Abstract
Background
The incidence and prevalence of hypothyroidism are increasing and the threshold for the treatment of hypothyroid as well as individuals without evident thyroid disease with thyroid hormone is declining.
Objective
To investigate endocrinologists' use of thyroid hormones in hypothyroid and euthyroid patients in Italy, a country where different formulations of levothyroxine (LT4; tablet, liquid solution and soft-gel capsule) are available on the market.
Methods
Members of the Associazione Medici Endocrinologi (Italian Association of Clinical Endocrinologists) were invited to participate in a web-based survey investigating the topic.
Results
A total of 797 of 2,028 (39.3%) members completed all the sections of the survey; 98.7% declared that the treatment of choice for hypothyroidism is LT4. A significant minority (37.3%) indicated that LT4 may be considered in infertile euthyroid women seeking pregnancy and harbouring positive thyroperoxidase antibodies (TPOAb) and in goitre increasing in size (18.1%). LT4 + LT3 was considered by 43.2% for LT4-replaced patients and normal TSH, if they reported persistent symptoms. High percentages of respondents chose LT4 in a liquid solution or soft-gel capsules when taken together with other drugs interfering with LT4 absorption (81.8%), in patients with a history of celiac disease, malabsorption, lactose intolerance, intolerance to common excipients (96.6%), or unexplained poor biochemical control of hypothyroidism (74.4%), or in patients not able to adhere to ingesting LT4 fasted and/or separated from food/drink (98.9%). In total, 43.6% of responders would use LT4 in a liquid solution or soft-gel capsules for hypothyroid patients with biochemical euthyroidism on LT4, who had persistent symptoms.
Conclusions
The preferred treatment for hypothyroidism is LT4; LT3 + LT4 combination treatment is mainly considered in patients with persistent symptoms. A significant minority would offer LT4 to euthyroid women with positive TPOAb and infertility and to euthyroid patients with progressive simple goitre. Alternative LT4 formulations like liquid solution or soft-gel capsules are largely reserved for specific conditions (interfering drugs, actual or suspected malabsorption, inability to take LT4 in the fasting state, unexplained poor biochemical control of hypothyroidism).
Keywords: Levothyroxine, Hypothyroidism, Euthyroidism, Survey, European thyroid association
Introduction
Levothyroxine (LT4) prescription for hypothyroidism is steadily increasing as the threshold of thyroid-stimulating hormone (TSH) concentration for initiating treatment is progressively decreasing [1]. In the past decade, different LT4 formulations (generic or branded, in tablet form, soft-gel capsules or liquid solution) have become commercially available for the treatment of hypothyroidism [2]. Approved LT4 formulations, whether generic or branded, are generally reported as effective in the treatment of hypothyroidism, though differences in bioavailability have been noted [3]. Pricing of different formulations of LT4 varies widely. In addition to pricing of LT4, a number of other factors may influence cost-effectiveness: better patient adherence to LT4 treatment was associated with a significant reduction in all-cause and hypothyroidism-related costs [4]; compared to tablets, liquid solution of LT4 was associated with reduced number of TSH tests [5]; while switching from branded to generic LT4 resulted in lower drug costs, overall healthcare costs rose [6]. Moreover, switching from tablets to the more costly gel capsule formulation of LT4 in patients who experience intolerance or efficacy problems led to a decrease in the mean number of dose changes and improved symptom control [7]. Conversely, change in medication formulation from branded to cheaper generic LT4 seems to be associated with increased reporting of side effects and reduced efficacy [8, 9]. In addition to this, the prevalence of treated hypothyroid individuals is growing [10], and this is paralleled by an increase in the use of desiccated thyroid extract (DTE) and LT3 + LT4 combination therapy [11].
Increase in LT4 prescription may be driven by pressure from patients attributing weight gain, fatigue, mood disorders and poor memory to suboptimal treatment of hypothyroidism [12, 13]. A recent American survey, focusing on the controversial topic of use of T3, revealed that physicians' choice of treatment was strongly influenced by ongoing patient symptoms and characteristics and independent of biochemical control of hypothyroidism [14]. This is a concerning trend given the lack of evidence of superiority of T3 treatment and the risks related to subclinical hyperthyroidism, which include increased morbidity and mortality [15, 16, 17, 18].
