Summary
Background
Previous studies have found rotavirus vaccination to be highly cost-effective in low-income countries. However, updated evidence is now available for several inputs (ie, rotavirus disease mortality rates, rotavirus age distributions, vaccine timeliness, and vaccine efficacy by duration of follow-up), new rotavirus vaccines have entered the market, vaccine prices have decreased, and cost-effectiveness thresholds have been re-examined. We aimed to provide updated cost-effectiveness estimates to inform national decisions about the new introduction and current use of rotavirus vaccines in Gavi countries.
Methods
We calculated the potential costs and effects of rotavirus vaccination for ten successive birth cohorts in 73 countries previously and currently eligible for Gavi support, compared with no vaccination. We used a deterministic cohort model to calculate numbers of rotavirus gastroenteritis cases, outpatient visits, hospitalisations, and deaths between birth and 5 years, with and without rotavirus vaccination. We calculated treatment costs from the government and societal perspectives. The primary outcome measure was the incremental cost-effectiveness ratio (discounted US$ per disability-adjusted life-year averted). Country-specific model input parameters were based on the scientific literature, published meta-analyses, and international databases. We ran deterministic and probabilistic uncertainty analyses.
Findings
Over the period 2018–27, rotavirus vaccination has the potential to prevent nearly 600 000 deaths in Gavi countries. Averted outpatient visits and hospitalisations could lead to treatment savings of approximately $484·1 million from the government perspective and $878·0 million from the societal perspective. The discounted dollars per disability-adjusted life-year averted has a very high probability (>90%) of being less than 0·5 times the gross domestic product per capita in 54 countries, and less than 1·0 times gross domestic product per capita in 63 countries.
Interpretation
Rotavirus vaccination continues to represent good value for money across most Gavi countries despite lower rotavirus mortality estimates and more stringent willingness-to-pay thresholds.
Funding
Bill & Melinda Gates Foundation.
Introduction
Diarrhoeal diseases are estimated to cause over half a million deaths each year in children younger than 5 years.1, 2, 3 This proportion is roughly 10% of all deaths in this age group, with most deaths occurring in the world's poorest countries. A large proportion (24–37%) of these deaths are estimated to be caused by rotavirus.4, 5
The introduction of rotavirus vaccines has played an important role in contributing to declines in diarrhoeal mortality and morbidity.6 In 2009, WHO recommended introduction of rotavirus vaccination in all national immunisation programmes.7 Over 90 countries have introduced the vaccine, with low-income countries benefiting from the financial support of Gavi, the Vaccine Alliance.8, 9 These introductions have had a profound effect on public health, not just from a rotavirus mortality and morbidity perspective, but also by freeing health-care resources for other priorities in resource-constrained settings.6
In addition to the declining diarrhoea burden, the incomes of the world's poorest regions are also growing. For example, between 2005 and 2015, real income per capita increased by nearly 25% in sub-Saharan Africa.10 Growing incomes have the potential to increase living standards, reduce poverty, and enable governments to raise additional revenue. Conversely, as incomes grow, countries have less access to international financing mechanisms to support health and development objectives. International donors, such as Gavi, the Global Fund, and the World Bank International Development Association all offer financial support to countries, but this support is linked to country income.11, 12, 13 As a result, countries could face the same challenges with fewer resources to meet them. Furthermore, many of the countries that have yet to introduce rotavirus vaccination are no longer eligible for Gavi funding.9, 14 In all countries that have received financial support from Gavi, the government is expected to eventually incur the full costs of the programme when Gavi's support expires.
In 2012, Atherly and colleagues15 published an impact and cost-effectiveness analysis of rotavirus vaccination in countries eligible for Gavi's support. This, and many other studies, found rotavirus vaccination to be highly cost-effective in low-income and middle-income countries around the world.16, 17, 18, 19, 20 However, the guidance around cost-effectiveness thresholds used to interpret interventions has changed, calling for the use of more stringent thresholds that better reflect the financial constraints of these countries.21 In addition, estimates of rotavirus prevaccination mortality have decreased from 453 000 in 2008 to 215 000 in 2013.4, 22 Updated evidence is available for rotavirus age distributions, vaccine timeliness, vaccine efficacy, and rotavirus disease treatment costs. The price of rotavirus vaccines has also been decreasing as new products have entered the global market. All these factors are important variables in a cost-effectiveness study. The purpose of this Article is to assess the potential impact and cost-effectiveness of rotavirus vaccination across 73 countries (currently and previously eligible for Gavi support) as a result of these important global trends.
Research in context.
Evidence before this study
Rotavirus is a leading cause of childhood deaths caused by diarrhoea worldwide. Rotavirus vaccines have been available for the past 10 years and introduced in many countries, including countries receiving support from Gavi, the Vaccine Alliance. In countries where they are used, rotavirus vaccines have contributed to the decrease of rotavirus gastroenteritis cases and deaths. We searched PubMed, Google Scholar, and Web of Science from Jan, 2008 to April, 2019 using broad search terms associated with “rotavirus”, “vaccine”, “cost-effective”, “Gavi”, and “low- and middle-income country”. We supplemented identified articles with studies known to the authors. The cost-effectiveness of rotavirus vaccination has been shown in Gavi-eligible countries in several analyses and many analyses in specific low-income and middle-income countries. However, global trends have potentially affected the cost-effectiveness profile of rotavirus vaccines: economic growth has led to a decrease in international support as recipient countries grow wealthier; updated evidence is showing lower rotavirus mortality; the guidance around cost-effectiveness thresholds used to interpret interventions has changed, calling for the use of more stringent thresholds; new products have entered the market; and evidence for several model inputs has been updated.
Added value of this study
Our study provides an update on the cost-effectiveness of rotavirus vaccination in previous and current Gavi-eligible countries. We covered more vaccines than in previous studies, including two new, potentially more affordable rotavirus vaccines that entered the market in 2018. The study generates numbers of rotavirus cases, clinic visits, hospitalisations, deaths, and treatment costs averted by vaccination, by country and region. We also calculated costs for vaccination programmes for vaccines with different characteristics.
Implications of all the available evidence
Our study provides evidence that rotavirus vaccination is still a cost-effective investment in Gavi countries. Additional rotavirus burden could be averted with more countries adopting the vaccines. Policy makers in countries with reducing international support and who are looking for budget efficiencies should consider newly available products as they might offer more affordable options.
Methods
Study design
We examined the projected impact and cost-effectiveness of rotavirus vaccination in 73 countries previously or currently eligible for Gavi support, across all WHO regions (table 1). Results were generated and reported per country and then aggregated per WHO regions and for all Gavi countries. We calculated the potential costs and benefits of nationwide infant rotavirus vaccination, compared with no vaccination, for ten consecutive birth cohorts (2018–27) in 73 Gavi countries.
Table 1.
