Abstract
Important aspects of sepsis management include early diagnosis as well as timely and specific treatment in the first few hours of triage. However, diagnosis and differentiation from non-infectious causes often cause uncertainties and potential time delays. Correct use of antibiotics still represents a major challenge, leading to increased risk for opportunistic infections, resistances to multiple antimicrobial agents and toxic side effects, which in turn increase mortality and healthcare costs. Optimized procedures for reliable diagnosis and management of antibiotic therapy has great potential to improve patient care. Herein, biomarkers have been shown to improve infection diagnosis, help in early risk stratification and provide prognostic information which helps optimizing therapeutic decisions (“antibiotic stewardship”). In this context, the use of the blood infection marker procalcitonin (PCT) has gained much attention. There is still no gold standard for the detection of sepsis and use of conventional diagnostic approaches are restricted by some limitations. Therefore, additional tests are necessary to enable early and reliable diagnosis. PCT has good discriminatory properties to differentiate between bacterial and viral inflammations with rapidly available results. Further, PCT adds to risk stratification and prognostication, which may influence appropriate use of health-care resources and therapeutic options. PCT kinetics over time also improves the monitoring of critically ill patients with sepsis and thus influences decisions regarding de-escalation of antibiotics. Most importantly, PCT helps in guiding antibiotic use in patients with respiratory infection and sepsis by limiting initiation and by shortening treatment duration. To date, PCT is the best studied biomarker regarding antibiotic stewardship. Still, further research is needed to understand optimal use of PCT, also in combination with other remerging diagnostic tests for most efficient sepsis care.
Keywords: Antibiotic stewardship, biomarker, high risk setting, procalcitonin, sepsis
Introduction
Sepsis, defined as a life-threatening organ dysfunction caused by a dysregulated host-response to infection, is a worldwide highly prevalent syndrome, associated with significant morbidity and mortality (1). Important aspects of sepsis management are early diagnosis as well as timely and specific treatment (e.g., antibiotics) in the first few hours of triage (2). However, the correct diagnosis and differentiation form non-infectious causes is challenging. Moreover, the correct use of antibiotics, still represents a major issue for treating physicians. The incorrect application of antimicrobial therapies lead to an increased risk for opportunistic infections, resistances to multiple antimicrobial agents and toxic side effects, which not only increase mortality but also healthcare costs (3,4).
It has been estimated that 30–50% of antibiotics used during the hospital stay are unnecessary or inappropriate, because patients have non-bacterial infection or because the patients could have been treated with shorter courses (5). There is thus much potential to optimizing antibiotic treatment through more reliable diagnosis and better management of antibiotic therapy.
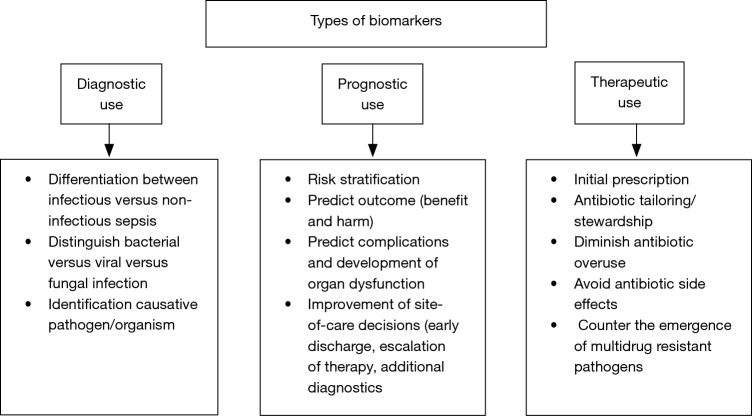
In this regard, the use of blood biomarkers has great potential to improve sepsis care (6). From a clinical point of view, biomarkers should be able to complement the clinical judgement and interpretation of available prognostic and diagnostic tests, in order to improve patients care. Clinical management of critically ill patients with severe infection and sepsis can be improved by shortening the time to diagnostic and treatment decision (i.e., differentiation between bacterial vs. viral vs. fungal infection and vs. non-infectious etiologies) (7). Furthermore, site-of-care decisions can be improved (e.g., early discharge or escalation of care) by an early risk stratification and the provision of prognostic information (8). Repeatedly measured biomarkers also help monitoring patients for tailoring therapy to individual needs of patients (antibiotic stewardship) (7,9) (Figure 1).
Figure 1.
Types of biomarkers and examples of their potential use.
In this context, the use of the host-response and blood infection marker procalcitonin (PCT) has gained much attention and has already been approved for guidance of antimicrobial therapies in patients with respiratory infection and sepsis (10-16). PCT is a precursor hormone of calcitonin, that is not detectable in healthy individuals. However, the production of PCT is upregulated in response to bacterial infections and can decrease rapidly during recovery (17,18). Thus, PCT provides important additional information, which are able to supplement clinical and diagnostic parameters (19). This in turn, has not only a high impact on decisions regarding treatment of patients with suspected infections or sepsis (20), but can also influence the duration of antibiotic treatment courses.