The aim of this survey was to identify current attitudes of Italian endocrinologists, relating to the treatment of hypothyroidism, in a typical European country where all LT4 formulations are available on the market and the patient is mainly managed by the endocrinologist.
Methods
We utilized a web-based survey constructed with Lime-Survey, an open-access platform that provides various question templates. The questionnaire included 12 questions. A total of 2,028 members of the Italian Association of Clinical Endocrinologists (Associazione Medici Endocrinologi [AME]) were sent an initial E-mail, including an electronic link to the questionnaire, followed by 2 reminders between March 1 and 30, 2019. Survey responses were collected and electronically stored by the survey service, which were accessible by password. The survey service automatically blocked repeat submissions from the same IP address. The entire survey is available (online suppl. Appendix 1, see www.karger.com/doi/10.1159/000502057).
Statistical Analysis
Summary statistics were prepared for responses to each question. We considered as valid for statistical evaluation only those questionnaires with complete demographic data from the respondents. Pearson's χ2 test or Fisher's exact test was used to compare frequencies (percentages) between categorical variables. A two-sided p value of <0.05 was considered as statistically significant. Data were analysed using IBM SPSS Statistics version 19 software (SPSS, Chicago, IL, USA). In all analyses, respondents stating that they did not know the answer to a given question were pooled in the response category with respondents who did not provide an answer.
Results
Sample Characteristics
A total of 882 (43.5%) members responded and 797 (39.3%) completed all the questions of the survey. Six hundred and twelve of the latter (76.8%) were also members of another Italian Scientific Society, 34 (4.3%) of the European Thyroid Association, and 11 (1.4%) of the American Thyroid Association. The demographic characteristics of the respondents (Table 1) were similar between respondents and the entire membership. Four hundred and sixty-one (57.8%) treated thyroid patients on a daily basis, 293 (36.8%) on a weekly basis, whereas only 43 (5.4%) rarely managed thyroid patients. More than 100 hypothyroid patients/year were treated by 470 (59%) respondents, 51–100 annually by 182 (22.8%), 10–50 by 121 (15.2%) and only 24 AME members (3%) rarely treated hypothyroid patients.
Table 1.
Characteristics of the 797 respondents
| Gender, n (%) | |
| Male | 327 (41) |
| Female | 470 (59) |
| Years in medical practice, n (%) | |
| <20 | 352 (44.2) |
| 21–40 | 371 (46.5) |
| >40 | 74 (9.3) |
| Specialisationa, n (%) | |
| Endocrinology | 734 (92.1) |
| Internal medicine | 130 (16.3) |
| Others | 90 (11.3) |
| Place of employmenta, n (%) | |
| Hospital | 385 (48.3) |
| Private | 287 (36) |
| District clinic | 163 (20.4) |
| University | 103 (12.9) |
The sum of percentages exceeds 100% because some respondents had >1 specialty and were employed in more than 1 place.
Treating Patients with Thyroid Hormones
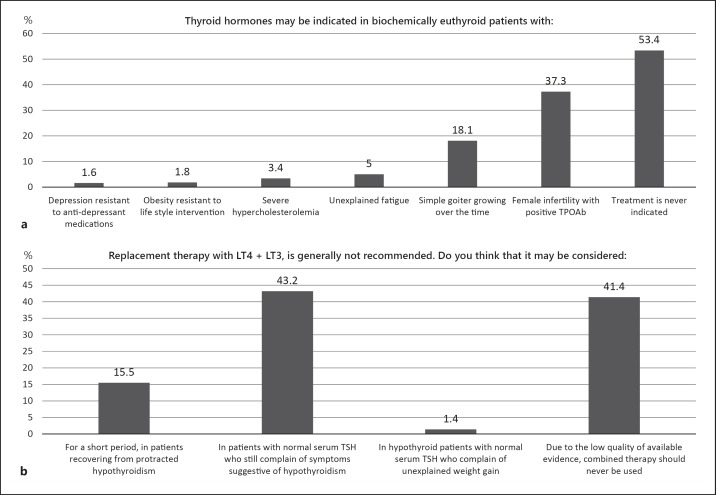
The questions shown in Figure 1a sought to explore the circumstances under which endocrinologists would consider therapy with thyroid hormones in patients without hypothyroidism. Just over half of the respondents (53.4%) answered that treatment with thyroid hormones is never indicated, but more than one-third (37.3%) would consider treatment with thyroid hormones in infertile females with positive thyroid antibodies, and nearly 20% would treat euthyroid patients with simple goitre that grew over time (never indicated versus. others; p = NS). Unrelated conditions (depression resistant to anti-depressant medications, obesity resistant to lifestyle intervention, severe hypercholesterolemia and unexplained fatigue) were rarely considered as indications for treatment. Nearly all respondents (98.7%) answered that the treatment of choice for hypothyroidism is LT4; very few would prescribe DTE, whereas T4 + T3 combination therapy was reserved for specific indications (LT4 vs. DTE + T4 + T3; p < 0.01). Ninety-nine per cent of physicians appear to have a major influence on the thyroid management of their patients, since in 48.3% of cases their patients were dispensed the type of LT4 recommended, and in 50.7% of cases they had control over the type of LT4 prescribed (patients dispensed the type of LT4 recommended + control over the type of LT4 prescribed versus no control + controlled by general practitioners; p < 0.01).
