Abstract
Objective:
To propose bismuth carbonate, a radiopacifying agent, as a new endodontic root repair material that was added to Portland cement (PC) at 2 wt%, 5 wt%, 10 wt% and 15 wt%, and physicochemical and biological properties of each formulation were evaluated in comparison to MTA-Angelus.
Methods:
Mixed and powder samples were analysed by scanning electron microscopy (SEM) and X-ray power diffraction (XRD), and the semiquantitative constitution of the powder was determined by energy-dispersive spectrometer (EDS). Setting time was evaluated by Vicat needle and radiopacity analysed with digital X-ray. The pH of all tested materials was observed after immersion in water for 3, 24, 48, 72 and 168 h (or 7 days). Solubility and calcium release were measured after immersion in water for 24 h. A multiparametric assay XTT-NR-CVDE was used to evaluate the cytotoxicity of the materials in human periodontal ligament (HPDL) fibroblasts. HPDL fibroblasts were exposed to PC 15% and mineral trioxide aggregate (MTA), and the expression of proinflammatory cytokines (IL1A, IL6, IL8, TNF) and bone formation genes (ALP, COL1, RUNX2) was evaluated by real-time PCR. Mineralisation of HPDL fibroblasts cocultured with PC, PC 15% and MTA was evaluated with Von Kossa staining.
Results:
PC-based groups presented more irregular and larger particles than MTA. PC and MTA showed similarities as observed by XRD and EDS. Setting time of PC-based groups was increased with the addition of bismuth carbonate. All tested materials were alkaline, and pH tended to reduce over time. All cements had solubility lower than recommended, with no difference between them (P>0.05) and showed calcium release. PC 15% had similar radiopacity when compared with MTA (P>0.05). Cell viability was higher for the tested materials than the positive control (P<0.001), but there was no difference when they were compared with negative control (P>0.05). Gene expression levels were similar for all tested groups (P>0.05). Analysed cements had positive Von Kossa staining.
Conclusion:
Overall, the addition of 15% of bismuth carbonate did not result in significant changes to its physicochemical and biological properties when compared with MTA, except for the setting time, and may be considered a potential substitute for MTA.
Keywords: Bismuth carbonate, mineral trioxide aggregate, portland cement, root repair material
HIGHLIGHTS.
PC and MTA had similar chemical constitution.
Setting time was increased by the addition of bismuth carbonate to the PC.
Radiopacity of PC plus 15 wt% of bismuth carbonate was similar to MTA.
PC-based groups and MTA were not cytotoxic to HPDL fibroblasts.
INTRODUCTION
Mineral trioxide aggregate (MTA) is a root repair material with Portland cement (PC) and bismuth oxide as major components (1). Several studies have shown that both MTA and PC present similar physicochemical and biological properties (2-5). PC has been proposed as an alternative material to MTA, but its major disadvantage is that it does not achieve the radiopacity level required to be distinguished from the surrounding anatomical structures, thus requiring the addition of a radiopacifying agent prior to its use as an endodontic material (6).
Previous studies have shown that bismuth oxide, the radiopacifying agent in ProRoot MTA and MTA-Angelus, decreased the mechanical properties of the cement (1, 7) and caused discolouration (8, 9). Therefore, bismuth carbonate has been proposed as radiopacifying agent in PC (6), but the physicochemical and biological properties of this combination have not yet been thoroughly investigated.
The aim of this study is to create a new PC-based material associated with bismuth carbonate to be used as an alternative to MTA. The physicochemical and biological properties of PC with varying concentrations of bismuth carbonate were compared with commercial MTA-Angelus.
MATERIALS AND METHODS
There was no need for ethics committee approval and informed consent for the present study.
Physicochemical properties
Water-to-powder ratio
Samples of PC (Construcola, Rio de Janeiro, Brazil) and PC mixed with 2 wt% (PC 2%), 5 wt% (PC 5%), 10 wt% (PC 10%) and 15 wt% (PC 15%) of bismuth carbonate [(BiO)2CO3] (B’Herzog, Rio de Janeiro, Brazil) were prepared. The water-to-powder ratio was established by weighing the amount of powder required for obtaining cement with ideal clinical consistency when mixed with 0.20 mL of distilled water. This procedure was repeated five times. MTA-Angelus (Angelus, Londrina, Brazil) was prepared according to the manufacturer’s instructions.
Setting time
Setting time was determined using a Vicat apparatus (Contenco, São José da Lapa, MG, Brazil), as recommended by ANSI/ADA 57 (10). Mixed cements were placed into stainless-steel rings (10 mm in diameter and 2 mm in height) and kept in a cabinet at 37°C±1°C and 95% relative humidity. The Vicat needle was lowered and placed in contact with the material surface for 5 s. This procedure was repeated every 60 s, until the indentation on the material surface was no longer visible. This test was repeated three times, and the setting time was established as the mean value obtained.
Scanning Electron Microscopy (SEM) and Energy-dispersive X-ray Spectroscopy (EDS)
Powder samples and freshly mixed cement placed into Teflon moulds (7.75 mm in diameter and 1.5 mm in height) were observed at the FEI Quanta™ 250 FEG (Field Emission Gun) (FEI, Germany) with a magnification of 1000´. Samples were placed on top of SEM conductive tapes and coated with a thin layer of carbon (Balzers CED 030, Bal-tec Union Ltd., Liechtenstein). The semiquantitative composition of the powder was done with an EDS Bruker e-Flash (FEI, Germany).
X-ray diffraction (XRD)
Powder and mixed samples of each group were placed onto the XRD apparatus of the diffractometer (PANalytical, X’pert Pro MPD model). Mixed samples were placed into a stainless-steel ring (10 mm in diameter and 1 mm in height) and incubated at 37°C±1°C and 95% relative humidity for 24 h. The CuKa X-ray source was used with a voltage of 45 kV and a current of 40 mA. The scan range was set at 5° to 80° in a continuous scan with a speed of 0.02°/s. The phase identification was executed with the X’Pert HighScore Plus, which compares the obtained patterns with the ICDD (International Center for Diffraction Data) database.