The use of PCT is evolving in the management of sepsis and several interventional studies and systematic reviews have analyzed and summarized the effects of PCT-guided strategies on antibiotic use and health outcomes. However, the there is no universal consensus on the optimal use of PCT in the setting of sepsis (21). The aim of this narrative review is to provide an overview about the current knowledge of PCT use in the treatment of critically ill patients with sepsis based on existing study results.
Procalcitonin as a diagnostic biomarker for bacterial infection and sepsis
Despite decades of research efforts, there is still no sepsis-specific treatment option available. Crucial for a successful treatment and positive outcomes, is an early diagnosis and differentiation from non-infectious causes, in order to rapidly start with antimicrobial therapy and fluid resuscitation (22). However, due to the fact, that clinical signs for a definite or suspected sepsis can be heterogeneous and often ambiguous, its diagnosis and treatment remains challenging. To date, no gold standard exists for the detection of sepsis caused by bloodstream infections (23). The use of conventional diagnostic approaches such as blood cultures and inflammatory blood markers [i.e., C-reactive protein (CRP), white blood count (WBC)] in patients with a clinical suspected infection or sepsis is restricted by some limitations (20).
The use of blood cultures for the identification of pathogens, can provide information about type of microorganism and susceptibility towards antibiotic therapy. However, only a small part of the analyzed cultures results positive and in around 40–90% of patients with an assumed systemic infection, the results are negative blood culture, with no growing pathogens (24-26). Moreover, the long time to results limits initial treatment decision making and contamination leads to suboptimal specificity of the obtained results. In order to improve diagnostic work-up, additional tests are appropriate, which are able to guarantee an early and reliable diagnosis.
One of the most investigated host-directed marker is PCT. Its synthesis pathway can vary depending on different inflammatory states. In healthy individuals, serum PCT is not detectable, since the protein is not released into the blood in absence of systematic inflammation (27-30). In case of a sepsis caused by bacterial infections, however, PCT synthesis is induced in practically all tissues and therefore, detectable in the blood. PCT synthesis is triggered by bacterial toxins, such as endotoxin and cytokines [e.g., interleukin (IL)-1beta, interleukin-6 and tumor necrosis factor (TNF)-alpha] (31). Due to cytokines released during viral infections that inhibit the production of TNF-alpha, PCT synthesis is not induced in the most viral infections (27-30). Moreover, PCT has a wide biological range, a short time to induction after bacterial stimulation and a long half-life (32). Thus, PCT has good discriminatory properties for the differentiation between bacterial and viral inflammations with rapidly available results.
PCT per se cannot isolate or detect specific pathogens, but the level of PCT may be useful to estimate the probability of a severe bacterial infection (26,33).
The diagnostic accuracy of PCT for sepsis has already been investigated in several observational studies, which however yielded divergent results. These conflicting results are most likely explainable by differences in the analyzed study population and different reference standard for infection used in the studies.
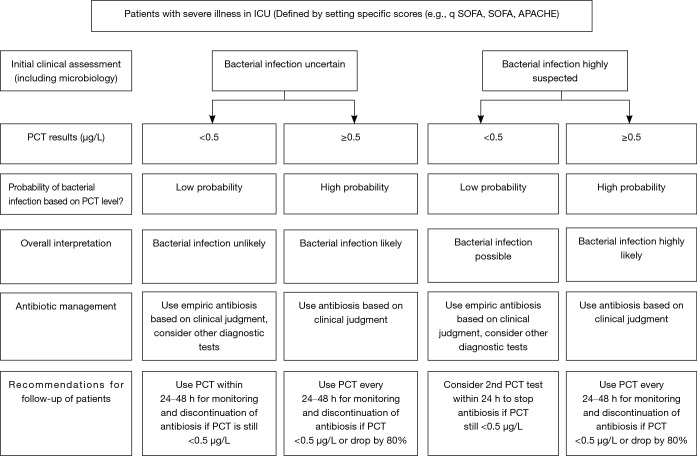
A meta-analysis conducted by Tang et al. in 2007, included 18 studies with 2,097 critically ill patients and showed a median sensitivity and specificity of 74% for PCT. Further, the calculated area under the summary receiver operating characteristic curve (SROC) was 0.78 (95% CI: 0.73–0.83), indicating that PCT cannot distinguish infectious from non-infections systemic inflammatory response syndrome (SIRS) with high certainty (34). In contrast, a more recent conducted meta-analysis including 30 high quality studies and 3,244 patients demonstrated with an ROC-curve of 0.85 (95% CI: 0.81–0.88) that PCT can differentiate effectively between sepsis and SIRS of non-infectious origin (18). However, the PCT levels should always be interpreted in a context with clinical presentation, medical history, physical examination and if available microbiological assessment of the patients (18). A more recently published international expert consensus, recommended PCT cut-off levels for critically ill patients in order to estimate the probability of bacterial infections and therefore, improve initial clinical assessment (Figure 2) (21). The presented guidelines recommend the use of cut-off ranges with higher and lower positive and negative predictive values for sepsis, instead of one general cut-off.
