Abstract
The use of L-cysteine modified silver nanoparticles (Cys-capped AgNPs) as a colorimetric probe for determination of vitamin B1 (thiamine) is described in the present work. This method is based on the measurement of red shift of localized surface plasmon resonance (LSPR) band of Cys-capped AgNPs in the region of 200–800 nm. The color of Cys-capped AgNPs was changed from yellow to colorless by the addition of vitamin B1. The mechanism for detection of vitamin B1 is based on the electrostatic interaction between positively charged vitamin B1, which causes the red shift of LSPR band from 390 nm to 580 nm. The interaction between Cys-capped AgNPs and vitamin B1 was theoretically explored by density function theory (DFT) using LANL2DZ basis sets with help of Gaussian 09 (C.01) program. The morphology, size distribution and optical properties of Cys-capped AgNPs were characterized by transmission electron microscope (TEM), UV-Visible spectrophotometry, Fourier transform infrared spectroscopy (FTIR) and dynamic light scattering (DLS) techniques. The method is linear in the range of 25–500 μg mL−1 with correlation coefficient (R2) 0.992 and limit of detection of 7.0 μg mL−1. The advantages of using Cys-capped AgNPs as a chemical sensor in colorimetry assay are being simple, low cost and selective for detection of vitamin B1 from food (peas, grapes and tomato) and environmental (river, sewage and pond) water samples.
Keywords: Food science, Environmental science, Materials chemistry, Cys-capped silver nanoparticles, Electrostatic interaction, Aggregation, Colorimetric probe, Vitamin B1, Food and environmental water samples
Food science; Environmental science, Materials chemistry, Cys-capped silver nanoparticles; Electrostatic interaction; Aggregation, Colorimetric probe; Vitamin B1; Food and environmental water samples
1. Introduction
Vitamin B1 is a water-soluble organic compound that is used in biological and pharmaceutical fields [1, 2]. The chemical name of vitamin B1 is 3-[(4-Amino –2–methylpyrimidin-5–yl)methyl]-5-(2-hydroxyethyl)-4-methylthiazolium chloride hydrochloride (C12H17ClN4OS, HCl), which contains an amino pyrimidine ring and a thiazole ring with hydroxyl ethyl and methyl side chains connected by a methylene bridge. Vitamins are a varied group of organic compounds, which are essential ingredients of food required for normal development, maintenance and functioning of human and animal bodies [3, 4]. On the basis of these characteristic behaviours these compounds are classified into two important groups such as water soluble groups (vitamins B and C) and fat soluble groups (vitamin A,E,D and K) [5]. Vitamin B1 (thiamine), a water-soluble compound, was first isolated in 1926 from rice bran [6, 7]. Vitamin B1 is coenzyme precursor, which involves in major cellular function such as degradation of carbon skeleton, sugar, carbohydrate metabolism process of cell, tissue maintenance, neuronal communication, cell membrane-dynamics and activation of immune system. It is used for treatment of beriberi and different forms of polyneuritis [8, 9, 10, 11, 12, 13]. Vitamin B1 is widely distributed in different foods such as pulse, meat, legumes (beans, peas), cereal products (whole grains cereals, oatmeal, wheat bran) as well as nuts, and milk [14, 15]. The human diet regularly does not contain the appropriate amount of vitamins needed for the normal development and maintenance of body functions [16, 17]. Therefore, due to the critical role of vitamin B1 in food, qualitative and quantitative analyses are major problems and a challenging work for food manufacturers [18, 19]. The determination of vitamins B1 in various food samples is slightly difficult due to the chemical instability and difficulty of matrices in which they generally exist. Thus, the development of new analytical method for the determination of vitamin B1 in food and environmental water samples is necessary. There have been various techniques reported for determination of vitamin B1 such as conventional colorimetry [1, 20], polarography/voltametry [21, 22, 23], high performance liquid chromatography (HPLC) [2, 24], fluorimetry [25, 26], electrochemical and potentiometry [3, 27]. These methods have major disadvantages such as poor sensitivity, high detection limit, time consuming, small sample throughput, and require large amount of organic solvents for the sample preparation. The spectrophotometric (colorimetric) method is rapid, simple and provides a lower cost, which is available in small laboratories as well as for the on-site analysis of food and environmental samples. Thus, in the present work, colorimetric method for determination of vitamin B1 in food and environmental water samples is investigated.
In recent years, metal nanoparticles have been paid attention because of their special features such as localized surface plasmon resonance (LSPR), quantum effects, chemical properties, chemical stability and catalytic activity which vary from their bulk properties of metals [28, 29, 30]. Metal nanoparticles such as silver (Ag0) and gold (Au0) are generally used in pharmaceuticals and electronics, and as biosensors and catalysts due to their versatile features like low toxicity, good biocompatibility, good stability, distance-dependent optical property, high extinction coefficient and strong LSPR properties [31, 32, 33, 34]. However, there are several researcher have suggest that silver nanoparticles can allegedly cause adverse effects on humans as well as the environment. It is estimated that tonnes of silver are released into the environment from industrial wastes and it is believed that the toxicity of silver in the environment is majorly due to free silver ions in the aqueous phase [35]. It is well-known that metallic Ag is not thermodynamically stable under most environmental conditions and therefore it reacts with organic ligands because of its small particle size. The kinetics of corrosion of AgNPs are expected to be faster than for bulk silver, reducing greatly the life time of the metallic state of Ag in nature [36]. LSPR is a specific property of NPs that is related to conduction of free electron when electromagnetic light interacts with size dependent NPs [37]. The aggregation of AgNPs results the red-shift, expansion of plasmon band and inter-particle surface plasmon coupling, which is used for sensing of vitamin B1 in food samples [38, 39]. The thiolate organic compounds have been applied for organising self-assembly and change in surface of noble metal nanoparticles [40, 41, 42]. L-cysteine (Cys) is a non-essential amino acid containing-SH group, which can bind on the surface of NPs. The carboxylic (COOH) and amino (-NH2) groups present in L-cysteine stabilizes the NPs [43]. The functional group provides a hydrophilic interface during the interaction of analytes and NPs surface [44, 45]. Thus, we have developed a new simple method for selective determination of vitamin B1 using Cys-capped AgNPs in food and environmental water samples using plasmonic colorimetric probe. The quantity of silver nanoparticles used as a chemical sensor in this work was only 1000 μL for determination of vitamin B1 sample solution, which is at least 100-fold less compared to others methods.