Countries considered in the analysis by WHO region and Gavi transition phase (2018)
| African region | Region of the Americas | Eastern Mediterranean region | European region | South-East Asia region | Western Pacific region | |
|---|---|---|---|---|---|---|
| Initial self-financing | Benin; Burkina Faso; Burundi; Central African Republic; Chad; Comoros; DR Congo; Eritrea; Ethiopia; Guinea; Guinea-Bissau; Liberia; Madagascar; Malawi; Mali; Mozambique; Niger; Rwanda; Senegal; Sierra Leone; South Sudan*; Tanzania; The Gambia; Togo; Uganda; Zimbabwe | Haiti | Afghanistan; Somalia | .. | Nepal; North Korea† | .. |
| Preparatory transition | Cameroon; Côte d'Ivoire; Ghana; Kenya; Lesotho; Mauritania; Zambia | .. | Djibouti; Pakistan; Sudan; Yemen | Kyrgyzstan†; Tajikistan | Bangladesh; Myanmar | Cambodia |
| Accelerated transition | Nigeria; São Tomé and Príncipe | Nicaragua† | .. | Uzbekistan | India | Laos; Papua New Guinea; Solomon Islands; Vietnam† |
| Fully self-financing | Angola; Republic of Congo | Bolivia; Cuba; Guyana; Honduras | .. | Armenia†; Azerbaijan; Georgia; Moldova†; Ukraine | Bhutan; Indonesia; Sri Lanka; Timor-Leste | Kiribati; Mongolia† |
Country not included in Atherly et al.15
Countries with medium under-5 mortality over the period 2010–15. Different vaccine efficacy assumptions were applied to countries with medium under-5 mortality and high under-5 mortality over the period 2010–15; all other countries are considered to have high mortality.
Rotavirus gastroenteritis cases, outpatient visits, hospitalisations (hospital admission), deaths, and costs were projected over the first 5 years of life. During the period of analysis, the vaccinated individuals could or could not become ill with rotavirus disease. If they got rotavirus disease, it could be non-severe or severe. Non-severe disease was defined as recovery with or without outpatient care (clinic visit). Severe disease was defined as recovery or death with or without outpatient or inpatient care. We did not consider informal care in this analysis.
Costs and benefits were examined from both the government and societal perspectives and are discounted at 3% per year. Monetary units were presented in 2015 US$. Key outputs of the analysis included aversions of deaths, disability-adjusted life-years (DALYs), cases, hospitalisations and outpatient visits, and health costs as a result of rotavirus vaccination. Additional outputs included the total costs of vaccination and our primary outcome measure, the incremental cost-effectiveness ratio (ICER), expressed as discounted US$ per DALY averted. To allow for comparison of ICERs with a uniform willingness-to-pay threshold that we applied to all countries, gross domestic product (GDP) and population values for each country were used to calculate values for individual countries, regions (WHO regions), and all Gavi countries' GDP per capita. We then used model-generated ICERs and compared the ICER with cost-effectiveness thresholds of 0·5 times and 1·0 times GDP per capita in all examined countries.
Impact and cost-effectiveness model
We used a Microsoft Excel-based static cohort model with a finely disaggregated age structure (weeks of age up to 5 years) to calculate numbers of rotavirus gastroenteritis cases, clinic visits, hospitalisations, and deaths expected to occur between birth and age 5 years, with and without rotavirus vaccination (UNIVAC version 1.3.41).23 Methods used to calculate the direct effects of vaccination have been described in detail elsewhere.24, 25 In brief, for each week of age, the expected number of disease events (ie cases, visits, hospitalisations, deaths) were multiplied by the expected coverage (adjusted for vaccine timeliness) and efficacy (adjusted for duration of follow-up) of each dose of vaccination. Health-care costs were calculated by multiplying the expected numbers of clinic visits and hospitalisations by the average cost per clinic visit and hospitalisation, from a government and societal perspective. Vaccination costs were calculated by multiplying the total number of doses administered by a wastage factor and other assumptions about price and the costs of delivery. More details on input parameters and values for each country are included later in this Article.
Disease burden
We estimated approximately 10 000 symptomatic rotavirus gastroenteritis cases per 100 000 children aged younger than 5 years per year on the basis of a global systematic review and meta-analysis by Bilcke and colleagues.26 We used WHO region estimates of the proportion of all-cause gastroenteritis cases that are severe (defined as children with moderate or severe dehydration), as a proxy for the proportion of rotavirus gastroenteritis cases that were severe (and non-severe).27 To calculate numbers of rotavirus deaths in each country (without vaccination), we estimated means (and 95% CIs) using country-specific estimates from three difference sources (Institute for Health Metrics and Evaluation, Maternal Child Epidemiology Estimation, and WHO US Centers for Diseases Control and Prevention) for the year 2015.1, 3, 4 We elected to use the mean because the range reported by the three different sources was from 158 000 to 202 000 deaths a year in children younger than 5 years for the group of 73 countries. Comparison and discussion of methods and results from the three sources have been published elsewhere.5 If a country had already introduced the vaccine in 2015, then the mortality for the most recent prevaccination year was used, using WHO–UNICEF joint estimates of national immunisation coverage to determine the most recent prevaccine year.28 In absence of vaccination, we assumed that rotavirus mortality would decrease at the same rate as all-cause mortality for children younger than 5 years of age. Rotavirus age distributions were based on a systematic review and statistical analysis of over 90 hospital datasets.29 We assumed that 20% of severe rotavirus gastroenteritis cases would require a hospital admission and further reduced this proportion to account for those without access to hospital, using coverage of the first dose of diphtheria–tetanus–pertussis vaccine (DTP1) as a proxy for access to care. This method generated rates of rotavirus gastroenteritis hospitalisations that were consistent with prevaccination rates previously reported (around 350 per 100 000 per year, among children younger than 5 years).30, 31, 32, 33, 34, 35, 36 We assumed that 100% of severe rotavirus gastroenteritis cases and 10% of non-severe cases would require a clinic visit, and again used DTP1 coverage to adjust for access to care. DALY weights were taken from the 2013 Global Burden of Disease study,37 using values reported for moderate diarrhoea as a proxy of non-severe rotavirus gastroenteritis and for severe diarrhoea as a proxy of severe rotavirus gastroenteritis. We assumed a duration of illness of 4 days for non-severe rotavirus gastroenteritis and 6 days for severe rotavirus gastroenteritis cases and explored longer and shorter durations in probabilistic analysis.38 Input values and ranges for DALY weights and duration of illness are available in the appendix.
Vaccine preference, coverage, and efficacy
Four rotavirus vaccines prequalified by WHO at end of 2018 were considered in the analysis. These four vaccines were Rotarix (manufactured by GlaxoSmithKline, Rixensart, Belgium), RotaTeq (manufactured by Merck and Co, Kenilworth, NJ, USA), Rotavac (manufactured by Bharat Biotech, Hyderabad, India), and Rotasiil (manufactured by Serum Institute, Pune, India).39 Rotarix was administered in a two-dose schedule whereas the other vaccines were administered in a three-dose schedule. Our base-case scenario explored all 73 countries with the vaccine they were using in 2018 for countries already using rotavirus vaccines, and a randomly allocated vaccine (Rotavac or Rotasiil) for countries that were not using rotavirus vaccine at the time of analysis.9 This process did not imply a preference for any vaccine but ensured that new products were represented in this analysis. We assumed the use of both Rotavac and Rotasiil in India was a 50–50 distribution countrywide. We also ran so-called what-if scenarios in which all 73 countries used the same product.