Figure 2.
Procalcitonin use in patients with severe illness in the ICU. Please note: caution in patients with immunosuppression (including HIV), cystic fibrosis, pancreatitis. trauma, pregnancy, high volume transfusion, malaria; PCT-guided stewardship should not be applied to patients with chronic infections (e.g., abscess, osteomyelitis, endocarditis). ICU, intensive care unit; PCT, procalcitonin.
Procalcitonin as a prognostic biomarker for the risk assessment in patients with severe infection and sepsis
The assessment of a patient’s individual risk profile and the ability to predict their outcomes, is a further key aspect in the setting of severe infections. Risk stratification and prognostication are important prerequisites, in order to appropriately apply health-care resources and available therapeutic options. This may help to find those patients who are most likely to benefit from targeted and extensive therapy without causing unnecessary harm. The Sequential Organ Failure Assessment (SOFA), which is based on the recently updated definition of sepsis, represents a complex tool mainly appropriate for patients in the intensive care unit. Clinical risk scores, such as APACHE or SAPS II, are only validated when used with admission values and are also limited by practicality issues (35,36). This in turn, reflects the need for newly available biomarkers that are measurable with high precision and reproducibility, respond to clinical recovery and provide real-time information (37).
Numerous biomarkers, which reflect the complex pathophysiology of sepsis, have been identified and evaluated in regard to their prognostic value. In this context, PCT and especially its kinetics is one of the most studied biomarkers. In fact, PCT kinetics over time has shown to improve the monitoring of critically ill patients with sepsis (38-44). Since decreasing PCT values correlate with good outcomes and increasing values are associated with adverse outcomes which also include mortality, PCT kinetics have demonstrated prognostic implications (45-49). PCT kinetics also showed a correlation with severity of illness (20). A Finnish observational study identified PCT concentrations being higher in more severe cases of already advanced sepsis. Moreover, it was shown that a substantial decrease in PCT concentration was more relevant for survival prediction compared to absolute values (46). The analysis of retrospective data from two independent US critical care institutions indicated a high prognostic power for the 72-hour PCT kinetics for predicting sepsis mortality (50). A PCT decrease >80% within 72 h after initial assessment had a negative predictive value of around 90% for the exclusion of ICU mortality, which probably can help to identify patients with a reduced risk, for whom a therapy de-escalation or an early ICU discharge could be considered. In contrast, no decrease or an increase of PCT in the same timeframe had a positive predictive value of around 50%, indicating patients at high risk who probably require treatment escalation (50). These results were also confirmed by the MOSES-Study, a prospective multicenter FDA study, conducted in different U.S based hospitals (17).
However, to demonstrate that prognostic information can also result in improved clinical outcomes of patients, has poses several challenges. This was reflected in a large interventional study, which showed that survival was not improved by a PCT-guided diagnostic and therapeutic management escalation. Moreover, the use of broad-spectrum antibiotics was prolonged which, in turn, had a negative impact on organ function and lengths of stay in the ICU (45).
A further important aspect to consider in patients with sepsis is the presence of renal impairment and the reduced glomerular filtration rate that may lower PCT clearance and levels thus may be higher than assumed (51,52).
Procalcitonin as a therapeutic biomarker for antibiotic stewardship in patient with severe infection and sepsis
Early empirical antibiotic therapy has demonstrated to be highly effective for the reduction of mortality and morbidity in sepsis (53,54). However, a prolonged and unnecessary (e.g., in the setting of viral infection) antibiotic therapy, exposes patients to a high risk for adverse drug reactions without any additional therapeutic benefit. Furthermore, antibiotic overuse, still represents an important risk factor for the development of antibiotic-resistant bacteria (55,56). For many physicians, determining the duration of an antibiotic therapy is a challenging decision, due to the fact that clinical signs and symptoms lack sensitivity and specificity to ensure differentiation between self-limited and mild viral infections from more severe bacterial infections. In recent years, there has been great interest in biomarkers that are able to indicate the risk for bacterial infection in a short time after admission and thus, can help to reduce antibiotic overuse and potentially diminish antibiotic associated side effects, mortality and treatment failure (12,57). The use of PCT for this propose has recently been approved by the US Food and Drug Administration (FDA) (21). This decision was based on several randomized controlled trials which have analyzed infections of different severity in various clinical settings ranging from primary care to ICU and have investigated and demonstrated the efficacy and safety of PCT-guided decision-making with regard to antibiotics (42,57,58). Table 1 shows a summary of previously published randomized controlled trials investigating PCT-guided antibiotic therapies in critically ill patients.