Fig. 1.
Use of LT4 in euthyroid subjects (a). Use of LT3 + LT4 in different conditions (b). TPOAb, thyroperoxidase antibodies; LT4, levothyroxine.
Using Different LT4 Formulations
In Italy, the treatment of choice for hypothyroidism remains LT4. Interestingly, in specific situations, Italian endocrinologists prefer LT4 as a liquid solution or as soft-gel capsules over other formulations in order to achieve satisfactory control of hypothyroidism (Table 2). These alternative formulations are preferred to tablets by the vast majority not only in the presence of interfering drugs (liquid solution + soft-gel capsules versus tablets and/or “no major changes expected”; p < 0.01) but also in the presence of celiac disease, malabsorption, lactose intolerance, or intolerance to excipients (liquid solution + soft-gel capsules versus tablets and/or “no major changes expected”; p < 0.01) and when the patient is unable to take LT4 in the fasting state and separate it from food/drink (liquid solution + soft-gel capsules versus tablets and/or “no major changes expected”; p < 0.01). Liquid solution or soft-gel capsules were also preferred by 3/4 of respondents, for patients on generic LT4 who have unexplained poor biochemical control of hypothyroidism, while 22.5% would suggest branded tablets (liquid solution + soft-gel capsules versus branded tablets; p < 0.01). Finally, in patients treated with generic LT4 tablets who despite good biochemical control of hypothyroidism had persistent symptoms, nearly half (42.9%) would choose liquid solution or soft-gel capsules, although half of physicians (50.3%) expected no major changes with the different formulations (liquid solution + soft-gel capsules versus “no major changes expected”; p = NS).
Table 2.
LT4 formulations preferred by respondents in different clinical scenarios
| Tablets, n (%) | Soft-gel capsules, n (%) | Liquid solution, n (%) | Branded tablets, n (%) | “I expect no major changes with the different formulations”, n (%) | |
|---|---|---|---|---|---|
| Interfering drugs may influence the stability of therapy. Which LT4 preparation is in your experience less likely to be subject to variable absorption? | 34 (4.3) | 255 (32) | 397 (49.8) | 0 (0) | 111 (13.9) |
| Which of the following preparations of LT4 would you prescribe in case of a first diagnosis of hypothyroidism, when the patient self-reports intolerance to various foods raising the possibility of celiac disease, malabsorption, lactose intolerance or intolerance to excipients? | 9 (1.1) | 246 (30.9) | 524 (65.7) | 0 (0) | 18 (2.3) |
| Which of the following preparations of LT4 would you prescribe for a patient established on generic LT4 who has unexplained poor biochemical control of hypothyroidism? | 0 (0) | 229 (29.7) | 364 (45.7) | 179 (22.5) | 25 (3.1) |
| Which of the following preparations of LT4 would you prescribe for a patient with poor biochemical control who is unable (due to busy lifestyle) to take LT4 fasting and separate from food/drink? | 3 (0.4) | 255 (32) | 533 (66.9) | 0 (0) | 6 (0.7) |
| Which of the following preparations of LT4 would you prescribe for a patient established on generic T4 who has good biochemical control of hypothyroidism but continues to have symptoms? | 0 (0) | 154 (19.3) | 188 (23.6) | 54 (6.8) | 401 (50.3) |
LT4, levothyroxine.
