Abstract
Hydrogen sulfide (H2S) is a signalling molecule that regulates neuronal transmission, vascular tone, cytoprotection, inflammatory responses, angiogenesis, and oxygen sensing. Some of these functions have recently been ascribed to its oxidized form polysulfides (H2Sn), which can be produced by 3‐mercaptopyruvate sulfurtransferase (MPST), also known as a H2S‐producing enzyme. H2Sn activate ion channels, tumour suppressors, transcription factors, and protein kinases. H2Sn S‐sulfurate (S‐sulfhydrate) cysteine residues of these target proteins to modify their activity by inducing conformational changes through the formation of a disulfide bridge between the two cysteine residues involved. The chemical interaction between H2S and NO also generates H2Sn, which may be a chemical entity that exerts the synergistic effect of H2S and NO. MPST also produces redox regulators cysteine persulfide (CysSSH), GSH persulfide (GSSH), and persulfurated proteins. In addition to MPST, haemoproteins such as haemoglobin, myoglobin, neuroglobin, and catalase as well as SOD can produce H2Sn, and sulfide quinone oxidoreductase and cysteinyl tRNA synthetase can make GSSH and CysSSH. This review focuses on the recent progress in the study of the production and physiological roles of these persulfurated and polysulfurated molecules.
Linked Articles
This article is part of a themed section on Hydrogen Sulfide in Biology & Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.4/issuetoc
Abbreviations
- CARS
cysteinyl tRNA synthetase
- CBS
cystathionine β‐synthase
- CSE
cystathionine γ‐lyase
- CysSSH
cysteine persulfide
- DAO
d‐aminoacid oxidase
- GSSH
GSH persulfide
- H2S
hydrogen sulfide
- H2Sn
hydrogen polysulfides
- HNO
nitroxyl
- Keap1
kelch ECH‐associating protein 1
- 3MP
3‐mercaptopyruvate
- MPST
3‐mercaptopyruvate sulfurtransferase
- Nrf2
nuclear factor erythroid 2‐related factor 2
- PD
Parkinson's disease
- PIP2
phospholipid phosphatidylinositol (4,5)‐biphosphate
- PTEN
tumour suppressor phosphatase and tensin homologue
- SQR
sulfur quinon oxidoreductase
- SSNO−
nitrosopersulfide
- TRPA1
transient receptor potential ankyrin 1
1. INTRODUCTION
Survivors of hydrogen sulfide (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9532) toxicity have memory loss in common (Reiffenstein, Hulbert, & Roth, 1992), and exposure of animals to H2S induces changes in the levels of neurotransmitters in the brain (Warenycia, Smith, Blashko, Kombian, & Reiffenstein, 1989). The endogenous levels of H2S measured in the brains of rats, bovines, and humans, suggested H2S has a physiological role in the brain, although these measurements were later found to be overestimated because of the method used (Goodwin et al., 1989; Savage & Gould, 1990; Warenycia, Goodwin, et al., 1989). H2S facilitates the induction of hippocampal LTP, a synaptic model of memory formation, by enhancing the activity of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 (Abe & Kimura, 1996). The effect was thought to be induced by the reducing activity of H2S on the disulfide bond at the hinge of a ligand‐binding domain of the receptors. This mechanism is based on the discovery of the enhancing effect of dithiothreitol (DTT) on NMDA receptors (Aizenman, Lipton, & Loring, 1989). However, H2S induced LTP more efficiently than DTT. This observation suggested that H2S has an additional effect to its reduction of NMDA receptors (Abe & Kimura, 1996).
H2S induced Ca2+ influx in astrocytes, a type of glia, which surround the synapses and release gliotransmitters to the synaptic clefts to modulate neuronal transmission (Nagai, Tsugane, Oka, & Kimura, 2004). Subsequently, polysulfides (H2Sn), oxidized forms of H2S, were found to be much more potent than H2S at inducing Ca2+ influx by activating transient receptor potential ankyrin 1 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485) channels, and endogenous H2Sn were identified in the brain (Kimura et al., 2013; Nagai, Tsugane, Oka, & Kimura, 2006; Oosumi et al., 2010; Streng et al., 2008). The activation of TRPA1 channels in astrocytes induces the release of a gliotransmitter http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4171, which enhances the activity of NMDA receptors, leading to the induction of LTP (Shigetomi, Jackson‐Weaver, Huckstepp, O'Dell, & Khakh, 2013; Shigetomi, Tong, Kwan, Corey, & Khakh, 2012). The activation of TRPA1 channels by H2Sn may be a mechanism in addition to the reduction of NMDA receptors by H2S for the induction of LTP (Kimura, 2015; Kimura et al., 2013).
Various other proteins whose activities are regulated by H2Sn have been identified: a tumour suppressor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2497 (PTEN; Greiner et al., 2013), a transcription factor complex http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2757 (Keap1)/nuclear factor‐like 2 (http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=Nrf2&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database; Koike, Ogasawara, Shibuya, Kimura, & Ishii, 2013), PKG1α (Stubbert et al., 2014), and GAPDH (Jarosz et al., 2015). A mechanism of H2S activation on target proteins has been proposed to be mediated by S‐sulfuration (S‐sulfhydration) of their cysteine residues leading to their conformational changes (Mustafa et al., 2009). However, it was later suggested that persulfurated molecules such as H2Sn S‐sulfurate cysteine residues, whereas H2S reacts with oxidized cysteine residues to S‐sulfurate them (Kimura, 2015).
A cytoprotective effect of H2S has been found in various tissues/organs. In neurons, H2S increases the intracellular concentrations of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737, a major intracellular antioxidant, by facilitating the transport of cystine and cysteine, a substrate for GSH production, and also by enhancing the activity of glutamate cysteine ligase, also known as γ‐glutamyl cysteine synthetase, a rate‐limiting enzyme for GSH production (Kimura, Dargusch, Schubert, & Kimura, 2006; Kimura, Goto, & Kimura, 2010; Kimura & Kimura, 2004). In the heart, H2S suppresses oxygen consumption by cardiac mitochondria and preserves mitochondrial function and membrane integrity against ischaemia–reperfusion injury through the activation of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1249 (Elrod et al., 2007; King et al., 2014). H2S protects the kidney against apoptosis and inflammation caused by ischaemia–reperfusion injury by suppressing the phosphorylation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=288 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1619, while recovering the expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2844 (Bos et al., 2009; Tripatara et al., 2008). Several clinical trials and preclinical studies on H2S‐based therapeutic agents are in progress (Wallace & Wang, 2015).
By increasing GSH levels different from that of H2S, H2Sn protect neurons from oxidative stress. H2Sn release Nrf2 from the Keap1/Nrf2 complex to translocate it to the nucleus to up‐regulate the transcription of antioxidant genes including those of enzymes involved in the production of GSH (Koike et al., 2013). In contrast, sulfite, a further oxidized molecule of H2S and H2Sn, protects neurons by converting extracellular cystine to cysteine, which is more efficiently transported into cells than cystine and used for the production of GSH (Kimura et al., 2010; Kimura, Shibuya, & Kimura, 2018).
3‐Mercaptopyruvate sulfurtransferase (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279) produces H2S from http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5118 (3MP) by interacting with thioredoxin or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6738 (Mikami, Shibuya, Kimura, Ogasawara, & Kimura, 2011). We found endogenous H2Sn in the brain and that the levels of persulfurated and polysulfurated molecules are increased in cells expressing MPST, whereas those are decreased in the brain of MPST knockout mice (Kimura et al., 2013; Kimura et al., 2015; Kimura et al., 2017; Koike et al., 2017). Hylin and Wood (1959) demonstrated that protein‐bound sulfane sulfur can be produced from 3MP. MPST was found to produce free sulfane sulfur such as H2Sn, cysteine persulfide (CysSSH), GSH persulfide (GSSH), and persulfurated proteins (Kimura et al., 2015; Kimura et al., 2017; Koike et al., 2017; Nagahara, Koike, Nirasawa, Kimura, & Ogasawara, 2018). SOD and catalase can also generate H2Sn (Olson et al., 2017; Olson et al., 2018), sulfide quinone oxidoreductase (SQR) generates GSSH (Landry, Ballou, & Banerjee, 2017; Mishanina, Yadav, Ballou, & Banerjee, 2015), and cysteinyl tRNA synthetase (CARS) produces CysSSH (Akaike et al., 2017).
H2S weakly relaxes vascular smooth muscle, whereas in the presence of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509, it exerts much greater effects (Hosoki, Matsuki, & Kimura, 1997). It is the first description of the synergistic effect of H2S with NO, and a similar effect was also reported in the ileum (Teague, Asiedu, & Moore, 2002). Concerning a mechanism for the synergy between H2S and NO, several potential chemical entities generated by the interaction of H2S and NO have been proposed: nitrosothiol (Whiteman et al., 2006), thionitrous acid (Filipovic et al., 2012), nitroxyl (HNO; Eberhardt et al., 2014), nitrosopersulfide (SSNO−; Cortese‐Krott et al., 2014), and H2Sn (Cortese‐Krott et al., 2015; Eberhardt et al., 2014; Miyamoto et al., 2017; Moustafa & Habara, 2016).
Another crosstalk between H2S and NO has also been demonstrated. H2S facilitates the activity of endothelial NOS to increase the production of NO, which exerts cytoprotective effect on various tissues (Bir et al., 2012; Coletta et al., 2012; King et al., 2014; Minamishima et al., 2009).
1.1. S‐sulfurated molecules‐bound sulfane sulfur
There are two forms of sulfur that can release H2S in cells. Acid‐labile sulfur, which releases H2S under acidic conditions, is mostly in iron–sulfur cluster at the active sites of enzymes belonging to the respiratory chain (Ishigami et al., 2009; Ogasawara, Ishii, Togawa, & Tanabe, 1993; Warenycia, Goodwin, et al., 1989). Another form is bound sulfane sulfur, which releases H2S under reducing conditions, including H2Sn, CysSSH, GSSH, and persulfurated proteins (Figure 1a; Ishigami et al., 2009; Ogasawara et al., 1993; Warenycia et al., 1990). Extracellularly applied H2S and H2Sn as well as those produced by MPST in cells are stored as bound sulfane sulfur, which may be involved in the cellular signalling as well as redox homeostasis (Ishigami et al., 2009; Kimura et al., 2017; Shibuya et al., 2009; Shibuya et al., 2013).
Figure 1.
