Abstract
Introduction
An important economic activity in Colombia is agricultural production and farmers are frequently exposed to pesticides. Occupational exposure to pesticides is associated with an increased incidence of various diseases, including cancer, Parkinson’s disease, Alzheimer’s disease, reproductive disorders, and birth defects. However, although high genotoxicity is associated with these chemicals, information about the type and frequency of specific chromosomal alterations (CAs) and the level of chromosomal instability (CIN) induced by exposure to pesticides is scarce or absent.
Methods
In this study, CAs and CIN were assessed in peripheral blood lymphocytes (PBLs) from five farmers occupationally exposed to pesticides and from five unexposed individuals using GTG-banding and molecular cytogenetic analysis.
Results
A significant increase in clonal and non-clonal chromosomal alterations was observed in pesticide-exposed individuals compared with unexposed individuals (510±12,2 vs 73±5,7, respectively; p<0.008). Among all CAs, monosomies and deletions were more frequently observed in the exposed group. Also, a high frequency of fragilities was observed in the exposed group.
Conclusion
Together, these findings suggest that exposure to pesticides could be associated with CIN in PBLs and indicate the need for the establishment of educational programs on safety precautions when handling pesticides, such as wearing gloves, masks and boots, changing clothes and maintaining proper hygiene, among others. Further evaluation in other similar studies that include a greater number of individuals exposed to pesticides is necessary.
Keywords: pesticides, occupational exposure, chromosomal instability, clonal chromosomal alteration, non-clonal chromosomal alteration
Introduction
In Colombia, one of the most important economic activities is agricultural production and farmers are frequently exposed to pesticides. Pesticides play an important role in the control of agricultural pests, and include several categories of fungicides, insecticides, herbicides, and others, with organophosphorus pesticides being the most frequently used.1
Exposure to pesticides is concerning because many studies have associated occupational exposure to these chemicals with an increased incidence of various diseases, including cancer, Parkinson’s disease, Alzheimer’s disease, reproductive disorders, and birth defects.2 Exposure to pesticides can induce oxidative stress by increased production of free radicals, which accumulate in cells and can cause gene mutations and chromosomal aberrations.3–5
In recent years, genotoxicity from exposure to pesticides has been extensively investigated in cell lines and animal models. However, although many cases of pesticide poisoning throughout the world are documented every year, data concerning chromosomal damage in occupationally exposed people is limited.6 Chromosomal alterations (CAs) related to pesticide exposure have been identified in several populations, and while some significant differences in the frequency of CAs in exposed individuals compared to unexposed controls have been reported,7–12 other studies have not observed any association.13,14 Furthermore, although some studies have shown the induction of CAs in humans, they have mainly reported alterations such as associations of satellites (between acrocentric chromosomes), gaps,15 ruptures, sister-chromatid exchanges,16 and micronuclei, so the level of chromosomal instability (CIN) induced by exposure to pesticides is poorly documented. Furthermore, information about the type and frequency of specific CAs induced by exposure to pesticides is scarce or absent.
The assessment of chromosomal damage in occupationally exposed humans is useful for measuring the genetic risk in the exposed population,17,18 and also an important step in the early detection of diseases when control measures could prove effective. CAs in peripheral blood lymphocytes (PBLs) reflect sensitivity to both exogenous and endogenous genotoxic substances and could be used as biomarkers of chromosomal damage and possible risk of developing diseases, including cancer. Further, these analyses are considered reliable and are an important tool to estimate both biological and genetic risk factors, related to pesticide exposure.19–21 The aim of this study was to evaluate CIN in farmers occupationally exposed to pesticides in the department of Cundinamarca, Colombia.
Materials and Methods
Study Population
The study was carried out on a group of five individuals from the department of Cundinamarca, Colombia who were routinely “exposed” to pesticides. The exposed individuals consisted of men and women between 51 and 66-years-old and who had been exposed to pesticides through work for at least 12 months.
The unexposed group consisted of five healthy men and women, without indication of previous occupational exposure to pesticides. The group had a similar age range (between 52 and 63 years old), sex distribution and lifestyle habits as the exposed group (Tables 1 and 2).
Table 1.
General Characteristics of the Groups Studied
| Exposed | Unexposed | |
|---|---|---|
| Number | 5 | 5 |
| Age (mean ± SD) | 57 ± 6.2 | 56.4 ±5.5 |
| Gender (n) | ||
| Male | 3 | 3 |
| Female | 2 | 2 |
| Exposure months (mean ± SD) | 154.8 ±152.2 | 0 |
| Smoking status (n) | ||
| Smokers | 1 | 1 |
| Non-smokers | 4 | 4 |
| Drinking status (n) | ||
| Drinkers | 3 | 4 |
| Non-drinkers | 2 | 1 |
Abbreviation: SD, Standard Deviation.
Table 2.
Detailed Characteristics and Percentages of Chromosome Variants (CVs) and Chromosomal Alterations (CAs) Identified in the Exposed and Unexposed Groups
| Age (Years) | Exposure (Months) | Habits | % of CVs and CAs | ||
|---|---|---|---|---|---|
| Smoking | Drinking | ||||
| Exposed | |||||
| E1 | 66 | 120 | – | – | 41 |
| E2 | 63 | 60 | – | 1/week | 26 |
| E3 | 52 | 48 | – | – | 32 |
| E4 | 51 | 126 | – | 1/month | 32 |
| E5 | 53 | 420 | + | 1/month | 19 |
| Mean | 57 | 154.8 | |||
| Median | 53 | 120 | |||
| Unexposed | |||||
| C1 | 63 | – | – | 1/month | 9 |
| C2 | 62 | – | – | 1/month | 4 |
| C3 | 52 | – | – | – | 10 |
| C4 | 52 | – | – | 1/six months | 4 |
| C5 | 53 | – | – | 1/week | 8 |
| Mean | 56.4 | ||||
| Median | 54.7 | ||||
Each individual was personally interviewed and filled in a routine questionnaire to record possible confounding factors such as diseases, age, smoking and drinking habits, exposure to pesticides, duration of exposure to pesticides and the use of protection devices (Table 1). Individuals who had suffered from cancer or had received radiotherapy, chemotherapy, or other recent prolonged medical treatment were excluded.
Data from the five exposed individuals were compared with those of the unexposed individuals. This study was approved by Ethics Committee of Universidad Pedagógica y Tecnológica de Colombia, Tunja (Colombia) and was conducted in accordance with the Declaration of Helsinki. Before blood sampling, a written informed consent was obtained from each participating subject.
Blood Sampling
Peripheral blood samples from exposed and unexposed individuals were collected in blood collection tubes containing heparin by venous puncture. The samples were labeled, transported to the laboratory, and immediately processed.
Metaphase Spreads and G-Banding Using Trypsin and Giemsa Stain
Metaphases were obtained using standard harvesting protocols for banding and molecular cytogenetic analysis. Briefly, 1 mL of heparinized peripheral blood were cultured in duplicates in 5 mL RPMI-1640 medium (Sigma, St. Louis, MO, USA), supplemented with 100 µL phytohemaglutinin-M (Gibco, Life Technologies, Nebraska, USA) and 10% fetal bovine serum (FBS) (Sigma). The cultures were incubated for 72 h at 37°C in 5% CO2 atmosphere. After 72 h, N-Deacetyl-N-methylcolchicine solution (0.0001 g/mL final conc.) (Sigma) was added to cultures 25 min before cell harvesting. Then, cells were treated with KCl solution at a concentration of 0.075 M (hypotonic solution), fixed with Carnoy’s fixative (3:1 methanol to acetic acid) three times and spread on glass slides. Thus obtained, the chromosomal preparations were banded with GTG-Banding using trypsin solution (0.25%) (Gibco) and Giemsa stain (Sigma).
