equilibration period of 60 min, the endothelial maintenance or removal were confirmed by the administration of ACh (10 μM) to KCl (25 mM)‐precontracted vascular rings. As regards the endothelium‐intact aortic rings, a relaxation ≥70% of the KCl‐induced contraction was considered representative of a suitable presence of functional endothelium; while, the rings, exhibiting a relaxation <70%, were discarded. Conversely, for the endothelium‐removed aortic rings, a relaxation <10% of the KCl‐induced contraction represented an acceptable lack of the endothelial layer; while, the rings, showing a relaxation ≥10%, were discarded.
In the first set of experiments, designed to evaluate the direct vasorelaxing activity of erucin, after about 45 min from the confirmation of the endothelium presence/removal, the aortic rings were contracted with KCl 25 mM and when the contraction had reached a stable plateau, erucin was added cumulatively (100 nM–100 μM). When required, the inhibitor of the biosynthesis of endothelial http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509), Nω‐nitro‐L‐arginine methyl ester (L‐NAME; Sigma‐Aldrich) 100 μM, was added 20 min before KCl. Preliminary experiments demonstrated that the KCl (25 mM)‐induced contractions remained in a stable tonic state for at least 60 min. The maximal vasorelaxing efficacy (Emax) was presented as the maximal vasorelaxing response achieved with the highest concentration of erucin(100 μM) and expressed as a percentage (%) of the contractile tone induced by KCl. Potency is expressed as pEC50, calculated as negative logarithm of the molar concentration of erucin evoking an effect = 50% of Emax. The parameters for efficacy and potency are expressed as mean ± SEM, from aortae of six animals (n = 6) for each different treatment.
In a separate set of experiments, the inhibition of noradrenaline (NA)‐induced vasoconstriction by erucin was evaluated. In particular, 45 min after the confirmation of endothelium removal, the endothelium‐denuded aortic rings were contracted with 60mM KCl, as a reference depolarizing stimulus evoking an almost full contraction of vascular smooth muscle. After a plateau state had been reached, the preparations were washed, and after 45 min of re‐equilibration, rings were exposed to different concentrations of erucin (10, 30, and 100 μM) or the corresponding vehicle (DMSO 1%) for 20 min. Thereafter, a cumulative concentration–response curve for NA (1 nM–1 μM) was performed. In order to rule out a potential toxic effect and/or to test the viability of the vascular musculature and the “reversibility” of the inhibitory effect of erucin, rings were extensively washed. Then, the concentration–response curve to NA (1 nM–1 μM) was repeated. NA concentration–vasocontraction response curves were analysed by the Hill equation, and the efficacy of NA expressed as maximal contractile response, calculated as a percentage (%) of the contractile tone previously induced by 60 mM KCl. The parameter of potency pE50, was calculated as the negative logarithm of the molar concentration of NA evoking a half‐maximal response. The results for the efficacy and potency of NA are expressed as mean ± SEM, of six experiments (n = 6) for each different treatment.
2.6. Measurement of coronary flow
Adult male normotensive Wistar rats (300–350 g) were anaesthetized with an overdose of sodium thiopental (100 mg·kg−1 i.p.); hearts were quickly excised and placed in Krebs solution (NaHCO3, 25 mM; NaCl, 118.1 mM; KCl, 4.8 mM; MgSO4, 1.2 mM; CaCl2·2H2O, 1.6 mM; KH2PO4, 1.2 mM; and glucose, 11.5 mM) at 4°C bubbled with clioxicarb to stop the heart contracting and reduce oxygen consumption. Then, the hearts were rapidly mounted in a Langendorff apparatus (Radnoti, Monrovia, USA) and perfused with Krebs solution gassed with clioxicarb at 37°C at constant pressure (70–80 mmHg). Coronary flow (CF) was volumetrically recorded every 5 min, expressed as ml·min−1, and normalized to the heart weight (g). After a 20 min equilibration period, some hearts were selected to determine the effects of erucin on the basal flow: briefly, the hearts were perfused with Krebs solution containing erucin at cumulatively increasing concentrations (0.1, 1, 10, and 100 μM, for 20 min, each concentration). Another set of experiments was dedicated to demonstrate the effects of erucin on CF of hearts precontracted with angiotensin II (AngII). These hearts, after the initial 20 min of equilibration, were perfused with AngII 0.1 μM, and at the onset of a stable coronary spasm (observed as a reduction in the CF), cumulatively increasing concentrations of erucin (0.1, 1, 10, and 100 μM, for 20 min, each concentration) were administered (in the constant presence of AngII 0.1 μM). Preliminary experiments demonstrated that perfusion with AngII 0.1 μM caused a rapid fall in the CF, stable for at least 2 hr. Changes in CF are expressed as percentage (%) of the basal CF. Experiments were carried out on hearts from six animals (n = 6) for each different treatment.
2.7. In vivo measurement of blood pressure on normotensive and spontaneously hypertensive rats
The effects of erucin and its vehicle (DMSO 1 ml·kg−1) on BP were tested on 12‐week‐old male normotensive (Wistar) and spontaneously hypertensive rats (SHR; 300–350 g), which represents one of the best models for essential hypertension. Briefly, rats were anaesthetized with sodium thiopental 60 mg·kg−1 i.p. and after the administration of the anaesthetic, they were kept on a heated platform (about 30°C) for 20 min to induce a slight vasodilatation of the tail artery, in order to allow an easier recording of the basal systolic BP (Psys) with the “tail‐cuff” method by a BP recorder (BP‐2000 Blood Pressure Analysis System, Series II, Visitech System, Apex NC, USA). Basal level of Psys was recorded for 20 min, at 5 min intervals. Then, erucin 10 mg·kg−1 or the corresponding vehicle were administered i.p. to different groups (normotensive vs. SHR), each composed of six rats. Starting from the administration of the test compounds, the Psys values were recorded, for 2 hr after 10, 30, 60, 90, and 120 min. Basal Psys was expressed as a mean of the four measurements carried out in each rat before the administration of test compounds. Change in Psys, recorded after erucin or vehicle administration, is expressed as a percentage of the basal Psys and also calculated as mean value of the five recordings (at 10, 30, 60, 90, and 120 min) carried out after drug administration. BP measurements were carried out in six animals per group (n = 6 for each treatment both in normotensive and SHR groups).