Coverage of each dose of rotavirus vaccine is based on the WHO–UNICEF estimates of national immunisation coverage.28 The average of DTP1 and DTP3 coverage is used as a proxy for DTP2 coverage. Coverage rates are considered constant throughout the analysis. Data for vaccine coverage timeliness were taken from Clark and colleagues.25 Assumptions about vaccine efficacy and waning were based on pooled data from published randomised controlled trials of rotavirus vaccines that are described elsewhere.40 In brief, in settings with medium under-5 mortality (defined as 13·5–28·1 deaths per 1000 livebirths) pooled efficacy was 82% (95% credibility interval 74–92%) after 2 weeks of follow-up and 77% (67–84) after 12 months, based on 11 observations. In settings with high under-5 mortality (defined as >28·1 deaths per 1000 livebirths) pooled efficacy was 66% (95% credibility interval 48–81) after 2 weeks of follow-up and 44% (27–59) after 12 months, based on 24 observations. We did not apply any age restriction to the vaccine schedule.41
Vaccine price and delivery costs
Given the importance of Gavi's support to countries, we examined costs of vaccine programmes with and without a Gavi subsidy for the vaccine. The costs with a Gavi subsidy was reflected in the government's perspective as this cost is to the country only. The costs without a Gavi subsidy, representing the cost to countries and to Gavi, were reflected in the societal perspective. As such, the government perspective used each country's cofinancing share based on the Gavi transition policy.11, 42 The societal perspective reflected each vaccine price: $2·29 per dose for Rotarix ($6·50 for countries procuring through the Pan American Health Organization revolving fund), $3·20 for RotaTeq, $0·85 for Rotavac, and $0·95 for Rotasiil.43 The countries that did not introduce rotavirus vaccine when they were still eligible for Gavi support are not automatically accessing Gavi negotiated prices. For these countries, prices were estimated from the WHO vaccine, price, and procurement database (table 2).44, 45
Table 2.
Vaccine preference, vaccine price per dose, cofinancing, and vaccine introduction year per country
| Vaccine preference | Vaccine price per dose (US$) | Average cofinancing per dose over 2018–27 period (US$) | Introduction year | |
|---|---|---|---|---|
| Afghanistan | Rotarix | 2·29 | 0·20 | 2018 |
| Angola | Rotarix | 2·29 | 2·29 | 2014 |
| Armenia | Rotarix | 2·29 | 2·29 | 2012 |
| Bolivia | Rotarix | 6·50 | 6·50 | 2008 |
| Burkina Faso | RotaTeq | 3·20 | 0·13 | 2013 |
| Burundi | Rotarix | 2·29 | 0·20 | 2013 |
| Cameroon | Rotarix | 2·29 | 0·90 | 2014 |
| Côte d'Ivoire | RotaTeq | 3·20 | 1·56 | 2017 |
| Djibouti | Rotarix | 2·29 | 0·53 | 2014 |
| Eritrea | Rotarix | 2·29 | 0·23 | 2014 |
| Ethiopia | Rotarix | 2·29 | 0·21 | 2013 |
| Georgia | Rotarix | 2·29 | 2·29 | 2013 |
| Ghana | Rotarix | 2·29 | 0·67 | 2012 |
| Guinea-Bissau | Rotarix | 2·29 | 0·20 | 2015 |
| Guyana | Rotarix | 6·50 | 6·50 | 2010 |
| Haiti | Rotarix | 6·50 | 0·20 | 2013 |
| Honduras | Rotarix | 6·50 | 6·50 | 2009 |
| India* | Rotavac/Rotasiil | 0·85/0·95 | 0·85/0·95 | 2017 |
| Kenya | Rotarix | 2·29 | 0·87 | 2014 |
| Kiribati | Rotarix | 6·20 | 6·20 | 2015 |
| Lesotho | Rotarix | 2·29 | 0·46 | 2017 |
| Liberia | Rotarix | 2·29 | 0·20 | 2016 |
| Madagascar | Rotarix | 2·29 | 0·20 | 2014 |
| Malawi | Rotarix | 2·29 | 0·20 | 2012 |
| Mali | RotaTeq | 3·20 | 0·13 | 2014 |
| Mauritania | Rotarix | 2·29 | 0·55 | 2014 |
| Moldova | Rotarix | 2·29 | 2·29 | 2012 |
| Mozambique | Rotarix | 2·29 | 0·20 | 2015 |
| Nicaragua | Rotarix | 6·50 | 5·98 | 2006 |
| Niger | Rotarix | 2·29 | 0·20 | 2014 |
| Pakistan | Rotarix | 2·29 | 1·25 | 2017 |
| Republic of Congo | Rotarix | 2·29 | 2·29 | 2014 |
| Rwanda | Rotarix | 2·29 | 0·20 | 2012 |
| São Tomé and Príncipe | RotaTeq | 3·20 | 2·36 | 2016 |
| Senegal | Rotarix | 2·29 | 0·20 | 2014 |
| Sierra Leone | Rotarix | 2·29 | 0·20 | 2014 |
| Sudan | Rotarix | 2·29 | 1·34 | 2011 |
| Tajikistan | Rotarix | 2·29 | 0·60 | 2015 |
| Tanzania | Rotarix | 2·29 | 0·20 | 2012 |
| The Gambia | Rotarix | 2·29 | 0·20 | 2013 |
| Togo | Rotarix | 2·29 | 0·20 | 2014 |
| Uganda | Rotarix | 2·29 | 0·20 | 2018 |
| Uzbekistan | Rotarix | 2·29 | 2·29 | 2014 |
| Yemen | Rotarix | 2·29 | 0·68 | 2012 |
| Zambia | Rotarix | 2·29 | 0·74 | 2013 |
| Zimbabwe | Rotarix | 2·29 | 0·20 | 2014 |
These data are for countries already using rotavirus vaccines at the end of 2018; countries not using rotavirus vaccines at the end of 2018 were randomly allocated one of the newly prequalified vaccines.
Assuming that 50% of immunised children are receiving Rotavac and the other 50% Rotasiil.
The incremental delivery cost is based on work by the Immunization Costing Action Network. This network completed a systematic review of the cost of immunisation programmes and developed a unit cost repository.46 The repository was searched for incremental costs per dose without vaccine cost and returned values for several low-income and lower-middle-income country studies and antigens. We elected to use these values adjusted to 2015 US$: $1·25 for low-income countries and $1·86 for lower-middle-income and upper-middle-income countries. This data input captured all programmatic costs linked to delivering the vaccine, including training costs, staff time, and vaccine storage and distribution. As it covers a wide range of parameters, we varied this data input in probabilistic analysis.
In addition to vaccine price and incremental delivery cost, we accounted for a 5% wastage rate for single dose vaccines and supplies: 10% for Rotasiil and 25% for Rotavac to reflect the multidose presentations. We accounted for the procurement of safety bags with a capacity of 100 doses and a unit cost of $0·80. International handling was estimated at 3·5% of the vaccine price and international transportation at 6·0%.47 All inputs used to model cost of the vaccination programme are shown in the appendix.
Health service costs
Treatment costs for inpatient and outpatient episodes of rotavirus across all 73 Gavi countries were not available. We used modelled estimates of direct medical, direct non-medical, and indirect costs for both inpatient and outpatient episodes. The cost estimation methods are described in detail elsewhere (unpublished). In summary, we generated country-specific direct medical costs using service delivery unit cost estimates from the WHO cost-effectiveness and strategic planning tool (WHO CHOICE)48 along with commodity costs. For inpatients costs, we used country-specific estimates of bed day costs at a secondary-level hospital, assuming 4 days of hospital stay, use of six oral rehydration solution packets per day for the duration of hospital stay, and two intravenous solutions. For outpatient costs, WHO CHOICE data for a primary hospital and six packets of oral rehydration solution per day for 2 days were assumed. To estimate direct non-medical costs, we first derived the share of direct medical to direct non-medical costs from the literature. We then used the share of direct non-medical cost from the literature and our estimate of direct medical cost to calculate the direct non-medical cost in each country. Indirect costs were calculated by multiplying the average GDP per capita per day with the average number of days lost to providing care for a patient with diarrhoea. We assumed inpatient caretakers lost one productive day and outpatient caretakers lost a quarter of a productive day based on an unpublished analysis of data from the GEMS study.49 Only direct medical costs were used in calculating health service costs from the government perspective. We added direct medical costs, direct non-medical costs, and indirect costs in calculating health-service costs from the societal perspective. Country-specific health-care costs values are available in the appendix.
