Abstract
Guinea pigs are a premier small animal model for infectious disease research, and chronic indwelling venous access ports may be used to facilitate various procedures. Here we report catheter-related lesions in 5 uninfected Dunkin–Hartley guinea pigs with chronic jugular vein catheters used for imaging studies. Three guinea pigs were found dead with no premonitory signs. At necropsy, there was severe bilateral pulmonary atelectasis due to 20 to 29 mL of pleural effusion resulting from catheter-related thrombosis and cranial vena cava syndrome. In addition, one of these 3 guinea pigs had a polymicrobial catheter infection with abscessation. A 4th clinically normal guinea pig was euthanized at the end of the study, having spontaneously lost its catheter 7 mo prior, and had 17 mL of pleural effusion. The 5th guinea pig was euthanized following pooling of contrast material around the distal catheter in the cranial vena cava on CT. By histology, affected animals had recent and remote thrombosis or fibrosis (or both) of the cranial vena cava and right atrial wall, with osseous and cartilaginous metaplasia. Cranial vena cava syndrome should be considered as a differential for dyspnea or death in chronically catheterized laboratory animals.
Abbreviations: FDG, 2-fluoro-2-deoxy-d-glucose; PET, positron emission tomography
Guinea pigs are important research animal models, although their use has declined somewhat in recent years, in part due to limited reagent availability (for example, species-specific validated antibodies), only a single available inbred strain (13/N), and—until very recently—difficulty in creating genetic mutants. Despite these limitations, guinea pigs nonetheless remain a frequently used animal model for infectious disease studies, particularly because of their size, cost, and tractable nature.10,21 Guinea pigs are commonly used in filovirus research, including therapeutic agent development, because they develop histopathologic lesions and serum biochemical changes, including coagulopathies, similar to those observed in humans and NHP.5,27 Other key uses of guinea pigs as animal models of noninfectious research include for pathophysiology of the inner ear, osteoarthritis, asthma, and cardiac arrhythmias.13,23,26,32
Chronic vascular cannulation of laboratory animals is a common technique to allow repetitive long-term vascular sampling (including invasive pressure measurements) and intravenous delivery of small molecules, imaging contrast agents and tracers, and other reagents.8 Functional obstruction or occlusion of the cranial vena cava (cranial vena cava syndrome) and chylothorax have previously been reported as an adverse consequence of central venous catheters and peripherally inserted central catheters in humans, rhesus macaques, and dogs.2,7,20,22 Additional reported complications in laboratory animals have included physical trauma caused by the catheters (for example, perforation), infections, formation of bland (uninfected) or septic thromboemboli with or without infarction, and aneurysms and dissections.6,11,14,18,28
Case Study
Eight SPF Dunkin–Hartley (Crl:HA, strain code 051) guinea pigs (Cavia porcellus; 4 sows and 4 boars; age, 40 d) with surgically implanted right jugular vein catheters were obtained from Charles River Laboratories (Wilmington, MA). The guinea pigs were acclimated to the Maximum Containment (BSL4) Laboratory at the United States NIH/National Institute of Allergy and Infectious Diseases, Division of Clinical Research, Integrated Research Facility at Fort Detrick. Animals were housed in IVC (One Cage, Lab Products, Seaford, DE) on paper bedding (TEK-Fresh 7099, Envigo, Madison, WI) and provided food and water without restriction. The water is treated by a reverse-osmosis system (RO8600, Edstrom Industries, Waterford, WI). All animals are fed a high-fiber guinea pig diet (Teklad 2041, Envigo). Environmental conditions were controlled, with a temperature setting of 72 °F (22.2 °C), humidity at 50%, and a 12:12-h light:dark cycle. MR-compatible miniature vascular access ports (PinPorts, Instech Laboratories, Plymouth Meeting, PA) were attached to the external portion of the catheter for catheter maintenance and venous access. Catheters were flushed weekly with heparinized saline (10 U/mL) and locked with heparinized glycerol (500 U/mL). All work was conducted in a Maximum Containment (BSL4) Laboratory at the Integrated Research Facility at Fort Detrick that is fully AAALAC-accredited. All animal experiments were performed in accordance with animal study protocols approved by the Division of Clinical Research Animal Care and Use Committee. Protocols were compliant with the US Department of Agriculture Animal Welfare Act regulations1 and adhered to the recommendations stated in the Guide for the Care and Use of Laboratory Animals.12
Guinea pigs were used for imaging development studies including positron emission tomography (PET) and CT.5 For imaging, guinea pigs were anesthetized with isoflurane (Patterson Veterinary, Greeley, CO) 1% to 4% in grade D breathing air in an anesthesia chamber and then by mask and a calibrated vaporizer. Contrast agents included Isovue-300 (Bracco Diagnostics, Monroe, NJ) for CT imaging and 2-fluoro-2-deoxy-d-glucose (18F-FDG; Cardinal Health, Dublin, OH) for PET imaging. Imaging agents were administered by IV infusion. Isovue-300 (2 mL/kg) was administered through intravenous infusion by using the miniature access ports. [18F]FDG was administered in a subset of 5 animals (guinea pigs 3 through 6 and 8) to optimize PET and CT imaging techniques for future studies in this species and to compare kinetics between intravenous and intramuscular uptake of the radiotracer.
Gross pathology.
Three guinea pigs (2 boars and one sow) were found dead with no premonitory signs (animals 1 through 3; Table 1). All 3 had obvious caudal bulging of the diaphragm into the abdominal cavity. The 2 boars each had 27 mL or 29 mL or more of blood-tinged, milky, turbid (chylous) pleural effusion (Figure 1 A through C). The sow had more than 20 mL of blood-tinged, turbid, serous pleural effusion, with more than 1.5 mL of pericardial effusion. All 3 of these animals had marked and diffuse pulmonary atelectasis (Figure 1 D), although the lungs still floated in formalin. Guinea pig 3 had obvious fibrin deposition, hyperemia, and thickening of the wall of the jugular and cranial vena cava around the catheter.
Table 1.
Animal demographics and pathologic findings
| Animal | Disposition, duration of catheterization | Sex | Pleural effusion | Pulmonary atelectasis | Other gross findings | Thrombosis | Mural remodeling in right atrium and cranial vena cava | Other histologic findings |
| 1 | Found dead, 151 d | M | >27 mL, blood-tinged, chylous | Yes | Postmortem intestinal rupture | Cranial vena cava, subacute | Yes | Mild to moderate pulmonary edema |
| 2 | Found dead, 184 d | M | >29 mL, blood-tinged, chylous | Yes | None | Catheter sheath | Yes | Moderate pulmonary edema |
| 3 | Found dead, 244 d | F | >20 mL, blood-tinged, serous | Yes | >1.5 mL, blood-tinged, serous pericardial effusion | No | Yes | Jugular and cranial vena caval phlebitis and mural abscess with bacteria; thromboemboli in lungs; tricuspid valve normal |
| 4 | Euthanized due to cranial vena cava thrombosis (CT imaging), 198 d | F | None | No | None | Right atrium and jugular, chronic | Yes | Chronic sublingual sialoadenitis |
| 5 | Euthanized at end of study, 381 d | F | None | No | None | No | Yes | None |
| 6 | Euthanized at end of study, 381 d | M | None | No | None | No | Yes | None |
| 7 | Euthanized at end of study, 262 da | M | >17 mL, blood-tinged, serous | Yes | None | No | Yes | None |
| 8 | Euthanized at end of study, 169 db | F | None | No | None | Yes | No | None |
Catheter was spontaneously removed at approximately 7 mo prior to death.
Catheter was spontaneously removed at approximately 10 mo prior to death.
Figure 1.
