Abstract
Background and Purpose
ATB‐346 is a hydrogen sulfide (H2S)‐releasing anti‐inflammatory and analgesic drug. Animal studies demonstrated negligible gastrointestinal (GI) damage despite marked inhibition of COX activity and significant analgesic and anti‐inflammatory effects. In humans, ATB‐346 (250 mg once daily) was found to inhibit COX to the same extent as naproxen (550 mg twice daily).
Experimental Approach
Two hundred forty‐four healthy volunteers completed a 2‐week, double‐blind study, taking either ATB‐346 (250 mg once daily) or naproxen (550 mg twice daily), with upper GI ulceration being examined endoscopically.
Key Results
Forty‐two per cent of the subjects taking naproxen developed at least one ulcer (≥3‐mm diameter), while only 3% of the subjects taking ATB‐346 developed at least one ulcer. The two drugs produced comparable and substantial (>94%) suppression of COX activity. Subjects in the naproxen group developed more ulcers per subject than ATB‐346‐treated subjects and a greater incidence of larger ulcers (≥5‐mm diameter). The incidence of dyspepsia, abdominal pain, gastro‐oesophageal reflux, and nausea was lower with ATB‐346 than with naproxen. Subjects treated with ATB‐346 had significantly higher plasma levels of H2S than those treated with naproxen.
Conclusions and Implications
This Phase 2B study provides unequivocal evidence for a marked reduction of GI toxicity of the H2S‐releasing analgesic/anti‐inflammatory drug, ATB‐346, as compared to the conventional dose of naproxen that produced equivalent suppression of COX.
Linked Articles
This article is part of a themed section on Hydrogen Sulfide in Biology & Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.4/issuetoc
What is already known
In animal studies, hydrogen sulfide‐releasing NSAIDs have been shown to be effective in reducing pain/inflammation without causing significant gastrointestinal (GI) damage.
What this study adds
This human study demonstrates that at an effective anti‐inflammatory dose of ATB‐346 given to healthy human subjects for 2 weeks does not cause significant upper gastrointestinal ulceration.
What is the clinical significance
NSAIDs are among the most commonly used drugs, but they can cause life‐threatening ulceration and bleeding. Through the release of hydrogen sulfide ATB‐346 can provide effective pain relief without causing significant GI damage.
Abbreviations
- GI
gastrointestinal
- H2S
hydrogen sulfide
- NSAID
nonsteroidal anti‐inflammatory drug
1. INTRODUCTION
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are among the most widely used drugs because they are very effective at reducing pain, fever, and inflammation. The ability of NSAIDs to cause gastrointestinal (GI) damage and bleeding has been recognized for almost a century, and many drugs have been developed with the aim of reducing the incidence and severity of this damage. For example, histamine H2 receptor antagonists and proton pump inhibitors, which suppress gastric acid secretion, have been shown to significantly reduce the incidence of NSAID‐induced damage in the stomach and proximal duodenum (Goldstein et al., 2003; Yeomans et al., 1998). The key mechanism underlying the ability of NSAIDs to cause gastro‐duodenal ulceration is inhibition of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=269 enzymes (Vane, 1971). COX enzymes play key roles in the production of prostaglandins, which contribute significantly to maintaining the integrity of the lining of the GI tract (Robert, 1979; Wallace, 2008). There are two forms of COX, referred to as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1375 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376. They produce the same prostaglandin (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4483) from http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2391. Selective inhibitors of COX‐2 were developed in the 1990s and were promoted as a solution to the NSAID‐gastropathy problem (Silverstein et al., 2000). However, their use was found to be associated with significant adverse cardiovascular events, resulting in the withdrawal of several selective COX‐2 inhibitors from the market. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2892 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7220, which are among the least selective of the COX‐2 inhibitors, have remained on the market. Patients taking celecoxib are often prescribed low‐dose aspirin to counteract the detrimental cardiovascular effects of that drug. However, co‐administration of aspirin with selective COX‐2 inhibitors has been shown to abolish any beneficial GI effects of the COX‐2 inhibitor (Silverstein et al., 2000). Some recent studies suggest that detrimental cardiovascular effects may be encountered with conventional NSAIDs as frequently as with the selective COX‐2 inhibitors (Nissen et al., 2016). However, on the basis of several Phase 4 randomized controlled trials of NSAIDs to date, a relatively low dose of celecoxib administered to low‐risk subjects was found to be associated with approximately the same cardiovascular risk as NSAIDs with less COX‐2 inhibitory activity but at the cost of not controlling arthritic pain as effectively (Antman, 2017).
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9532 is a gaseous mediator produced throughout the body and by some of the bacteria that reside within the GI tract (see reviews by Kimura, 2015, and Wallace & Wang, 2015). Over the past 20 years, a substantial body of evidence has been generated to demonstrate that H2S plays key roles in regulating numerous physiological processes. In the GI tract, H2S has been shown to reduce inflammation and accelerate healing of damaged tissue (such as ulcers), while suppression of H2S production in the GI tract results in impaired healing of tissue injury and exacerbation of inflammation (Blackler, De Palma, et al., 2015; Blackler, Motta, et al., 2015; Blackler, Syer, Bolla, Ongini, & Wallace, 2012; Flannigan, Ferraz, Wang, & Wallace, 2013; Motta et al., 2015; Wallace, Dicay, McKnight, & Martin, 2007; Wallace, Vong, McKnight, Dicay, & Martin, 2009).
Several years ago, we began to test the hypothesis that linking an H2S‐releasing molecule to an NSAID would reduce the GI toxicity of the NSAID without reducing the ability of the drug to reduce inflammation and pain. Extensive studies in laboratory animals demonstrated that the delivery of H2S from these experimental drugs did indeed reduce or completely prevent GI ulceration and bleeding, while still exerting anti‐inflammatory and analgesic effects (Blackler, De Palma, et al., 2015; Wallace, Caliendo, Santagada, & Cirino, 2010). The GI safety of these “H2S‐NSAIDs” was maintained even in animal models in which the integrity of the GI tract was intentionally and drastically impaired (Blackler et al., 2012; Wallace et al., 2010; Wallace, Vaughan, Dicay, MacNaughton, & de Nucci, 2018).