Alternative scenarios and probabilistic analysis
In addition to our base-case scenario covering countries with the vaccine they were using in 2018, or a randomly allocated vaccine (Rotavac or Rotasiil) for countries that were not using rotavirus vaccine at the time of analysis, we also explored alternative scenarios looking at the use of Rotarix, Rotavac, and Rotasiil in all Gavi countries. We elected to exclude the use of RotaTeq for non-introducing countries because of the manufacturer's announcement in 2018 to withdraw from the Gavi market.50 Inputs for these alternative scenarios are available in the appendix.
We ran probabilistic simulations to account for uncertainty in the parameter inputs. We calculated the proportion of those simulations with an ICER below different possible willingness-to-pay thresholds to indicate the probability that the vaccine would be cost-effective at each threshold. For each country, we generated 1000 runs of results on the basis of randomly selected data inputs using a specified distribution, within a range of low and high values for all study parameters. The complete set of lower and higher input ranges as well as distributions for each input are available in the appendix.
Role of the funding source
The funder was not involved in the study design, data analysis, interpretation, or reporting of results. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Over the period 2018–27 in Gavi countries, without discounting future health benefits, rotavirus vaccination has the potential to avert 158·6 million cases of rotavirus gastroenteritis, 80·7 million outpatient visits, 7·9 million hospitalisations, 576 567 deaths, and 14·7 million DALYs (table 3). Of the cases, visits, and hospitalisations averted, 42% would be in the African region, 41% in the South-East Asian region, and 9% in the Eastern Mediterranean region. Of deaths averted, 65% would be in the African region, 23% in the South-East Asian region, and 10% in the Eastern Mediterranean region.
Table 3.
Health and economic benefits over a 10-year period (2018–27) for the base-case scenario
| African region | Region of the Americas | Eastern Mediterranean region | European region | South-East Asia region | Western Pacific region | All Gavi countries | |
|---|---|---|---|---|---|---|---|
| Averted rotavirus burden | |||||||
| Cases | 64 941 257 | 2 279 644 | 14 727 951 | 3 514 830 | 66 895 132 | 6 202 581 | 158 561 393 |
| Visits | 23 883 590 | 938 830 | 5 922 875 | 859 706 | 15 531 821 | 1 674 650 | 48 811 472 |
| Hospitalisations | 2 826 609 | 78 392 | 392 566 | 93 906 | 2 096 962 | 244 059 | 5 732 494 |
| Deaths | 376 560 | 3293 | 57 927 | 2547 | 130 824 | 5417 | 576 567 |
| DALYs* | 9 407 363 | 89 129 | 1 488 332 | 73 308 | 3 472 376 | 152 446 | 14 682 955 |
| Averted health-care costs (US$) | |||||||
| Government perspective* | 192 412 959 | 15 216 757 | 26 438 853 | 14 047 862 | 186 275 477 | 49 726 662 | 484 118 569 |
| Societal perspective* | 351 173 250 | 26 076 818 | 51 733 623 | 24 427 446 | 345 072 153 | 79 549 803 | 878 033 093 |
| Vaccine programme costs (US$) | |||||||
| With Gavi subsidy* (cost to country only) | 1 211 762 128 | 105 071 230 | 375 178 351 | 93 259 104 | 2 360 479 712 | 208 622 785 | 4 354 373 309 |
| Without Gavi subsidy* (cost to country and to Gavi) | 2 187 656 303 | 129 326 733 | 550 683 257 | 104 171 153 | 2 459 017 468 | 217 671 893 | 5 648 526 807 |
| Cost per DALY averted (US$) | |||||||
| Government perspective*† | 108 (29–568) | 1008 (71–3389) | 234 (42–463) | 1081 (196–5396) | 626 (242–4529) | 1042 (137–2661) | 264 (202–428) |
| Societal perspective* | 195 | 1158 | 335 | 1088 | 609 | 906 | 325 |
| Cost per DALY averted (government perspective) as a proportion of GDP per capita‡ | 0·09 | 0·30 | 0·17 | 0·50 | 0·33 | 0·49 | 0·16 |
DALYs=disability-adjusted life-years. GDP=gross domestic product.
Discounted values.
Figures in parentheses show 95% uncertainty intervals (2·5th and 97·5th percentiles of 1000 simulations).
GDP per capita in current US$ calculated for each region.
In terms of economic benefits, outpatient visits and hospitalisations averted represent $484·1 million from the government perspective and $878·0 million from the societal perspective (table 3). Most of the costs from the government perspective are averted in the African region, with 40%, 38% in the South-East Asia region, and 10% in the Western Pacific region. The total vaccination programme cost across all countries is estimated to be $4·4 billion, assuming a Gavi subsidy to countries, and about $5·6 billion without considering Gavi subsidy on vaccine prices. The regional distribution of this cost also differs depending on the inclusion or exclusion of Gavi support, reflecting regions where countries are receiving more support. Without cofinancing, 44% of the global vaccine programme cost would be in the South-East Asia region, 39% in the African region, and 10% in the Eastern Mediterranean region. Accounting for Gavi subsidy, 54% of the cost is attributed to countries in the South-East Asia region, 28% in the African region, and 9% in the Eastern Mediterranean region.
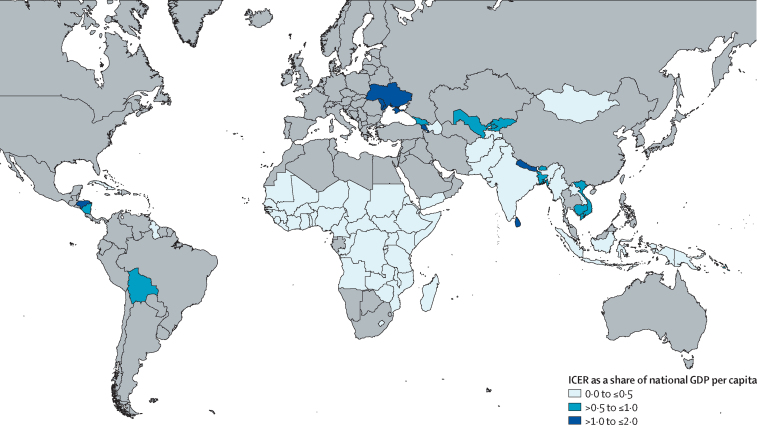
From the societal perspective, incremental cost-effectiveness ratios expressed in US$ per DALY averted ranged from $195 for the African region to $1158 for the region of the Americas. Overall from the societal perspective, the cost per DALY averted in Gavi countries is $325. From the government perspective, the cost per DALY averted ranges from $108 for the African region to $1081 for the European region. Overall from the government perspective, the cost per DALY averted in Gavi countries is $264 (figure 1, Table 3, Table 4).
Figure 1.
Map displaying country ICER as a share of GDP per capita from the government perspective
GDP=gross domestic product. ICER=incremental cost-effectiveness ratio.
Table 4.