GTG-Banding Cytogenetic Analysis
Characterization of CIN by using G-Banding cytogenetic was performed on a total of 544 metaphases. Image acquisition and karyotyping of metaphases were performed using an Olympus microscope with cytogenetic software, Cytovision System 7.4 (Leica Biosystems Richmond, VA, USA). Fragilities (fra), variation in length of heterochromatic segments on the long arms of chromosomes 1 (1qh+), 9 (9qh+) and 16 (16qh+); inversion of chromosome 9 [inv(9)]; chromosomal breaks (chrb) and chromatid breaks (chrb), and CAs including structural (SCAs) and numerical chromosomal alterations (NCAs) were evaluated. All chromosome variations and CAs were described according to the International System for Human Cytogenomic Nomenclature (ISCN) 2016.22
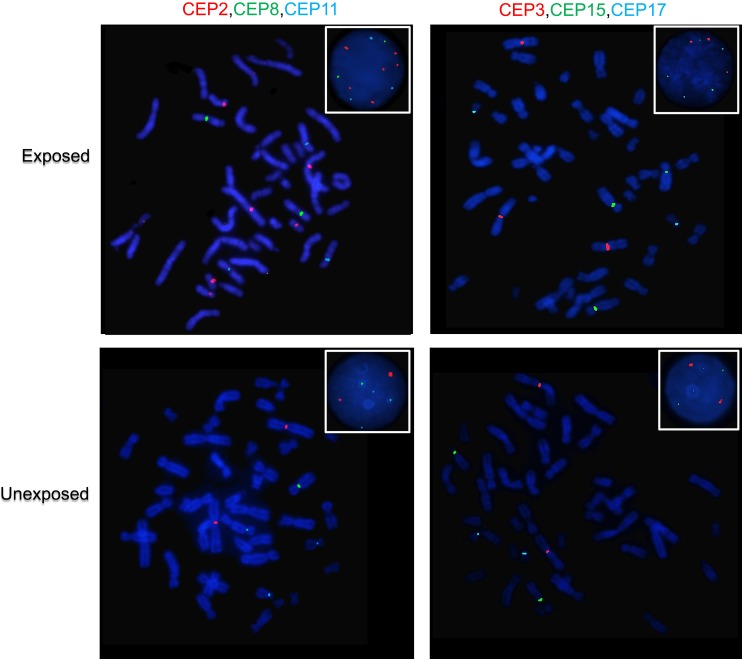
Fluorescence in situ Hybridization (FISH) and CIN Evaluation
CIN was evaluated on the metaphase and nuclei spreads obtained previously by FISH using six centromeric probes (CEP) for chromosomes 2, 3, 8, 11, 15 and 17 (all from Cytocell, Cambridge) and standard procedures. Briefly, slides were dehydrated in ethanol series before hybridization with FISH probes. Three-color FISH was performed on nuclei/metaphase spreads for chromosomes 2, 8 and 11, and for chromosomes 3, 15 and 17, using centromeric probes labeled with different spectrum colors: spectrum orange for CEP2 and CEP3; spectrum green for CEP8 and CEP17; and spectrum aqua for CEP11 and CEP15. After the addition of the probe mix, the slides were codenatured in the Top Brite System (Resnova, Italy) at 75ºC for 2 mins and hybridized overnight at 37ºC. Slides were then washed, dehydrated, and counterstained with 4′,6-diamidino-2-phenylindole (Cytocell). Ten randomly selected areas of each exposed and unexposed individual were acquired using an Olympus microscope with the cytogenetic software Cytovision System 7.4 (Leica Biosystems Richmond, Inc.). CIN was assessed in a minimum of 100 intact and non-overlapping nuclei/metaphases for each chromosome.
Although some studies have shown that the use of probes for only two chromosomes is enough to differentiate diploid from aneuploid tumors,23 we decided to use six probes since the use of more than two probes allows the identification of clonal populations with greater certainty.24
Data Analysis
Fisher’s exact test, Student’s t-test and Wilcoxon test were performed to compare the GTG-banding cytogenetic data with parametric and non-parametric distribution, respectively. Normality of the data was evaluated by the Shapiro–Wilk test. Data from the exposed individuals were compared with those of the unexposed individuals. p values less than 0.05 were considered significant (*p≤0.05 **p≤0.01). All statistical analyses were performed using IBM-SPSS Statistics Developer (Version 21.0 IBM Company, Chicago, IL). The CIN rate for each exposed and unexposed individual was defined first by calculating the percentage of nuclei with a CEP signal number different to the modal number (most common chromosome number in a tumor cell population) for each individual chromosome and then calculating the mean CIN percentage of all chromosomes analyzed.25,26 According to the level of CIN, each exposed and unexposed individual was classified as having low CIN (CIN<25%) or high CIN (CIN≥25%).27,28
Results
Characteristics of Study Groups
GTG-Banding and molecular cytogenetics were used in order to evaluate chromosomal alterations and CIN in a group of farmers exposed to pesticides and in a control group. General and detailed characteristics of the groups studied (exposed and unexposed) are presented in Tables 1 and 2, respectively. For the exposed group, the median time of exposure to pesticides was 120 months and the median age was 53 years (Table 2). In both groups, exposed and unexposed, a low prevalence of smoking and alcoholic beverage consumption was reported. The results are expressed as the mean ± standard deviation (SD) (Tables 1 and 2). Pesticides to which farmers were mainly exposed included fungicides, insecticides and herbicides (Table 3).
Table 3.
Pesticides Most Commonly Used by Exposed Individuals
| Pesticide | Active Ingredient | Commercial Name |
|---|---|---|
| Fungicide | Mancozeb + Cymoxanil | Curathane; Curzate M-8; Cymozeb |
| Mancozeb | Manzate | |
| Cymozeb | Cymoxanil and Mancozeb | |
| Propineb | Antracol | |
| Propineb; Cymoxanil | Fitoraz | |
| Insecticide | Imidacloprid | Confidor |
| Profenofos | Curacron | |
| Lambda-cyhalothrin | Karate Zeon SC | |
| Carbosulfan | Eltra 48 | |
| Carbofuran | Furadan | |
| Chlorpyrifos | Lorsban | |
| Herbicide | Paraquat | Gramoxone |
GTG-Banding Cytogenetic Results
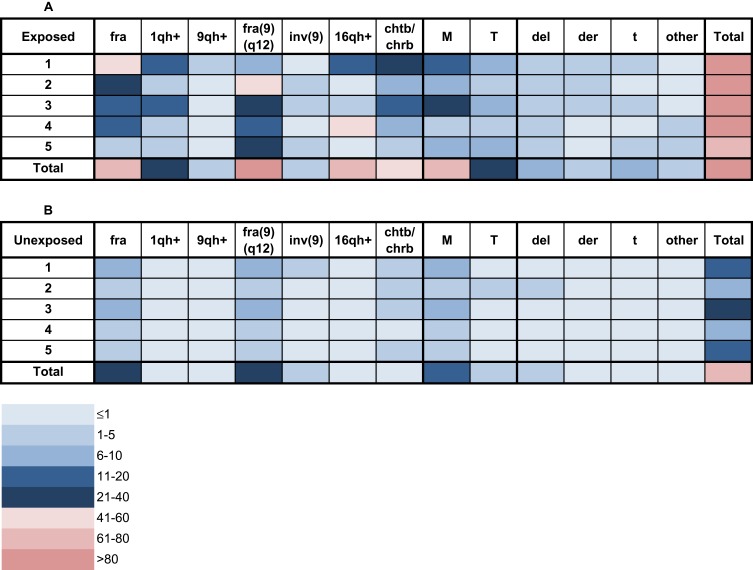
To define fragilities (fra), chromosome variants (CVs) (increase in length of heterochromatic segments of chromosomes 1, 9 and 16), chromosome breaks (chrb), chromatid breaks (chtb), and clonal (CCA) and non-clonal (NCCA) chromosomal alterations (numerical and structural chromosomal alterations), between 20 and 95 metaphases with good chromosome morphology and chromosome dispersion, were analyzed from individuals of both groups (exposed and unexposed). In total 544 metaphases were analyzed. GTG-banding cytogenetic analysis for both, exposed and unexposed groups, demonstrated a modal diploid number (2n). As shown in the Figures 1 and 2, significantly high frequencies for fragilities, variation in length of heterochromatic segments on the long arms of chromosomes 1 (1qh+), 9 (9qh+) and 16 (16qh+); inversion of chromosome 9 [inv(9)]; chromosomal breaks (chrb) and chromatid breaks (chrb), and CAs including structural (SCAs) and numerical chromosomal alterations (NCAs), were found in the exposed group compared with those observed in the unexposed group (510 and 73, respectively) (p≤ 0.008; Wilcoxon test) (Table 4).
