Abstract
Background and Purpose
A clinical need exists for targeted, safe, and effective sulfide donors. We recently reported that ammonium tetrathiomolybdate (ATTM) belongs to a new class of sulfide‐releasing drugs. Here, we investigated the cellular uptake mechanisms of this drug class compared to sodium hydrosulfide (NaHS) and the effects of a thiometallate tungsten congener of ATTM, ammonium tetrathiotungstate (ATTT).
Experimental Approach
In vitro H2S release was determined by headspace gas sampling of vials containing dissolved thiometallates. Thiometallate and NaHS bioactivity was assessed by spectrophotometry‐derived sulfhaemoglobin formation. Cellular uptake dependence on the anion exchange protein (AE)‐1 was investigated in human red blood cells. ATTM/glutathione interactions were assessed by LC–MS/MS. Rodent pharmacokinetic and pharmacodynamic studies focused on haemodynamics and inhibition of aerobic respiration.
Key Results
ATTM and ATTT both exhibit temperature‐, pH‐, and thiol‐dependence of sulfide release. ATTM/glutathione interactions revealed the generation of inorganic and organic persulfides and polysulfides. ATTM showed greater ex vivo and in vivo bioactivity over ATTT, notwithstanding similar pharmacokinetic profiles. Cellular uptake mechanisms of the two drug classes are distinct; thiometallates show dependence on AE‐1, while hydrosulfide itself was unaffected by inhibition of this pathway.
Conclusions and Implications
The cellular uptake of thiometallates relies upon a plasma membrane ion channel. This advances our pharmacological knowledge of this drug class, and further supports their utility as cell‐targeted sulfide donor therapies. Our results indicate that, as a more stable form, ATTT is better suited as a copper chelator. ATTM, a superior sulfide donor, may additionally participate in intracellular redox recycling.
Linked Articles
This article is part of a themed section on Hydrogen Sulfide in Biology & Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.4/issuetoc
Abbreviations
- AE‐1
Anion exchange protein‐1
- ATN‐224
bis‐choline tetrathiomolybdate
- ATTM
ammonium tetrathiomolybdate
- ATTT
ammonium tetrathiotungstate
- GSSH
glutathione persulfide
- GSSSH/GSSSSH
glutathione polysulfide(s)
- H2DIDS
4′diisothiocyanato‐dihydrostilbene‐2,2′‐disulfonic acid
- H2S
hydrogen sulfide gas
- HS−
hydrosulfide anion
- MoS42−
tetrathiomolybdate ion
- MRM
multiple reaction monitoring
- NaHS
sodium hydrosulfide
- Na2S
sodium sulfide
- PaCO2
arterial partial pressure of carbon dioxide
- PaO2
arterial partial pressure of oxygen
- PK/PD
pharmacokinetics/pharmacodynamics
- sHb
sulfhaemoglobin
- tHb
total Hb
- WS42−
tetrathiotungstate ion.
What is already known
A clinical need exists for safe and cell‐targeted sulfide donors to treat ischaemia/reperfusion injury.
The copper chelator, ammonium tetrathiomolybdate (ATTM), is a sulfide donor with novel properties.
What this study adds
We discovered that the cellular uptake mechanism of thiometallates rely on anion exchanger‐1 in human erythrocytes.
ATTM undergoes significant thiol interactions that promote sulfide release and generate per‐ and polysulfides.
What is the clinical significance
Our mechanistic evidence suggests that thiometallates act via a targeted intracellular mechanism of action.
The potential participation of thiometallates in redox recycling could be applicable to other oxidative pathologies.
1. INTRODUCTION
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9532 (comprising H2S and HS−) is the third endogenous gasotransmitter alongside NO and carbon monoxide and acts as a signalling molecule across numerous physiological systems (Kimura, 2014; Szabo et al., 2014). Given exogenously in preclinical studies, sulfide protected against diverse pathological conditions, ranging from circulatory (Wang, Wang, Guo, & Zhu, 2011), neurodegenerative (Zhang & Bian, 2014) and arthritic (Wu et al., 2016) disorders to diabetes (Wu et al., 2009), pain (Di Cesare Mannelli et al., 2017), and cancer (Lee et al., 2014). Our particular interest in sulfide relates to its ability to transiently reduce cellular respiration by inhibition of mitochondrial cytochrome C oxidase (Szabo et al., 2014). This, and the consequent reduction of mitochondria‐derived ROS production, confers protection in states of ischaemia/reperfusion injury, hypoxia, and circulatory shock (Blackstone & Roth, 2007; Elrod et al., 2007; Morrison et al., 2008).
Notwithstanding more than a decade of promising preclinical research, only a handful of sulfide‐based therapies have entered clinical trials (Wallace, Vaughan, Dicay, MacNaughton, & de Nucci, 2017); none have yet proven successful in randomised phase 2/3 studies. Numerous delivery methods have been investigated, focusing initially on the use of simple sulfur salts such as sodium sulfide (Na2S). However, these salts release sulfide in a rapid, uncontrolled fashion with resulting implications for safety and efficacy. More recently, drug design of sulfide‐based therapeutics has advanced significantly, with identification of numerous slow‐release sulfide “donors” and sulfur‐hybrid molecules. These aim for more controlled sulfide delivery and improved targeting to its intended site of action (Szabo & Papapetropoulos, 2017).
We and others recently reported that inorganic thiometallates represent a new class of sulfide‐releasing drugs (Dyson et al., 2017; Xu et al., 2016) that confer protection against ischaemia/reperfusion injury (Dyson et al., 2017). These thiometallates consist of a transition metal core and four covalently bound sulfur atoms, and cleavage of the metal–sulfur bonds enables these molecules to act as slow‐release sulfide donors. The archetypical thiometallate, tetrathiomolybdate, was first synthesised nearly 200 years ago (Berzelius, 1826). The ammonium salt, ATTM ([NH4]2MoS4), has proven efficacy as a copper chelator, having been developed and used off‐label for the treatment of Wilson's disease (Brewer et al., 2009). Notably, the rate(s) of hydrolysis of molecules from this class depend on numerous intrinsic and extrinsic factors (Lee, Schulman, Stiefel, & Lee, 2007); this ultimately determines their suitability as sulfide donors or copper chelators.
Given the potential utility of thiometallates for indications not necessarily related to the depletion of copper, we investigated other compounds within this class. Here, we report on the chemistry and pharmacology of the tungsten analogue, ATTT ([NH4]2WS4), in comparison to its lighter homologue and the “standard” sulfide generator, sodium hydrosulfide (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6278). In view of the need for sulfide mimetics that are better targeted, we explored mechanisms of cellular uptake by both thiometallates and NaHS. We hypothesised that (a) thiometallates utilise non‐selective anion channels to gain intracellular access due to their ionic nature in solution and (b) ATTT would represent a new member of the sulfide‐releasing thiometallate drug class. Finally, we elaborate on two methods developed as part of this work, and provide a detailed description of our H2S release assay and the measurement of sulfhaemoglobin, and present pilot data on the interaction of ATTM with oxidised and reduced http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737.
2. METHODS
2.1. In vitro H2S release
As noted above, free (biologically active and physiologically relevant) sulfide constitutes H2S gas and the hydrosulfide (HS−) anion (May, Batka, Hefter, Königsberger, & Rowland, 2018). The assay, developed in‐house and described in detail herein, relies on the detection of free H2S that is measured by a commercially available H2S detector (Z900XP, Environmental Sensors, Boca Raton, FL, USA). Further elaboration on the protocol is provided in Supplementary Data S1. In brief, the compound under investigation is dissolved in PBS to 10× stock solutions and rapidly diluted (0.5 into 4.5 ml) into airtight Falcon tubes (50 ml; Corning Science Mexico, Reynosa, Mexico) containing PBS. The PBS is typically pre‐warmed to 37°C but can be adjusted as necessary (e.g., to different temperatures and pH levels or to contain thiols, other adjuvants, or alternative matrices). The compound is then incubated in a water bath (typically for 1 hr at 37°C). Withdrawal of 5 ml of headspace gas is done over 10 s and this is passed through the detector using a three‐way tap to accommodate the syringe (closed to room air until aspiration of the gas); this is attached to the detector inlet, as depicted in the inset of Figure 1a.