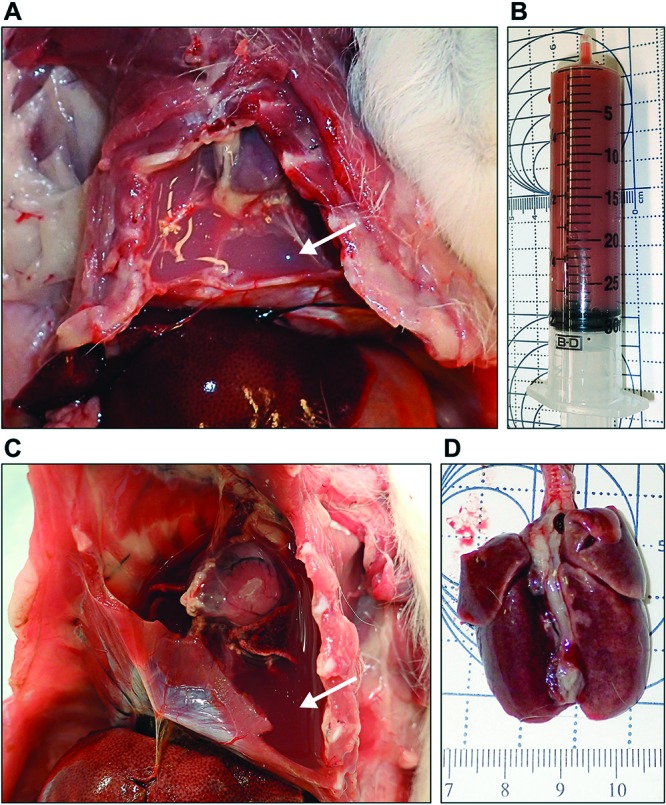
(A and B) Guinea pig 1. There is more than 27 mL of chylous effusion filling the pleural spaces and obscuring the heart and lungs (A, arrow). (B) Syringe shows the volume and chylous nature of the effusion. (C and D). Guinea pig 2. There is more than 29 mL of chylous pleural effusion (C, arrow) with severe and diffuse atelectasis of the lungs (D).
In addition, another sow (no. 4) was euthanized after a fibrinous catheter sheath was identified due to contrast pooling in the cranial vena cava on CT imaging. This animal lacked grossly evident lesions, but gross dissection of the right jugular vein, cranial vena cava, and right atrium was limited. A 3rd boar (animal no. 7) was euthanized at the end of the study, with no clinical abnormalities noted. This guinea pig had spontaneously lost or removed its catheter approximately 7 mo prior to necropsy and consequently was shifted to a training protocol. At necropsy there was more than 17 mL of blood-tinged, turbid, serous pleural effusion with pulmonary atelectasis. The remaining 3 guinea pigs were euthanized at the end of the study; there were no gross abnormalities. One of these animals (no. 8) had spontaneously lost or removed her catheter approximately 10 mo prior. No facial swelling or edema was noted in any of the animals. Fluid analyses of effusions, including specific gravity and cytology, were not practical within the BSL4 facility, due to the absence of appropriate equipment within containment and the requirement to irradiate unfixed specimens prior to removal from containment. Bacterial cultures were impractical in animals with pleural effusions that were found dead and had significant autolysis; one of these animals had postmortem intestinal rupture.
Tissues were fixed for 72 h in 10% neutral buffered formalin before automated processing (Tissue-Tek VIP-6, Sakura Finetek USA, Torrance, CA) followed by paraffin embedding (Tissue-Tek model TEC, Sakura). Samples for slides were cut at 4 µm on a microtome (model 2245, Leica), stained with hematoxylin and eosin, and coverslipped. Additional slides were stained with Brown and Brenn Gram stain and Masson trichrome stain. Gross necropsies and slide examinations were performed by a single board-certified veterinary pathologist (ACVP diplomate). All images were captured by using a microscope (model DM3000, Leica Microsystems, Buffalo Grove, IL), digital camera (model DFC 500, Leica) and Application Suite version 4.10.0 (Leica).
Histopathologic findings.