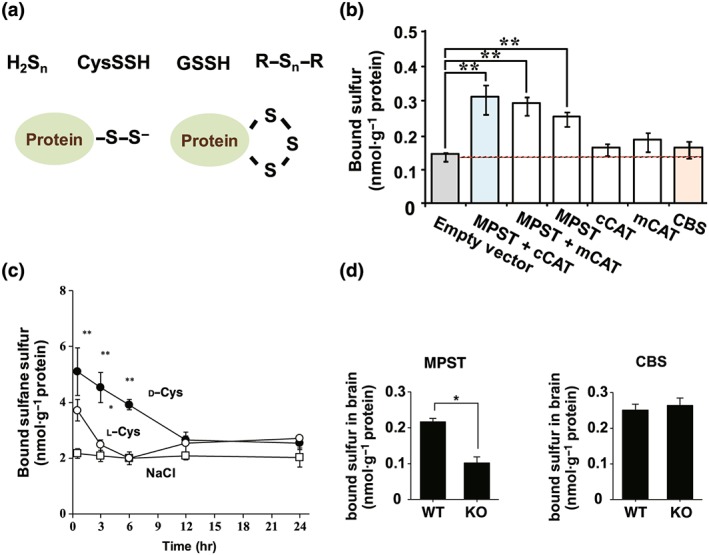
Endogenous hydrogen sulfide (H2S), hydrogen polysulfides (H2Sn), cysteine persulfide (CysSSH), GSH persulfide (GSSH), and persulfurated proteins produced by 3‐mercaptopyruvate sulfurtransferase (MPST) are stored as bound sulfane sulfur in cells and tissues. (a) Various forms of bound sulfane sulfur, which releases H2S under reducing conditions (Ishigami et al., 2009; Ogasawara, Isoda, & Tanabe, 1994). (b) Cells expressing MPST contain greater levels of bound sulfane sulfur than control cells (Shibuya et al., 2009). (c) The administration of d‐cysteine or l‐cysteine, which is metabolized by d‐amino acid oxidase or cysteine aminotransferase, respectively, to 3‐mercaptopyruvate (3MP), to mice increased the levels of bound sulfane sulfur in tissues expressing these enzymes (Shibuya et al., 2013). (d) Brains of the wild‐type (WT) mice contain approximately twice as much bound sulfane sulfur as those in MPST knockout (KO) mice (Kimura et al., 2017). Figures in Shibuya et al. (2009), Shibuya et al. (2013), and Kimura et al. (2017) were modified. CBS: cystathionine β‐synthase
Bound sulfane sulfur was initially identified as nonacid labile H2S liberated by DTT from brains of H2S‐poisoned animals (Warenycia et al., 1990), and it was later designated as bound sulfur (Ogasawara et al., 1993). Tissue homogenates absorbed H2S, which contained a trace amount of H2Sn, and stored it as bound sulfane sulfur (Ishigami et al., 2009). Endogenous H2S, H2Sn, CysSSH, GSSH, and persulfurated proteins produced by MPST can also be stored as bound sulfane sulfur in cells and tissues. This is based on the following observations: (a) cells expressing MPST contain greater levels of bound sulfane sulfur than control cells, whereas those expressing MPST defective mutants do not (Figure 1b; Kimura et al., 2015; Kimura et al., 2017; Shibuya et al., 2009). (b) The administration of d‐ or l‐cysteine, which is metabolized by d‐amino acid oxidase or cysteine aminotransferase (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279#1445), respectively, to 3MP, to mice increased the levels of bound sulfane sulfur in tissues expressing these enzymes (Figure 1c; Shibuya et al., 2013). (c) The brains of wild‐type mice contain approximately twice as much of bound sulfane sulfur as those in MPST knockout mice (Figure 1d; Kimura et al., 2017).
Bound sulfane sulfur or S‐sulfurated molecules has been proposed as an intracellular H2S storage that may release H2S in response to neuronal activity (Ishigami et al., 2009). In the presence of endogenous concentrations of cysteine, GSH, or dihydrolipoic acid at pH 8.4, H2S is released from lysates of neurons and astrocytes (Ishigami et al., 2009; Mikami et al., 2011). When neurons are repetitively excited, sodium ion enters and potassium ions are released from cells, leading to high extracellular potassium concentrations, which cause depolarization of surrounding astrocytes (Somjen, 1979). The Na+/HCO3 − cotransporter is activated in astrocytes to recover from depolarization and restore them to their quiescent condition, and HCO3 − causes alkalinization of the cells (Brookes & Turner, 1994). Although approximately 10% of astrocytes reached pH 8.4 in 10 mM K+, H2S released in the superfusion medium has not been successfully measured (Ishigami et al., 2009).
1.2. Cellular signalling by S‐sulfuration (S‐sulfhydration)
S‐sulfuration of cysteine residues in target proteins that changes their activities was initially reported for serine dehydratase (Kato, Ogura, & Suda, 1966). In the presence of cystine together with cystathionine γ‐lyase (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279#1444), or the addition of elemental sulfur to the reaction mixture, the activity of serine dehydratase was suppressed. In this experiment, cystine and CSE were applied to produce CysSSH (Cavallini, Marco, Mondavi, & Mori, 1960), which S‐sulfurated serine dehydratase. However, this may not occur in cells, as CSE is a cytosolic enzyme, whereas cystine only exists in the extracellular milieu not in cytosol (Yadav et al., 2016). The activities of several other enzymes, such as homoserine dehydratase (Pestaña & Sols, 1970), ornithine decarboxylase (Murakami, Kameji, & Hayashi, 1984), tyrosine aminotransferase (Hargrove & Wichman, 1987), and cytochrome P‐450 reductase (Ogasawara, Isoda, & Tanabe, 1998), were found to be suppressed by S‐sulfuration.
The existence of the endogenous S‐sulfurated molecules has been found by Warenycia et al. (1990) as these release H2S when DTT is applied to brain homogenates. They also found that DTT is significantly protective against H2S poisoning. Their proposed mechanism is that cysteine disulfide bonds in proteins may be reduced by H2S to persulfides, which is toxic to cells, whereas the liberation of persulfides by DTT leads to detoxification (Warenycia et al., 1990). However, after H2S reduces disulfide bond to persulfide, which can be further reduced by H2S to thiol, then DTT can no longer exert any protective effect on H2S poisoning. Nevertheless, some of the cysteine residues may be oxidized to Cys‐SOH or nitrosylated to Cys‐SNO, and these cysteine residues can be S‐sulfurated by H2S to CysSSH, which can be reduced by DTT to Cys‐SH and to a lesser extent by H2S (Figure 2; Kabil, Motl, & Banerjee, 2014; Kimura, 2016; Kimura et al., 2017; Mishanina, Libiad, & Banerjee, 2015). H2S inhibits http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=766, which catabolizes monoamines, resulting in an increase in the levels of catecholamines and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5 in the brain (Warenycia, Smith, et al., 1989).
Figure 2.
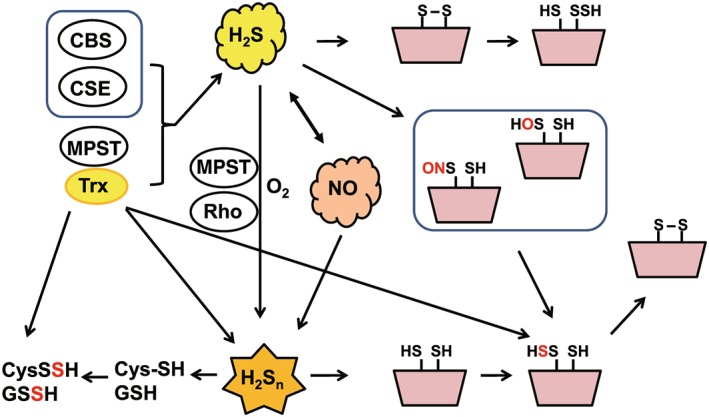
Production of hydrogen sulfide (H2S), hydrogen polysulfides (H2Sn), and other persulfurated molecules and their modification of target proteins. H2S is produced by cystathionine β‐synthase (CBS), cystathionine γ‐lyase (CSE), and 3‐mercaptopyruvate sulfurtransferase (MPST) and reduces cysteine disulfide bonds in the target proteins to modify their activity, whereas it S‐sulfurates oxidized cysteine residues such as Cys‐SOH or Cys‐SNO. H2Sn, which are produced by MPST and also by the chemical interaction between H2S and NO, S‐sulfurate cysteine residues of the target protein (Kimura et al., 2015; Miyamoto et al., 2017). H2Sn can also be produced by the oxidation of H2S where MPST and rhodanese (Rho) may be also be involved (Kimura et al., 2015). MPST can produce cysteine persulfide (CysSSH) and GSH persulfide (GSSH) directly or through the production of H2Sn (Kimura et al., 2017). The figure is a reprint of Kimura (2018)
S‐sulfuration (S‐sulfhydration) has been proposed by Snyder and colleagues as a mode of action of H2S (Mustafa et al., 2009). It is compared with the effects of protein phosphorylation. Both S‐sulfuration and phosphorylation induce conformational changes in the target proteins to modify their activity. Phosphorylation is the covalent addition of a phosphate group to serine, threonine, and tyrosine residues by specific kinases, and two negative charges carried by each phosphate group, which attract positively charged amino acid residues located nearby, leading to a conformational change (Figure 3a). In contrast, S‐sulfuration can add one negative charge to the target protein. Cys‐SH has a pKa of 8.29, and the predicted pKa for CysSSH is 4.34 (Cuevasanta et al., 2015). At physiological pH, CysSSH dissociates to CysSS− and H+, whereas Cys‐SH exists as a nondissociated form. S‐sulfuration is induced by either diffusion of H2S (or H2Sn) or transsulfuration by MPST (Figure 3b). The addition of molecular mass of 32 by S‐sulfuration is less effective to induce a conformational change compared with that of 95 by phosphorylation. Therefore, S‐sulfuration of one cysteine residue may lead to the formation of a disulfide bond with another cysteine residue to induce greater and more effective conformational changes than a simple S‐sulfuration (Kimura, 2015).
Figure 3.