Figure 1.
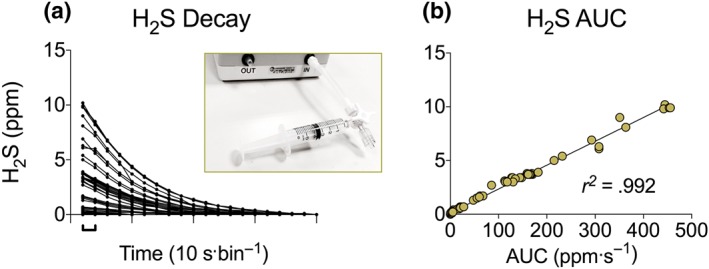
H2S release test. Comparison of (a) H2S values over time and (b) the AUC during washout. Inset picture in (a) shows the apparatus for H2S detection. Values recorded are in p.p.m
The Z900XP H2S detector displays a reading every 10 s. During the first series of experiments, we recorded both peak H2S gas levels (in p.p.m.) and values during the washout phase until the meter displayed zero. A direct correlation was seen when comparing the peak H2S value (Figure 1a) against the sum of the washout readings (decay) over time (AUC; Figure 1b). As such, it is appropriate to present only the peak H2S gas level. In the current study, we adjusted the environment into which thiometallate stocks were diluted as follows: Temperature was set to either 4, 21, 37, or 50°C and solution pH to 4.5, 7.4, or 10. As we previously observed sulfide released by ATTM to be enhanced by the presence of thiols, a further set contained reduced glutathione (GSH) (5‐mmol·L−1 final concentration). Final concentrations of drug solutions (1‐ to 100‐mmol·L−1 total sulfur) and time of incubation (30–180 min) were also adjusted under standard conditions (pH 7.4, 37°C).
2.2. Thiol interactions
We further investigated the interaction of ATTM with glutathione using H2S generation following incubation with either reduced or oxidised glutathione (GSH or GSSG respectively; 5 mmol·L−1). We additionally monitored the fall in absorbance of ATTM at 468 nm (A468; indicative of breakdown of the molecule and liberation of sulfide) using a microplate reader and BioTek (Gen5) software (Synergy 2, North Star Scientific, Sandy, Beds, UK). For this study, ATTM (175 μmol·L−1) ± GSH or GSSG (both 5 mmol·L−1) were combined in conditions designed to replicate an intracellular environment (pH 6.8 and 37°C). A468 was assessed every 10 min for 800 min. In our final assessment of ATTM/glutathione interactions, the formation of hydrogen disulphide, inorganic polysulfides, and organic per/polysulfides (up to S4) were assessed by LCMS/MS. We additionally studied Na2S and the oxomolybdate, [NH4]2MoO4 as controls. Further details are described in Supplementary Data S2.
2.3. Sulfhaemoglobin formation
To assess plasma membrane transport and subsequent intracellular biological activity, we examined the formation of sulfhaemoglobin in blood spiked with either ATTT, ATTM, or NaHS. The assay developed and described in detail herein is based on the various forms of Hb (oxy‐, deoxy‐, carboxy‐, met‐, and sulf‐) having distinct absorbance signatures. Figure 2a shows that oxyhaemoglobin is highly absorbent at 541 and 577 nm. The sulfhaemoglobin spectrum, while flatter at these wavelengths, has an additional absorbance peak at 620 nm. Notably, there is no interference from ATTM or ATTT, or the colourless NaHS, at these wavelengths.
Figure 2.
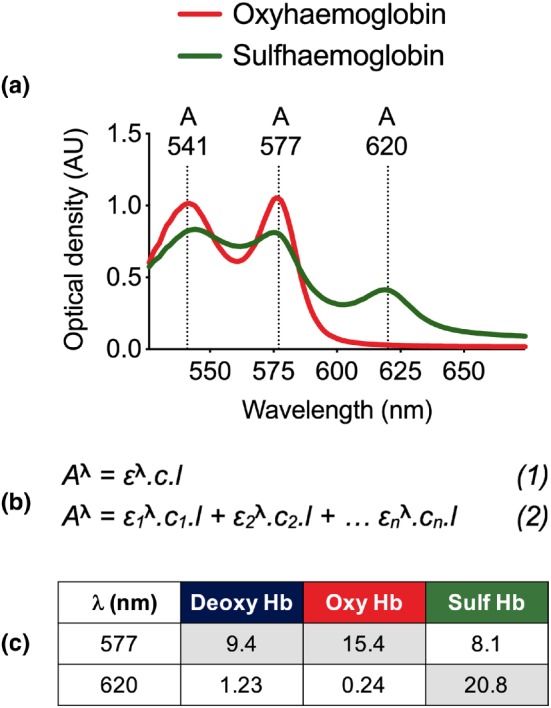
Sulfhaemoglobin assay. (a) Spectrophotometry‐derived absorbance spectrum of oxyhaemoglobin and sulfhaemoglobin. (b) Equations for the calculation of absorbance for (1) one and (2) an infinite number (n) of dissolved chromophores. (c) Extinction coefficients for deoxy Hb, oxy Hb, and sulf Hb (Zwart et al., 1981). Coefficients required for calculation of total‐ and sulfhaemoglobin are shown in shaded cells. λ, wavelength. Deoxy Hb, deoxyhaemoglobin; Oxy Hb, oxyhaemoglobin; Sulf Hb, sulfhaemoglobin
Absorbance of any given chromophore is calculated according to the Beer–Lambert law (Figure 2b, Equation 1) where A is absorbance at a particular wavelength (λ), c is concentration of the chromophore, l is the pathlength, and ε is an extinction co‐efficient (known also as millimolar lineic absorbance) specific to the wavelength and chromophore(s) of interest. The contribution of ≥2 chromophores to absorbance can be derived according to Equation 2 (Figure 2b). For oxy‐, deoxy‐, and sulfhaemoglobin, ε values at 577 and 620 nm are shown in Figure 2c, obtained from Zwart et al. (1981).
Calculation of sulfhaemoglobin can be broadly divided into two components. The first requires an assessment of the relative contributions of oxy‐ and deoxyhaemoglobin to spectrophotometer‐derived total Hb (tHb) measurements in either untreated or baseline samples. This is accomplished using Equation 2 (Figure 2b) and ε values at 577 nm (Figure 2c). To assess the fractions of oxy‐ and deoxyhaemoglobin, we used a blood gas analyser (ABL90 FLEX, Radiometer, Crawley, West Sussex, UK). In studies where blood gas analyses were not available, the following standard values were used for arterial blood: partial pressure of oxygen (13 kPa), deoxyhaemoglobin (5%), and oxyhaemoglobin (95%). The second component concerns manual measurement of spectrophotometer‐derived sulfhaemoglobin (sHb) in treated samples, achieved using absorbance and ε values at 620 nm. The fraction of sulfhaemoglobin is then calculated as sHb/tHb that we herein express as a percentage.
We used fresh venous whole blood obtained from consenting healthy human volunteers, collected into EDTA‐containing (20 ml) syringes (final concentration; 2.5 mmol·L−1). This venous blood (typically 15 ml) was subsequently transferred to 50‐ml Falcon tubes (with room air in the Falcon headspace) and placed on a rotary shaker for 10 min; this enables oxygenation to a representative arterial partial pressure of oxygen (13 kPa). Whole blood (400 μl) was then incubated with 50 μl of either ATTM, ATTT, or NaHS and 50 μl of PBS. In non‐drug treated samples (used both as negative controls and as a reference for total Hb), the drug was replaced with PBS. All samples were incubated at 37°C and followed two treatment protocols: a dose–response and a time course. For the dose–response study, upon dilution in blood, and having accounted for the four sulfur atoms of the thiometallates, final concentrations of total sulfur were 1–100 mmol·L−1, with incubation time set at 1 hr. For the time course, all groups were set at the highest concentration (100‐mmol·L−1 total sulfur) and incubated for 15–360 min. After incubation, a 1/20 dilution in PBS was performed, and 100 μl was transferred to 96 well microplates. Absorbance values were derived using the above microplate reader and software, with calculation of sulfhaemoglobin performed as above.