Table 1. Summary of Procalcitonin randomized trials for infections in critically ill patients.
| First author (year) (Ref), trial name | Setting, type of study (country) | Number of participant and type of infection | PCT Algorithm | Outcomes |
|---|---|---|---|---|
| Nobre (2008) (40) | Medical-Surgical ICU, Single center (Switzerland) |
79 patients with suspected severe sepsis/septic shock | Only discontinuation: antibiotic stop if PCT decreased 90% from initial value, but not before day 3 (if baseline <1 µg/L) or day 5 (if baseline ≥1 µg/L) | • No different in mortality or recurrent infections |
| • Reduction of ICU LOS by 2 days (P=0.03) | ||||
| • Reduction of 4 days in median duration of antibiotic therapy (P=0.003) | ||||
| Hochreiter (2009) (44) | 1 Surgical ICU, Single Centre (Germany) | 110 sepsis patients with SIRS and documented infection | Only discontinuation: Antibiotic stop if PCT <1 µg/L or decrease to 25–35% of initial value over 3 days | • No differences in treatment success, SOFA Score and hospital mortality |
| • Reduction of ICU LOS by 2.2 day (P=0.046) | ||||
| • Antibiotic reduction: Mean 5.9 vs. 7.9 days (P<0.001) | ||||
| Schroeder (2009) (41) | 1 Surgical ICU, Single Centre (Germany) | 27 patients with severe sepsis | Only discontinuation: antibiotic stop if PCT<1 µg/L or decrease to <35% of initial value over 3 days | • No differences in SAPS II, SOFA Score, hospital mortality |
| • Antibiotic reduction: Mean 6.6 vs. 8.3 days (P<0.001) | ||||
| Bouadma (2010) (38) PRORATA | 5 Medical ICUs and 2 surgical ICUs, Multicenter (France) | 621critically ill patients with assumed/proven bacterial infection | Initiation and discontinuation: antibiotic therapy strongly discouraged (<0.25 µg/L), discouraged (0.25–0.5 µg/L), encouraged (0.5–1 µg/L), strongly encouraged (>1 µg/L) à daily PCT measurements required. Discontinuation: if PCT decreased ≥80% from the initial peak | • No differences in non-inferiority analysis regarding 28- and 60-day mortality |
| • Trend towards increased 60 days mortality (+3.8%) | ||||
| • No differences in infection relapse, superinfection, mechanical ventilation, ICU and hospital LOS | ||||
| • Antibiotic reduction: Mean 11.6 vs. 14.3 days of therapy (P<0.0001) | ||||
| Annane (2013) (10) | 8 ICUs, Multicentre (France) | 58 severe sepsis patients without overt source of infection and negative blood culture | Initiation and discontinuation: withhold or stop antibiotic therapy (<0.25 µg/L); antibiotic therapy strongly discouraged (0.25–0.5 µg/L), recommended (0.5–5 µg/L), strongly recommended (≥0.5 µg/L). Higher cut-off values used for post-surgical patients | • No difference in day 5 mortality, ICU or hospital LOS or mortality, SOFA score at day 3 or 5 (Study terminated early due to low incidence of eligible patients) |
| • Nonsignificant trend in antibiotic reduction: 67% vs. 81% of patients on antibiotics at day 5 (P=0.24) | ||||
| Deliberato (2013) (13) | ICU, Single Centre (Brazil) |
81 sepsis patients with microbiologically confirmed bacterial infection | Only discontinuation: antibiotic stop if: PCT decreased >90% from the initial peak or PCT <0.5 µg/L | • No differences in in-hospital mortality, ICU mortality, primary infection relapse rate, ICU LOS and median hospital LOSpat |
| • Antibiotic reduction: median antibiotic therapy duration: 9 vs.13 days (P=0.008) | ||||
| Shehabi (2014) (59) ProGUARD | 11 ICUs, Multicentre (Australia) | 394 patients with suspected bacterial infection/sepsis | Only discontinuation: stop antibiotic therapy if: PCT <0.1 µg/L, or 0.1–0.25 µg/L and infection is highly unlikely, or subsequent PCT declines ≥90% from baseline (daily PCT measurements) | • No differences in ventilation time, ICU and hospital LOS, hospital and 90-day mortality) |
| • Nonsignificant trend in antibiotic reduction: median 9 vs. 11 days antibiotic therapy (P=0.58) | ||||
| de Jong (2016) (12) SAPS | ICUs at 15 hospitals, Multicentre (Netherlands) | 1,546 critically ill patients with assumed infection | Only discontinuation: stop antibiotic therapy if PCT decreased to ≥80% of the peak value or ≤0.5 µg/L (daily PCT measurements) | • Decreased 28-daily mortality (20% vs. 25%, P=0.0122) and 1-year mortality (36% vs. 43%, P=0.0188) |
| • No differences in hospital and ICU LOS or requirement for additional antibiotics within 28 days | ||||
| • 5% vs. 3% rate of reinfections by the same pathogen (P=0.0492). | ||||
