Abstract
Background
The levothyroxine absorption test for evaluation of pseudomalabsorption in patients with primary hypothyroid is not standardised. An individual in whom a workup for malabsorption is warranted remains undefined.
Methods
Twenty-five euthyroid, 25 newly diagnosed hypothyroid, 25 treated hypothyroid with normalised TSH, and 25 hypothyroid subjects with elevated TSH despite adequate dose of levothyroxine for more than 6 months, and 10 euthyroid subjects with true malabsorption were administered levothyroxine (10 μg/kg or maximum 600 μg) to study its absorption profile by measuring free T4 level at hourly intervals for 5 h. Results: Free T4 peaked at 3 h with marginal insignificant decline at 4 h in all groups. The increments of free T4 (between baseline and 3 h) of the four groups (except malabsorption) were not statistically different. The mean increment of free T4 in true malabsorption was 0.39 ng/dL (95% CI: 0.29–0.52) and it was 0.78 ng/dL (95% CI: 0.73–0.85) (10.4 pmol/L) for other groups combined together. The cut off of free T4 increment at 3 h from baseline above 0.40 ng/dL had a sensitivity of 97% and specificity of 80% (AUC 0.904, p < 0.001) to exclude true malabsorption.
Conclusion
Subjects with elevated TSH on adequate dose of LT4 can be reliably diagnosed to be non-adherent to treatment with levothyroxine absorption test. The incremental value above 0.40 ng/dL (5.14 pmol/L) at 3 h may be useful to identify individuals where workup of malabsorption is unwarranted.
Keywords: Pseudomalabsorption, Levothyroxine absorption test, Primary hypothyroidism, Compliance
Introduction
Hypothyroid patients with minimal endogenous thyroid function usually require LT4 doses of 1.6–1.8 µg/kg of body weight [1]. The primary site of absorption is small intestine, especially in duodenum and jejunum [2]. However, absorption pattern is different in euthyroid subjects as compared to hypothyroid subjects. Benvenga et al.[3] reported that absorption peak of levothyroxine was more than 70%, which occurs at around 2 h in euthyroid subjects, 3 h in primary hypothyroidism, and free T4 (FT4) rises linearly in first 60–90 min before reaching a plateau. Approximately 62–82% of orally administered levothyroxine is absorbed, and it occurs within the first 3 h of intake after an overnight fast [4].
However in clinical practice, it is not infrequently seen that clinical and biochemical hypothyroidism persists even after an adequately prescribed doses of LT4 [5]. The most common explanation for this elevated TSH is non-adherence/noncompliance, despite repeated counselling [5]. Even after ruling out of important organic causes, e.g. concomitant medications (e.g., ferrous sulphate, calcium carbonate, antacid), intrinsic gastrointestinal disorders [6] (e.g., celiac disease, atrophic gastritis, tropical sprue, resection of small intestine, diabetic diarrhoea), pharmacokinetics/pharmacodynamics modifying drugs (e.g., rifampicin, carbamazepine, phenytoin, amiodarone), anti-T4 antibodies [7], this phenomenon persists [1]. This is termed as pseudomalabsorption of levothyroxine [8]. Altered gut microbiota is also known to modify enterohepatic cycling of iodothyronine by decreasing the absorption of iodothyronine secreted through bile and thereby may affect pharmacological homeostasis of administered LT4 [9].
If non-adherence is suspected, but not volunteered, the options to confirm the possibility are limited. Weekly supervised dose of levothyroxine may be a useful option, but seldom practically useful. Switching over to liquid preparations of LT4 could be useful, though it is not easily available [10]. Assessment of absorption of levothyroxine is necessary before undertaking an extensive evaluation for malabsorption syndromes. Only few case reports/series have described relatively good absorption of LT4 on supervised high dose, suggesting that evaluation for malabsorption is unnecessary [11, 12, 13, 14, 15].
Several protocols of the levothyroxine absorption test (LAT) have been described in the literature [16]. However, there are no well-designed clinical studies of absorption of levothyroxine in different subgroups. We undertook this study to determine the absorption profile of levothyroxine after an oral administration of high dose in pseudomalabsorption subjects and compared this with euthyroid, newly diagnosed hypothyroid and treated hypothyroid subjects. We also looked at the absorption profile of levothyroxine in subjects with documented malabsorption and attempted to find out a cutoff or an increment in FT4 on the LAT that could reliably identify patients who are noncompliant and do not warrant further evaluation for true malabsorption.