Figure 1.
Frequency of chromosome variants (CVs) and chromosomal alterations (CAs) in peripheral blood lymphocytes (PBLs) from farmers exposed to pesticides (A) and from unexposed individuals (B). The frequency of each CV and CA is indicated for each exposed and unexposed individual using a color code for each category according to the legend at the bottom.
Abbreviations: fra, fragilities; 1qh+, increase in length of the heterochromatic segment on the long arm of chromosome 1; 9qh+, increase in length of the heterochromatic segment on the long arm of chromosome 9; fra(9)(q12), fragility in the long arm of chromosome 9, region 1 and band 2; inv(9), inversion of chromosome 9; 16qh+, increase in length of the heterochromatic segment on the long arm of chromosome 16; chtb/chrb, chromatidic/chromosomic break; M, monosomies; T, trisomies; del, chromosomal deletions; der, derivative chromosomes; t, chromosomal translocations; other, other structural chromosomal alterations.
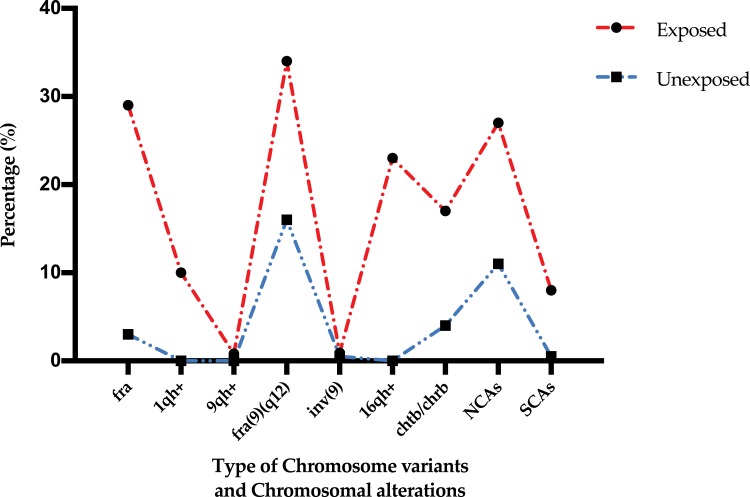
Figure 2.
Percentages of chromosome variants and chromosomal alterations observed in the exposed and unexposed groups.
Abbreviations: fra, fragilities; 1qh+, increase in length of the heterochromatic segment on the long arm of chromosome 1; 9qh+, increase in length of the heterochromatic segment on the long arm of chromosome 9; inv(9), inversion of chromosome 9; 16qh+, increase in length of the heterochromatic segment on the long arm of chromosome 16; chtb/chrb, chromatidic/chromosomic break; NCAs, numerical chromosomal alterations; SCAs, structural chromosomal alterations.
Table 4.
Frequencies and Percentages of Chromosome Variants (CVs) and Chromosomal Alterations (CAs) Identified in the Exposed and Unexposed Groups
| CVs and CAs | Number of Individuals | Number of Alterations | |||
|---|---|---|---|---|---|
| Exposed n (%) | Unexposed n (%) | Exposed n (%) | Unexposed n (%) | p+ | |
| fra | 5 (100) | 5 (100) | 97 (28) | 6 (3) | 0.00001** |
| 1qh+ | 5 (100) | 0 (0) | 35 (10) | 0 (0) | 0.0015** |
| 9qh+ | 1 (20) | 0 (0) | 3 (0.9) | 0 (0) | 1 |
| fra(9)(q12) | 5 (100) | 5 (100) | 115 (34) | 33 (16) | 0.0052** |
| inv(9) | 2 (40) | 1 (20) | 3 (0.9) | 1 (0.5) | 0.6212 |
| 16qh+ | 3 (60) | 0 (0) | 79 (23) | 0 (0) | 0.000001** |
| chtb/chrb | 5 (100) | 4 (80) | 59 (17) | 9 (4) | 0.0046** |
| NCAs | 5 (100) | 5 (100) | 93 (27) | 23 (11) | 0.0063** |
| SCAs | 5 (100) | 1 (20) | 26 (8) | 1 (0.5) | 0.0349* |
| Total | 510 | 73 | |||
| Mean | 16.5 | 3.9 | |||
| SD | 12.2 | 5.7 | |||
| p++ | 0.008** | ||||
Notes: *Statistically significant difference relative to the unexposed group at p≤0.05. **Statistically significant difference relative to the unexposed group at p≤0.01 (p+: Fisher’s exact test; p++: Wilcoxon test).
Abbreviation: SD, standard deviation.
In addition, we assessed the effect of smoking and alcohol consumption as confounding factors on the frequency of CVs, chromosome breaks (chrb), chromatid breaks (chtb), and clonal (CCA) and non-clonal (NCCA) chromosomal alterations (numerical and structural chromosomal alterations) in all study subjects. Our results indicate that neither alcohol consumption nor cigarette smoking increase the frequency of CVs and CAs in any of the groups studied, exposed and unexposed (Table 2).
Fragilities
A high frequency of fra was found in the exposed group (212 fragilities) (Figure 1A) compared with the unexposed group (39 fragilities) (Figure 1B). In the exposed and unexposed groups, fra(9)(q12) was the most frequent (115/212 and 33/39, respectively) (Table 4, Figure 3A) and was present in 100% of the exposed and unexposed individuals (Table S1). Comparison of the presence of fra between exposed and unexposed groups showed a significant difference (p≤0.005) (Table 4). In both exposed and unexposed groups many of the fragilities were non-clonal (Table S1).
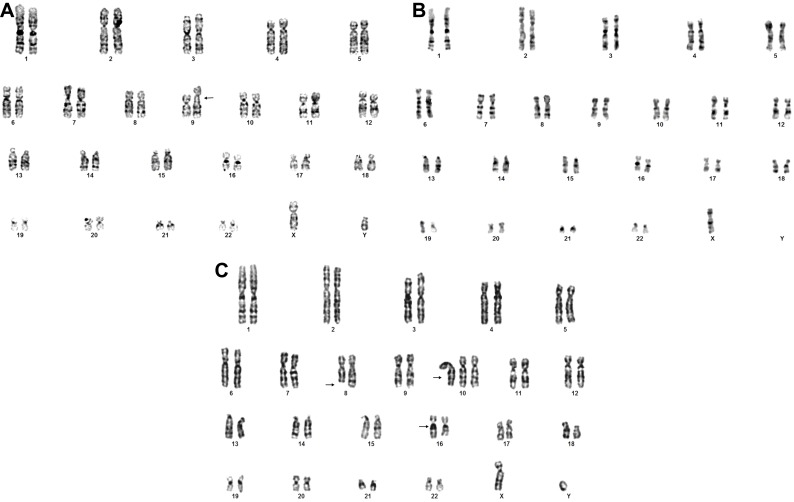
Figure 3.
Representative karyotypes of exposed individual showing: (A) Fragility of the long arm of chromosome 9: 46,XY,fra(9)(q12). (B) Monosomy of chromosome X:45,X,-X. (C) Numerical and structural chromosomal alterations: 47,XY,del(8)(q23),+10,16qh+. Arrows indicate chromosomal alterations.
Variation in Length of Heterochromatic Segments
High frequency of increase in the length of heterochromatic segments on the long arms of chromosomes 1 (1qh+) and 16 (16qh+) was identified in 100% and 60% of the exposed individuals, respectively, and in none of the unexposed individuals (Table 4 and Figures 1 and 2). Comparison of the presence of 1qh+ and 16qh+ between exposed and unexposed groups showed significant differences (p≤0.001, Fisher’s exact test) (Tables 4 and S2).
Chromatid and Chromosomic Breakage (Chtb/Chrb)
Also, higher frequencies of chrb and chtb were observed in the exposed group (59 breaks) compared with those observed in the unexposed group (9 breaks) (Table 4). In the exposed group the chromosomes most affected by such changes were chromosomes 9, 1, 5 and 6 (Table S3). The frequency of chrb and chtb between exposed and unexposed groups showed statistically significant differences (p≤0.004) (Table 4).