| • Median antibiotic consumption of 7.5 vs. 9.3 daily defined doses (P<0.0001), | ||||
| • Median antibiotic treatment duration 5 vs. 7 days (P<0.0001) | ||||
| Bloos (2016) (11) SISPCT | 33 ICUs, Multicentre (Germany) | 1,089 patients with severe sepsis or septic shock | Discontinuation or “Alert”: PCT measurement on days 0, 4, 7, 10 and 14. On day 4: change antibiotics or intensify source control efforts if PCT not decreased by ≥50% form baseline value. Other days: Antibiotic treatment stop if PCT <1 µg/L or decreased by ≥50% from previous value | • No differences in 28-day mortality (25.6% vs. 28.2%, P=0.34) and procedures for infection source control or diagnosis |
| • 4.5% reduction in antibiotic exposure per 1,000 ICU days (823 vs. 862 days, P=0.02) |
ICU, intensive care unit; PCT, procalcitonin; LOS, length of stay; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Score.
Nobre et al. conducted one of the first randomized “proof of concept” study investigating the effect and safety of PCT-guided therapy in patients with sepsis requiring intensive care. Using a PCT-guided algorithm the exposure to antibiotics could be reduced without causing any harm or negative outcome (40). Subsequent large, multicenter studies, including the PRORATA trial (38,60) and the Stop Antibiotics on Procalcitonin Guidance Study (SAPS) (12), validated the use of PCT-guided therapy and showed that PCT can help to reduce antibiotic exposure by shortening treatment duration. In case of critically ill patients with a high probability for bacterial infection early empiric antibiotic therapy is crucial and PCT is mainly used for treatment cessation, based on its kinetics. PCT use in patients with sepsis treated in the ICU was investigated in a recent meta-analysis, including 11 studies and 4,482 patients. The analysis demonstrated a significant reduction in mean treatment duration (from 10.4 to 9.3 days, P=0.001) (61). Further, it was shown that the mortality rate was lower in the PCT-guided group compared to the control group (21.1% vs. 23.7%, P=0.03). Subgroup analyses based on sepsis 3 definition, sepsis severity, presence of renal failure as well as in different types of infection revealed similar effects. The observed positive effects are probably due to the lower risk for antibiotics associated toxic effects in the PCT-guided group. Despite a not yet fully understood pathophysiological mechanism, several observational analyses have reported a reduced risk of treatment failure and mortality which was associated with early antibiotic de-escalation in patients with sepsis (62,63).
Consistent with the results mentioned above, a meta-analysis investigating PCT use in septic patients with positive blood cultures indicated a significantly shorter antibiotic therapy duration for the patients guided with PCT (−2.86 days, 95% CI: −4.88 to 0.84; P=0.006) as well as a trend towards lower mortality (16.6% vs. 20.0%; P=0.263) (64).
Despite the available evidence regarding PCT-guided therapy de-escalation, the use of PCT-guided antibiotic therapy escalation is not yet recommended. A randomized trial analyzing 1,200 critically ill patients in nine multidisciplinary intensive care units in Denmark, demonstrated that therapy escalation did not improve outcome when PCT-algorithms were used (45).
A more widespread use of PCT in critically ill patients was limited by the fact, that a commonly accepted algorithm for the utilization of PCT in those patients was long lacking (21). However, the applied PCT-protocols used in the different studies were all similar and based on the same concept: The initiation of an antibiotics therapy was recommended in all patients with a suspected sepsis based on the clinical grounds and PCT kinetics over time were used for recommendations concerning an early discontinuation of the antibiotic therapy (58). PCT cut-offs of <0.5 µg/L or a decrease of 80–90% from the peak level were considered to indicate recovery and in such cases discontinuation of antibiotic treatment was favorable. According to the current body of evidence an international expert group recently published a consensus algorithm for the PCT use in patients with suspected bacterial infections. The proposed algorithm was utilized in various interventional trials, which all resulted in significantly reduced antibiotic exposure, without increasing mortality or adverse event rates. However, in particular for ICU trials, adherence rates to the PCT protocol were very variable (38,40).