2.8. Data and statistical analysis
All the experimental data were analysed by a computer fitting procedure (GraphPad Prism 6.0, La Jolla, CA, USA, RRID:SCR_002798) and expressed as mean ± SEM of at least six independent experiments. All the group sizes were designed to be homogeneous. ANOVA and Student's t‐test were selected as statistical analyses. When required, the Bonferroni post hoc test was used. P < 0.05 was considered representative of significant statistical differences. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
2.9. Materials
Cell culture reagents Medium 231 (Cat.no.: M‐231‐500) and Smooth Muscle Growth Supplement (Cat.no.: S‐007‐25) were from Thermo Fisher while penicillin/streptomycin solution (Cat.no.: P4333), gelatin (Cat.no.: G1890, CAS Number: 9000‐70‐8), DADS (Cat.no.: SMB00378, CAS Number: 2179‐57‐9), DMSO (Cat.no.: D 2650, CAS Number: 67‐68‐5), DiBac4(3) (Cat.no: D8189, CAS Number: 70363‐83‐6), NS1619 (Cat.no.: N170, CAS Number: 153587‐01‐0), ACh chloride (Cat.no: A9101, CAS Number: 60‐31‐1), KCl (Cat.no.: P9333, CAS Number: 7447‐40‐7), L‐NAME (Cat.no.: N5751, CAS Number 51298‐62‐5), NA hydrochloride (Cat.no: A7256, CAS Number 55‐27‐6), AngII (Cat.no.: A9525, CAS Number 4474‐91‐3), and all the salts and glucose (Cat.no.: G8270, CAS Number 50‐99‐7) used for preparation of Buffer standard, Tyrode, and Krebs solutions were from Sigma‐Aldrich. WSP‐1 (Cat.no.: 11179, CAS Number: 1352750‐34‐5) was from Cayman Chem. Purified erucin (CAS Number: 4430‐36‐8) was kindly provided by the Italian Council for Agricultural Research and Economics, Research Centre for Cereal and Industrial Crops (Bologna, Italy).
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017; Alexander, Striessnig et al., 2017).
3. RESULTS
3.1. Erucin exhibits intracellular H2S‐release in HASMCs
Erucin was able to release H2S inside cells. Indeed, the addition of several concentrations of erucin to HASMCs caused a significant, gradual, and concentration‐dependent increase in WSP‐1 fluorescence (indicating the generation of intracellular H2S), until the reaching of a plateau, (Figure 2a). The AUC of the H2S‐release was also calculated and highlighted a clear concentration‐dependent release. The amount of H2S released from the highest concentration of erucin (300 μM) reached about 70% of the value obtained after the administration of the reference H2S‐donor DADS (300 μM; Figure 2b).
Figure 2.
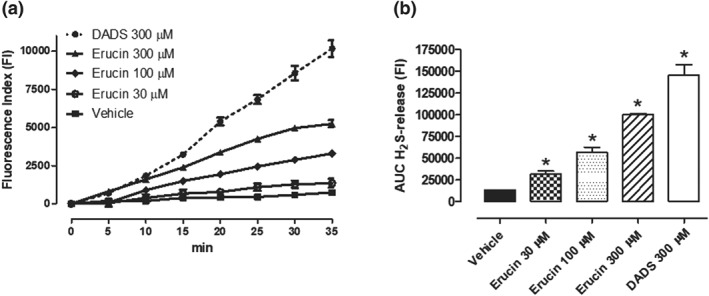
Fluorometric recording of erucin‐mediated H2S‐release inside HASMCs. (a) Time course of the fluorometric recordings of H2S released by vehicle, erucin 30, 100, and 300 μM and DADS 300 μM, during 35 min of observation: the increase in H2S is expressed as FI. (b) The histograms show the total amount of H2S released by vehicle, erucin 30, 100, and 300 μM and DADS 300 μM, in the 35 min of observation time, expressed as AUC. The vertical bars represent SEM, six different experiments were performed, each with six replicates (n = 6). The asterisks indicate a statistically significant difference from vehicle (*P < 0.05)
3.2. Hyperpolarizing effect of erucin on HASMC membrane
The hyperpolarizing effect of erucin on HASMCs pre‐incubated with DiBac4(3) was recorded for 35 min until a plateau was obtained, and compared with the effect of a well‐known hyperpolarizing drug, that is, the BKCa activator, NS1619 10 μM (Figure 3a). As shown in Figure 3a and in the correlated AUC graph (Figure 3b), the hyperpolarizing effect of erucin on HASMC membrane was significant versus vehicle, concentration‐dependent and, at the highest concentration (300 μM), it was also comparable (106 ± 2% of the NS1619 hyperpolarizing effect) with that of the reference drug, confirming that the novel H2S‐donor erucin is a hyperpolarizing drug.
Figure 3.
