Abstract
Stage III N2 nonsmall cell lung cancer (NSCLC) is a complex disease with poor treatment outcomes. For patients in whom the disease is considered technically resectable, the main treatment options include surgery (with neoadjuvant or adjuvant chemotherapy/neoadjuvant chemoradiotherapy (CRT)) or CRT followed by adjuvant immunotherapy (dependent on programmed death ligand 1 status). As there is no clear evidence demonstrating a survival benefit between these options, patient preference plays an important role. A lack of a consensus definition of resectability of N2 disease adds to the complexity of the decision-making process. We compared 10 international guidelines on the treatment of NSCLC to investigate the recommendations on preoperatively diagnosed stage III N2 NSCLC. This comparison simplified the treatment paths to multimodal therapy based on surgery or radiotherapy (RT). We analysed factors relevant to decision-making within these guidelines. Overall, for nonbulky mediastinal lymph node involvement there was no clear preference between surgery and CRT. With increasing extent of mediastinal nodal disease, a tendency towards multimodal treatment based on RT was identified. In multiple scenarios, surgery or RT-based treatments are feasible and patient involvement in decision-making is critical.
Short abstract
For many patients with stage III N2 NSCLC, radiotherapy or surgery are options and should be discussed with the patient http://bit.ly/2Z39MW5
Introduction
Stage III nonsmall lung cancer (NSCLC) with ipsilateral and/or subcarinal mediastinal lymphatic spread (N2) represents a potentially curable disease, although prognosis remains poor with a 5-year overall survival (OS) in the range of 15–40% [1–3]. The optimal treatment remains controversial due to a high degree of heterogeneity among stage III N2 patients and a lack of a universally agreed definition on resectability. The two main multimodal treatment options in this setting are chemoradiotherapy (CRT) (with or without adjuvant immunotherapy) or surgery (with neoadjuvant or adjuvant chemotherapy (Ch)/neoadjuvant CRT).
These treatment options may both be available for several decision criteria combinations, especially since there is no clear randomised evidence suggesting one treatment regime is superior over another in terms of OS [4, 5]. In such a setting, patient preference becomes even more important and the focus of the decision-making process should include the patient experience, influenced by side effects and logistical considerations (e.g. having to stay in the hospital with surgery or receiving daily treatments for several weeks with radiotherapy (RT)). As shared decision-making is a complex process with various potential pitfalls [6], patient decision aids will become increasingly helpful in these settings [7, 8]. Various factors may influence the availability and suitability of available treatments [9] and a multitude of clinical decision criteria can be implemented [10].
In clinical practice, it is important to recognise situations where the patient can be offered multiple treatment options. The aim of this work was to identify which disease characteristics are essential in current international guidelines for stage III N2 NSCLC and how they impact decision-making.
Methods
Guidelines available in English and German (based on spoken languages of authors) and published after 2009 were identified. The web-based search conducted in spring 2019 included: national guidelines NSCLC, stage III N2 NSCLC and guidelines NSCLC. Current valid guidelines (American College of Chest Physicians (ACCP) 2013 [11], British Thoracic Society (BTS) 2010 [12], European Society for Medical Oncology (ESMO) 2015/2017 [13, 14], National Institute for Health and Care Excellence (NICE) 2011/2019 [15, 16], National Comprehensive Cancer Network (NCCN) 2018 [17, 18], Spanish Society of Medical Oncology (SEOM) [19], German S3 Guideline on Treatment of NSCLC [20], Chinese [21], Irish [22] and Australian [23]) were identified, reviewed, and summarised analogous to a previous summary of five selected guidelines [24]. The analysed guidelines were weighted equally and not evaluated for their methodological quality. As such, statements regarding the quality of evidence for specific recommendations and their consensus were not made.
The recommendations on stage III N2 NSCLC within the guidelines were reduced to recommendations towards a definitive surgical approach (with neoadjuvant or adjuvant Ch/CRT), a definitive CRT approach or no preference (no specific recommendation for either RT or surgery and both being options). Decision criteria implemented were analysed and standardised if their meaning was not changed, as described by Panje et al. [25–27]. Universal criteria such as the ability to give informed consent were not included in this analysis. In several instances, trial participation was a recommended option; this was excluded from the decision tree analysis.