Since 2013, Antibe Therapeutics Inc. (“Antibe”) has been developing H2S‐releasing NSAIDs for treatment of a variety of human diseases, including arthritis. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9534 is an H2S‐releasing derivative of naproxen. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5230 is among the most widely used NSAID for treatment of arthritis and other conditions characterized by inflammation and pain. In animal studies, ATB‐346 was observed to inhibit COX‐2 significantly more than an equimolar dose of naproxen (Wallace et al., 2010). In a Phase 1 clinical trial, ATB‐346 was again found to be much more potent (approximately sixfold) as an inhibitor of COX than naproxen, as well as suppressing COX activity for much longer than was observed with naproxen. In 2016, Antibe performed a Phase 2 clinical trial, in osteoarthritis patients, to test the hypothesis that ATB‐346 was more potent and long acting than naproxen. The study involved 12 osteoarthritis patients taking ATB‐346 (250 mg) once daily for 10 days, with measurements of the patients' level of pain during the 10‐day period. The amount of naproxen in a once daily dose of ATB‐346 is one sixth the amount of the usual daily dose of naproxen. The study demonstrated that ATB‐346 produced a substantial and statistically significant (P < 0.001) reduction in pain (Wallace et al., 2018). The degree of pain relief observed after 4 days of treatment was comparable to what has been observed in clinical trials with naproxen and celecoxib in osteoarthritis patients (Boucher, 2008; Wittenberg et al., 2006), and pain relief was further reduced after 10 days of treatment with ATB‐346 (Wallace et al., 2018). COX enzyme activity was substantially inhibited (by ~85%) following administration of ATB‐346 (Wallace et al., 2018).
Having established that once daily administration of ATB‐346 at a dose of 250 mg could produce substantial relief of pain in osteoarthritis patients, we then undertook the present study to determine if there was a significant increase in GI safety of this dose of ATB‐346 versus an equi‐effective dose of naproxen. This involved endoscopic examination of the upper GI tract in healthy volunteers before and after a 14‐day course of treatment with ATB‐346 or naproxen. To participate in the study, the volunteers had to be healthy, ≥18 to ≤65 years of age, with no prior history of significant GI disease, arthritis, or bleeding disorders. The main exclusion criteria were subjects with abnormal baseline laboratory values deemed to be clinically significant; subjects with a history GI ulcers, GI bleeding, or any clinically significant GI disease; use of NSAIDs within 14 days prior to study entry; subjects taking gastroprotective drugs or drugs affecting GI motility; and subjects positive for Helicobacter pylori. Those subjects meeting these inclusion and exclusion criteria were randomized to the two groups, with drugs/placebo taken orally: ATB‐346 (250 mg) in the morning and placebo in the evening, or sodium naproxen (550 mg) in the morning and in the evening.
2. METHODS
2.1. Primary objective
The primary objective of this study was to evaluate the GI safety of a 14‐day dosing regimen of ATB‐346 in comparison to naproxen in reducing endoscopically detected gastric and/or duodenal ulcers of at least 3‐mm diameter with unequivocal depth.
2.2. Secondary objectives
The secondary objectives of this study were to determine
the incidence of one or more gastric and/or duodenal ulcers of at least 5‐mm diameter with unequivocal depth;
the number of ulcers or erosions in the stomach/duodenum (erosions are very small breaks in the lining of the stomach and small intestine that are superficial and widely deemed as “clinically insignificant.” However, they remain a common endpoint in endoscopy trials of NSAIDs, so they were evaluated in this trial);
the incidence of dyspepsia leading to discontinuation of study treatment;
changes from baseline haematocrit; and
changes from baseline in ex vivo whole blood TxB2 synthesis.
2.3. Clinical trial design
Healthy volunteers (male and female) were recruited by Topstone Research Ltd., initially to a single clinic, but later expanded to three additional clinics in Ontario, Canada (to increase the rate of recruitment). The study consisted of three phases: a screening, pretreatment phase of 21 days, a blinded treatment phase of 14 days, and a follow‐up visit 14 days after the end of treatment.
For the screening, pretreatment phase, subject eligibility was determined, and those who consented to participate in the study underwent medical history and physical examinations, clinical laboratory evaluations, and a screening endoscopy to confirm the absence of erosions and ulcers in the gastroduodenal mucosa. Additional exclusion criteria included subjects with abnormal baseline laboratory values deemed to be clinically significant by the investigator, as well as abnormal laboratory values related to clinically significant GI, hepatic or renal disease, or any other conditions known to significantly impact or interfere with the absorption, distribution, metabolism, or elimination of the drugs being investigated. Also, any subject positive for H. pylori (urea breath test) at the screening visit was excluded from participation in the study.
At randomization, blood samples were obtained from eligible subjects for whole blood thromboxane and haematocrit analyses. The drugs were administered in capsules that were physically identical so that neither the subject nor the physician knew which drug the subject was taking. The subjects took the drug they were assigned for 14 days. The second endoscopy was performed on the 14th day of the study. The endoscopist (blind to which drug the subject was taking) looked for ulcers in the stomach and duodenum. The number of ulcers with a diameter of greater than 3 mm was recorded, as was the number of ulcers with a diameter of greater than 5 mm. The number of erosions in the stomach and duodenum was also recorded. Blood samples were taken for measurement of haematocrit prior to and upon completion of the 14‐day treatment period. Additional blood samples were taken prior to the first dose and on Days 7 and 14 of dosing for measurement of plasma naproxen levels (measured by LC‐MS).
This clinical trial was registered with http://ClinicalTrials.gov (NCT03291418).
2.4. Plasma H2S
Plasma sulfide levels were determined using monobromobimane (MBB) derivatization followed by HPLC separation and fluorescent quantification of the sulfide‐dibimane product. All procedures were carried out in the dark to avoid photo‐induced decomposition of MBB derivatives. Twenty‐five microlitres of a plasma sample were mixed with 66 μl of the MBB working solution (10 μl of 100 mM MBB in acetonitrile mixed with 650 μl of 200 mM HEPES buffer, pH 8.2). The derivatization proceeded at 20°C for exactly 10 min. The reaction was stopped by adding 5 μl of 50% trichloroacetic acid to the samples, followed by vigorous vortex mixing. The samples were then centrifuged (3,000 g for 5 min), and the supernatants were stored in the autosampler in vials at 5°C.