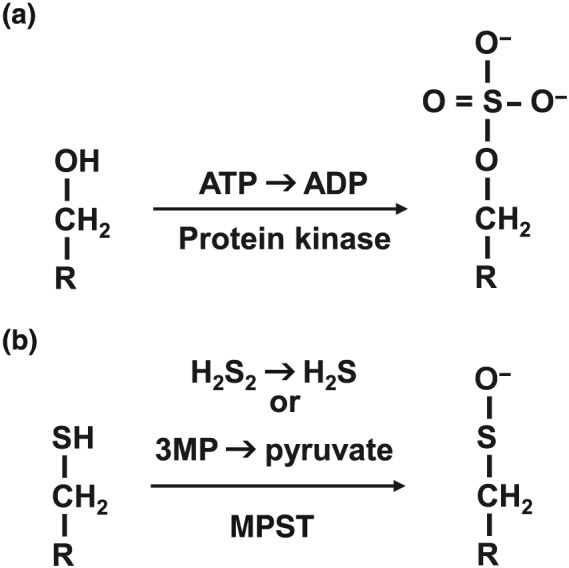
Both S‐sulfuration and phosphorylation induce conformational changes in the target proteins to modify their activity. (a) Phosphorylation is the covalent addition of a phosphate group to serine, threonine, and tyrosine residues by specific kinases and the two negative charges carried by each phosphate group attract positively charged amino acid residues located nearby, leading to a conformational change. In contrast, S‐sulfuration adds one negative charge to the target protein. (b) It is induced by either diffusion of hydrogen sulfide (H2S) (or hydrogen polysulfides [H2Sn]) or transsulfuration by 3‐mercaptopyruvate sulfurtransferase (MPST). The addition of molecular mass of 32 by S‐sulfuration is less effective to induce a conformational change compared with that of 95 by phosphorylation. Therefore, S‐sulfuration of one cysteine residue may lead to the formation of a disulfide bond with another cysteine residues to induce greater and more effective conformational changes than a simple S‐sulfuration (Kimura, 2015). 3MP, 3‐mercaptopyruvate
Mustafa et al. (2009) examined the liver lysates treated with NaHS and extracted the S‐sulfurated proteins by the modified biotin switch assay with an antibody against biotin that were analysed by LC/MS/MS. They detected 39 S‐sulfurated proteins including GAPDH, β‐tubulin, and actin. These three proteins were not S‐sulfurated in the liver of CSE knockout mice, suggesting that H2S and other molecules produced by CSE may S‐sulfurate specific proteins (Mustafa et al., 2009). Several enzymes have been demonstrated to produce persulfurated diffusible molecules including H2Sn, CysSSH, and GSSH, which in turn are able to S‐sulfurate proteins. Because it is difficult for diffusible molecules to S‐sulfurate specific cysteine residues, it is interesting to know how the persulfurated molecules produced from different enzymes selectively S‐sulfurate specific target proteins. Even if most cysteine residues are S‐sulfurated, a few of them may be responsible and involved in the conformational changes of the target proteins. For example, 23 cysteine residues are located at the amino terminus of TRPA1 channels (Wang, Cvetkov, Chance, & Moiseenkova‐Bell, 2012), whereas only two cysteine residues are responsible for the sensitivity to H2Sn (Hatakeyama, Takahashi, Tominaga, Kimura, & Ohta, 2015).
Because molecules in the same oxidation state (S atom in both H2S and thiol is −2) are not able to react with each other, S‐sulfuration of cysteine residues by H2S is not theoretically correct. Cysteine residues can be S‐sulfurated by H2Sn and other persulfurated molecules that contain sulfur with an oxidation state of −1 or 0. In contrast, Cys‐SOH and Cys‐SNO can be S‐sulfurated by H2S (Mishanina, Libiad, & Banerjee, 2015). For example, cysteine residues of parkin whose disruption is the most common cause of inherited Parkinson's disease (PD) are S‐nitrosylated in the brain of PD patients, whereas those of healthy individuals are S‐sulfurated (Chung et al., 2004; Vandiver et al., 2013). Although S‐sulfurated cysteine residues in parkin expressed in HEK293 cells were identified, those in the brains of PD patients were just total levels quantitatively measured but which cysteine residues are S‐sulfurated was not identified (Vandiver et al., 2013). If the same cysteine residues are S‐nitrosylated or S‐sulfurated, a role of H2S must be critical, for H2S S‐sulfurates the S‐nitrosylated cysteine residues (Cys‐SNO) more efficiently than the intact cysteine residues (Cys‐SH). Intact cysteine residues are S‐sulfurated by H2Sn rather than H2S (Kabil et al., 2014).
The identification of endogenous H2Sn and other persulfide and polysulfide species in tissues confirmed S‐sulfuration as a mechanism for the regulation of target proteins activity (Ida et al., 2014; Kimura et al., 2013; Kimura et al., 2015; Kimura et al., 2017; Koike et al., 2017; Nagahara et al., 2018). Endogenous H2Sn were initially identified in the brain and demonstrated to activate TRPA1 channels (Kimura et al., 2013;Nagai et al., 2006 ; Oosumi et al., 2010), which were previously reported to be activated by H2S (Nagai et al., 2004; Streng et al., 2008). The activities of a tumour suppressor PTEN (Greiner et al., 2013) and PKG1α (Stubbert et al., 2014) were found to be regulated by H2Sn. Although it is controversial whether S‐sulfuration activates or inhibits its activity, GAPDH is regulated by S‐sulfuration (Jarosz et al., 2015; Mustafa et al., 2009).
Two cysteine residues are responsible for the regulation of some of these target proteins; TRIPA1 channels: cys422 and cys634; PTEN: cys71 and cys124; Keap1: cys266 and cys613; and PKG1α: a dimer formation of the monomer induced by each cysteine residue (Figure 4; Greiner et al., 2013; Hatakeyama et al., 2015; Hourihan, Kenna, & Hayes, 2013; Kimura et al., 2013; Koike et al., 2013; Stubbert et al., 2014). The potential mechanism for changes in their activities by S‐sulfuration is that either one of cysteine residues is S‐sulfurated to react with the remaining thiol to produce a cysteine disulfide bond, which may induce enough conformational changes to modify their activity. Alternatively, both cysteine residues are equally S‐sulfurated. However, compared with the formation of a disulfide bond, it may induce less conformational change to regulate the activity of target proteins.
Figure 4.
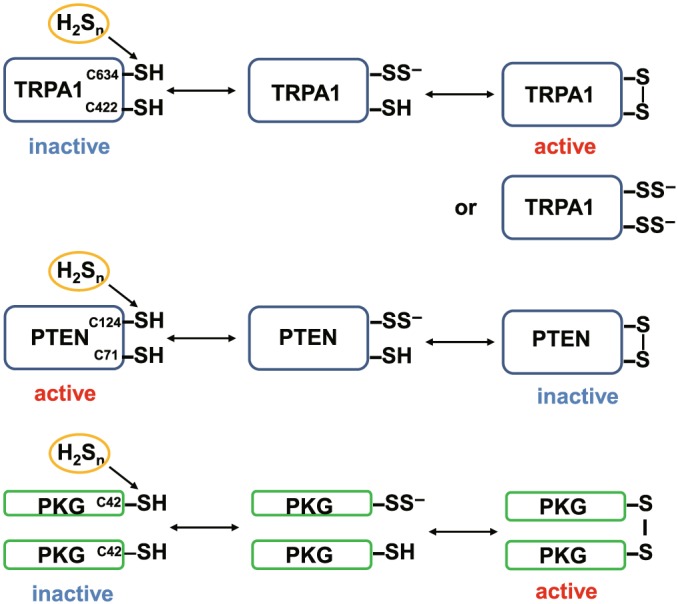
Two cysteine residues are responsible for the regulation of some target proteins by S‐sulfuration. The cys422 and cys634 at the amino terminus of TRPA1 channels are sensitive to H2Sn to induce Ca2+ influx (Hatakeyama et al., 2015; Kimura et al., 2013). Either one of the cysteine residues is S‐sulfurated to react with the remaining thiol to produce a cysteine disulfide bond, which may induce enough conformational changes to modify their activity. Alternatively, both cysteine residues are equally S‐sulfurated. However, compared with the formation of a disulfide bond, it may induce less conformational change to regulate the activity of the channels. When either the cys71 or cys124 of PTEN is S‐sulfurated, a cysteine disulfide bond is produced that then inactivates its activity (Greiner et al., 2013). A dimer formation by each cysteine residue of the monomer protein kinase G1α (PKG1α) activates the enzyme (Stubbert et al., 2014)
It has been reported that S‐sulfuration of one cysteine residue turns on the activity of http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=74. KATP channels are activated through S‐sulfuration of cys43 in its Kir subunits by H2S that enhances the binding of Kir to phospholipid phosphatidylinositol (4,5)‐biphosphate (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2387) while suppressing the binding to ATP (Mustafa et al., 2011). A replacement of cys43 to serine enhances the binding to ATP whereas decreases that to PIP2 (Mustafa et al., 2011). Because cys43 is located within the ATP‐binding region and adjacent to the PIP2 binding region of Kir subunits, a subtle change of S‐sulfuration with one cysteine residue may be effective enough to regulate the activity of KATP channels. Because cys43 is located in the intracellular side of cells, cys43 can be a reduced form and S‐sulfuration may not be induced by H2S but by sulfane sulfurs such as H2Sn. If cys43 is oxidized or nitrosylated, it can be S‐sulfurated by H2S.
1.3. Production of H2Sn
Since the identification of H2Sn as potent inducers of Ca2+ influx in astrocytes, their existence in cells has been examined (Nagai et al., 2006; Oosumi et al., 2010). Endogenous H2Sn have been identified in the brain, and subsequently, MPST has been found as the enzyme producing them (Kimura et al., 2013; Koike et al., 2017).
MPST produces H2S in the presence of reducing substances such as DTT (Shibuya et al., 2009). Considering the structure of leach MPST, which consists of MPST and thioredoxin domains, it has been predicted that an endogenous reducing substance(s) in mammals must be thioredoxin or related molecules (Williams, Kelly, Mottram, & Coombs, 2003). In the presence of thioredoxin or dihydrolipoic acid, MPST efficiently produces H2S from 3MP (Mikami et al., 2011; Nagahara, Yoshii, Abe, & Matsumura, 2007; Yadav, Yamada, Chiku, Koutmos, & Banerjee, 2013). MPST also produces H2S2 and H2S3 as well as H2S (Kimura et al., 2015). Brain cell suspensions prepared from the wild‐type mice produce H2S2 and H2S3, whereas those prepared from MPST knockout mice generated much less H2S2 and H2S3 (Kimura et al., 2015). A potential mechanism has been proposed in which MPST receives sulfur from 3MP to produce a per or polysulfur chain at its active site that is reduced to H2S, H2S2, and H2S3 probably depending on the intensity of interaction with thioredoxin (Kimura, 2016). Nagahara et al. elucidated the predicted mechanism: trisulfide is predominantly produced at the sulfurated catalytic‐site cysteine of MPST, and H2S2 is the first to be released, followed subsequently, by H2S and H2S3 (Nagahara, 2018; Nagahara et al., 2018).
Alkylating agents have been used to measure the levels of per and polysulfurated molecules. However, the reaction is a competition between alkylation of these molecules and their interchange reaction. Bogdandi et al. (2019) drew attention to this by applying just one of the alkylating agents monobromobimane; monobromobimane was applied at alkaline pH. We previously reported that at alkaline pH, the levels of S1‐dibimane are increased in a time‐dependent manner, whereas those of S2‐ and S3‐dibimane are decreased. In contrast, all three dibimane adducts are stable at pH 7.0 for up to 60 min (Koike et al., 2017). In the reaction at pH 7.0, the concentrations of H2S2 and H2S in the brain were approximately 0.026 and 0.030 μmol·g−1 protein, respectively, whereas at pH 9.5, they were 0.019 and 0.111 μmol·g−1 protein, respectively, shifting greatly to H2S from H2S2 (Koike et al., 2017). It is necessary to apply this agent at physiological neutral pH.