The final decision criteria implemented included: resectability (potentially resectable or not), lymph node extent (single station, multistation and single zone, multizone), and lymph node volume (bulky, nonbulky). In order to ensure comparability, simplifications of terminology were performed, while attempting to keep the original statements as verbatim as possible. The resectability criterion used here includes the possibility of surgical tumour removal with an R0 margin based on preoperative imaging, as well as the required physical fitness for radical operative treatment. In the BTS and Irish guidelines, lymph nodes are grouped by zones according to the International Association for the Study of Lung Cancer (IASLC) lymph node map presented in the American Joint Committee on Cancer (AJCC) 7th edition, whereas others (e.g. ESMO, German guidelines) used nodal stations. For the purposes of this analysis the extent of mediastinal lymph node involvement was categorised as: 1) single station and therefore single zone; 2) multistation, but limited to single zone; and 3) multizone. The obvious limitation is that the primary multistation nodal involvement described in a guideline using nodal stations as a descriptor could fall within a single zone or multiple zones (stations 2 and 4 single zone, stations 4 and 7 multizone).
Morphological characteristics of the lymph nodes were categorised into simplified categories: bulky and nonbulky. There are different descriptions used to characterise nonbulky lymph nodes. These include lymph node diameter less than 3 cm, easily measurable and defined lymph nodes free of major mediastinal structures including the trachea and great vessels, or low-volume lymph nodes [12, 17, 18, 21, 28].
The treatment of stage III N2 NSCLC is clearly multimodal. In multimodal treatment based on surgical approach, to further simplify the comparison, there was no distinction between neoadjuvant and adjuvant Ch as the benefit from preoperative Ch is similar to that of postoperative Ch and either approach is justified [17, 18].
The resulting decision trees were analysed for consensus and dissent with the objective consensus methodology [25, 29, 30].
Results
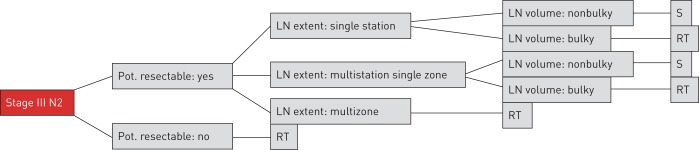
Ten guidelines were identified and converted into decision trees, as shown in the example in figure 1.
FIGURE 1.
Example of the simplified decision tree representing the 2010 British Thoracic Society guideline. Pot.: potentially; LN: lymph node; RT: radiotherapy; S: surgery.
A total of three simplified criteria were used in the analysed guideline (table 1).
TABLE 1.
The use of simplified decision criteria per analysed guideline
| Criterion | ACCP | BTS | ESMO | NICE | NCCN | SEOM | German | Chinese | Irish | Australian |
| Resectability | • | • | • | • | • | • | • | • | • | • |
| Lymph node volume | • | • | • | • | • | • | • | |||
| Lymph node extent | • | • | • | • | • | • |
ACCP: American College of Chest Physicians; BTS: British Thoracic Society; ESMO: European Society for Medical Oncology; NICE: National Institute for Health and Care Excellence; NCCN: National Comprehensive Cancer Network; SEOM: Spanish Society of Medical Oncology.
Discussion
The review of the existing guidelines revealed RT-based approaches as the most commonly recommended treatment (based on these simplified criteria). For several parameter combinations, multimodal treatment based on surgery is a recommended alternative, and in several instances, this was the treatment of choice.
The management of stage III N2 NSCLC is undergoing rapid evolution with the introduction of immunotherapy. New evidence is being collected in ongoing clinical trials. The implementation in international treatment guidelines is progressing; however, this has not directly affected the decision between surgical and RT-based approaches.