Practical consideration for the use of procalcitonin testing
Figure 2 (Flowchart PCT) provides a practical guide for the rational use of PCT in a high risk setting in conjunction with clinical assessment, including interpretation of PCT and recommendations for antibiotic use (21,58).
The interventional non-inferiority proACT trial revealed low adherence rates to the PCT protocol, indicating a shortcoming of experience in the use of PCT as well as in its interpretation in a clinical context. In order to gain more confidence with PCT measurements, repeated education for antibiotic stewardship could be advantageous for physicians. This statement was also confirmed by a retrospective cohort study of Broyles et al. Through education-based antibiotic stewardship, which includes also the use of PCT measurements, a reduction of antibiotic prescriptions and lower resistance rates could be achieved. Moreover, an association with improved outcomes like lower readmission rates, shorter length of stay and lower Clostridium difficile infections was observed (65).
Limitation of procalcitonin
PCT studies are limited to the use in patients with respiratory infections and sepsis. Data about the use of PCT in immunosuppressed patients including patients with HIV, cystic fibrosis, pancreatitis, trauma, pregnancy and high volume transfusion are very low. Moreover, it should be noted that some non-infectious disorders, such as C-cell carcinoma or trauma, can lead to a systemic inflammation resulting in elevated PCT levels. Further, the use of PCT-guided stewardship is not recommended in patients suffering from a chronic infection such as osteomyelitis or endocarditis, since observational studies were unable to identify any benefit and interventional investigations in this context are still lacking (66).
Conclusion and outlook
An early diagnosis and the initiation of an appropriate antibiotic treatment are still the cornerstones of effective sepsis care. In this respect, PCT has shown promising results for the treatment of patients with sepsis. However, it should be noted that PCT values are not intended to replace good clinical practice, but should be used as a complementary tool combined with available clinical and diagnostic parameters. In order to estimate the probability of bacterial infections, it is recommended to use cut-off ranges with higher and lower positive and negative predictive values for the identification of sepsis, instead of one general cut-off. A further important consideration is the quality of the used PCT assays (67). The use of high-sensitive PCT assays should be preferred in clinical practice since the use of semi-quantitative assays is not able to detect an increased PCT in lower ranges. The prognostic information derived from PCT kinetics can influence further procedure with regard to diagnostic testing, but also therapeutic decisions and timing of patients discharge (40,60). In high risk situation the use of PCT should not delay or inhibit the start of empirical treatments, but should rather be used for treatment termination in case PCT is <0.5 µg/L or decreased by 80–90% of the peak level. To date, integration of the host-response marker PCT into a comprehensive clinical assessment seems to be a promising approach to reduce diagnostic uncertainties and antibiotic overuse. Still, further research is needed to understand optimal use of PCT, also in combination with other remerging diagnostic tests for most efficient sepsis care.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflict of Interest: Prof. Schuetz reports receiving grants form bioMerieux Thermo Fischer and Roche Diagnostics (paid to the institution).
References
- 1.Bracht H, Hafner S, Weiss M. Sepsis Update: Definition and Epidemiology. Anasthesiol Intensivmed Notfallmed Schmerzther 2019;54:10-20. 10.1055/a-0625-5492 [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 3.Zilahi G, McMahon MA, Povoa P, et al. Duration of antibiotic therapy in the intensive care unit. J Thorac Dis 2016;8:3774-80. 10.21037/jtd.2016.12.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jee Y, Carlson J, Rafai E, et al. Antimicrobial resistance: a threat to global health. Lancet Infect Dis 2018;18:939-40. 10.1016/S1473-3099(18)30471-7 [DOI] [PubMed] [Google Scholar]
- 5.Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014;63:194-200. [PMC free article] [PubMed] [Google Scholar]
- 6.Schuetz P, Aujesky D, Muller C, et al. Biomarker-guided personalised emergency medicine for all - hope for another hype? Swiss Med Wkly 2015;145:w14079. [DOI] [PubMed] [Google Scholar]
- 7.Schuetz P, Raad I, Amin DN. Using procalcitonin-guided algorithms to improve antimicrobial therapy in ICU patients with respiratory infections and sepsis. Curr Opin Crit Care 2013;19:453-60. 10.1097/MCC.0b013e328363bd38 [DOI] [PubMed] [Google Scholar]