Monitoring Thyroid Hormone Treatment
After starting LT4 replacement treatment for hypothyroidism, or switching from one formulation to another, or from a branded to a generic product, about 50% would recheck TSH in 8 weeks and about 40% in 4–6 weeks (8 vs. 4–6 weeks; p = NS).
Combination Treatment with LT4 + LT3
LT4 + LT3 combination treatment was considered by about 40% when symptoms persisted notwithstanding normal TSH concentration, although the same percentage stated that available evidence does not support combination treatment (LT3 + LT4 versus “no evidence”; p = NS); other indications were scarcely considered for combination treatment (p = NS; Fig. 1b).
Discussion
Clinical Indications for Treatment with Thyroid Hormones
This survey confirms that in Italy LT4 is the treatment of choice for hypothyroidism. Furthermore, depression, obesity, unexplained fatigue and hypercholesterolemia are not regarded as indications for thyroid hormone treatment. Such practice is in accordance with the available evidence [19, 20, 21].
In contrast with current guidelines, a significant minority (more than a third) of endocrinologists would consider LT4 treatment in euthyroid female patients with infertility associated with chronic autoimmune thyroiditis. Infertility has been associated with positive thyroperoxidase antibodies, especially in women with ovulatory dysfunction, but a large prospective study and a recently published randomized clinical trial refuted this association and any benefit of LT4 treatment [22, 23].
About a fifth of endocrinologists would treat simple goitre growing over the time with LT4 in euthyroid patients. Robust evidence demonstrates that clinically significant size reduction is rarely obtained with LT4 treatment, that any favourable effect ceases after the withdrawal of the suppressive treatment and that protracted subclinical hyperthyroidism is potentially associated with adverse effects [24]. So, most patients with simple goitre do not benefit from this therapy and guidelines discourage such use [25]. Randomized clinical trials showed that goitre size is at best modestly reduced by LT4, whereas a greater effect may be obtained by triiodothyroacetic acid or radioactive iodine, the latter being boosted in its effect by recombinant human thyrotropin [26, 27]. Overall, the responses of Italian endocrinologists about indications for treatment with thyroid hormones were satisfactory but deviations from evidence-based practice were noted and need to be addressed.
Choice of LT4 Formulation
This issue is of interest because in Italy (unlike many other European countries), different LT4 formulations (tablet, soft-gel capsules, liquid solution) have been commercially available. In patients without malabsorption or concomitant drug intake that may interfere with absorption, the use of LT4 liquid solution or soft-gel capsules was reported to achieve improved control of TSH and better quality of life [28, 29]. The liquid solution was found effective also when taken at the same time as breakfast or simultaneously with interfering drugs like calcium, iron and proton-pump inhibitors [30, 31]. The liquid solution also led to a satisfactory control of hypothyroidism when used in enteral feeding tubes and in patients who had bariatric surgery [32, 33]. These results are consistent with the faster absorption of liquid solutions and, probably to a lesser extent of soft-gel capsules, compared to tablets [34]. On the basis of these data, even if high-quality evidence is limited, liquid solutions or soft-gel capsules are also recommended by 3/4 of Italian endocrinologists for a patient established on generic LT4 who has unexplained poor biochemical control of hypothyroidism. However, the inclination to suggest liquid solutions or soft-gel capsules declined to about 50% when symptoms persist despite adequate biochemical control on generic LT4 tablets.
In our sample of endocrinologists, liquid solution or soft-gel capsules are largely reserved for specific conditions (interfering drugs, actual or suspected malabsorption, inability to take LT4 in the fasting state, unexplained poor biochemical control of hypothyroidism) and studies suggest that these formulations might also guarantee a better biochemical control of hypothyroidism; on the other hand, they are more expensive. In this view, a cost-effective analysis based on real-world data as well as studies aiming at optimizing patients' adherence would be of interest.
Once LT4 (generic or branded) has been prescribed, the endocrine societies suggest monitoring thyroid function maintaining the same product and avoiding switching from one to another [35]. Since 2007 the Food and Drug Administration has required that LT4 preparations maintain 95–105% of their stated potency and that when a generic or branded LT4 preparation meets these criteria the LT4 preparations can be substituted, one for another by the pharmacy [36, 37]. The American Thyroid Association recommends that patients should remain on a given LT4 product for as long as possible, and if a change in the product is made, then thyroid function should be rechecked [38]. That is of particular importance in specific categories of subjects like thyroid cancer and congenital hypothyroidism patients, in whom a narrow therapeutic range is desired [39, 40]. Overall, adequate follow-up has been demonstrated by 90% of Italian endocrinologists, who after starting LT4 replacement treatment, or switching from one formulation to another, or from a branded to a generic product, would recheck TSH within 8 weeks.
