Abstract
Background and Purpose
In preclinical studies, cannabidiol (CBD) mitigates fear memories by facilitating their extinction or interfering with their generalization and reconsolidation. The brain regions and mechanisms underlying these effects, and their temporal window, are still poorly understood. Here, we have investigated related questions in the dorsal hippocampus (DH) during contextual fear consolidation.
Experimental Approach
Adult male Wistar rats received CBD (10–30 pmol) intra‐DH immediately, 1 or 3 hr after fear conditioning. Effects of CBD on consolidation were inferred behaviourally and by analysing expression of the activity‐regulated, cytoskeleton‐associated (Arc) protein. The contribution of anandamide, CB1, CB2, 5‐HT1A, A2A, and PPARγ receptors was also assessed.
Key Results
CBD impaired memory consolidation when given immediately or 1 hr after fear conditioning, but not after 3 hr. Expression of Arc protein in DH was reduced by systemic CBD treatment in both cases. Immediately after fear conditioning, CBD effects were abolished by CB1 or CB2 receptor blockade, partly reduced by 5‐HT1A or A2A antagonism, and remained unchanged after antagonism of PPARγ receptors. One hour after fear conditioning, CBD effects were prevented only by PPARγ receptor antagonism. Also, inhibition of fatty acid amide hydrolase by URB597, impaired memory consolidation when infused immediately, but not 1 hr after fear conditioning.
Conclusions and Implications
CBD disrupts memory consolidation up to 1 hr after fear conditioning, allowing an extended window of opportunity to mitigate aversive memories after their acquisition. Our results suggest time‐dependent participation of anandamide, CB1, CB2 and PPARγ receptors in the DH, during this process.
Abbreviations
- Arc
activity‐regulated cytoskeleton‐associated protein
- CBD
cannabidiol
- DH
dorsal hippocampus
- FAAH
fatty acid amide hydrolase
What is already known
Cannabidiol given immediately after fear conditioning impaired memory generalization.
Effects of cannabidiol on fear generalization are mediated by CB1 and CB2 receptors.
What does this study add
Cannabidiol effects on memory consolidation are associated with reduced Arc expression in the dorsal hippocampus.
Cannabidiol effects on memory consolidation rely on different mechanisms of action across the consolidation time‐window.
What is the clinical significance
There is an extended time‐window to mitigate fearful memories after their acquisition with cannabidiol.
The PPARγ receptor could be investigated as a new target to mitigate aversive memories
1. INTRODUCTION
Classically, the memory consolidation time‐window lasts 6 hr (McGaugh, 2000), a period in which cellular and molecular events occur to stabilize and store a newly acquired long‐term memory (Lamprecht & LeDoux, 2004; McGaugh, 2000). Most studies investigating the effects of pharmacological interventions on memory consolidation are conducted at very short intervals after the acquisition, that is, 0–10 min (Lunardi et al., 2017; Schmidt et al., 2017). Few have explored longer intervals of the consolidation time‐window. In general, they have reported that either 1 hr (Igaz, Vianna, Medina, & Izquierdo, 2002) or 3 hr (Gafford, Parsons, & Helmstetter, 2005) after acquisition, the memory is less susceptible to interference. In any case, the mechanisms underlying the memory consolidation over its time‐window are still poorly understood. The study of these mechanisms is relevant because several psychiatric disorders, such as post‐traumatic stress disorder, seem to involve abnormal memory consolidation of the aversive events (Ehlers, Hackmann, & Michael, 2004).
The systemic administration of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150 (CBD), the major compound from Cannabis sp. devoid of psychotomimetic effects, immediately after memory acquisition, has been reported to impair the contextual fear memory generalization, an effect depending on the activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors located in the dorsal hippocampus (DH; Stern et al., 2017). It has been proposed that fear overgeneralization, a feature of post‐traumatic stress disorder, is associated with memory consolidation (Gazarini, Stern, Piornedo, Takahashi, & Bertoglio, 2015; Stern et al., 2017). Several studies support the involvement of the DH in fear memory processing, especially during the consolidation phase (Izquierdo & Medina, 1997). However, it is unknown whether, when, and for how long, CBD administered into the DH would impair the memory consolidation process.
Various mechanisms have been associated with the effects of CBD (Campos et al., 2017). For example, the cannabinoid CB1 and CB2, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1 receptors have been involved in the effects of CBD on fear memory and anxiety‐related responses, respectively (Bitencourt, Pamplona, & Takahashi, 2008; Lee, Bertoglio, Guimarães, & Stevenson, 2017; Stern, Gazarini, Takahashi, Guimarães, & Bertoglio, 2012). Moreover, the adenosine https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=19 receptor and the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 receptor, a member of a family of nuclear receptors, have been associated with CBD‐induced neuronal plasticity and learning and memory changes in animal models of neurodegenerative diseases (Esposito et al., 2011; Jahrling, Hernandez, Denner, & Dineley, 2014; Simões et al., 2016). All these receptors are expressed in the DH (Moreno, Farioli‐Vecchioli, & Cerù, 2004). However, their relative contributions to CBD‐induced effects on fear memory consolidation have not yet been explored.
The main objective of this study was to investigate the effects of CBD administered intra‐DH immediately, 1 hr, or 3 hr after a contextual fear conditioning protocol. We observed that CBD impairs memory consolidation when given immediately or 1 hr after memory acquisition. This effect is accompanied by a reduction in the expression of activity‐regulated cytoskeleton‐associated protein (Arc), a product of an immediate early gene necessary for memory consolidation (Besnard, Laroche, & Caboche, 2014), in the DH. We also investigated the involvement of CB1, CB2, 5‐HT1A, A2A, and PPARγ receptors in the CBD‐induced impairments of memory consolidation. the effects of CBD on memory consolidation depended on the activation of CB1 and CB2, but not 5‐HT1A, A2A, or PPARγ, receptors in the DH immediately after fear conditioning. In contrast, when given 1 hr after memory acquisition, the effects of CBD were mediated by activation of PPARγ receptors in the DH. Selective inhibition of fatty acid amide hydrolase (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1400) by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4339 impaired memory consolidation when infused immediately, but not 1 hr, after fear conditioning.
2. METHODS
2.1. Animals
All animal care and experimental procedures were approved by the local Committee on the Care and Use of Laboratory Animals (authorization number 1048). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Three‐month‐old male Wistar rats (270‐320 g) were obtained from the local breeding facilities. The animals were housed in groups of five in Plexiglas cages measuring 60 × 25 × 25 cm. They were kept in the animal facility under controlled temperature (22 ± 2 °C) and illumination (12‐hr cycle, lights on at 7 a.m.) conditions and had free access to water and standard laboratory chow.
2.2. Drugs
CBD (Phytoplant, Spain; 10–30 pmol or 10 mg·kg−1), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3317 (antagonist of CB1 receptors; Tocris, USA; 0.5 nmol), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=750 (antagonist of CB2 receptors; Tocris, USA; 0.1 nmol), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=405 (antagonist of A2A receptors; Sigma, USA; 10 nM), and URB597 (FAAH inhibitor; Tocris, USA; 0.1 μg) were dissolved in 0.9% NaCl containing 5.0% Tween® 80 (Sigma‐Aldrich, USA). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=80 (antagonist of 5‐HT1A receptors; Sigma‐Aldrich, USA; 0.14 nmol) was dissolved in 0.9% NaCl. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3442 (antagonist of PPARγ receptors; Sigma, USA; 32 pmol) was dissolved 0.9% NaCl containing 5.0% DMSO. The dose selection was based on pilot experiments or previously published studies (Dall'Igna, Porciúncula, Souza, Cunha, & Lara, 2003; De Paula Soares & Zangrossi, 2004; Denner et al., 2012; Segev et al., 2018; Stern et al., 2012, 2017). All solutions were prepared immediately before use and injected into the DH in a volume of 0.5 μl per side or i.p. in a volume of 1.0 ml·kg−1. In all experiments, the vehicle solution of the control group was 0.9% NaCl containing 5.0% Tween® 80.
2.3. Stereotaxic surgery and drug infusion
The rats were anaesthetized with 1.0 ml·kg−1 of xylazine (10 mg·ml−1; Carlier, Brazil) and ketamine (100 mg·ml−1; Sespo, Brazil), together with local anaesthesia (3% lidocaine with noradrenaline 1:50,000; Dentsply, Brazil) given i.p., and positioned in the stereotaxic frame. Two stainless steel guide cannulas (length = 9.0 mm and outer diameter = 0.7 mm) were implanted bilaterally aimed at the CA1 region of the DH following the coordinates (AP = −3.8 mm from bregma, L = ±2.5 mm from the central suture, and DV = −1.8 mm from the skull bone) from the rat brain atlas by Paxinos and Watson (2009). They were fixed to the skull with acrylic resin and two stainless steel screws. To prevent occlusion, a stylet was introduced inside each guide cannula. At the end of the surgery, the animals received 0.4 ml of ibuprofen orally (20 mg·ml−1, Natulab, Brazil).