Materials and Methods
In this cross-sectional study, five subgroups of subjects aged between 18 and 60 years were recruited. Relatives or spouses of subjects were tested for thyroid function, and consecutive 25 subjects (male [M] 5, female [F] 20) having normal thyroid function test were included as “euthyroid” group (group A).
Consecutive 25 subjects (M 8, F 17) with newly diagnosed hypothyroid (TSH >10 mU/L with FT4 below normal reference range) were included as “newly diagnosed hypothyroid group” (group B).
The third group consisted of consecutive 25 follow-up subjects with hypothyroidism (M 5, F 20), whose TSH were within normal limits, termed as “treated hypothyroid group” (group C).
Twenty-five subjects (M 7, F 18) with documented hypothyroidism for several months or years, who despite an adequate daily dose of LT4 (>2.5 µg/kg) for more than 6 months, still had an elevated TSH (>10 mU/L) were taken as “pseudomalabsorption” group (group D). All were assessed, and none of them were on drugs known to interfere with levothyroxine absorption/clearance. All were negative for celiac antibody, and persons with known gastritis or malabsorptive pathology were excluded.
Ten subjects (M 4, F 6) with malabsorption with documented abnormality in D-Xylose but with normal thyroid function were included as “true malabsorption group” (group E). They were negative for H. pylori infection and celiac antibody. They are not known to have a problem of levothyroxine absorption but have generalised malabsorption. Six subjects were suffering from malabsorption related to Crohn's disease, 1 had tropical sprue, and 3 had idiopathic malabsorption. All persons were normotensive and had no exertional/effort intolerance and normal baseline ECG.
Protocol
We used the short absorption test over 5 h and measured FT4 as it changes quickly and helps predict absorption of an orally administered dose of levothyroxine. Patients were advised to come in overnight fasting state and were not allowed to eat anything during the test. Those already taking LT4 (group C and D) were advised not to take the medicine on the day of testing. Basal samples were taken between 8 and 9 am for TSH and FT4. A supervised dose of LT4 (10 µg/kg or 600 µg, whichever was lower) of the same brand (Thyronorm, Abbott Pharmaceuticals) was given and observed until swallowing. Blood was collected hourly for 5 h for FT4 measurement. Serum samples were immediately stored at −20°C for subsequent analysis.
Hormone Assays
Serum TSH and FT4 were estimated by chemiluminescence technique using commercially available kits from Siemens Diagnostics (Germany) with Immulite-1000 analyzer. Analytical sensitivity and total precision (as given by providers) for TSH were 0.01 µIU/mL and 2.2%, respectively, and for FT4 were 0.35 ng/dL and 2.7%, respectively. Laboratory reference range for TSH was 0.4–4 mU/L, for FT4 was 0.8–1.9 ng/dL, and inter-assay CV were 8.9 and 5.5%, respectively (as determined locally).
We also calculated qualitative percentage of levothyroxine absorbed. In the indirect non-isotope method, as used by Sun et al.[17], volume of distribution (Vd) is incorporated to calculate the percent LT4 absorption as LT4 absorbed (%) = (peak Δtotal T4 [μg/dL] × Vd [dL]/administered dose of LT4 [μg]) × 100. Vd is calculated by the formula (Vd = 0.442 × body mass index). In this study, a very strong correlation was found between increment in total T4 and FT4 (r = 0.88, p< 0.001). Hence we used the same formula by using peak delta FT4.
Statistical Package for the Social Sciences (SPSS version 21.0; SPSS Inc.) was used for data processing and analysis. FT4 was presented as mean ± standard deviation. Comparison of FT4 at different hours in same group was done by Student's paired t test. Intergroup comparison of increment in FT4 was done by one-way ANOVA. The cutoff for FT4 increment was calculated by the ROC curve.
Results
The baseline characteristics of the subjects are presented in Table 1. The peak FT4 occurred at 3 h in all groups. It was not significantly different from FT4 at 4 h in any group and further declined at 5 h in all. Hence, we used the FT4 at 3 h to indicate the peak value (Cmax) for FT4.
Table 1.