A large nonadherent acute fibrin thrombus was present in the cranial vena cava of guinea pig 1, filling approximately 60% of the luminal cross-sectional area (Figure 2 A and B). A fibrin catheter sheath was present in the cranial vena cava of guinea pig 2.19 Guinea pig 3 had chronic–active suppurative phlebitis of the right jugular vein and cranial vena cava, with a focally extensive mural abscess containing gram-negative rods and gram-positive cocci. Guinea pig 4 had 2 nonocclusive, mature, organized thrombi in the jugular vein and cranial vena cava. For all animals except no. 8 there was extensive remodeling of the right atrial wall, including mature endocardial and myocardial fibrosis (which was occasionally transmural), endocardial hyperplasia, and well-differentiated myocardial nodules of hyaline cartilage and bone with marrow elements (cartilaginous and osseous metaplasia). Mural remodeling, including abundant mature medial fibrosis (Figure 2 C and D) extended into the cranial vena cava in all guinea pigs except no. 8 (Figure 2 E and F); guinea pig no. 8 was normal except for a small, organized, mural thrombus at the atriocaval junction. In addition, 6 of the 7 animals with chest imaging demonstrated calcification in the area of the distal cranial vena cava or right atrium on CT (Figure 3 A through C). Pulmonary thromboemboli were present in a single animal (no. 3) only; this animal had concurrent bacterial thrombophlebitis and abscessation. In another animal (no. 4), a single medium pulmonary artery was effaced by granulomatous inflammation with foreign material (embolized hairshafts). None of the animals had any evidence of suppuration or neutrophilic inflammation of the visceral pleura consistent with bacterial infection of the pleural space.
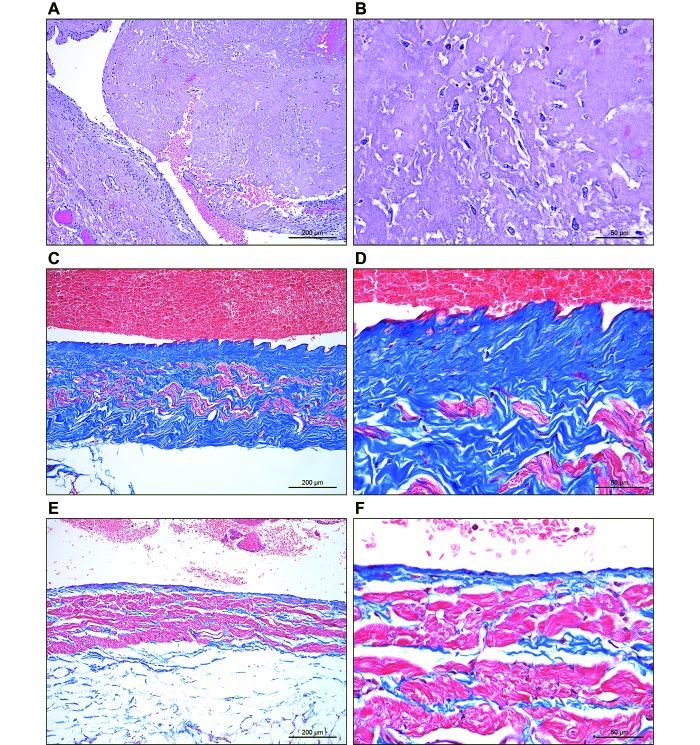
Figure 2.
(A and B) Guinea pig 1. A large, nonadherent thrombus with nascent reendothelialization and organization fills the cranial vena cava. The vein wall is at the lower left in panel A. (B) A higher-magnification image shows the nonseptic fibrinous nature of the thrombus with ingrowth of fibroblasts and macrophages. (C and D) Guinea pig 7. Diffuse mature, mural fibrosis (blue) expands and replaces the wall of the cranial vena cava. Masson trichrome stain. (E and F) Guinea pig 8. Normal cranial vena cava with tunica media composed of bundles of smooth muscle (red) with scant intervening collagen (blue). Masson trichrome stain.
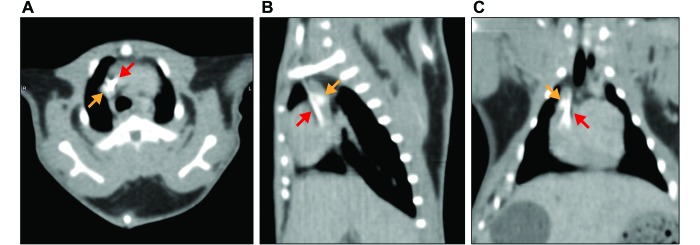
Figure 3.
