Abstract
Background
Long noncoding RNAs (lncRNAs) have been identified as key players in promoting tumourigenesis in osteosarcoma. LncRNA OR3A4 (OR3A4) has been reported as an oncogene in a number of tumours. However, the clinical value of OR3A4 in osteosarcoma and the role of OR3A4 in osteosarcoma progression are still unknown.
Methods
The expression levels of OR3A4 in the tumour tissue of osteosarcoma patients and osteosarcoma cell lines were detected by RT-PCR. Kaplan-Meier analysis and log-rank test were performed to evaluate the relationship between the level of OR3A4 expression and the prognosis of osteosarcoma patients. We investigated the association between the tissue expression levels of OR3A4 and different clinicopathological characteristics of osteosarcoma patients by χ2 tests. Bioinformatic databases and luciferase reporter assays were used to predict and validate the target microRNA of OR3A4. Finally, the role of OR3A4 in the proliferation and invasion of osteosarcoma cells was tested by MTT and Transwell assays, respectively.
Results
We observed that the expression level of OR3A4 was upregulated in the tumour tissue of osteosarcoma patients (p < 0.001) and osteosarcoma cell lines (p < 0.01) compared with the normal adjacent tissue and a normal human foetal osteoblastic cell line, respectively. The survival curve revealed that patients with high expression levels of OR3A4 had lower overall survival. Increased OR3A4 expression in osteosarcoma patients was associated with distant metastasis (p = 0.02) and advanced clinical stage (p < 0.001). In addition, bioinformatics analysis and luciferase reporter assays verified the complementary binding between OR3A4 and miR-1227-5p. Furthermore, we found that OR3A4 acted as a miR-1227-5p “sponge” to modulate osteosarcoma cell proliferation and invasion via downregulation of miR-1227-5p.
Conclusion
OR3A4 promotes osteosarcoma cell proliferation and invasion by sponging miR-1227-5p, which might be related to the metastasis of osteosarcoma and could be used as a potential prognostic biomarker and therapeutic target in osteosarcoma.
Keywords: Osteosarcoma invasion, OR3A4, miR-1227-5p, Prognosis
1. Introduction
Osteosarcoma is one of the most common primary bone tumours and mainly occurs in adolescents and young adults [1]. It is pathologically characterized by spindle cells and abnormal osteoid formation. Extreme malignancy and a strong tendency to metastasize are important features of this tumour. Lung metastasis is frequently observed among most newly diagnosed patients who might develop pulmonary symptoms within one year [2,3]. Despite improvements in combined surgery, chemotherapy and radiotherapy, the 5-year survival rate of patients with advanced osteosarcoma is still unsatisfactory. It has been reported that the overall 5-year survival rate of osteosarcoma patients is 60–70% [4]. Therefore, it is imperative to determine the underlying molecular mechanisms of osteosarcoma development and progression, which might contribute to the discovery of new biomarkers for osteosarcoma early diagnosis and improve the prognosis of osteosarcoma patients.
Long noncoding RNAs (lncRNAs) are a group of RNAs that are more than 200 nucleotides in length and have limited or no protein-coding ability [5], [6], [7], [8]. Previous studies have shown that lncRNAs are important factors in several cellular processes, including cell proliferation, differentiation, migration, invasion, metabolism and apoptosis [9,10]. Aberrant expression of lncRNAs has been observed in various human cancers, such as glioma, breast cancer, bladder cancer, hepatocellular carcinoma and lung cancer, and increasing evidence has demonstrated that it is involved in cancer metastasis [11]. In addition, the expression of lncRNAs in serum samples of patients has been used to diagnose different kinds of human cancers, which suggests that lncRNAs are potential diagnostic markers of cancer [12,13]. Recently, lncRNA OR3A4, which stands for lncRNA olfactory receptor, family 3, subfamily A, member 4, was detected to be abnormally expressed in gastric cancer and breast cancer and to function as an oncogene, promoting tumourigenesis, metastasis and angiogenesis [14,15]. However, there is no record of the relationship between OR3A4 and osteosarcoma.
In the present study, we detected the expression of OR3A4 in osteosarcoma tissues and osteosarcoma cell lines. In addition, we analysed the role of OR3A4 in the proliferation and invasion of osteosarcoma cells. To further clarify the mechanism underlying the promoting effect of OR3A4 on osteosarcoma cell metastasis, we predicted and validated the target miRNA of OR3A4. Finally, the effect of the target miRNA on OR3A4 inducing osteosarcoma cell metastasis was explored.