Baseline characteristics of subjects who had undergone levothyroxine absorption test
| Characteristics | Group A (euthyroid) (n = 25) | Group B (newly diagnosed hypothyroid) (n = 25) | Group C (treated hypothyroid) (n = 25) | Group D (pseudomalabsorption) (n = 25) | Group E (true malabsorption) (n = 10) |
|---|---|---|---|---|---|
| Age, mean ± SD, years | 32±14.3 | 34.6±13.1 | 34.5±12.7 | 31.6±14.1 | 31.3±9.3 |
| Male/female | 5/20 | 8/17 | 5/20 | 7/18 | 4/6 |
| BMI, mean ± SD, kg/m2 | 23.13±4.8 | 22.94±5.5 | 25.41±4.7 | 22.95±6.3 | 18.13±1.3 |
| Goiter | 6 (23%) | 11 (42.3%) | 5 (17.8%) | 15 (44.1%) | 3 (30%) |
| Family history of thyroid disease | 4 (l5.3%) | 5 (19.2%) | 4 (14.2%) | 10 (29.4%) | 3 (30%) |
| Baseline TSH, mean ± SD, mIU/L | 2.49±1.41 | 48.44±25.99 | 1.57±1.31 | 26.18±9.94 | 2.98±1.11 |
| Baseline TSH, IQR, mIU/L | 1.38–3.63 | 14.42–68.23 | 0.68–3.81 | 11.63–39.81 | 2.11–3.71 |
| Hypothyroidism duration, mean ± SD, years | NA | NA | 7.9±7.4 | 7.4±6.7 | NA |
Table 2 shows the concentration of FT4 (ng/dL) in different subgroups from baseline up to 5 h after a loading dose of LT4 10 µg/kg (maximum 600 µg) and increment in FT4 level from baseline to 3 h.
Table 2.
Concentration of FT4 (ng/dL) in different subgroups from baseline up to 5 h after loading dose of LT4 10 µg/kg (max 600 µg) and increment in free T4 level from baseline to 3 h
| Categories | 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | p value 3 h vs. 4 h | p value 0 h vs. 3 h | ∆FT4 0 h to 3 h | p value: ∆T4 between first 4 groups |
|---|---|---|---|---|---|---|---|---|---|---|
| Group A (euthyroid) (n = 25) | 1.07±0.15 | 1.45±0.29 | 1.73±0.35 | 1.89±0.23 | 1.79±0.76 | 1.68±0.33 | NS | <0.001 | 0.78±0.16 | NS* |
| Group B (newly diagnosed hypothyroid) (n = 25) | 0.83±0.25 | 1.07±0.36 | 1.40±0.46 | 1.56±0.41 | 1.48±0.39 | 1.41±0.46 | NS | <0.001 | 0.76±0.33 | |
| Group C (treated hypothyroid) (n = 25) | 1.23±0.24 | 1.69±0.23 | 1.95±0.41 | 2.07±0.35 | 2.00±0.38 | 1.90±0.47 | NS | <0.001 | 0.78±0.28 | |
| Group D (pseudo-malabsorption) (n = 25) | 1.19±0.23 | 1.55±0.43 | 1.80±0.58 | 1.89±0.39 | 1.85±0.41 | 1.84±0.33 | NS | <0.001 | 0.81±0.27 | |
| Group E (true malabsorption (n = 10) | 1.26±0.26 | 1.53±0.47 | 1.66±0.53 | 1.66±0.39 | 1.63±0.43 | 1.60±0.41 | NS | <0.001 | 0.39±0.17 |
p value for ∆T4 between first 4 groups as a whole vs. group E is <0.001.
The mean increment in FT4 from baseline to 3 h (ΔFT4 0–3 h) in different groups except true malabsorption was similar and was 0.78 ± 0.16 ng/dL (euthyroid), 0.76 ± 0.33 ng/dL (newly diagnosed hypothyroid), 0.78 ± 0.28 ng/dL (treated hypothyroid), 0.81 ± 0.27 ng/dL (pseudomalabsorption). With true malabsorption, it was 0.39 ± 0.17 ng/dL (95% CI: 0.29–0.52). Combining all groups except true malabsorption, the mean increment in FT4 level during same period was 0.78 ± 0.16 ng/dL (95% CI: 0.73–0.85). There was no significant difference between different groups (except true malabsorption) in the increment of FT4 (p = 0.91). However, there was significant difference when all four groups as a whole (except true malabsorption) were compared with the true malabsorption group (p < 0.001).