2.4. Cellular uptake studies
We considered that the thiometallates would prevail in their respective ionic forms (i.e., MoS4 2−, WS4 2−) upon dissolution. Additionally, the majority (85% at physiological pH and temperature) of the free sulfide generated by NaHS, or liberated by the thiometallates, would be present as the hydrosulfide anion. We thus investigated the role of the human erythrocyte http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=170#904 as a putative cellular uptake mechanism. Whole human blood (400 μl) was pretreated for 30 min with either 50 μl of the AE‐1 inhibitor, H2DIDS (4′diisothiocyanato‐dihydrostilbene‐2,2′‐disulfonic acid; 0.5 mmol·L−1), or an equivalent volume of PBS. Following pretreatment, 50 μl of either ATTT, ATTM, or NaHS were added (100‐mmol·L−1 total sulfur), and samples were incubated for either 15 min (NaHS) or 180 min (thiometallates) at 37°C, prior to measurement of sulfhaemoglobin.
In separate studies using the two thiometallates (1.25 mmol·L−1; final concentration), blood samples (with or without H2DIDS pretreatment) were incubated for 180 min then centrifuged (1,900× g, 1 min). Excess drug was removed by twice substituting the supernatant with an equivalent volume of PBS. Blood cells were then lysed by subjecting samples to three freeze–thaw cycles and filtered to remove plasma membrane fragments and proteins (10‐kDa nominal molecular weight limit microfilters; MerckMillipore, Watford, Herts, UK). Extracellular (from the initial supernatant) and intracellular (following lysis and filtration) drug levels were assessed by spectrophotometry. Absorbance peaks at 392 and 468 nm were used, respectively, for ATTT and ATTM, and concentrations were determined by comparison against standard curves. Studies were not performed using NaHS as it has no distinct colouration. All experiments utilising H2DIDS were carried out in amber Eppendorf tubes due to the light sensitivity of this molecule.
2.5. Pharmacokinetic/pharmacodynamic studies
To compare the in vivo pharmacology of the thiometallates, we performed pharmacokinetic/pharmacodynamic (PK/PD) studies. These experiments were performed according to local ethics committee and UK Home Office guidelines under the Animals (Scientific Procedures) Act 1986. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Male Wistar rats (approximately 300‐g body weight) were used as mice markedly reduce their metabolism within hours in response to systemic challenges, whereas rats more closely mimic human responses (Zolfaghari, Pinto, Dyson, & Singer, 2013). As drug responses often vary between species, the use of mice would likely confound a translational evaluation of metabolism‐modifying agents.
Twelve animals were purchased from Charles River (Margate, Kent UK) and certified healthy and pathogen‐free. One week prior to experimentation, animals were housed in standard cages of four individuals on a 12‐hr light/dark cycle, with food and water ad libitum. All animals were anaesthetised with isoflurane in room air (Abbott, Maidenhead, Berks, UK); 5% for induction, 2% for surgical procedures, and 1.5% for maintenance. They were placed on a heated mat (Harvard Apparatus, Cambridge, Cambs, UK) to maintain rectal temperature at 37°C. The left common carotid artery and right internal jugular vein were cannulated using 0.96 mm outside diameter polyvinyl chloride tubing catheter (Scientific Commodities Inc., Lake Havasu City, AZ, USA). The arterial line was connected to a pressure transducer (Powerlab; AD Instruments, Chalgrove, Oxon, UK) for continuous monitoring of mean arterial BP, and the venous line was used for administration of fluids and drugs. The bladder was cannulated through a keyhole laparotomy to measure urine output and renal excretion of thiometallates. Anaesthesia was then switched to i.p. sodium pentobarbitone 10 mg·kg−1 (Pentoject; Animalcare, York, Yorks, UK), allowing BP guided tracheal intubation with 2.08‐mm external diameter polythene tubing (Portex Ltd, Hythe, UK) and subsequent mechanical ventilation using a small animal ventilator (Physiosuite, Kent Scientific, Torrington, USA). Ventilator settings were as follows: fraction of inspired oxygen, 0.21; tidal volume, 10 ml·kg−1; respiratory rate, 80 min−1; positive end‐expiratory pressure, 3‐cmH2O. These settings ensured adequate post‐surgical gas exchange and consistent minute volumes across all animals used. Perioperative analgesia was provided by buprenorphine, 0.05 mg·kg−1 s.c. (Reckitt Benckiser, Slough, Berks, UK). In these non‐recovery experiments, the animals were killed at the end of the experiment using i.v. sodium pentobarbitone.
Following surgery and a 1‐hr stabilisation period, animals received increasing i.v. bolus doses of ATTT or ATTM (1–100 mg·kg−1, n = 6 per group). Drugs were dissolved in normal (0.9%) saline and administered (within 2 min) in a volume of 2 ml·kg−1 over 10 s. H2S in exhaled breath was monitored by connecting the ventilator exhaust to the (above) H2S detector. Measurements were collected at baseline, then as follows after each dose: BP and exhaled H2S (peak change or level) within 30 s, cardiac function (by echocardiography) within 1 min, blood sampling to determine plasma drug concentration at 2 min, and arterial blood gas analysis (to measure partial pressures of O2 [PaO2] and CO2 [PaCO2], glucose, lactate, and acid/base changes) at 27 min. Doses were escalated every 30 min. After the highest dose, the half‐life of both drugs was assessed by sampling at 2 min (for maximal concentration) then at regular intervals up to 1‐hr post‐dose. Urine was collected hourly. Absorbance of plasma or urine samples was assessed using the microplate reader at the wavelength specific to each drug (as above) and concentrations derived by comparison against standard curves. To determine fractional renal excretion, a mass‐balance approach was utilised. This was accomplished by calculation of the number of moles of each drug excreted in urine, expressed as a fraction against the number of moles injected.
2.6. Data and statistical analysis
Data are presented as mean ± SEM or median, quartiles, and range. Group sizes were n = 5–14 for in vitro and human blood studies and n = 6 for in vivo experiments. Correlations were performed using a non‐linear regression, straight line model. PK data were analysed using a biexponential decay curve and least squares fitting method. Parametric data were analysed using repeated measures one‐ or two‐way ANOVA followed by Bonferroni's post hoc testing, as appropriate. Non‐parametric data were analysed using the Mann–Whitney U‐test. All statistical analyses were two‐tailed and performed using Prism 7.0.1 software (GraphPad Software, CA, USA; RRID:SCR_002798). The data and statistical analysis comply with recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Multiplicity adjusted P values < 0.05 were considered statistically significant.
2.7. Materials
We sourced thiometallates (Sigma‐Aldrich, Gillingham, Kent, UK) and NaHS (Alfa Aesar, Heysham, Lancs, UK) from specific suppliers as they provide, respectively, good consistency between batches and the purest form available commercially (Hughes, Centelles, & Moore, 2009). Although we have found a low level of variability across numerous thiometallate batches, we would urge other researchers to establish their own H2S release assays if using these molecules as sulfide donors. Particular attention should be drawn to storing and handling these materials in a moisture‐ and oxygen‐free environment. H2DIDS was obtained from Thermofisher (Hemel Hempstead, Herts, UK). All other chemicals and reagents were obtained from Sigma‐Aldrich.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. In vitro H2S gas release
We report here the novel finding that ammonium tetrathiotungstate is a new member of the sulfide‐releasing inorganic thiometallate drug class. However, it releases only half the amount of free sulfide compared to ATTM under standard incubation conditions (Figure 3a; n = 25, pooled data from the control arms of b–f). This pattern was maintained under different experimental environments (all n = 5). Both thiometallates release sulfide in a concentration‐dependent (Figure 3b; P < 0.05) and linear fashion over time (Figure 3c; P < 0.05). Like ATTM, ATTT releases sulfide in a temperature (P < 0.05), thiol (P < 0.05), and pH‐dependent (P < 0.05) manner (Figure 3d–f). Warmer and more acidic conditions, and the presence of thiols, promote liberation of sulfide from these molecules.
Figure 3.