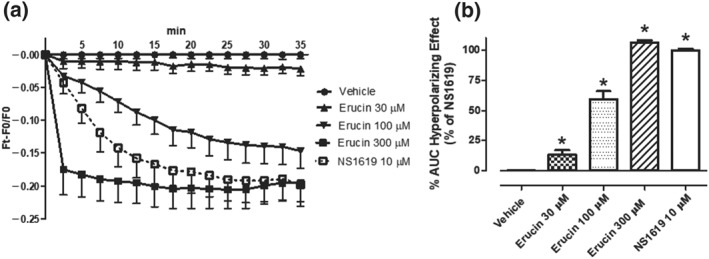
Hyperpolarizing effects in HASMCs. (a) Time‐course spectrofluorometric recording of changes in HASMCs membrane potential, followed for 35 min, expressed as (Ft − F0)/F0 and induced by vehicle, erucin 30, 100, and 300 μM and the hyperpolarizing reference drug, NS1619 10 μM. (b) Corresponding AUC of the hyperpolarizing effect of vehicle, erucin 30, 100, and 300 μM and NS1619 10 μM. Data are expressed as mean ± SEM. Six different experiments were performed, each with six replicates (n = 6). The asterisks indicate a significant difference from the effect evoked by vehicle (*P < 0.05)
3.3. Direct vasorelaxing effect on rat aortic rings
Erucin evoked endothelium‐independent vasorelaxing effects, since it caused vasodilatation of endothelium‐denuded rat aortic rings precontracted with KCl (Emax = 35 ± 6%; pEC50 = 4.95 ± 0.07). However, this vasorelaxing effect was significantly increased when erucin was tested on endothelium‐intact rat aortic rings precontracted with KCl (Emax = 56 ± 4%; pEC50 = 5.63 ± 0.08). In order to confirm a possible influence of the endothelial function on the vasorelaxing effect of erucin, its vascular effects were also evaluated in endothelium‐intact rat aortic rings pre‐incubated with L‐NAME 100 μM, an inhibitor of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1249 biosynthesis. The experiments performed in the presence of L‐NAME showed a reduction in the maximal vasorelaxing effect (Emax = 26 ± 7; pEC50 = 5.27 ± 0.11) leading to a curve being obtained that was almost superimposable on the curve produced by erucin in endothelium‐denuded rat aortic rings (Figure 4).
Figure 4.
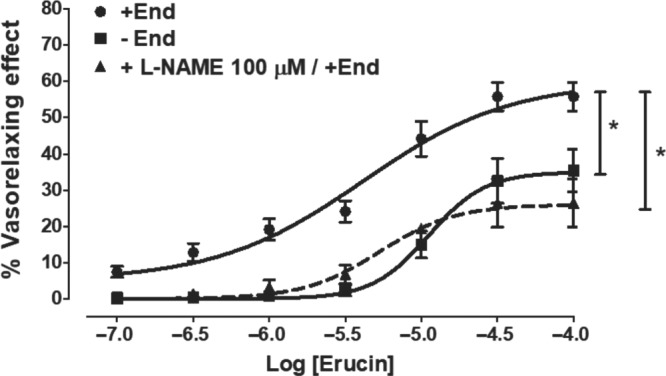
Direct vasorelaxing effects. Concentration–response curves, relative to the vasorelaxing effects evoked by erucin, in endothelium‐intact and ‐denuded rat aortic rings, precontracted with KCl 25 mM. In endothelium‐intact aortic rings, the vasorelaxing effects have been recorded either in the absence or presence of L‐NAME 100 μM (inhibitor of the NO‐biosynthesis). The vertical bars indicate the SEM. Six different experiments were performed, each with six replicates (n = 6). The asterisks indicate a significant difference from the curve obtained on endothelium‐intact aortic rings (*P < 0.05)
3.4. Inhibition of NA‐induced vasoconstriction
Besides its direct vasodilating effect, erucin also inhibited the NA‐evoked vasoconstriction. In the vehicle pretreated endothelium‐denuded aortic rings, NA induced a sustained vasoconstriction, with an Emax of 100 ± 2 and a potency value (pEC50) of 7.44 ± 0.04. Pretreatment with increasing concentrations of erucin (10, 30, and 100 μM) caused a progressive inhibition of both the potency and efficacy of NA. Indeed, pre‐incubation of the aortic rings with erucin (10, 30, and 100 μM) led to concentration‐dependent and significant inhibitory effects. In particular, the increasing concentrations of erucin produced a similar reduction in the efficacy of NA by about 25–30%, but different effects on NA potency (pEC50) of 7.20 ± 0.10, 6.94 ± 0.06, and 6.69 ± 0.04, for erucin 10, 30, and 100 μM respectively (Figure 5a). Noteworthy, the inhibitory effect of erucin at the different concentrations was fully reversible; indeed, after an organ washout, a full recovery of the contractile effects of NA was observed (Figure 5b).
Figure 5.
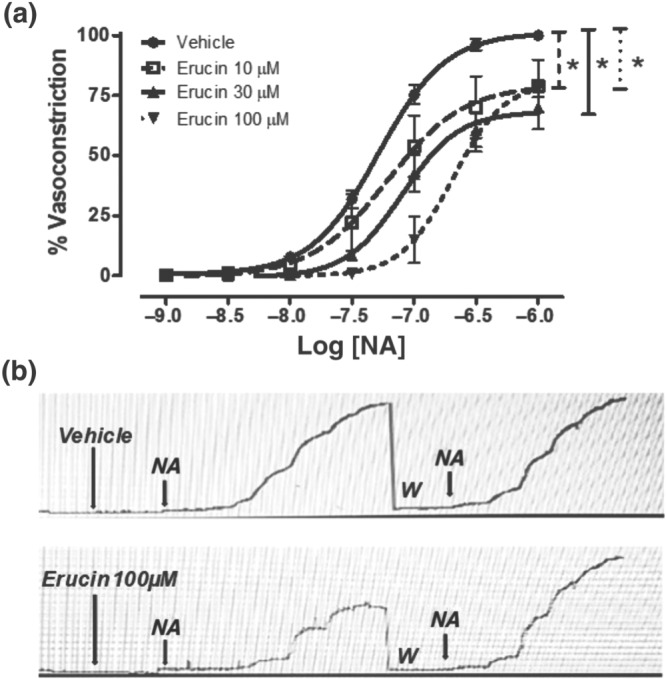