After 10 days, the animals received a bilateral infusion with dental needles (outer diameter = 0.3 mm) introduced through the guide cannulas until their tips were 1.5 mm below the cannula ends. During 1 min, either the vehicle or drug was injected using two microsyringes connected to an infusion pump (Insight, Brazil). A polyethylene catheter was interposed between the upper end of the dental needles and the microsyringes. The displacement of an air bubble inside the polyethylene was used to monitor the drug flow. The needles were removed 30 s after the end of injections. In cases receiving pretreatment, the second infusion was performed 10 min after the first one.
After the end of each experiment, the rats were anaesthetized, as described above and Evans Blue (0.5 μl per side) was injected through the guide cannulas for subsequent evaluation of the drug infusion site. The brains were removed and immersed in a 10% formalin solution. Brain slices (100 μm thick) were obtained in a vibratome (Leica, Germany), and the injection site was determined. Animals were only included in the analysis when both sides of the DH were tagged by the Evans Blue (Figure 1).
Figure 1.
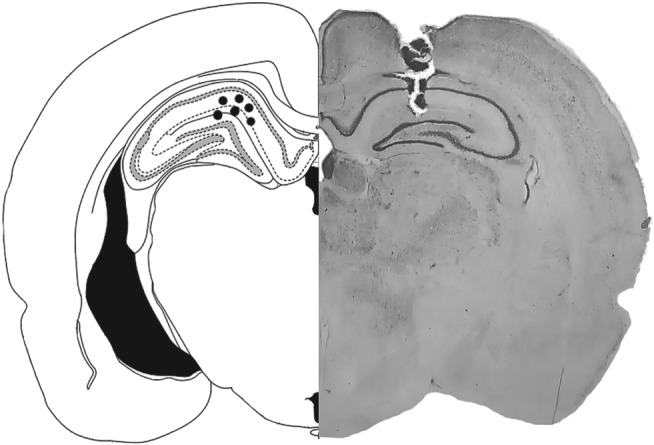
Representative infusion site (right) and schematic diagram (left) adapted from Paxinos and Watson (2009; Bregma—3.8 mm) showing the placement of drug infusions into the dorsal hippocampus (filled circles)
2.4. Behavioural procedures, experiments, and data collection
General procedures were conducted as previously described (Stern et al., 2017). The behavioural testing was conducted under 70 lux, from 1 to 5 p.m. to minimize possible circadian effects on learning and memory processing.
Fear conditioning was performed in a rectangular chamber (Context A; 35 × 20 × 30 cm), with aluminium sidewalls and a front wall and ceiling‐door made of clear plexiglass acrylic. Its grid floor, made of stainless steel bars, was connected to a circuit board and a shock generator (Insight, Brazil) to enable the delivery of controlled electrical shocks as detailed subsequently. In all experiments, the animals were placed in Context A and allowed to freely explore it for 3 min, as an initial familiarization session. They were then returned to their home cage.
On the next day, each animal was again placed in Context A for fear conditioning, during which it received, after an initial 30‐s delay, the unconditioned stimulus (three electrical footshocks of 0.8 or 1.0 mA, for 3 s, with a 30‐s inter‐trial period). The animal remained in this chamber for another 30 s before returning to its home cage. The animals of each cage were randomly allocated in the groups so that each cage had animals of different groups. The use of two different intensities was based on a prior study (Stern et al., 2017) showing that animals subjected to stereotaxic surgery require higher footshock intensity to present freezing time values similar to those without stereotaxic surgery.
In Experiment 1A, to investigate whether intra‐DH CBD could interfere with the contextual fear memory consolidation, 21 animals were randomly allocated to three groups (n = 7 rats each group) based on the treatment (vehicle, CBD 10 or 30 pmol) given immediately after pairing the Context A with three footshocks.
In Experiment 1B, to investigate whether CBD could interfere with Arc protein expression in the DH, 18 contextually fear‐conditioned animals were randomly allocated to two groups based on the systemic treatment VEH (n = 8) or CBD 10 mg·kg−1 (n = 10) given immediately after Context A‐footshock pairing. An additional group (n = 8) of naive, non‐conditioned animals was used to assess the basal Arc expression in the DH. The animals were killed immediately after being removed from the vivarium (naive group) or 90 min after treatment (VEH and CBD groups).
In Experiment 2A, to investigate whether CBD could still affect the contextual fear memory consolidation when administered into the DH at a later time point of the consolidation time‐window, 20 animals were randomly allocated to two groups based on the treatment VEH (n = 11) or CBD 30 pmol (n = 9) given 1 hr after Context A‐footshock pairing.
In Experiment 2B, to investigate whether CBD could still reduce Arc protein expression in the DH when given at a later time point of the consolidation time‐window, 20 contextually fear‐conditioned animals were randomly allocated to two groups based on the systemic treatment VEH (n = 9) or CBD 10 mg·kg−1 (n = 11) given 1 hr after Context A‐footshock pairing. An additional group (n = 7) of naive, non‐conditioned animals was used to assess the basal DH Arc expression. Animals were killed immediately after being removed from the vivarium (naive group) or 90 min after treatment (VEH and CBD groups), that is, 150 min after the conditioning procedure.
In Experiment 3, to investigate whether CBD could still affect contextual fear memory consolidation when injected into the DH at a later time point of the consolidation time‐window, 14 animals were randomly allocated to two groups (n = 7 rats each group) based on the treatment (vehicle or CBD 30 pmol) given 3 hr after Context A‐footshock pairing.
In Experiment 4, to investigate the receptors involved in the impairment of memory consolidation when CBD was injected into the DH immediately after contextual fear conditioning, 111 animals were randomly allocated to 12 groups based on the pretreatment with vehicle, AM251 (0.5 nmol), AM630 (0.1 nmol), WAY100635 (0.14 nmol), ZM241385 (10 nM), or GW9662 (32 pmol) and the treatment (VEH or 30 pmol of CBD). Pretreatment and treatment were given immediately after Context A‐footshock pairing and 10 min later, respectively. The group sample sizes (n) were as follows: VEH‐VEH = 9; VEH‐CBD = 11; AM251‐VEH = 10; AM251‐CBD = 8; AM630‐VEH = 9; AM630‐CBD = 9; WAY‐VEH = 10; WAY‐CBD = 10; ZM‐VEH = 7; ZM‐CBD = 8; GW‐VEH = 10; and GW‐CBD = 10.
In Experiment 5, to investigate the receptors involved in the impairment of memory consolidation when CBD was injected into the DH 1 hr after contextual fear conditioning, 104 animals were randomly allocated to 12 groups based on the pretreatment with vehicle, AM251 (0.5 nmol), AM630 (0.1 nmol), WAY100635 (0.14 nmol), ZM241385 (10 nM), or GW9662 (32 pmol) and the treatment (VEH or 30 pmol of CBD). Pretreatment and treatment administrations were performed 1 hr after Context A‐footshock pairing and 10 min later, respectively. The group sample sizes (n) were as follows: VEH‐VEH = 8; VEH‐CBD = 8; AM251‐VEH = 8; AM251‐CBD = 10; AM630‐VEH = 7; AM630‐CBD = 9; WAY‐VEH = 8; WAY‐CBD = 9; ZM‐VEH = 7; ZM‐CBD = 9; GW‐VEH = 10; and GW‐CBD = 11.
In Experiment 6A, to investigate whether URB597 given into the DH would interfere with contextual fear memory consolidation, 19 animals were randomly allocated to two groups based on the treatment VEH (n = 9) or URB597 0.1 μg (n = 10) given immediately after Context A‐footshock pairing.
In Experiment 6B, to investigate whether URB597 could affect the contextual fear memory consolidation when injected into the DH 1 hr later, 19 animals were randomly allocated to two groups (VEH[n = 9] or URB597 0.1 μg [n = 10]). The drug injections were performed 1 hr after Context A‐footshock pairing.
In the behavioural experiments, the assessment of treatment effects on fear memory consolidation was performed on the following day by re‐exposing the animals to the conditioning Context A for 3 min in the absence of the unconditioned stimulus (Test 1). To investigate whether the effects of CBD are long‐lasting, in some cases, the animals were again re‐exposed to the conditioning Context A (Test 2) one week after Test 1.