The qualitative percentage of T4 absorbed at 3 h in different groups was 150 ± 37% (euthyroid), 141 ± 40% (newly diagnosed hypothyroid), 162 ± 71% (treated hypothyroid), 160 ± 58% (pseudomalabsorption). In true malabsorption, it was 76 ± 28%. There was no significant difference between different groups (except true malabsorption) (p = 0.506).
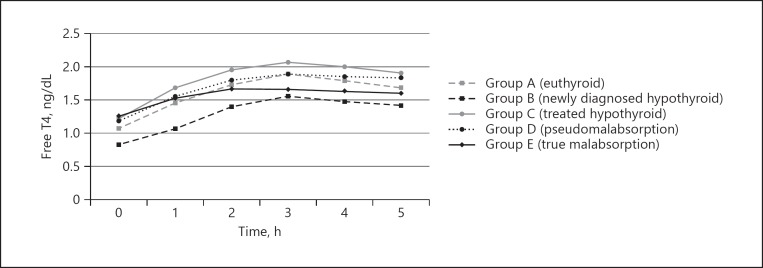
Figure 1 shows the graphical presentation of FT4 levels (LAT curve) in euthyroid, newly diagnosed hypothyroid, treated hypothyroid, pseudomalabsorption, and true malabsorption after the loading dose of levothyroxine. We also calculated a cutoff to exclude true malabsorption based on the increment of FT4 at 3 h from baseline in LAT by the ROC curve. In this model, an increment of FT4 at 3 h of >0.40 ng/dL (5.14 pmol/L) had a sensitivity of 97% and specificity of 80% to rule out true malabsorption (AUC 0.904, p< 0.001) (online suppl. Figure; see www.karger.com/doi/10.1159/000504218 for all online suppl. material).
Fig. 1.
Levothyroxine absorption test curve. Free T4 levels in euthyroid, newly diagnosed hypothyroid, treated hypothyroid, pseudomalabsorption, and true malabsorption subjects after levothyroxine loading dose.
Discussion
Levothyroxine absorption is described in the literature in a small number of subjects with different doses and different protocols. Several small studies reported good absorption of levothyroxine in such patients with a bolus dose of 1,000 μg, but there was no suggestion for any cutoff for LT4 increment to rule out pseudomalabsorption [12, 13, 15]. Though TSH was measured in a study as a marker of levothyroxine absorption [18], we did not measure TSH hourly, as it takes 4–6 weeks to get normalized.
An increment of at least of 2.5 times the baseline FT4 to suggest pseudomalabsorption in the LT4 absorption test was also reported [14]. However, this may be inappropriate as it is dependent on a pre-test FT4 level. Subjects in the pseudomalabsorption group are heterogeneous and may vary from grossly nonadherent (hence biochemically and possibly clinically presenting like treatment-naïve hypothyroid) to infrequently nonadherent subjects (hence presenting as biochemically mildly hypothyroid). Moreover, some patients may actually take LT4 for some days before the scheduled day of test; this may raise the baseline FT4 to yield a fallacious result. Hence, we used absolute increment in FT4 levels instead of multiple of increment from baseline values. It may be noted that in our study, the mean FT4 in the hypothyroid group was 0.83 ng/dL, which is higher than that expected in subjects with overt hypothyroidism. When we recruited hypothyroid subjects for the study, we did so on the basis of baseline thyroid function test results that they presented with. We tried to perform the LAT as early as possible after recruitment. However, few patients had already inadvertently started levothyroxine for few days before the LAT, and this resulted in a higher than expected baseline FT4 in the hypothyroid group.
A mean increment in FT4 in pseudomalabsorption of 11.6 pmol/L (0.9 ng/dL) with a 54% rise in FT4 at 2 h is also reported [19]. However, this study does not suggest any cutoff value below which investigation for malabsorption is warranted. This is true for other studies as none of them included true malabsorption subjects. Sun et al.[17] also reported that mean FT4 increased from a baseline of 0.61 ng/dL to a peak value of 1.8 ng/dL. The primary importance of their study reflects that there was a strong correlation between maximum changes in FT4 versus the maximum change in total T4. They have suggested that FT4 may be used interchangeably with total T4 as a qualitative assessment of suspected malabsorption. We also followed this principle and measured FT4 for absorption of levothyroxine.