Radiopacity
Samples were mixed and placed into a stainless-steel ring (10 mm in diameter and 1 mm in height), followed by an incubation at 37°C±1°C and 95% relative humidity for 24 h as previously described (11). Briefly, samples were positioned onto an occlusal phosphor plate with an aluminium stepwedge. FocusTM X-ray (Instrumentarium Dental, Tuusula, Finland) was used to obtain radiographic images of all samples. Three samples were prepared per group. Three distinct points from the samples and stepwedge were analysed with AxioVision Rel. 4.6 Software (Zeiss, Jena, Germany) regarding the grey pixel values and an average were obtained. The average pixel values were converted into mm of Al, as previously described (12).
pH Analysis
Freshly mixed samples were placed into plastic tube (1.0 mm of internal diameter and 1 cm of length) with an endodontic file, as previously described (11). Briefly, samples were placed in a glass vial with 10 mL of distilled water and kept in the incubator for 3, 24, 48, 72 and 168 h (or 7 days). pH was evaluated with a pH meter (Accumet basic AB 15, Fisher Scientific, Pittsburgh, PA) after each period, and the sample was immersed in fresh water. Five samples were prepared for each group.
Solubility and Calcium Ion Release
Mixed samples were placed into Teflon moulds (7.75 mm in diameter and 1.5 mm in height), and a nylon thread was attached to the cement surface. After incubation at 37°C±1°C and 95% relative humidity for 24 h, the samples were weighted, and the nylon thread was attached to the vial’s lid, and the mould was immersed in the vial with 7.5 mL of distilled water and kept in suspension for 24 h. Next, samples were dried and placed into a desiccator. After 24 h, they were weighted, and the solubility was calculated as a function of the weight loss. Five samples were prepared for each group. The concentration of the calcium ion released on the distilled water after the solubility assay was measured with the ICP-2100-DV (Perkin Elmer, Waltham, MA).
Biological properties
Preparation of cement elutes (Extract media)
Samples were mixed and inserted into 1000-μL pipette tips (VWR, Radnor, PA), as previously described (11, 13). Briefly, the tip with the cement was attached to the lid of a microcentrifuge tube so that 1 mm of the tip was immersed in containing 0.5 mL of Dulbecco’s Modified Eagle Medium (DMEM) (ATCC, Manassas, VA) supplemented with 10% (v/v) heat-inactivated foetal bovine serum (FBS) (Gibco, Grand Island, NY), 100 U/mL penicillin (PEN) and 100 mg/mL streptomycin (STREP) (Sigma-Aldrich, Saint Louis, MO). The samples were incubated at 37°C, 95% humidity and 5% CO2 for 24 and 48 h. After each incubation period, the samples were removed from the media and the extracted media were briefly vortexed, transferred to a new microcentrifuge tube and frozen at -20°C until further use.
Cell culture
Human periodontal ligament (HPDL) fibroblasts were kindly provided by Dr. Isabel Gay, Department of Periodontics, University of Texas Health Science Center at Houston School of Dentistry. Cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL PEN and 100 mg/mL STREP and incubated at 37°C, 95% humidity and 5% CO2.
Cell viability assays
HPDL fibroblasts were seeded at a density of 2 ´104 cells/well in a 96-well plate and incubated for 24 h, as published elsewhere (11, 13). Briefly, 200 μL of extract media was added to the wells and incubated for 24 h. Fresh media were used as a negative control and 0.1% sodium dodecyl sulfate (SDS) (Bio- Rad, Hercules, CA) as a positive control. All experiments were done in triplicates and in three independent reactions.
A multiparametric assay kit (In Cytotox XTT-NR-CVDE, Xenometrix, Allschwill, Switzerland) was used to evaluate the cytotoxicity of the materials performed following the manufacturer’s recommendations, and the results were analysed using an ELISA plate reader (Dynex Technologies, Chantilly, VA).
Gene expression assays
HPDL fibroblasts were seeded at a density of 100.000 cells/well in 6-well plate and incubated for 24 h. Next, culture media were replaced by the 24-h extract media of PC 15% and MTA. Cells cultured with fresh media were used as controls. Following incubation for 24 h, RNA extraction and cDNA synthesis were performed using the PowerSYBR® Green Cells-to-CT kit (Ambion, Warrington, UK) according to the manufacturer’s instructions.
We evaluated the mRNA expression of the following proinflammatory cytokine [interleukin-1α (IL1A), interleukin-6 (IL6), interleukin-8 (IL8) and tumour necrosis factor (TNF)] and bone formation [alkaline phosphatase (ALP), collagen type 1 (COL1) and runt-related transcription factor 2 (RUNX2)] genes. Primer sequences are presented in Table 1. Real-time quantitative polymerase chain reaction (PCR) was performed in a ViiA7 Sequence Detection System (Life Technologies, Foster City, CA) using SYBR® Green chemistry (Life Technologies, Foster City, CA). Reaction conditions were: 95°C (10 min), 40 cycles at 95°C (15 s), 60°C (1 min), 95°C (15 s), then 60°C (1 min) and a final stage at 95°C (15 s). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a housekeeping gene for normalisation. Reactions were performed in a final volume of 20 μL in triplicate experiments. Data were obtained as threshold cycle (Ct) values. Expression levels were calculated using the comparative 2-ΔΔCt method (14).
TABLE 1.