Freezing behaviour, defined as the total absence of body and head movements except for those associated with breathing, was recorded as an index of fear memory. The freezing time was quantified in seconds by a trained observer (inter‐ and intra‐observer reliabilities of >90%) blind to the experimental groups and expressed as the percentage of total session time.
2.5. Evaluation of Arc expression by western blotting
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology. The DH was quickly removed and stored at −80 °C. The DH from a group of naive animals was used to record the basal expression of the Arc protein. For protein extraction, the tissues were homogenized in 0.6 ml of solubilization buffer (10‐mM EDTA, 100‐mM Tris pH 7.5, 0.2% protease inhibitor cocktail [PROMEGA], and 1% Triton X‐100). Insoluble material was removed by centrifugation (20 min, 6,613 g, 4 °C). The supernatant protein concentration was determined colorimetrically (Bradford Protein Assay, Bio‐Rad). Tissue extracts (500 μl) were denatured in boiling water for 5 min in Laemmli buffer containing 200 mM of DTT. Protein extracts were separated by SDS‐PAGE, transferred onto a nitrocellulose membrane (0.45 μm; BIO‐RAD), blocked with basal solution (20‐mM Tris pH 7.6, 137‐mM NaCl, and 0.025% Tween® 20) containing 3% BSA (Sigma, USA) for 2 hr, and then incubated with monoclonal primary antibody anti‐Arc 1:500 (Santa Cruz Biotechnology Cat# sc‐17839, RRID:AB_626696) overnight and secondary antibody anti‐mouse 1:5,000 (Santa Cruz Biotechnology Cat# sc‐516102, RRID:AB_2687626) for 1 hr. For evaluation of protein loading, all membranes were stripped and reblotted with monoclonal primary anti‐α‐tubulin antibody 1:1,000 (Santa Cruz Biotechnology Cat# sc‐134237, RRID:AB_2212295). The membranes were stripped because the MW of Arc and α‐tubulin are 45 and 55 kDa, respectively. Therefore, separate assays were performed for each protein, and consequently, they were individually represented in the Figures 2b and 3b.
Figure 2.
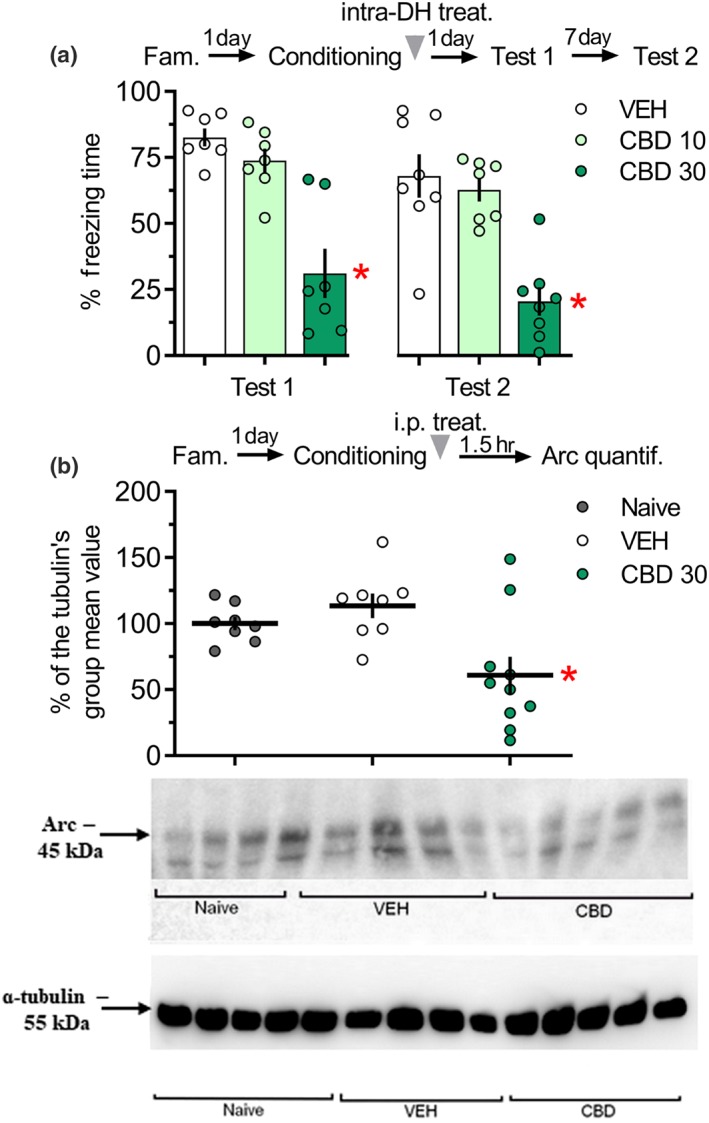
CBD infused into the DH immediately after fear conditioning impaired fear memory consolidation and the systemic administration of CBD reduced Arc expression in the DH. (a) The group that received CBD (30 pmol in 0.5 μl per side) into the DH, immediately after conditioning, presented for less freezing times than the control group, during Tests 1 and 2. *P < .05, significantly different from vehicle group in the same session. n = 7 rats in each group. (b) Ninety minutes after fear conditioning, there was a reduction in Arc expression of CBD‐treated rats, compared with controls. Data are presented as individual units and mean ± SEM; naive = 8 rats per group; vehicle = 8 rats per group; CBD = 10 rats per group. *P < .05, significantly different from vehicle group. The arrowhead indicates the time of drug injection after fear conditioning. Arc, activity‐regulated cytoskeleton‐associated protein; CBD, cannabidiol; DH, dorsal hippocampus
Figure 3.
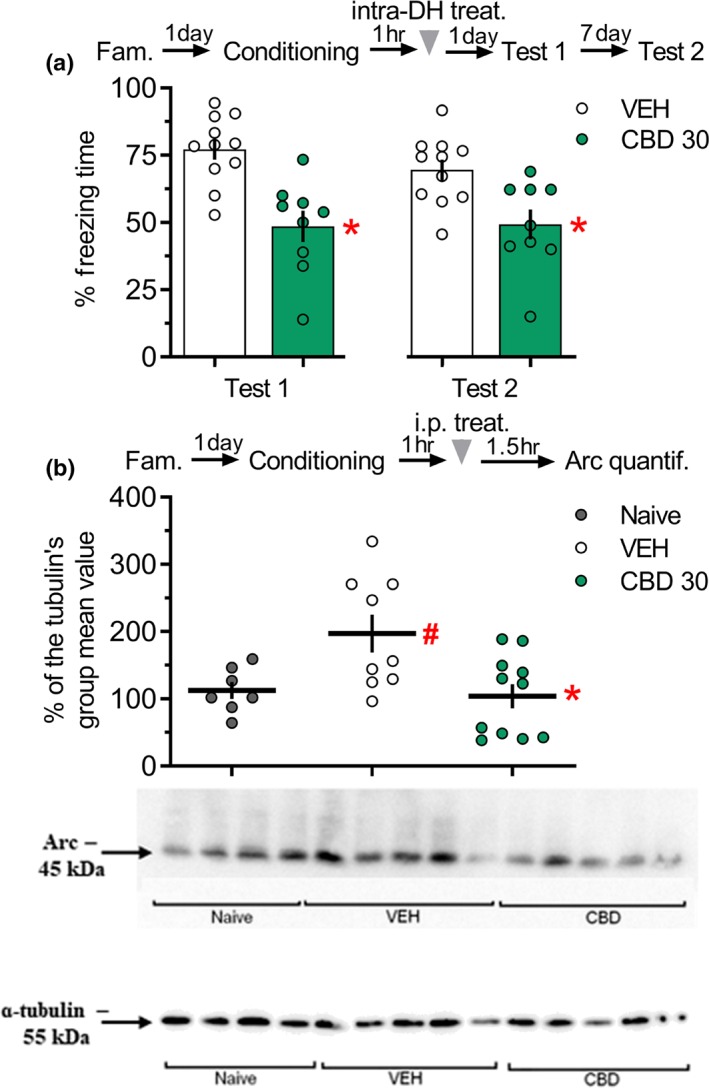
CBD infused into the DH 1 hr after fear conditioning impaired fear memory consolidation and the systemic administration of CBD reduced Arc expression in the DH. (a) The group that received CBD (30 pmol in 0.5 μl per side) into the DH, 1 hr after fear conditioning presented less freezing time than control during Tests 1 and 2. *P < .05, significantly different from vehicle group in the same session. Vehicle = 11 rats per group; CBD = 9 rats per group. (b) One hundred fifty minutes after fear conditioning, there was an increase in Arc expression of vehicle‐treated rats when compared to basal. CBD reduced this expression when administered 1 hr after fear conditioning. Data are presented as individual units and mean ± SEM;.naive = 7 rats per group; vehicle = 9 rats per group; CBD = 11 rats per group. *P < .05, significantly different from vehicle group. # P < .05, significantly different from naive group (baseline). The arrowhead indicates the time of drug injection after fear conditioning. Arc, activity‐regulated cytoskeleton‐associated protein; CBD, cannabidiol; DH, dorsal hippocampus
After incubation with the appropriate secondary antibody conjugated with Western ECL Substrate (Bio‐Rad), membranes were developed by chemiluminescence. Quantitative analysis was performed by densitometry using Scion Image software (Scion Corporation, USA). The intensities were normalized to corresponding values for α‐tubulin expression and expressed with relative value to the basal expression (naive group expression), according to Alexander et al. (2018).