(A) Axial, (B) sagittal, and (C) dorsoventral postcontrast CT images from guinea pig no. 3 at 25 d after catheter implantation, showing calcification in the right atrium–cranial vena cava junction (orange arrows) and collection of contrast material in the cranial vena cava, indicating a fibrinous sheath around the distal end of the catheter tip (red arrows).
Discussion
Cranial (superior) vena cava syndrome results from the obstruction, stenosis, or occlusion of the cranial vena cava through various processes, including thrombosis and neoplasia.22,24 Pleural effusions may occur in a significant proportion of patients, because increased venous hydrostatic pressure is thought to restrict pleural lymphatic drainage, thus resulting in fluid accumulation.7,24,25,30 In addition, direct or indirect damage to the thoracic duct may occur. Other typical features of cranial vena cava syndrome can include edema of the face and neck, dyspnea, and dilated collateral veins of the thorax. Surgical ligation of the cranial vena cava has been described as an experimental animal model of superior vena cava syndrome in dogs.3 In those studies, initial pleural effusions were bloody and nonchylous, with later development of chylous and bloody effusions in approximately half of the animals, with frequent death from respiratory embarrassment within several weeks. In the present study, occlusion or stenosis of the cranial vena cava due to acute thrombosis or chronic mural fibrosis (or both) were thought to impair thoracic lymphatic drainage in 4 guinea pigs, leading to significant pleural effusions that were fatal in 3 animals. Caval syndrome associated with fibrosis of intravenous pacemaker leads has been previously described in people and in dogs.15,29 Due to the relatively sedentary nature of guinea pigs, combined with the inability to auscultate animals in a BSL4 environment,16 premonitory clinical signs of disease were not observed. No pleural effusions were identified by PET or CT; however, none of the animals with catheters that died were imaged within 46 d of death, and the boar with pleural effusion that had spontaneously lost its catheter had not been imaged for 287 d. Animals were imaged a variable number of times, ranging from only once (CT with contrast only, guinea pigs 1 and 2, at 126 and 159 d before necropsy, respectively) to 10 times over the course of 1 y (PET–CT and CT with contrast, guinea pig 5). Although we cannot exclude the possibility that imaging contrast agent or radiotracer may have contributed to lesion development, both agents are widely used in human medicine and generally considered safe.
The histology of the jugular vein, cranial vena cava and right atrium in animals with long-term venous catheters has been described previously in other species and is largely consistent with what was observed in these animals.14,18,31 Lesions of the right atrium and cranial vena cava were minimal in the sow that had lost her catheter after only 4 mo on the study (approximately 1 y before euthanasia). The frequent presence of cartilaginous and osseous metaplasia in the right atrium is likely a unique feature of guinea pigs, a species in which similar metaplasia is common in the lung and ciliary body.9,17 Unilateral chronic inflammation and atrophy of the sublingual salivary gland was present in a single sow (no. 4) but has been observed in other catheterized guinea pigs at this institution (data not shown) and is interpreted as a complication of surgery. The presence of ectatic salivary ducts in some animals is consistent with inadvertent ligation at the time of catheter placement.
Overall, jugular vein cannulation was reasonably well tolerated, with no adverse events observed until the animals had been on study for approximately 5 mo and only a single catheter infection. Cranial vena cava syndrome causing pleural effusion and respiratory embarrassment should be considered in any laboratory animal with a central venous catheter displaying dyspnea or found dead, even if the catheter was previously removed.
Acknowledgments
We thank Nejra Isic, Maureen Abbott, and Amanda Hischak for histology support; Jiro Wada for figure preparation; and Phil Sayre, Chris Bartos, Dr Ji Hyun Lee, and Dr David Thomasson for imaging support. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services (DHHS) or of the institutions and companies affiliated with the authors. This work was funded in part through Battelle Memorial Institute's prime contract with the US National Institute of Allergy and Infectious Diseases (NIAID) under contract no. HHSN272200700016I. LED performed this work as an employee of Battelle Memorial Institute. Subcontractors to Battelle Memorial Institute who performed this work are TKC, RB, and KC, all of whom are employees of Charles River Laboratories (contact Daniela Pusl, daniela.pusl@nih.gov).