2. Materials and methods
2.1. Patients and specimens
A total of 98 osteosarcoma patients who underwent surgical resection were recruited at the Department of Pathology at the First Affiliated Hospital of Hainan Medical University and the First Affiliated Hospital of Xinjiang Medical University from 2011 to 2013. None of the patients received chemotherapy or radiotherapy, and all patients signed a written informed consent form before the surgery. All patients underwent routine follow-up after surgery until death or the last follow-up period, and the median follow-up was 44 months. To preserve the specimens appropriately, osteosarcoma tumours and normal adjacent specimens were immediately frozen in liquid nitrogen and stored at −80 °C as soon as they were excised. The diagnosis and clinical stage of the osteosarcoma patients were classified according to the Tumor Node Metastasis (TNM) Classification of Malignant Tumors (sixth edition) from the Union for International Cancer Control (UICC) by two experienced pathologists [16]. The Ethical Committee for Clinical Research approved the protocol for this study.
2.2. Cell culture and cell transfection
The human osteosarcoma cell lines U2OS, HOS, and MG-63 and the normal human foetal osteoblastic cell line hFOB were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS. Cells were maintained in a humidified incubator at 37 °C with 5% CO2. MiR-1227-5p inhibitor and si-OR3A4 were synthesized and purchased from Shanghai GenePharma Co., Ltd. Cell transfection was performed with Lipofectamine 3000 (Invitrogen) following the manufacturer's instructions.
2.3. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from osteosarcoma tumours, normal adjacent specimens and cell lines by TRIzol reagent (Invitrogen) following the manufacturer's instructions. The expression levels of OR3A4 were quantified with SYBR Master Mix (TAKARA, Tokyo, Japan) on a LightCycler 480 (Roche Applied Science, Mannheim, Germany). U6 levels were used as the internal controls. Relative mRNA expression was analysed using the ΔΔCt method. The primers were as follows: OR3A4-F: ACTGCTAGTGGAAGACAGCC, OR3A4-R: GTTTCCATAAGGATGGCCGC; U6-F: ATTGGAACGATACAGAGAAGATT, and U6-R: GGAACGCTTCACGAA -TTTG.
2.4. Cell proliferation assay
Osteosarcoma cells and hFOB cells were cultured in 96-well plates at a density of 3.0 × 103 cells/well for three days. The MTT assay was used to measure cell proliferation according to the manufacturer's instructions. Before analysis, 20 ml methylthiazolyldiphenyl-tetrazolium bromide (MTT) reagent (2.5 mg/ml) was added to each well, and the plates were incubated for 4 h at 37 °C. Then, the medium was removed, and the cells were solubilized in 200 µl of dimethyl sulfoxide for analysis at 492 nm on a microplate reader (Thermo Scientific, Waltham, MA, USA).
2.5. Transwell invasion assay
Transwell chambers with an 8-µm pore size (Corning, Corning, NY, USA) were used to observe osteosarcoma cell invasion. Two hundred microliters of osteosarcoma cell suspension containing 1 × 105 cells in serum-free medium was seeded into the upper chamber, which was coated with Matrigel (BD Biosciences, San Diego, CA, USA). Then, 800 µl of DMEM supplemented with 10% FBS was added to the lower chamber. After 48 h of incubation, the cells on the top side of the membrane were removed by a swab. The invasive cells on the basal side were fixed and stained with 0.1% crystal violet solution and were counted using a high-power microscope (Olympus Corporation, Tokyo, Japan).
2.6. Target gene prediction
To predict miRNAs that interact with OR3A4, miRDB14 (http://mirdb.org/), DIANA IncBase v212 (http://diana.imis.athena-innovation.gr/) and RegRNA2.0 (http://regrna2.mbc.nctu.edu.tw/detection.html) were used.
2.7. Luciferase reporter assay
To verify the interaction between OR3A4 and miR-1227-5p, wild-type pmirGLO-OR3A4 and different mutant pmirGLO-OR3A4 vectors were constructed by GenePharma, Shanghai, China. The miR-1227-5p mimic, miR-1227-5p inhibitor and negative control (NC) mimic and inhibitor were purchased from GenePharma, Shanghai, China. One hundred microliters of HEK293T cell suspension containing 8 × 103 cells was seeded in 96-well plates and incubated at 37 °C with 5% CO2 for 24 h. Lipofectamine 3000 was used to co-transfect the wild-type or mutant luciferase reporter plasmids and miR-1227-5p mimic or NC in cells. Following 48 h, a Dual-Luciferase Reporter Assay System (Promega, USA) was used to measure the luciferase activity following the manufacturer's protocol.