The effects of various concentrations (0.5–98 mM) of other alkylating agents http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6271 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5335 were also examined by incubating them with per and polysulfide molecules for 3 hr (Bogdandi et al., 2019). In our study, after the addition of 10 μM Na2S3 to 100 μM Cys and 1 mM GSH, most of Na2S3 was reacted with Cys and GSH in 15 s, and all Na2S3 was completely consumed after 3 min to produce per and polysulfurated cysteine and GSH (Kimura et al., 2017). These observations suggest that 3 hr of exposure of per and polysulfurated molecules to the alkylating agents is too long, as a lot of exchange reactions may proceed. Further studies are required to properly measure the endogenous levels of these molecules.
1.4. Production of CysSSH and GSSH
Cavallini et al. initially demonstrated the production of CysSSH by CSE from cystine, and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=279#1443 was recently reported to have a similar activity to CSE (Cavallini et al., 1960; Ida et al., 2014). However, CSE and CBS are localized to the cytoplasm where cysteine is a dominant form compared to cystine. For example, the endogenous concentrations of cystine in the liver and lung are approximately 0.20 and 0.05 μM, respectively, and those in the heart and brain are under detectable levels (Ida et al., 2014). For these reasons, it is controversial for CBS and CSE to produce CysSSH in cells (Yadav et al., 2016). It is intriguing to know whether the cystine/glutamate antiporter, which transports cystine into cells, is coupled with or closely localized to CSE and CBS at the plasma membrane to enable both enzymes to access to cystine (Sato, Tamba, Ishii, & Bannai, 1999).
When H2Sn were produced by MPST, we found that the levels of cysteine and GSH were decreased in the reaction mixture. It is because production of CysSSnH and GSSnH consumes cysteine and GSH (Kimura et al., 2017). In the reaction with lysates of cells expressing MPST, CysSSnH (1 < n < 5) and GSSnH were produced, whereas only CysSSH and GSSH were found in cells. The reaction mixture of cell lysates we used contained 1/100 of physiological concentrations of cysteine and GSH. This observation suggested that the production of CysSSnH and GSSnH depends on the concomitant concentrations of cysteine and GSH. CysSSH and GSSH are mainly produced in the presence of physiological concentrations of cysteine and GSH, and the production of CysSSnH and GSSnH with longer sulfur chains requires lower concentrations of cysteine and GSH than those present in physiological conditions (Kimura et al., 2017).
One of the potential mechanisms of the production of CysSSH and GSSH by MPST is that H2S2 produced by MPST reacts with cysteine and GSH to generate CysSSH and GSSH (Kimura et al., 2017; Nagahara et al., 2018). The observation that the endogenous concentrations of H2S2 are almost equivalent to those of H2S and that H2S2 and H2S3 produced by MPST readily react with cysteine and GSH to produce CysSSH and GSSH supports this mechanism (Figures 2 and 5a; Kimura et al., 2013; Kimura et al., 2015; Kimura et al., 2017; Koike et al., 2017; Nagahara et al., 2018). Alternatively, MPST transfers sulfur to cysteine and GSH to generate CysSSH and GSSH without the involvement of H2S2 or H2S3 (Figures 2 and 5b). It has been reported that MPST receives sulfur from 3MP and can transfer back to another 3MP to generate 3MP persulfide (Vachek & Wood, 1972; Yadav et al., 2013). However, because of ketone in the structure, 3MP easily releases sulfur, whereas 3MP is a less efficient sulfur acceptor than cysteine. For this reason, CysSSH is more likely to be produced compared with 3MP persulfide by MPST (Kimura et al., 2017; Vachek & Wood, 1972).
Figure 5.
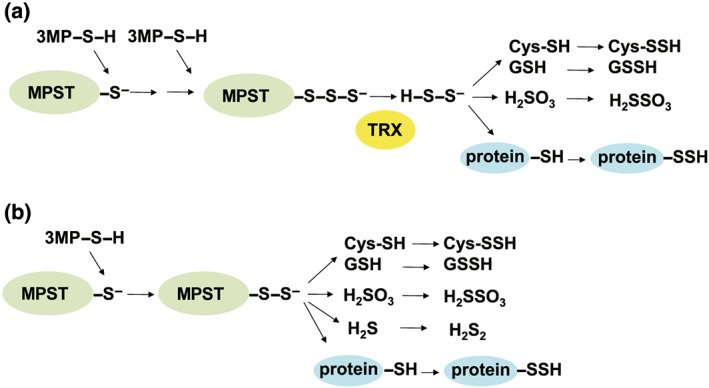
A potential mechanism for the production of cysteine persulfide (CysSSH) and GSH persulfide (GSSH) by 3‐mercaptopyruvate sulfurtransferase (MPST). (a) Hydrogen polysulfide (H2S2) produced by MPST reacts with cysteine and GSH to generate CysSSH and GSSH (Kimura et al., 2017; Nagahara et al., 2018). The observations that the endogenous concentrations of H2S2 are almost equivalent to those of hydrogen sulfide (H2S) and that H2S2 and H2S3 produced by MPST readily react with cysteine and GSH to produce CysSSH and GSSH support this mechanism (Kimura et al., 2013; Kimura et al., 2015; Kimura et al., 2017; Koike et al., 2017; Nagahara, 2018; Nagahara et al., 2018). (b) Alternatively, MPST transfers sulfur to cysteine and GSH to generate CysSSH and GSSH without the involvement of H2S2 or H2S3. The figure is a reprint from Kimura et al. (2017)
MPST, which is localized to both cytosol and mitochondria, is also known as tRNA thiouridine modification protein (Frasdorf, Radon, & Leimkuhler, 2014; Nagahara, Okazaki, & Nishino, 1995). It had been demonstrated that this enzyme transfers sulfur from 3MP to tRNA in the brain and liver (Wong, Harris, & Jankowicz, 1974; Wong, Harris, & Morris, 1975). Thio modification of uridine is required for accurate deciphering of the genetic code and the stabilization of tRNA structure (Frasdorf et al., 2014). The human cytosolic isoform of MPST thiolates cytosolic tRNA, whereas the mitochondrial form, which can also localize to cytosol and thiolate tRNA, supplies sulfur for iron–sulfur cluster formation in mitochondria (Frasdorf et al., 2014).
Mitochondrial MPST has been proposed to be involved in the formation of cellular energy formation complimenting and balancing the bioenergetic role of Krebs cycle‐derived electron donors (Modis, Coletta, Erdelyi, Papapetropoulos, & Szabo, 2013). H2S produced by MPST may regulate mitochondrial electron transport and oxidative phosphorylation. Suppression of MPST activity by siRNA decreased basal energetic parameters such as ATP formation and oxygen consumption (Modis et al., 2013).
Another enzyme CARS, which adenylates cysteine by consuming ATP and attaches it to tRNA, was recently proposed to have additional activity to sulfurate cysteine to produce CysSSH (Akaike et al., 2017). A proposed mechanism is that CARS takes sulfur from cysteine and in turn sulfurates cysteine to produce CysSSH. It is quite similar to MPST, which transfers sulfur from 3MP to cysteine to generate CysSSH (Kimura et al., 2017; Vachek & Wood, 1972).
Three enzymes are known to produce H2S: CBS, CSE, and MPST, and CARS has recently been reported to produce H2S (Akaike et al., 2017; Chiku et al., 2009; Shibuya et al., 2009; Singh, Padovani, Leslie, Chiku, & Banerjee, 2009; Stipanuk & Beck, 1982). Although some of the observations are controversial, the levels of some of these enzymes are interrelated with each other. The levels of MPST are up‐regulated in the liver of CSE knockout mice (Shirozu et al., 2014), whereas those in the heart are not significantly different between CSE knockout and wild‐type mice (King et al., 2014). This discrepancy may be due to the difference in tissues. Another example is that knockdown of CARS in fibrosarcoma cells up‐regulates the expression of CBS (Hayano, Yang, Corn, Pagano, & Stockwell, 2016), whereas heterozygous CARS knockout mouse liver does not change it probably due to a weak effect of heterozygous knockout on the expression of other enzymes (Akaike et al., 2017).
Persulfide molecules have also been proposed to be produced via other pathways. The intracellular concentrations of H2S are well regulated by a balance of its biosynthesis and clearance (Kimura, 2012; Vitvitsky, Kabil, & Banerjee, 2012). H2S is mainly metabolized in mitochondria. The first step is the formation of persulfide at Cys379 in SQR, and then sulfane sulfur is transferred to acceptors such as GSH, sulfite, sulfide, cysteine, and homocysteine to generate GSSH, H2SSO3, H2S2, CysSSH, and Hcy‐SSH respectively (Landry et al., 2017). This pathway is coupled with oxidative phosphorylation where two electrons are transferred to SQR‐bound http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5184 to generate dihydroflavin adenine dinucleotide and further to coenzyme Q (Goubern, Andriamihaja, Nübel, Blachier, & Bouillaud, 2007). On the basis of the high intracellular concentrations of GSH, Landry et al. (2017) proposed that GSH must be a dominant acceptor of sulfur to produce GSSH as a major persulfide product via this pathway.
Haemoproteins such as haemoglobin, myoglobin, neuroglobin, and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2979 can also oxidize H2S to produce polysulfides. Haemoglobin is a haematoporphyrin built of four polypeptide chains. Centrally located iron (Fe3+) of haemoglobin binds to H2S to generate sulfhaemoglobin (Fe2+). The ratio of sulfide and oxygen may determine the oxidation products. When the sulfide concentrations are low, thiosulfate may be the predominant product, whereas polysulfides are favoured at high concentrations of sulfide (Vitvitsky, Yadav, Kurthen, & Banerjee, 2015). Myoglobin, a monomeric haem protein found in most animal muscles, oxidizes H2S to thiosulfate and polysulfides similarly to haemoglobin (Bostelaar et al., 2016).
Neuroglobin, which is primarily expressed in neurons, oxidizes H2S but much less efficiently compared with haemoglobin and myoglobin (Ruetz et al., 2017). Because the expression of SQR in the brain is very low (Linden et al., 2012), it is predicted that neuroglobin plays a role in H2S oxidation in the brain (Ruetz et al., 2017).
Catalase oxidizes H2S to generate H2Sn in the presence of O2, whereas it produces H2S in hypoxia (Olson et al., 2017). When oxygen levels decrease, the catalase activity turns from H2Sn production via H2S oxidation to H2S production. As catalase has been identified as one of the highly S‐sulfurated proteins (Mustafa et al., 2009), its activity may well be regulated by H2S, H2Sn, and other persulfurated molecules.