Results by country in the base case scenario (all countries using rotavirus vaccine from 2018 to 2027)
| Number of fully immunised children | Averted cases of rotavirus | Averted deaths by rotavirus | Total health-care costs averted government perspective (US$)* | Vaccine programme costs (US$)* | Vaccine programme costs with Gavi subsidy (US$)* | Cost per DALY averted government perspective (US$)*† | GDP per capita (US$) | ||
|---|---|---|---|---|---|---|---|---|---|
| Afghanistan | Rotarix | 7 762 176 | 1 580 540 | 7305 | 1 790 959 | 54 638 902 | 20 878 029 | 102 (68–174) | 562 |
| Angola | Rotarix | 9 358 710 | 2 242 237 | 30 528 | 21 333 511 | 77 669 464 | 77 669 464 | 74 (36–119) | 3309 |
| Armenia | Rotarix | 307 794 | 106 670 | 9 | 473 387 | 2 499 384 | 2 499 384 | 4337 (2225–6420) | 3615 |
| Azerbaijan | Rotavac | 1 296 082 | 256 505 | 183 | 1 388 389 | 10 954 903 | 10 954 903 | 1824 (678–3460) | 3879 |
| Bangladesh | Rotasiil | 27 821 356 | 6 381 993 | 6038 | 12 508 907 | 224 858 236 | 168 951 095 | 911 (565–1219) | 1359 |
| Benin | Rotavac | 3 409 188 | 915 805 | 6618 | 2 419 236 | 23 113 488 | 13 917 319 | 69 (37–100) | 789 |
| Bhutan | Rotavac | 130 982 | 31 051 | 16 | 130 410 | 1 101 703 | 1 101 703 | 1993 (851–3758) | 2774 |
| Bolivia | Rotarix | 2 458 502 | 552 970 | 820 | 4 601 265 | 40 490 928 | 40 490 928 | 1599 (868–2513) | 3105 |
| Burkina Faso | Rotateq | 6 938 849 | 1 741 437 | 11 105 | 4 990 861 | 92 210 089 | 26 332 567 | 76 (30–134) | 627 |
| Burundi | Rotarix | 4 369 163 | 975 496 | 6008 | 1 609 641 | 30 075 387 | 11 492 083 | 66 (35–97) | 286 |
| Cambodia | Rotasiil | 3 093 143 | 693 787 | 646 | 1 760 655 | 25 019 452 | 17 315 754 | 855 (480–1222) | 1270 |
| Cameroon | Rotarix | 7 659 052 | 1 813 529 | 13 867 | 7 230 672 | 61 673 034 | 39 524 515 | 93 (55–128) | 1375 |
| Central African Republic | Rotavac | 733 714 | 269 033 | 3419 | 421 169 | 6 240 284 | 3 628 809 | 38 (16–68) | 382 |
| Chad | Rotasiil | 2 812 216 | 766 128 | 12 178 | 1 373 576 | 21 493 685 | 12 650 864 | 39 (20–61) | 664 |
| Comoros | Rotasiil | 228 838 | 59 906 | 194 | 154 074 | 1 534 044 | 902 903 | 150 (77–227) | 775 |
| Côte d'Ivoire | Rotateq | 7 677 006 | 2 171 684 | 9889 | 22 224 658 | 122 376 764 | 79 727 547 | 239 (141–336) | 1535 |
| Cuba | Rotasiil | 1 116 234 | 508 721 | 19 | 6 191 925 | 8 998 257 | 8 998 257 | 1681 (98–4332) | 7602 |
| DR Congo | Rotasiil | 27 297 657 | 7 268 535 | 53 013 | 18 669 915 | 177 176 708 | 104 282 116 | 64 (28–98) | 449 |
| Djibouti | Rotarix | 178 289 | 40 238 | 121 | 127 781 | 1 441 579 | 786 340 | 212 (125–304) | 1862 |
| Eritrea | Rotarix | 1 539 050 | 261 276 | 770 | 421 978 | 10 611 584 | 4 155 848 | 188 (91–303) | 583 |
| Ethiopia | Rotarix | 26 078 179 | 5 162 506 | 16 375 | 7 580 039 | 187 304 858 | 72 057 918 | 152 (85–217) | 707 |
| Georgia | Rotarix | 421 489 | 192 937 | 12 | 738 532 | 3 395 328 | 3 395 328 | 3581 (1805–5246) | 3866 |
| Ghana | Rotarix | 8 085 885 | 1 775 985 | 6344 | 6 160 965 | 64 090 055 | 37 213 746 | 192 (110–265) | 1513 |
| Guinea | Rotavac | 2 600 878 | 695 933 | 2897 | 1 178 462 | 18 533 035 | 10 777 108 | 130 (77–184) | 662 |
| Guinea-Bissau | Rotarix | 584 936 | 127 634 | 848 | 320 995 | 4 118 150 | 1 573 583 | 59 (25–96) | 642 |
| Guyana | Rotarix | 141 621 | 30 965 | 46 | 223 693 | 2 335 070 | 2 335 070 | 1736 (1303–2282) | 4529 |
| Haiti | Rotarix | 1 642 083 | 380 229 | 1557 | 609 246 | 27 623 504 | 4 702 378 | 103 (50–157) | 740 |
| Honduras | Rotarix | 1 920 553 | 422 385 | 391 | 2 083 282 | 31 797 350 | 31 797 350 | 2667 (1978–3549) | 2361 |
| India | Rotavac/Rotasiil | 210 355 220 | 46 083 379 | 106 007 | 117 772 708 | 1 761 981 734 | 1 761 981 734 | 588 (428–747) | 1710 |
| Indonesia | Rotasiil | 35 606 711 | 8 613 368 | 11 969 | 41 943 398 | 320 037 163 | 320 037 163 | 866 (463–1306) | 3570 |
| Kenya | Rotarix | 14 630 923 | 3 225 501 | 8877 | 8 233 918 | 117 831 196 | 73 903 636 | 283 (182–383) | 1455 |
| Kiribati | Rotarix | 25 503 | 5 365 | 14 | 23 395 | 406 410 | 406 410 | 1080 (646–1646) | 1587 |
| Kyrgyzstan | Rotavac | 1 219 828 | 466 414 | 232 | 2 902 866 | 10 203 078 | 7 256 136 | 625 (147–1107) | 1078 |
| Laos | Rotasiil | 1 066 947 | 265 434 | 1481 | 2 476 970 | 9 945 424 | 9 205 425 | 177 (99–243) | 2339 |
| Lesotho | Rotarix | 550 446 | 126 078 | 594 | 403 802 | 4 419 060 | 2 325 045 | 133 (79–178) | 1040 |
| Liberia | Rotarix | 1 481 187 | 352 291 | 1278 | 587 456 | 10 701 360 | 4 089 089 | 106 (56–164) | 455 |
| Madagascar | Rotarix | 7 244 209 | 1 622 511 | 6370 | 2 465 080 | 50 490 355 | 19 292 831 | 102 (45–176) | 402 |
| Malawi | Rotarix | 6 138 614 | 1 487 654 | 6147 | 3 486 309 | 42 520 140 | 16 247 338 | 81 (38–121) | 300 |
| Mali | Rotateq | 5 370 633 | 1 528 415 | 7623 | 2 536 699 | 80 689 518 | 23 042 622 | 107 (42–197) | 780 |