2.8. Statistical analysis
All the results are presented as the means ± SD. Statistical differences were analysed using SPSS 20.0 statistical software (IBM Corporation, USA). The p-values were calculated using a two-tailed Student's t-test and Pearson's χ2 tests. Kaplan–Meier curves and the log-rank test were used for the survival rate. p < 0.05 was considered significant.
3. Results
3.1. The expression of OR3A4 was upregulated in osteosarcoma and associated with poor prognosis
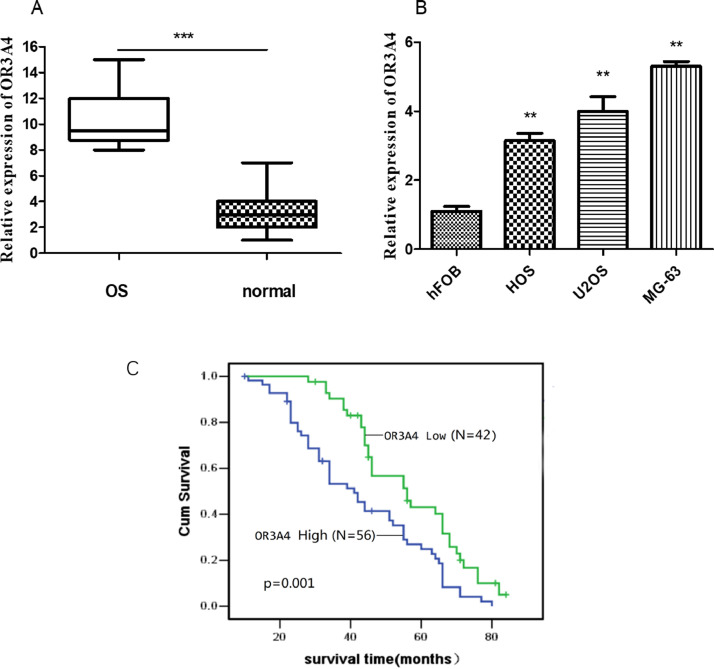
The expression of OR3A4 in 98 osteosarcoma tumour tissues and normal adjacent tissues was examined using qRT-PCR. As shown in Fig. 1(A), the expression of OR3A4 in osteosarcoma tumour tissues was significantly higher than that of the normal adjacent tissues (p < 0.001). To confirm the expression of OR3A4 in osteosarcoma, the expression levels of OR3A4 in osteosarcoma cell lines (U2OS, HOS, MG-63) and a normal human foetal osteoblastic cell line (hFOB) were observed by qRT-PCR. Fig. 1(B) shows that the expression levels of OR3A4 were significantly upregulated in U2OS, HOS and MG-63 cells compared with hFOB cells (p < 0.01), which was consistent with the tissue results. Kaplan-Meier analysis and log-rank test were applied to evaluate the relationship between the expression levels of OR3A4 and the prognosis of osteosarcoma patients (Fig. 1(C)). The survival curve revealed that patients with high expression level of OR3A4 had lower overall survival, suggesting that high OR3A4 expression was a significant predictor of a poorer prognosis than low expression. In brief, OR3A4 was verified to be upregulated in osteosarcoma tissue and cell lines, and high OR3A4 expression was related to poor prognosis.
Fig. 1.
OR3A4 was upregulated in osteosarcoma tissue and cell lines, which was correlated with poor prognosis. (A) The expression of OR3A4 in osteosarcoma patient tissue was significantly higher than that in normal adjacent tissue. (B) Higher expression of OR3A4 was found in osteosarcoma cell lines (U2OS, HOS and MG-63) than in the control cell line (hFOB). (C) Kaplan–Meier analysis showed that patients with high OR3A4 expression had significantly lower survival than patients with low OR3A4 expression. **p < 0.01, ***p < 0.001, compared to the control group.