Primer sequences
| Gene | Forward and reverse primers |
|---|---|
| GAPDH | 5’- ACA ACT TTG GTA TCG TGG AAG G -3’ |
| 5’- GCC ATC ACG CCA CAG TTT C -3’ | |
| IL-1A | 5’- AGA TGC CTG AGA TAC CCA AAA CC -3’ |
| 5’- CCA AGC ACA CCC AGT AGT CT -3’ | |
| IL-6 | 5’- ACT CAC CTC TTC AGA ACG AAT TG -3’ |
| 5’- CCA TCT TTG GAA GGT TCA GGT TG -3’ | |
| IL-8 | 5’- ACT GAG AGT GAT TGA GAG TGG AC -3’ |
| 5’- AAC CCT CTG CAC CCA GTT TTC -3’ | |
| TNF | 5’- GAG GCC AAG CCC TGG TAT G -3’ |
| 5’- CGG GCC GAT TGA TCT CAG C -3’ | |
| ALP | 5’- CCA CAA GCC CGT GAC AGA -3’ |
| 5’- GCG GCA GAC TTT GGT TTC -3’ | |
| COL 1 | 5’- GCC CTG TCT GCT TCC TGT A -3’ |
| 5’- TTT GGG TTG GTT GTC TGT TT -3’ | |
| Runx 2 | 5’- CTT CAT TCG CCT CAC AAA C -3’ |
| 5’- GTC ACT GCG CTG AAG A -3’ |
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, IL1A: Interleukin-1α, IL6: Interleukin 6, IL8: Interleukin 8, TNF: Tumour necrosis factor, ALP: Alkaline phosphatase, COL1: Collagen type 1, RUNX2: Runt-related transcription factor 2
Von Kossa staining
HPDL fibroblasts were seeded at a density of 50.000 cells/well in a 6-well plate and incubated for 24 h (15). After the incubation period, media were replaced by an osteogenic media consisting of DMEM supplemented with 10% FBS, 100 U/mL PEN, 100 μg/mL STREP, 50 μg/mL ascorbic acid (Sigma-Aldrich, Saint Louis, MO), 10 nmol/L dexamethasone (Sigma-Aldrich, Saint Louis, MO) and 10 mmol/L B-glycerophosphate (Sigma-Aldrich, Saint Louis, MO) (16). PC, PC 15% and MTA were mixed, placed into Teflon rings (3.5 mm in diameter and 3.5 mm in thickness) and incubated for 24 h at 37°C, 95% humidity and 5% CO2. Teflon rings containing the test materials were then placed on insert wells (3.0 μm) (Corning, Lowell, MA), into the 6-well plate and incubated for 15 days (15). The experiment was performed in duplicates. The Von Kossa staining (American MasterTech, Lodi, CA) was done according to the manufacturer’s instructions and analysed using an IX-71 inverted microscope (Olympus, Center Valley, PA).
Statistical analysis
All data were submitted to a normality test, which indicated the use of parametric tests (data not shown). The water-to-powder ratio, solubility and calcium ion release were analysed by analysis of variance (ANOVA). ANOVA and Tukey’s were used to analyse setting time, pH variation, radiopacity and cytotoxicity. For mRNA expression, results were depicted as the mean average mRNA expression of triplicate measurements, normalised to the endogenous gene expression of GAPDH. Statistical analysis was performed using ANOVA followed by Tukey’s multiple comparison test as implemented in GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA). The significance level was established at 5% (P<0.05).
RESULTS
Water-to-Powder Ratio
Table 2 presents the mean and standard deviation of powder required to obtain cement with ideal consistency when mixed with 0.20 mL of distilled water for the groups of PC pure and associated with 2 wt%, 5 wt%, 10 wt% and 15 wt% of bismuth carbonate. Statistically significant differences were not observed between the groups (P<0.05).
TABLE 2.
Radiographic studies on the prevalence of pulp stones in several populations
| Sample preparation (g) | Setting time (min) | Radiopacity (mm Al) | Solubility (%) | Calcium concentration (mg/l) | |
|---|---|---|---|---|---|
| PC | 0.55±0.02a | 92.0±1.00a | 1.59±0.05a | 1.83±0.42a | 31.86±5.6a |
| PC 2% | 0.52±0.01a | 106.0±1.00b | 2.06±0.15a | 1.89±0.91a | 30.55±7.0a |
| PC 5% | 0.53±0.04a | 106.3±1.15b | 2.88±0.23b | 2.44±1.78a | 28.32±4.0a |
| PC 10% | 0.54±0.02a | 125.3±2.08c | 3.68±0.39c | 1.86±1.8a | 30.61±8.3a |
| PC 15% | 0.56±0.03a | 123.3±1.53c | 4.46±0.05d | 1.44±0.31a | 28.30±2.2a |
| MTA | - | 22.0±1.00d | 4.86±0.19d | 2.86±1.76a | 25.25±4.5a |
PC: Portland cement, PC 2%: Portland cement plus 2 wt% of bismuth carbonate, PC 5%: Portland cement plus 5 wt% of bismuth carbonate, PC 10%: Portland cement plus 10 wt% of bismuth carbonate, PC 15%: Portland cement plus 15 wt% of bismuth carbonate, MTA: mineral trioxide aggregate, Different superscript letter represent statistical differences between groups (P<0.05)
Setting time
Table 2 shows the mean setting time and standard deviation, in minutes, for all groups. Significant differences were observed between the following groups: MTA and PC, MTA and PC 2%, MTA and PC 5%, MTA and PC 10%, MTA and PC 15%, PC and PC 2%, PC and PC 5%, PC and PC 10%, PC and PC 15%, PC 2% and PC 10%, PC 2% and PC 15%, PC 5% and PC 10%, and PC 5% and PC 15% (P<0.05). No differences were found between groups PC 2% and PC 5% and PC 10% and PC 15% (P>0.05).