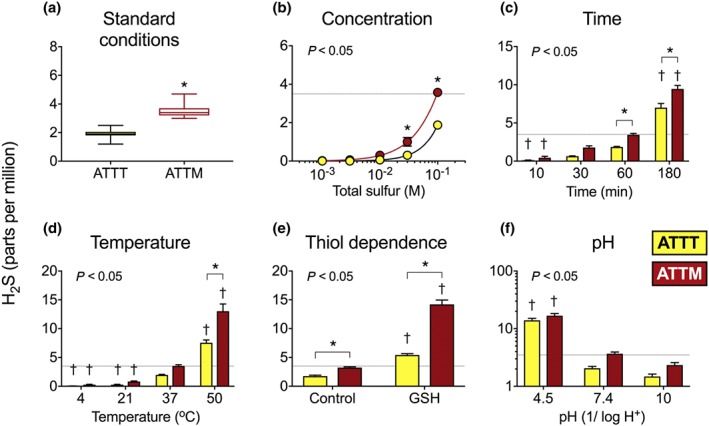
In vitro H2S release from ATTM and ATTT under different experimental conditions. In (a), standard conditions indicate that drugs (100‐mmol·L−1 total sulfur; n = 25; pooled data from the control arms of b–f) were incubated for 1 hr at physiological pH (7.4) and temperature (37°C). (b) Concentration‐dependence of drugs incubated for 1 hr (n = 5). Effects of time, temperature, the presence of thiols (GSH), and variations in pH are shown, respectively, in (c–f); all n = 5. The dotted lines reflect typical H2S gas levels (4 ppm) obtained from ATTM (100‐mmol·L−1 total sulfur) following 1‐hr incubation at normal physiological pH and temperature. Statistics: two‐way ANOVA followed by Bonferroni's test. Stated P values are the result of the overall ANOVA. *P < 0.05 between ATTM and ATTT and † P < 0.05 for each drug compared to standard conditions
3.2. Thiol interactions
Results of further investigations into the interaction between ATTM and glutathione are shown in Figure S1. H2S generation was significantly enhanced (P < 0.05) by glutathione, regardless of its oxidation state (Figure S1A; n = 5). The breakdown kinetics of ATTM were further monitored by temporal decreases in absorbance at 468 nm. Figure S1B shows the kinetics of the reaction (untreated; n = 14) that were significantly (overall ANOVA; P < 0.05) enhanced by the addition of either GSH (n = 8) or GSSG (n = 6). Finally, preliminary data of ATTM/glutathione interactions, at either 21 or 37°C, revealed the formation of glutathione persulfide (GSSH; S2) and polysulfides (S3–S4; Figure S1C); in addition, hydrogen disulfide and inorganic polysulfides were detected (data not shown). As expected, this reaction was also observed with Na2S, while the oxomolybdate, [NH4]2MoO4, showed only spontaneous generation of glutathione persulfide that was two to three orders of magnitude lower compared to that seen with ATTM; the source of sulfur to form GSSH in these experiments is unclear but likely originates from metal‐driven desulfuration reactions.
3.3. Sulfhaemoglobin formation and cellular uptake
We assessed (intracellular) sulfhaemoglobin formation as an indicator of plasma membrane transport to examine cellular uptake mechanisms. All three drugs generated sulfhaemoglobin in a concentration‐dependent manner (Figure 4a; n = 5). Sulfhaemoglobin formation by the thiometallates was also dependent on time, with ATTM exhibiting significantly (P < 0.05) more bioactivity than ATTT between 60 and 360 min (Figure 4b; n = 5). By contrast to both ATTM and ATTT, NaHS produced sulfhaemoglobin at significantly (P < 0.05) higher levels in the dose‐response study and was significantly (P < 0.05) quicker in the time course experiment (Figure 4a,b). Pretreatment of blood with the AE‐1 inhibitor H2DIDS significantly reduced sulfhaemoglobin formation by both ATTT (Figure 4c; P < 0.05; n = 9) and ATTM (Figure 4d; P < 0.05; n = 6 with H2DIDS, n = 9 without). AE‐1 inhibition had no effect on sulfhaemoglobin formation in NaHS‐treated samples (Figure 4e; P = 0.29; n = 7).
Figure 4.
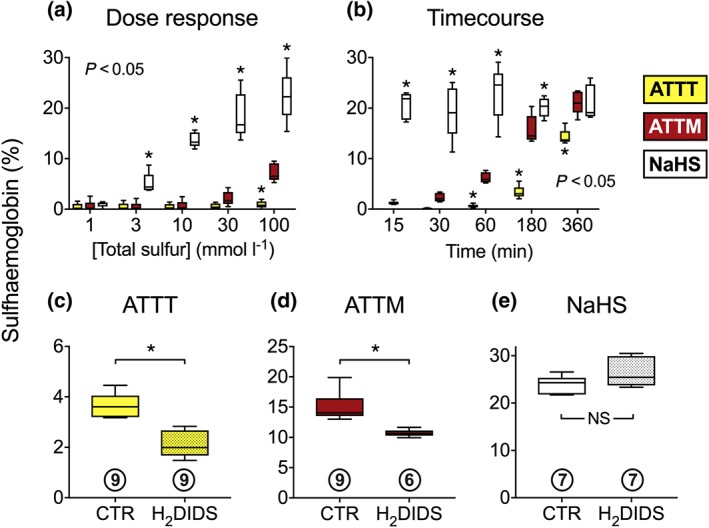
Formation of sulfhaemoglobin in human erythrocytes. A dose‐response (incubation for 1 hr) and time course (all drugs at 100‐mmol·L−1 total sulfur) are shown in (a) and (b), respectively; all n = 5. The sensitivity of sulfhaemoglobin formation to (30 min) pretreatment with the anion exhanger‐1 inhibitor (H2DIDS; 4′diisothiocyanato‐dihydrostilbene‐2,2′‐disulfonic acid; 0.5 mmol·L−1) is shown in panels (c–e). Sample sizes are shown in circles beneath each data set. Total sulfur for each drug was 100 mmol·L−1. Incubation time was 3 hr for the thiometallates (in c and d) and 15 min for NaHS (e). Statistics: (a and b) two‐way ANOVA followed by Bonferroni's test. Stated P values are the result of the overall ANOVA. *P < 0.05 versus ATTM (multiple comparisons). c–e, *P < 0.05, unpaired non‐parametric t‐test. NS, not significant
The distinct spectral signatures of the thiometallates (Figure 5a) allow for their quantification using basic absorbance assays. To confirm the use of the AE‐1 transporter by thiometallates using an alternative approach, we assessed the disappearance from the plasma of blood treated with these drugs and, in the same samples, the appearance of drugs inside erythrocytes. A similar pattern was observed for both thiometallates, whereby pre‐incubation with H2DIDS reduced both the disappearance from plasma (Figure 5b, ATTT; Figure 5c, ATTM; both P < 0.05; all n = 5) and detection of their presence intracellularly (Figure 5d, ATTT, Figure 5e, ATTM; both P < 0.05; all n = 5). The measurement most sensitive to H2DIDS treatment was the intracellular appearance of the drugs, with AE‐1 inhibition reducing ATTT and ATTM concentrations by 73% and 65%, respectively.
Figure 5.