In the present work, L-cysteine modified AgNP is used for the detection of vitamin B1 in food and water samples using plasmonic colorimetric sensor and visual (naked eye) methods under the optimized conditions. These methods are based on color change and red shift of LSPR band from 390 nm to 580 nm, which are due to the interaction of vitamin B1 towards the L-cysteine through strong electrostatic interaction perturbing the stability of AgNPs that further directed the aggregation of particles. The interaction between Cys-capped AgNPs and vitamin B1 is theoretically explored by density function theory (DFT) using Gaussian 09 (C.01) program. The size, shape, diameter distribution, and optical properties of AgNPs are investigated by transmission electron microscope (TEM), dynamics light scattering (DLS) and Fourier transform infrared spectroscopic (FTIR). The selectivity of colorimetric sensor for detection of vitamin B1 is verified by spiking different diverse chemical substances present in food and water samples using Cys-capped AgNPs. The analytical parameters such as linearity range, precision and accuracy, selectivity, recovery % and limit of detection are evaluated for validation of present method. Finally, Cys-capped AgNPs is used for the determination of vitamin B1 in food and environmental water samples (peas, grapes and tomato).
2. Experimental design
2.1. Chemicals, reagents and solution preparation
All chemicals and reagents used were of analytical reagent grade. Sodium hydroxide (NaOH), sodium borohydride (NaBH4) and L-cysteine were purchased from Hi-Media (AR reagent, 99.0% Mumbai, India). Spectroscopic grade potassium bromide (KBr), silver nitrate (AgNO3), thiamine hydrochloride (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), pantothenic acid (vitamin B5), pyridoxine hydrochloride (vitamin B6), folic acid (vitamin B9) and ascorbic acid (vitamin C) were obtained from Sigma-Aldrich (ACS reagent, ≥99%,MA, USA). The structure of thiamine and L-cysteine are given in Figure 1. Ultrapure water (18.2 MΩ cm) was used for preparing all aqueous solutions. The stock solutions (1000 μg mL−1) of all vitamins were prepared by dissolving a proper amount of substance in ultrapure water. The working standard solutions were prepared by the suitable dilution of the standard stock solution.
Figure 1.
The chemical structure of (a) vitamin B1 and (b) L-cysteine.
2.2. Synthesis of Cys-capped AgNPs
Cys-capped AgNPs were prepared by the reduction of silver salt (AgNO3) with sodium borohydride (NaBH4) as described in given literatures [46, 47, 48, 49]. A 100 mL of 1.0 ×10−3 M aqueous solution of AgNO3 was taken in a 250 mL conical flask. A 1.0 mg of NaBH4 was added as a reducing agent in the above solution to produce light yellow color solution on constant stirring at 250 rpm under the room temperature. Next, 2.0 mL of 1.0×10−3 M L-cysteine was added to the colloidal AgNPs solution to form an intense deep yellow color showing the formation of Cys-capped AgNPs. The synthesized Cys-capped AgNPs was stored in refrigerator at 5 °C, which even after a month's time period showed no sign of any aggregation due to the desired stability of AgNPs.
2.3. Samples collection and preparation for determination of vitamin B1 using Cys-capped AgNPs
Food samples (peas, grapes and tomato) were collected from different sites of Chhattisgarh, India. After collection, the samples were dried and grinded into fine powder using a mortar and pestle and kept in a refrigerator until analysis. A 10 g sample was refluxed with 40 mL organic solvent for 4 h using Soxhlet apparatus. The extracted sample was centrifuged at 10,000 rpm for 10 min to eliminate any debris present in sample and then filtered using Whatman filter paper (0.45μm pore size) for analysis. The water samples were collected in polyethylene bottles from Raipur city, Chhattisgarh. The water samples were directly filtered through Whatman filter paper to prevent the adsorption of any chemical substances on the surface of suspended elements and kept in a freezer at 5 °C until analysis.
2.4. Procedure for determination of vitamin B1 using colorimetric techniques
Schematic procedure for detection of vitamin B1 using Cys-capped AgNPs with colorimetric analysis is shown in Figure 2. An aliquot of 1.0 mL from 1000 μg mL−1 of standard solution of vitamin B1 or filtered samples was taken into a 10 mL of glass vial and the pH of the sample solution was maintained at 5.0 using 0.1 M HCl and 0.1 M NaOH solutions. The total volume of solution mixture was made up to 5.0 mL with ultrapure water. Then, 1.0 mL of Cys-capped AgNPs was added to the sample solution under the optimized condition. The solution mixture was kept for 5 min of reaction time at room temperature. The color change as well as signal intensity of aggregated Cys-capped AgNPs with vitamin B1 solution mixture was monitored with a UV-Vis spectrophotometer in the range of 200–800 nm.
Figure 2.
Original images of food samples taken by using of xiaomiredmi note 7 pro (A) pea (pisum sativum) (B) tomato (solanum lycopersicum) (C) grape (vitis vinifera) and analytical procedure for analysis.
2.5. Instruments
UV-Vis spectrophotometer (Cary 60 UV-Vis, Agilent Technologies) was used for measurement of LSPR absorption intensity in the range of 200–800 nm for the determination of vitamin B1 using Cys-capped AgNPs in food and environmental samples. The extraction of vitamin B1 from food samples was performed with Soxhlet extraction apparatus (Borosil, India). The size and shape of Cys-capped AgNPs and aggregated Cys-capped AgNPs with vitamin B1 were recorded by TEM and DLS. The surface modification and structural characterization of Cys-capped AgNPs and aggregated Cys-capped AgNPs with vitamin B1 was confirmed by using FTIR spectrometer (Nicolet is10, Thermo Fisher, Scientific Madison, USA). A Systronics digital pH meter (type-335) was employed for pH measurement of solution. Thermo Fisher Scientific Barnstead Smart2pure water system (Conductivity 18.2 Ω−1) was used to obtain ultrapure water for solution preparations. The Gaussian 09 (C.01) software with LANL2DZ basis sets density function theory (DFT) was used for the optimization and interaction between Cys-capped AgNPs and vitamin B1.