- 8.Schuetz P, Koller M, Christ-Crain M, et al. Predicting mortality with pneumonia severity scores: importance of model recalibration to local settings. Epidemiol Infect 2008;136:1628-37. 10.1017/S0950268808000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuetz P, Christ-Crain M, Muller B. Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections--hope for hype? Swiss Med Wkly 2009;139:318-26. [DOI] [PubMed] [Google Scholar]
- 10.Annane D, Maxime V, Faller JP, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open 2013;3. doi: . 10.1136/bmjopen-2012-002186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloos F, Trips E, Nierhaus A, et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern Med 2016;176:1266-76. 10.1001/jamainternmed.2016.2514 [DOI] [PubMed] [Google Scholar]
- 12.de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016;16:819-27. 10.1016/S1473-3099(16)00053-0 [DOI] [PubMed] [Google Scholar]
- 13.Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis 2013;76:266-71. 10.1016/j.diagmicrobio.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 14.Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care 2017;7:114. 10.1186/s13613-017-0338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iankova I, Thompson-Leduc P, Kirson NY, et al. Efficacy and Safety of Procalcitonin Guidance in Patients With Suspected or Confirmed Sepsis: A Systematic Review and Meta-Analysis. Crit Care Med 2018;46:691-8. 10.1097/CCM.0000000000002928 [DOI] [PubMed] [Google Scholar]
- 16.Lamping F, Jack T, Rubsamen N, et al. Development and validation of a diagnostic model for early differentiation of sepsis and non-infectious SIRS in critically ill children - a data-driven approach using machine-learning algorithms. BMC Pediatr 2018;18:112. 10.1186/s12887-018-1082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuetz P, Birkhahn R, Sherwin R, et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med 2017;45:781-9. 10.1097/CCM.0000000000002321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:426-35. 10.1016/S1473-3099(12)70323-7 [DOI] [PubMed] [Google Scholar]
- 19.Mitsuma SF, Mansour MK, Dekker JP, et al. Promising new assays and technologies for the diagnosis and management of infectious diseases. Clin Infect Dis 2013;56:996-1002. 10.1093/cid/cis1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sager R, Kutz A, Mueller B, et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med 2017;15:15. 10.1186/s12916-017-0795-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med 2019;57:1308-18. 10.1515/cclm-2018-1181 [DOI] [PubMed] [Google Scholar]
- 22.Vijayan AL, Vanimaya, Ravindran S, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care 2017;5:51. 10.1186/s40560-017-0246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care 2016;20:89. 10.1186/s13054-016-1266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laukemann S, Kasper N, Kulkarni P, et al. Can We Reduce Negative Blood Cultures With Clinical Scores and Blood Markers? Results From an Observational Cohort Study. Medicine (Baltimore) 2015;94:e2264. 10.1097/MD.0000000000002264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34:344-53. 10.1097/01.CCM.0000194725.48928.3A [DOI] [PubMed] [Google Scholar]
- 26.Muller B, Schuetz P, Trampuz A. Circulating biomarkers as surrogates for bloodstream infections. Int J Antimicrob Agents 2007;30 Suppl 1:S16-23. 10.1016/j.ijantimicag.2007.06.032 [DOI] [PubMed] [Google Scholar]
- 27.Becker KL, Nylen ES, White JC, et al. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004;89:1512-25. 10.1210/jc.2002-021444 [DOI] [PubMed] [Google Scholar]
- 28.Christ-Crain M, Muller B. Procalcitonin in bacterial infections--hype, hope, more or less? Swiss Med Wkly 2005;135:451-60. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol 2010;48:2325-9. 10.1128/JCM.00655-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res 2000;49 Suppl 1:S57-61. [PubMed] [Google Scholar]
- 31.Linscheid P, Seboek D, Schaer DJ, et al. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med 2004;32:1715-21. 10.1097/01.CCM.0000134404.63292.71 [DOI] [PubMed] [Google Scholar]
- 32.Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994;79:1605-8. [DOI] [PubMed] [Google Scholar]
- 33.Cuquemelle E, Soulis F, Villers D, et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med 2011;37:796-800. 10.1007/s00134-011-2189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang BM, Eslick GD, Craig JC, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007;7:210-7. 10.1016/S1473-3099(07)70052-X [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73. 10.1097/01.CCM.0000117317.18092.E4 [DOI] [PubMed] [Google Scholar]
- 36.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327. 10.1097/01.CCM.0000298158.12101.41 [DOI] [PubMed] [Google Scholar]
- 37.Schuetz P, Wolbers M, Christ-Crain M, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care 2010;14:R106. 10.1186/cc9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375:463-74. 10.1016/S0140-6736(09)61879-1 [DOI] [PubMed] [Google Scholar]