2.6. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). The results are expressed as mean ± SEM. After testing for data normality, the freezing times scored from behavioural experiments and the Arc/α‐tubulin relation from western blotting were subjected to separate one‐way, two‐way, or repeated‐measures ANOVA. When ANOVA revealed a significant interaction between independent variables, the F values from the main effects were omitted. The Newman–Keuls test was used for post hoc comparisons only when F values achieved statistical significance (P < .05). When there were only two groups and no context re‐exposure was performed (URB597 experiment), an unpaired Student's t test was used. The effect size was calculated using the formula for Hedges' g to reflect the mean differences between the two groups (n ≤ 20 per group) that could be dissimilar in size. A g ≥ 0.8 was considered a large effect size (Ellis, 2010). The statistical significance level was set at P < .05. For statistical analysis, Statistic 12 (StatSoft, EUA) was used, and GraphPad Prism 8 (GraphPad Prism, EUA) was used for graphing.
The a priori sample size determined by power analysis was of eight animals per group (α = .05; β = .20; and standardized effect size or Cohen's d = 1.0). The group sizes were equal by design, but due to experimental losses (e.g., when treatment was infused outside the target brain region), in a few cases, they were unequal. We have replaced the exclusions to attempt to keep the study balanced and to maintain its power.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019; Alexander et al., 2019; Alexander et al., 2019).
3. RESULTS
3.1. CBD impairs memory consolidation and reduces Arc expression in the DH when administered immediately after contextual fear conditioning
In Experiment 1A, the one‐way repeated‐measures ANOVA showed significant effects of the treatment and the Context A re‐exposure, but not the interaction between these factors. As shown in Figure 2a, the group treated with 30 pmol of CBD presented for less freezing times than the controls during Test 1 and Test 2, indicating a persistent memory consolidation impairment. The group treated with 10 pmol of CBD and the controls had similar values.
In Experiment 1B, the one‐way ANOVA showed significant effects of the experimental group for Arc expression. As shown in Figure 2b, CBD‐treated animals presented lower values than the controls which did not differ from the naive group.
3.2. CBD also impairs memory consolidation and reduces Arc expression in the DH when administered 1 hr after contextual fear conditioning
In Experiment 2A, the one‐way repeated‐measures ANOVA showed significant effects of the treatment, but not the Context A re‐exposure or the interaction between these factors. As shown in Figure 3a, the CBD‐treated group presented for less freezing times than the controls during Test 1 and Test 2, indicating a persistent memory consolidation impairment.
In Experiment 2B, the one‐way ANOVA showed significant effects of the experimental group for Arc expression. As shown in Figure 3b, the VEH group presented higher values than the naive and the CBD‐treated animals. The naive and CBD groups had similar values.
3.3. CBD no longer impairs memory consolidation when administered into the DH 3 hr after contextual fear conditioning
In Experiment 3, the one‐way repeated‐measures ANOVA showed no significant effects of the treatment, the Context A re‐exposure or the interaction between these factors. As shown in Table 1, CBD‐treated animals presented freezing times similar to the controls, during Tests 1 and 2, indicating no CBD‐induced changes in memory consolidation.
Table 1.
Infusing CBD (30 pmol) into the dorsal hippocampus 3 hr after contextual fear conditioning induced no changes in memory consolidation
| Session group | Test 1 | Test 2 |
|---|---|---|
| VEH | 73.3 ± 4.9 | 55.4 ± 7.4 |
| CBD | 66.2 ± 6.9 | 62.22 ± 6.0 |
Note. The CBD group presented freezing times similar to those in the VEH group during Tests 1 and 2 performed 1 and 7 days later, respectively. Values are expressed as mean ± SEM of the % freezing time (n = 7 per group). CBD, cannabidiol.
3.4. The memory consolidation impairing effects of CBD injected immediately after fear conditioning depends on the activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors
In Experiment 4, the two‐way ANOVA showed significant effects of the interaction between pretreatment and treatment for time spent freezing during the test. As shown in Figure 4, the VEH‐CBD and GW9662‐CBD groups presented for less freezing times than their respective controls. In contrast, both the AM251‐CBD and AM630‐CBD groups had similar values relative to their respective control group. The AM251‐CBD and AM630‐CBD groups presented for higher freezing times than the VEH‐CBD group, which had values similar to the WAY100635‐CBD, ZM241385‐CBD, and GW9662‐CBD groups. Altogether, the impairment of memory consolidation when CBD was infused intra‐DH immediately after fear conditioning was mediated mostly by activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors.
Figure 4.
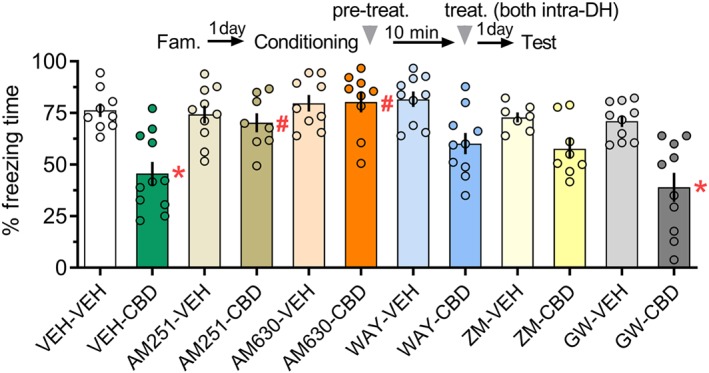
The impairment of memory consolidation induced by infusing CBD (30 pmol) into the DH immediately after fear conditioning was mediated by activation of CB1 and CB2 receptors. The CBD‐treated group presented for less freezing times than controls during Test 1. This effect was prevented by pretreatment with the CB1 receptor antagonist/inverse agonist AM251 (0.5 nmol) or the CB2 receptor antagonist/inverse agonist AM630 (0.1 nmol). Pretreatment with the 5‐HT1A receptor antagonist WAY100635 (WAY; 0.14 nmol) or the A2A receptor antagonist ZM241385 (ZM; 10 nM) partly reduced the effects of CBD. The PPARγ antagonist GW6992 (GW; 32 pmol) did not affect the CBD‐induced changes. The arrowhead indicates the time of drug injection after fear conditioning. Data are presented as individual units and mean ± SEM; VEH‐VEH = 9 rats per group; VEH‐CBD = 11 rats per group; AM251‐VEH = 10 rats per group; AM251‐CBD = 8 rats per group; AM630‐VEH = 9 rats per group; AM630‐CBD = 9 rats per group; WAY‐VEH = 10 rats per group; WAY‐CBD = 10 rats per group; ZM‐VEH = 7 rats per group; ZM‐CBD = 8 rats per group; GW‐VEH = 9 rats per group; and GW‐CBD = 11 rats per group. *P < .05, significantly different from the control group. # P < .05, significantly different from the VEH‐CBD group. CBD, cannabidiol; DH, dorsal hippocampus
3.5. The impairment of memory consolidation by CBD injected 1 hr after fear conditioning was mediated by activation of PPARγ receptors
In Experiment 5, the two‐way ANOVA showed significant effects of the interaction between pretreatment and treatment, for freezing during the test. As shown in Figure 5, the VEH‐CBD group presented for less freezing times than the VEH‐VEH group. The significant CBD‐induced reduction in freezing time was similarly observed in animals pretreated with AM251, AM630, WAY100635, or ZM241385. In contrast, the GW9662‐VEH and GW9662‐CBD groups had similar values. The GW9662‐CBD group presented for more freezing times than the VEH‐CBD group which had values similar to the AM251‐CBD, AM630‐CBD, WAY100635‐CBD, and ZM241385‐CBD groups. Altogether, the impairment of memory consolidation when CBD was infused intra‐DH, 1 hr after fear conditioning, depended mostly on PPARγ receptor activation.