3. Results and discussion
3.1. Selection of AgNPs as a chemical sensor for detection of vitamin B1
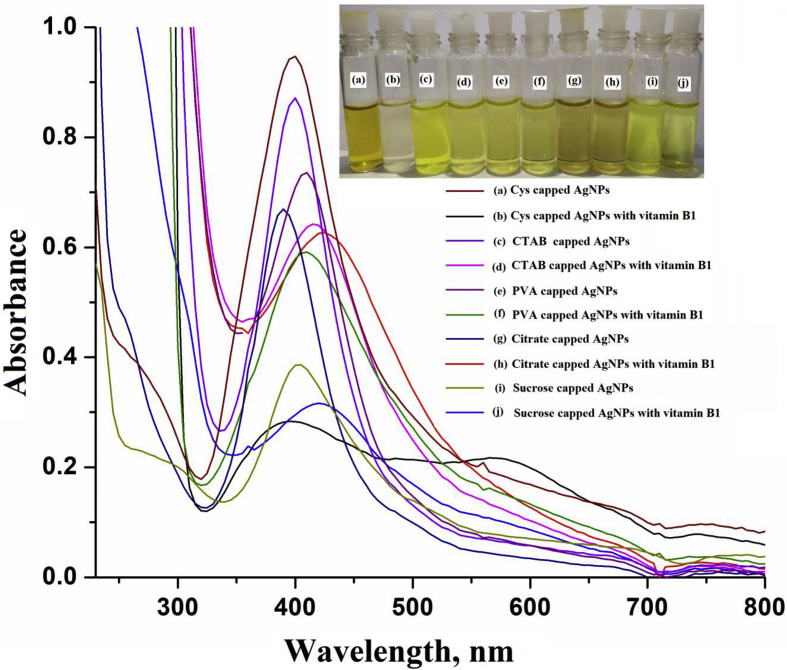
AgNPs modified with L-cysteine was chosen for selective detection of vitamin B1 owing to its chemical stability and suitable optical and electronic properties. In addition, the high surface area to volume ratio of AgNPs is found to be good for surface interactions with vitamin B1 molecules that causes the shift of LSPR absorption band in colorimetric analysis. The LSPR absorption band of the NPs was shifted to a higher wavelength from 390 nm to 580 nm when vitamin B1 was added into the NPs due to the aggregation of particles (Figure 3a-b). In addition, the yellow colored mono dispersed Cys-capped AgNPs in aqueous medium turned colorless after addition of vitamin B1only and not with the addition of other tested biomolecules. The high extinction coefficient and LSPR property of Cys-capped AgNPs has been used as a sensing probe for detection of variety of analytes by many researchers [42]. The high signal intensity was obtained of Cys-capped AgNPs as compared to other functionalized NPs. The results are depicted in Figure 3(c-j). The LSPR of NPs is dependent on the particle size, shape and the inter-particle distance, the adsorbed species on the surface NPs and the dielectric constant of the surrounding medium [50, 51, 52]. Based on the long-time storage property at room temperature without aggregation of particles in presence of analyte, the Cys-capped AgNPs is more stable than gold (Au0) and copper (Cu0) NPs. The morphological and color changes occurring at the surface of Cys-capped AgNPs followed by the change in LSPR absorption band in UV–vis spectra were observed for sensing the target analyte from the sample solution. Therefore, we exploited the use of Cys-capped AgNPs for selective colorimetric detection of vitamin B1 by taking the advantage of LSPR of NPs in the UV-Vis region.
Figure 3.
Images of glass vials containing Cys-capped AgNPs and Cys-capped AgNPs with vitamin B1 (a,b), CTAB-capped AgNPs and CTAB-capped AgNPs with vitamin B1 (c,d), PVA-capped AgNPs and PVA-capped AgNPs with vitamin B1 (e,f), citrate-capped AgNPs and citrate-capped AgNPs with vitamin B1 (g,h), sucrose-capped AgNPs and sucrose-capped AgNPs with vitamin B1 (i,j) and their respective UV–Vis absorption spectra.
3.2. Characterization of Cys-capped AgNPs
In the present work, UV-Vis spectrophotometric technique is used for characterization and quantification of complex mixture on the basis of LSPR band through the color change [17, 28]. The dispersed Cys-capped AgNPs and aggregated AgNPs with vitamin B1 were characterized by FTIR, and DLS techniques. UV-Vis spectrophotometry was used to determine the plasmon resonance band of NPs. A strong band at 390 nm showed the formation of Cys-capped AgNPs and the addition of vitamin B1 into the NPs caused the aggregation of particles resulting in a change of solution color from intense yellow to colorless. The plasmon band centred at 390 nm was found shifted to the right side forming a second peak at 580 nm, indicated in Figure 4(a-b). The appearance of second peak in the spectra could be attributed to the complex formed due to the aggregation of particles. The stabilization of prepared AgNPs colloidal solution was done by the use of a stabilizing agent (L-cysteine) bearing negative charge. The prevalence of electrostatic interaction between the neighbouring AgNPs inhibited the aggregation of NPs. The morphology, size and particle distribution of Cys-capped AgNPs and aggregated NPs with vitamin B1 in aqueous solution were carried out with TEM measurements. Figure 5(a) shows the morphology of the dispersed Cys-capped AgNPs in terms of size and shape to be 8.0 nm and spherical, respectively. Figure 5(b) shows indication towards morphological variations and aggregation of Cys-capped AgNPs upon addition of vitamin B1 with average particle size less than 10.0 nm. These results strongly suggested that the introduction of vitamin B1 to the AgNPs induced the aggregation of NPs. The FTIR spectra of dispersed Cys-capped AgNPs and aggregated AgNPs with vitamin B1 were performed on the basis of functional groups which are present in chemical substances. The bands observed at 1700 cm−1 and 2948 cm−1 for S–H and N–H symmetric stretching, respectively, were due to the combination bands in the ring for L-cysteine as pure compound. The FTIR spectra of the Cys-capped AgNPs with vitamin B1 showed a small band shift and broadening of the bands from 1654 cm−1 to 1665 cm−1. This was due to the C=N+ and C=N symmetric stretching for pyrimidine and benzene ring in vitamin B1, resulting from the aggregation AgNPs [39]. The small band shift from 1700 cm−1 to 1750 cm−1 for Cys-capped AgNPs was obtained in the presence of vitamin B1. The IR absorption band at 1750 cm−1 was observed for L-cysteine molecules and Cys-capped AgNPs due to the Ag–S bond interaction. The results are shown in Figure 5(c-f). Therefore, the Cys-capped AgNPs was selected as a chemical sensor for determination of vitamin B1 in food and environmental water samples. Further, the size dispersal for the addition of vitamin B1 into the Cys-capped AgNPs was established by DLS instrument. The average hydrodynamic diameter of Cys-capped AgNPs was 57.8 ± 6.4 nm confirming the excellent dispersion property of AgNPs in aqueous solution (Figure 5g). However, the average hydrodynamic diameter of Cys-capped AgNPs resulted in four-fold enhancement in the size of NPs (190.0 ± 8.0 nm) in the presence of vitamin B1 caused by the aggregation induced by the addition of analytes as shown in Figure 5(h). The obtained results strongly suggested that the AgNPs were capped with L-cysteine molecules.