3.2. Increased tissue expression of OR3A4 was associated with the clinicopathological characteristics of osteosarcoma patients
To further explore the role of OR3A4 in osteosarcoma, a preliminary analysis was performed to identify whether the expression of OR3A4 in osteosarcoma patient tissue was associated with the clinicopathological parameters of osteosarcoma patients. According to the median value of OR3A4 expression, patients were divided into high and low OR3A4 expression groups. As shown in Table 1, the results showed that osteosarcoma with high OR3A4 expression tended to present distant metastasis (p = 0.02) and a more advanced clinical stage (p < 0.001), which indicated that OR3A4 played an important role in osteosarcoma progression. However, no significant association was found between OR3A4 expression and other clinical features, such as age, gender and tumour size.
Table 1.
Relationship between OR3A4 expression and clinical features of osteosarcoma.
| Variable | Number of cases | OR3A4 expression |
χ2 | p | |
|---|---|---|---|---|---|
| High(n = 56) | Low(n = 42) | ||||
| Gender | |||||
| Male | 47 | 27 | 20 | 0.003 | 0.953 |
| Female | 51 | 29 | 22 | ||
| Age (years) | |||||
| < 20 | 50 | 25 | 25 | 2.127 | 0.145 |
| ≥20 | 48 | 31 | 17 | ||
| Tumor size (cm) | |||||
| < 5 cm | 49 | 30 | 19 | 0.667 | 0.414 |
| ≥5 cm | 49 | 26 | 23 | ||
| Tumor metastasis | |||||
| Presence | 78 | 49 | 29 | 5.03 | 0.02 |
| Absence | 20 | 7 | 13 | ||
| Clinical stage | |||||
| I/II | 40 | 10 | 30 | 28.51 | <0.001 |
| III/IV | 58 | 46 | 12 | ||
3.3. Downregulation of OR3A4 significantly inhibited the proliferation and invasion of osteosarcoma cells
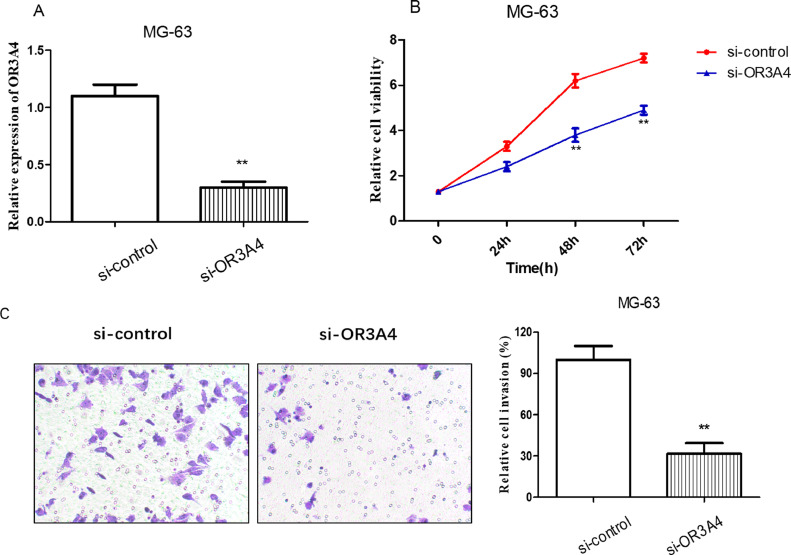
Next, the RNAi method was used to knock down endogenous OR3A4 expression. si-control and si-OR3A4 were developed to further investigate the biological roles of OR3A4 in osteosarcoma cells. As shown in Fig. 2(A), qRT-PCR analysis was conducted at 48 h after transfection, and compared with that in the si-control, the expression of OR3A4 in MG-63 cells transfected with si-OR3A4 was significantly reduced. We used the MTT assay and Transwell invasion assay to assess the potential roles of OR3A4 in osteosarcoma cancer progression. The proliferation of MG-63 cells transfected with si-OR3A4 was inhibited compared with that of cells transfected with si-control (p < 0.01) (Fig. 2(B)). Consistent with the results of the MTT assay, transfection with si-OR3A4 resulted in a significantly decreased MG-63 cell invasion (p < 0.01) (Fig. 2(C)). Collectively, these results suggest that OR3A4 may play an oncogenic role in osteosarcoma progression.
Fig. 2.
Downregulation of OR3A4 inhibited the proliferation and invasion of osteosarcoma cells. (A) qRT-PCR was used to detect the expression of OR3A4 in the MG-63 cell line transfected with si-OR3A4. (B) MTT assay was performed to analyse the effect of OR3A4 on MG-63 cell proliferation. (C) Transwell assay was conducted to determine the effect of OR3A4 on MG-63 cell invasion **p < 0.01 compared to the control group.