Numerical (NCAs) and Structural Chromosomal Alterations (SCAs)
In the exposed and unexposed groups, 119 NCAs and 24 SCAs were observed, respectively (Table 4). In the exposed group, NCAs (93/119) were more frequent than SCAs (26/119) (Figures 1 and 2). Similar results were observed in the unexposed group, where NCAs (23/24) were also more frequent than SCAs (1/24). When exposed and unexposed groups were compared, the frequency of NCAs and SCAs showed significant differences (p≤0.006 and p≤0.03, respectively) (Table 4).
Numerical Chromosomal Alterations
The NCAs identified in both groups were mainly monosomies, while trisomies were less frequently observed and were non-clonal (NCCAs). Note that in the exposed group, monosomy of the X chromosome (Figure 3B) was most frequently found in exposed females, but not exposed males, followed by monosomies of chromosomes 10 and 20, which showed statistically significant differences compared with the monosomies observed in the unexposed group (p≤0.006) (Tables 4 and S4). Additionally, between 1 and 3 marker chromosomes (mar) were identified in all exposed individuals, which were absent in the unexposed group (Table S4).
Structural Chromosomal Alterations
A higher frequency of non-clonal SCAs was identified in the exposed group (26/119) compared with those observed in the unexposed group (1/24). SCAs were observed in all individuals of the exposed group (100%), including dicentric chromosomes (dic), deletions (del), translocations (t), inversions (inv), derivative chromosomes (der) and ring chromosomes (r), while in the unexposed group only one deletion in one individual was observed (Table 6). Among all SCAs, deletions were most frequently found (10/26) in all exposed individuals, followed by translocations (6/26) and derivative chromosomes (5/26) (Figure 1 and Table 5). Note that the chromosome most affected by SCAs in the exposed group was chromosome 8, where deletions and translocations were prevalent (Table 5 and Figure 3C).
Table 6.
Frequency and Percentage of Chromosome Variants (CVs) and Chromosomal Alterations (CAs) Identified in Paired Exposed/Unexposed Individuals
| No | Exposed | Unexposed | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 1 | 138 | 41 | 18 | 9 | 0.0001** |
| 2 | 89 | 26 | 9 | 4 | 0.0001** |
| 3 | 109 | 32 | 21 | 10 | 0.0002** |
| 4 | 109 | 32 | 9 | 4 | 0.0001** |
| 5 | 65 | 19 | 16 | 8 | 0.037* |
Notes: *Statistically significant difference relative to the unexposed group at p≤0.05. **Statistically significant difference relative to the unexposed group at p≤0.01 (Fisher’s exact test).
Table 5.
Type and Frequency of Structural Chromosomal Alterations (SCAs) Observed in Exposed (E) and Unexposed (C) Groups. The Frequency of Each SCAs Is Indicated for Each Exposed and Unexposed Individual Using a Color Code for Each Category According to the Legend to the Right
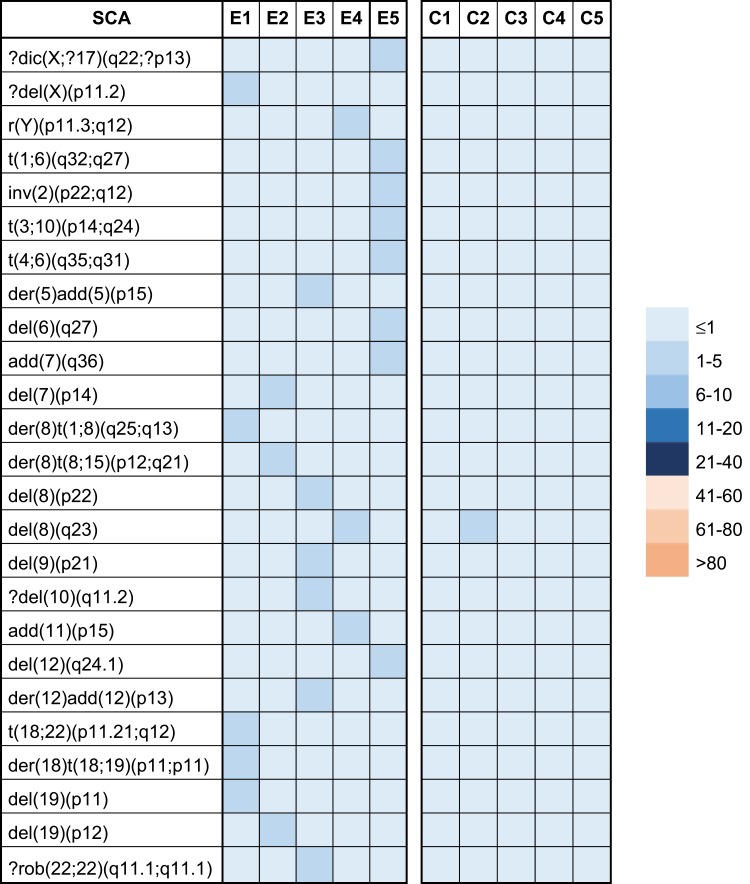 |
Abbreviations: dic, dicentric chromosome; del, chromosome deletion; r, ring chromome; t, chromosome translocation; inv, chromosome inversion; add, additional material of unknown origin; rob, Robertsonian translocation.
Comparison of the presence of fragilities, variation in length of heterochromatic segments on the long arms of chromosomes 1, 9 and 16, chromosomal and chromatid breaks, and CAs between paired exposed/unexposed individuals showed statistically significant differences (Table 6).
Molecular Cytogenetic (Fluorescence in situ Hybridization) Results
In order to better quantify the level of CIN in exposed compared to unexposed individuals, we assessed CIN by using centromeric FISH in 100 interphase nuclei and some metaphases. The CIN rate for each exposed and unexposed individual was defined first by calculating, for each of the six chromosomes separately, the percentage of nuclei with a CEP signal number different to the modal number, and then calculating the mean CIN percentage of all six chromosomes analyzed (Table S5).
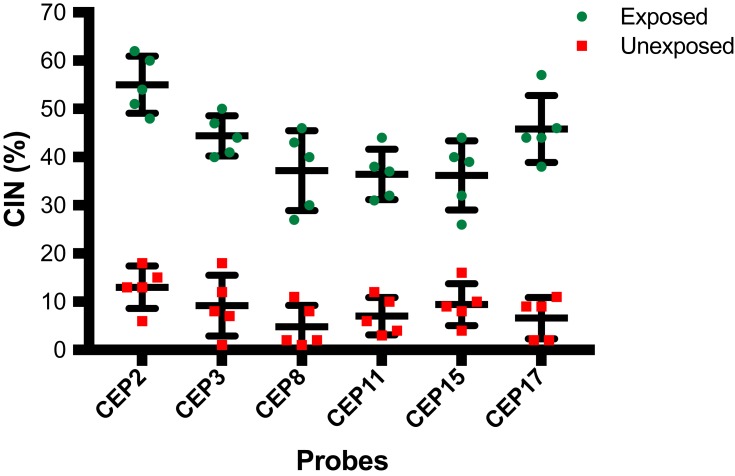
Exposed individuals showed a high CIN (≥25%) compared with a low CIN (≤14%) observed in unexposed individuals (Figures 4 and 5, and Table S5). More specifically, in exposed individuals, CIN ranged between 41% and 45%, while in non-exposed individuals, CIN ranged between 4% and 14% (Figures 4 and 5). These results suggest that pesticides can induce aneuploidy, which is indicative of numerical CIN.
Figure 4.
Percentage of Chromosomal Instability (CIN) observed in peripheral blood lymphocytes (PBLs) from farmers exposed to pesticides and from unexposed individuals. CIN was evaluated on nuclei spreads by using FISH with six centromeric probes (CEP) for chromosomes 2, 3, 8, 11, 15 and 17. The CIN rate for each exposed and unexposed individual was defined first by calculating the percentage of nuclei with a CEP signal number different to the modal number (most common chromosome number in a tumor cell population) for each individual chromosome and then calculating the mean CIN percentage of all chromosomes analyzed. The bars indicate the mean and standard deviation of each probe in each group (exposed and unexposed).
Figure 5.