Figure 4.
UV–Vis absorption spectra of dispersed Cys-capped AgNPs (a) and aggregated Cys-capped AgNPs after addition of vitamin B1 (b).
Figure 5.
TEM images of dispersed Cys-capped AgNPs (a) and aggregated Cys-capped AgNPs after addition of vitamin B1 (b); FTIR spectra of pure compound of cysteine (c), dispersed Cys-capped AgNPs (d), pure vitamin B1(e) and aggregated Cys-capped AgNPs after the addition of vitamin B1(f); DLS measurements showing the size distribution of Cys-capped AgNPs (g) and Cys-capped AgNPs with vitamin B1 (h).
3.3. Mechanism for the detection of vitamin B1 using the Cys-capped AgNPs as a chemical sensor
In the present work colorimetric procedure is employed for selective determination of vitamin B1 using Cys-capped AgNPs from different food and environmental samples. The different vitamins such as B1, B2, B3, B5, B6, B9 and C were chosen to demonstrate the selective determination of vitamin B1 with Cys-capped AgNPs. For this, all the vitamins and NPs were separately taken in 10 mL glass vial in the volume ratio of 1:1 while maintaining the pH of sample to 5.0 and kept at room temperature for 5 min of reaction time.
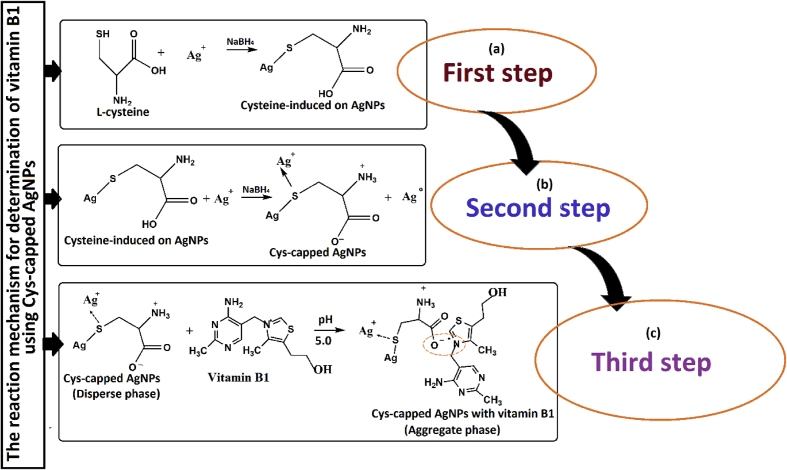
The NPs solution with vitamins such as B2, B3, B5, B6, B9 and C showed a LSPR absorption peak at 390 nm, which were found similar to UV–Vis spectrum of disperse Cys-capped AgNPs (Figure 6a-g). However, on addition of vitamin B1 into the solution of Cys-capped AgNPs, the plasmon band at 390 nm was shifted along with appearance of new peak at about 580 nm, Figure 6(h). The color of the sample solution was changed from yellow to colorless and shift of LSPR absorption band was obtained due to the aggregation of the particles after addition of vitamin B1 only and not with other biomolecules. Thus, the change in solution color from yellow to colorless and appearance of a new LSPR absorption band at 580 nm for vitamin B1 with NPs were used as a colorimetric assay method for selective determination of vitamin B1 in food and environmental samples. UV–Vis spectroscopy results confirmed the aggregation of AgNPs that produced consistent LSPR band around 580 nm as shown in Figure 6(h). The negative charge containing L-cysteine molecules at the surface of AgNPs prevent the aggregation of these AgNPs with the similar structures carrying negative charges due to the electrostatic repulsion. The color change and absorption intensity of AgNPs were decreased after the addition of vitamin B1. The reason for red shift and lowering of absorption intensity are owing to electrostatic interactions among positively charged vitamin B1 molecules and negatively charged Cys-capped AgNPs [53, 54, 55]. Addition of L-cysteine into the AgNPs in solution as a stabilizing or capping agent was done to avoid the accumulations of nanoparticles from aggregation or attaching with other particle components. It is described that when colloidal particles are smaller in size, they absorb visible light through SPR motion [4, 56]. The reactions between cysteine and AgNPs as well as Cys-capped AgNPs with vitamin B1 were completed into three steps for determination of vitamin B1. Step-I-II, the amine and carboxylic acid functional groups of L-cysteine onto colloidal AgNPs surface from hydrogen bond which provides cross-linked structure to the colloidal NPs for stabilized the AgNPs in aqueous solution. The terminal thiol part of L-cysteine binds on the surface of NPs through Ag–S bond to generate the Cys modified AgNPs. An electrostatic repulsion takes place between AgNPs since NPs are enclosed by molecules of L-cysteine. Since work function of silver atom is among the highest (4.26–4.75 eV), they can bind electron rich sites strongly. This generation of cumulative negative charge through NPs sphere, and this best own an isolated nature and stability to NPs. L-cysteine has carboxylate, amine and thiol sites to act as legating absorbing grips. Step-III, vitamin B1 holds positive charge (+ve) on nitrogen atom which can powerfully interact with the negative charge (-ve) site of oxygen (O–H) of thiol moiety of Cys-capped AgNPs and therefore it forms bridging between vitamin B1 and Cys-capped AgNPs due to the aggregation [7, 55, 57, 58]. The results are shown in Figure 7 step I-III. Utilizing these properties, we define a new and facile colorimetric sensor for the detection of vitamin B1 in real samples. The DFT calculation showed interactions of AgNPs with sulphur of L-cysteine in a square planar geometry having bond length of Ag–S (3.400 A0), bond angle of Ag–S–Ag (900) with energy of -333.7491 and 1478.3385 a.u (Figure 8a-e). Thus, the Cys-capped AgNPs interacted with an electrostatic force of attraction between the positive charge of nitrogen on vitamin B1 and negative charge of oxygen (O–H group) of L-cysteine, particularly in acidic pH 5.0. The average bond distance between N+ and O− and the total energy were theoretically calculated to be 2.5175 A0 and -4560.0764 a.u., respectively (Figure 8a-e). The electrostatic potential (ESP) is one of the most important parameters to measure for any molecule's interaction characteristics, especially non-covalent interactions. Here, we have shown the positive (yellow color) and negative (oval, orange) parts of the molecules which varies the SCF total charge density. All the data were calculated using DFT- LANL2DZ method using Gaussian 09 (C.01) program [59]. The results are shown in Figure 8 (e) and Figure 9.