3.4. MiR-1227-5p is the target miRNA of OR3A4
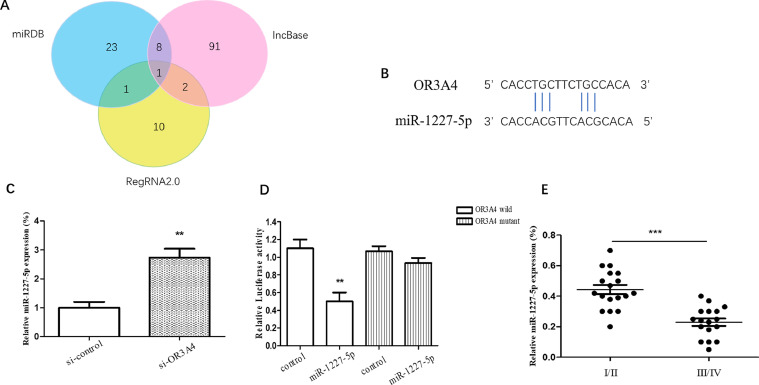
Thus, to further investigate the oncogenic role of OR3A4 in osteosarcoma, we predicted the potential target miRNAs of OR3A4 using bioinformatics analysis. RegRNA2.0, miRDB and DIANA IncBase v2 identified 14, 33 and 102 miRNAs that may interact with OR3A4, respectively (Fig. 3(A)). MiR-1227-5p was found to be the target miRNA of OR3A4. The complementary binding sites of miR-1227-5p and OR3A4 are shown in Fig. 3(B). The qRT-PCR results showed that the expression of miR-1227-5p1227-5p was elevated after transfection with si-OR3A4 in MG-63 cells (Fig. 3(C)). The luciferase reporter assay results showed that the miR-1227-5p mimic inhibited the relative luciferase activity compared with the control mimic in the OR3A4 wild-type group, while there was no difference between the miR-1227-5p mimic and the control mimic in the OR3A4 mutant group (Fig. 3(D)). The clinical data showed that miR-1227-5p was significantly decreased in clinical stage III-IV compared with clinical stage I-II (Fig. 3(E)).
Fig. 3.
MiR-1227-5p was the potential target microRNA of OR3A4, which was predicted and validated by bioinformatics analysis and luciferase reporter assay. (A) A flow chart to screen the miRNAs based on the diagrams. (B) The complementary binding site of OR3A4 and miR-1227-5p was predicted by bioinformatics. (C) qRT-PCR was performed to observe the expression of miR-1227-5p in MG-63 cells transfected with si-OR3A4. (D) Luciferase reporter assay was used to confirm the predicted binding. (E) The expression of miR-1227-5p in clinical stages I/II and III/IV of osteosarcoma was detected by qRT-PCR. **p < 0.01, ***p < 0.001, compared to the control group.
3.5. OR3A4 accelerated osteosarcoma cell proliferation and invasion by targeting miR-1227-5p
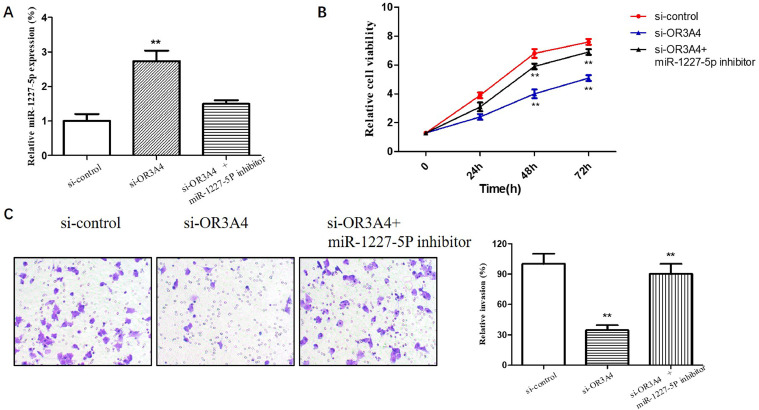
Bioinformatic analysis and luciferase reporter assays were used to predict and verify the complementary binding of OR3A4 and miR-1227-5p. The result was confirmed by qRT-PCR. Thus, we performed further experiments to verify the competing endogenous RNA (ceRNA) regulatory mechanism of OR3A4 targeting miR-1227-5p. As shown in Fig. 4(A), the expression of miR-1227-5p was elevated in the si-OR3A4 transfected group but significantly decreased in the si-OR3A4 and miR-1227-5p inhibitor co-transfected group (p < 0.01). MTT assays and Transwell invasion assays showed that the miR-1227-5p1227-5p inhibitor prominently reversed the inhibitory effect of si-OR3A4 on osteosarcoma cell proliferation and invasion (p < 0.01, p < 0.01) (Fig. 4(B) and (C)). All of the rescue experiments suggested that si-OR3A4 and miR-1227-5p inhibitor exerted opposite effects on osteosarcoma metastasis, indicating a ceRNA role of OR3A4 in regulating miR-1227-5p.