Most of these studies used 1,000 μg of LT4 with minimally reported side effects. This could be due to exclusion of susceptible individuals in whom angina and cardiac arrhythmias might have been precipitated. However, these studies have not included sufficiently large numbers of patients, and therefore the safety of the high dose of levothyroxine administered cannot be guaranteed. Also, this dose may not be necessary at all. In the dose equivalence study of levothyroxine as evaluated by Food and Drug Administration (FDA), LT4 dose used was 600 µg [1]. Hence, in our study, the highest dose of LT4 used was 600 μg, and patients were followed up for 4 weeks with no issues of tolerability.
The mean increment in FT4 level from baseline to 3 h in the different groups except true malabsorption was similar in our study, and it was 0.78 ± 0.16 ng/dL. However, in true malabsorption, the increment was 0.39 ± 0.17 ng/dL. We did not use the ratio of 3 h FT4 to baseline FT4 as an indicator of efficiency of absorption as it is likely to be confounded by baseline FT4, particularly in long-term hypothyroid in whom the ratio may actually appear large due to persistently low FT4, thereby yielding false positive results. Also, this may yield a false negative result in the pseudomalabsorption group if they inadvertently/deliberately consume levothyroxine for some days before the LAT test, raising FT4 at baseline. The cutoff of FT4 increment at 3 h from baseline above 0.40 ng/dL (5.14 pmol/L) had a sensitivity of 97% and specificity of 80% to exclude true malabsorption.
Amongst the four groups (except the true malabsorption), the LAT curve for the treated hypothyroid group attained the highest levels. This probably reflects the fact that LT4 treatment reduces TBG (thyroxine binding globulin) [20] allowing a significant portion of absorbed LT4 to be available in free state. On the contrary, the LAT curve of the hypothyroid subjects achieved lowest levels despite similar absorption pattern as depicted by similar ΔFT4 at 3 h. Chronically decreased secretion of intrinsic T4 is probably the main explanation for this as they have a lower baseline. Elevated TBG in untreated hypothyroid [20] could also be the explanation. LAT curve for pseudomalabsorption group attained intermediate levels, almost coinciding with the euthyroid group. We appreciated that the pseudomalabsorption group is not homogeneous and consists of some who are frequent defaulters, who are likely to have an initial level and absorption pattern similar to untreated hypothyroid and some who are occasional defaulters, who are likely to have a pattern partially similar to treated hypothyroid. Hence combining these heterogeneous subjects in one group may have caused the curve to almost overlap in the euthyroid group with a normal baseline FT4 for reasons explained above.
Limitations of the Study
The number of true malabsorption subjects was small compared to other groups. We did not perform a D-Xylose test in the pseudomalabsorption group and used the definition available in the literature. However, all persons were followed for the next 3 months after adequate counselling and were found to have statistically significant decrease in TSH with LT4 replacement.
Conclusion
This study establishes normative data for the absorption of levothyroxine in different subjects. Peak increment FT4 was a good tool for identifying normal absorption in subjects with pseudomalabsorption. Mean increment in FT4 from baseline to 3 h of 0.78 ± 0.16 ng/dL suggests pseudomalabsorption due to frequent nonadherence or noncompliance to therapy. For a true malabsorption patient, it was 0.39 ± 0.17 ng/dL. However, a peak rise in FT4 of <0.40 ng/dL (5.14 pmol/L) at 3 h warrants evaluation for malabsorptive pathology.
Statement of Ethics
Written informed consent was taken from all subjects. The study was approved by Institutional Ethics Committee of Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
Endocrine Society of Bengal, Kolkata, India. Vide Grant No.: IPGMER/Endo/9/2016.
Author Contributions
Conceptualisation of the Study and overseeing: S.G. Patient selection and conduct of test: S.P., K.B., R.S. Data analysis: K. Bh. Manuscript preparation: P.M. Overall supervising and manuscript editing with valuable inputs: S.C.
Supplementary Material
Supplementary data
Acknowledgement
We sincerely acknowledge Endocrine Society of Bengal, Kolkata, India, for financial support to conduct the study. We are also grateful to Ms. Sutapa Chakraborty, the coordinator for this project.