Figure 5.
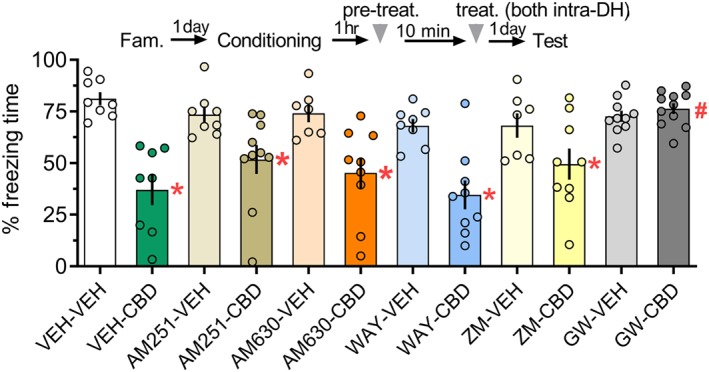
The impairment of memory consolidation induced by infusing CBD (30 pmol) into the DH 1 hr after fear conditioning was mediated by the activation of PPARγ receptors. The CBD‐treated group presented shorter freezing times than controls during Test 1. This effect was prevented by pretreatment with the PPARγ antagonist GW6992 (GW; 32 pmol). Pretreatment with the CB1 receptor antagonist/inverse agonist AM251 (0.5 nmol), the CB2 receptor antagonist/inverse agonist AM630 (0.1 nmol), the 5‐HT1A receptor antagonist WAY100635 (WAY; 0.14 nmol), or the A2A receptor antagonist ZM241385 (ZM; 10 nM) did not affect the CBD‐induced changes in memory consolidation. The arrowhead indicates the time of drug injection 1 hr after fear conditioning. Data are presented as individual units and mean ± SEM; VEH‐VEH = 8 rats per group; VEH‐CBD = 8 rats per group; AM251‐VEH = 8 rats per group; AM251‐CBD = 10 rats per group; AM630‐VEH = 7 rats per group; AM630‐CBD = 9 rats per group; WAY‐VEH = 8 rats per group; WAY‐CBD = 9 rats per group; ZM‐VEH = 7 rats per group; ZM‐CBD = 9 rats per group; GW‐VEH = 10 rats per group; and GW‐CBD = 11 rats per group. *P < .05, significantly different from the respective control group. # P < .05, significantly different from the VEH‐CBD group. CBD, cannabidiol; DH, dorsal hippocampus
3.6. URB597 impairs memory consolidation when administered in the DH immediately after contextual fear conditioning, but not 1 hr later
In Experiment 6A, the unpaired Student's t test showed significant effects of the treatment during the test. As shown in Figure 6a, the URB597‐treated group presented less freezing time than the controls, indicating impairment of memory consolidation.
Figure 6.
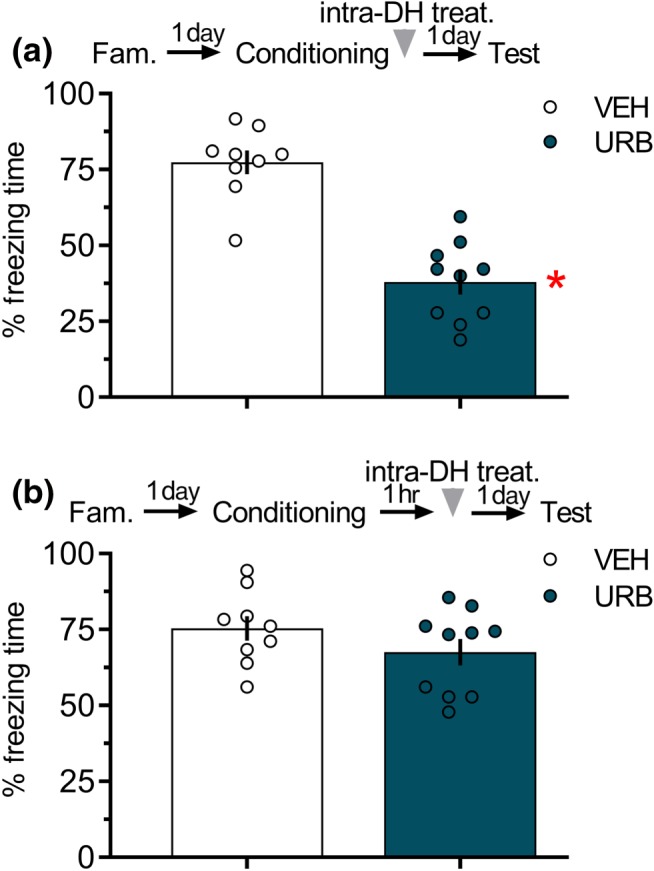
Inhibition of fatty acid amide hydrolase by URB597 infusion into the DH immediately, but not 1 hr, after fear conditioning impaired fear memory. (a) The group that received URB597 (0.1 μg in 0.5 μl per side) into the DH immediately after conditioning presented shorter freezing times than control during Test 1. The arrowhead indicates the time of drug injection after fear conditioning. Data are presented as individual units and mean ± SEM; VEH = 9 rats per group and URB = 10 rats per group. (b) The group that received URB597 (0.1 μg in 0.5 μl per side) into the DH 1 hr after conditioning did not differ from the controls. The arrowhead indicates the time of drug injection after fear conditioning. Data are presented as individual units and mean ± SEM; VEH = 9 rats per group and URB = 10 rats per group. * P < .05, significantly different from vehicle group in the same session. DH, dorsal hippocampus
In Experiment 6B, the unpaired Student's t test showed no significant effects of the treatment during the test. As shown in Figure 6b, the URB597‐treated group presented freezing times, similar to those in the controls, indicating no changes in memory consolidation.
4. DISCUSSION
Infusing CBD (30 pmol) into the DH immediately after contextual fear conditioning reduced freezing times in animals exposed to the paired Context A, 1 day (Test 1) and 7 days (Test 2) later, indicating a persistent memory consolidation impairment. This result is in line with results reported after systemic administration of CBD (Stern et al., 2017). However, when infused into the prelimbic cortex immediately after contextual fear conditioning, CBD did not change memory consolidation (Rossignoli et al., 2017). Altogether, it seems that CBD acts primarily in the DH to interfere with the consolidation process at its early stage.
CBD (30 pmol) injection into the DH 1 hr after contextual fear conditioning reduced freezing during Tests 1 and 2, also indicating a persistent memory consolidation impairment. However, when given 3 hr later, the CBD no longer affected the consolidation process. Of note, the more intense the fear conditioning protocol, the narrower the consolidation time‐window will be in the DH. Thus, using an intense fear conditioning protocol, the consolidation time‐window would be less than 6 hr, remaining susceptible to experimental intervention for only 3 hr (Casagrande et al., 2018). A strong fear conditioning stimulus was adopted in the present work. This situation, therefore, could have shortened the consolidation time‐window, explaining the lack of the effects of CBD when given 3 hr after fear conditioning. At later time intervals, other brain areas than the DH might be involved in the impairment of memory consolidation by CBD. Indeed, infusing CBD into the prelimbic cortex 5 hr after acquisition has been reported to impair the consolidation of contextual memory (Rossignoli et al., 2017).
Immediate early genes such as Arc have been studied as markers of activation and plasticity in brain areas associated with memory consolidation, including the DH (Besnard et al., 2014; Lee, Everitt, & Thomas, 2004). Systemic administration of CBD either immediately or 1 hr after contextual fear conditioning reduced the levels of Arc protein expression in the DH. These findings suggest that the effects of CBD on memory consolidation involve changes in synaptic plasticity in the DH. Corroborating this proposal, the anxiolytic‐like effect of chronic treatment with CBD in stressed animals was associated with dendritic remodelling and increased neurogenesis in the hippocampus (Fogaça, Campos, Coelho, Duman, & Guimarães, 2018). However, further studies are needed to investigate if the reduced Arc expression in the DH and the decrease in contextually conditioned freezing observed after CBD administration are causally related.