The question of resectability and how it is defined is essential to decision-making for stage III N2 NSCLC. Preoperative surgical evaluation can be challenging and depends on an individual surgeon's expertise. There are no definitive criteria to preoperatively confirm the resectability of disease. This is in part a subjective decision based on a surgeon's individual judgement at being able to achieve a clear resection margin.
In many cases of preoperatively diagnosed N2 disease, neoadjuvant Ch or CRT are performed with the aim of tumour downsizing and/or controlling local disease. Neoadjuvant treatment may also come with a risk of forming mediastinal soft tissue fibrosis, potentially complicating subsequent hilar and mediastinal dissections. Therefore, when multimodal regimes are considered, even closer interdisciplinary collaboration by an experienced team is essential.
There are also reports of neoadjuvant treatment with immunotherapy in NSCLC [31, 32]. Bott et al. [31] confirmed a major pathological response in 9 of 20 (45%) patients treated with nivolumab in NSCLC stage I–IIIA being enrolled. In this report, more than a half of the minimally invasive intended operations were converted to thoracotomy due to hilar inflammation and fibrosis. While there was no operative mortality, perioperative morbidity was present and mostly due to atrial arrhythmia. Similarly, Forde et al. [32] reported a major pathological response on histologic examination in 45% of examined tumours. Interestingly, only two patients had a radiologic partial response, due to postulated immune-cell infiltration into the tumour, rather than true tumour mass. To define the role of neoadjuvant immunotherapy long-term follow-up of currently recruiting phase III studies, such as ClinicalTrials.gov identifier: NCT02998528, need to be evaluated. The purpose of this neoadjuvant study is to compare nivolumab plus Ch and Ch alone in terms of safety and effectiveness, and to describe nivolumab plus ipilimumab's safety and effectiveness in treating resectable NSCLC. The estimated completion date is November 2028.
Not surprisingly, for unresectable tumours RT-based approaches are recommended. There are randomised controlled trial data confirming a significant improvement with adjuvant immunotherapy following definitive CRT in unresectable stage III N2 NSCLC [33]. The definition of unresectable in this trial was left to the discretion of local tumour boards and was likely to have been highly variable. How immunotherapy in both RT-based multimodality treatment and surgical-based multimodality treatment will impact future trends remains undefined but is expected to become a critical aspect in future decision-making as evidence develops.
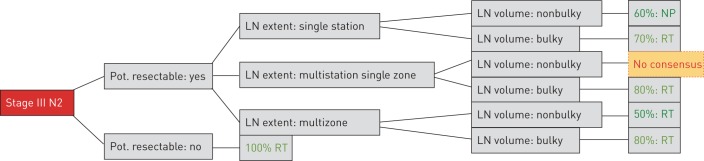
In general, with increasing volume or extent of N2 disease, there is an increasing trend towards therapy based on RT. There is no consensus on a unified cut-off for nodal volume related to surgery. In patients with low-volume mediastinal N2 disease (especially single station) surgery was a common recommendation. Four of the analysed guidelines clearly recommended surgery in potentially resectable tumours with single station nonbulky involvement. In this setting, the other six guidelines declared no preference between surgery and RT. In potentially resectable tumours with multistation but single-zone, nonbulky mediastinal involvement, significant variation was observed: four guidelines recommended surgery, two recommended RT and four declared no preference (figures 2 and 3). For resectable tumours with bulky lymph nodes the overwhelming majority of analysed guidelines recommended a RT-based approach. The recommendation towards surgery in a few decision trees for this particular constellation might potentially be due to a lack of explicitly naming bulky disease within selected guidelines.
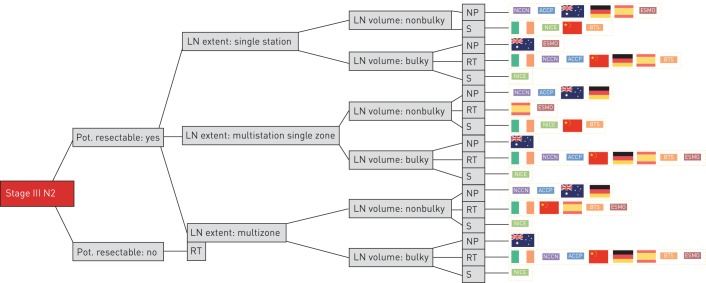
FIGURE 2.