References
- 1.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 2.Bashir RA, Callejas AM, Osiovich HC, Ting JY. 2016. Percutaneously inserted central catheter-related pleural effusion in a level III neonatal intensive care unit: a 5-year review (2008-2012). JPEN J Parenter Enteral Nutr 41:1234–1239. 10.1177/0148607116644714. [DOI] [PubMed] [Google Scholar]
- 3.Blalock A, Cunningham RS, Robinson CS. 1936. Experimental production of chylothorax by occlusion of the superior vena cava. Ann Surg 104:359–364. 10.1097/00000658-193609000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrum R, Keith L, Bartos C, St Claire M, Lackemeyer MG, Holbrook MR, Janosko K, Barr J, Pusl D, Bollinger L, Wada J, Coe L, Hensley LE, Jahrling PB, Kuhn JH, Lentz MR. 2016. Safety precautions and operating procedures in an (A)BSL4 laboratory: 4. medical imaging procedures. J Vis Exp 116 1–7. 10.3791/53601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross RW, Fenton KA, Geisbert JB, Ebihara H, Mire CE, Geisbert TW. 2015. Comparison of the pathogenesis of the Angola and RAVN strains of Marburg virus in the outbred guinea pig model. J Infect Dis 212 Suppl 2:S258–S270. 10.1093/infdis/jiv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DaRif CA, Rush HG. 1983. Management of septicemia in rhesus monkeys with chronic indwelling venous catheters. Lab Anim Sci 33:90–94. [PubMed] [Google Scholar]
- 7.Dhande V, Kattwinkel J, Alford B. 1983. Recurrent bilateral pleural effusions secondary to superior vena cava obstruction as a complication of central venous catheterization. Pediatrics 72:109–113. [PubMed] [Google Scholar]
- 8.Dowall S, Taylor I, Yeates P, Smith L, Rule A, Easterbrook L, Bruce C, Cook N, Corbin-Lickfett K, Empig C, Schlunegger K, Graham V, Dennis M, Hewson R. 2013. Catheterized guinea pigs infected with Ebola Zaire virus allows safer sequential sampling to determine the pharmacokinetic profile of a phosphatidylserine-targeting monoclonal antibody. Antiviral Res 97:108–111. 10.1016/j.antiviral.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Griffith JW, Sassani JW, Bowman TA, Lang CM. 1988. Osseous choristoma of the ciliary body in guinea pigs. Vet Pathol 25:100–102. 10.1177/030098588802500119. [DOI] [PubMed] [Google Scholar]
- 10.Hickey AJ. 2011. Guinea pig model of infectious disease—viral infections. Curr Drug Targets 12:1018–1023. 10.2174/138945011795677827. [DOI] [PubMed] [Google Scholar]
- 11.Hysell DK, Abrams GD. 1967. Complications in the use of indwelling vascular catheters in laboratory animals. Lab Anim Care 17:273–280. [PubMed] [Google Scholar]
- 12.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 13.Kraus VB, Huebner JL, DeGroot J, Bendele A. 2010. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage 18 Suppl 3:S35–S52. 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilbert J, Burnett R. 2003. Main vascular changes seen in the saline controls of continuous infusion studies in the cynomolgus monkey over an eight-year period. Toxicol Pathol 31:273–280. 10.1080/01926230390204306. [DOI] [PubMed] [Google Scholar]
- 15.Madkaiker AN, Krishna N, Jose R, Balasubramoniam KR, Murukan P, Baquero L, Varma PK. 2016. Superior vena cava syndrome caused by pacemaker leads. Ann Thorac Surg 101:2358–2361. 10.1016/j.athoracsur.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Mazur S, Holbrook MR, Burdette T, Joselyn N, Barr J, Pusl D, Bollinger L, Coe L, Jahrling PB, Lackemeyer MG, Wada J, Kuhn JH, Janosko K. 2016. Safety precautions and operating procedures in an (A)BSL4 laboratory: 2. General practices. J Vis Exp 116: 1–7. 10.3791/53600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McInnes EF. 2012. Hamsters and guinea pigs, p 73–79. Chapter 5. In: Background lesions in laboratory animals. St Louis (MO): Saunders. [Google Scholar]