To investigate how CBD impaired fear memory consolidation when given immediately and 1 hr after fear conditioning, we used selective antagonists of various receptors that have been associated with the effects of CBD (Campos et al., 2017). Pretreatment with AM251 or AM630 immediately after fear conditioning totally prevented the CBD effect on memory consolidation. These results agree with earlier findings showing that either systemic or intra‐DH antagonism of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors prevents the systemic effects of CBD on memory generalization (Stern et al., 2017). Besides, they are in line not only with the results showing the contribution of CB1 and/or CB2 receptors to consolidating aversive (Shoshan & Akirav, 2017) and non‐aversive memories (Clarke et al., 2008) but also with those showing that CBD‐induced facilitation in extinction learning and impairment of fear memory reconsolidation involves the activation of CB1 receptors (Bitencourt et al., 2008; Stern et al., 2012, 2017). It is worth mentioning that CBD is not a direct agonist of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors but can inhibit the metabolism of the endocannabinoid anandamide by the enzyme FAAH (Bisogno et al., 2001). The interference by CBD on memory consolidation reported here, therefore, could be indirectly mediated by the endogenous https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptor agonist, anandamide (Gonsiorek et al., 2000). The affinity of anandamide for CB1 receptors is higher than for CB2 receptors, where it acts as a partial agonist with low intrinsic activity (Gonsiorek et al., 2000). Of note, infusing the FAAH inhibitor URB597 into the DH immediately after fear conditioning also induced a memory consolidation impairment, which would support the participation of anandamide, at the early phase of this process (Busquets‐Garcia et al., 2011; Stern et al., 2017).
Neither https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR nor https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptor blockade prevented CBD‐induced memory consolidation disruption when the drug was administered 1 hr after fear conditioning. This result could be explained by the fact that the anandamide level reaches its peak in the DH as early as 10 min after a high‐intensity shock (Morena et al., 2014). Moreover, the anandamide effects are short‐lived due to rapid hydrolysis or reuptake into the synaptic terminals (Giuffrida, Beltramo, & Piomelli, 2001). In agreement with this observation, infusing URB597 into the DH 1 hr after fear conditioning no longer changed memory consolidation, suggesting that anandamide was not involved with memory consolidation at this stage.
Pretreatment with WAY100635 partly prevented the effects of CBD on memory consolidation when given immediately, but not at 1 hr, after fear conditioning. The dose of WAY100635 used in our experiments was based on reports showing that it could block the facilitatory effect on inhibitory avoidance of the 5‐HT1A receptor agonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7 in rats (De Paula Soares & Zangrossi, 2004) and was above its Ki. However, it cannot be ruled out that a higher dose of WAY100635 would completely prevent the effects of CBD on consolidation when given immediately after fear conditioning. There is evidence of active trafficking and recycling of 5‐HT1A receptors in the DH after contextual fear conditioning (Sase, Stork, Lubec, & Li, 2015). However, 5‐HT1A receptors are believed to play a more significant role in memory acquisition (Homberg, 2012). Indeed, animals with decreased 5‐HT neurotransmission present deficits in memory acquisition in the object recognition task. However, when 8‐OH‐DPAT or the antagonist WAY100635 was administered into the DH immediately after an auditory fear conditioning, no differences in memory consolidation were observed (Stiedl, Misane, Spiess, & Ogren, 2000). Corroborating the proposed role of 5‐HT1A receptors in memory acquisition, the blockade of these receptors in the nucleus accumbens prevents impairment of the acquisition of olfactory fear memory by CBD (Norris et al., 2016). Of note, CBD‐induced changes in contextual fear memory reconsolidation and extinction have also not been prevented by 5‐HT1A antagonism (Do Monte, Souza, Bitencourt, Kroon, & Takahashi, 2013; Stern et al., 2012). In contrast, activation of 5‐HT1A receptors mediated the anxiolytic‐like effects of CBD following acute treatment (Fogaça, Reis, Campos, & Guimarães, 2014). Intriguingly, in chronically stressed animals, the anxiolytic‐like effects of CBD were not associated with 5‐HT1A, but rather with the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors (Fogaça et al., 2018). Altogether, these findings indicate that multiple mechanisms could be involved with the effects of CBD on anxiety and learning and memory processes.
Pretreatment with ZM241385 also partly prevented the effects of CBD when infused immediately, but not 1 hr, after fear conditioning. The concentration of ZM241385 we used showed neuroprotective effects in previous work (Dall'Igna et al., 2003) and was in the Ki range of the drug. However, it is possible that a higher concentration would have totally prevented the CBD‐induced impairments in memory consolidation when given immediately after fear conditioning. CBD inhibits adenosine reuptake (Carrier, Auchampach, & Hillard, 2006), acting indirectly at adenosine receptors. There is evidence showing that the antagonism of A2A receptors blocks the effects of CBD in animal models of hypoxic‐ischaemia and multiple sclerosis (Castillo, Tolón, Fernández‐Ruiz, Romero, & Martinez‐Orgado, 2010; Mecha et al., 2013). In the object recognition task, administration of CBD, 10 min after training, impaired memory consolidation only in the presence of a CB1 receptor antagonist, and this effect was prevented by antagonism of A2A receptors (Aso, Fernández‐dueñas, López‐cano, Taura, & Watanabe, 2019). This finding might be explained by the existence of A2A and CB1 receptor heteromers (Ferré et al., 2010). Importantly, unlike the present study, in the work by Aso et al. (2019), all the drugs were systemically injected, and the antagonists were administered before memory acquisition. Consequently, interference with this memory phase cannot be excludedt. The partial involvement of A2A receptors in the CBD‐induced effects observed here, therefore, might be explained by differences in the protocols adopted and the administration routes. Moreover, the role of A2A receptors in modulating fear memory seems to depend on the brain area studied. For instance, both genetic and pharmacological inhibition of A2A receptors in the basolateral amygdala impaired the acquisition and retrieval of contextual fear memory in mice (Simões et al., 2016). Deletion of striatal A2A receptors increased contextual and tone fear conditioning. However, when the deletion was extended to the forebrain, only the tone fear conditioning was attenuated. When hippocampal A2A receptors were deleted, there was an impairment in contextual fear conditioning (Wei et al., 2014). Although these findings do suggest a role for A2A receptors in fear conditioning, their relative contribution in different memory phases remain unknown. Moreover, acquisition and consolidation mechanisms might overlap at very short intervals after learning, in the object recognition test. It is not known if the same happens in fear conditioning (Akkerman, Blokland, & Prickaerts, 2015). Thus, we cannot exclude the possibility that the partial reversal of the effects of CBD, induced by 5‐HT1A or A2A blockade immediately after fear conditioning, might be mediated by residual participation of these receptors in the acquisition.
PPARγ blockade did not induce any effect by itself and did not prevent the CBD‐impairing effects on memory consolidation when administered immediately after fear conditioning. However, GW9662 totally prevented the effects of CBD on consolidation, when administered 1 hr after fear conditioning. The involvement of PPARγ receptors in the effects of CBD has been described in animal models involving neuroinflammation. CBD reduced the neuroinflammation and promoted neurogenesis in animal models of Alzheimer's disease through PPARγ activation (Esposito et al., 2011; Fernández‐Ruiz et al., 2013). The impairing effect of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4865 on LTP in hippocampal slices was prevented by CBD infusion, an effect mediated by PPARγ, but not by CB1, 5‐HT1A, or A2A receptors (Hughes & Herron, 2018). Moreover, the protective effects of CBD in https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=86‐induced dyskinesia and LPS‐stimulated microglial activation were also mediated through PPARγ receptors (Sonego et al., 2018). Of potential relevance to this discussion are the results suggesting the involvement of the immune system and microglial activation in Pavlovian fear memory acquisition, consolidation, and extinction (Adamsky et al., 2018; Young et al., 2018). Activation of PPARγ receptors promotes the inhibition of the NF‐κB signalling pathway, which mediates pro‐inflammatory mechanisms and the late phase of LTP (Ryan et al., 2012). Therefore, it is possible to suggest that 1 hr after fear conditioning, there is an engagement of inflammatory‐related mechanisms in memory consolidation. Further, the PPARγ receptor is activated by endocannabinoids such as anandamide. Activation of membrane CB1 receptors triggers an intracellular cascade that activates the PPARγ receptors (Pistis & O'Sullivan, 2017). Therefore, a temporal shift in the mechanism observed here might depend on the initial activation of the endocannabinoid system. However, FAAH inhibition 1 hr after fear conditioning failed to impair memory consolidation. As CBD can be transported intracellularly by fatty acid‐binding proteins (Elmes et al., 2015), it is possible that the memory effects observed when the drug was administered 1 hr after fear conditioning depend on the direct activation of PPARγ receptors. Further studies are necessary to examine this possibility.
In summary, the present results show that multiple mechanisms mediate the interference by CBD with memory consolidation in the DH. The recruitment of these mechanisms is time‐dependent, involving an initial activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56&familyId=13&familyType=GPCR and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57&familyId=13&familyType=GPCR receptors, followed by a later engagement of PPARγ receptors. These findings bring new insights on how to mitigate fearful memories, highlighting the importance of exploiting the consolidation time‐window.