Figure 6.
Photographic image of glass vials containing solution mixtures of dispersed Cys-capped AgNPs (a) and Cys-capped AgNPs after addition of different vitamins (50 μg mL−1): vitamin B2 (b), vitamin B3 (c), vitamin B5 (d), vitamin B6 (e), vitamin B9 (f), vitamin C (g) and vitamin B1 (h) with their UV-Vis spectra using colorimetric probe at pH 5.0 for 5 min reaction time at room temperature.
Figure 7.
Possible reaction pathways for vitamin B1 adsorption onto Cys-capped AgNPs (a, b) terminal thiol part of L-cysteine binds on the surface of NPs through Ag–S bond (c) vitamin B1 containing positive charge (+ve) on nitrogen atom interact with the negative charge (-ve) site of oxygen (O–H) of thiol moiety of Cys-capped AgNPs due to the agglomeration of particles.
Figure 8.
(a) Optimized cyclic structure of L-cysteine, (b)Optimized structure of L-cysteine capped silver (c) HOMO-LUMO of L-cysteine-Ag interacted with vitamin B1 taking 0.020 and SCF electron density 0.00040 for MO = 376, 377 (hydrogen atoms are omitted for clarity), (d) HOMO-LUMO diagram with counter taking is value 0.020 red lobe represent positive and green lobe present negative and (e) HOMO-LUMO diagram of vitamin B1 and silver atom.
Figure 9.
HOMO-LUMO of L-cysteine-Ag interacted with vitamin B1 taking 0.020 and SCF electron density 0.00040 for MO = 376, 377 with red and green lobe represent positive and negative, respectively due to determination of vitamin B1.
The color of solution mixture was captured using Smartphone (xiaomiredmi note 7 pro) and then analysed by UV-Vis spectrophotometry. The color of Cys-capped AgNPs was changed from yellow to colorless in the presence of vitamin B1 that indicated the aggregation of NPs with analytes causing effective absorption of UV light. This is due to the aggregation of NPs caused by electrostatic force of interactions between vitamin B1 and L-cysteine ions of NPs verified through performing the additional experiments with metal ions such as alkali (K+ and Na+), alkaline earth (Mg2+, Ca2+), transition-metal ions (Zn2+, Hg2+, Cd2+) and other biomolecules containing only a positive charge. Neither the color change from yellow to colorless nor any LSPR absorption band shift of the NPs solution was obtained with K+ (400 mg L−1), Na+ (500 mg L−1), Mg2+ (100 mg L−1), Ca2+ ions (200 mg L−1), Zn2+ (200 mg L−1), Hg2+ (300 mg L−1), Cd2+ (100 mg L−1) ions and other tested biomolecules (100 mg L−1). These results showed that the metal ions and other biomolecules individually have no electrostatic force of interactions to aggregate the NPs. Similar type of work have been reported for the determination of vitamin B1 using multiwalled carbon nanotube [3, 60, 61, 62, 63]. We have also performed the interference studies with other diverse substances for selective determination of vitamin B1. Thus, AgNPs/colorimetric method is free from interferences of all tested ions and very selective for detection of vitamin B1 in various environmental and food samples. Controlled experiments were also performed for selective determination of vitamin B1 by addition of other vitamins (B2, B3, B5, B6, B9 and C) into the AgNPs solution at under the optimized conditions. There was no change in color of the solution as well as LSPR band position found when addition of other biomolecules (500 mg L−1) into the nanoparticles solution was done. The color observation of glass vial containing NPs is shown in Figure 6(a-h). This is due to the negative charge of L-cysteine present on the surface of the AgNPs preventing the extraction of negative or same charge of other vitamin such as B2, B3, B5, B6, B9 and vitamin C. Vitamin B1 contains an amino pyrimidine ring and thiazole ring with hydroxyl ethyl and methyl side chains connected by a methylene bridge. The amino pyrimidine ring (C5H5N+) is very sensitive for electrostatic interaction with negative charge of L-cysteine present on the surface of the AgNPs. Therefore, the color change and aggregation of Cys-capped AgNPs in the presence of vitamin B1 were confirmed by recording the UV–Vis spectra (Figure 4). The schematic mechanism for selective detection of vitamin B1 is shown in Figure 10a-c.
Figure 10.
The schematic illustration of Cys-capped AgNPs based sensing strategy for determination of vitamin B1 using colorimetric probe.