| Mauritania | Rotarix | 1 200 458 | 270 702 | 1537 | 870 930 | 9 887 444 | 5 457 313 | 118 (72–166) | 1102 |
| Mongolia | Rotavac | 598 514 | 234 615 | 133 | 976 855 | 4 904 458 | 4 904 458 | 997 (491–1628) | 3694 |
| Mozambique | Rotarix | 10 006 557 | 2 270 541 | 9103 | 4 751 912 | 70 827 392 | 27 063 800 | 97 (49–149) | 382 |
| Myanmar | Rotavac | 8 102 780 | 2 040 478 | 4888 | 4 479 595 | 68 695 435 | 49 153 966 | 351 (351–215) | 1196 |
| Nepal | Rotavac | 4 864 133 | 1 116 376 | 816 | 1 527 722 | 33 107 970 | 19 252 409 | 751 (370–1190) | 729 |
| Nicaragua | Rotarix | 1 092 186 | 384 373 | 460 | 1 507 347 | 18 081 624 | 16 747 246 | 1186 (828–1596) | 2151 |
| Niger | Rotarix | 8 811 793 | 2 098 720 | 19 320 | 3 657 328 | 63 969 987 | 24 443 523 | 43 (21–69) | 364 |
| Nigeria | Rotavac | 35 492 367 | 10 650 771 | 91 934 | 28 485 738 | 342 230 664 | 287 705 916 | 116 (69–169) | 2176 |
| North Korea | Rotavac | 3 272 406 | 1 214 173 | 779 | 2 212 329 | 22 063 877 | 12 830 293 | 467 (231–735) | .. |
| Pakistan | Rotarix | 37 967 843 | 8 098 797 | 23 446 | 12 209 306 | 308 853 983 | 222 737 186 | 343 (165–567) | 1444 |
| Papua New Guinea | Rotavac | 1 607 485 | 441 152 | 1145 | 1 412 872 | 14 741 493 | 14 223 924 | 432 (298–599) | 2500 |
| Moldova | Rotarix | 319 744 | 114 889 | 9 | 827 754 | 2 576 006 | 2 576 006 | 3433 (1681–5255) | 1900 |
| Republic of Congo | Rotarix | 1 536 206 | 348 359 | 1090 | 4 065 847 | 12 290 418 | 12 290 418 | 292 (97–560) | 1528 |
| Rwanda | Rotarix | 3 585 413 | 715 749 | 2539 | 2 031 404 | 24 601 057 | 9 419 779 | 112 (37–207) | 703 |
| São Tomé and Príncipe | Rotateq | 66 017 | 15 146 | 27 | 169 848 | 969 103 | 790 346 | 729 (453–1378) | 1715 |
| Senegal | Rotarix | 5 356 154 | 1 078 428 | 3025 | 3 505 164 | 36 891 487 | 14 096 578 | 134 (53–217) | 953 |
| Sierra Leone | Rotarix | 2 237 531 | 494 152 | 4068 | 1 096 614 | 15 995 382 | 6 111 983 | 51 (26–78) | 505 |
| Solomon Islands | Rotasiil | 168 506 | 39 695 | 35 | 179 991 | 1 363 103 | 1 275 262 | 1116 (604–1768) | 2005 |
| Somalia | Rotasiil | 2 731 238 | 791 252 | 7413 | 926 056 | 19 683 313 | 11 585 139 | 58 (31–90) | 434 |
| South Sudan | Rotasiil | 1 160 690 | 362 015 | 2364 | 615 357 | 8 780 072 | 5 167 745 | 77 (34–135) | 759 |
| Sri Lanka | Rotavac | 2 868 156 | 1 315 209 | 69 | 4 936 522 | 23 848 677 | 23 848 677 | 3938 (1801–6603) | 3835 |
| Sudan | Rotarix | 12 845 274 | 2 833 307 | 14 567 | 8 823 965 | 103 124 331 | 77 184 096 | 184 (121–245) | 2415 |
| Tajikistan | Rotarix | 2 291 676 | 515 367 | 1320 | 995 629 | 18 273 646 | 10 308 538 | 265 (159–371) | 796 |
| Gambia | Rotarix | 818 934 | 167 343 | 533 | 330 064 | 5 663 362 | 2 164 023 | 137 (41–276) | 473 |
| Timor-Leste | Rotasiil | 391 577 | 99 103 | 242 | 763 884 | 3 322 673 | 3 322 673 | 400 (163–726) | 1405 |
| Togo | Rotarix | 2 416 422 | 540 639 | 3061 | 1 293 933 | 16 683 379 | 6 374 873 | 67 (27–110) | 578 |
| Uganda | Rotarix | 15 696 079 | 3 497 987 | 9314 | 7 773 885 | 110 814 276 | 42 343 158 | 146 (76–212) | 580 |
| Ukraine | Rotasiil | 630 398 | 692 799 | 35 | 3 911 990 | 10 290 365 | 10 290 365 | 2609 (812–4 702) | 2072 |
| Tanzania | Rotarix | 22 592 798 | 5 235 956 | 12 673 | 12 913 994 | 154 758 157 | 66 128 551 | 160 (75–255) | 852 |
| Uzbekistan | Rotarix | 5 772 626 | 1 169 250 | 747 | 2 809 315 | 45 978 443 | 45 978 443 | 1979 (532–4606) | 2111 |
| Vietnam | Rotavac | 14 251 963 | 4 522 533 | 1963 | 42 895 925 | 161 291 552 | 161 291 552 | 1930 (410–5174) | 2171 |
| Yemen | Rotarix | 6 182 800 | 1 383 818 | 5075 | 2 560 785 | 62 941 149 | 42 007 560 | 303 (160–471) | 990 |
| Zambia | Rotarix | 6 573 203 | 1 560 484 | 6263 | 4 279 137 | 66 609 961 | 45 525 340 | 259 (196–316) | 1270 |
| Zimbabwe | Rotarix | 4 669 296 | 1 044 688 | 4766 | 2 768 787 | 41 811 906 | 21 871 830 | 159 (109–210) | 1029 |
DALY=disability-adjusted life-year. GDP=gross domestic product.
Discounted value.
Figures in parentheses show 95% uncertainty intervals (2·5th and 97·5th percentiles of 1000 simulations).
From the government perspective, regional ICERs represent only a small share of the GDP per capita, ranging from 0·09 times GDP per capita in the African region to 0·50 times GDP per capita in the European region. Across Gavi countries, the cost per DALY averted is approximately 0·16 times GDP per capita.
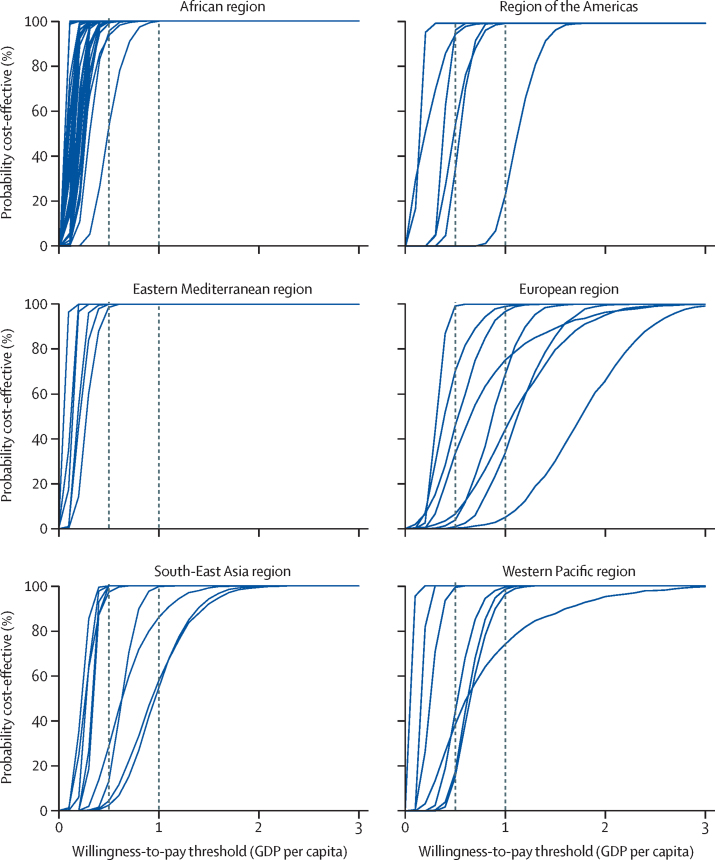
Results from the probabilistic analysis show that there is a very high probability (>90%) that the discounted US$ per DALY averted will be less than 0·5 times the national GDP per capita in 54 countries and less than 1·0 times GDP per capita in 63 countries. Countries where the probability of rotavirus vaccination being cost-effective is the lowest are in the Americas, Europe, and Western Pacific, which is consistent with the results of our deterministic analysis (figure 2).