References
- 1.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. American Thyroid Association Task Force on Thyroid Hormone Replacement Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hays MT. Localization of human thyroxine absorption. Thyroid. 1991;1((3)):241–8. doi: 10.1089/thy.1991.1.241. [DOI] [PubMed] [Google Scholar]
- 3.Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F. Delayed intestinal absorption of levothyroxine. Thyroid. 1995 Aug;5((4)):249–53. doi: 10.1089/thy.1995.5.249. [DOI] [PubMed] [Google Scholar]
- 4.Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009 Dec;23((6)):781–92. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017 Dec;40((12)):1289–301. doi: 10.1007/s40618-017-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal Malabsorption of Thyroxine. Endocr Rev. 2019 Feb;40((1)):118–36. doi: 10.1210/er.2018-00168. [DOI] [PubMed] [Google Scholar]
- 7.Vita R, Santaguida MG, Virili C, Segni M, Galletti M, Mandolfino M, et al. Serum Thyroid Hormone Antibodies Are Frequent in Patients with Polyglandular Autoimmune Syndrome Type 3, Particularly in Those Who Require Thyroxine Treatment. Front Endocrinol. 2017;28(8):212. doi: 10.3389/fendo.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ain KB, Refetoff S, Fein HG, Weintraub BD. Pseudomalabsorption of levothyroxine. JAMA. 1991 Oct;266((15)):2118–20. [PubMed] [Google Scholar]
- 9.Virili C, Centanni M. “With a little help from my friends” - The role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol Cell Endocrinol. 2017 Dec;458((458)):39–43. doi: 10.1016/j.mce.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 10.Virili C, Giovanella L, Fallahi P, Antonelli A, Santaguida MG, Centanni M, et al. Levothyroxine Therapy: Changes of TSH Levels by Switching Patients from Tablet to Liquid Formulation A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2018 Jan;9:10. doi: 10.3389/fendo.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damle N, Bal C, Soundararajan R, Kumar P, Durgapal P. A curious case of refractory hypothyroidism due to selective malabsorption of oral thyroxine. Indian J Endocrinol Metab. 2012 May;16((3)):466–8. doi: 10.4103/2230-8210.95716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balla M, Jhingan RM, Rubin DJ. Rapid levothyroxine absorption testing: a case series of nonadherent patients. Int J Endocrinol Metab. 2015 Oct;13((4)):e31051. doi: 10.5812/ijem.31051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Wilder N, Bravenboer B, Herremans S, Vanderbruggen N, Velkeniers B. Pseudomalabsorption of Levothyroxine: A Challenge for the Endocrinologist in the Treatment of Hypothyroidism. Eur Thyroid J. 2017 Feb;6((1)):52–6. doi: 10.1159/000452489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares RM, de Figueiredo RM, Dantas MM, Marinho MV, Sousa AG, da Câmara VL, et al. Rapid Levothyroxine (Lt4) Absorption Test for Diagnosis of Lt4 Pseudomalabsorption: Case Report and Proposal of a Cutoff Point. J Endocrinol Diabetes Obes. •••;4((1)):1083. [Google Scholar]
- 15.Lewandowski KC, Dąbrowska K, Komorowska-Dudek I, Lewiński A. A single bolus of high dose levothyroxine (L-T4) as a test in cases of suspected poor compliance to L-T4 therapy. Thyroid Res. 2015 Dec;8((1)):16. doi: 10.1186/s13044-015-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaled A, Sulaiman A, Seong KC, John HM. Thyroxine Absorption Test. Biomed J Sci & Tech Res. 2018;3((2)):1–4. [Google Scholar]
- 17.Sun GE, Pantalone KM, Faiman C, Gupta M, Olansky L, Hatipoglu B. The clinical utility of free thyroxine in oral levothyroxine absorption testing. Endocr Pract. 2014 Sep;20((9)):925–9. doi: 10.4158/EP13487.OR. [DOI] [PubMed] [Google Scholar]
- 18.Rdzak GM, Whitman LM, Inzucchi SE. Levothyroxine pseudo-malabsorption: testing and treatment in the outpatient setting. Ther Adv Endocrinol Metab. 2018 Jul;9((7)):217–22. doi: 10.1177/2042018818771433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker JN, Shillo P, Ibbotson V, Vincent A, Karavitaki N, Weetman AP, et al. A thyroxine absorption test followed by weekly thyroxine administration: a method to assess non-adherence to treatment. Eur J Endocrinol. 2013 May;168((6)):913–7. doi: 10.1530/EJE-12-1035. [DOI] [PubMed] [Google Scholar]
- 20.Benvenga S. ‘Thyroid hormone transport proteins and the physiology of hormone binding’. In: Braverman LE, Cooper DS, Werner &Ingbar's The Thyroid, editors. New Delhi: Wolter Kluer (India) / Lippincott Williams & Wilkins; 2013. p. p 98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data