A Thermo Ultimate 3000 HPLC system equipped with fluorescent detector was used with a Phenomenex Luna C18(2) column (200 × 4.6 mm, 3 μm) for the separation of the analytes. The fluorescence detector was set at 390 nm (excitation wavelength) and 475 nm (emission wavelength). The derivatized sample (10 μl) was injected and eluted using a 15‐min gradient profile consisting of 0.1% trifluoroacetic acid in water (A) and 0.1% trifluoroacetic acid in acetonitrile (B) at a 1‐ml·min−1 flow rate. The gradient profile began with 15% B followed by a linear increase to 35% B over 3 min and an isocratic step with 35% B for 6 min. The linear gradient was continued with a rapid increase to 90% B over 2 min and an isocratic wash for 1 min before returning to the initial composition of 15% B. The column was then regenerated for another 2 min before the next injection.
Concentrations of H2S were estimated based on calibration curves that were established using derivatized sodium sulfide solutions in water. A 50 μM derivatized stock solution was used to make the calibration standards by a serial dilution using the calibration blank solution, which was deionized water treated the same way as the standards. Plasma H2S levels were measured in the Day 7 samples from all subjects treated with ATB‐346 and from 10 subjects (selected using a random number generator) from the naproxen‐treated group (Day 7 samples). The demographics of this subset of naproxen‐treated subjects were six female/four male, of whom seven were categorized for race as “White,” one was “Asian,” one was “Black or African American,” and one was “Other: West Indian.” The mean age was 41.7, and the mean body weight was 62.5 kg.
2.5. Data and statistical analysis
For the primary endpoint of the study, the sample size was powered to obtain a significant difference in endoscopically observed gastric or duodenal ulcers between the two study groups (n = 120 subjects per group) at the end of 2 weeks of daily treatment. The study was powered based on a predicted incidence of ulceration in 30 (25%) of the naproxen‐treated subjects based on a review of several similar endoscopic clinical trials of naproxen that have been published (Bias, Buchner, Klesser, & Laufer, 2004; Goldstein, Aisenberg, Zakko, Berger, & Dodge, 2008; Goldstein, Johanson, Hawkey, Suchower, & Brown, 2007; Goldstein et al., 2003; Goldstein, Lowry, Lanza, Schwartz, & Dodge, 2006; Moberly et al., 2007; Scheiman et al., 2004; Simon et al., 1998; Wilder‐Smith, Jonzon, Fornstedt‐Wallin, Hedman, & Karlsson, 2006).
The study employed a safety analysis set, which included all subjects who had received at least one dose of the drug being investigated.
The primary endpoint was analysed using the conventional chi‐squared test with a significance level of P < 0.05 to determine any difference between naproxen and ATB‐346 in the incidence of endoscopically detected gastric or duodenal ulcers of at least 3‐mm diameter with unequivocal depth. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. Demographics
Of the 258 subjects enrolled, 244 (94.6%) completed the study: 126 subjects in the naproxen group and 118 subjects in the ATB‐346 group. In the ATB‐346 group, the mean age of trial subjects was 42.7 years, and 53.5% were female; 51.9% of the subjects were Caucasian, 29.5% were Asian, 14% were Black or African American, 0.8% were American Indian or Alaska Native, and 3.9% were “other.” With respect to ethnicity, 12.4% of subjects identified themselves as “Hispanic or Latino,” and 87.6% identified themselves as “Not Hispanic or Latino.”
In the naproxen group, the mean age of trial subjects was 40.7 years, and 55.8% were female; 54.3% of the subjects were Caucasian, 27.9% were Asian, 14% were Black or African American, 0.8% were American Indian or Alaska Native, and 3.1% were “other.” With respect to ethnicity, 9.3% of subjects identified themselves as “Hispanic or Latino,” and 90.7% identified themselves as “Not Hispanic or Latino.”
3.2. Primary endpoint
Of the 126 subjects treated with naproxen, 53 had at least one ulcer greater than 3 mm in diameter. Of the 118 subjects treated with ATB‐346, three had at least one ulcer greater than 3 mm in diameter. The incidence of ulcers between the two groups was significantly different (P < 0.05; Figure 1).
Figure 1.
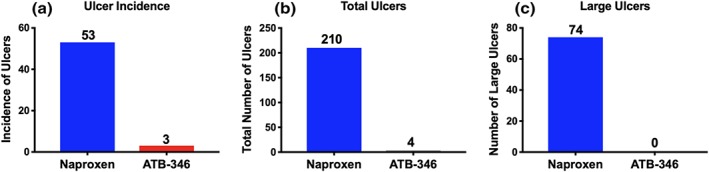
Subjects treated with naproxen developed significantly more gastroduodenal ulcers than those treated with ATB‐346. (a) The incidence of ulceration was markedly higher in the naproxen group than in the ATB‐346 group (P < 0.05). (b) The total number of ulcers in the naproxen group was >50 times that in the ATB‐346 group. (c) Ulcers greater than 5 mm in diameter were observed in 24% of naproxen‐treated subjects (average of 2.5 large ulcers per affected subject), while none were observed in the ATB‐346‐treated subjects
There were also far more gastric ulcers (50‐fold) in the naproxen group than in the ATB‐346 group. There was a total of 210 gastric ulcers observed in the 53 subjects that developed ulcers, while in the ATB‐346 group, there was a total of only four gastric ulcers in the three subjects that developed ulcers.
Ulceration of the duodenal mucosa was also observed in seven of the subjects treated with naproxen (with an average of 3.4 duodenal ulcers per subject), while duodenal ulcers were not observed in any of the subjects treated with ATB‐346.
3.3. Secondary endpoints
3.3.1. Large ulcers
Twenty‐four per cent of the naproxen‐treated subjects had gastric ulcers larger than 5 mm in diameter (with an average of 2.5 large ulcers per subject). There were no large gastric ulcers in the ATB‐346 group (Figure 1).
3.3.2. Erosions
These are small, superficial lesions (not penetrating through the muscularis mucosae) which are considered much less clinically significant than ulcers (Greaves, 2012). In the ATB‐346 group, there was an average of 1.7 erosions per subject versus 12.7 erosions per subject in the naproxen group.
3.3.3. Haematocrit
There were no significant changes in haematocrit (from baseline) between the two treatment groups at the end of the 2‐week treatment period. The mean haematocrits in the naproxen‐treated and ATB‐346‐treated groups were the same at the beginning and end of the trial (0.41 ± 0.04).