Copper/zinc SOD located in cytosol can produce H2S2 and to lesser extent H2S3 and H2S5 by oxidizing H2S in a concentration‐dependent manner with O2 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2448 as electron acceptors (Olson et al., 2018; Searcy, 1996; Searcy, Whitehead, & Maroney, 1995). The interesting characteristics of this pathway is that SOD oxidation prefers dissolved H2S over HS−, which rather inhibits the production of H2Sn. Two cysteine residues Cys57 and Cys146, which form intramolecular disulfide bridge, are involved in the conformational stability of SOD, and Cys111 has a low pKa and is nucleophilic under physiological conditions (Olson et al., 2018). SOD also has a polysulfide bridge structure (Nielsen, Tachibana, Hansen, & Winther, 2011). These cysteine residues can be the targets of H2S and H2Sn to elongate the sulfur chain. Mitochondrial manganese SOD (MnSOD) can also oxidize H2S to produce H2Sn. SODs are localized to both cytoplasm and mitochondria similar to MPST. Both cytoplasm and mitochondria may require H2S, H2Sn, and other persulfurated molecules for their proper function including signalling and redox homeostasis.
Peroxidases such as lactoperoxidase and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2789 can oxidize H2S in the presence of H2O2 (Garai et al., 2017; Nakamura, Nakamura, Yamazaki, & Morrison, 1984). Recently, it was reported that myeloperoxidase can even produce per or polysulfides even in the absence of H2O2 by utilizing oxygen in collaboration with SOD (Garai et al., 2017).
1.5. Crosstalk between H2S and NO and generation of H2Sn
H2S alone weakly relaxes vascular smooth muscle, but its effect is greatly augmented in the presence of NO (Hosoki et al., 1997). A similar synergistic effect of H2S with NO was observed in the twitch response of the ileum to the electrical stimulation (Teague et al., 2002). The studies on the crosstalk between H2S and NO can be categorized mainly into two groups: (a) the chemical interaction between H2S and NO produces products, which exert an effect greater than additive, and (b) H2S enhances the activity of NO‐producing enzymes, leading to a greater effect than that induced by NO alone.
Whiteman et al. (2006) demonstrated that the chemical interaction of H2S with NO produces a molecule, which they suggested is a nitrosothiol based on their observations with electroparamagnetic resonance, amperometry, and nitrite measurements. We demonstrated that H2Sn produced by the oxidation of H2S activate TRPA1 channels and that two cysteine residues at the amino terminus of the channels are targets of H2Sn (Hatakeyama et al., 2015; Kimura et al., 2013; Nagai et al., 2006; Oosumi et al., 2010). Filipovic and colleagues proposed that H2S can further react with S‐nitrosothiols to form thionitrous acid (HSNO), which can freely diffuse through membranes to release NO, and further oxidize to HNO and H2Sn (Eberhardt et al., 2014; Filipovic et al., 2012). HNO activates TRPA1 channels via a mechanism in which the oxidized one of two cysteine residues at the amino terminus of the channels reacts with the remaining cysteine residue to generate a disulfide bond, resulting in the conformational change for the activation of the channels that is similar to the mechanism for H2Sn described above.
Cortese‐Krott et al. (2015) reported that H2S and NO initiate a chemical reaction to produce SSNO−, H2Sn, and dinitrososulfite. SSNO− is resistant to the reduction by thiols as well as degradation by cyanide or cyanolysis and efficiently releases sulfane sulfur and NO to relax vascular smooth muscle to the same extent as H2Sn, whereas dinitrososulfite is a weak relaxant.
H2Sn, the H2S donor http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9808, and NO donor DEA NONOate all induce Ca2+ influx in peritoneal mast cells, which express H2S‐producing enzymes as well as NOSs (Moustafa & Habara, 2016). Based on these observations, Moustafa and Habara suggested that NO induces the production of H2S by activating H2S‐producing enzymes and vice versa and that the interaction of both molecules produces H2Sn to induce Ca2+ influx in mast cells.
We demonstrated that the major active products from H2S and NO are H2Sn (H2S2 and H2S3), which activate TRPA1 channels in dorsal root ganglion cells (Figure 2; Miyamoto et al., 2017). The active molecules produced from H2S and NO to induce biological activity in dorsal root ganglion cells were different from those identified in previous studies examined in this vasculature (Cortese‐Krott et al., 2015; Eberhardt et al., 2014; Miyamoto et al., 2017). The H2Sn and H2S/NO product that activate TRPA1 channels in dorsal root ganglion cells is degraded by thiols and cyanolysis, whereas SSNO− is resistant to thiols, and HNO is resistant to cyanolysis. These observations suggest that molecules produced by the chemical interaction of H2S and NO must be H2Sn rather than SSNO− and HNO that activate TRPA1 channels in the dorsal root ganglion neurons (Cortese‐Krott et al., 2015; Eberhardt et al., 2014; Miyamoto et al., 2017).
1.6. Perspective
H2S, H2Sn, CysSSH, GSSH, persulfurated proteins, and the enzymes that produce them have been identified. They are localized to either cytoplasm or mitochondria or both and involved in cellular signalling and redox homeostasis.
Because these endogenous molecules exchange sulfane sulfur with each other, it is difficult to determine which one is a real effecter under physiological conditions. H2S2 is the smallest among these molecules and is able to access the cysteine residues of target proteins more easily than the other larger persulfurated molecules. Nevertheless, H2S2 readily reacts with cysteine and GSH to produce CysSSH and GSSH. Considering the fact that H2S2 is such a highly reactive molecule and exists in the brain at almost the same concentrations as H2S (Koike et al., 2017), the rate of H2S2 production must be greater than that of H2S generation in cells.
The activation of target proteins by H2Sn can be reinstated by the redox conditions of the environment. For example, TRPA1 channels are activated by H2Sn via S‐sulfuration. Once H2Sn are consumed, the channel returns to the quiescent state through the reduction of its S‐sulfurated cysteine residues or disulfide bonds by the abundant GSH in cells.
Cellular stimuli that regulate the production of H2Sn and other persulfurated or polysulfurated molecules are not well understood. It is possible that the enzymatic production regulates their endogenous levels. Bound sulfane sulfur levels in the brains of MPST knockout mice are less than half of those of wild‐type mice, suggesting the shift of equilibrium of persulfurated and polysulfurated molecules by the enzymes involved in their production (Kimura et al., 2017). It is interesting to know whether the remaining half of bound sulfane sulfur in MPST knockout mice consists of molecules with totally different characteristics from the other half or the same molecules but produced by other enzymes.
Compared with the conventional signalling molecules such as amino acids, peptides, and proteins, H2Sn and other persulfurated molecules are very reactive and extremely unstable. Once they S‐sulfurate other molecules such as cysteine residues of target proteins, more stable H2S and other thiols can be released. H2S is metabolized by SQR and produces persulfurated molecules such as GSSH (Hildebrandt & Grieshaber, 2008; Mishanina, Libiad, & Banerjee, 2015), which are also further metabolized by persulfide dioxygenease and rhodanese to sulfate. Further studies are needed to elucidate the mechanism for the cessation of the signalling by per and polysulfide molecules.
The pathophysiology of these molecules has been reported. The parkin of PD brains is S‐nitrosylated, whereas that of normal individuals is S‐sulfurated (Vandiver et al., 2013). However, it is not known whether the same cysteine residues are S‐nitrosylated or S‐sulfurated. If the same cysteine residues are modified, H2S may have a critical role, as H2S can S‐sulfurate S‐nitrosylated cysteine residues. When different cysteine residues are modified, the balance of NO and H2S2 and other persulfurated molecules may play an important role in regulating the activity of parkin.
Human lung adenocarcinoma tissue expresses high levels of CBS, CSE, and MPST compared with adjacent lung tissue, and H2S activates mitochondrial DNA repair through S‐sulfuration of a specific DNA repair enzyme, exo/endonuclease G, and is also involved in maintaining cellular bioenergetics (Szczesny et al., 2016). The involvement of CBS/H2S in cell proliferation and cellular bioenergetics has also been demonstrated in colorectal cancer, ovarian cancer, and breast cancer (Bhattacharyya et al., 2013; Sen et al., 2015; Szabo et al., 2013). In contrast, CBS‐silenced glioma exhibited increased depth of invasion, vascular density, and cell proliferation with increased levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5085 and HIF‐2α expression, suggesting that CBS/H2S may suppress glioma (Takano et al., 2014). Further studies are awaited to clarify the mechanisms of responses to H2S that are conflicting depending on the type of cancer.
Wrobel et al. (2014) reported that the levels of sulfane sulfur (polysulfides) in gliomas with the highest grades of malignancy are greater than those of glioma‐free brain regions. A similar result was also reported in glioblastoma‐bearing ipsilateral hemispheres, which contain greater amounts of H2Sn than the glioblastoma‐free control hemispheres (Shiota et al., 2018). These observations suggest that sulfane sulfur accumulation may play an important role in glioma cell proliferation and that its dependence on sulfane sulfur is greater in higher grades of malignancy.
H2Sn and other per or polysulfurated molecules as well as H2S may be involved in the regulation of cancer cell growth. Altering the levels of these molecules and the activity of their target proteins may have a therapeutic benefit for diseases caused by redox disturbance including neurodegenerative disorders, vascular diseases, and cancer.
1.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017; Alexander, Peters et al., 2017; Alexander, Striessnig et al., 2017).
CONFLICT OF INTEREST
The author declares no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by the KAKENHI (26460115 and 17K08331), a Grant‐in‐Aid for Scientific Research, and the Strategic Research Program for Brain Sciences from the Japan Agency for Medical Research and Development, AMED, under Grant JP18dm0107085 to H.K.