Demonstration of all combinations of simplified criteria. Pot.: potentially; LN: lymph node; RT: radiotherapy; NP: no preference; S: surgery.
FIGURE 3.
Demonstration of the majority recommendations for each parameter combination. Pot.: potentially; LN: lymph node; RT: radiotherapy; NP: no preference; no consensus: a majority preference among the three options was not identified (four recommended surgery, two RT and four declared NP).
The Intergroup study (INT 0139) compared treatment outcomes after definitive Ch/RT and Ch/RT followed by surgery in pre-therapeutically diagnosed N2 disease considered to be resectable. A significantly better progression-free survival (PFS) was shown (29% versus 19%; p=0,02). However, OS was not significantly different (3-year OS 38% versus 33%). Based on the relatively high mortality after pneumonectomy (22%) compared to lobectomy (1%), it might be reasonable to perform definitive CRT if the surgery does require a pneumonectomy. In a post hoc analysis, the OS of lobectomy patients from the surgical arm was significantly better than the CRT arm. It is widely debated how much weight can be applied to this post hoc analysis, as excluding selected patients least suitable for RT might have resulted in similar improvements. Within this trial, some patients requiring pneumonectomy were operated in low-volume centres and may have contributed to the high pneumonectomy mortality. Lower mortality rates for post-induction pneumonectomy have since been published from high volume centres as reported by Casiraghi et al. [34] although these findings are not consistent, which was shown by d’Amato et al. [35]. These factors further cement the need for a multidisciplinary discussion with experienced clinicians.
The meta-analysis by Pöttgen et al. [36] found no significant difference between bimodal treatments related to OS. There was heterogeneity across studies at 2 years, resulting from the rate of performed pneumonectomies. The increased mortality following pneumonectomy was not confirmed in the ESPATUE trial. Comparisons between surgery and RT are complicated by early perioperative mortality of surgical patients with potential advantages in survival later on [37].
A meta-analysis by McElnay et al. [5] also compared trimodality treatment with CRT and surgery versus definitive CRT. It combined the data from the INT 0139 trial and a study from Sorensen et al. [39] comparing induction Ch followed by RT versus induction Ch followed by surgery followed by RT. Both studies were given equal weighting in the meta-analysis and the pooled hazard ratio for death in the surgery group was 0.87 (CI 0.75–1.01; p=0.068) with no statistical evidence of heterogeneity (I2=0%, p=0.976). Trimodal therapy was associated with a trend towards improved survival compared to bimodal treatments.
The 2019 NICE guidelines addressed the optimal management of potentially resectable stage III N2 NSCLC. Their analysis compared CRT followed by surgery (CRS), CRT, and chemotherapy and surgery (CS). The meta-analysis could not distinguish the odds of survival across the treatment groups at 4 and 5 years. However, there was a strong but statistically insignificant trend towards improved survival with CRS (in line with the trends from meta-analyses). CRS was associated with longer PFS than CRT/CS at 4 and 5 years (high quality evidence) with an average of a 4.5-month improvement. There were fewer grade 3+ adverse events with CRS than with CR/CS. The NICE guideline group also undertook cost-effectiveness modelling, concluding that CRT is more cost effective than CS (incremental cost-effectiveness ratio of £53 000 per quality-adjusted life-year) and CRS is more cost effective than CRT (incremental cost-effectiveness ratio of £17 800 per quality-adjusted life-year). This, therefore, is the first guideline to specifically recommend trimodality treatment over the others in resectable N2 NSCLC. The guideline group recommended that for patients fit enough for multimodality treatment and considered to be resectable, induction CRT followed by surgery should be considered. This is a highly significant change from previous NICE recommendations and a significant change in UK practice, where only 1.1% of N2 patients currently receive trimodality treatment [38]. Such practice would require careful and considered implementation within agreed protocols through high volume centres where outcomes are monitored closely.