Fig. 4.
OR3A4 accelerated osteosarcoma cell proliferation and invasion by targeting miR-1227-5p. (A) The expression levels of miR-1227-5p in MG-63 cells transfected with si-OR3A4 and miR-1227-5p inhibitor. (B) MTT assay was used to observe the role of si-OR3A4+miR-1227-5p inhibitor on MG-63 cell proliferation. (C) Transwell assay was used to detect the effect of si-OR3A4+miR-1227-5p inhibitor on MG-63 cell invasion. **p < 0.01 compared to the control group.
4. Discussion
The 5-year survival rate of osteosarcoma, the most common type of primary bone tumour, has improved over the past decade because of advances in surgical techniques [17]. However, due to the lack of effective biomarkers for early diagnosis and efficient targets for treatment, osteosarcoma-related morbidity remains high. Clinical evidence suggests that osteosarcoma cell proliferation and invasion to regional areas is a crucial stage in tumour development; however, the molecular mechanism of the regulation of osteosarcoma cell metastasis is unclear. Therefore, exploring the specific molecular mechanism might contribute to the identification of early diagnostic markers and new therapeutic targets of osteosarcoma.
Currently, noncoding RNAs (ncRNAs) have been reported to be involved in processes that are likely to induce osteosarcoma progression, including cell proliferation and invasion, and may serve as novel and effective therapeutic targets [18]. Studies have shown that lncRNAs, as one type of ncRNAs, play important roles in cancer progression and have been proven to be associated with the development of different tumour types, including breast cancer, cholangiocarcinoma, and prostate cancer. Previous evidence indicates that the expression of lncRNAs in cancer patient blood or urine is aberrant, suggesting that lncRNAs may serve as cancer diagnostic biomarkers [19], [20], [21]. OR3A4 is abnormally expressed and plays an oncogenic role in a series of tumours. For example, the expression of OR3A4 in hepatocellular carcinoma tissues and cell lines is upregulated, and OR3A4 participates in the angiogenesis of hepatocellular carcinoma by modulating the AGGF1/akt/mTOR pathway [22,23]. However, the role of OR3A4 in osteosarcoma remains unclear. In the present study, our results showed that the expression of OR3A4 was elevated in osteosarcoma tissues and cell lines compared with that in adjacent normal tissues and the normal human foetal osteoblastic cell line. Moreover, osteosarcoma patients with high OR3A4 expression tended to present distant metastasis and advanced clinical stage and had a significantly lower survival rate than patients with low OR3A4 expression, suggesting that OR3A4 could be a promising biomarker. Moreover, downregulation of OR3A4 reduced osteosarcoma cell proliferation and invasion. Our results showed that OR3A4 participated in osteosarcoma cell proliferation and invasion. Hence, the OR3A4 expression level may be an important indicator of the occurrence and progression of osteosarcoma.