Kimura H. Signalling by hydrogen sulfide and polysulfides via protein S‐sulfuration. Br J Pharmacol. 2020;177:720–733. 10.1111/bph.14579
REFERENCES
- Abe, K. , & Kimura, H. (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of Neuroscience, 16, 1066–1071. 10.1523/JNEUROSCI.16-03-01066.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman, E. , Lipton, D. A. , & Loring, R. H. (1989). Selective modulation of NMDA responses by reduction and oxidation. Neuron, 2, 1257–1263. 10.1016/0896-6273(89)90310-3 [DOI] [PubMed] [Google Scholar]
- Akaike, T. , Ida, T. , Wei, F.‐Y. , Nishida, M. , Kumagai, Y. , Alam, M. M. , … Motohashi, H. (2017). Cysteinyl‐tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nature Communications, 8, 1177 10.1038/s41467-017-01311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174(S1), S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(S1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174(S1), S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, S. , Saha, S. , Giri, K. , Lanza, I. R. , Nair, K. S. , Jennings, N. B. , … Mukherjee, P. (2013). Cystathionine beta‐synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE, 8, e79167 10.1371/journal.pone.0079167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bir, S. C. , Kolluru, G. K. , McCarthy, P. , Shen, X. , Pardue, S. , Pattillo, C. B. , Kevil, C. G. (2012). Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia‐inducible factor‐1α and vascular endothelial growth factor‐dependent angiogenesis. Journal of the American Heart Association, 1: e004093. 10.1161/JAHa.112.004093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdandi, V. , Ida, T. , Sutton, T. R. , Bianco, C. , Ditroi, T. , Koster, G. , … Nagy, P. (2019). Speciation of reactive sulfur species and their reactions with alkylating agents: do we have any clue about what is present inside the cell? British Journal of Pharmacology, 176, 646-670. 10.1111/bph.14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos, E. M. , Leuvenink, H. G. D. , Snijder, P. M. , Koosterhuis, N. J. , Hillebrands, J.‐L. , Leemans, J. C. , … van Goor, H. (2009). Hydrogen sulfide‐induced hypometabolism prevents renal ischemia/reperfusion injury. Journal of the American Society of Nephrology, 20, 1901–1905. 10.1681/ASN.2008121269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostelaar, T. , Vitvitsky, V. , Kumutima, J. , Lewis, B. E. , Yadav, P. K. , Brunold, T. C. , … Banerjee, R. (2016). Hydrogen sulfide oxidation by myoglobin. Journal of the American Chemical Society, 138, 8476–8488. 10.1021/jacs.6b03456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes, N. , & Turner, R. J. (1994). K+‐induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+–HCO3‐cotransport. The American Journal of Physiology, 267, C1633–C1640. 10.1152/ajpcell.1994.267.6.C1633 [DOI] [PubMed] [Google Scholar]
- Cavallini, D. , Marco, C. D. , Mondavi, B. , & Mori, B. G. (1960). The cleavage of cystine by cystathionase and the transulfuration of hypotaurine. Enzymologia, 22, 161–173. [PubMed] [Google Scholar]
- Chiku, T. , Padovani, D. , Zhu, W. , Singh, S. , Vitvitsky, V. , & Banerjee, R. (2009). H2S biogenesis by human cystathionine γ‐lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. The Journal of Biological Chemistry, 284, 11601–11612. 10.1074/jbc.M808026200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K. K. , Thomas, B. , Li, X. , Pletnikova, O. , Troncoso, J. C. , Marsh, L. , … Dawson, T. M. (2004). S‐nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science, 304, 1328–1331. 10.1126/science.1093891 [DOI] [PubMed] [Google Scholar]
- Coletta, C. , Papapetropoulos, A. , Erdelyi, K. , Olah, G. , Módis, K. , Panopoulos, P. , … Szabo, C. (2012). Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium‐dependent vasorelaxation. Proceedings of the National Academy of Sciences of the United States of America, 109, 9161–9166. 10.1073/pnas.1202916109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese‐Krott, M. M. , Fernandez, B. O. , Santos, J. L. , Mergia, E. , Grman, M. , Nagy, P. , … Feelisch, M. (2014). Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S‐nitrosothiols following reaction with sulfide. Redox Biology, 2, 234–244. 10.1016/j.redox.2013.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese‐Krott, M. M. , Kuhnle, G. G. C. , Dyson, A. , Fernandez, B. O. , Grman, M. , DuMond, J. F. , … Feelisch, M. (2015). Key bioactive reaction products of the NO/H2S interaction are S/N‐hybrid species, polysulfides, and nitroxyl. Proceedings of the National Academy of Sciences of the United States of America, 112, E4651–E4660. 10.1073/pnas.1509277112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevasanta, E. , Lange, M. , Bonanata, J. , Coitiño, E. L. , Ferrer‐Sueta, G. , Filipovic, M. R. , & Alvarez, B. (2015). Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. The Journal of Biological Chemistry, 290, 26866–26880. 10.1074/jbc.M115.672816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt, M. , Dux, M. , Namer, B. , Jiljkovic, J. , Cordasic, N. , Will, C. , … Filipovic, M. R. (2014). H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signaling pathway. Nature Communications, 5, 4381 10.1038/ncomms5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod, J. W. , Calvert, J. W. , Morrison, J. , Doeller, J. E. , Kraus, D. W. , Tao, L. , … Lefer, D. J. (2007). Hydrogen sulfide attenuates myocardial ischemia–reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America, 104, 15560–15565. 10.1073/pnas.0705891104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic, M. R. , Miljkovic, J. L. , Nauser, T. , Royzen, M. , Klos, K. , Shubina, T. , … Ivanović‐Burmazović, I. (2012). Chemical characterization of the smallest S‐nitrosothiol, HSNO; cellular cross‐talk of H2S and S‐nitrosothiols. Journal of the American Chemical Society, 134, 12016–12027. 10.1021/ja3009693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasdorf, B. , Radon, C. , & Leimkuhler, S. (2014). Characterization and interaction studies of two isoforms of the dual localized 3‐mercaptopyruvate sulfurtransferase TUM1 from humans. The Journal of Biological Chemistry, 289, 34543–34556. 10.1074/jbc.M114.605733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garai, D. , Ríos‐González, B. B. , Furtmüller, P. G. , Fukuto, J. M. , Xian, M. , López‐Garriga, J. , … Nagy, P. (2017). Mechanisms of myeloperoxidase catalyzed oxidation of H2S by H2O2 or O2 to produce potent protein Cys–polysulfide‐inducing species. Free Radical Biology and Medicine, 113, 551–563. 10.1016/j.freeradbiomed.2017.10.384 [DOI] [PubMed] [Google Scholar]
- Goodwin, L. R. , Francom, D. , Dieken, F. P. , Taylor, J. D. , Warenycia, M. W. , Reiffenstein, R. J. , & Dowling, G. (1989). Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. Journal of Analytical Toxicology, 13, 105–109. 10.1093/jat/13.2.105 [DOI] [PubMed] [Google Scholar]
- Goubern, M. , Andriamihaja, M. , Nübel, T. , Blachier, F. , & Bouillaud, F. (2007). Sulfide, the first inorganic substrate for human cells. The FASEB Journal, 21, 1699–1706. 10.1096/fj.06-7407com [DOI] [PubMed] [Google Scholar]
- Greiner, R. , Palinkas, Z. , Basell, K. , Becher, D. , Antelmann, H. , Nagy, P. , & Dick, T. P. (2013). Polysulfides link H2S to protein thiol oxidation. Antioxidants and Redox Signaling, 19, 1749–1765. 10.1089/ars.2012.5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove, J. L. , & Wichman, R. D. (1987). A cystine‐dependent inactivator of tyrosine aminotransferase co‐purifies with gamma‐cystathionase (cystine desulfurase). The Journal of Biological Chemistry, 262, 7351–7357. [PubMed] [Google Scholar]
- Hatakeyama, Y. , Takahashi, K. , Tominaga, M. , Kimura, H. , & Ohta, T. (2015). Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Molecular Pain, 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano, M. , Yang, W. S. , Corn, C. K. , Pagano, N. C. , & Stockwell, B. R. (2016). Loss of cysteinyl‐tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death and Differentiation, 23, 270–278. 10.1038/cdd.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt, T. M. , & Grieshaber, M. K. (2008). Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. The FEBS Journal, 275, 3352–3361. 10.1111/j.1742-4658.2008.06482.x [DOI] [PubMed] [Google Scholar]
- Hosoki, R. , Matsuki, N. , & Kimura, H. (1997). The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications, 237, 527–531. 10.1006/bbrc.1997.6878 [DOI] [PubMed] [Google Scholar]
- Hourihan, J. M. , Kenna, J. G. , & Hayes, J. D. (2013). The gasotransmitter hydrogen sulfide induces nrf2‐target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys‐226 and cys‐613. Antioxidants and Redox Signaling, 19, 465–481. 10.1089/ars.2012.4944 [DOI] [PubMed] [Google Scholar]
- Hylin, J. W. , & Wood, J. L. (1959). Enzymatic formation of polysulfides from mercaptopyruvate. The Journal of Biological Chemistry, 234, 2141–2144. [PubMed] [Google Scholar]
- Ida, T. , Sawa, T. , Ihara, H. , Tsuchiya, Y. , Watanabe, Y. , Kumagai, Y. , … Akaike, T. (2014). Reactive cysteine persulfides and S‐polythiolation regulate oxidative stress and redox signaling. Proceedings of the National Academy of Sciences of the United States of America, 111, 7606–7611. 10.1073/pnas.1321232111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami, M. , Hiraki, K. , Umemura, K. , Ogasawara, Y. , Ishii, K. , & Kimura, H. (2009). A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxidants and Redox Signaling, 11, 205–214. 10.1089/ars.2008.2132 [DOI] [PubMed] [Google Scholar]
- Jarosz, A. P. , Wei, W. , Gauld, J. W. , Auld, J. , Ozcan, F. , Aslan, M. , & Mutus, B. (2015). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) is inactivated by S‐sulfuration in vitro. Free Radical Biology and Medicine, 89, 512–521. 10.1016/j.freeradbiomed.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Kabil, O. , Motl, N. , & Banerjee, R. (2014). H2S and its role in redox signaling. Biochimica et Biophysica Acta, 1844, 1355–1366. 10.1016/j.bbapap.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, A. , Ogura, M. , & Suda, M. (1966). Control mechanism in the rat liver enzyme system converting l‐methionine to l‐cystine. 3. Noncompetitive inhibition of cystathionine synthetase–serine dehydratase by elemental sulfur and competitive inhibition of cystathionase–homoserine dehydratase by l‐cysteine and l‐cystine. Journal of Biochemistry, 59(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Kimura, H. (2012). Metabolic turnover of hydrogen sulfide. Frontiers in Physiology, 3, article 101, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. (2015). Signaling molecules: Hydrogen sulfide and polysulfide. Antioxidants and Redox Signaling, 22, 362–376. 10.1089/ars.2014.5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. (2016). Hydrogen polysulfide (H2Sn) signaling along with hydrogen sulfide (H2S) and nitric oxide (NO). Journal of Neural Transmission, 123, 1235–1245. 10.1007/s00702-016-1600-z [DOI] [PubMed] [Google Scholar]
- Kimura, H (2018) MPST produces redox regulators Cys‐SSH and GSSH as well as signaling molecules H2S and H2Sn . Atlas of Science July, 22.