Combination Treatment with LT3 + LT4
Combination treatment with LT3 + LT4 is discouraged by both American and European Guidelines, based on lack of indisputable benefits when compared to not only LT4 therapy alone but also paucity of long-term outcome data [15]. LT4 + LT3 combination therapy might be considered as an experimental approach in compliant LT4-treated hypothyroid patients who have persistent complaints despite serum TSH values within the reference range, provided other autoimmune diseases or comorbidities have been excluded [15]. Nearly all respondents considered LT4 the treatment of choice for hypothyroidism, while very few would prescribe LT4 + LT3 combination therapy. Notably, a trial of combination therapy was considered by about 40% of respondents in the presence of persistent symptoms suggestive of hypothyroidism notwithstanding TSH within the normal range, whereas the same percentage would not expect any improvement from this therapy. Regarding the question to treat patients with persistent symptoms with T4/T3 combination 43% was for and 41% against, meaning that this issue is still controversial and more evidence is needed on which subgroup an effect could be expected and also studies on DTE compared to T4/T3 combination therapy are needed.
Strengths of the present survey that render the obtained results reliable are (1) the high number of participants; (2) similar characteristics between respondents and the entire cohort of AME members; and (3) the responses came from clinical endocrinologists who routinely manage a large number of hypothyroid patients.
Limitations mainly relate to the virtual patient situation and the fact that the patients' views cannot be incorporated in the decision process. Whether a 39% response rate is representative can always be questioned, and whether these data can be extrapolated to other countries where the available spectrum of thyroid hormone replacement modalities may be different.
It is concluded that among Italian endocrinologists, the preferred treatment for hypothyroidism is LT4. Around 40% would, under certain conditions, consider LT3 + LT4 combination treatment but only for patients with a diagnosis of hypothyroidism who are biochemically euthyroid and complain of persistent symptoms. In a biochemically euthyroid patient, the only scenario when LT4 was considered by a significant number of physicians was female infertility with positive thyroperoxidase antibodies. Alternative LT4 formulations, like liquid solution or soft-gel capsules, were recommended for patients with suspected or proven malabsorption, use of interfering drugs, lifestyle issues and unexplained poor biochemical control of hypothyroidism.
Disclosure Statement
R.N. and R.A. have nothing to disclose. E.V.N., E.P., P.P., and L.H. have undertaken consultancy work for IBSA. IBSA had no role in the design of the survey, data analysis, data presentation, data interpretation or writing the manuscript; the authors did not receive remuneration by IBSA.
Author Contributions
R.N. was responsible for drafting the manuscript and data evaluation; R.A. was responsible for creating the questionnaire and data evaluation; E.V.N., E.P., and P.P. was responsible for drafting the manuscript; L.H. was responsible for creating the questionnaire and drafting the manuscript.