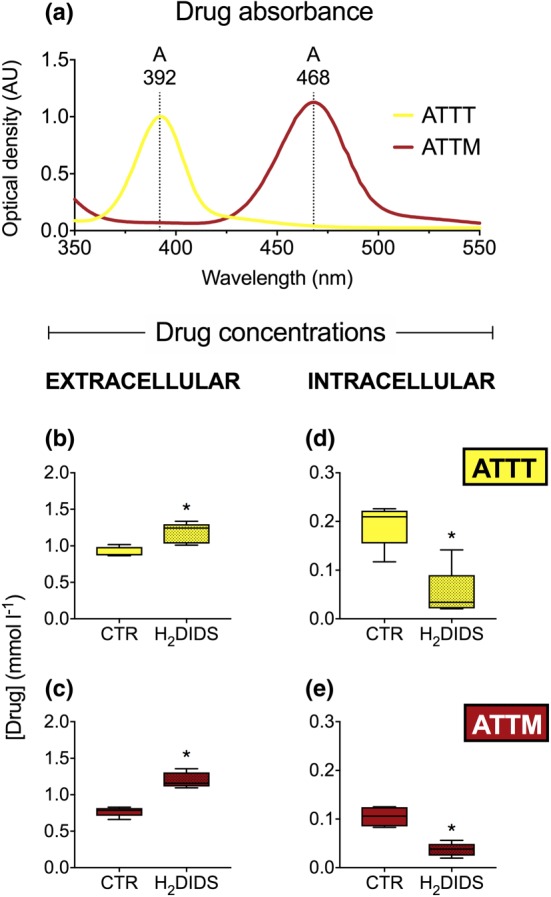
Extra‐ and intracellular concentrations of thiometallates. Absorbance spectrum of ATTT and ATTM is shown in (a) with λ MAX values of 392 and 468 nm, respectively. Drug concentrations detected extracellularly are shown in panels (b) and (c) (both n = 5). Corresponding intracellular measurements are shown in (d) and (e) (both n = 5). 4′diisothiocyanato‐dihydrostilbene‐2,2′‐disulfonic acid (H2DIDS; 0.5 mmol·L−1). *P < 0.05, unpaired non‐parametric t‐test
To confirm no direct chemical interaction occurred between thiometallates and H2DIDS in these experiments, we additionally performed a H2S release assay (n = 6; Figure S2A) and a spectrophotometric time course of ATTM absorbance (n = 6; Figure S2B) with and without H2DIDS. These results showed no chemical interaction between these molecules (P = 0.97 and 0.98, respectively).
3.4. PK/PD studies
Eleven of the 12 animals studied survived until the end of the experiment protocol. One (ATTM‐treated) animal died from an accidental air embolus. Final group sizes were six for ATTT and five for ATTM. Both thiometallates caused a dose‐dependent metabolic acidaemia, demonstrated by falls in arterial pH (Figure 6a) and base excess (the metabolic component of acid–base interactions; Figure 6b). No changes in the respiratory component, arterial PaCO2, were observed in these mechanically ventilated animals (Figure 6c). The magnitude of these metabolic changes was significantly (P < 0.05) pronounced in ATTM‐treated animals; this was mirrored by a dose‐dependent rise in blood lactate (Figure 6d; P < 0.05) and fall in blood glucose (Figure 6e; P < 0.05), indicative of a compensatory increase in glycolysis (anaerobic respiration). ATTT‐treated animals only showed a rise in arterial lactate at the highest dose (Figure 6d), and this was not accompanied by any change in blood sugar (Figure 6e). Dose‐dependent falls in BP (Figure 6f) were observed only at supra‐pharmacological doses (≥30 mg·kg−1). However, this reduction was transient (typically <2 min), did not differ significantly between drugs (P = 0.34), and did not affect cardiac output at any dose level (Figure 6g; P = 0.12). No exhaled H2S was detected over the dose range studied.
Figure 6.
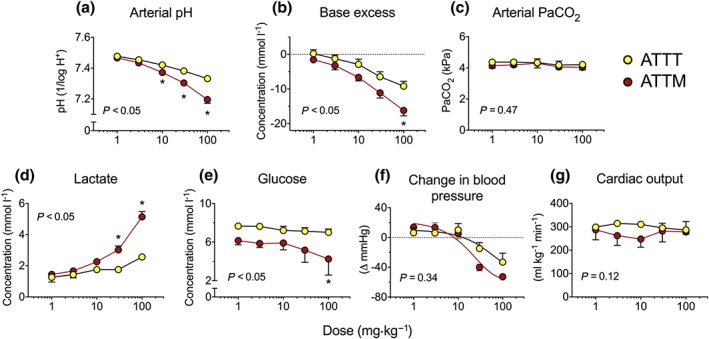
In vivo pharmacology of thiometallates. Panels (a–c), acid/base interactions with (a) arterial pH, (b) base excess (the metabolic component of acid/base status), and (c) arterial partial pressure of carbon dioxide (PaCO2; respiratory component). Blood lactate and glucose levels are shown in (d) and (e), respectively. Panel (f) shows maximal changes in mean arterial BP for each dose, while panel (g) depicts cardiac output, measured by echocardiographic pulsed‐wave Doppler. Group sizes: n = 6 for ATTT and n = 5 for ATTM. *P < 0.05; two‐way repeated measures ANOVA followed by Bonferroni's test. The P values stated are the result of the overall ANOVA
In order to determine if the PD differences between thiometallates (i.e., greater potency with ATTM) were due to the differences in drug handling, we performed PK analyses (Figure 7). Colouration of the thiometallates allowed for their quantification in blood and urine. Plasma concentrations of both drugs followed a direct linear correlation with the quantity administered in the dose range 1–100 mg kg−1 (Figure 7a). Assessment of the half‐life following administration of the highest dose revealed, as discovered previously for ATTM, a biexponential decay in plasma concentrations for both thiometallates (Figure 7b). Half‐lives were calculated for the fast (distribution) and slow (elimination) phases of these decay curves. These were, respectively, 1.28 and 23.4 min for ATTT, compared with 1.36 and 21.5 min for ATTM. An assessment of urine volume (Figure 7c; P = 0.93) and the number of moles in urine (data not shown) showed comparable results for ATTT and ATTM. Accordingly, percentage renal excretion was similar for both compounds (ATTT, 0.59 ± 0.11%; ATTM, 0.67 ± 0.23%; P = 0.75). Using acidification of the blood as a biomarker of mitochondrial inhibition, PK/PD analyses (Figure 7d) demonstrated that ATTM was twice as potent as ATTT; the PK/PD slope, that is, the relationship between plasma drug concentration and blood acidification (reduction in pH), was 0.14 ± 0.02 for ATTM and 0.07 ± 0.01 for ATTT (P < 0.05).
Figure 7.
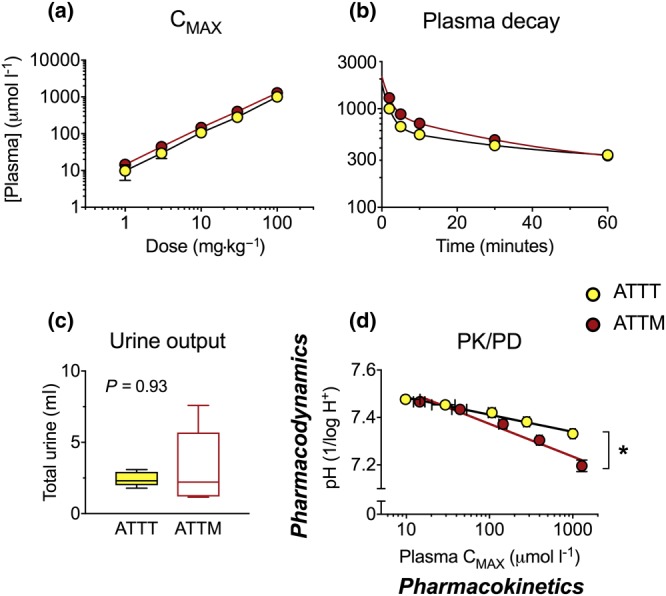
Pharmacokinetics. (a) The dependence of plasma concentration on i.v. dose level and (b) the decay in drug levels after the highest dose (100 mg·kg−1). (c) Urine output. NB, the number of moles in urine was the same for both compounds (data not shown). (d) The relationship between drug plasma levels (measured at 2‐min post‐administration) and subsequent (25 min later) changes in arterial pH. The slope in (d) is used as an indicator of drug potency. Group sizes: n = 6 for ATTT and n = 5 for ATTM. *P < 0.05; unpaired non‐parametric t‐test (urine output and slope in d). CMAX, maximum plasma concentration
4. DISCUSSION
The putative therapeutic utility of thiometallates has been investigated for over 40 years. The most frequently studied drug in this class, ATTM, has demonstrated preclinical efficacy in >10 diverse disease pathologies ranging from its initial indication, Wilson's disease, to Alzheimer's disease, pain, and cancer (Brewer, 2009, 2014). These studies assumed the mechanism of action to be via copper chelation, with examples that include the depletion of injurious levels of copper in Wilson's disease, to a reduction of copper‐dependent angiogenesis by solid tumours. However, in addition to its modulation of copper status, we and others recently shown ATTM to be a slow‐release sulfide donor (Dyson et al., 2017; Xu et al., 2016).