- Kimura, Y. , Dargusch, R. , Schubert, D. , & Kimura, H. (2006). Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxidants and Redox Signaling, 8, 661–670. 10.1089/ars.2006.8.661 [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , Goto, Y.‐I. , & Kimura, H. (2010). Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxidants and Redox Signaling, 12, 1–13. 10.1089/ars.2008.2282 [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , & Kimura, H. (2004). Hydrogen sulfide protects neurons from oxidative stress. The FASEB Journal, 18, 1165–1167. 10.1096/fj.04-1815fje [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , Koike, S. , Shibuya, N. , Lefer, D. , Ogasawara, Y. , & Kimura, H. (2017). 3‐Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine‐ and glutathione‐persulfide (Cys‐SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Scientific Reports, 7, 10459 10.1038/s41598-017-11004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y. , Mikami, Y. , Osumi, K. , Tsugane, M. , Oka, J.‐I. , & Kimura, H. (2013). Polysulfides are possible H2S‐derived signaling molecules in rat brain. The FASEB Journal, 27, 2451–2457. 10.1096/fj.12-226415 [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , Shibuya, N. , & Kimura, H. (2018). Sulfite protects neurons from oxidative stress. British Journal of Pharmacology. in press, DOI: 10.1111/bph.14373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y. , Toyofuku, Y. , Koike, S. , Shibuya, N. , Nagahara, N. , Lefer, D. , … Kimura, H. (2015). Identification of H2S3 and H2S produced by 3‐mercaptopyruvate sulfurtransferase in the brain. Scientific Reports, 5, 14774 10.1038/srep14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A. L. , Polhemus, D. , Bhushan, S. , Otsuka, H. , Kondo, K. , Nicholson, C. K. , … Lefer, D. J. (2014). Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase–nitric oxide dependent. Proceedings of the National Academy of Sciences of the United States of America, 111, 3182–3187. 10.1073/pnas.1321871111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike, S. , Kawamura, K. , Kimura, Y. , Shibuya, N. , Kiimura, H. , & Ogasawara, Y. (2017). Analysis of endogenous H2S and H2Sn in mouse brain by high‐performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radical Biology and Medicine, 113, 355–362. 10.1016/j.freeradbiomed.2017.10.346 [DOI] [PubMed] [Google Scholar]
- Koike, S. , Ogasawara, Y. , Shibuya, N. , Kimura, H. , & Ishii, K. (2013). Polysulfide exerts a protective effect against cytotoxicity caused by t‐buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Letters, 587, 3548–3555. 10.1016/j.febslet.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Landry, A. P. , Ballou, D. P. , & Banerjee, R. (2017). H2S oxidation by nanodisc‐embedded human sulfide quinone oxidoreductase. The Journal of Biological Chemistry, 292, 11641–11649. 10.1074/jbc.M117.788547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, D. R. , Furne, J. , Stoltz, G. J. , Abdel‐Rehim, M. S. , Levitt, M. D. , & Szurszewski, J. H. (2012). Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. British Journal of Pharmacology, 165, 2178–2190. 10.1111/j.1476-5381.2011.01681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami, Y. , Shibuya, N. , Kimura, Y. , Ogasawara, Y. , & Kimura, H. (2011). Thioredoxin and dihydrolipoic acid are endogenous reductants required for 3‐mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. The Biochemical Journal, 439, 479–485. 10.1042/BJ20110841 [DOI] [PubMed] [Google Scholar]
- Minamishima, S. , Bougaki, M. , Sips, P. Y. , Yu, J. D. , Minamishima, Y. A. , Elrod, J. W. , … Ichinose, F. (2009). Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3‐dependent mechanism in mice. Circulation, 120, 888–896. 10.1161/CIRCULATIONAHA.108.833491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishanina, T. V. , Libiad, M. , & Banerjee, R. (2015). Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nature Chemical Biology, 11, 457–464. 10.1038/nchembio.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishanina, T. V. , Yadav, P. K. , Ballou, D. P. , & Banerjee, R. (2015). Transient kinetic analysis of hydrogen sulfide oxidation catalyzed by human sulfide quinone oxidoreductase. The Journal of Biological Chemistry, 290, 25072–25080. 10.1074/jbc.M115.682369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, R. , Koike, S. , Takano, Y. , Shibuya, N. , Kimura, Y. , Hanaoka, K. , … Kimura, H. (2017). Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Scientific Reports, 7, 45995 10.1038/srep45995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis, K. , Coletta, C. , Erdelyi, K. , Papapetropoulos, A. , & Szabo, C. (2013). Intramitochondrial hydrogen sulfide production by 3‐mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. The FASEB Journal, 27, 601–611. 10.1096/fj.12-216507 [DOI] [PubMed] [Google Scholar]
- Moustafa, A. , & Habara, Y. (2016). Cross talk between polysulfide and nitric oxide in rat peritoneal mast cells. American Journal of Physiology. Cell Physiology, 310, C894–C902. 10.1152/ajpcell.00028.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, Y. , Kameji, T. , & Hayashi, S. (1984). Cysteine‐dependent inactivation of hepatic ornithine decarboxylase. The Biochemical Journal, 217, 573–580. 10.1042/bj2170573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa, A. K. , Gadalla, M. M. , Sen, N. , Kim, S. , Mu, W. , Gazi, S. K. , … Snyder, S. (2009). H2S signals through protein S‐sulfhydration. Science Signaling, 2, ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa, A. K. , Sikka, G. , Gazi, S. K. , Steppan, J. , Jung, S. M. , Bhunia, A. K. , … Snyder, S. H. (2011). Hydrogen sulfide as endothelium‐derived hyperpolarizing factor sulfhydrates potassium channels. Circulation Research, 109, 1259–1268. 10.1161/CIRCRESAHA.111.240242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, N. (2018). Multiple role of 3‐mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. British Journal of Pharmacology, 175, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, N. , Koike, S. , Nirasawa, T. , Kimura, H. , & Ogasawara, Y. (2018). Alternative pathway of H2S and polysulfides production from sulfurated catalytic‐cysteine of reaction intermediates of 3‐mercaptopyruvate sulfurtransferase. Biochemical and Biophysical Research Communications, 496, 648–653. 10.1016/j.bbrc.2018.01.056 [DOI] [PubMed] [Google Scholar]
- Nagahara, N. , Okazaki, T. , & Nishino, T. (1995). Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site‐directed mutagenesis. Journal of Biological Chemistry, 270, 16230–16235. [DOI] [PubMed] [Google Scholar]
- Nagahara, N. , Yoshii, T. , Abe, Y. , & Matsumura, T. (2007). Thioredoxin‐dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. Journal of Biological Chemistry, 282, 1561–1569. 10.1074/jbc.M605931200 [DOI] [PubMed] [Google Scholar]
- Nagai, Y. , Tsugane, M. , Oka, J. , & Kimura, H. (2004). Hydrogen sulfide induces calcium waves in astrocytes. The FASEB Journal, 18, 557–559. 10.1096/fj.03-1052fje [DOI] [PubMed] [Google Scholar]
- Nagai, Y. , Tsugane, M. , Oka, J.‐I. , & Kimura, H. (2006). Polysulfides induce calcium waves in rat hippocampal astrocytes. Journal of Pharmacological Sciences, 100, 200. [Google Scholar]
- Nakamura, S. , Nakamura, M. , Yamazaki, I. , & Morrison, M. (1984). Reactions of ferryl lactoperoxidase (compound II) with sulfide and sulfhydryl compounds. The Journal of Biological Chemistry, 259, 7080–7085. [PubMed] [Google Scholar]
- Nielsen, R. W. , Tachibana, C. , Hansen, N. E. , & Winther, J. R. (2011). Trisulfides in proteins. Antioxidants and Redox Signaling, 15, 67–75. 10.1089/ars.2010.3677 [DOI] [PubMed] [Google Scholar]
- Ogasawara, Y. , Ishii, K. , Togawa, T. , & Tanabe, S. (1993). Determination of bound sulfur in serum by gas dialysis/high‐performance liquid chromatography. Analytical Biochemistry, 215, 73–81. 10.1006/abio.1993.1556 [DOI] [PubMed] [Google Scholar]
- Ogasawara, Y. , Isoda, S. , & Tanabe, S. (1998). A labile sulfur in trisulfide affects cytochrome P‐450 dependent lipid peroxidation in rat liver microsomes. Toxicology Letters, 99, 191–198. [DOI] [PubMed] [Google Scholar]
- Ogasawara, Y. , Isoda, S. , & Tanabe, S. (1994). Tissue and subcellular distribution of bound and acid‐labile sulfur, and the enzymic capacity for sulfide production in the rat. Biological and Pharmaceutical Bulletin, 17, 1535–1542. 10.1248/bpb.17.1535 [DOI] [PubMed] [Google Scholar]
- Olson, K. R. , Gao, Y. , Arif, F. , Arora, K. , Patel, S. , DeLeon, E. R. , … Straub, K. D. (2018). Metabolism of hydrogen sulfide (H2S) and production of reactive sulfur species (RSS) by superoxide dismutase. Redox Biology, 15, 74–85. 10.1016/j.redox.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, K. R. , Gao, Y. , DeLeon, E. R. , Arif, M. , Arif, F. , Arora, N. , & Straub, K. D. (2017). Catalase as a sulfide–sulfur oxido‐reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biology, 12, 325–339. 10.1016/j.redox.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosumi, K. , Tsugane, M. , Ishigami, M. , Nagai, Y. , Iwai, T. , Oka, J.‐I. , & Kimura, H. (2010). Polysulfide activates TRP channels and increases intracellular Ca2+ in astrocytes. Neuroscience Research, 685, e109–e222. [Google Scholar]
- Pestaña, A. , & Sols, A. (1970). Reversible inactivation by elemental sulfur and mercurials of rat liver serine dehydratase and certain sulfhydryl enzymes. Biochemical and Biophysical Research Communications, 39, 522–529. 10.1016/0006-291X(70)90609-1 [DOI] [PubMed] [Google Scholar]
- Reiffenstein, R. J. , Hulbert, W. C. , & Roth, S. H. (1992). Toxicology of hydrogen sulfide. Annual Review of Pharmacology and Toxicology, 32, 109–134. 10.1146/annurev.pa.32.040192.000545 [DOI] [PubMed] [Google Scholar]
- Ruetz, M. , Kumutima, J. , Lewis, B. E. , Filipovic, M. R. , Lehnert, N. , Stemmler, T. L. , & Banerjee, R. (2017). A distal ligand mutes the interaction of hydrogen sulfide with human neuroglobin. The Journal of Biological Chemistry, 292, 6512–6528. 10.1074/jbc.M116.770370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, H. , Tamba, M. , Ishii, T. , & Bannai, S. (1999). Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. The Journal of Biological Chemistry, 274, 11455–11458. 10.1074/jbc.274.17.11455 [DOI] [PubMed] [Google Scholar]