GI disorders were reported more frequently in subjects treated with naproxen (n = 36, 28%) than in subjects receiving ATB‐346 (n = 20, 15%). Twenty‐two subjects (17.5%) receiving sodium naproxen experienced GI/dyspepsia‐related symptoms during the study period compared to 10 subjects (8.5%) receiving ATB‐346. The other principal adverse events reported were abdominal pain/distension (6.2% in the naproxen group and 1.6% in the ATB‐346 group), gastro‐oesophageal reflux disease (4.7% naproxen and 0% ATB‐346), and nausea (3.1% naproxen and 0% ATB‐346; Figure 2). There was no significant difference in the incidence of constipation/diarrhoea in the two groups (7.0% ATB‐346 and 5.5% naproxen).
Figure 2.
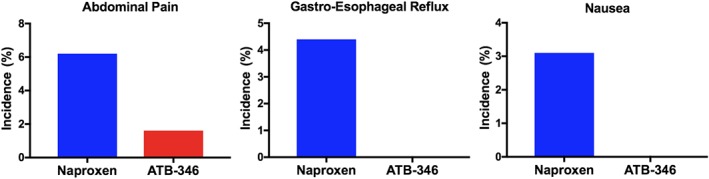
Treatment with ATB‐346 was associated with lower rates of gastrointestinal symptoms, such as abdominal pain, gastro‐esophageal reflux, and nausea, than was observed with naproxen treatment
3.4. COX inhibition
Thromboxane is a substance produced mainly by blood platelets, via the enzyme COX. COX is the main target enzyme for the anti‐inflammatory and analgesic effects of NSAIDs, so measuring the effects of naproxen and ATB‐346 on whole blood Tx generation is a good index of the extent of inhibition of COX that has been achieved after administration of an NSAID (Vane, 1971). As mentioned above, COX‐1 and COX‐2 produce the same PG (PGH2). As shown in Figure 3, both ATB‐346 and naproxen profoundly suppressed COX activity (>94%) after 1 and 2 weeks of treatment, with no significant differences between the two groups.
Figure 3.
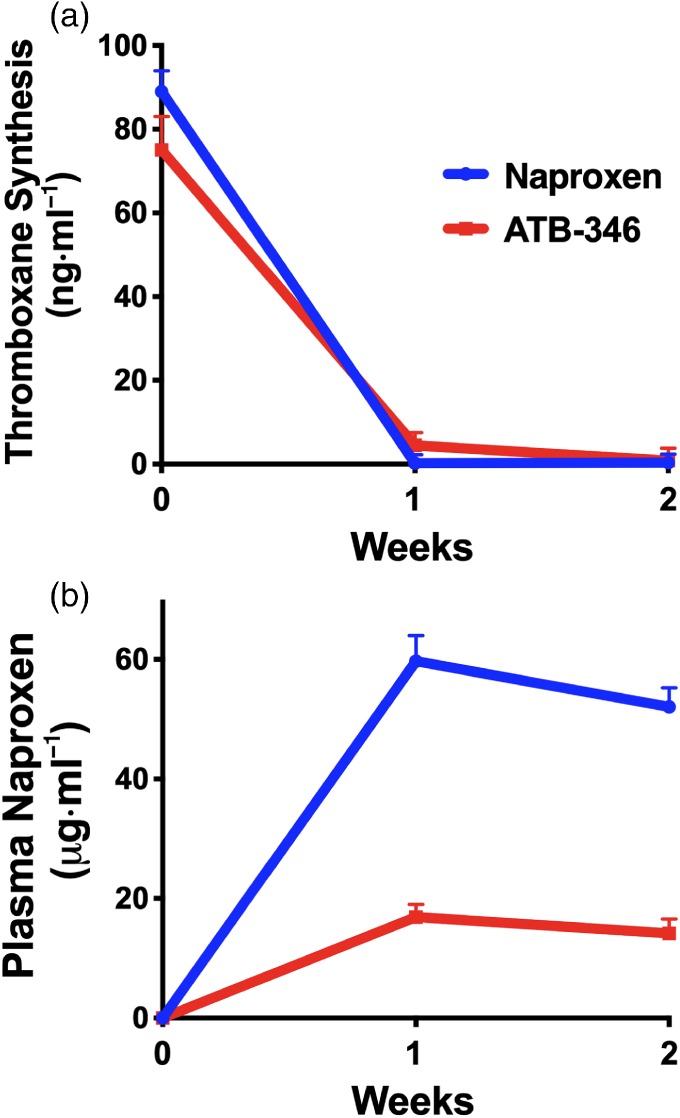
(a) Treatment with naproxen or ATB‐346 produced substantial (>95%) inhibition of whole blood thromboxane (TxB2) synthesis—an assay for COX activity. There were no significant differences between the two groups at any time point. (b) Plasma levels of naproxen were substantially lower in the subjects treated with ATB‐346 than in the subjects treated with naproxen. Results are shown as mean ± SEM
3.5. Plasma naproxen levels
After 1 and 2 weeks of administration of naproxen to healthy subjects, the mean plasma naproxen concentrations were 59.7 and 52.1 μg·ml−1, respectively (Figure 3). At the same time points in the group treated with ATB‐346, the mean plasma naproxen concentrations were 16.9 and 14.2 μg·ml−1, respectively. This difference was anticipated since the 250 mg dose of ATB‐346 contains approximately one sixth the amount of naproxen as the 550 mg, twice‐daily dose of naproxen. Also, the pharmacokinetics of naproxen derived from ATB‐346 differ substantially from that following administration of naproxen itself (Wallace et al., 2018).
3.6. Plasma H2S levels
Plasma levels of H2S levels in subjects treated with ATB‐346 were significantly greater (P < 0.05) than those in subjects treated with naproxen (Figure 4). Plasma H2S levels were measured by a modified MBB method (see Section 2), which has been extensively optimized and validated in the laboratory of Dr. Peter Nagy, by dissecting all potential parameters that could influence the values measured (Ditrói et al., unpublished observations). It is important to emphasize that because sulfide is irreversibly alkylated during this protocol (as with most other techniques used to measure sulfide concentration), only a part of the values measured represents free sulfide. The other part is a small fraction (small under our tightly controlled conditions) of the biomolecule‐bound sulfide pool, which is captured during incubation of the samples with MBB (Bogdandi et al., 2019). As we proposed and demonstrated experimentally (Nagy et al., 2014), all procedures that irreversibly bind or consume free sulfide from biological samples will trigger the liberation of sulfide from the endogenous sulfide pool. Hence, this biomolecule‐bound sulfide pool serves as a sulfide buffer system by responding to concentration changes via shifting chemical equilibria (Nagy et al., 2014). Importantly, when sulfide is consumed in a biological process, the sulfide pool will also likely serve as a slow endogenous sulfide donor system and maintain steady‐state sulfide levels via similar mechanisms. Therefore, the differences measured by these methods are highly relevant and represent the sum of differences in (a) free sulfide levels and (b) the ability of the sulfide pool to release sulfide.