The comparison between surgery and RT after induction Ch showed no differences in OS [1]. However, the Chinese, BTS and the NICE guidelines are the only ones to recommend surgical resection over RT with disease involvement of two nodal stations if involved lymph nodes are all smaller than 3 cm. The Irish guideline only considers surgery in nonbulky single-zone N2 disease. This statement is based on studies suggesting a survival benefit with a Ch plus surgery protocol. The BTS guideline restricts the mediastinal involvement to single zone, whereas the Chinese does not differentiate this issue any further. The only criterion described in the NICE guideline is resectability. Due to the simplification used to compare the guidelines within the decision trees, all potentially resectable tumours are therefore treated with surgery. We are aware of potential limitations of the interpretation of the comparison due to the lack of a more specific description of mediastinal lymph nodes status. It is controversial whether surgery should be recommended in multizone bulky N2 NSCLC disease. The used criteria on mediastinal lymph node extent does not allow for a differentiated categorisation between single zone and single station. This is why the Chinese guideline is interpreted as recommending surgery for multistation single-zone disease and RT for multistation multizone disease. The Chinese guideline used two “sets” of enlarged, not fused lymph nodes to define a cut-off. For the purpose of this analysis, we categorised this as multistation single zone. The Australian guideline was also simplified; the recommended treatments were RT with surgery, reserved for rare cases with low-volume mediastinal lymph node involvement.
While some of these simplifications can be questioned, the authors believe that specialty bias did not play a significant role, as we included two thoracic surgeons and two radiation oncologists. The big picture of this result is that multiple options are available in multiple situations; this result would probably not change by changing individual interpretations or by including potentially missed guidelines.
The large variability of recommendations among these guidelines may have multiple potential reasons. These may include different methodologies used to develop the guidelines, local traditions, incomplete definition of decision criteria (e.g. only broadly specified lymph node involvement, lacking definition of resectability) as well as different time of guideline publication 2009–2019 and different studies included in analyses.
The evidence base in this setting has several severe limitations. A number of key trials (Intergroup and European Organisation for Research and Treatment of Cancer) date from a time before routine positron emission tomography, CT and endoscopic nodal staging and advances in surgical and RT techniques are not accounted for. Some trials, such as the ESPATUE trial had a heterogeneous study population (e.g. one-third of patients with T4 N0/1) further hampering definitive conclusions on the management of N2 disease.
Many patients with resectable stage III N2 NSCLC have a number of treatment options available to them, assuming adequate physiological reserve for treatment: definitive CRT (± adjuvant immunotherapy), surgical resection followed by adjuvant Ch, induction Ch followed by surgery and induction CRT followed by surgery. The majority of guidelines note that no treatment has been shown to be superior to another and therefore multiple options exist. Furthermore, a clear definition of resectability remains elusive. There are randomised controlled data, meta-analyses and guideline group network meta-analyses (NICE) that suggest an improvement in PFS with CRT followed by surgery over definitive CRT for resectable tumours. There are meta-analyses data confirming a strong suggestion towards improved OS and post hoc analysis data suggesting a significant OS improvement if lobectomy is performed. All of this information is followed by a series of questions and controversies. Additionally, significant questions remain on how to interpret the data in the era of immunotherapy after definitive CRT. Definitive evidence for the optimal treatment of resectable stage III N2 NSCLC remains elusive.
Conclusion
The optimal strategy in resectable III N2 NSCLC remains unresolved. Most guidelines consider surgical approaches to be a treatment option, and in several instances, surgery is the preferred treatment option in patients with low-volume mediastinal nodal involvement. In more advanced cases, most guidelines recommend multimodal treatment based on RT. Immunotherapy has an established role following CRT in unresectable disease and continues to be explored in resectable disease in clinical trials. Patient preference should be central to decision-making where both surgical and RT-based options are available.
Footnotes
Conflict of interest: P.M. Putora has nothing to disclose.