Further, we verified the size, shape and morphologies of Cys-capped AgNPs and aggregated AgNPs with and without vitamin B1 in aqueous solution by performing TEM measurements (Figure 5a-b). We observed from Figure 5(a) that in the absence of vitamin B1, Cys-capped AgNPs are roughly sphere-shaped with an average size of ~8 ± 2 nm Figure 5(b) shows indication for the morphological variations and aggregation of Cys-capped AgNPs depending upon the addition of vitamin B1 and the average particle size <10 nm was observed. We also confirmed the size and percentage distribution of Cys-capped AgNPs in presence and absence of vitamin B1 using DLS measurements and results are shown in Figure 5(g-h). An observation of Figure 5(g) showed that the size of Cys-capped AgNPs in the absence of vitamin B1 is 57.8 nm. However, in presence of vitamin B1 the dynamic size of Cys-capped AgNPs increases from 57.8 to 190.0 nm, as shown in Figure 5(h). The increase in hydrodynamic diameter is attributed to analyte persuaded to get aggregation and a decrease in electrostatic interaction between analyte and Cys-capped AgNPs. The results from DLS and TEM measurements were close to the results of UV-Vis analysis. Further, we also confirmed FTIR spectroscopic studies for characterization and identification of vitamin B1 with and without using Cys-capped AgNPs [64, 65]. The FTIR spectra of the vitamin B1 showed sharp peaks at 1658.25 cm−1, 1613.08 cm−1, 2910.88 cm−1 and 3490.76 cm−1, which corresponded to C=N+, C=N, CH3 asymmetric stretching and NH2 stretching frequencies, respectively. The FTIR absorption spectra of Cys-capped AgNPs were recorded using a DRS-FTIR in the range of 400–4000 cm−1. The bands observed in the FTIR spectra of Cys-capped AgNPs were identified as the following: peak at 1550–1745 cm−1 (C=O stretching), 1389, 1200–1250 cm−1 (C–O stretching), 3187 cm−1 (O–H stretching), 2988 cm−1 (N–H stretching), 1054 cm−1 (C–NH2 stretching), 600–800 cm−1 (C–S stretching) and 2550 cm−1 (S–H stretching) [66, 67, 68]. The S–H band (2565 cm−1 symmetry stretching) was very less intense in IR spectroscopic analysis of the Cys-capped AgNPs, which is attributed to the Ag–S interaction. These results confirmed the formation of Cys-capped AgNPs. The FTIR spectra of the vitamin B1 with Cys-capped AgNPs, showed a small shift and broadening of the bands from 1654 cm−1 to 1665 cm−1. This is due to the C=N+ and C=N symmetric stretching for pyrimidine ring in vitamin B1, resulting from the aggregation AgNPs [46]. The aggregation is promoted by the electrostatic force of attraction between NH2 group of L-cysteine and the aromatic ring of vitamin B1 molecule. The band observed at 2131 cm−1 was due to combination bands in the substituted benzene ring. FTIR spectra of Cys-capped AgNPs and Cys-capped AgNPs in the presence of vitamin B1 are shown in Figure 5(c-f). The obtained results strongly suggested the capping of AgNPs with L-cysteine molecules as well as the aggregation in presence of vitamin B1. Therefore, the Cys-capped AgNPs was selected as a chemical sensor for selective determination of vitamin B1 in real samples.
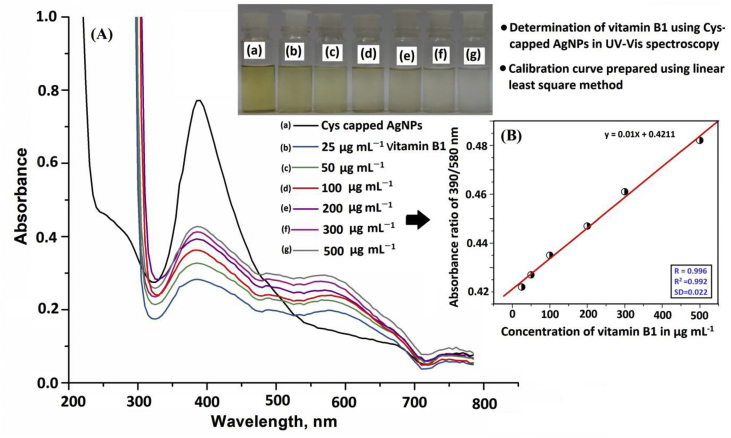
Further, the different concentrations of vitamin B1 solutions ranging from 25–500 μg mL−1 were used to test the sensitivity level of this method (Figure 11A).The absorption intensity at 390 nm as observed for the Cys-capped AgNPs (6.0 μM, at optimum experimental condition) dropped down drastically on addition of vitamin B1 but simultaneously there emerged a new peak at 580 nm due to the LSPR phenomena. However, on addition of increased concentrations of vitamin B1 the absorption intensities increased at both the wavelength values with progressive change in solution color from yellow to colorless. The significant decreasing absorption intensity and red shift is attributed to electrostatic interactions between the negatively charged Cys-capped AgNPs and the positively charged vitamin B1 molecules [69, 70, 71]. This leads to the charge screening effect resulting in decrease in interparticle distances among the isolated nanoparticles [72, 73]. Therefore, the absorption ratio at 390/580 nm was taken for the quantitative analysis of vitamin B1 in food and environmental water samples.
Figure 11.
(A) Photographic images of glass vials containing solution mixture of Cys-capped AgNPs (a) and different concentrations of vitamin B1: 25 μg mL−1(b), 50 μg mL−1(c), 100 μg mL−1(d), 200 μg mL−1 (e), 300 μg mL−1 (f) and 500 μg mL−1 (g) with their UV-Vis spectra with pH 5.0 for 5 min reaction time at room temperature and (B) calibration curve for different concentrations of vitamin B1.
3.4. Optimization for determination of vitamin B1
The several parameters such as effect of pH, stirring rate, reaction time and concentration of NPs were optimized in present work for determination of vitamin B1. The efficient detection of vitamin B1 was obtained when pH sample solution was 5.0 at stirring rate of 250 rpm for 5 min of reaction time using 6.0 μM concentrations of AgNPs. The results are given in Figure 12 and Figure 13(a-d).
Figure 12.