- 39.Coelho LM, Salluh JI, Soares M, et al. Patterns of c-reactive protein RATIO response in severe community-acquired pneumonia: a cohort study. Crit Care 2012;16:R53. 10.1186/cc11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008;177:498-505. 10.1164/rccm.200708-1238OC [DOI] [PubMed] [Google Scholar]
- 41.Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009;394:221-6. 10.1007/s00423-008-0432-1 [DOI] [PubMed] [Google Scholar]
- 42.Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012;55:651-62. 10.1093/cid/cis464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theodorou VP, Papaioannou VE, Tripsianis GA, et al. Procalcitonin and procalcitonin kinetics for diagnosis and prognosis of intravascular catheter-related bloodstream infections in selected critically ill patients: a prospective observational study. BMC Infect Dis 2012;12:247. 10.1186/1471-2334-12-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hochreiter M, Kohler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 2009;13:R83. 10.1186/cc7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011;39:2048-58. 10.1097/CCM.0b013e31821e8791 [DOI] [PubMed] [Google Scholar]
- 46.Karlsson S, Heikkinen M, Pettila V, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care 2010;14:R205. 10.1186/cc9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest 2012;141:1063-73. 10.1378/chest.11-2430 [DOI] [PubMed] [Google Scholar]
- 48.Alan M, Grolimund E, Kutz A, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Intern Med 2015;278:174-84. 10.1111/joim.12341 [DOI] [PubMed] [Google Scholar]
- 49.Schuetz P, Kutz A, Grolimund E, et al. Excluding infection through procalcitonin testing improves outcomes of congestive heart failure patients presenting with acute respiratory symptoms: results from the randomized ProHOSP trial. Int J Cardiol 2014;175:464-72. 10.1016/j.ijcard.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 50.Schuetz P, Maurer P, Punjabi V, et al. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care 2013;17:R115. 10.1186/cc12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014;141:43-51. 10.1309/AJCP4GV7ZFDTANGC [DOI] [PubMed] [Google Scholar]
- 52.Heredia-Rodriguez M, Bustamante-Munguira J, Fierro I, et al. Procalcitonin cannot be used as a biomarker of infection in heart surgery patients with acute kidney injury. J Crit Care 2016;33:233-9. 10.1016/j.jcrc.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 53.Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136:1237-48. 10.1378/chest.09-0087 [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. 10.1097/01.CCM.0000217961.75225.E9 [DOI] [PubMed] [Google Scholar]
- 55.Ohl CA, Luther VP. Antimicrobial stewardship for inpatient facilities. J Hosp Med 2011;6 Suppl 1:S4-15. 10.1002/jhm.881 [DOI] [PubMed] [Google Scholar]
- 56.Lawrence KL, Kollef MH. Antimicrobial stewardship in the intensive care unit: advances and obstacles. Am J Respir Crit Care Med 2009;179:434-8. 10.1164/rccm.200809-1394CP [DOI] [PubMed] [Google Scholar]
- 57.Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018;18:95-107. 10.1016/S1473-3099(17)30592-3 [DOI] [PubMed] [Google Scholar]
- 58.Schuetz P, Chiappa V, Briel M, et al. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 2011;171:1322-31. 10.1001/archinternmed.2011.318 [DOI] [PubMed] [Google Scholar]
- 59.Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med 2014;190:1102-10. 10.1164/rccm.201408-1483OC [DOI] [PubMed] [Google Scholar]
- 60.Landman GW, Kleefstra N. Procalcitonin in intensive care units: the PRORATA trial. Lancet 2010;375:1606; author reply 1606-7. 10.1016/S0140-6736(10)60697-6 [DOI] [PubMed] [Google Scholar]
- 61.Wirz Y, Branche A, Wolff M, et al. Management of Respiratory Infections with Use of Procalcitonin: Moving toward More Personalized Antibiotic Treatment Decisions. ACS Infect Dis 2017;3:875-9. 10.1021/acsinfecdis.7b00199 [DOI] [PubMed] [Google Scholar]
- 62.Schuetz P, Mueller B. Biomarker-guided de-escalation of empirical therapy is associated with lower risk for adverse outcomes. Intensive Care Med 2014;40:141. 10.1007/s00134-013-3139-x [DOI] [PubMed] [Google Scholar]
- 63.Garnacho-Montero J, Gutierrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014;40:32-40. 10.1007/s00134-013-3077-7 [DOI] [PubMed] [Google Scholar]
- 64.Meier MA, Branche A, Neeser OL, et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis 2019;69:388-96. 10.1093/cid/ciy917 [DOI] [PubMed] [Google Scholar]
- 65.Broyles MR. Impact of Procalcitonin-Guided Antibiotic Management on Antibiotic Exposure and Outcomes: Real-world Evidence. Open Forum Infect Dis 2017;4:ofx213. 10.1093/ofid/ofx213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent JL, Quintairos ESA, Couto L, Jr, et al. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016;20:257. 10.1186/s13054-016-1403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuetz P, Bretscher C, Bernasconi L, et al. Overview of procalcitonin assays and procalcitonin-guided protocols for the management of patients with infections and sepsis. Expert Rev Mol Diagn 2017;17:593-601. 10.1080/14737159.2017.1324299 [DOI] [PubMed] [Google Scholar]