AUTHOR CONTRIBUTIONS
A.M.R. and T.R.S. conducted the experiments. All authors analysed the data, elaborated on the work design, interpreted the results, and wrote the paper.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
Our study was supported by Brazilian grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2017/24304‐0), Fundação Araucária (convênio 006/2017), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 409615/2016‐1), and CAPES (Finance code 001). FAPESP, Fundação Araucária, CNPq, and CAPES had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication. We thank Dr. Ana Paula S. Dornellas for helping us to conduct the western blotting study; Dr. Joice Maria da Cunha from the Department of Pharmacology, Federal University of Parana for kindly donating WAY100635; Dr. Rui Daniel S. Prediger from the Department of Pharmacology, Federal University of Santa Catarina for kindly donating ZM241385; and Phytoplant for kindly donating cannabidiol.
Raymundi AM, da Silva TR, Zampronio AR, Guimarães FS, Bertoglio LJ, Stern CAJ. A time‐dependent contribution of hippocampal CB1, CB2 and PPARγ receptors to cannabidiol‐induced disruption of fear memory consolidation. Br J Pharmacol. 2020;177:945–957. 10.1111/bph.14895
REFERENCES
- Adamsky, A. , Kol, A. , Kreisel, T. , Doron, A. , Ozeri‐Engelhard, N. , Melcer, T. , … Goshen, I. (2018). Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell, 174(1), 59–71. 10.1016/j.cell.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Akkerman, S. , Blokland, A. , & Prickaerts, J. (2015). Possible overlapping time frames of acquisition and consolidation phases in object memory processes: A pharmacological approach. Learning and Memory, 23(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Alistair, P. , … CGTP Collaborators (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso, E. , Fernández‐dueñas, V. , López‐cano, M. , Taura, J. , & Watanabe, M. (2019). Adenosine A2A‐cannabinoid CB1 receptor heteromers in the hippocampus: Cannabidiol blunts Δ9‐tetrahydrocannabinol‐induced cognitive impairment. Molecular Neurobiology, 56, 5382–5391. 10.1007/s12035-018-1456-3 [DOI] [PubMed] [Google Scholar]
- Besnard, A. , Laroche, S. , & Caboche, J. (2014). Comparative dynamics of MAPK/ERK signalling components and immediate early genes in the hippocampus and amygdala following contextual fear conditioning and retrieval. Brain Structure and Function, 219(1), 415–430. 10.1007/s00429-013-0505-y [DOI] [PubMed] [Google Scholar]
- Bisogno, T. , Hanus, L. , De Petrocellis, L. , Tchilibon, S. , Ponde, D. E. , Brandi, I. , … Mechoulam, R. (2001). Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology, 134(4), 845–852. 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt, R. M. , Pamplona, F. A. , & Takahashi, R. N. (2008). Facilitation of contextual fear memory extinction and anti‐anxiogenic effects of AM404 and cannabidiol in conditioned rats. European Neuropsychopharmacology, 18(12), 849–859. 10.1016/j.euroneuro.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Busquets‐Garcia, A. , Puighermanal, E. , Pastor, A. , de la Torre, R. , Maldonado, R. , & Ozaita, A. (2011). Differential role of anandamide and 2‐arachidonoylglycerol in memory and anxiety‐like responses. Biological Psychiatry, 70(5), 479–486. 10.1016/j.biopsych.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Campos, A. C. , Fogaça, M. V. , Scarante, F. F. , Joca, S. R. L. , Sales, A. J. , Gomes, F. V. , … Guimarães, F. S. (2017). Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Frontiers in Pharmacology, 8, 269–287. 10.3389/fphar.2017.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier, E. J. , Auchampach, J. A. , & Hillard, C. J. (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proceedings of the National Academy of Sciences of the United States of America, 103(20), 7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande, M. A. , Haubrich, J. , Pedraza, L. K. , Popik, B. , Quillfeldt, J. A. , & De Oliveira Alvares, L. (2018). Synaptic consolidation as a temporally variable process: Uncovering the parameters modulating its time‐course. Neurobiology of Learning and Memory, 150, 42–47. 10.1016/j.nlm.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Castillo, A. , Tolón, M. R. , Fernández‐Ruiz, J. , Romero, J. , & Martinez‐Orgado, J. (2010). The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic‐ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiology of Disease, 37(2), 434–440. 10.1016/j.nbd.2009.10.023 [DOI] [PubMed] [Google Scholar]
- Clarke, J. R. , Rossato, J. I. , Monteiro, S. , Bevilaqua, L. R. M. , Izquierdo, I. , & Cammarota, M. (2008). Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long‐term memory. Neurobiology of Learning and Memory, 90(2), 374–381. 10.1016/j.nlm.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Igna, O. P. , Porciúncula, L. O. , Souza, D. O. , Cunha, R. A. , & Lara, D. R. (2003). Neuroprotection by caffeine and adenosine A2A receptor blockade of β‐amyloid neurotoxicity. British Journal of Pharmacology, 38(7), 1207–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paula Soares, V. , & Zangrossi, H. (2004). Involvement of 5‐HT1A and 5‐HT2 receptors of the dorsal periaqueductal gray in the regulation of the defensive behaviors generated by the elevated T‐maze. Brain Research Bulletin, 64(2), 181–188. 10.1016/j.brainresbull.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Denner, L. A. , Rodriguez‐Rivera, J. , Haidacher, S. J. , Jahrling, J. B. , Carmical, J. R. , Hernandez, C. M. , … Dineley, K. T. (2012). Cognitive enhancement with rosiglitazone links the hippocampal PPAR and ERK MAPK signaling pathways. The Journal of Neuroscience, 32(47), 16725–16735. 10.1523/JNEUROSCI.2153-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Monte, F. H. , Souza, R. R. , Bitencourt, R. M. , Kroon, J. A. , & Takahashi, R. N. (2013). Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behavioural Brain Research, 250(1), 23–27. 10.1016/j.bbr.2013.04.045 [DOI] [PubMed] [Google Scholar]
- Ehlers, A. , Hackmann, A. , & Michael, T. (2004). Intrusive re‐experiencing in post‐traumatic stress disorder: Phenomenology, theory, and therapy. Memory, 12(4), 403–415. 10.1080/09658210444000025 [DOI] [PubMed] [Google Scholar]
- Ellis, P. D. (2010). The essential guide to effect sizes: Statistical power, meta‐analysis, and the interpretation of research results. Cambridge University Press. [Google Scholar]
- Elmes, M. W. , Kaczocha, M. , Berger, W. T. , Leung, K. , Ralph, B. P. , Wang, L. , … Deutsch, D. G. (2015). Fatty acid‐binding proteins (FABPs) are intracellular carriers for Δ9‐tetrahydrocannabinol (THC) and cannabidiol (CBD). The Journal of Biological Chemistry, 290(14), 8711–8721. 10.1074/jbc.M114.618447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, G. , Scuderi, C. , Valenza, M. , Togna, G. I. , Latina, V. , De Filippis, D. , … Steardo, L. (2011). Cannabidiol reduces Aβ‐induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE, 6(12), 1–8, e28668 10.1371/journal.pone.0028668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Ruiz, J. , Sagredo, O. , Pazos, M. R. , García, C. , Pertwee, R. , Mechoulam, R. , & Martínez‐Orgado, J. (2013). Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? British Journal of Clinical Pharmacology, 75(2), 323–333. 10.1111/j.1365-2125.2012.04341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré, S. , Lluís, C. , Justinova, Z. , Quiroz, C. , Orru, M. , Navarro, G. , … Goldberg, S. R. (2010). Adenosine‐cannabinoid receptor interactions. Implications for striatal function. British Journal of Pharmacolology, 160(3), 443–453. 10.1111/j.1476-5381.2010.00723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça, M. V. , Campos, A. C. , Coelho, L. D. , Duman, R. S. , & Guimarães, F. S. (2018). The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology, 135, 22–33. 10.1016/j.neuropharm.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Fogaça, M. V. , Reis, F. M. C. V. , Campos, A. C. , & Guimarães, F. S. (2014). Effects of intra‐prelimbic prefrontal cortex injection of cannabidiol on anxiety‐like behavior: Involvement of 5HT1A receptors and previous stressful experience. European Neuropsychopharmacology, 24(3), 410–419. 10.1016/j.euroneuro.2013.10.012 [DOI] [PubMed] [Google Scholar]