Inhibition of NA‐induced vasoconstriction. (a) Concentration–response curves to NA, obtained in endothelium‐denuded rat aortic rings pre‐incubated with vehicle or erucin at the concentrations of 10, 30, and 100 μM. The effects are expressed as a % of the contractile responses previously induced by the administration of KCl 60 mM. The vertical bars indicate the SEM. Six different experiments were performed, each with six replicates (n = 6). The asterisks indicate a significant difference from the NA‐induced vasoconstriction curve obtained on endothelium‐denuded aortic rings pre‐incubated with the vehicle (*P < 0.05). (b) Representative microdynamometric tracings obtained in two different aortic rings, demonstrating that the inhibitory effects of erucin were fully reversible. In the upper panel, one aortic ring was pre‐incubated with vehicle (20 min); thereafter, cumulative increasing concentrations of NA, evoking concentration‐dependent vasoconstriction, were added. The aortic ring was submitted to washout (W) and adequate equilibration time to recover again the resting tension. Then, the aortic ring was treated again with vehicle (20 min); thereafter, cumulative increasing concentrations of NA, evoking concentration‐dependent vasoconstriction, were added again. In the lower panel, the other aortic ring was pre‐incubated with erucin 100 μM (the highest concentration, for 20 min); thereafter, cumulative increasing concentrations of NA, evoking reduced concentration‐dependent vasoconstriction, were added. The ring was submitted to washout (W) and an adequate equilibration time to recover again the resting tension. Finally, the aortic ring was treated with vehicle (20 min); thereafter, cumulative increasing concentrations of NA, evoking concentration‐dependent vasoconstriction, were added again. In the lower panel, complete recovery of the vasoconstriction effects of NA is evident
3.5. Evaluation of the functional effects on the CF
The basal CF in Langendorff‐perfused rat hearts was 13.08 ± 1.92 ml·min−1·g−1. The administration of 0.1, 1, 10, and 100 μM erucin did not affect the basal values of CF (Figure 6a). A different trend was observed when AngII (0.1 μM) was administered to the rat hearts. Indeed, in the presence of AngII, the CF was significantly reduced to 81.8 ± 3.3% of the basal value, and the administration of 0.1, 1, 10, and 100 μM erucin, in the continuous presence of AngII, led to a progressive increase in CF, which was finally restored to the basal CF value, when 100 μM erucin was added (101.9 ± 4.1% of the CF basal value; Figure 6b).
Figure 6.
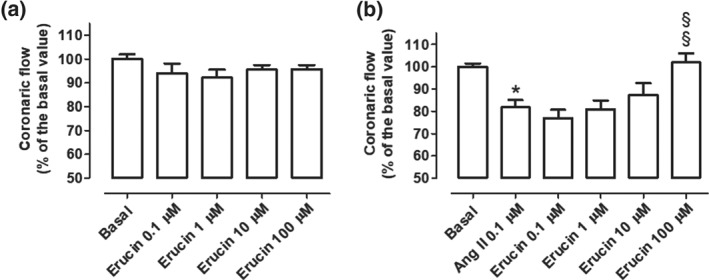
Effects on coronary flow. Changes in CF (expressed as a % of the basal CF) induced by the perfusion of increasing concentrations of erucin, in the absence (a) or in the constant presence (b) of AngII, as a co‐administration. The vertical bars indicate the SEM. Six different experiments were performed, (n = 6). The asterisks indicate a significant difference from the basal CF (*P < 0.05) while the symbols §indicate a significant difference from the CF recorded in the presence of AngII 0.1 μM (§ P < 0.05)
3.6. Effect of erucin on systolic BP in SHR and normotensive rats
On the basis of previous results obtained in vascular smooth muscle, in vivo BP measurements have been carried out in SHR and normotensive rats. Normotensive Wistar rats showed basal Psys of 133 ± 2 mmHg, and i.p. administration of vehicle did not alter the basal Psys value. The administration of erucin 10 mg·kg−1 i.p. caused only a slight decrease in Psys, which was significantly different from the vehicle at three recording times (10, 30, and 120 min; Figure 7a). However, the overall mean change in Psys was not significant (−9.98 ± 6.2%; Figure 7c). This behaviour was completely different when erucin and vehicle were administered into SHRs. Indeed, SHRs exhibited basal Psys of 177 ± 3 mmHg and, as already observed for normotensive rats, the i.p. administration of vehicle did not alter the basal Psys. In contrast, the administration of erucin 10 mg·kg−1 i.p. to SHRs caused a clear and significant decrease in Psys both in the curve of time recordings and in the bars reporting the overall mean change in Psys (−23.9 ± 3.8%; Figure 7b,c).
Figure 7.