Representative FISH images of an exposed and an unexposed individual. Metaphase spreads are shown and boxes indicate representative interphase nuclei for each case. In the exposed individual, more than two signals are observed for chromosomes 2, 3, 8, 11, 15 and 17, which is indicative of chromosomal instability. While in the unexposed individual, for each of the chromosomes indicated above, a normal number of signals (two signals) are observed. Three-color FISH was performed on nuclei spreads for chromosomes 2, 8 and 11 and, chromosomes 3, 15 and 17 using centromeric probes (CEP) labeled with different spectrum colors: spectrum orange for CEP2 and CEP3; spectrum green for CEP8 and CEP17; and spectrum aqua for CEP11 and CEP15.
Discussion
In Colombia, one of the most important economic activities is agricultural production, which results in farmers being exposed to pesticides. Many of these pesticides are carcinogenic and mutagenic. It is well known that chromosomal alterations are causal events in the development of neoplasms.29 Therefore, cytogenetic damage may reflect an increase in cancer risk.21,30 However, although many studies have reported high genotoxicity associated with these chemicals, in Colombia, information about the type and frequency of specific CAs and the level of CIN induced by exposure to pesticides is scarce or absent. Considering the above, the aim of our study was to evaluate CAs and CIN induced by pesticides in peripheral blood lymphocytes (PBLs) from farmers occupationally exposed to pesticides.
The results obtained using GTG-Banding and FISH analysis of a large number of metaphases, allowed us to identify previously unreported CVs and CAs in farmers exposed to pesticides. The mean number of CVs and CAs observed in the exposed individuals was seven times higher than that in the unexposed individuals. The above suggests a possible cytogenetic effect of pesticides on occupationally exposed farmers in the Department of Cundinamarca, Colombia. Numerical and structural CIN was also observed in both, exposed and unexposed groups, with a higher and statistically significant prevalence in the exposed group. However, it is important to highlight that many of the numerical and structural alterations observed were NCCAs.
In the exposed group, the monosomies were the CAs most frequently observed compared with those observed in the unexposed group. Monosomies have been correlated with hematological malignancies in several recent studies.31 For instance, monosomy of chromosome X, observed at high frequency in two exposed individuals, has been correlated with autoimmune disease in females,32,33 autoimmune thyroid disease and systemic sclerosis.34 Furthermore, a high frequency of monosomy X was found in peripheral leukocytes from patients with primary biliary cirrhosis.35 Interestingly, it has been proposed that xenobiotics, including pesticides, may be environmental triggers of primary biliary cirrhosis in genetically susceptible individuals.36–38 The above studies demonstrate a possible link between pesticide exposure, chromosome X monosomy and disease. In addition, chromosome 10 trisomy, observed in 40% of exposed and in 0% of non-exposed subjects, has been reported as a non-random anomaly in myeloid disorders.39,40 Aneuploidy, defined as the loss or gain of complete chromosomes, is a characteristic of tumor cells associated with cellular stress.41
Regarding structural chromosomal alterations, this type of alterations may alter the relative dosage of genes on the affected chromosomes, which could lead to the development of diseases, including cancer. In fact, it has been reported that unbalanced chromosomal abnormalities, in which there is a net gain or loss of genetic material, lead to the development of many human genetic disorders.42
In addition, a significant increase in the frequency of fragilities (fra), increase in length of heterochromatic segments on the long arms of chromosome 1 (1qh+) and chromosome 16 (16qh+) and chromosomal and chromatidic breakages (chrb/chtb) were also observed in the exposed compared with the unexposed group. Fragilities are unstable regions of the genome43 that can lead to the formation of complex CAs, including sister chromatid exchanges,44 duplications,45 intrachromosomal gene amplification,46 deletions and translocations,47,48 among others. All the above CAs have been associated with the development of cancer.49,50 In fact, many tumor suppressor genes and oncogenes have been located within fragile sites.51 Among the fragilities identified in this study, fra(9)(q12) was the most frequently observed in the exposed individuals. Interestingly, this fragility was observed by us in high frequency, in a Colombian population with breast cancer52 and also in individuals occupationally exposed to paint removers.53 Therefore, we suggest that fra(9)(q12) could be considered as a cytogenetic biomarker of chromosomal damage associated with pesticide exposure.
With respect to the increase in the length of heterochromatin segments, Atkin, et al54 suggested susceptibility to malignancy associated mainly with heteromorphisms in chromosome 1. In addition, several groups have reported the presence of heterochromatin variation on chromosomes 1, 9 and 16, in patients with various malignant tumors including ovarian, breast and hematological disorders, among others.55–57 To highlight that, scoring of chromosomal breakages has been used to monitor populations with increased cancer risk due to carcinogen exposure.58–60 Moreover, chromosomal breakages generally produce non-recurrent chromosomal aberrations (NCCAs),61 which are indicative of CIN.
In addition, CIN was also evaluated by using FISH. This method allowed us to identify, in each exposed and exposed individual, the variations in the modal number of chromosomes 2, 3, 8, 11, 15 and 17. Our results show that individuals exposed to pesticides have a high frequency of aneuploidy (high CIN) compared to low aneuploidy (low CIN) observed in unexposed individuals. Some studies have shown that a wide range of chemicals, classified as aneugens, are capable of inducing aneuploidy both in vitro and in vivo.62 Thus, it has been reported that exposure to low concentrations of potentially aneugenic chemicals, such as pesticides, can induce carcinogenesis.63 The mechanisms through which pesticides induce aneuploidy are not well known. Aneugenic chemicals may lead to chromosome loss and non-disjunction by interacting with a variety of cell components and activities, such as synthesis and functioning of the spindle fibres,64 activity of the centrosome and modification of centromeres.65 Additionally, it has been reported that chemically induced aneuploidy may be extremely relevant for environmental carcinogenesis.66 To our knowledge, this is the first study to assess numerical CIN using six centromeric FISH probes in individuals occupationally exposed to pesticides.
Together, our results show that exposure to pesticides is associated with a significant increase in CIN. CIN is characterized by cell-to-cell variability in the number or structure of chromosomes in a given cell population,67 and by the presence of clonal (CCAs) and non-clonal alterations (NCCAs). Considering that CCAs are recurrent chromosomal alterations (alterations found at least twice in a population of 20–40 mitosis), and NCCAs are present in a population of cells in a non-recurrent manner,68 our results not only suggest that pesticides induce CIN but also high levels of heterogeneity. In fact, it has been reported that NCCAs reflect a more unstable system and are a stronger indicator of CIN.67,69 In addition, CCAs and NCCAs can lead to expansion of chromosomal alterations and, therefore, to a general increase in heterogeneity. Both CIN and heterogeneity reflect the instability of the system and may predispose cells to acquire additional CAs and, therefore, to a higher risk of malignant transformation.70,71 In fact, a linear trend between CAs in PBLs and subsequent cancer risk has been shown by several prospective cancer studies.29,72–75
Most of the farmers in our study were exposed to complex mixtures of pesticides. Considering that pesticides come in many different formulations due to variations in the active ingredient’s solubility, ability to control the pest, and ease of handling and transport, it is very difficult to determine whether the chromosomal damage observed in exposed individuals is due to a specific pesticide. Furthermore, variable combinations of products are often used.17 In addition, pesticides generally have different biological modes of action. For example, it has been reported that mancozeb, one of the pesticides used by farmers in this study, interferes with the synthesis, metabolism, transportation, and elimination of hormones, which results in decreased natural hormone concentrations.76 Paraquat, besides being the second most widely used herbicide,77 also has been associated with an increased risk of Parkinson’s disease.78 Another of the pesticides used by the exposed group is Carbosulfan. This insecticide is a potent genotoxic agent and a potent germ cell mutagen.79 In fact, it has been reported that the exposure of mice to this insecticide increases the formation of bone marrow micronuclei, chromosomal abnormalities and sperm alterations.77
The high frequency of CVs and CAs observed in the exposed group might result from pesticide-induced oxidative stress. Oxidative stress is known to cause DNA damage, which in turn may cause health disorders including Parkinson’s disease,78 endocrine disruption,80 respiratory and reproductive disorders,81 Hodgkin’s disease, non-Hodgkin lymphoma,82 leukemia, Burkitt lymphoma, ovarian cancer, neuroblastoma, soft tissue sarcoma,83 and cancers of the lung, rectum, stomach, bladder, colon and breast.84,85
Considering that the mutagenic risk of various cigarette components is considered a confounding factor that can influence the frequency of CVs and CAs, we analyze whether smoking and alcohol consumption in both the exposed and unexposed individuals affect the frequency of chromosomal damage. Our results show that smoking does not increase the frequency of chromosomal damage in the exposed 5 (E5), the only exposed individual who indicated being a smoker. This result may be due to the fact that the average daily cigarette intake of E5 is 3, so according to the criteria considered by Calderón-Ezquerro et al (2007),86 the E5 can be classified as a light smoker (<19 ± 3.88 cigarettes/day), so this amount is insufficient to cause an effect on PBLs. Additional studies found no effect of smoking habit on workers exposed to pesticides and whose intake was between 22 and 25 cigarettes/day.87 With regard to alcohol consumption, our results showed no associations between alcohol consumption and increased in the frequency of CVs and CAs. Similar findings have been previously reported, which indicate that the increase in the frequency of chromosomal damage is not related to alcohol consumption in people exposed to pesticides.88
Conclusions
The results obtained from the analysis of a large number of metaphases for using GTG-Banding and FISH allowed us to identify previously unreported chromosome variants (CVs) and CAs in farmers exposed to pesticides. The results of our study, although conducted on a small number of individuals, suggest a deleterious effect of pesticides on chromosomes as well as the association between them with a significant increase in CIN. Considering that CIN can predispose cells to additional chromosomal alterations and, therefore, to an increased risk of developing diseases, the establishment of educational programs on safety precautions when handling pesticides, such as wearing boots and masks, gloves, changing clothes and maintaining proper hygiene, among others, is urgent and necessary. Our study provides relevant information for further evaluation with a greater number of individuals.