SEM and EDS
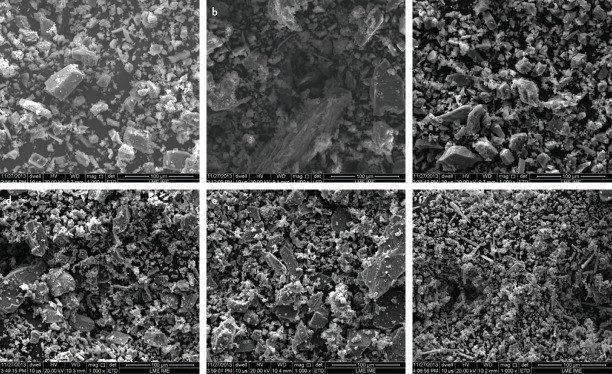
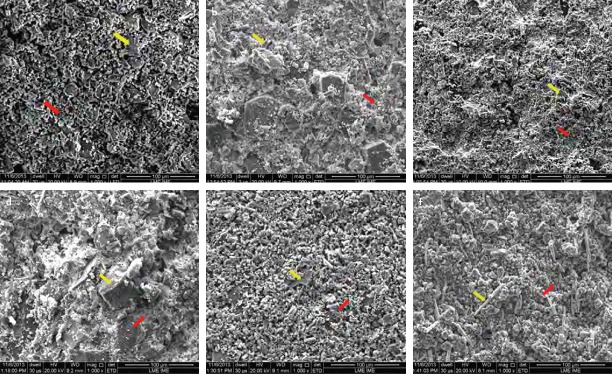
Scanning electron micrographs revealed that PC presented larger and more irregular particles, whereas MTA presented as more homogenous and with smaller particles (Fig. 1). Freshly mixed samples showed the presence of crystalline (yellow arrow) and amorphous (red arrow) phases in all samples (Fig. 2). The hydration process of analysed materials occurs through the dissolution of the powder followed by crystallisation, which consists of the formation of cubic and needle-shaped crystals.
Figure 1.
Scanning electron micrograph of powder samples of PC (a), PC 2% (b), PC 5% (c), PC 10% (d), PC 15% (e) and MTA (f) (magnification, 1000×)
Figure 2.
Scanning electron micrograph of freshly mixed samples of PC (a), PC 2% (b), PC 5% (c), PC 10% (d), PC 15% (e) and MTA (f) (magnification, 1000×). Presence of crystalline (yellow arrow) and amorphous (red arrow) phases in all samples (magnification, 1000×)
EDS showed that the major chemicals components of PC were calcium, oxygen, silicon and aluminium. MTA-Angelus and PCbased materials showed the same constituents plus bismuth.
XRD
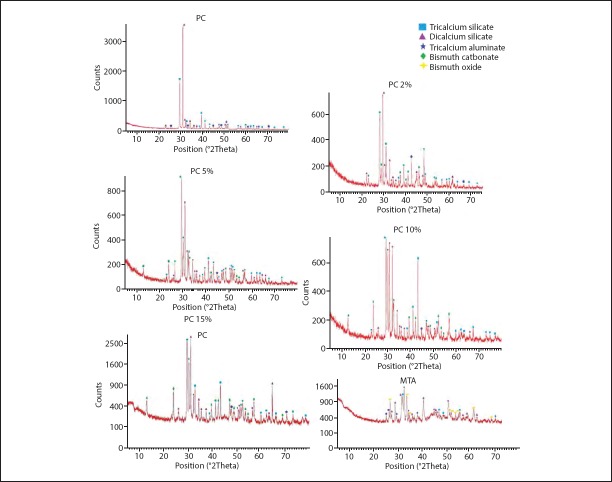
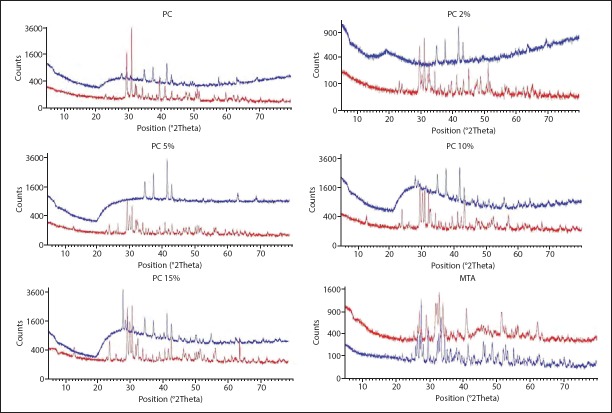
XRD patterns of the powder forms of all groups are presented in Figure 3. The main constitution of PC was tricalcium silicate (JCPDS 01-073-0599), dicalcium silicate (JCPDS 01-083-0460) and tricalcium aluminate (JCPDS 00-038-1429). The PC-based materials had the same constituents plus bismuth carbonate (JCPDS 01-073-6504) and MTA-Angelus had bismuth oxide (JCPDS 01-076-1730). The powder form of all cements showed more peaks, and they were more intense compared to the hydrated form (Fig. 4).
Figure 3.
Results of XRD analysis of the powder form of PC, PC 2%, PC 5%, PC 10%, PC 15% and MTA
Figure 4.
XRD analysis comparing powder and hydrated forms of PC, PC 2%, PC 5%, PC 10%, PC 15% (E) and MTA (F) (red-powder sample; blue-hydrated sample)
Radiopacity
The results for the mean value and standard deviation of the radiopacity in mm Al of the tested materials are shown in Table 2. No statistical significant differences were observed between PC and PC 2% and PC 15% and MTA (P>0.05).
pH
The pH of all tested cements was alkaline (Table 3) and tended to decrease over time.
TABLE 3.