The interest in sulfide as a potential therapeutic surged following publication in 2005 of a landmark study in which mice breathing H2S entered a profound and reversible “suspended animation‐like state” (Blackstone, Morrison, & Roth, 2005). However, unsuccessful clinical trials using basic sulfur salts encouraged the notion that delivery of sulfide needs to be better targeted. This drove the design of more sophisticated sulfide‐releasing molecules (Szabo & Papapetropoulos, 2017; Yang et al., 2017) that, once activated, aim to release sulfide in a more controlled manner. We contended that the combination of chemically and biologically relevant factors promoting sulfide release by ATTM is unique (Dyson et al., 2017). These characteristics help to ensure that sulfide release is better targeted to its intended intracellular site of action. Coupled with the historical use of this drug class over many decades in humans, this has led us to investigate its utility in modifying reperfusion injury in a clinically relevant setting.
Given this therapeutic potential of ATTM, we explored other molecules within this drug class and, as remains largely unknown for other sulfide donors, potential mechanisms of cellular uptake. We examined the tungsten congener of ATTM, and in line with our hypothesis, we confirmed that ATTT also acts as a sulfide donor. In accordance with the similarities in chemical composition, and the proximity of molybdenum and tungsten in the periodic table, several parallels were seen with regard to their sulfide release profile. ATTT releases sulfide in a controlled fashion over time when dissolved in aqueous solution, and like ATTM, this is accelerated by heat, acidity, and the presence of thiols. However, across this wide range of environmental conditions, ATTT releases only around half the quantity of sulfur as sulfide compared to ATTM. In keeping with our findings, Lee et al. (2007) demonstrated that rates of hydrolysis of ATTT, at various pH levels, are significantly slower than those of ATTM. Hydrolysis of thiometallates, particularly in acidic environments, generates a vast array of polynuclear species. For example, following dilution of ATTM with equimolar HCl, the most abundant form (>40%) was the polynuclear anion, Mo3S9 2−, and this trimeric formation liberates free sulfide (Quagraine, Georgakaki, & Coucouvanis, 2009).
Unlike hydrolysis, the mechanism(s) of enhanced sulfide release from thiometallates by thiols requires further study. Focusing on ATTM, we expand on our previous findings here by demonstrating that not only reduced thiols but also their oxidised forms (shown here with GSSG) promote the release of sulfide from thiometallates. It is notable that ATTM and ATTT lie on a redox knife edge; they have highly oxidised metal centres bound to electron‐rich (and hence oxidisable) ligands and, as such, are able to interact with other compounds in a variety of redox states. In preliminary experiments, we showed that ATTM can interact with thiols to generate hydrogen disulfide and glutathione persulfide, as well as organic and inorganic polysulfides. These findings enrich our basic knowledge of ATTM; beyond acting as a metabolic‐modulating sulfide donor via partial inhibition of cytochrome C oxidase, “bioactivation” of thiometallates by thiols, with subsequent per‐ and polysulfide formation, raises the potential for significant interactions that involve intracellular redox biology. With regard to the putative efficacy of this compound for oxidative pathologies such as ischaemia/reperfusion injury, the ability of ATTM to react with oxidised thiols to generate powerful reducing molecules (intracellular redox recycling) is a novel, tantalising, and intriguing aspect of its molecular mode of action. The importance of this feature in physiological and pathophysiological settings will be subject to further investigation.
In view of the increased appreciation of the need for cell‐targeted sulfide donors, there is a surprisingly small amount of literature on cellular uptake mechanisms. Although there are considerable biological differences between water and gaseous hydrogen sulfide, their similarity in chemical structure has prompted the exploration of the ability of H2S to use plasma membrane aquaporins (Mathai et al., 2009). The authors found that no facilitator was required and, in accordance with the known lipid solubility of sulfide, concluded that simple diffusion occurs without enablement by membrane channels (Mathai et al., 2009). However, physiologically relevant free sulfide is maintained in equilibrium in two forms: H2S ↔ HS− with a pKa value of 6.76 (Liu et al., 2012). This indicates that around 85% of physiological sulfide is present as the hydrosulfide anion. As such, (and, notably, in addition to free diffusion) a role for the most ubiquitous ion transporter in human erythrocytes, AE‐1, has been suggested (Jennings, 2013).
The mechanism of cellular uptake by thiometallates has not been previously explored. Further to the rationale for studying the transfer of sulfide by ion exchange, the ionic nature of ATTT and ATTM following dissolution suggested that an anion exchanger would be a strong candidate. We initially used the formation of intracellular sulfhaemoglobin in intact human erythrocytes as an indicator of plasma membrane transport. Accordingly, pre‐incubation of human blood with the AE‐1 inhibitor, H2DIDS, prevented, at least in part, the formation of sulfhaemoglobin. Not surprisingly native sulfide (from NaHS) did not exclusively use this channel as diffusion permits rapid cellular entry (Jennings, 2013). To confirm the role of AE‐1 in cellular thiometallate uptake, we also measured intra‐ and extracellular concentrations of these drugs using a basic absorbance assay. H2DIDS treatment reduced both extracellular disappearance and intracellular detection of both thiometallates. Given that the formation of sulfhaemoglobin could not be fully prevented, it is conceivable that some free extracellular sulfide cleaved from these molecules may gain access by diffusion and contribute to its formation. Other ion channels and/or additional unstudied mechanisms may also be involved. However, given the substantial inhibition (65–73%) of intracellular detection, we consider that the AE‐1 is an important mechanism governing the targeted intracellular delivery of this class of drugs.
Although we focused our attention on the human erythrocyte AE‐1, this channel is present in various forms elsewhere (Redzic, 2011; Walsh & Stewart, 2010), including the α‐intercalated cells of the kidney, endothelial cells of the blood brain barrier, and a truncated variant in rat heart. This suggests that the AE‐1 uptake mechanism may be relevant for other target tissues.
As we have previously assessed the utility of thiometallates for the putative treatment of reperfusion injury, we examined the pharmacology of ATTT and ATTM in vivo, with a particular focus on haemodynamics and biomarkers of inhibition of aerobic respiration. The known hypotensive effect of sulfide‐releasing drugs, particularly at high concentrations, was observed here. This is attributed to free‐circulating sulfide that occurs via multiple putative mechanisms described in detail elsewhere (Liu et al., 2012). However, this hypotensive effect was transient in nature (<2 min), only present after supra‐pharmacological dosing and occurred in the absence of detectable exhaled H2S (indicative of high quantities of free‐circulating sulfide).
We monitored blood acid/base status and lactate levels as biomarkers of mitochondrial electron transport chain inhibition. By inhibiting oxidative phosphorylation, cells augment both net ATP hydrolysis and glycolysis. Both of these processes release H+ ions that contribute to (metabolic) acidaemia, and the latter to an excess of glycolysis‐derived pyruvate that, unable to enter the mitochondrion, undergoes dehydrogenation to lactate. Given the enhanced stability profile of ATTT, these effects were less pronounced than those observed with ATTM. We confirmed that these metabolic disparities were not due to differences in drug handling. There was a remarkably similar PK profile for both drugs; on comparison of the PK/PD relationship, we showed that ATTM is twice as potent as ATTT as a modulator of oxidative respiration.
The proposed mechanism of copper chelation by the thiometallates is the formation of a tripartite complex between the thiometallate anion, a metal (mainly copper, but to a lesser extent, iron, cobalt, and other metal ions), and a protein (e.g., albumin; Hou, Dick, Zeng, & Brewer, 2007). In addition to the nature of the metal, the hydrolysis rates of these molecules are also determined by the cation (Lee et al., 2007). In a previous study, we showed that ATN‐224, the bis‐choline salt of tetrathiomolybdate, released less sulfide compared to ATTM in vitro (Dyson et al., 2017). As a result, it was less effective as a metabolic modulator in vivo. This is considered desirable, as ATN‐224 is undergoing clinical development as a copper‐sequestering agent for Wilson's disease and shows some utility for treating cancer (Brewer, 2009, 2014; Lowndes et al., 2008). Our results here suggest that ATTT falls into the same category as ATN‐224, being better suited as a copper chelator over a sulfide donor. While this could potentially lessen its utility in ischaemia/reperfusion injury, hypoxia, and circulatory shock states, it could provide additional benefit in pathologies sensitive to depletion of copper.