Previous studies have shown that OR3A4 influences biological functions in gastric cancer cells by regulating the activation of PDLIM2, MACC1, NTN4, and GNB2L1. OR3A4 could enhance ovarian cancer cell metastasis and invasion by suppressing the expression of KLF6 [24]. The classic regulatory mechanism of lncRNAs in various biological functions is acting as a ceRNA [25]. Increasing evidence has shown that lncRNAs regulate cell biological functions by targeting miRNAs that bind to the 3′-UTR of functional mRNAs to act as a miRNA “sponge” or ceRNA [26,27]. Here, bioinformatic analysis was applied to predict the target miRNAs of OR3A4, and the results showed that miR-1227-5p was the potential target miRNA of OR3A4, which was verified by luciferase reporter assay and RT-PCR. MiR-1227-5p is a miRNA that was recently found to be involved in tumourigenesis and metastasis. The downregulated expression of miR-1227-5p in breast cancer is related to lymph node metastasis. A study showed that miR-1227-5p served as a biomarker for the diagnosis of pancreatic and biliary tract cancers [28,29]. Our results showed that the tissue expression of miR-1227-5p was significantly decreased in clinical stage III-IV compared with clinical stage I-II. This result is consistent with a previous study in breast cancer [29]. Our present study found that the expression of miR-1227-5p was elevated in the si-OR3A4 transfected group but significantly decreased in the si-OR3A4 and miR-1227-5p inhibitor co-transfected group. The miR-1227-5p inhibitor prominently reversed the inhibition of si-OR3A4 on osteosarcoma cell proliferation and invasion. The rescue experiments indicated that OR3A4 promoted osteosarcoma cell proliferation and invasion by targeting miR-1227-5p.
In summary, our study demonstrated for the first time the upregulated expression of OR3A4 in osteosarcoma and verified that miR-1227-5p was the target miRNA of OR3A4 in osteosarcoma. The discovery of the role of OR3A4 and miR-1227-5p in osteosarcoma cell proliferation and invasion could lead to the development of osteosarcoma treatment.
CRediT authorship contribution statement
Yang Changcheng: Conceptualization, Methodology, Software, Writing - original draft. Cai Xingrui: Data curation, Writing - original draft, Methodology. Yu Mengsi: Data curation, Methodology, Visualization, Resources, Investigation. Wang Bangmin: Data curation, Resources, Visualization. Wang Song: . He Zhihui: Formal analysis, Investigation. Zeng Jiangzheng: Software, Validation, Data curation. Zhang Boke: Conceptualization, Writing - review & editing, Project administration. Lu Yanda: Conceptualization, Supervision, Funding acquisition.
Declaration of Competing Interest
None.
Acknowledgment
This work is supported by Natural Science Foundation of Hainan Province (No. 814353). The granting agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.12.023.
Contributor Information
Boke Zhang, Email: zhbk2011@163.com.
Yanda Lu, Email: yandalu986@163.com.
Appendix. Supplementary materials
References
- 1.T.A. Marko, B.J. Diessner, L.G. Spector, Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison, Pediatr. Blood Cancer. 63(6) (2016) 1006–1011, doi: 10.1002/pbc.25963. [DOI] [PMC free article] [PubMed]
- 2.Bacci G., Longhi A., Versari M., Mercuri M., Briccoli A., Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 3.Gill J., Ahluwalia M.K., Geller D., Gorlick R. New targets and approaches in osteosarcoma. Pharmacol. Ther. 2013;137(1):89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Omer N., Le Deley M.C., Piperno-Neumann S. Phase-II trials in osteosarcoma recurrences: a systematic review of past experience. Eur. J. Cancer. 2017;75:98–108. doi: 10.1016/j.ejca.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Li Z., Shen J., Chan M.T., Wu W.K. TUG1: a pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016;49(4):471–475. doi: 10.1111/cpr.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu G., Li B., Sun L., An C. MicroRNA-153 inhibits osteosarcoma cells proliferation and invasion by targeting TGF-beta2. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Zhao W., Dong S., Duan B. HOTAIR is a predictive and prognostic biomarker for patients with advanced gastric adenocarcinoma receiving fluorouracil and platinum combination chemotherapy. Am. J. Transl. Res. 2015;7(7):1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 8.Xin Y., Li Z., Shen J., Chan M.T., Wu W.K. CCAT1: a pivotal oncogenic long non-coding RNA in human cancers. Cell Proli. 