- 18.Mesfin GM, Higgins MJ, Brown WP, Rosnick D. 1988. Cardiovascular complications of chronic catheterization of the jugular vein in the dog. Vet Pathol 25:492–502. 10.1177/030098588802500613. [DOI] [PubMed] [Google Scholar]
- 19.O'Farrell L, Griffith JW, Lang CM. 1996. Histologic development of the sheath that forms around long-term implanted central venous catheters. JPEN J Parenter Enteral Nutr 20:156–158. 10.1177/0148607196020002156. [DOI] [PubMed] [Google Scholar]
- 20.Olson LC, Anver MR. 1979. Chylothorax in rhesus monkeys with intravenous catheters. Lab Anim Sci 29:791–796. [PubMed] [Google Scholar]
- 21.Padilla-Carlin DJ, McMurray DN, Hickey AJ. 2008. The guinea pig as a model of infectious diseases. Comp Med 58:324–340. [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer KG, King LG, Van Winkle TJ. 1998. Clinical manifestations and associated disease syndromes in dogs with cranial vena cava thrombosis: 17 cases (1989–1996). J Am Vet Med Assoc 213:220–224. [PubMed] [Google Scholar]
- 23.Ricciardolo FL, Nijkamp F, De Rose V, Folkerts G. 2008. The guinea pig as an animal model for asthma. Curr Drug Targets 9:452–465. 10.2174/138945008784533534. [DOI] [PubMed] [Google Scholar]
- 24.Rice TW. 2007. Pleural effusions in superior vena cava syndrome: prevalence, characteristics, and proposed pathophysiology. Curr Opin Pulm Med 13:324–327. 10.1097/MCP.0b013e32812144aa. [DOI] [PubMed] [Google Scholar]
- 25.Rice TW, Rodriguez RM, Light RW. 2006. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 85:37–42. 10.1097/01.md.0000198474.99876.f0. [DOI] [PubMed] [Google Scholar]
- 26.Ruppert S, Vormberge T, Igl BW, Hoffmann M. 2016. ECG telemetry in conscious guinea pigs. J Pharmacol Toxicol Methods 81:88–98. 10.1016/j.vascn.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 27.St Claire MC, Ragland DR, Bollinger L, Jahrling PB. 2017. Animal models of ebolavirus infection. Comp Med 67:253–262. [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor WM, Grady AW. 1998. Catheter-tract infections in rhesus macaques (Macaca mulatta) with indwelling intravenous catheters. Lab Anim Sci 48:448–454. [PubMed] [Google Scholar]
- 29.Van De Wiele CM, Hogan DF, Green HW, 3rd, Parnell NK. 2008. Cranial vena caval syndrome secondary to transvenous pacemaker implantation in two dogs. J Vet Cardiol 10:155–161. 10.1016/j.jvc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Walker RW. 1991. Recurrent pleural effusions following superior vena cava thrombosis. Anaesthesia 46:704–704. 10.1111/j.1365-2044.1991.tb09750.x. [DOI] [PubMed] [Google Scholar]
- 31.Weber K, Mowat V, Hartmann E, Razinger T, Chevalier HJ, Blumbach K, Green OP, Kaiser S, Corney S, Jackson A, Casadesus A. 2011. Pathology in continuous infusion studies in rodents and non-rodents and ito (infusion technology organisation)—recommended protocol for tissue sampling and terminology for procedure-related lesions. J Toxicol Pathol 24:113–124. 10.1293/tox.24.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young YH. 2018. Inner ear test battery in guinea pig models—a review. Acta Otolaryngol 138:519–529. 10.1080/00016489.2017.1419576. [DOI] [PubMed] [Google Scholar]