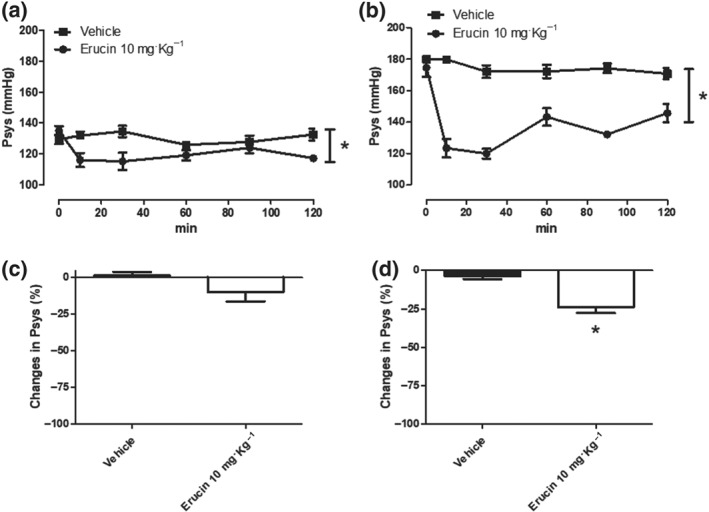
Effects on blood pressure. Time‐course of changes in systolic blood pressure values, expressed in mmHg in normotensive (a) and SHR rats (b) after i.p. administration of vehicle (DMSO 1 ml·kg−1) or erucin 10 mg·kg−1, recorded for 2 hr, at 10, 30, 60, 90, and 120 min. The vertical bars indicate the SEM. Six different experiments were performed for each treatment (n = 6). The asterisks indicate a significant difference from Psys trend curve obtained after the administration of vehicle (*P < 0.05). Overall mean change in Psys (expressed as a % of the basal Psys values) in normotensive (c) and SHRs (d), recorded in the 2 hr after i.p. administration of vehicle (DMSO 1 ml·kg−1) or erucin 10 mg·kg−1. The vertical bars indicate the SEM. Six different experiments were performed for each treatment (n = 6). The asterisks indicate a significant difference from changes in Psys (%) obtained after the administration of vehicle (*P < 0.05)
4. DISCUSSION
After it was discovered that H2S has multiple pathophysiological roles and the potential therapeutic application of H2S‐donor drugs in “H2S‐poor” pathologies was realized, much effort has been devoted to the characterization of new H2S‐releasing chemical moieties. In this broad panorama of heterogeneous chemical structures, we identified the isothiocyanate group as an interesting moiety. Indeed, in previous studies, we found that some synthetic aryl‐isothiocyanates behaved as H2S donors and caused clear H2S‐mediated vasorelaxing effects. Noteworthy, the release of H2S from these aryl‐isothiocyanates was not a spontaneous process but was triggered by the presence of organic thiols (e.g., http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4782 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737), as often documented for other classes of H2S donors, such as allyl polysulfides (Benavides et al., 2007), thioamides (Martelli, Testai, Citi, et al., 2013), iminotioethers (Barresi et al., 2017), or GYY4137 (one of the best characterized H2S donors; Martelli, Testai, Citi, et al., 2013). This thiol‐dependent profile was considered as a useful feature of a “smart” H2S donor (Calderone, Martelli, Testai, Citi, & Breschi, 2016), as it would ensure that the release of H2S does not occur spontaneously and in an undifferentiated way but is particularly facilitated in just the cell cytosol, where the highest concentrations of organic thiols are present: GSH (in 1–10 mM range) and cysteine (in 30–200 μM range; Tian et al., 2014)
In this context, we have previously demonstrated that erucin, a natural isothiocyanate, typically present in many edible cruciferous plants, behaved as a smart H2S donor, since it exhibited slow, long‐lasting, and thiol‐dependent release of H2S; however, this result was obtained in a “cell‐free” experimental model in vitro (Citi et al., 2014). Therefore, the first objective of this work was to test the ability of erucin to enter vascular smooth muscle cells and promote the generation of H2S using the intracellular pool of organic thiols. DADS was used as a reference H2S donor for this experiment, since it shares some physicochemical analogies with erucin (relatively low molecular weight, no charge, lipophilicity). In contrast, the use of other well‐known H2S donors, such as the inorganic sulfides, did not seem to be appropriate, because of the large differences in terms of physicochemical properties and of H2S‐releasing rates. As detected by the spectrofluorimetric assay with the WSP‐1 probe, the incubation of HASMCs with erucin promoted a slow and progressive increase in H2S levels, in a concentration‐dependent way. In particular, incubation with the highest concentration of erucin (300 μM) led to an intracellular production of H2S which was about 75% of that produced by an equimolar concentration of DADS. However, it should be noted that the functional group isothiocyanate can release a single H2S molecule; instead, DADS generates two H2S molecules, as elegantly demonstrated by Benavides et al. (2007).
Next, we evaluated whether erucin evoked typical H2S‐mediated vascular responses. As reported above, H2S is a pivotal regulator of vascular tone, through its vasorelaxing effect. Indeed, by means of “S‐sulfhydration,” that is, the post‐translational modification of cysteine residues of target proteins (Paul & Snyder, 2012), H2S promotes multiple mechanisms of action responsible for its vasodilator activity, against the vasoconstrictor effects evoked by different stimuli, which were either receptor‐mediated or non‐receptor‐mediated. Among them, the modulation of several types of vascular potassium channel, with consequent membrane hyperpolarization in vascular smooth muscle cells, plays a relevant role. For instance, H2S‐mediated vasorelaxation was very early attributed to the activation of vascular http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=442 (Kir6.2) (Zhao, Zhang, & Lu, 2001). In addition, the activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=81 has been more recently proposed as a further important and peculiar mechanism accounting for the vasorelaxing effects of H2S (Martelli, Testai, Breschi, et al., 2013; Schleifenbaum et al., 2010). Therefore, we decided to evaluate the effects produced by erucin on the membrane potential of HASMCs cells. In this kind of experiment, NS1619 was selected as a reference drug, because it is a powerful activator of vascular http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=380 (KCa1.1), which can promote a massive hyperpolarization of the cell membrane (Calderone, 2002). Through the potential‐sensitive probe DiBac4(3), it was shown that erucin causes a concentration‐dependent membrane hyperpolarization in HASMCs, thus exhibiting typical H2S‐mediated vascular effects. Noteworthy, the hyperpolarizing effects produced by the highest concentration of erucin (300 μM) were equivalent to those produced by the reference drug. The identification of the specific potassium channel type(s) involved in this hyperpolarizing effect will be the object of future electrophysiological studies.
Concerning the H2S‐mediated functional effects, erucin exhibited endothelium‐independent vasorelaxing activity, since it evoked concentration‐dependent vasorelaxation of endothelium‐denuded aortic rings. The vasorelaxing effects were further increased when erucin was administered to endothelium‐intact rat aortic rings. However, when erucin was added to endothelium‐intact rat aortic rings pretreated with L‐NAME (inhibitor of eNOS), the vasorelaxing effects were almost equivalent to those recorded in the endothelium‐denuded vessels. Thus, the H2S‐mediated vasorelaxing effect of erucin is endothelium‐independent but is significantly improved by the presence of endothelium and in particular by the presence of endothelial NO. Indeed, data on the possible interactions between H2S and NO are numerous and sometimes conflicting (Cirino, Vellecco, & Bucci, 2017; Nagpure & Bian, 2016). However, it is currently accepted that the overall interplay between the two gasotransmitters (at physiological concentrations) is positive and acts through multiple mechanisms, such as the formation of active hybrid species, the H2S‐induced mobilization of NO from stable “pools,” the increased activity of eNOS through calcium mobilization and PI3K/Akt signalling, and the synergistic effects on cGMP (NO activates the guanylate cyclase, while H2S inhibits http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1304 and activates http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=287; Szabo, 2016). Alongside its direct vasodilator effects, erucin significantly inhibited the vasoconstriction induced by the adrenoceptor agonist NA on endothelium‐denuded rat aortic rings. Noteworthy, these effects were reversible, confirming the involvement of pharmacological mechanisms of action, rather than possible tissue damage induced by toxicity.