Acknowledgments
We thank Dr. Nelson Rangel for excellent assistance with statistical analysis and for critical reading of the manuscript. We thank Jeremy Allen, PhD, from Edanz Group for editing a draft of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bolognesi C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat Res. 2003;543(3):251–272. doi: 10.1016/S1383-5742(03)00015-2 [DOI] [PubMed] [Google Scholar]
- 2.Kaur K, Kaur R. Occupational pesticide exposure, impaired DNA repair, and diseases. Indian J Occup Environ Med. 2018;22(2):74–81. doi: 10.4103/ijoem.IJOEM_45_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostafalou S, Abdollahi M. Pesticides: an update of human exposure and toxicity. Arch Toxicol. 2017;91(2):549–599. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez AF, Parron T, Tsatsakis AM, Requena M, Alarcon R, Lopez-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 5.Koureas M, Tsezou A, Tsakalof A, Orfanidou T, Hadjichristodoulou C. Increased levels of oxidative DNA damage in pesticide sprayers in thessaly region (Greece). Implications of pesticide exposure. Sci Total Environ. 2014;496:358–364. doi: 10.1016/j.scitotenv.2014.07.062 [DOI] [PubMed] [Google Scholar]
- 6.Ji BT, Silverman DT, Stewart PA, et al. Occupational exposure to pesticides and pancreatic cancer. Am J Ind Med. 2001;39(1):92–99. doi: [DOI] [PubMed] [Google Scholar]
- 7.Dulout FN, Pastori MC, Olivero OA, et al. Sister-chromatid exchanges and chromosomal aberrations in a population exposed to pesticides. Mutat Res. 1985;143(4):237–244. doi: 10.1016/0165-7992(85)90087-9 [DOI] [PubMed] [Google Scholar]
- 8.De Ferrari M, Artuso M, Bonassi S, et al. Cytogenetic biomonitoring of an Italian population exposed to pesticides: chromosome aberration and sister-chromatid exchange analysis in peripheral blood lymphocytes. Mutat Res. 1991;260(1):105–113. doi: 10.1016/0165-1218(91)90086-2 [DOI] [PubMed] [Google Scholar]
- 9.Rupa DS, Reddy PP, Sreemannarayana K, Reddi OS. Frequency of sister chromatid exchange in peripheral lymphocytes of male pesticide applicators. Environ Mol Mutagen. 1991;18(2):136–138. doi: 10.1002/em.2850180209 [DOI] [PubMed] [Google Scholar]
- 10.Balaji M, Sasikala K. Cytogenetic effect of malathion in in vitro culture of human peripheral blood. Mutat Res. 1993;301(1):13–17. doi: 10.1016/0165-7992(93)90050-6 [DOI] [PubMed] [Google Scholar]
- 11.Carbonell E, Xamena N, Creus A, Marcos R. Cytogenetic biomonitoring in a Spanish group of agricultural workers exposed to pesticides. Mutagenesis. 1993;8(6):511–517. doi: 10.1093/mutage/8.6.511 [DOI] [PubMed] [Google Scholar]
- 12.Brega SM, Vassilieff I, Almeida A, et al. Clinical, cytogenetic and toxicological studies in rural workers exposed to pesticides in Botucatu, Sao Paulo, Brazil. Cad Saude Publica. 1998;14(Suppl 3):109–115. doi: 10.1590/S0102-311X1998000700011 [DOI] [PubMed] [Google Scholar]
- 13.Carbonell E, Puig M, Xamena N, Creus A, Marcos R. Sister chromatid exchange in lymphocytes of agricultural workers exposed to pesticides. Mutagenesis. 1990;5(4):403–405. doi: 10.1093/mutage/5.4.403 [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Arroyo S, Noriega-Aldana N, Osorio A, Galicia F, Ling S, Villalobos-Pietrini R. Sister-chromatid exchange analysis in a rural population of Mexico exposed to pesticides. Mutat Res. 1992;281(3):173–179. doi: 10.1016/0165-7992(92)90005-3 [DOI] [PubMed] [Google Scholar]
- 15.Prabhavathy Das G, Pasha Shaik A, Jamil K. Cytotoxicity and genotoxicity induced by the pesticide profenofos on cultured human peripheral blood lymphocytes. Drug Chem Toxicol. 2006;29(3):313–322. doi: 10.1080/01480540600653093 [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Arroyo S, Diaz-Sanchez Y, Meneses-Perez MA, Villalobos-Pietrini R, De Leon-rodriguez J. Cytogenetic biomonitoring in a Mexican floriculture worker group exposed to pesticides. Mutat Res. 2000;466(1):117–124. doi: 10.1016/S1383-5718(99)00231-4 [DOI] [PubMed] [Google Scholar]
- 17.Bull S, Fletcher K, Boobis AR, Battershill JM. Evidence for genotoxicity of pesticides in pesticide applicators: a review. Mutagenesis. 2006;21(2):93–103. doi: 10.1093/mutage/gel011 [DOI] [PubMed] [Google Scholar]
- 18.Remor AP, Totti CC, Moreira DA, Dutra GP, Heuser VD, Boeira JM. Occupational exposure of farm workers to pesticides: biochemical parameters and evaluation of genotoxicity. Environ Int. 2009;35(2):273–278. doi: 10.1016/j.envint.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 19.Silins I, Hogberg J. Combined toxic exposures and human health: biomarkers of exposure and effect. Int J Environ Res Public Health. 2011;8(3):629–647. doi: 10.3390/ijerph8030629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentile N, Manas F, Bosch B, Peralta L, Gorla N, Aiassa D. Micronucleus assay as a biomarker of genotoxicity in the occupational exposure to agrochemicals in rural workers. Bull Environ Contam Toxicol. 2012;88(6):816–822. doi: 10.1007/s00128-012-0589-8 [DOI] [PubMed] [Google Scholar]
- 21.Bonassi S, Hagmar L, Stromberg U, et al. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. European Study Group on Cytogenetic Biomarkers and Health. Cancer Res. 2000;60(6):1619–1625. [PubMed] [Google Scholar]
- 22.ISCN 2016. An International System for Human Cytogenomic Nomenclature (2016). Vol. 149 Basel (Switzerland): Karger; 2016. [Google Scholar]
- 23.Takami S, Kawasome C, Kinoshita M, Koyama H, Noguchi S. Chromosomal instability detected by fluorescence in situ hybridization in Japanese breast cancer patients. Clin Chim Acta. 2001;308(1–2):127–131. doi: 10.1016/S0009-8981(01)00473-9 [DOI] [PubMed] [Google Scholar]
- 24.Farabegoli F, Santini D, Ceccarelli C, Taffurelli M, Marrano D, Baldini N. Clone heterogeneity in diploid and aneuploid breast carcinomas as detected by FISH. Cytometry. 2001;46(1):50–56. doi: 10.1002/(ISSN)1097-0320 [DOI] [PubMed] [Google Scholar]
- 25.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386(6625):623–627. doi: 10.1038/386623a0 [DOI] [PubMed] [Google Scholar]
- 26.Munro AF, Twelves C, Thomas JS, Cameron DA, Bartlett JM. Chromosome instability and benefit from adjuvant anthracyclines in breast cancer. Br J Cancer. 2012;107(1):71–74. doi: 10.1038/bjc.2012.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawauchi S, Furuya T, Ikemoto K, et al. DNA copy number aberrations associated with aneuploidy and chromosomal instability in breast cancers. Oncol Rep. 2010;24(4):875–883. doi: 10.3892/or.2010.875 [DOI] [PubMed] [Google Scholar]