Supplementary Material
Supplementary data
References
- 1.Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014 Jan;174((1)):32–9. doi: 10.1001/jamainternmed.2013.11312. [DOI] [PubMed] [Google Scholar]
- 2.Fallahi P, Ferrari SM, Ruffilli I, Ragusa F, Biricotti M, Materazzi G, et al. Advancements in the treatment of hypothyroidism with L-T4 liquid formulation or soft gel capsule: an update. Expert Opin Drug Deliv. 2017 May;14((5)):647–55. doi: 10.1080/17425247.2016.1227782. [DOI] [PubMed] [Google Scholar]
- 3.Carswell JM, Gordon JH, Popovsky E, Hale A, Brown RS. Generic and brand-name L-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J Clin Endocrinol Metab. 2013 Feb;98((2)):610–7. doi: 10.1210/jc.2012-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018 Sep;21((9)):912–9. doi: 10.1080/13696998.2018.1484749. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara R, Ientile V, Arcoraci V, Ferrajolo C, Piccinni C, Fontana A, et al. Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population-based study during the years 2009-2015. Endocrine. 2017 Oct;58((1)):143–52. doi: 10.1007/s12020-017-1242-4. [DOI] [PubMed] [Google Scholar]
- 6.Khandelwal N, Johns B, Hepp Z, Castelli-Haley J. The economic impact of switching from Synthroid for the treatment of hypothyroidism. J Med Econ. 2018 May;21((5)):518–24. doi: 10.1080/13696998.2018.1443110. [DOI] [PubMed] [Google Scholar]
- 7.Ernst FR, Sandulli W, Elmor R, Welstead J, Sterman AB, Lavan M. Retrospective Study of Patients Switched from Tablet Formulations to a Gel Cap Formulation of Levothyroxine: Results of the CONTROL Switch Study. Drugs R D. 2017 Mar;17((1)):103–15. doi: 10.1007/s40268-016-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faasse K, Cundy T, Gamble G, Petrie KJ. The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosom Med. 2013 Jan;75((1)):90–6. doi: 10.1097/PSY.0b013e3182738826. [DOI] [PubMed] [Google Scholar]
- 9.Faasse K, Cundy T, Petrie KJ. Medicine and the Media. Thyroxine: anatomy of a health scare. BMJ. 2009 Dec;339(dec29 1):b5613. doi: 10.1136/bmj.b5613. [DOI] [PubMed] [Google Scholar]
- 10.Razvi S, Korevaar TI, Taylor P. Trends, Determinants, and Associations of Treated Hypothyroidism in the United Kingdom, 2005-2014. Thyroid. 2019 Feb;29((2)):174–82. doi: 10.1089/thy.2018.0251. [DOI] [PubMed] [Google Scholar]
- 11.Michaelsson LF, Medici BB, la Cour JL, Selmer C, Røder M, Perrild H, et al. Treating Hypothyroidism with Thyroxine/Triiodothyronine Combination Therapy in Denmark: Following Guidelines or Following Trends? Eur Thyroid J. 2015 Sep;4((3)):174–80. doi: 10.1159/000437262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, et al. An Online Survey of Hypothyroid Patients Demonstrates Prominent Dissatisfaction. Thyroid. 2018 Jun;28((6)):707–21. doi: 10.1089/thy.2017.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burch HB, Burman KD, Cooper DS, Hennessey JV. A 2013 survey of clinical practice patterns in the management of primary hypothyroidism. J Clin Endocrinol Metab. 2014 Jun;99((6)):2077–85. doi: 10.1210/jc.2014-1046. [DOI] [PubMed] [Google Scholar]
- 14.Jonklaas J, Tefera E, Shara N. Physician Choice of Hypothyroidism Therapy: Influence of Patient Characteristics. Thyroid. 2018 Nov;28((11)):1416–24. doi: 10.1089/thy.2018.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J. 2012 Jul;1((2)):55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Over- and Under-Treatment of Hypothyroidism Is Associated with Excess Mortality: A Register-Based Cohort Study. Thyroid. 2018 May;28((5)):566–74. doi: 10.1089/thy.2017.0517. [DOI] [PubMed] [Google Scholar]
- 17.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur J Endocrinol. 2019 Jun;180((6)):407–16. doi: 10.1530/EJE-19-0006. [DOI] [PubMed] [Google Scholar]
- 18.Mazziotti G, Formenti AM, Frara S, Olivetti R, Banfi G, Memo M, et al. High Prevalence of Radiological Vertebral Fractures in Women on Thyroid-Stimulating Hormone-Suppressive Therapy for Thyroid Carcinoma. J Clin Endocrinol Metab. 2018 Mar;103((3)):956–64. doi: 10.1210/jc.2017-01986. [DOI] [PubMed] [Google Scholar]
- 19.Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid. 2014 May;24((5)):802–8. doi: 10.1089/thy.2013.0555. [DOI] [PubMed] [Google Scholar]
- 20.Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. Effects of Altering Levothyroxine (L-T4) Doses on Quality of Life, Mood, and Cognition in L-T4 Treated Subjects. J Clin Endocrinol Metab. 2018 May;103((5)):1997–2008. doi: 10.1210/jc.2017-02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SY, Park SE, Jung SW, Jin HS, Park IB, Ahn SV, et al. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin Endocrinol (Oxf) 2017 Jul;87((1)):87–96. doi: 10.1111/cen.13345. [DOI] [PubMed] [Google Scholar]