Images of glass vials containing Cys-capped AgNPs (a) after the addition of vitamin B1 (50 μg mL-1) with different pH of solutions mixture: 3.0 (b), 5.0 (c), 7.0 (d), 9.0 (e) and 11.0 (f) with their respective UV-Vis spectra using colorimetric probe for 5 min reaction time at room temperature.
Figure 13.
(a) Effect of pH when stirring rate was 250 rpm for 5-min reaction time; (b) Effect of reaction time when stirring rate was 250 rpm; (c) effect of stirring rate when reaction time 5-min and pH 5.0; (d) effect of concentration of AgNPs when stirring rate was 250 rpm for 5-min reaction time at pH 5.0.
3.4.1. Effect of pH
Images of glass vials containing Cys-capped AgNPs after the addition of vitamin B1 with different pH of solutions mixture and their UV-Vis absorbance bands are shown in Figure 12. The effect of pH on the interaction between AgNPs and vitamin B1 was studied over the pH range from 2.0-11.0 (Figure 13a) and the pH adjustment were performed by addition of appropriate amounts of diluted HCl or NaOH solution. As illustrated by Figure 13a the absorbance increased in the pH range of 2.0–5.0. As we can be seen, low absorbance at pH < 5.0 might be due to the competition of proton with Cys capped AgNPs for interaction with vitamin B1 and in the higher pH might be due to Cys-capped AgNPs was unstable. Thus, pH 5.0 was selected for further experiments.
3.4.2. Effect of reaction time
The effect of the reaction time was also investigated in the range of 1.0–5.0 min for determination of vitamin B1 from sample solution. The reaction time is also a key factor, affecting colorimetric results. It can be seen that the mean color intensity gradually increased from 1.0 to 5.0 min, and remained steady at 5.0 min, indicating that the etching of the Cys-capped AgNPs was completed within 5 min. Thus, the reaction time was chosen at 5.0 min for further experiment (Figure 13b).
3.4.3. Effect of AgNPs concentration
The effect of AgNPs concentration different stages in the range of 2.0–12.0 μM were studied for determination of vitamin B1 in colorimetric method is shown in Figure 13c. The absorbance of vitamin B1 increased with increasing concentration of AgNPs up to 2.0–12.0 μM there was no increase in absorbance. The use of higher concentrations of Cys-capped AgNPs (more than 6.0 μM) was not good because of fast flocculation of AgNPs followed by settling on the bottom of the glass vial. Hence, an AgNPs concentration of 6.0 μM final concentration was used for determination of vitamin B1 in further investigations.
3.4.4. Effect of stirring rate
Different stirring rate were also studied using a magnetic stirrer for determination of vitamin B1 for chemical reaction between vitamin B1 and L-cysteine molecules present on the surface of AgNPs. Stirring rate from 50 to 250 rpm was applied for aggregation with vitamin B1 and Cys-capped AgNPs using 5 min at pH 5.0. The results are displayed in Figure 13d. The absorbance value in UV-Vis spectrophotometry of the analyte was increased with increasing stirring rate up to 250 rpm, beyond decreased absorbance. Thus, the stirring rate was chosen at 250 rpm for further experiment.
3.5. Effect of diverse substances
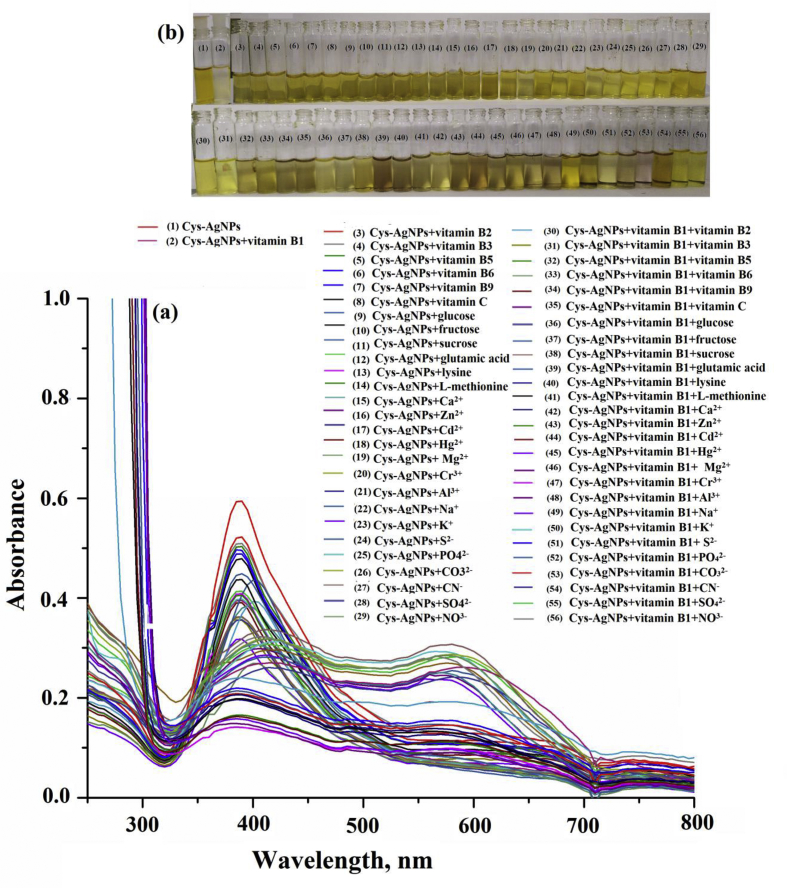
The effect of various diverse substances and other biomolecules that may be present in water and food samples is evaluated for selective determination of vitamin B1 with and without using Cys-capped AgNPs. Selectivity describes whether analytical method could discriminate interference of similar group of compounds in the presence of analytes which is determined by relative absorbance value by colorimetric/AgNPs method. The possible interfering chemical substances with their tolerance limits in the determination of vitamin B1 are given in Table 1 and Figure 14. The UV-Vis absorption band of Cys-capped AgNPs remained unchanged in the presence of other vitamins and amino acids at the optimized conditions, while only vitamin B1 displayed the decrease in color change from yellow to colorless as well as red shift of LSPR absorption band (Figure 15). The ratio of absorbance intensities at 390–580 nm was used to assess the degree of Cys-capped AgNPs aggregation in colorimetric measurements. Therefore, the colorimetric method was found free from interference of diverse substances and other biomolecules which are commonly associated with the determination of vitamin B1 from food and environmental water samples.