Figure 4.
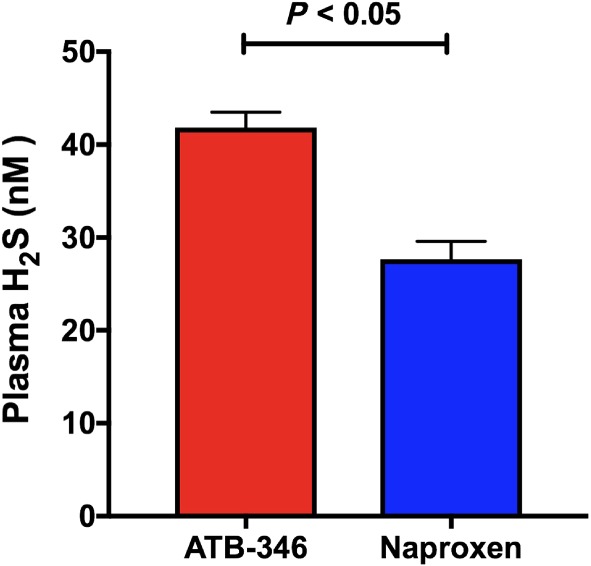
Plasma levels of hydrogen sulfide (H2S) were significantly higher in subjects treated with ATB‐346 (n = 122) than in subjects treated with naproxen (n = 10, randomly selected). Results are shown as mean ± SEM
3.7. Liver‐related effects
Blood levels of liver enzymes (including alanine transaminase and aspartate transaminase) were measured on Days 7 and 14 of treatment and at 2 weeks post‐treatment (Day 28). Over the 14‐day treatment period, clinically insignificant, treatment‐related transient elevations in liver transaminases were observed in 7% of subjects receiving ATB‐346 and 7% of subjects receiving naproxen. One subject receiving ATB‐346 had clinically significant, treatment‐related, transient transaminase elevations during this period. At the 2‐week post‐treatment assessment, 5.4% of the ATB‐346‐treated subjects had clinically significant, treatment‐related, transient transaminase elevations that had resolved or were resolving. Cumulative data from the three clinical trials in which ATB‐346 has been administered at 250 mg once daily for 10–14 days reveal a 4.7% overall incidence of clinically significant increases in liver transaminases. This is comparable to what has been observed with the use of NSAIDs such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2714, naproxen, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7273 (~4%) and well below that observed with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239 (39%; National Institute of Health, n.d.). One naproxen‐treated subject had clinically significant elevations of alkaline phosphatase and total bilirubin at the end of treatment (Day 14).
3.8. BP effects
NSAIDs can cause significant elevations of systemic BP (hypertension), which is an important risk factor for stroke and cardiovascular disease (Mackenzie & MacDonald, 2010). BP measurements were made at various points throughout the study period. No significant changes in systolic or diastolic BP were observed among subjects in either treatment group (Figure 5).
Figure 5.
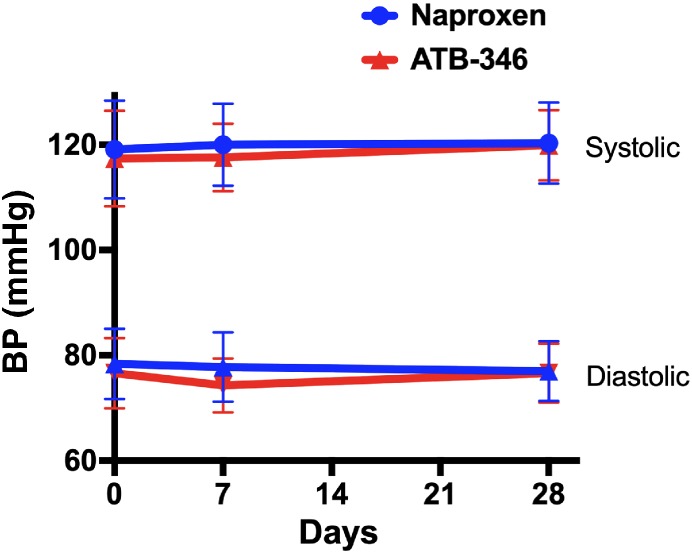
Treatment with naproxen (550 mg twice daily; n = 126) or ATB‐346 (250 mg daily; n = 118) did not significantly affect systolic or diastolic BP. Subjects were treated from Day 1 to Day 14, with a 2‐week follow‐up. Results are shown as mean ± SD
4. DISCUSSION
GI bleeding and ulceration continue to be the major limitation to the use of NSAIDs for treating the pain and inflammation associated with numerous disorders, including osteoarthritis, rheumatoid arthritis, and gout. Despite the introduction of drugs that suppress gastric acid secretion (e.g., proton pump inhibitors and histamine H2 receptor antagonists) and NSAIDs that exhibit selectivity for the COX‐2 enzyme, NSAID gastroenteropathy remains the most common adverse effect of this class of drugs. There are also significant and dangerous polypharmacy issues surrounding the use of NSAIDs with low‐dose aspirin and drugs that suppress gastric acid secretion (Lanas et al., 2009; Wallace, 2013; Wallace et al., 2011).
ATB‐346 is a novel anti‐inflammatory drug developed by Antibe Therapeutics Inc. that combines two key actions: suppression of COX activity and release of H2S (Wallace et al., 2010). The latter provides GI protection from the adverse effects of suppression of COX. The ability of ATB‐346 to inhibit COX without causing significant GI damage has been demonstrated in extensive laboratory studies (Blackler et al., 2012; Blackler, De Palma, et al., 2015; Blackler, Motta, et al., 2015; Flannigan et al., 2013; Motta et al., 2015; Wallace et al., 2007; Wallace et al., 2010; Wallace et al., 2018). Importantly, the GI safety of ATB‐346 was demonstrated in several models of impaired GI mucosal defence, which more closely approximate the susceptibility of patients to the GI‐damaging effects of NSAIDs. However, until now, the GI safety of ATB‐346 had not been directly examined in humans.