- 22.Plowden TC, Schisterman EF, Sjaarda LA, Zarek SM, Perkins NJ, Silver R, et al. Subclinical Hypothyroidism and Thyroid Autoimmunity Are Not Associated With Fecundity, Pregnancy Loss, or Live Birth. J Clin Endocrinol Metab. 2016 Jun;101((6)):2358–65. doi: 10.1210/jc.2016-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception. N Engl J Med. 2019 Apr;380((14)):1316–25. doi: 10.1056/NEJMoa1812537. [DOI] [PubMed] [Google Scholar]
- 24.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, et al. Thyroid Studies Collaboration Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012 May;172((10)):799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. AACE/ACE/AME Task Force on Thyroid Nodules AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ASSOCIAZIONE MEDICI ENDOCRINOLOGI MEDICAL GUIDELINES FOR CLINICAL PRACTICE FOR THE DIAGNOSIS AND MANAGEMENT OF THYROID NODULES—2016 UPDATE. Endocr Pract. 2016 May;22((5 Supplement 1)):622–39. doi: 10.4158/EP161208.GL. [DOI] [PubMed] [Google Scholar]
- 26.Brenta G, Schnitman M, Fretes O, Facco E, Gurfinkel M, Damilano S, et al. Comparative efficacy and side effects of the treatment of euthyroid goiter with levo-thyroxine or triiodothyroacetic acid. J Clin Endocrinol Metab. 2003 Nov;88((11)):5287–92. doi: 10.1210/jc.2003-030095. [DOI] [PubMed] [Google Scholar]
- 27.Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012 Dec;33((6)):920–80. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 28.Guglielmi R, Grimaldi F, Negro R, Frasoldati A, Misischi I, Graziano F, et al. Shift from Levothyroxine Tablets to Liquid Formulation at Breakfast Improves Quality of Life of Hypothyroid Patients. Endocr Metab Immune Disord Drug Targets. 2018;18((3)):235–40. doi: 10.2174/1871530318666180125155348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cappelli C, Pirola I, Daffini L, Formenti A, Iacobello C, Cristiano A, et al. A Double-Blind Placebo-Controlled Trial of Liquid Thyroxine Ingested at Breakfast: results of the TICO Study. Thyroid. 2016 Feb;26((2)):197–202. doi: 10.1089/thy.2015.0422. [DOI] [PubMed] [Google Scholar]
- 30.Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metab. 2014 Dec;99((12)):4481–6. doi: 10.1210/jc.2014-2684. [DOI] [PubMed] [Google Scholar]
- 31.Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Deliv. 2017 Apr;14((4)):467–72. doi: 10.1080/17425247.2017.1290604. [DOI] [PubMed] [Google Scholar]
- 32.Pirola I, Daffini L, Gandossi E, Lombardi D, Formenti A, Castellano M, et al. Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J Endocrinol Invest. 2014 Jun;37((6)):583–7. doi: 10.1007/s40618-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 33.Fallahi P, Ferrari SM, Camastra S, Politti U, Ruffilli I, Vita R, et al. TSH Normalization in Bariatric Surgery Patients After the Switch from L-Thyroxine in Tablet to an Oral Liquid Formulation. Obes Surg. 2017 Jan;27((1)):78–82. doi: 10.1007/s11695-016-2247-4. [DOI] [PubMed] [Google Scholar]
- 34.Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung. 2012 Dec;62((12)):631–6. doi: 10.1055/s-0032-1329951. [DOI] [PubMed] [Google Scholar]
- 35.American Association of Clinical Endocrinologists the Endocrine Society, and the American Thyroid Association Joint Position Statement on the Use and Interchangeability of Thyroxine Products, 2004 www.thyroid.org/thyroxine-products-joint-position-statement. [Google Scholar]
- 36.U.S. Food and Drug Administration FDA acts to ensure thyroid drugs don't lose potency before expiration date. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161259.htm.
- 37.U.S. Food and Drug Administration Questions and answers on levothyroxine sodium products. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161266.htm.
- 38.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. American Thyroid Association Task Force on Thyroid Hormone Replacement Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24((12)):1670–751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE. Congenital Hypothyroidism Consensus Conference Group European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014 Feb;99((2)):363–84. doi: 10.1210/jc.2013-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26((1)):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data