- Savage, J. C. , & Gould, D. H. (1990). Determination of sulfide in brain tissue and rumen fluid by ion‐interaction reversed‐phase high‐performance liquid chromatography. Journal of Chromatography, 526, 540–545. 10.1016/S0378-4347(00)82537-2 [DOI] [PubMed] [Google Scholar]
- Searcy, D. G. (1996). HS−: O2 oxidoreductase activity of Cu, Zn superoxide dismutase. Archives of Biochemistry and Biophysics, 334, 50–58. 10.1006/abbi.1996.0428 [DOI] [PubMed] [Google Scholar]
- Searcy, D. G. , Whitehead, J. P. , & Maroney, M. J. (1995). Interaction of Cu, Zn superoxide dismutase with hydrogen sulfide. Archives of Biochemistry and Biophysics, 318, 251–263. 10.1006/abbi.1995.1228 [DOI] [PubMed] [Google Scholar]
- Sen, S. , Kawahara, B. , Gupta, D. , Tsai, R. , Khachatryan, M. , Roy‐Chowdhuri, S. , … Chaudhuri, G. (2015). Role of cystathionine β‐synthase in human breast cancer. Free Radical Biology and Medicine, 86, 228–238. 10.1016/j.freeradbiomed.2015.05.024 [DOI] [PubMed] [Google Scholar]
- Shibuya, N. , Koike, S. , Tanaka, M. , Ishigami‐Yuasa, M. , Kimura, Y. , Ogasawara, Y. , … Kimura, H. (2013). A novel pathway for the production of hydrogen sulfide from d‐cysteine in mammalian cells. Nature Communications, 4, 1366 10.1038/ncomms2371 [DOI] [PubMed] [Google Scholar]
- Shibuya, N. , Tanaka, M. , Yoshida, M. , Ogasawara, Y. , Togawa, T. , Ishii, K. , & Kimura, H. (2009). 3‐Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants and Redox Signaling, 11, 703–714. 10.1089/ars.2008.2253 [DOI] [PubMed] [Google Scholar]
- Shigetomi, E. , Jackson‐Weaver, O. , Huckstepp, R. T. , O'Dell, T. J. , & Khakh, B. S. (2013). TRPA1 channels are regulators of astrocyte basal calcium levels and long‐term potentiation via constitutive d‐serine release. The Journal of Neuroscience, 33, 10143–10153. 10.1523/JNEUROSCI.5779-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi, E. , Tong, X. , Kwan, K. Y. , Corey, D. P. , & Khakh, B. S. (2012). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT‐3. Nature Neuroscience, 15, 70–80. 10.1038/nn.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota, M. , Naya, M. , Yamamoto, T. , Hishiki, T. , Tani, T. , Takahashi, H. , … Suematsu, M. (2018). Gold‐nanofève surface‐enhanced Raman spectroscopy visualizes hypotaurine as a robust anti‐oxidant consumed in cancer survival. Nature Communications, 9, 1561 10.1038/s41467-018-03899-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirozu, K. , Tokuda, K. , Marutani, E. , Lefer, D. , Wang, R. , & Ichinose, F. (2014). Cystathionine γ‐lyase deficiency protects mice from galactosamine/lipopolysaccharide‐induced acute liver failure. Antioxidants and Redox Signaling, 20, 204–216. 10.1089/ars.2013.5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. , Padovani, D. , Leslie, R. A. , Chiku, T. , & Banerjee, R. (2009). Relative contributions of cystathionine beta‐synthase and gamma‐cystathionase to H2S biogenesis via alternative trans‐sulfuration reactions. The Journal of Biological Chemistry, 284, 22457–22466. 10.1074/jbc.M109.010868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen, G. G. (1979). Extracellular potassium in the mammalian central nervous system. Annual Review of Physiology, 41, 159–177. 10.1146/annurev.ph.41.030179.001111 [DOI] [PubMed] [Google Scholar]
- Stipanuk, M. H. , & Beck, P. W. (1982). Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. The Biochemical Journal, 206, 267–277. 10.1042/bj2060267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng, T. , Axelsson, H. E. , Hedlund, P. , Andersson, D. A. , Jordt, S. E. , Bevan, S. , … Zygmunt, P. M. (2008). Distribution and function of the hydrogen sulfide‐sensitive TRPA1 ion channel in rat urinary bladder. European Urology, 53, 391–399. 10.1016/j.eururo.2007.10.024 [DOI] [PubMed] [Google Scholar]
- Stubbert, D. , Prysyazhna, O. , Rudyk, O. , Scotcher, J. , Burgoyne, J. R. , & Eaton, P. (2014). Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension, 64, 1344–1351. 10.1161/HYPERTENSIONAHA.114.04281 [DOI] [PubMed] [Google Scholar]
- Szabo, C. , Coletta, C. , Chao, C. , Módis, K. , Szczesny, B. , Papapetropoulos, A. , & Hellmich, M. R. (2013). Tumor‐derived hydrogen sulfide, produced by cystathionine‐β‐synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proceedings of the National Academy of Sciences of the United States of America, 110, 12474–12479. 10.1073/pnas.1306241110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny, B. , Marcatti, M. , Zatarain, J. R. , Druzhyna, N. , Wiktorowicz, J. E. , Nagy, P. , … Szabo, C. (2016). Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Scientific Reports, 6, 36125 10.1038/srep36125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, N. , Sarfraz, Y. , Gilkes, D. M. , Chaturvedi, P. , Xiang, L. , Suematsu, M. , … Semennza, G. L. (2014). Decreased expression of cystathionine b-synthase promotes glioma tumorigenesis. Molecular Cancer Research, 12, 1398–1406. 10.1158/1541-7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague, B. , Asiedu, S. , & Moore, P. K. (2002). The smooth muscle relaxant effect of hydrogen sulphide in vitro: Evidence for a physiological role to control intestinal contractility. British Journal of Pharmacology, 137, 139–145. 10.1038/sj.bjp.0704858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripatara, P. , Patel, N. S. A. , Collino, M. , Gallicchio, M. , Kieswich, J. , Castiglia, S. , … Thiemermann, C. (2008). Generation of endogenous hydrogen sulfide by cystathionine γ‐lyase limits renal ischemia/reperfusion injury and dysfunction. Laboratory Investigation, 88, 1038–1048. 10.1038/labinvest.2008.73 [DOI] [PubMed] [Google Scholar]
- Vachek, H. , & Wood, J. L. (1972). Purification and properties of mercaptopyruvate sulfurtransferase of Escherichia coli . Biochimica et Biophysica Acta, 258, 133–146. 10.1016/0005-2744(72)90973-4 [DOI] [PubMed] [Google Scholar]
- Vandiver, M. S. , Paul, B. D. , Xu, R. , Karuppagounder, S. , Rao, F. , Snowman, A. M. , … Snyder, S. H. (2013). Sulfhydration mediates neuroprotective actions of parkin. Nature Communications, 4, 1626 10.1038/ncomms2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky, V. , Kabil, O. , & Banerjee, R. (2012). High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxidants and Redox Signaling, 17, 22–31. 10.1089/ars.2011.4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky, V. , Yadav, P. K. , Kurthen, A. , & Banerjee, R. (2015). Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. Journal of Biological Chemistry, 290, 8310–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, J. L. , & Wang, R. (2015). Hydrogen sulfide‐based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nature Reviews. Drug Discovery, 14, 329–345. 10.1038/nrd4433 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Cvetkov, T. L. , Chance, M. R. , & Moiseenkova‐Bell, V. Y. (2012). Identification of in vivo disulfide conformation of TRPA1 ion channel. The Journal of Biological Chemistry, 287, 6169–6176. 10.1074/jbc.M111.329748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenycia, M. W. , Goodwin, L. R. , Benishin, C. G. , Reiffenstein, R. J. , Francom, D. M. , Taylor, J. D. , & Dieken, F. P. (1989). Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochemical Pharmacology, 38, 973–981. 10.1016/0006-2952(89)90288-8 [DOI] [PubMed] [Google Scholar]
- Warenycia, M. W. , Goodwin, L. R. , Francom, D. M. , Dieken, F. P. , Kombian, S. B. , & Reiffenstein, R. J. (1990). Dithiothreitol liberates non‐acid labile sulfide from brain tissue of H2S‐poisoned animals. Archives of Toxicology, 64, 650–655. 10.1007/BF01974693 [DOI] [PubMed] [Google Scholar]
- Warenycia, M. W. , Smith, K. A. , Blashko, C. S. , Kombian, S. B. , & Reiffenstein, R. J. (1989). Monoamine oxidase inhibition as a sequel of hydrogen sulfide intoxication: Increases in brain catecholamine and 5‐hydroxytryptamine levels. Archives of Toxicology, 63, 131–136. 10.1007/BF00316435 [DOI] [PubMed] [Google Scholar]
- Whiteman, M. , Li, L. , Kostetski, I. , Chu, S. H. , Siau, J. L. , Bhatia, M. , & Moore, P. K. (2006). Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and Biophysical Research Communications, 343, 303–310. 10.1016/j.bbrc.2006.02.154 [DOI] [PubMed] [Google Scholar]
- Williams, R. A. , Kelly, S. M. , Mottram, J. C. , & Coombs, G. H. (2003). 3‐Mercaptopyruvate sulfurtransferase of Leishmania contains an unusual C‐terminal extension and is involved in thioredoxin and antioxidant metabolism. The Journal of Biological Chemistry, 278, 1480–1486. 10.1074/jbc.M209395200 [DOI] [PubMed] [Google Scholar]
- Wong, T. W. , Harris, M. A. , & Jankowicz, C. A. (1974). Transfer ribonucleic acid sulfurtransferase isolated from rat cerebral hemispheres. Biochemistry, 13, 2805–2812. 10.1021/bi00711a004 [DOI] [PubMed] [Google Scholar]
- Wong, T. W. , Harris, M. A. , & Morris, H. P. (1975). The presence of an inhibitor of RNA sulfurtransferase in Morris hepatomas. Biochemical and Biophysical Research Communications, 65, 1137–1145. 10.1016/S0006-291X(75)80504-3 [DOI] [PubMed] [Google Scholar]
- Wrobel, M. , Czubak, J. , Bronowicka‐Adamska, P. , Jurkowska, H. , Adamek, D. , Papla, B. (2014). Is development of high‐grade gliomas sulfur‐dependent? Molecules, 19, 21350–21362. 10.3390/molecules191221350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, P. K. , Martinov, M. , Vitvitsky, V. , Seravalli, J. , Wedmann, R. , Filipovic, M. R. , & Banerjee, R. (2016). Biosynthesis and reactivity of cysteine persulfides in signaling. Journal of the American Chemical Society, 138, 289–299. 10.1021/jacs.5b10494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, P. K. , Yamada, K. , Chiku, T. , Koutmos, M. , & Banerjee, R. (2013). Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. The Journal of Biological Chemistry, 288, 20002–20013. 10.1074/jbc.M113.466177 [DOI] [PMC free article] [PubMed] [Google Scholar]