Figure 2.
Cost-effectiveness acceptability curves for 72 Gavi countries,* displayed per WHO region
Curves are country-specific and show the probability in the base-case scenario for rotavirus vaccination to be cost-effective from the government perspective (accounting for Gavi subsidy) at different thresholds. Vertical dotted lines represent thresholds of 0·5 times and 1·0 times GDP per capita. GDP=gross domestic product. *North Korea was excluded because of the absence of data for GDP per capita.
Four countries stand out as having less than 50% probability of rotavirus vaccine being cost-effective at 1·0 times GDP per capita threshold: Armenia, Honduras, Moldova, and Ukraine. These four countries are fully self-financing countries. The appendix contains the results of our alternative scenarios, showing ICERs for all different vaccines in each country.
Discussion
Rotavirus vaccination is an impactful and cost-effective intervention for a disease that causes around 200 000 deaths in children younger than 5 years each year. This analysis serves as an important reminder to continue to prioritise immunisation in the context of efforts to achieve universal health coverage, health equity, and other important priorities. Immunisation should continue to be an essential component of these efforts, especially for countries that face decreasing international financial assistance and pressure to achieve additional objectives.
In previous studies, the cost per DALY averted for all Gavi-supported countries was reported to be $42.15, 16 Although the ICERs presented here are higher than in previous analyses, rotavirus vaccination is likely to be cost-effective across most Gavi countries, even in countries not receiving any support and not accessing lower, Gavi-like vaccine prices. Comparison with previous studies is not straightforward because of differing methods and assumptions; however, results are in line with the various trends affecting rotavirus cost-effectiveness, including decreases in rotavirus mortality and reduced donor support. A comparison of the results presented in this Article and the previous analysis15 shows that the largest changes in ICERs across Gavi countries are due to changes in rotavirus burden estimates and increases in the prices countries pay for vaccines as they transition from Gavi support.
Like previous analyses, this analysis also shows that the health benefits of rotavirus vaccination are concentrated in the highest-burden regions.15, 16 In addition, many of the countries that are most quickly transitioning from Gavi support are also the ones with a lower burden of disease. Unsurprisingly, rotavirus vaccination is less cost-effective in some lower-burden regions that pay higher vaccine prices (Figure 1, Figure 2). The geographies in which rotavirus vaccination is least cost-effective because of a lower burden and higher vaccine costs are those that appear to benefit most from the availability of new rotavirus vaccine products with lower prices. Such products are appearing on the market as the burden is falling and country vaccine costs are rising, and as countries shoulder a larger share of their vaccine costs, these new lower-cost vaccine products have the potential to reduce or to mitigate the effects of declining international support. A full product comparison by country is beyond the scope of this analysis, but additional analyses might illustrate economic benefits for lower-cost products in countries with less access to Gavi support.
Finally, this analysis and comparison to previous work is being undertaken in the context of evolving guidance on cost-effectiveness thresholds. Previous cost-effectiveness analyses have relied on the guidance from the WHO World Health Report, using 3·0 times the GDP per capita as a threshold to characterise cost-effective interventions, and 1·0 times the GDP per capita for highly cost-effective interventions.51 This guidance has been updated since 2012, highlighting the need to account for additional dimensions when framing cost-effectiveness results such as affordability, feasibility, and other country-specific factors.52 Attempts to refine these norms have resulted in more stringent thresholds.53 Although we were unable to apply a country-contextualised threshold in a global analysis, we did apply more stringent willingness-to-pay thresholds. Vaccination has always been considered one of the best buys in public health and the evolving norms in interpreting cost-effectiveness results have not fundamentally changed the outcome of our analysis. Rotavirus vaccination still represents good, if not excellent, value for money, which is an important message for donors such as Gavi and country governments.
This analysis includes several limitations worth noting. First, we used a transparent and widely used static cohort model to estimate only the direct effect among vaccinated children. Excluding other indirect (herd immunity) effects is likely to underestimate the potential impact and value of rotavirus vaccines, so results from this Article should be viewed as conservative estimates. Second, this is a global analysis. Although we explored rotavirus vaccination for 73 countries, several input values used for modelling were average values at a global or regional level and not country-specific, so results should be interpreted cautiously. A country study involving detailed country engagement would probably yield improved data inputs leading to more accurate results. Third, several data inputs are uncertain. In the absence of a reliable measure of treatment-seeking rates and access to care, we used proportions of cases seeking care and DTP1 coverage rate as an indicator for access to care and ran a plausibility check against the limited country-specific data available in the published literature. Further, although we searched a comprehensive database to inform cost estimates of incremental vaccine programmes, data were not available for many countries and we were not able to differentiate these costs by vaccine product.46 In addition, we projected each country's Gavi eligibility status into the future based on current Gavi status, the Gavi transition policy, and projected International Monetary Fund growth rates. Although we believe our projections are reasonable, economic growth is difficult to project and deviations from projections will influence Gavi transitions, vaccine prices, and country-specific results. Finally, this Article addresses the impact and cost-effectiveness of rotavirus vaccination. Although value for money is a crucial consideration, affordability is also essential and can be examined through a budget impact analysis. Although crucial to decision making for a country, budget impacts are beyond the scope of this analysis. However, our finding that rotavirus vaccine is likely to be less cost-effective in countries with less international support highlights the importance of affordability and the need for such analyses.
Overall, rotavirus vaccination offers strong value for money across Gavi countries despite important global trends contributing to higher cost-effectiveness ratios. Countries transitioning away from Gavi support should explore newly prequalified vaccines as an option that might provide enhanced value for money. Countries that have yet to introduce rotavirus vaccination should actively consider the potential benefits and cost-effectiveness of rotavirus vaccination as a step to achieving broader health goals.
Contributors
FD, CP, and AC designed the study. AC developed the model. RB, CS, and JT provided data for the analysis. FD, AC, and RB performed the data collection and data analysis. FD developed the tables and figures and wrote the draft manuscript. LK developed the maps. DA, CP, JT, and UP revised the manuscript and provided scientific inputs. All authors edited and approved the final manuscript.