This randomized Phase 2 study demonstrated superior safety and GI outcomes of ATB‐346, compared to naproxen, in healthy subjects. A dramatic reduction in ulcer incidence (94%) was observed in subjects treated with ATB‐346. Naproxen and ATB‐346 produced profound and comparable suppression of COX activity. COX is the main target for drugs of the NSAID class that are the primary medications used to treat the symptoms of osteoarthritis. As observed previously (Wallace et al., 2018), plasma naproxen levels in subjects treated with ATB‐346 were considerably lower than those in subjects treated with naproxen. A 250‐mg tablet of ATB‐346 contains only one sixth the amount of naproxen that was administered each day to the subjects treated with naproxen. However, as demonstrated in a previous Phase 2 clinical trial, this dose of ATB‐346, given only once a day, produced marked reduction of pain in osteoarthritis patients (Wallace et al., 2018). Also, as shown in the present study and in the previous Phase 1 and Phase 2 clinical trials, once‐daily dosing with ATB‐346 produced profound suppression of COX activity, comparable to what was observed with twice‐daily dosing with 550‐mg sodium naproxen. The effectiveness of once‐daily dosing of ATB‐346 is a favourable commercial characteristic with respect to patient compliance.
In addition to the profound reduction in ulcer formation with ATB‐346 versus naproxen, subjects treated with ATB‐346 exhibited significantly lower incidences of several GI symptoms associated with the use of conventional NSAIDs. For example, the incidence of GI/dyspepsia‐related symptoms, abdominal pain, gastro‐oesophageal reflux disease, and nausea was either markedly reduced or completely absent in the subjects treated with ATB‐346 versus those treated with naproxen. This is a significant finding because these types of symptoms can often lead to patient non‐compliance. The reduced dyspepsia with ATB‐346 may be related to the ability of this drug to rapidly stimulate bicarbonate secretion in the stomach and duodenum, as has been observed in rats (Blackler, Gemici, Manko, & Wallace, 2014; Takeuchi et al., 2012).
A significant elevation of plasma H2S levels was observed in the subjects treated with ATB‐346, versus those treated with naproxen. Plasma levels of H2S in the naproxen‐treated subjects (on Day 7, when steady‐state plasma naproxen levels would have been achieved) were 28 ± 2 nM. The finding that subjects treated with ATB‐346 exhibited an increase in plasma H2S levels of ~50% (P < 0.05) serves as confirmation that the drug produced a biologically relevant elevation of this mediator in humans, which could contribute to both its analgesic and cytoprotective effects. Previous studies in animal models have demonstrated significant analgesic effects of H2S donors (Cenac et al., 2016; Fiorucci et al., 2007; Wallace et al., 2015).
Studies in rats (Wallace et al., 2010; Wallace et al., 2015) and humans (unpublished) have shown that ATB‐346 is very rapidly metabolized after oral administration, and inhibition of COX activity can be observed almost immediately. It is likely that there is rapid formation of at least one active metabolite of ATB‐346. The blood concentrations of naproxen generated from ATB‐346 are not sufficient to account for the COX inhibition that is observed (with respect to both potency and duration of inhibition). Analgesic effects of naproxen are observed with plasma levels of >40 μg·ml−1 (typical steady‐state levels of naproxen with 550 mg twice‐daily dosing are ~40–70 μg·ml−1; Sevelius, Runkel, Segre, & Bloomfield, 1980). In the present studies, the plasma levels of naproxen in the naproxen‐treated subjects were ~50–60 μg·ml−1. With ATB‐346 treatment, steady‐state naproxen levels in plasma were only ~14–17 μg·ml−1, well below the threshold to produce significant analgesic effects (Sevelius et al., 1980). Analysis of ATB‐346 metabolism in humans has revealed the presence of several naproxen‐like structures with possible inhibitory effects on COX. These ATB‐346 metabolites are presently being characterized to establish a better understanding of the mechanisms contributing to the substantial increase in potency and duration of activity of the drug.
In summary, this Phase 2B endoscopy trial has demonstrated a dramatic reduction in upper GI ulcer formation in subjects taking ATB‐346 versus naproxen, the latter being among the most commonly used drugs for treatment of osteoarthritis and other painful conditions. The two drugs produced comparable suppression of COX activity (a biomarker for pain relief and reduced inflammation). Plasma H2S levels were significantly elevated in the subjects treated with ATB‐346. Future studies will be aimed at determining if lower doses of ATB‐346 can produce significant reductions in osteoarthritis‐associated pain and inflammation.
CONFLICT OF INTEREST
J.L.W. and D.J.V. are employees of Antibe Therapeutics Inc. A.G.B. and G.d.N. are members of the Scientific Advisory Board of Antibe Therapeutics.
AUTHOR CONTRIBUTIONS
J.L.W. contributed to the design of the study, collection of data, analysis of data, and writing of manuscript. P.N., T.D., and A.G.B. contributed to the collection of data, analysis of data, and writing of manuscript. T.D.F., T.A., and M.N.M. contributed to the collection of data and analysis of data. D.J.V. and G.d.N. contributed to the design of the study, collection of data, and analysis of data.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical reporting as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
P. N. is grateful for financial support from the Hungarian National Research, Development and Innovation Office under Grants KH17_126766 and K 129286.