With respect to ATTM, we have identified a safe and effective lead candidate for the treatment of ischaemia/reperfusion injury. Its sulfide release profile is better suited to this indication over other molecules (ATTT, bis‐choline tetrathiomolybdate) within this class. Importantly, given the appreciated need for sulfide drugs that are better targeted, we have demonstrated for the first time that thiometallates use non‐selective ion transporters to gain access to intracellular compartments. Moreover, the sulfide release profile of these drugs (sensitive to thiols and low pH) indicates that more sulfide is released intracellularly, particularly in ischaemic cells that exhibit lower intracellular pH levels and have a greater need for treatment.
CONFLICT OF INTEREST
A.D. and M.S. are developing thiometallates for the treatment of reperfusion injury.
AUTHOR CONTRIBUTIONS
T.D., D.Z., N.S., M.M., and A.D. did the experimental work. T.D., M.M., M.F., M.S., and A.D. wrote the manuscript. T.D. and A.D. performed the data analysis and statistics. A.D. did the data conception and designed and supervised the study. G.H., M.M., M.F., M.S., and A.D. did the academic input and knowledge transfer. All authors have read and approved the final manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Supplementary information S1 In vitro H2S gas release
Supplementary information S2 Detection of hydrogen di‐/polysulfides and glutathione per‐/polysulfides
Figure S1 Thiol interactions. (A) H2S release from ATTM following incubation with reduced (GSH) or oxidised (GSSG) glutathione (n=5). Values recorded are in parts per million (ppm). (B) Degradation of ATTM ± GSH (n=8) or GSSG (n=5) monitored by absorbance at 468 nm (l468). Untreated samples are n=14. (C) pilot data shows generation of glutathione persulfide and organic polysulfides following incubation of ATTM, the oxomolybdate, [NH4]2MoO4 and Na2S with either GSH or GSSG. Due to lack of authentic standards, data are shown as ‘peak areas’. *p<0.05, 1‐ or 2‐way ANOVA followed by Bonferroni's test. Stated p‐values are the result of overall ANOVA. AU, arbitrary units;
Figure S2 Chemical interaction of ATTM with H2DIDS. (A) H2S release from ATTM. Standard conditions apply; incubation of ATTM (25 mmol l‐1; 100 mmol l‐1 total sulfur) for 1h at physiological temperature and pH, with or without H2DIDS (0.5 mmol l‐1) (n=6). (B) Degradation of ATTM (175 μmol l‐1) ± H2DIDS (3.5 μmol l‐1) (n=6) monitored by absorbance at 468 nm (l468). Conditions here were designed to replicate an intracellular environment (37oC, pH 6.8). In both experiments, the ratio of ATTM to H2DIDS reflects that used in the sulfhaemoglobin studies. Statistics: (A) unpaired non‐parametric T‐test, (B) 2‐way ANOVA followed by Bonferroni's test. Stated p‐values are the result of the T‐test and overall ANOVA. AU, arbitrary units; H2DIDS, 4''diisothiocyanato‐dihydrostilbene‐ 2,2'‐disulfonic acid; ppm, parts per million; λ, wavelength.
Supporting information item
ACKNOWLEDGEMENTS
We acknowledge Dr. Alireza Mani (University College London, UK) for his advice regarding micropore filters and assessment of intracellular drug levels and Prof. Mike Jennings (University of Arkansas, USA) for his advice on the preparation and use of H2DIDS.
Durham T, Zander D, Stomeo N, et al. Chemistry, pharmacology, and cellular uptake mechanisms of thiometallate sulfide donors. Br J Pharmacol. 2020;177:745–756. 10.1111/bph.14670
REFERENCES
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The concise guide to pharmacology 2017/18: Transporters. British Journal of Pharmacology, 174(Suppl 1), S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzelius, J. J. (1826). Ueber die Schwefelsalze. Annalen der Physik, 83, 261–288. 10.1002/andp.18260830702 [DOI] [Google Scholar]
- Blackstone, E. , Morrison, M. , & Roth, M. B. (2005). H2S induces a suspended animation–like state in mice. Science, 308, 518–518. 10.1126/science.1108581 [DOI] [PubMed] [Google Scholar]
- Blackstone, E. , & Roth, M. B. (2007). Suspended animation‐like state protects mice from lethal hypoxia. Shock, 27, 370–372. 10.1097/SHK.0b013e31802e27a0 [DOI] [PubMed] [Google Scholar]
- Brewer, G. J. (2009). The use of copper‐lowering therapy with tetrathiomolybdate in medicine. Expert Opinion on Investigational Drugs, 18, 89–97. [DOI] [PubMed] [Google Scholar]
- Brewer, G. J. (2014). The promise of copper lowering therapy with tetrathiomolybdate in the cure of cancer and in the treatment of inflammatory disease. Journal of Trace Elements in Medicine and Biology, 28, 372–378. 10.1016/j.jtemb.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Brewer, G. J. , Askari, F. , Dick, R. B. , Sitterly, J. , Fink, J. K. , Carlson, M. , … Lorincz, M. T. (2009). Treatment of Wilson's disease with tetrathiomolybdate: V. control of free copper by tetrathiomolybdate and a comparison with trientine. Translational Research, 154, 70–77. 10.1016/j.trsl.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Bond, R. A. , Spina, D. , Ahluwalia, A. , Alexander, S. P. A. , Giembycz, M. A. , … McGrath, J. C. (2015). Experimental design and analysis and their reporting: New guidance for publication in BJP. British Journal of Pharmacology, 172, 3461–3471. 10.1111/bph.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare Mannelli, L. , Lucarini, E. , Micheli, L. , Mosca, I. , Ambrosino, P. , Soldovieri, M. V. , … Ghelardini, C. (2017). Effects of natural and synthetic isothiocyanate‐based H2S‐releasers against chemotherapy‐induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology, 121, 49–59. 10.1016/j.neuropharm.2017.04.029 [DOI] [PubMed] [Google Scholar]
- Dyson, A. , Dal‐Pizzol, F. , Sabbatini, G. , Lach, A. B. , Galfo, F. , Dos Santos Cardoso, J. , … Singer, M. (2017). Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLoS Medicine, 14, e1002310 10.1371/journal.pmed.1002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod, J. W. , Calvert, J. W. , Morrison, J. , Doeller, J. E. , Kraus, D. W. , Tao, L. , … Lefer, D. J. (2007). Hydrogen sulfide attenuates myocardial ischemia‐reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America, 104, 15560–15565. 10.1073/pnas.0705891104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, G. , Dick, R. , Zeng, C. , & Brewer, G. J. (2007). Antitumor and antiinflammatory effects of tetrathiotungstate in comparison with tetrathiomolybdate. Translational Research, 149, 260–264. 10.1016/j.trsl.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Hughes, M. N. , Centelles, M. N. , & Moore, K. P. (2009). Making and working with hydrogen sulfide. Free Radical Biology and Medicine, 47, 1346–1353. 10.1016/j.freeradbiomed.2009.09.018 [DOI] [PubMed] [Google Scholar]
- Jennings, M. L. (2013). Transport of H2S and HS− across the human red blood cell membrane: Rapid H2S diffusion and AE1‐mediated Cl−/HS− exchange. American Journal of Physiology. Cell Physiology, 305, C941–C950. 10.1152/ajpcell.00178.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. (2014). Production and physiological effects of hydrogen sulfide. Antioxidants & Redox Signaling, 20, 783–793. 10.1089/ars.2013.5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, V. E. , Schulman, J. M. , Stiefel, E. I. , & Lee, C. C. (2007). Reversible precipitation of bovine serum albumin by metal ions and synthesis, structure and reactivity of new tetrathiometallate chelating agents. Journal of Inorganic Biochemistry, 101, 1707–1718. 10.1016/j.jinorgbio.2007.07.015 [DOI] [PubMed] [Google Scholar]