Declaration of interests
FD and CP report grants from the Bill & Melinda Gates Foundation over the course of the study. All other authors declare no competing interests. The findings and conclusions in this Article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Material
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Barber R, Phillips DE, Lopez AD, Murray CJL. Causes of child death: comparison of MCEE and GBD 2013 estimates—Authors' reply. Lancet. 2015;385:2462–2464. doi: 10.1016/S0140-6736(15)61133-3. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Oza S, Hogan D. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 4.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62:S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 5.Clark A, Black R, Tate J. Estimating global, regional and national rotavirus deaths in children aged <5 years: current approaches, new analyses and proposed improvements. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215:1666–1672. doi: 10.1093/infdis/jix186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strategic Advisory Group of Experts Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009;84:533–537. [Google Scholar]
- 8.Gavi. the Vaccine Alliance Rotavirus vaccine support. https://www.gavi.org/support/nvs/rotavirus
- 9.International Vaccine Access Center VIEW-hub report: global vaccine introduction and implementation. Baltimore, MD. 2017. https://www.dropbox.com/s/mt9mhc11zaqeusc/IVAC_VIEW-hub_Report 2017Mar_External.pdf?dl=0
- 10.The World Bank Gross national income (GNI) per capita in constant US$ 2010. https://data.worldbank.org/indicator/NY.GNP.PCAP.KD?locations=ZG
- 11.Gavi. the Vaccine Alliance Eligibility and transition policy. https://www.gavi.org/about/programme-policies/eligibility-and-transition
- 12.The Global Fund Eligibility. 2019. https://www.theglobalfund.org/en/funding-model/before-applying/eligibility
- 13.International Development Association Borrowing countries. 2019. http://ida.worldbank.org/about/borrowing-countries
- 14.Gavi. the Vaccine Alliance Eligibility. 2019. https://www.gavi.org/support/sustainability/eligibility [DOI] [PubMed]
- 15.Atherly DE, Lewis KDC, Tate J, Parashar UD, Rheingans RD. Projected health and economic impact of rotavirus vaccination in GAVI-eligible countries: 2011–2030. Vaccine. 2012;30(suppl 1):A7–A14. doi: 10.1016/j.vaccine.2011.12.096. [DOI] [PubMed] [Google Scholar]
- 16.Atherly D, Dreibelbis R, Parashar UD, Levin C, Wecker J, Rheingans RD. Rotavirus vaccination: cost-effectiveness and impact on child mortality in developing countries. J Infect Dis. 2009;200:S28–S38. doi: 10.1086/605033. [DOI] [PubMed] [Google Scholar]
- 17.Rheingans R, Amaya M, Anderson JD, Chakraborty P, Atem J. Systematic review of the economic value of diarrheal vaccines. Hum Vaccin Immunother. 2014;10:1582–1594. doi: 10.4161/hv.29352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotirum S, Vutipongsatorn N, Kongpakwattana K, Hutubessy R, Chaiyakunapruk N. Global economic evaluations of rotavirus vaccines: a systematic review. Vaccine. 2017;35:3364–3386. doi: 10.1016/j.vaccine.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 19.Kim S-Y, Sweet S, Slichter D, Goldie SJ. Health and economic impact of rotavirus vaccination in GAVI-eligible countries. BMC Public Health. 2010;10:253. doi: 10.1186/1471-2458-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider S, Chaikledkaew U, Thavorncharoensap M, Youngkong S, Islam MA, Thakkinstian A. Systematic review and meta-analysis of cost-effectiveness of rotavirus vaccine in low-income and lower-middle-income countries. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marseille E, Larson B, Kazi D, Kahn J, Rosen S. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 23.Pan American Health Organization. WHO Provac Toolkit. https://www.paho.org/provac-toolkit/tools/about-univac
- 24.Clark A, Jauregui B, Griffiths U. TRIVAC decision-support model for evaluating the cost-effectiveness of Haemophilus influenzae type b, pneumococcal and rotavirus vaccination. Vaccine. 2013;31(suppl 3):C19–C29. doi: 10.1016/j.vaccine.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 25.Clark A, Tate J, Parashar U, et al. Mortality reduction benefits and intussusception risks of rotavirus vaccination in 135 low-income and middle-income countries: a modelling analysis of current and alternative schedules. Lancet Glob Health7: e1541–52. [DOI] [PMC free article] [PubMed]
- 26.Bilcke J, Van Damme P, Van Ranst M, Hens N, Aerts M, Beutels P. Estimating the incidence of symptomatic rotavirus infections: a systematic review and meta-analysis. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker CLF, Rudan I, Liu L. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO WHO/UNICEF estimates of national immunization coverage. 2017. https://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html
- 29.Hasso-Agopsowicz M, Ladva CN, Lopman B. Global review of the age distribution of rotavirus disease in children aged <5 years before the introduction of rotavirus vaccination. Clin Infect Dis. 2019;69:10071–10078. doi: 10.1093/cid/ciz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen C, Armero Guardado JA, Alberto P. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30(suppl 1):6–10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- 31.Bahl R, Ray P, Subodh S. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis. 2005;192(suppl 1):114–119. doi: 10.1086/431497. [DOI] [PubMed] [Google Scholar]
- 32.Omore R, Khagayi S, Ogwel B. Rates of hospitalization and death for all-cause and rotavirus acute gastroenteritis before rotavirus vaccine introduction in Kenya, 2010–2013. BMC Infect Dis. 2019;19:47. doi: 10.1186/s12879-018-3615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rheingans RD, Constenla D, Antil L, Innis BL, Breuer T. Economic and health burden of rotavirus gastroenteritis for the 2003 birth cohort in eight Latin American and Caribbean countries. Rev Panam Salud Publica. 2007;21:192–204. doi: 10.1590/s1020-49892007000300002. [DOI] [PubMed] [Google Scholar]
- 34.Carlos CC, Inobaya MT, Bresee JS. The burden of hospitalizations and clinic visits for rotavirus disease in children aged <5 years in the Philippines. J Infect Dis. 2009;200(suppl 1):174–181. doi: 10.1086/605044. [DOI] [PubMed] [Google Scholar]
- 35.Fischer TK, Anh DD, Antil L. Health care costs of diarrheal disease and estimates of the cost-effectiveness of rotavirus vaccination in Vietnam. J Infect Dis. 2005;192:1720–1726. doi: 10.1086/497339. [DOI] [PubMed] [Google Scholar]
- 36.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomon JA, Haagsma JA, Davis A. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention . Rotavirus. In: Hamborsky J, Kroger A, Wolfe C, editors. Epidemiology and prevention of vaccine preventable diseases. 13th edn. Public Health Foundation; Washington DC: 2015. https://www.cdc.gov/vaccines/pubs/pinkbook/rota.html [Google Scholar]
- 39.WHO WHO prequalified vaccines. 2018. https://extranet.who.int/gavi/PQ_Web/Default.aspx?nav=2
- 40.Clark A, van Zandvoort K, Flasche S. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis. 2019;19:717–727. doi: 10.1016/S1473-3099(19)30126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO Rotavirus vaccines WHO position paper—January, 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 42.Gavi. the Vaccine Alliance Gavi, the Vaccine Alliance co-financing policy version 2.0. 2016. https://www.gavi.org/library/gavi-documents/policies/gavi-co-financing-policy
- 43.Gavi. the Vaccine Alliance Detailed product profiles (DPPs) for WHO prequalified vaccines. 2019. http://www.gavi.org/library/gavi-documents/supply-procurement/detailed-product-profiles
- 44.WHO MI4A/V3P: vaccine pricing: Gavi fully self-financing & accelerated transition countries. https://www.who.int/immunization/programmes_systems/procurement/v3p/platform/module2/Factsheet_vacc_pricing_Gavi_transitioning.pdf
- 45.WHO MI4A/V3P: vaccine purchase database. https://www.who.int/immunization/programmes_systems/procurement/v3p/platform/module1/en
- 46.Immunization Costing Action Network Unit cost repository for immunization delivery. 2018. https://immunizationeconomics.org/ican-idcc
- 47.UNICEF Supplies and logistics: handling fees. 2015. https://www.unicef.org/supply/index_62330.html
- 48.WHO Cost effectiveness and strategic planning (WHO-CHOICE) https://www.who.int/choice/cost-effectiveness/en
- 49.Kotloff KL, Nataro JP, Blackwelder WC. Burden and etiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 50.Doucleff M. Merck pulls out of agreement to supply life-saving vaccine to millions of kids. NPR Morning Ed. 2018. https://www.npr.org/sections/goatsandsoda/2018/11/01/655844287/merck-pulls-out-of-agreement-to-supply-life-saving-vaccine-to-millions-of-kids?t=1543839217599
- 51.WHO The World Health Report 2002—reducing risks, promoting healthy life. Geneva. 2002. https://www.who.int/whr/2002/en/whr02_en.pdf?ua=1 [DOI] [PubMed]
- 52.Bertram MY, Lauer JA, De Joncheere K. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94:925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19:929–935. doi: 10.1016/j.jval.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.