Wallace JL, Nagy P, Feener TD, et al. A proof‐of‐concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide‐releasing anti‐inflammatory drug. Br J Pharmacol. 2020;177:769–777. 10.1111/bph.14641
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman, E. M. (2017). Evaluating the cardiovascular safety of nonsteroidal anti‐inflammatory drugs. Circulation, 135, 2062–2072. 10.1161/CIRCULATIONAHA.117.027288 [DOI] [PubMed] [Google Scholar]
- Bias, P. , Buchner, A. , Klesser, B. , & Laufer, S. (2004). The gastrointestinal tolerability of the LOX/COX inhibitor, licofelone, is similar to placebo and superior to naproxen therapy in healthy volunteers: Results from a randomized, controlled trial. The American Journal of Gastroenterology, 99, 611–618. 10.1111/j.1572-0241.2004.04133.x [DOI] [PubMed] [Google Scholar]
- Blackler, R. , Syer, S. , Bolla, M. , Ongini, E. , & Wallace, J. L. (2012). Gastrointestinal‐sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS ONE, 7, e35196 10.1371/journal.pone.0035196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackler, R. W. , De Palma, G. , Manko, A. , Da Silva, G. J. , Flannigan, K. L. , Bercik, P. , … Wallace, J. L. (2015). Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide‐releasing NSAID. American Journal of Physiology. Gastrointestinal and Liver Physiology, 308, G994–G1003. 10.1152/ajpgi.00066.2015 [DOI] [PubMed] [Google Scholar]
- Blackler, R. W. , Gemici, B. , Manko, A. , & Wallace, J. L. (2014). NSAID‐gastroenteropathy: New aspects of pathogenesis and treatment. Current Opinion in Pharmacology, 19, 11–16. 10.1016/j.coph.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Blackler, R. W. , Motta, J. P. , Manko, A. , Workentine, M. , Bercik, P. , Surette, M. G. , & Wallace, J. L. (2015). Hydrogen sulphide protects against NSAID‐enteropathy through modulation of bile and the microbiota. British Journal of Pharmacology, 172, 992–1004. 10.1111/bph.12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdandi, V. , Ida, T. , Sutton, T. Y. , Biaco, C. , Ditroi, T. , Koster, G. , … Nagy, P. (2019). Speciation of reactive sulfur species and their reactions with alkylating agents: Do we have any clue about what is present inside the cell? British Journal of Pharmacology, 176(4), 646–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher M. A Bayesian meta‐analysis of longitudinal data in placebo controlled studies with naproxen. (2008) http://www.page-meeting.org/pdf_assets/8269-Bayesian%20Meta%20Analysis%20Final.pdf
- Cenac, N. , Castro, M. , Desormeaux, C. , Colin, P. , Sie, M. , Ranger, M. , & Vergnolle, N. (2016). A novel orally administered trimebutine compound (GIC‐1001) is anti‐nociceptive and features peripheral opioid agonistic activity and hydrogen sulphide‐releasing capacity in mice. European Journal of Pain, 20, 723–730. 10.1002/ejp.798 [DOI] [PubMed] [Google Scholar]
- Fiorucci, S. , Orlandi, S. , Mencarelli, A. , Caliendo, G. , Santagada, V. , Distrutti, E. , … Wallace, J. L. (2007). Enhanced activity of a hydrogen sulphide‐releasing derivative of mesalamine (ATB‐429) in a mouse model of colitis. British Journal of Pharmacology, 150, 996–1002. 10.1038/sj.bjp.0707193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannigan, K. L. , Ferraz, J. G. , Wang, R. , & Wallace, J. L. (2013). Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: A site‐specific, pro‐resolution mechanism. PLoS ONE, 8, e71962 10.1371/journal.pone.0071962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. L. , Aisenberg, J. , Zakko, S. F. , Berger, M. F. , & Dodge, W. E. (2008). Endoscopic ulcer rates in healthy subjects associated with use of aspirin (81 mg q.d.) alone or coadministered with celecoxib or naproxen: A randomized, 1‐week trial. Digestive Diseases and Sciences, 53, 647–656. 10.1007/s10620-007-9903-4 [DOI] [PubMed] [Google Scholar]
- Goldstein, J. L. , Johanson, J. F. , Hawkey, C. J. , Suchower, L. J. , & Brown, K. A. (2007). Clinical trial: Healing of NSAID‐associated gastric ulcers in patients continuing NSAID therapy—A randomized study comparing ranitidine with esomeprazole. Alimentary Pharmacology & Therapeutics, 26, 1101–1111. 10.1111/j.1365-2036.2007.03460.x [DOI] [PubMed] [Google Scholar]
- Goldstein, J. L. , Kivitz, A. J. , Verburg, K. M. , Recker, D. P. , Palmer, R. C. , & Kent, J. D. (2003). A comparison of the upper gastrointestinal mucosal effects of valdecoxib, naproxen and placebo in healthy elderly subjects. Alimentary Pharmacology & Therapeutics, 18, 125–132. 10.1046/j.1365-2036.2003.01650.x [DOI] [PubMed] [Google Scholar]
- Goldstein, J. L. , Lowry, S. C. , Lanza, F. L. , Schwartz, H. I. , & Dodge, W. E. (2006). The impact of low‐dose aspirin on endoscopic gastric and duodenal ulcer rates in users of a non‐selective non‐steroidal anti‐inflammatory drug or a cyclo‐oxygenase‐2‐selective inhibitor. Alimentary Pharmacology & Therapeutics, 23, 1489–1498. 10.1111/j.1365-2036.2006.02912.x [DOI] [PubMed] [Google Scholar]
- Greaves, P. (2012). Histopathology of preclinical toxicity studies (Fourth ed.). Amsterdam: Academic Press. [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. (2015). Physiological roles of hydrogen sulfide and polysulfides. Handbook of Experimental Pharmacology, 230, 61–81. 10.1007/978-3-319-18144-8_3 [DOI] [PubMed] [Google Scholar]
- Lanas, A. , García‐Rodríguez, L. A. , Polo‐Tomás, M. , Ponce, M. , Alonso‐Abreu, I. , Perez‐Aisa, M. A. , … Garcia, S. (2009). Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. The American Journal of Gastroenterology, 104, 1633–1641. 10.1038/ajg.2009.164 [DOI] [PubMed] [Google Scholar]
- Mackenzie, I. S. , & MacDonald, T. M. (2010). Treatment of osteoarthritis in hypertensive patients. Expert Opinion on Pharmacotherapy, 11, 393–403. 10.1517/14656560903496422 [DOI] [PubMed] [Google Scholar]
- Moberly, J. B. , Harris, S. I. , Riff, D. S. , Dale, J. C. , Breese, T. , McLaughlin, P. , … Truitt, K. E. (2007). A randomized, double‐blind, one‐week study comparing effects of a novel COX‐2 inhibitor and naproxen on the gastric mucosa. Digestive Diseases and Sciences, 52, 442–450. 10.1007/s10620-006-9521-6 [DOI] [PubMed] [Google Scholar]
- Motta, J. P. , Flannigan, K. L. , Agbor, T. A. , Beatty, J. K. , Blackler, R. W. , Workentine, M. L. , … Wallace, J. L. (2015). Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflammatory Bowel Diseases, 21, 1006–1117. 10.1097/MIB.0000000000000345 [DOI] [PubMed] [Google Scholar]
- Nagy, P. , Palinkas, Z. , Nagy, A. , Budai, B. , Toth, I. , & Vasas, A. (2014). Chemical aspects of hydrogen sulfide measurement in physiological samples. Biochimica et Biophysica Acta, 1840, 867–891. [DOI] [PubMed] [Google Scholar]
- National Institute of Health : LiverTox database: Retrieved from https://livertox.nlm.nih.gov/Diclofenac.htm, n.d.