- Lee, Z. W. , Teo, X. Y. , Tay, E. Y. W. , Tan, C. H. , Hagen, T. , Moore, P. K. , & Deng, L. W. (2014). Utilizing hydrogen sulfide as a novel anti‐cancer agent by targeting cancer glycolysis and pH imbalance. British Journal of Pharmacology, 171, 4322–4336. 10.1111/bph.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.‐H. , Lu, M. , Hu, L.‐F. , Wong, P. T.‐H. , Webb, G. D. , & Bian, J.‐S. (2012). Hydrogen sulfide in the mammalian cardiovascular system. Antioxidants & Redox Signaling, 17, 141–185. 10.1089/ars.2011.4005 [DOI] [PubMed] [Google Scholar]
- Lowndes, S. A. , Adams, A. , Timms, A. , Fisher, N. , Smythe, J. , Watt, S. M. , … Harris, A. L. (2008). Phase I study of copper‐binding agent ATN‐224 in patients with advanced solid tumors. Clinical Cancer Research, 14, 7526–7534. 10.1158/1078-0432.CCR-08-0315 [DOI] [PubMed] [Google Scholar]
- Mathai, J. C. , Missner, A. , Kugler, P. , Saparov, S. M. , Zeidel, M. L. , Lee, J. K. , & Pohl, P. (2009). No facilitator required for membrane transport of hydrogen sulfide. Proceedings of the National Academy of Sciences of the United States of America, 106, 16633–16638. 10.1073/pnas.0902952106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, P. M. , Batka, D. , Hefter, G. , Königsberger, E. , & Rowland, D. (2018). Goodbye to S2 − in aqueous solution. Chemical Communications, 54, 1980–1983. 10.1039/C8CC00187A [DOI] [PubMed] [Google Scholar]
- Morrison, M. L. , Blackwood, J. E. , Lockett, S. L. , Iwata, A. , Winn, R. K. , & Roth, M. B. (2008). Surviving blood loss using hydrogen sulfide. The Journal of Trauma, 65, 183–188. 10.1097/TA.0b013e3181507579 [DOI] [PubMed] [Google Scholar]
- Quagraine, E. , Georgakaki, I. , & Coucouvanis, D. (2009). Reactivity and kinetic studies of [NH4]2MoS4 in acidic aqueous solution: Possible relevance to the angiostatic function of the MoS4 2− ligand. Journal of Inorganic Biochemistry, 103, 143–155. 10.1016/j.jinorgbio.2008.09.015 [DOI] [PubMed] [Google Scholar]
- Redzic, Z. (2011). Molecular biology of the blood‐brain and the blood‐cerebrospinal fluid barriers: Similarities and differences. Fluids and Barriers of the CNS, 8, 3 10.1186/2045-8118-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, C. , & Papapetropoulos, A. (2017). International Union of Basic and Clinical Pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacological Reviews, 69, 497–564. 10.1124/pr.117.014050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, C. , Ransy, C. , Módis, K. , Andriamihaja, M. , Murghes, B. , Coletta, C. , … Bouillaud, F. (2014). Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms: Biochemistry of H2S and mitochondrial function. British Journal of Pharmacology, 171, 2099–2122. 10.1111/bph.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, J. L. , Vaughan, D. , Dicay, M. , MacNaughton, H. K. , & de Nucci, G. (2017). Hydrogen sulfide‐releasing therapeutics: Translation to the clinic. Antioxidants & Redox Signaling, 28, 1533–1540. [DOI] [PubMed] [Google Scholar]
- Walsh, S. B. , & Stewart, G. W. (2010). Anion exchanger 1: Protean function and associations. The International Journal of Biochemistry & Cell Biology, 42, 1919–1922. 10.1016/j.biocel.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Wang, Q. , Guo, H. , & Zhu, H. Z. (2011). Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: A mechanism through cardiac mitochondrial protection. Bioscience Reports, 31, 87–98. 10.1042/BSR20100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Yang, W. , Jia, X. , Yang, G. , Duridanova, D. , Cao, K. , & Wang, R. (2009). Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Laboratory Investigation, 89, 59–67. 10.1038/labinvest.2008.109 [DOI] [PubMed] [Google Scholar]
- Wu, W.‐J. , Jia, W.‐W. , Liu, X.‐H. , Pan, L.‐L. , Zhang, Q.‐Y. , Yang, D. , … Zhu, Y. Z. (2016). S‐propargyl‐cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2‐ARE signaling pathway. Redox Biology, 10, 157–167. 10.1016/j.redox.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, S. , Yang, C.‐T. , Meng, F.‐H. , Pacheco, A. , Chen, L. , & Xian, M. (2016). Ammonium tetrathiomolybdate as a water‐soluble and slow‐release hydrogen sulfide donor. Bioorganic & Medicinal Chemistry Letters, 26, 1585–1588. 10.1016/j.bmcl.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. , Chen, L. , Xu, S. , Day, J. J. , Li, X. , & Xian, M. (2017). Recent development of hydrogen sulfide releasing/stimulating reagents and their potential applications in cancer and glycometabolic disorders. Frontiers in Pharmacology, 8, 664 10.3389/fphar.2017.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , & Bian, J.‐S. (2014). Hydrogen sulfide: A neuromodulator and neuroprotectant in the central nervous system. ACS Chemical Neuroscience, 5, 876–883. 10.1021/cn500185g [DOI] [PubMed] [Google Scholar]
- Zolfaghari, P. S. , Pinto, B. B. , Dyson, A. , & Singer, M. (2013). The metabolic phenotype of rodent sepsis: Cause for concern? Intensive Care Medicine Experimental, 1, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart, A. , Buursma, A. , van Kampen, E. J. , Oeseburg, B. , van der Ploeg, P. H. , & Zijlstra, W. G. (1981). A multi‐wavelength spectrophotometric method for the simultaneous determination of five haemoglobin derivatives. Journal of Clinical Chemistry and Clinical Biochemistry, 19, 457–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information S1 In vitro H2S gas release
Supplementary information S2 Detection of hydrogen di‐/polysulfides and glutathione per‐/polysulfides
Figure S1 Thiol interactions. (A) H2S release from ATTM following incubation with reduced (GSH) or oxidised (GSSG) glutathione (n=5). Values recorded are in parts per million (ppm). (B) Degradation of ATTM ± GSH (n=8) or GSSG (n=5) monitored by absorbance at 468 nm (l468). Untreated samples are n=14. (C) pilot data shows generation of glutathione persulfide and organic polysulfides following incubation of ATTM, the oxomolybdate, [NH4]2MoO4 and Na2S with either GSH or GSSG. Due to lack of authentic standards, data are shown as ‘peak areas’. *p<0.05, 1‐ or 2‐way ANOVA followed by Bonferroni's test. Stated p‐values are the result of overall ANOVA. AU, arbitrary units;
Figure S2 Chemical interaction of ATTM with H2DIDS. (A) H2S release from ATTM. Standard conditions apply; incubation of ATTM (25 mmol l‐1; 100 mmol l‐1 total sulfur) for 1h at physiological temperature and pH, with or without H2DIDS (0.5 mmol l‐1) (n=6). (B) Degradation of ATTM (175 μmol l‐1) ± H2DIDS (3.5 μmol l‐1) (n=6) monitored by absorbance at 468 nm (l468). Conditions here were designed to replicate an intracellular environment (37oC, pH 6.8). In both experiments, the ratio of ATTM to H2DIDS reflects that used in the sulfhaemoglobin studies. Statistics: (A) unpaired non‐parametric T‐test, (B) 2‐way ANOVA followed by Bonferroni's test. Stated p‐values are the result of the T‐test and overall ANOVA. AU, arbitrary units; H2DIDS, 4''diisothiocyanato‐dihydrostilbene‐ 2,2'‐disulfonic acid; ppm, parts per million; λ, wavelength.
Supporting information item