- Gafford, G. M. , Parsons, R. G. , & Helmstetter, F. J. (2005). Effects of post‐training hippocampal injections of midazolam on fear conditioning. Learning & Memory, 12(6), 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazarini, L. , Stern, C. A. , Piornedo, R. R. , Takahashi, R. N. , & Bertoglio, L. J. (2015). PTSD‐like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. International Journal of Neuropsychopharmacology, 18(1), 1–9, pyu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida, A. , Beltramo, M. , & Piomelli, D. (2001). Mechanisms of endocannabinoid inactivation: Biochemistry and pharmacology. The Journal of Pharmacology and Experimental Therapeutics, 298(1), 7–14. [PubMed] [Google Scholar]
- Gonsiorek, W. , Lunn, C. , Fan, X. , Narula, S. , Lundell, D. , & Hipkin, R. W. (2000). Endocannabinoid 2‐arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Molecular Pharmacology, 57(5), 1045–1050. [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg, J. R. (2012). Serotonergic modulation of conditioned fear. Scientifica (Cairo), 2012, 1–16, 821549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, B. , & Herron, C. E. (2018). Cannabidiol reverses deficits in hippocampal LTP in a model of Alzheimer's disease. Neurochemical Research, 44(3), 703–713. [DOI] [PubMed] [Google Scholar]
- Igaz, L. M. , Vianna, M. R. M. , Medina, J. H. , & Izquierdo, I. (2002). Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear‐motivated learning. The Journal of Neuroscience, 22(15), 6781–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, I. , & Medina, J. H. (1997). Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiology of Learning and Memory, 68(3), 285–316. [DOI] [PubMed] [Google Scholar]
- Jahrling, J. B. , Hernandez, C. M. , Denner, L. , & Dineley, K. T. (2014). PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer's disease‐related cognitive enhancement. The Journal of Neuroscience, 34(11), 4054–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , Altman, D. G. , & NC3Rs Reporting Guidelines Working Group (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160(7), 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht, R. , & LeDoux, J. (2004). Structural plasticity and memory. Nature Reviews Neuroscience, 5(1), 45–54. [DOI] [PubMed] [Google Scholar]
- Lee, J. L. C. , Bertoglio, L. J. , Guimarães, F. S. , & Stevenson, C. W. (2017). Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety‐related and substance abuse disorders. British Journal of Pharmacology, 174(19), 3242–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. L. C. , Everitt, B. J. , & Thomas, K. L. (2004). Independent cellular processes for consolidation and reconsolidation. Science, 304(5672), 839–844. [DOI] [PubMed] [Google Scholar]
- Lunardi, P. , Sachser, R. M. , Sierra, R. O. , Pedraza, L. K. , Medina, C. , De la Fuente, V. , … De Oliveira Alvares, L. (2017). Effects of hippocampal LIMK inhibition on memory acquisition, consolidation, retrieval, reconsolidation, and extinction. Molecular Neurobiology, 55(2), 958–967. 10.1007/s12035-016-0361-x [DOI] [PubMed] [Google Scholar]
- McGaugh, J. L. (2000). Memory—A century of consolidation. Science, 287(5451), 248–251. [DOI] [PubMed] [Google Scholar]
- Mecha, M. , Feliú, A. , Iñigo, P. M. , Mestre, L. , Carrillo‐Salinas, F. J. , & Guaza, C. (2013). Cannabidiol provides long‐lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiology of Disease, 59, 141–150. [DOI] [PubMed] [Google Scholar]
- Morena, M. , Roozendaal, B. , Trezza, V. , Ratano, P. , Peloso, A. , Hauer, D. , … Campolongo, P. (2014). Endogenous cannabinoid release within prefrontal‐limbic pathways affects memory consolidation of emotional training. Proceedings of the National Academy of Sciences of the United States of America, 111(51), 18333–18338. 10.1073/pnas.1420285111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S. , Farioli‐Vecchioli, S. , & Cerù, M. P. (2004). Immunolocalization of peroxisome proliferator‐activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience, 123(1), 131–145. [DOI] [PubMed] [Google Scholar]
- Norris, C. , Loureiro, M. , Kramar, C. , Zunder, J. , Renard, J. , Rushlow, W. , & Laviolette, S. R. (2016). Cannabidiol modulates fear memory formation through interactions with serotonergic transmission in the mesolimbic system. Neuropsychopharmacology, 41(12), 2839–2850. 10.1038/npp.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G. , & Watson, C. (2009). The rat brain in stereotaxic coordinates, compact (sixth ed.). San Diego: Academic Press. [Google Scholar]
- Pistis, M. , & O'Sullivan, S. E. (2017). The role of nuclear hormone receptors in cannabinoid function. Advances in Pharmacology, 80, 291–328. 10.1016/bs.apha.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Rossignoli, M. T. , Lopes‐Aguiar, C. , Ruggiero, R. N. , Do Val da Silva, R. A. , Bueno‐Junior, L. S. , Kandratavicius, L. , … Romcy‐Pereira, R. N. (2017). Selective post‐training time window for memory consolidation interference of cannabidiol into the prefrontal cortex: Reduced dopaminergic modulation and immediate gene expression in limbic circuits. Neuroscience, 350, 85–93. 10.1016/j.neuroscience.2017.03.019 [DOI] [PubMed] [Google Scholar]
- Ryan, M. M. , Ryan, B. , Kyrke‐Smith, M. , Logan, B. , Tate, W. P. , Abraham, W. C. , & Williams, J. M. (2012). Temporal profiling of gene networks associated with the late phase of long‐term potentiation in vivo. PLoS ONE, 7(7), 1–14, e40538 10.1371/journal.pone.0040538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sase, S. , Stork, O. , Lubec, G. , & Li, L. (2015). Contextual fear conditioning modulates hippocampal AMPA‐, GluN1‐ and serotonin receptor 5‐HT1A‐containing receptor complexes. Behavioural Brain Research, 278, 44–54. [DOI] [PubMed] [Google Scholar]
- Schmidt, S. D. , Furini, C. R. G. , Zinn, C. G. , Cavalcante, L. E. , Ferreira, F. F. , Behling, J. A. K. , … Izquierdo, I. (2017). Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiology of Learning and Memory, 142(Pt A), 48–54. 10.1016/j.nlm.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Segev, A. , Korem, N. , Mizrachi Zer‐Aviv, T. , Abush, H. , Lange, R. , Sauber, G. , … Akirav, I. (2018). Role of endocannabinoids in the hippocampus and amygdala in emotional memory and plasticity. Neuropsychopharmacology, 43(10), 2017–2027. 10.1038/s41386-018-0135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan, N. , & Akirav, I. (2017). The effects of cannabinoid receptors activation and glucocorticoid receptors deactivation in the amygdala and hippocampus on the consolidation of a traumatic event. Neurobiology of Learning and Memory, 144, 248–258. [DOI] [PubMed] [Google Scholar]
- Simões, A. P. , Machado, N. J. , Gonçalves, N. , Kaster, M. P. , Simões, A. T. , Nunes, A. , … Cunha, R. A. (2016). Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology, 41(12), 2862–2871. 10.1038/npp.2016.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonego, A. B. , Prado, D. S. , Vale, G. T. , Sepulveda‐Diaz, J. E. , Cunha, T. M. , Tirapelli, C. R. , … Guimarães, F. S. (2018). Cannabidiol prevents haloperidol‐induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain, Behavior, and Immunity, 74, 241–251. 10.1016/j.bbi.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Stern, C. A. J. , da Silva, T. R. , Raymundi, A. M. , de Souza, C. P. , Hiroaki‐Sato, V. A. , Kato, L. , … Bertoglio, L. J. (2017). Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors. Neuropharmacology, 125, 220–230. 10.1016/j.neuropharm.2017.07.024 [DOI] [PubMed] [Google Scholar]
- Stern, C. A. J. , Gazarini, L. , Takahashi, R. N. , Guimarães, F. S. , & Bertoglio, L. J. (2012). On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology, 37(9), 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl, O. , Misane, I. , Spiess, J. , & Ogren, S. O. (2000). Involvement of the 5‐HT1A receptors in classical fear conditioning in C57BL/6J mice. The Journal of Neuroscience, 20(22), 8515–8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, C. J. , Augusto, E. , Gomes, C. A. , Singer, P. , Wang, Y. , Boison, D. , … Chen, J. F. (2014). Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biological Psychiatry, 75(11), 855–863. 10.1016/j.biopsych.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M. B. , Howell, L. L. , Hopkins, L. , Moshfegh, C. , Yu, Z. , Clubb, L. , … Marvar, P. J. (2018). A peripheral immune response to remembering trauma contributes to the maintenance of fear memory in mice. Psychoneuroendocrinology, 94, 143–151. 10.1016/j.psyneuen.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