Conflict of interest: P. Leskow has nothing to disclose.
Conflict of interest: F. McDonald has nothing to disclose.
Conflict of interest: T. Batchelor reports personal fees from Medtronic, Johnson & Johnson and AstraZeneca, outside the submitted work.
Conflict of interest: M. Evison has nothing to disclose.
References
- 1.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007; 99: 442–450. doi: 10.1093/jnci/djk093 [DOI] [PubMed] [Google Scholar]
- 2.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009; 374: 379–386. doi: 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015; 33: 4194–4201. doi: 10.1200/JCO.2015.62.6812 [DOI] [PubMed] [Google Scholar]
- 4.Evison M, Clive A, Castle L, et al. Resectable clinical N2 non-small cell lung cancer; what is the optimal treatment strategy? An update by the British Thoracic Society Lung Cancer Specialist Advisory Group. J Thorac Oncol 2017; 12: 1434–1441. doi: 10.1016/j.jtho.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 5.McElnay PJ, Choong A, Jordan E, et al. Outcome of surgery versus radiotherapy after induction treatment in patients with N2 disease: systematic review and meta-analysis of randomised trials. Thorax 2015; 70: 764–768. doi: 10.1136/thoraxjnl-2014-206292 [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir S, Finkelstein EA. Cognitive bias: the downside of shared decision making. JCO Clin Cancer Inform 2018; 2: 1–10. doi: 10.1200/CCI.18.00011 [DOI] [PubMed] [Google Scholar]
- 7.McAlpine K, Lewis KB, Trevena LJ, et al. What is the effectiveness of patient decision aids for cancer-related decisions? A systematic review subanalysis. JCO Clin Cancer Inform 2018; 2: 1–13. doi: 10.1200/CCI.17.00148 [DOI] [PubMed] [Google Scholar]
- 8.Ankolekar A, Dekker A, Fijten R, et al. The benefits and challenges of using patient decision aids to support shared decision making in health care. JCO Clin Cancer Inform 2018; 2: 1–10. doi: 10.1200/CCI.18.00013 [DOI] [PubMed] [Google Scholar]
- 9.Panje CM, Glatzer M, Siren C, et al. Treatment options in oncology. JCO Clin Cancer Inform 2018; 2: 1–10. doi: 10.1200/CCI.18.00017 [DOI] [PubMed] [Google Scholar]
- 10.Glatzer M, Panje CM, Siren C, et al. Decision making criteria in oncology. Oncology 2018: 1–9. doi: 10.1159/000492272 [DOI] [PubMed] [Google Scholar]
- 11.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: Suppl. 5, e314S–e340S. doi: 10.1378/chest.12-2360 [DOI] [PubMed] [Google Scholar]
- 12.Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010; 65: Suppl. 3, iii1–ii27. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015; 26: 1573–1588. doi: 10.1093/annonc/mdv187 [DOI] [PubMed] [Google Scholar]
- 14.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: Suppl. 4, iv1–iv21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 15.Baldwin DR, White B, Schmidt-Hansen M, et al. Diagnosis and treatment of lung cancer: summary of updated NICE guidance. BMJ 2011; 342: d2110. doi: 10.1136/bmj.d2110 [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence Clinical Guidelines. Lung cancer: diagnosis and management. London, NICE, 2019. [Google Scholar]
- 17.Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 2018; 16: 807–821. doi: 10.6004/jnccn.2018.0062 [DOI] [PubMed] [Google Scholar]
- 18.Ettinger DS, Wood DE, Aisner DL, et al. Non–small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 504–535. doi: 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- 19.Majem M, Juan O, Insa A, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol 2019; 21: 3–17. doi: 10.1007/s12094-018-1978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF). Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms, Langversion 1.0. http://leitlinienprogramm-onkologie.de/Lungenkarzinom.98.0.html Date last accessed: 5 December 2018. Date last updated: 2018.