- 28.Talamo A, Chalandon Y, Marazzi A, Jotterand M. Clonal heterogeneity and chromosomal instability at disease presentation in high hyperdiploid acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2010;203(2):209–214. doi: 10.1016/j.cancergencyto.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 29.Hagmar L, Bonassi S, Stromberg U, et al. Chromosomal aberrations in lymphocytes predict human cancer: a report from the European Study Group on Cytogenetic Biomarkers and Health (ESCH). Cancer Res. 1998;58(18):4117–4121. [PubMed] [Google Scholar]
- 30.Baris D, Silverman DT, Brown LM, et al. Occupation, pesticide exposure and risk of multiple myeloma. Scand J Work Environ Health. 2004;30(3):215–222. doi: 10.5271/sjweh.782 [DOI] [PubMed] [Google Scholar]
- 31.Lumley M, Barker H, Murray JA. Benzene in petrol. Lancet. 1990;336(8726):1318–1319. doi: 10.1016/0140-6736(90)93002-7 [DOI] [PubMed] [Google Scholar]
- 32.Invernizzi P, Miozzo M, Battezzati PM, et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363(9408):533–535. doi: 10.1016/S0140-6736(04)15541-4 [DOI] [PubMed] [Google Scholar]
- 33.Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M. Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun. 2009;33(1):12–16. doi: 10.1016/j.jaut.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 34.Invernizzi P, Miozzo M, Selmi C, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. J Immunol. 2005;175(1):575–578. doi: 10.4049/jimmunol.175.1.575 [DOI] [PubMed] [Google Scholar]
- 35.Bianchi I, Lleo A, Bernuzzi F, Caliari L, Smyk DS, Invernizzi P. The X-factor in primary biliary cirrhosis: monosomy X and xenobiotics. Auto Immun Highlights. 2012;3(3):127–132. doi: 10.1007/s13317-012-0043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: convenient and inconvenient truths. Hepatology. 2008;47(2):737–745. doi: 10.1002/hep.22042 [DOI] [PubMed] [Google Scholar]
- 37.Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30(8):415–420. doi: 10.1016/j.it.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 38.Smyk D, Rigopoulou EI, Baum H, Burroughs AK, Vergani D, Bogdanos DP. Autoimmunity and environment: am I at risk? Clin Rev Allergy Immunol. 2012;42(2):199–212. doi: 10.1007/s12016-011-8259-x [DOI] [PubMed] [Google Scholar]
- 39.Estalilla O, Rintels P, Mark HF. Trisomy 10 in leukemia. Cancer Genet Cytogenet. 1998;101(1):68–71. doi: 10.1016/S0165-4608(97)00056-3 [DOI] [PubMed] [Google Scholar]
- 40.Ohyashiki K, Kodama A, Nakamura H, et al. Trisomy 10 in acute myeloid leukemia. Cancer Genet Cytogenet. 1996;89(2):114–117. doi: 10.1016/0165-4608(95)00263-4 [DOI] [PubMed] [Google Scholar]
- 41.Williams BR, Amon A. Aneuploidy: cancer’s fatal flaw? Cancer Res. 2009;69(13):5289–5291. doi: 10.1158/0008-5472.CAN-09-0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theisen A, Shaffer LG. Disorders caused by chromosome abnormalities. Appl Clin Genet. 2010;3:159–174. doi: 10.2147/TACG.S8884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glover TW. Instability at chromosomal fragile sites. Recent Results Cancer Res. 1998;154:185–199. doi: 10.1007/978-3-642-46870-4_11 [DOI] [PubMed] [Google Scholar]
- 44.Glover TW, Stein CK. Induction of sister chromatid exchanges at common fragile sites. Am J Hum Genet. 1987;41(5):882–890. [PMC free article] [PubMed] [Google Scholar]
- 45.Hellman A, Zlotorynski E, Scherer SW, et al. A role for common fragile site induction in amplification of human oncogenes. Cancer Cell. 2002;1(1):89–97. doi: 10.1016/S1535-6108(02)00017-X [DOI] [PubMed] [Google Scholar]
- 46.Coquelle A, Pipiras E, Toledo F, Buttin G, Debatisse M. Expression of fragile sites triggers intrachromosomal mammalian gene amplification and sets boundaries to early amplicons. Cell. 1997;89(2):215–225. doi: 10.1016/S0092-8674(00)80201-9 [DOI] [PubMed] [Google Scholar]
- 47.Durkin SG, Glover TW. Chromosome fragile sites. Annu Rev Genet. 2007;41:169–192. doi: 10.1146/annurev.genet.41.042007.165900 [DOI] [PubMed] [Google Scholar]
- 48.Re A, Cora D, Puliti AM, Caselle M, Sbrana I. Correlated fragile site expression allows the identification of candidate fragile genes involved in immunity and associated with carcinogenesis. BMC Bioinformatics. 2006;7:413. doi: 10.1186/1471-2105-7-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent-Salomon A, Benhamo V, Gravier E, et al. Genomic instability: a stronger prognostic marker than proliferation for early stage luminal breast carcinomas. PLoS One. 2013;8(10):e76496. doi: 10.1371/journal.pone.0076496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debacker K, Kooy RF. Fragile sites and human disease. Hum Mol Genet. 2007;16(2):R150–R158. doi: 10.1093/hmg/ddm136 [DOI] [PubMed] [Google Scholar]
- 51.Popescu NC. Genetic alterations in cancer as a result of breakage at fragile sites. Cancer Lett. 2003;192(1):1–17. doi: 10.1016/S0304-3835(02)00596-7 [DOI] [PubMed] [Google Scholar]
- 52.Rondón M, Caicedo J, Robledo J. Establishment of chromosomic abnormalities and amplified DNA sequences in breast cancer. Revista Ciencias de la Salud. 2006;4(2):15. [Google Scholar]
- 53.Villalba-Campos M, Chuaire-Noack L, Sanchez-Corredor MC, Rondon-Lagos M. High chromosomal instability in workers occupationally exposed to solvents and paint removers. Mol Cytogenet. 2016;9:46. doi: 10.1186/s13039-016-0256-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atkin NB. Chromosome 1 heteromorphism in patients with malignant disease: a constitutional marker for a high-risk group? Br Med J. 1977;1(6057):358. doi: 10.1136/bmj.1.6057.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shabtai F, Halbrecht I. Risk of malignancy and chromosomal polymorphism: a possible mechanism of association. Clin Genet. 1979;15(1):73–77. doi: 10.1111/j.1399-0004.1979.tb02029.x [DOI] [PubMed] [Google Scholar]
- 56.Aguilar L, Lisker R, Ruz L, Mutchinick O. Constitutive heterochromatin polymorphisms in patients with malignant diseases. Cancer. 1981;47(10):2437–2439. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 57.Petkovic I. Constitutive heterochromatin of chromosomes No. 1, 9, and 16 in 90 patients with malignant disease and 91 controls. Cancer Genet Cytogenet. 1983;10(2):151–158. doi: 10.1016/0165-4608(83)90119-X [DOI] [PubMed] [Google Scholar]
- 58.Wu X, Zheng YL, Hsu TC. Mutagen-induced chromatid breakage as a marker of cancer risk. Methods Mol Biol. 2005;291:59–67. doi: 10.1385/1-59259-840-4:059 [DOI] [PubMed] [Google Scholar]
- 59.Bonassi S, Abbondandolo A, Camurri L, et al. Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Preliminary results of an Italian cohort study. Cancer Genet Cytogenet. 1995;79(2):133–135. doi: 10.1016/0165-4608(94)00131-T [DOI] [PubMed] [Google Scholar]
- 60.Bonassi S, Znaor A, Norppa H, Hagmar L. Chromosomal aberrations and risk of cancer in humans: an epidemiologic perspective. Cytogenet Genome Res. 2004;104(1–4):376–382. doi: 10.1159/000077519 [DOI] [PubMed] [Google Scholar]