Means and standard deviations of pH values for all groups at different time periods
| PC | PC 2% | PC 5% | PC 10% | PC 15% | MTA | |
|---|---|---|---|---|---|---|
| 3 hours | 9.90±0.04a | 10.01±0.14a | 9.71±0.31a,b | 9.77±0.13a,b | 9.47±0.09b,c | 9.37±0.17c |
| 24 hours | 8.53±0.06a,c | 8.57±0.99a,b,c | 8.37±0.10a | 8.92±0.39b,c | 8.40±0.04a | 8.85±0.23c |
| 48 hours | 7.92±0.22a | 8.56±0.33b | 8.05±0.30a | 8.28±0.16a,b | 7.97±0.16a | 8.15±0.10a,b |
| 72 hours | 8.08±0.08a,b | 8.12±0.13a | 7.95±0.11a,b | 7.88±0.12a,b | 7.82±0.19b | 8.05±0.13a,b |
| 7 days | 7.98±0.15a | 8.04±0.09a | 7.98±0.12a | 7.90±0.12a | 7. 86±0.08a | 8.03±0.06a |
PC: Portland cement, PC 2%: Portland cement plus 2 wt% of bismuth carbonate, PC 5%: Portland cement plus 5 wt% of bismuth carbonate, PC 10%: Portland cement plus 10 wt% of bismuth carbonate, PC 15%: Portland cement plus 15 wt% of bismuth carbonate, MTA: mineral trioxide aggregate, Different superscript letter represent statistical differences between groups (P<0.05)
Solubility and Calcium Ion Release
Table 2 presents the solubility as a function of the weight loss and the calcium ion release for all groups. There were no statistical differences between the groups for both assays (P>0.05).
Cell viability assays
The cell viability was calculated in function of the negative control] Cell viability%=mean optical density (OD) of test sample´ 100/mean OD of negative control]. A reduction in cell viability by more than 30% is considered a cytotoxic effect, according to the ISO standard number 10993-5 (17). In this study, the cell viability remained above 70% for all tested materials considering the three assay parameters (XTT, NR, and CVDE). No significant differences were observed between each of the test materials and the negative control group (P>0.05). On the other hand, the differences in cell viability between each test material and the positive control group (SDS) were statistically significant (P<0.001) (Fig. 5).
Figure 5.
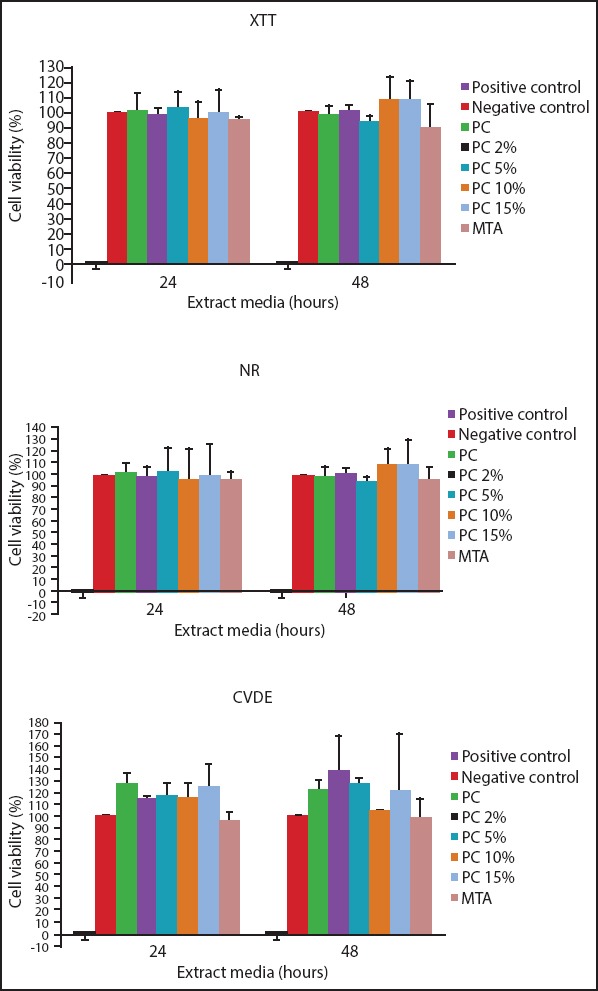
Results of XTT (2,3-bis (2-methoxy-4-nitro-5-sulfopheny)-2 Htetrazolium-5-carboxyanilide inner salt), NR (neutral red) and CVDE (crystal violet dye elution) cytotoxicity assays on HPDL fibroblasts exposed to PC, PC 2%, PC 5%, PC 10%, PC 15% and MTA. Culture media were used as negative control and 0.1% SDS as positive control. Note that cell viability was greater than 70% for all the tested materials as required by the International Standardization Organization (ISO) (dashed line) in all three assay parameters
*: significantly different from all groups
mRNA expression
No statistically significant differences were observed for the expression of the proinflamatory cytokines IL1A, IL6, IL8 and TNF and bone formation genes ALP, COL1 and RUNX2 in HPDL cells treated with PC 15% or MTA or control cells (Fig. 6).
Figure 6.
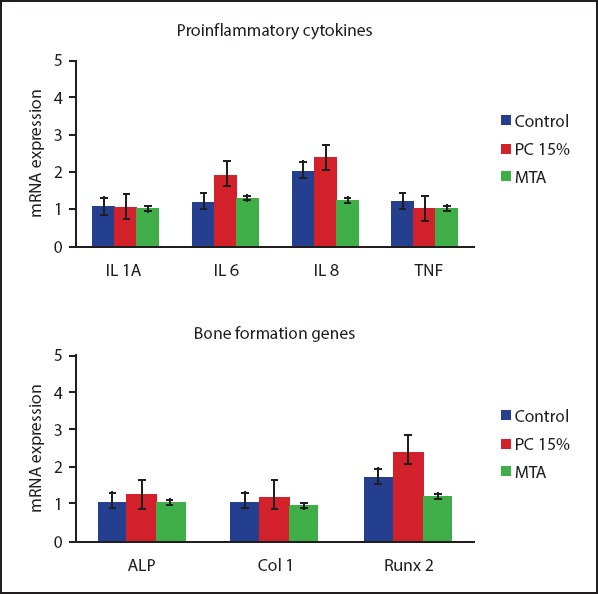
Results of gene expression assays for proinflammatory cytokines (IL1A, IL6, IL8 and TNF) and mineralisation genes (ALP, COL1 and RUNX2) in HPDL fibroblasts treated with MTA and PC 15%. No significant differences were found for cytokine or mineralisation gene expression between the groups in comparison to control tissues. Although not significant, PC15% showed higher expression of RUNX2 compared to MTA
Von Kossa staining
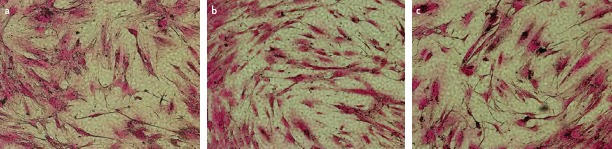
The presence of calcified mineral deposits, represented as dark nodules, was observed in the HPDL cells treated with PC, PC 15% and MTA (Fig. 7).