- Nissen, S. E. , Yeomans, N. D. , Solomon, D. H. , Lüscher, T. F. , Libby, P. , Husni, M. E. , … PRECISION Trial Investigators (2016). Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. The New England Journal of Medicine, 375, 2519–2529. 10.1056/NEJMoa1611593 [DOI] [PubMed] [Google Scholar]
- Robert, A. (1979). Cytoprotection by prostaglandins. Gastroenterology, 77, 761–767. [PubMed] [Google Scholar]
- Scheiman, J. M. , Cryer, B. , Kimmey, M. B. , Rothstein, R. I. , Riff, D. S. , & Wolfe, M. M. (2004). A randomized, controlled comparison of ibuprofen at the maximal over‐the‐counter dose compared with prescription‐dose celecoxib on upper gastrointestinal mucosal injury. Clinical Gastroenterology and Hepatology, 2, 290–295. 10.1016/S1542-3565(04)00057-6 [DOI] [PubMed] [Google Scholar]
- Sevelius, H. , Runkel, R. , Segre, E. , & Bloomfield, S. S. (1980). Bioavailability of naproxen sodium and its relationship to clinical analgesic effects. British Journal of Clinical Pharmacology, 10, 259–263. 10.1111/j.1365-2125.1980.tb01753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein, F. E. , Faich, G. , Goldstein, J. L. , Simon, L. S. , Pincus, T. , Whelton, A. , … Geis, G. S. (2000). Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study. JAMA, 284, 1247–1255. 10.1001/jama.284.10.1247 [DOI] [PubMed] [Google Scholar]
- Simon, L. S. , Lanza, F. L. , Lipsky, P. E. , Hubbard, R. C. , Talwalker, S. , Schwartz, B. D. , … Geis, G. S. (1998). Preliminary study of the safety and efficacy of SC‐58635, a novel cyclooxygenase 2 inhibitor: Efficacy and safety in two placebo‐controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis and Rheumatism, 41, 1591–1602. [DOI] [PubMed] [Google Scholar]
- Takeuchi, K. , Aihara, E. , Kimura, M. , Dogishi, K. , Hara, T. , & Hayashi, S. (2012). Gas mediators involved in modulating duodenal HCO3 − secretion. Current Medicinal Chemistry, 19, 43–54. 10.2174/092986712803413962 [DOI] [PubMed] [Google Scholar]
- Vane, J. R. (1971). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin‐like drugs. Nature: New Biology, 231, 232–235. [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. (2008). Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn't the stomach digest itself? Physiological Reviews, 88, 1547–1565. 10.1152/physrev.00004.2008 [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. (2013). Polypharmacy of osteoarthritis: The perfect intestinal storm. Digestive Diseases and Sciences, 58, 3088–3093. 10.1007/s10620-013-2777-8 [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , Blackler, R. W. , Chan, M. V. , Da Silva, G. J. , Elsheikh, W. , Flannigan, K. L. , … Buret, A. G. (2015). Anti‐inflammatory and cytoprotective actions of hydrogen sulfide: Translation to therapeutics. Antioxid Redox Signal, 22, 398–410. [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , Caliendo, G. , Santagada, V. , & Cirino, G. (2010). Markedly reduced toxicity of a hydrogen sulphide‐releasing derivative of naproxen (ATB‐346). British Journal of Pharmacology, 159, 1236–1246. 10.1111/j.1476-5381.2009.00611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, J. L. , Dicay, M. , McKnight, W. , & Martin, G. R. (2007). Hydrogen sulfide enhances ulcer healing in rats. The FASEB Journal, 21, 4070–4076. 10.1096/fj.07-8669com [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , Syer, S. , Denou, E. , de Palma, G. , Vong, L. , McKnight, W. , … Ongini, E. (2011). Proton pump inhibitors exacerbate NSAID‐induced small intestinal injury by inducing dysbiosis. Gastroenterology, 141, 1314–1322. 10.1053/j.gastro.2011.06.075 [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , Vaughan, D. , Dicay, M. , MacNaughton, W. K. , & de Nucci, G. (2018). Hydrogen sulfide‐releasing therapeutics: Translation to the clinic. Antioxidants & Redox Signaling, 28, 1533–1540. [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , Vong, L. , McKnight, W. , Dicay, M. , & Martin, G. R. (2009). Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology, 137, 569–578. 10.1053/j.gastro.2009.04.012 [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , & Wang, R. (2015). Hydrogen sulfide‐based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nature Reviews. Drug Discovery, 14, 329–345. 10.1038/nrd4433 [DOI] [PubMed] [Google Scholar]
- Wilder‐Smith, C. H. , Jonzon, B. , Fornstedt‐Wallin, B. , Hedman, A. , & Karlsson, P. (2006). Dose‐effect comparisons of the CINOD AZD3582 and naproxen on upper gastrointestinal tract mucosal injury in healthy subjects. Scandinavian Journal of Gastroenterology, 41, 264–273. 10.1080/00365520510024197 [DOI] [PubMed] [Google Scholar]
- Wittenberg, R. H. , Schell, E. , Krehan, G. , Maeumbaed, R. , Runge, H. , Schluter, P. , … Trechsel, U. (2006). First‐dose analgesic effect of the cyclo‐oxygenase‐2 selective inhibitor lumiracoxib in osteoarthritis of the knee: A randomized, double‐blind, placebo‐controlled comparison with celecoxib [NCT00267215]. Arthritis Research & Therapy, 8, R35 10.1186/ar1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans, N. D. , Tulassay, Z. , Juhász, L. , Rácz, I. , Howard, J. M. , van Rensburg, C. J. , … Hawkey, C. J. (1998). A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid suppression trial: Ranitidine versus omeprazole for NSAID‐associated ulcer treatment (ASTRONAUT) study group. The New England Journal of Medicine, 338, 719–726. 10.1056/NEJM199803123381104 [DOI] [PubMed] [Google Scholar]