- 61.Heng HH, Liu G, Bremer S, Ye KJ, Stevens J, Ye CJ. Clonal and non-clonal chromosome aberrations and genome variation and aberration. Genome. 2006;49(3):195–204. doi: 10.1139/g06-023 [DOI] [PubMed] [Google Scholar]
- 62.Parry JM, Parry EM, Bourner R, et al. The detection and evaluation of aneugenic chemicals. Mutat Res. 1996;353(1–2):11–46. doi: 10.1016/0027-5107(95)00242-1 [DOI] [PubMed] [Google Scholar]
- 63.Bolt HM, Foth H, Hengstler JG, Degen GH. Carcinogenicity categorization of chemicals-new aspects to be considered in a European perspective. Toxicol Lett. 2004;151(1):29–41. doi: 10.1016/j.toxlet.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 64.Zijno A, Marcon F, Leopardi P, Crebelli R. Analysis of chromosome segregation in cytokinesis-blocked human lymphocytes: non-disjunction is the prevalent damage resulting from low dose exposure to spindle poisons. Mutagenesis. 1996;11(4):335–340. doi: 10.1093/mutage/11.4.335 [DOI] [PubMed] [Google Scholar]
- 65.Renzi L, Pacchierotti F, Russo A. The centromere as a target for the induction of chromosome damage in resting and proliferating mammalian cells: assessment of mitomycin C-induced genetic damage at kinetochores and centromeres by a micronucleus test in mouse splenocytes. Mutagenesis. 1996;11(2):133–138. doi: 10.1093/mutage/11.2.133 [DOI] [PubMed] [Google Scholar]
- 66.Mattiuzzo M, Fiore M, Ricordy R, Degrassi F. Aneuploidy-inducing capacity of two widely used pesticides. Carcinogenesis. 2006;27(12):2511–2518. doi: 10.1093/carcin/bgl102 [DOI] [PubMed] [Google Scholar]
- 67.Heng HH, Bremer SW, Stevens JB, et al. Chromosomal instability (CIN): what it is and why it is crucial to cancer evolution. Cancer Metastasis Rev. 2013;32(3–4):325–340. doi: 10.1007/s10555-013-9427-7 [DOI] [PubMed] [Google Scholar]
- 68.Giam M, Rancati G. Aneuploidy and chromosomal instability in cancer: a jackpot to chaos. Cell Div. 2015;10:3. doi: 10.1186/s13008-015-0009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rangel N, Forero-Castro M, Rondon-Lagos M. New insights in the cytogenetic practice: karyotypic chaos, non-clonal chromosomal alterations and chromosomal instability in human cancer and therapy response. Genes (Basel). 2017;8(6). doi: 10.3390/genes8060155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pikor L, Thu K, Vucic E, Lam W. The detection and implication of genome instability in cancer. Cancer Metastasis Rev. 2013;32(3–4):341–352. doi: 10.1007/s10555-013-9429-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Lan Q, Guo W, et al. Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis. 2011;32(4):605–612. doi: 10.1093/carcin/bgq286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lan Q, Zhang L, Li G, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306(5702):1774–1776. doi: 10.1126/science.1102443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jang JE, Min YH, Yoon J, et al. Single monosomy as a relatively better survival factor in acute myeloid leukemia patients with monosomal karyotype. Blood Cancer J. 2015;5:e358. doi: 10.1038/bcj.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farkas G, Szekely G, Vass N, Kiss K, Gundy S. Rate of spontaneous numerical chromosome aberrations in Hungarian healthy population. Magy Onkol. 2015;59(3):198–204. [PubMed] [Google Scholar]
- 75.Vodenkova S, Polivkova Z, Musak L, et al. Structural chromosomal aberrations as potential risk markers in incident cancer patients. Mutagenesis. 2015;30(4):557–563. doi: 10.1093/mutage/gev018 [DOI] [PubMed] [Google Scholar]
- 76.Akhtar N, Kayani SA, Ahmad MM, Shahab M. Insecticide-induced changes in secretory activity of the thyroid gland in rats. J Appl Toxicol. 1996;16(5):397–400. doi: 10.1002/(ISSN)1099-1263 [DOI] [PubMed] [Google Scholar]
- 77.Sabarwal A, Kumar K, Singh RP. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ Toxicol Pharmacol. 2018;63:103–114. doi: 10.1016/j.etap.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 78.Brouwer M, Huss A, van der Mark M, et al. Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ Int. 2017;107:100–110. doi: 10.1016/j.envint.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 79.Giri S, Giri A, Sharma GD, Prasad SB. Mutagenic effects of carbosulfan, a carbamate pesticide. Mutat Res. 2002;519(1–2):75–82. doi: 10.1016/S1383-5718(02)00114-6 [DOI] [PubMed] [Google Scholar]
- 80.Mazur CS, Marchitti SA, Zastre J. P-glycoprotein inhibition by the agricultural pesticide propiconazole and its hydroxylated metabolites: implications for pesticide-drug interactions. Toxicol Lett. 2015;232(1):37–45. doi: 10.1016/j.toxlet.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 81.Kirkhorn SR, Schenker MB. Current health effects of agricultural work: respiratory disease, cancer, reproductive effects, musculoskeletal injuries, and pesticide-related illnesses. J Agric Saf Health. 2002;8(2):199–214. doi: 10.13031/2013.8432 [DOI] [PubMed] [Google Scholar]
- 82.Luo D, Zhou T, Tao Y, Feng Y, Shen X, Mei S. Exposure to organochlorine pesticides and non-Hodgkin lymphoma: a meta-analysis of observational studies. Sci Rep. 2016;6:25768. doi: 10.1038/srep25768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polanco Rodriguez AG, Riba Lopez MI, DelValls Casillas TA, Araujo Leon JA, Mahjoub O, Prusty AK. Monitoring of organochlorine pesticides in blood of women with uterine cervix cancer. Environ Pollut. 2017;220(Pt B):853–862. doi: 10.1016/j.envpol.2016.10.068 [DOI] [PubMed] [Google Scholar]
- 84.Bonner MR, Freeman LE, Hoppin JA, et al. Occupational exposure to pesticides and the incidence of lung cancer in the agricultural health study. Environ Health Perspect. 2017;125(4):544–551. doi: 10.1289/EHP456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal NR. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults. Environ Health Perspect. 2010;118(1):60–66. doi: 10.1289/ehp.0900919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calderon-Ezquerro C, Sanchez-Reyes A, Sansores RH, et al. Cell proliferation kinetics and genotoxicity in lymphocytes of smokers living in Mexico City. Hum Exp Toxicol. 2007;26(9):715–722. doi: 10.1177/0960327107083451 [DOI] [PubMed] [Google Scholar]
- 87.Ergene S, Celik A, Cavas T, Kaya F. Genotoxic biomonitoring study of population residing in pesticide contaminated regions in Goksu Delta: micronucleus, chromosomal aberrations and sister chromatid exchanges. Environ Int. 2007;33(7):877–885. doi: 10.1016/j.envint.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 88.Sailaja N, Chandrasekhar M, Rekhadevi PV, et al. Genotoxic evaluation of workers employed in pesticide production. Mutat Res. 2006;609(1):74–80. doi: 10.1016/j.mrgentox.2006.06.022 [DOI] [PubMed] [Google Scholar]