Figure 7.
Images of Von Kossa staining results in HPDL fibroblasts cocultured with (A) PC, (B) PC plus 15 wt% of bismuth carbonate (PC 15%) c and (C) MTA-Angelus. Note the formation of mineral deposits (dark nodules) in all groups (original magnification, 10×)
DISCUSSION
Root repair material must have a radiopacity level that allows the dentist to distinguish the material from the surrounding anatomical structures. Because PC does not have the required radiopacity level, a radiopacifying agent should be added in order to be used as an endodontic material (6). Alternative materials, including bismuth carbonate and others, have been proposed to substitute bismuth oxide (6); therefore, bismuth carbonate was chosen as the radiopacifying agent for this study.
As there were no statistical differences for the water-to-powder ratios between the groups, the arithmetic mean was made with the means of each group in order to obtain only one water-to-powder ratio for all PC groups. Therefore, the water-to-powder ratio used in all tests of this study was 0.54 g of cement powder to 0.20 mL of distilled water. This water-to-powder ratio is in accordance with previous study (3).
The setting time of a root repair material is an important factor because it allows the clinician to plan the clinical intervention. In order to avoid being washed out, the ideal setting time should be reduced. However, an extremely reduced setting time may result in difficult handling during application of the material (4, 18). According to the manufacturer, the final setting time of white MTA-Angelus is 15 min. In the present study, the final setting time obtained for the MTA was 22 min, similar to those obtained in other studies (18-20).
PC-based groups had higher setting time than MTA-Angelus and increased proportionally with the amount of bismuth carbonate, which was also observed by other studies (21, 22). The addition of radiopacifying agents increases the setting time because it prevents cement from particle packing, and therefore the time for the formation of the cement matrix will be increased (21). Nevertheless, studies have shown that the addition of nanoparticles of some radiopacifying agents to PC has decreased the setting time of the mixture (23, 24). Li et al. (23) suggested that the hydration reaction is accelerated due to the presence of heterogeneous nucleation sites on the nanoparticles surface.
SEM images showed that the PC-based groups had larger and more irregular particles when compared to MTA, corroborating with the findings of Hwang et al. (4, 5). The difference in particle shape could explain the higher setting times for the PC-based groups, as smaller particles have larger surface area, and therefore faster setting time (25). EDS results from this study are in accordance with previous findings (4, 5, 26) and further confirm that MTA and PC have the same major components, which are calcium, oxygen, silicon and aluminium.
The similarity between PC and MTA was also observed by the XRD, which is in agreement with numerous reports (26). The hydrated forms of all cements had less intense peaks than the powder forms, as described elsewhere (1, 27). During the cement hydration, the components are dissolved in water, which results in reduction of peak intensity as well as in the appearance of an amorphous phase (1, 27), which fails to be identified by the XRD analysis.
ANSI/ADA specification n°57 (10) establishes that an endodontic material must have a radiopacity above 3 mm Al. The obtained results showed that the PC associated with 0 wt%, 2 wt% and 5 wt% bismuth carbonate did not have the required radiopacity to meet the criteria of an endodontic material. However, if associated with 10 wt% or 15 wt% of bismuth carbonate, the required radiopacity by ANSI/ADA was achieved (3.68±0.39 and 4.46±0.05 mm of Al, respectively). Statistical analysis showed no difference between the radiopacity levels of MTA and PC 15%.
The pH of all tested materials was alkaline, varying from 7.5 to 10.2. Other studies using similar methodology present similar findings (18, 20). Higher pH values have also been reported, and discrepancies among study results may be explained, in part, by the different methodologies used (2). The alkaline pH in these materials promotes an antimicrobial action in the surrounding tissues creating an unfavourable environment for bacteria survival (28).
ISO 6876 (29) recommends that an endodontic material should have solubility lower than 3%, which was observed for all groups. Even though no statistical significant differences were found between the groups, the solubility of the MTA was higher than PC, which was also observed by Ricci Vivan et al. (18).
All groups showed calcium ion release, and this release may justify the good biological properties of the MTA and PCbased groups (18, 20).
The model selected to obtain extract media for the biological property assays simulated a clinical scenario, and the use of HPDL cells also reflects a relevant clinical scenario (11, 13). The multiparametric cytotoxicity assay kit used allows the evaluation of the material toxic effects in the same cells through three different parameters: mitochondrial metabolism and respiratory toxicity (XTT); lysosomal integrity and membrane permeability (NR); and cell proliferation and presence of DNA (CVDE). This assay provides information on the specific biological mechanisms through which the cements may be cytotoxic (11, 13, 30, 31).