The role of endogenous H2S in the physiological regulation of CF is still an underdeveloped field of research. Recent experimental findings indicate that endogenous H2S may have a modest role in regulating canine coronary microvascular resistance (Casalini et al., 2014), while the presence of H2S‐producing enzymes (in particular, mercaptopyruvate sulfotransferase) was observed in rodent coronary arteries (Kuo et al., 2016). However, irrespective of the potential role of endogenous H2S, in the above experimental studies, the administration of exogenous H2S donors always caused a reduction in vascular resistance and a significant increase in CF. This feature had been observed previously for synthetic aryl‐isothiocyanate H2S donors (Martelli et al., 2014; Testai et al., 2015). These effects suggest a possible utility for H2S donors in coronary artery diseases. In the present study, the possible vasorelaxing effects of erucin were also confirmed in the coronary arteries. The perfusion with erucin did not change the flow of coronary arteries with a basal vascular tone, since the vascular smooth muscle of these vessels is likely to be already relaxed. In contrast, when the coronary vessels were precontracted with AngII, erucin promoted a significant increase in the flow in concentration‐dependent fashion, bringing it back to baseline levels.
Although there is a paucity of data on H2S and human hypertension, many experimental studies indicate that an increase in H2S levels induced by suitable H2S donors may be a rational and effective therapeutic approach for hypertensive patients (Van Goor, van den Born, Hillebrands, & Joles, 2016). Indeed, different chemotypes of H2S donors exhibit antihypertensive effects in different experimental models of hypertension. For instance, early on it was observed that administration of the well‐known H2S‐donor GYY4137 caused a marked decrease in Psys in SHRs, while it caused lower (albeit significant) hypotensive effects in normotensive animals (Li et al., 2008). Sodium hydrosulfide (rapid H2S donor) has also been shown to exhibit antihypertensive effects in SHRs (Ahmad et al., 2012). Novel H2S‐donor iminothioethers have been recently reported to cause a significant reduction in Psys in a rat experimental model of hypertension, induced by pharmacological inhibition of eNOS (Barresi et al., 2017). Therefore, in order to translate the above experimental observations towards a reliable experimental model of pathology and therefore to identify a possible clinical application of erucin, in the last step of this study we evaluated the effects of erucin on the arterial pressure of normotensive and hypertensive rats. In normotensive animals, inhibitors of H2S biosynthesis are known to increase BP (Yan et al., 2004), suggesting that the vascular regulatory mechanisms are fully activated by endogenous H2S. Consistently, in our study, erucin had only a negligible influence on the BP of normotensive rats. In contrast, the expression/activity of H2S‐producing enzymes is significantly reduced in SHRs and thus the H2S‐mediated regulatory mechanisms are defective (Bucci et al., 2014; Yan et al., 2004). Noteworthy, in SHRs, the administration of erucin (i.e., the H2S supplementation with this H2S donor) caused a marked reduction in Psys, bringing it back to the normotensive levels.
5. CONCLUSIONS
The isothiocyanate erucin is naturally present in some edible cruciferous plants, commonly used in human nutrition and in functional diets, and this would exclude serious toxicity concerns. Several studies have already examined the main pharmacokinetic characteristics of erucin, highlighting its appreciable oral bioavailability and providing a detailed description of its metabolites. In these studies, no significant toxicity of erucin or erucin‐derived metabolites has been reported. In the present study, we demonstrated for the first time that erucin can be considered as an original H2S donor, with interesting vascular effects and, in particular, with significant BP lowering effects in hypertensive animals and negligible effects in normotensive ones. Therefore, an adequate pharmaceutical development of erucin or of improved erucin analogues may actually pave the way for a new class of H2S‐donor antihypertensive drugs, with reliable “druggability” and thus with the real possibility of clinical use.
AUTHOR CONTRIBUTIONS
A.M. and E.Pi. performed the research. A.M. and V.Ca. designed the research study. E.Pa., L.U., and L.L. provided purified Erucin. L.T., V.Ci., and O.L.M. contributed to preliminary studies. A.M. and V.Ca. wrote the manuscript. A.M., V.Ca., L.D.M., C.G., M.C.B., and M.B. analysed the data and revised the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.13405 acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
We are grateful to Dr Jessica Silicani, Dr Elena D'Asaro, and Dr Federica Battaglia for technical assistance. This work has been funded by the Italian MIUR (Ministry of Instruction, University and Research) in the programme “Project of Relevant National Interest” (PRIN, 2015).
Martelli A, Piragine E, Citi V, et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S‐releasing properties. Br J Pharmacol. 2020;177:824–835. 10.1111/bph.14645
Contributor Information
Alma Martelli, Email: alma.martelli@unipi.it.
Vincenzo Calderone, Email: vincenzo.calderone@unipi.it.