- 21.Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015; 121: Suppl. 17, 3165–3181. doi: 10.1002/cncr.29550 [DOI] [PubMed] [Google Scholar]
- 22.Department of Health Diagnosis, Staging and Treatment of Lung Cancer (NCEC National Clinical Guideline No. 16). http://health.gov.ie/national-patient-safety-office/ncec/national-clinical-guidelines Date last accessed: 15 December 2018. Date last updated: 2017.
- 23.Cancer Council Australia Lung Cancer Guidelines Working Party Clinical practice guidelines for the treatment of lung cancer. Sydney, Cancer Council Australia, 2017. [Google Scholar]
- 24.Evison M, McDonald F, Batchelor T. What is the role of surgery in potentially resectable N2 non-small cell lung cancer? Thorax 2018; 73: 1105–1109. [Google Scholar]
- 25.Panje CM, Glatzer M, von Rappard J, et al. Applied Swarm-based medicine: collecting decision trees for patterns of algorithms analysis. BMC Med Res Methodol 2017; 17: 123. doi: 10.1186/s12874-017-0400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumstein V, Betschart P, Abt D, et al. Surgical management of urolithiasis – a systematic analysis of available guidelines. BMC Urol 2018; 18: 25. doi: 10.1186/s12894-018-0332-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hundsberger T, Schoser B, Leupold D, et al. Comparison of recent pivotal recommendations for the diagnosis and treatment of late-onset Pompe disease using diagnostic nodes—the Pompe disease burden scale. J Neurol 2019; 266: 2010–2017. doi: 10.1007/s00415-019-09373-2 [DOI] [PubMed] [Google Scholar]
- 28.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: Suppl. 5, e211S–e250S. doi: 10.1378/chest.12-2355 [DOI] [PubMed] [Google Scholar]
- 29.Putora PM, Panje CM, Papachristofilou A, et al. Objective consensus from decision trees. Radiat Oncol 2014; 9: 270. doi: 10.1186/s13014-014-0270-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putora PM, Glatzer M, Belderbos J, et al. Prophylactic cranial irradiation in stage IV small cell lung cancer: selection of patients amongst European IASLC and ESTRO experts. Radiother Oncol 2019; 133: 163–166. doi: 10.1016/j.radonc.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 31.Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019; 158: 269–276. doi: 10.1016/j.jtcvs.2018.11.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378: 1976–1986. doi: 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 34.Casiraghi M, Guarize J, Sandri A, et al. Pneumonectomy in stage IIIA-N2 NSCLC: should it be considered after neoadjuvant chemotherapy? Clin Lung Cancer 2019; 20: 97–106.doi: 10.1016/j.cllc.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 35.d'Amato TA, Ashrafi AS, Schuchert MJ, et al. Risk of pneumonectomy after induction therapy for locally advanced non-small cell lung cancer. Ann Thorac Surg 2009; 88: 1079–1085. doi: 10.1016/j.athoracsur.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 36.Pottgen C, Eberhardt W, Stamatis G, et al. Definitive radiochemotherapy versus surgery within multimodality treatment in stage III non-small cell lung cancer (NSCLC) - a cumulative meta-analysis of the randomized evidence. Oncotarget 2017; 8: 41670–41678. doi: 10.18632/oncotarget.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rusthoven CG, Palma DA, Senan S, et al. The head start effect: will acute and delayed postoperative mortality lead to improved survival with stereotactic body radiation therapy for operable stage I non-small-cell lung cancer? J Clin Oncol 2017; 35: 1749–1751. doi: 10.1200/JCO.2016.72.0003 [DOI] [PubMed] [Google Scholar]
- 38.Adizie J, Khakwani A, Beckett P, et al. Stage III non-small cell lung cancer management in England. Clin Oncol 2019; 31: 688–696. doi: 10.1016/j.clon.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 39.Sorensen JB, Ravn J, Pilegaard HK, et al. Surgery for NSCLC stages T1-3N2M0 having preoperative pathologically verified N2 involvement: a prospective randomized multinational phase III trial by the Nordic Thoracic Oncology Group. J Clin Oncol 2013; 31: 7504. [Google Scholar]