Results for the cell viability assays showed no statistically significant differences between the tested materials and negative control group for both 24- and 48-h experimental periods, suggesting that the addition of bismuth carbonate up to a 15 wt% concentration to PC is biocompatible and not damaging to HPDL cells. All of the tested materials maintained cell viability and proliferation, and no evidence of damage to the cell membrane and DNA was found. Corroborating with our findings, previous studies have shown that ProRoot MTA, MTA-Angelus and PC were not cytotoxic, allowing a cell viability of 70% or higher, as required by the ISO guidelines (4, 30-33).
Additional biological properties, such as anti-inflammatory and osteoconductive properties, are also desirable for MTA-substitute materials. Once the PC 15% provided excellent radiopacity and cell biocompatibility, we decided to analyse the effects of PC 15% on the expression of inflammatory cytokine and bone formation genes, as well as its ability to induce mineralisation.
No significant differences were observed for mRNA expression of the proinflammatory cytokines IL1A, IL6, IL8 and TNF between MTA, PC 15%, and control groups. These results are in agreement with Pereda et al. (34) who reported that MTA-Angelus did not induce higher cytokine gene expression in murine macrophages in comparison to controls. Similarly, the expression of ALP, COL1 and RUNX2, markers of fibroblast differentiation and bone formation, was also not significantly different between the groups, although the expression of RUNX2 in the cells exposed to PC 15% was notably higher. In a previous study, the incubation of HPDL cells with ProRoot MTA for 5 days resulted in higher COL1 and RUNX2 mRNA expression than in controls, and it was suggested that ProRoot MTA is able to induce the differentiation of PDL cells to cementoblast/osteoblast (35). Pérard et al. (36) also evaluated the expression of bone formation genes in dental pulp cells in the presence of ProRoot MTA for 7 days and observed decrease in COL1 expression when compared to the control, whereas no differences were found for the expression of RUNX2 and ALP. Any discrep-ancies in the results of our study and previous reports may be related to differences in the time of material exposure to the cells and the different effects observed for cell dif-ferentiation.
Nonetheless, using Von Kossa staining, we observed the pres-ence of mineralisation nodules in the HPDL fibroblasts cocul-tured with PC, PC 15% and MTA. These results are consistent with previous studies, where mineral deposits were found in cells cultured with ProRoot MTA (15, 37, 38).
CONCLUSION
The results of the present study demonstrated that the addition of 15 wt% of bismuth carbonate to Portland cement did not result in sig-nificant changes to its physicochemical properties when compared to MTAAngelus, except for the setting time, while maintaining cell viability and proliferation, and inducing min-eralisation and new bone formation. Therefore, it may be con-sidered a potential substitute for MTA.
Acknowledgements
The authors wish to thank Dr. Isabel Gay at the University of Texas Health Science Center School of Dentistry Department of Periodontics for kindly providing HPDL cells, Min Zhao and Lillian Cameron for invaluable technical assistance, and Dr. Jose Mauro Granjeiro for many helpful discussions. This work was performed with financial support from Brazilian Agencies CAPES (BEX: 1099/12-4) to Leticia Souza, CNPq and FAPERJ. Addi-tional funding was provided by UTSD start up funds to Dr. Ariadne Letra.
Footnotes
Conflict of interest: No conflict of interest was declared by the authors.
Peer-review: Externally peer-reviewed.
Financial Disclosure: This work was performed with financial support from Brazilian Agencies CAPES (BEX: 1099/12-4) to Leticia Souza, CNPq and FAPERJ. Additional funding was provided by UTSD start-up funds to Dr. Ariadne Letra.
Authorship contributions: Concept – C.N.E., H.P.L., A.L.; Design – C.N.E., A.L., L.C.S., M.Y., R.S., H.P.L.; Supervision – L.C.S.; Resources – R.S., C.N.E., A.L.; Materials – R.S., H.P.L., A.L., C.N.E.; Data Collection and/or Processing – L.C.S., M.Y.; Analysis and/or interpretation – L.C., M.Y.; Literature search – L.C.S., M.Y.; Writing – L.C.S.; Critical Review – C.N.E., A.L.
REFERENCES
- 1.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41(5):408–17. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 2.Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006;32(3):193–7. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Min KS, Kim HI, Park HJ, Pi SH, Hong CU, Kim EC. Human pulp cells response to Portland cement in vitro. J Endod. 2007;33(2):163–6. doi: 10.1016/j.joen.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Hwang YC, Kim DH, Hwang IN, Song SJ, Park YJ, Koh JT, et al. Chemical constitution, physical properties, and biocompatibility of experimentally manufactured Portland cement. J Endod. 2011;37(1):58–62. doi: 10.1016/j.joen.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, et al. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):e96–102. doi: 10.1016/j.tripleo.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Hungaro Duarte MA, de Oliveira El Kadre GD, Vivan RR, Guerreiro Tanomaru JM, Tanomaru Filho M, de Moraes IG. Radiopacity of portland cement associated with different radiopacifying agents. J Endod. 2009;35(5):737–40. doi: 10.1016/j.joen.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Coomaraswamy KS, Lumley PJ, Hofmann MP. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J Endod. 2007;33(3):295–8. doi: 10.1016/j.joen.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Marciano MA, Duarte MA, Camilleri J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin Oral Investig. 2015;19(9):2201–9. doi: 10.1007/s00784-015-1466-8. [DOI] [PubMed] [Google Scholar]
- 9.Marconyak LJ, Jr, Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, et al. A Comparison of Coronal Tooth Discoloration Elicited by Various Endodontic Reparative Materials. J Endod. 2016;42(3):470–3. doi: 10.1016/j.joen.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 10.ANSI/ADA Specification N°57, Endodontic sealing material. Chicago, IL: ANSI/ADA; 2000. [Google Scholar]