2016;49(3):255–260. doi: 10.1111/cpr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Zheng L., Wang Q., Hu Y.W. Emerging roles and mechanisms of long noncoding RNAs in atherosclerosis. Int. J. Cardiol. 2017;228:570–582. doi: 10.1016/j.ijcard.2016.11.182. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B., Wang D., Ji T.F., Shi L., Yu J.L. Overexpression of lncRNA anril up-regulates vegf expression and promotes angiogenesis of diabetes mellitus combined with cerebral infarction by activating nf-kappab signaling pathway in a rat model. Oncotarget. 2017;8(10):17347–17359. doi: 10.18632/oncotarget.14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheetham S.W., Gruhl F., Mattick J.S., Dinger M.E. Long noncoding RNAs and the genetics of cancer. Br. J. Cancer. 2013;108(12):2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renganathan A., Felley-Bosco E. Long noncoding RNAs in cancer and therapeutic potential. Adv. Exp. Med. Biol. 2017;1008:199–222. doi: 10.1007/978-981-10-5203-3_7. [DOI] [PubMed] [Google Scholar]
- 13.Li H., Yu B., Li J. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5(8):2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X., Yang Z., Zhi Q. Long noncoding rna OR3A4 promotes metastasis and tumorigenicity in gastric cancer. Oncotarget. 2016;7(21):30276–30294. doi: 10.18632/oncotarget.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G., Hu X., Zhou G. Long non-coding rna OR3A4 promotes proliferation and migration in breast cancer. Biomed. Pharmacother. 2017;96:426–433. doi: 10.1016/j.biopha.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Moore D.D., Luu H.H. Osteosarcoma. Cancer Treat. Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 17.Longhi A., Fabbri N., Donati D. Neoadjuvant chemotherapy for patients with synchronous multifocal osteosarcoma: results in eleven cases. J. Chemother. 2001;13(3):324–330. doi: 10.1179/joc.2001.13.3.324. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q., Yu H., Yin Q., Hu X., Zhang C. lncRNA-NEF is downregulated in osteosarcoma and inhibits cancer cell migration and invasion by downregulating miRNA-21. Oncol. Lett. 2019;17(6):5403–5408. doi: 10.3892/ol.2019.10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi Y., Luo Q., Song Y. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1alpha regulation. J. Cell. Biochem. 2019;120(11):19019–19030. doi: 10.1002/jcb.29225. [DOI] [PubMed] [Google Scholar]
- 20.Wang K.W., Dong M. Role of circular RNAs in gastric cancer: recent advances and prospects. World. J. Gastrointest. Oncol. 2019;11(6):459–469. doi: 10.4251/wjgo.v11.i6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J., Peng X., Dai Y. The long non-Coding rna (lncRNA) AGAP2-AS1 is upregulated in ovarian carcinoma and negatively regulates lncRNA MEG3. Med. Sci. Monit. 2019;25:4699–4704. doi: 10.12659/MSM.914766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao J., Yu H., Ma Z. LINC00339 promotes growth and invasiveness of hepatocellular carcinoma by the miR-1182/SKA1 pathway. Onco. Targets Ther. 2019;12:4481–4488. doi: 10.2147/OTT.S207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Xiao X., Chang R., Zhang C. Comprehensive bioinformatics analysis identifies lncRNA HCG22 as a migration inhibitor in esophageal squamous cell carcinoma. J. Cell. Biochem. 2020;121(1):468–481. doi: 10.1002/jcb.29218. [DOI] [PubMed] [Google Scholar]
- 24.Guo F.F., Jiang M.M., Hong L.L. Long non-coding rna OR3A4 promotes metastasis of ovarian cancer via inhibiting KLF6. Eur. Rev. Med. Pharmacol. Sci. 2019;23(6):2360–2365. doi: 10.26355/eurrev_201903_17380. [DOI] [PubMed] [Google Scholar]
- 25.Liu G., Shi H., Deng L. Circular RNA circ-FOXM1 facilitates cell progression as ceRNA to target ppdpf and MACC1 by sponging miR-1304-5p in non-small cell lung cancer. Biochem. Biophy. Res .Commun. 2019;513(1):207–212. doi: 10.1016/j.bbrc.2019.03.213. [DOI] [PubMed] [Google Scholar]
- 26.Xia T., Liao Q., Jiang X. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci. Rep. 2014;4:6088. doi: 10.1038/srep06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang S., Li Z., Weng X. The role of lncRNA RP11-154D6 in steroid-induced osteonecrosis of the femoral head through BMSC regulation. J. Cell Biochem. 2019;120(10):18435–18445. doi: 10.1002/jcb.29161. [DOI] [PubMed] [Google Scholar]
- 28.Kojima M., Sudo H., Kawauchi J. MicroRNA markers for the diagnosis of pancreatic and biliary-tract cancers. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Wang Y.W., Zhu W.J. A 4-microRNA signature predicts lymph node metastasis and prognosis in breast cancer. Hum. Pathol. 2018;76:122–132. doi: 10.1016/j.humpath.2018.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.