REFERENCES
- Achelrod, D. , Wenzel, U. , & Frey, S. (2015). Systematic review and meta‐analysis of the prevalence of resistant hypertension in treated hypertensive populations. American Journal of Hypertension, 28(3), 355–361. 10.1093/ajh/hpu151 [DOI] [PubMed] [Google Scholar]
- Ahmad, A. , Sattar, M. A. , Rathore, H. A. , Khan, S. A. , Lazhari, M. I. , Afzal, S. , … Johns, E. J. (2015). A critical review of pharmacological significance of hydrogen sulfide in hypertension. Indian J Pharmacol, 47, 243–247. 10.4103/0253-7613.157106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, F. U. , Sattar, M. A. , Rathore, H. A. , Abdullah, M. H. , Tan, S. , Abdullah, N. A. , & Johns, E. J. (2012). Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Renal Failure, 34(2), 203–210. 10.3109/0886022X.2011.643365 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174(S1), S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi, E. , Nesi, G. , Citi, V. , Piragine, E. , Piano, I. , Taliani, S. , … Martelli, A. (2017). Iminothioethers as hydrogen sulfide donors: From the gasotransmitter release to the vascular effects. Journal of Medicinal Chemistry, 60, 7512–7523. 10.1021/acs.jmedchem.7b00888 [DOI] [PubMed] [Google Scholar]
- Benavides, G. A. , Squadrito, G. L. , Mills, R. W. , Patel, H. D. , Isbell, T. S. , Patel, R. P. , … Kraus, D. W. (2007). Hydrogen sulfide mediates the vasoactivity of garlic. Proceedings of the National Academy of Sciences of the United States of America, 104(46), 17977–17982. 10.1073/pnas.0705710104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci, M. , & Cirino, G. (2011). Hydrogen sulphide in heart and systemic circulation. Inflammation & Allergy Drug Targets, 10(2), 103–108. 10.2174/187152811794776204 [DOI] [PubMed] [Google Scholar]
- Bucci, M. , Vellecco, V. , Cantalupo, A. , Brancaleone, V. , Zhou, Z. , Evangelista, S. , … Cirino, G. (2014). Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovascular Research, 102, 138–147. 10.1093/cvr/cvu026 [DOI] [PubMed] [Google Scholar]
- Calderone, V. (2002). Large‐conductance, Ca(2+)‐activated K(+) channels: Function, pharmacology and drugs. Current Medicinal Chemistry, 9(14), 1385–1395. 10.2174/0929867023369871 [DOI] [PubMed] [Google Scholar]
- Calderone, V. , Martelli, A. , Testai, L. , Citi, V. , & Breschi, M. C. (2016). Using hydrogen sulfide to design and develop drugs. Expert Opinion on Drug Discovery, 11(2), 163–175. 10.1517/17460441.2016.1122590 [DOI] [PubMed] [Google Scholar]
- Casalini, E. D. , Goodwill, A. G. , Owen, M. K. , Moberly, S. P. , Berwick, Z. C. , & Tune, J. D. (2014). Contribution of hydrogen sulfide to the control of coronary blood flow. Microcirculation, 21(2), 104–111. 10.1111/micc.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino, G. , Vellecco, V. , & Bucci, M. (2017). Nitric oxide and hydrogen sulfide: The gasotransmitter paradigm of the vascular system. British Journal of Pharmacology, 174(22), 4021–4031. 10.1111/bph.13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi, V. , Martelli, A. , Testai, L. , Marino, A. , Breschi, M. C. , & Calderone, V. (2014). Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae. Planta Medica, 80, 610–613. [DOI] [PubMed] [Google Scholar]
- Clarke, J. D. , Hsu, A. , Riedl, K. , Bella, D. , Schwartz, S. J. , Stevens, J. F. , & Ho, E. (2011). Bioavailability and inter‐conversion of sulforaphane and Erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross‐over study design. Pharmacological Research, 64(5), 456–463. 10.1016/j.phrs.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheibi, S. , Jeddi, S. , Kashfi, K. , & Ghasemi, A. (2018). Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochemical Pharmacology, 149, 42–59. 10.1016/j.bcp.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, J. (2013). Epidemiology of hypertension. Clinical Queries: Nephrology, 2, 56–61. 10.1016/j.cqn.2013.04.005 [DOI] [Google Scholar]
- Kuo, M. M. , Kim, D. H. , Jandu, S. , Bergman, Y. , Tan, S. , Wang, H. , … Santhanam, L. (2016). MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. American Journal of Physiology. Heart and Circulatory Physiology, 310(1), H71–H79. 10.1152/ajpheart.00574.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Whiteman, M. , Guan, Y. Y. , Neo, K. L. , Cheng, Y. , Lee, S. W. , … Moore, P. K. (2008). Characterization of a novel, water‐soluble hydrogen sulfide‐releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation, 117(18), 2351–2360. 10.1161/CIRCULATIONAHA.107.753467 [DOI] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Breschi, M. C. , Lawson, K. , McKay, N. G. , Miceli, F. , … Calderone, V. (2013). Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacological Research, 70, 27–34. 10.1016/j.phrs.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Citi, V. , Marino, A. , Bellagambi, F. G. , Ghimenti, S. , … Calderone, V. (2014). Pharmacological characterization of the vascular effects of aryl isothiocyanates: Is hydrogen sulfide the real player? Vascular Pharmacology, 60, 32–41. 10.1016/j.vph.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Citi, V. , Marino, A. , Pugliesi, I. , Barresi, E. , … Calderone, V. (2013). Arylthioamides as H2S donors: L‐cysteine‐activated releasing properties and vascular effects in vitro and in vivo. ACS Medicinal Chemistry Letters, 4, 904–908. 10.1021/ml400239a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Marino, A. , Breschi, M. C. , Da Settimo, F. , & Calderone, V. (2012). Hydrogen sulphide: Biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Current Medicinal Chemistry, 19(20), 3325–3336. 10.2174/092986712801215928 [DOI] [PubMed] [Google Scholar]