- 11.Souza LC, Yadlapati M, Dorn SO, Silva R, Letra A. Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J Appl Oral Sci. 2015;23(4):383–9. doi: 10.1590/1678-775720150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho-Junior JR, C-SLCASM Consani S, Sousa-Neto MD. Radiopacity of root filling materials using digital radiography. Int Endod J. 2007;40:514–20. doi: 10.1111/j.1365-2591.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- 13.Yadlapati M, Souza LC, Dorn S, Garlet GP, Letra A, Silva RM. Deleterious effect of triple antibiotic paste on human periodontal ligament fibroblasts. Int Endod J. 2014;47(8):769–75. doi: 10.1111/iej.12216. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Guven EP, Tasli PN, Yalvac ME, Sofiev N, Kayahan MB, Sahin F. In vitro comparison of induction capacity and biomineralization ability of mineral trioxide aggregate and a bioceramic root canal sealer. Int Endod J. 2013;46(12):1173–82. doi: 10.1111/iej.12115. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Yang G, Fan M. Effects of homeobox gene distal-less 3 on proliferation and odontoblastic differentiation of human dental pulp cells. J Endod. 2012;38(11):1504–10. doi: 10.1016/j.joen.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 17.International Standards Organization (ISO). ISO 10993-5:2009. Biological evaluation of medical devices - Part 5:Tests for in vitro cytotoxicity. Geneva, Switzerland: International Standards Organization; 2009. [Google Scholar]
- 18.Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernardineli N, Garcia RB, et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(2):250–6. doi: 10.1016/j.tripleo.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009;35(4):550–4. doi: 10.1016/j.joen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Massi S, Tanomaru-Filho M, Silva GF, Duarte MA, Grizzo LT, Buzalaf MA, et al. pH, calcium ion release, and setting time of an experimental mineral trioxide aggregate-based root canal sealer. J Endod. 2011;37(6):844–6. doi: 10.1016/j.joen.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int Endod J. 2010;43(3):231–40. doi: 10.1111/j.1365-2591.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 22.Cutajar A, Mallia B, Abela S, Camilleri J. Replacement of radiopacifier in mineral trioxide aggregate;characterization and determination of physical properties. Dent Mater. 2011;27(9):879–91. doi: 10.1016/j.dental.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Deacon AD, Coleman NJ. The impact of zirconium oxide nanoparticles on the hydration chemistry and biocompatibility of white Portland cement. Dent Mater J. 2013;32(5):808–15. doi: 10.4012/dmj.2013-113. [DOI] [PubMed] [Google Scholar]
- 24.Coleman NJ, Li Q. The impact of zirconium oxide radiopacifier on the early hydration behaviour of white Portland cement. Mater Sci Eng C Mater Biol Appl. 2013;33(1):427–33. doi: 10.1016/j.msec.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Asgary S KS, Sohilipour E. Particle size of two endodontic biomaterials and Portland cement. Biointerface Res Appl Chem. 2011;1:83–88. [Google Scholar]
- 26.Belio-Reyes IA, Bucio L, Cruz-Chavez E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder diffraction. J Endod. 2009;35(6):875–8. doi: 10.1016/j.joen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25(5):787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira IR, Pandolfelli VC, Jacobovitz M. Chemical, physical and mechanical properties of a novel calcium aluminate endodontic cement. Int Endod J. 2010;43(12):1069–76. doi: 10.1111/j.1365-2591.2010.01770.x. [DOI] [PubMed] [Google Scholar]
- 29.International Standards Organization (ISO) ISO 687:2011. Dentistry - Root canal sealing materials. Geneva, Switzerland: International Standards Organizations; 2011. [Google Scholar]
- 30.De-Deus G, Canabarro A, Alves GG, Marins JR, Linhares AB, Granjeiro JM. Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int Endod J. 2012;45(6):508–13. doi: 10.1111/j.1365-2591.2011.02003.x. [DOI] [PubMed] [Google Scholar]
- 31.De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J Endod. 2009;35(10):1387–90. doi: 10.1016/j.joen.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues EM, Cornelio ALG, Mestieri LB, Fuentes ASC, Salles LP, Rossa-Junior C, et al. Human dental pulp cells response to mineral trioxide aggregate (MTA) and MTA Plus:cytotoxicity and gene expression analysis. Int Endod J. 2017;50(8):780–89. doi: 10.1111/iej.12683. [DOI] [PubMed] [Google Scholar]
- 33.Tanomaru-Filho M, Andrade AS, Rodrigues EM, Viola KS, Faria G, Camilleri J, et al. Biocompatibility and mineralized nodule formation of Neo MTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017 doi: 10.1111/iej.12780. doi:10.1111/iej.12780. [DOI] [PubMed] [Google Scholar]
- 34.Pereda GO, Fudinaga AC, Beltran HS, Peroni LA, Stach-Machado D. Inflammatory and bone regulators expression in murine macrophages under exposure of commercial and experimental mineral trioxide aggregate. Aust Dent J. 2012;57(3):284–91. doi: 10.1111/j.1834-7819.2012.01701.x. [DOI] [PubMed] [Google Scholar]
- 35.Hakki SS, Bozkurt SB, Ozcopur B, Purali N, Belli S. Periodontal ligament fibroblast response to root perforations restored with different materials:a laboratory study. Int Endod J. 2012;45(3):240–8. doi: 10.1111/j.1365-2591.2011.01968.x. [DOI] [PubMed] [Google Scholar]
- 36.Perard M, Le Clerc J, Watrin T, Meary F, Perez F, Tricot-Doleux S, et al. Spheroid model study comparing the biocompatibility of Biodentine and MTA. J Mater Sci Mater Med. 2013;24(6):1527–34. doi: 10.1007/s10856-013-4908-3. [DOI] [PubMed] [Google Scholar]
- 37.Guven EP, Yalvac ME, Sahin F, Yazici MM, Rizvanov AA, Bayirli G. Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod. 2011;37(5):650–6. doi: 10.1016/j.joen.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, Monnouchi S, et al. Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod. 2010;36(4):647–52. doi: 10.1016/j.joen.2009.12.024. [DOI] [PubMed] [Google Scholar]