- Meng, G. , Ma, Y. , Xie, L. , Ferro, A. , & Ji, Y. (2015). Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. British Journal of Pharmacology, 172(23), 5501–5511. 10.1111/bph.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat, A. , Redwood, S. R. , Qureshi, A. C. , Spertus, J. A. , & Williams, B. (2012). Resistant hypertension. BMJ, 345, e7473 10.1136/bmj.e7473 [DOI] [PubMed] [Google Scholar]
- Nagpure, B. V. , & Bian, J. S. (2016). Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxidative Medicine and Cellular Longevity, 6, 6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubiap, J. J. , Nansseu, J. R. , Nyaga, U. F. , Sime, P. S. , Francis, I. , & Bigna, J. J. (2018). Global prevalence of resistant hypertension: A meta‐analysis of data from 3.2 million patients. Heart, 105, 98–105. 10.1136/heartjnl-2018-313599 [DOI] [PubMed] [Google Scholar]
- Paul, B. D. , & Snyder, S. H. (2012). H₂S signalling through protein sulfhydration and beyond. Nature Reviews. Molecular Cell Biology, 13, 499–507. 10.1038/nrm3391 [DOI] [PubMed] [Google Scholar]
- Pierdomenico, S. D. , Lapenna, D. , Bucci, A. , Di Tommaso, R. , Di Mascio, R. , Manente, B. M. , … Mezzetti, A. (2005). Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. American Journal of Hypertension, 18, 1422–1428. 10.1016/j.amjhyper.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Platz, S. , Piberger, A. L. , Budnowski, J. , Herz, C. , Schreiner, M. , Blaut, M. , … Rohn, S. (2015). Bioavailability and biotransformation of sulforaphane and Erucin metabolites in different biological matrices determined by LC‐MS‐MS. Analytical and Bioanalytical Chemistry, 407(7), 1819–1829. 10.1007/s00216-015-8482-z [DOI] [PubMed] [Google Scholar]
- Sarafidis, P. A. , Georgianos, P. , & Bakris, G. L. (2013). Resistant hypertension—Its identification and epidemiology. Nature Reviews. Nephrology, 9, 51–58. 10.1038/nrneph.2012.260 [DOI] [PubMed] [Google Scholar]
- Schleifenbaum, J. , Köhn, C. , Voblova, N. , Dubrovska, G. , Zavarirskaya, O. , Gloe, T. , … Gollasch, M. (2010). Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. Journal of Hypertension, 28, 1875–1882. 10.1097/HJH.0b013e32833c20d5 [DOI] [PubMed] [Google Scholar]
- Sun, N. L. , Xi, Y. , Yang, S. N. , Ma, Z. , & Tang, C. S. (2007). Plasma hydrogen sulfide and homocysteine levels in hypertensive patients with different blood pressure levels and complications. Zhonghua Xin Xue Guan Bing Za Zhi, 35, 1145–1148. [PubMed] [Google Scholar]
- Sundström, J. , Arima, H. , Jackson, R. , Turnbull, F. , Rahimi, K. , Chalmers, J. , … Neal, B. (2015). Effects of blood pressure reduction in mild hypertension: A systematic review and meta‐analysis. Annals of Internal Medicine, 162, 184–191. 10.7326/M14-0773 [DOI] [PubMed] [Google Scholar]
- Szabo, C. (2016). Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. American Journal of Physiology. Cell Physiology, 312, C3–C15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, C. , & Papapetropoulos, A. (2017). International union of basic and clinical pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacological Reviews, 69(4), 497–564. 10.1124/pr.117.014050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testai, L. , D'Antongiovanni, V. , Piano, I. , Martelli, A. , Citi, V. , Duranti, E. , … Calderone, V. (2015). Different patterns of H2S/NO activity and cross‐talk in the control of the coronary vascular bed under normotensive or hypertensive conditions. Nitric Oxide, 47, 25–33. 10.1016/j.niox.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Tian, M. , Guo, F. , Sun, Y. , Zhang, W. , Miao, F. , Liu, Y. , … Wong, W. Y. (2014). A fluorescent probe for intracellular cysteine overcoming the interference by glutathione. Organic & Biomolecular Chemistry, 12(32), 6128–6633. 10.1039/C4OB00382A [DOI] [PubMed] [Google Scholar]
- Van Goor, H. , van den Born, J. C. , Hillebrands, J. L. , & Joles, J. A. (2016). Hydrogen sulfide in hypertension. Current Opinion in Nephrology and Hypertension, 25(2), 107–113. 10.1097/MNH.0000000000000206 [DOI] [PubMed] [Google Scholar]
- Wang, C. , Han, J. , Xiao, L. , Jin, C. E. , Li, D. J. , & Yang, Z. (2014). Role of hydrogen sulfide in portal hypertension and esophagogastric junction vascular disease. World Journal of Gastroenterology, 20, 1079–1087. 10.3748/wjg.v20.i4.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Ahmad, S. , Cai, M. , Rennie, J. , Fujisawa, T. , Crispi, F. , … Ahmed, A. (2013). Dysregulation of hydrogen sulfide producing enzyme cystathionine γ‐lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation, 127, 2514–2522. 10.1161/CIRCULATIONAHA.113.001631 [DOI] [PubMed] [Google Scholar]
- Xie, X. , Atkins, E. , Lv, J. , Bennett, A. , Neal, B. , Ninomiya, T. , … Rodgers, A. (2016). Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta‐analysis. Lancet, 387, 435–443. 10.1016/S0140-6736(15)00805-3 [DOI] [PubMed] [Google Scholar]
- Yan, H. , Du, J. , & Tang, C. (2004). The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochemical and Biophysical Research Communications, 313, 22–27. 10.1016/j.bbrc.2003.11.081 [DOI] [PubMed] [Google Scholar]
- Yang, G. , & Wang, R. (2015). H2S and blood vessels: An overview. Handbook of Experimental Pharmacology, 230, 85–110. 10.1007/978-3-319-18144-8_4 [DOI] [PubMed] [Google Scholar]
- Yang, G. , Wu, L. , Jiang, B. , Yang, W. , Qi, J. , Cao, K. , … Wang, R. (2008). H2S as physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ‐lyase. Science, 322, 587–590. 10.1126/science.1162667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, W. , Zhang, J. , & Lu, Y. (2001). The vasorelaxant effect of H(2)S as a novel endogenous gaseous K (ATP) channel opener. The EMBO Journal, 20, 6008–6016. 10.1093/emboj/20.21.6008 [DOI] [PMC free article] [PubMed] [Google Scholar]