Table 1.
Effect of foreign species on colorimetric determination of vitamin B1 by using Cys-capped AgNPs.
| Foreign species/ions | Tolerance limit (mg L−1) |
|---|---|
| Na+, K+ | 500 |
| Mg2+, CN−, SO42−, NO3− | 400 |
| Vitamin B3 | 300 |
| Vitamin B5 Vitamin B6, Ca2+, Zn2+,Cd2+, Hg2+, S2− | 200 |
| Vitamin C, Glucose, Fructose, Sucrose, Glutamic acid, L-lysine, L-Methionine | 100 |
| Vitamin B2, Vitamin B9, Cr3+, Al3+, CO32−, PO43− | 50 |
Figure 14.
Effect of the diverse substances in the presence of the Cys-capped AgNPs and Cys-capped AgNPs with analytes for selective determination of vitamin B1.
Figure 15.
UV-Vis absorption spectra of different diverse substances and other biomolecules, metal ions and anions in the presence of the Cys-capped AgNPs and Cys-capped AgNPs with target analytes for selective determination of vitamin B1 from food and environmental samples (a,b).
3.6. Analytical evaluation for determination of vitamin B1 using Cys-capped AgNPs
Important parameters such as linearity range, limit of detection (LOD), limit of quantification (LOQ), correlation coefficient (R2), selectivity, accuracy and precision for the determination of vitamin B1 were estimated to determine the plausibility of using Cys-capped AgNPs as a chemical sensor. The calibration curve was prepared for vitamin B1 by adding different concentrations of vitamin B1 (25, 50, 100, 200, 300 and 500 μg mL−1) in different glass vials containing 1.0 mL of Cys-capped AgNPs at pH 5.0 and the total volume was maintained at 5.0 mL with ultrapure water. Different concentrations of vitamin B1 solutions ranging from 25–500 μg mL−1 were used to test the sensitivity level of this method. The vitamin B1 properly binds on the surface of functionalized AgNPs and captured in colorimetric probe with above concentrations range. The results are shown in Figure 11A. The color intensity of solution mixture was measured by UV–Vis spectrophotometer. It was seen that the intensity of LPSR absorption bands obtained at 580 nm increased when the concentration of vitamin B1was increased. The relative signal intensity obtained at A390/A580 nm was used to prepare the standard calibration curve. A good linearity was obtained in the range of 25–500 μg mL−1 vitamin B1 with a correlation coefficient (R2) of 0.992, as shown in Figure 11B. The LOD was also determined by adding a minimum amount of vitamin B1 into the NPs solution and using three times the standard deviation with the slope of the curve (3SD/slope) [74, 75]. LOQ is the lowest concentration of analyte at 10 × SD by acceptable precision at the similar concentration, i.e., LOQ = 10SD/slope [76]. The value of LOD and LOQ in the present work were calculated to be 7.0 g mL−1 and 13.8 μg mL−1, respectively. The precision of the method was obtained by calculating the relative standard deviation percentage (RSD %) by six replicate analysis of the samples under the optimized conditions. The RSD for determination of vitamin B1 was found to be 5.0 % showing a good precision of the method for determination of vitamin B1 in sample solution. The reason behind not including negative and positive experiments is that, in the present work we have not used any extracting solvent or buffer solution since biomolecules are soluble in water. We have developed an advanced method for extraction of biomolecules without the involvement of any toxic solvent which is based on physico-chemical adsorption of biomolecule on the surface of nanoparticle.
3.7. Application for determination of vitamin B1 in real samples using Cys-capped AgNPs
The Cys-capped AgNPs was successfully used for the determination of vitamin B1 in food and environmental water samples obtained from Raipur city, Chhattisgarh, India. An aliquot of filtered water/food sample (1.0 mL) was added into a glass vial containing 1.0 mL of Cys-capped AgNPs at pH 5.0. The solution mixture was kept at 5 min reaction time while maintaining the pH of sample solution. Cys-capped AgNPs, AgNPs-enriched with vitamin B1 was directly used for colorimetric analyses. LSPR absorption peak obtained at 580 nm in colorimetric analysis was used for determination of vitamin B1 from food and environmental water samples. In order to determine the applicability and consistency of the proposed method, three food samples including peas, tomato and grapes were selected for the determination of vitamin B1. To verify the utility of the colorimetric method, the concentration of vitamin B1 in various food and water samples were detected. The color of AgNPs solution clearly changed from yellow to colorless upon the addition of samples with different dilution ratios. The LSPR absorption peak showed red shift from 390 to 580 nm due to the aggregation of Cys-capped AgNPs and their ratio of absorption was used to calculate the concentration of vitamin B1 using linear least square equation. In the present work, the quality control experiment was carried out for each samples consisting of a linear calibration standard in matrix, a blank and a spiked samples for the compounds. Total 50 numbers of food and water samples were tested those were obtained from Raipur city, India. Out of all samples, six kinds of food and three kinds of water samples were found positive towards the presence of vitamin B1 in significant concentrations. The results on the vitamin B1 contents of all positive samples are shown in Table 2. The concentrations of vitamin B1 in water and food samples were found in the range of 10.9–115.1 μg mL−1 for colorimetric assay (Table 2 and Table 3). The recovery percentage (%) was also calculated to determine the accuracy of the proposed method. The accuracy of the method, found by means of recovery evaluates, gave acceptable results. The recovery % was calculated by spiking two different concentrations of vitamin B1 to the actual sample. A good % recovery range, i.e., 95.4–99.6 % found for the determination of vitamin B1 in food and environmental water samples using Cys-capped AgNPs displayed remarkable selectivity of chemical sensor for detection of the target analyte. According to ICH Q2 (R1) standard guidelines, the specificity is defined as the ability of the method to a single analyte in the presence of mixture of similar components. There were no significant interferences and no changes of LSPR band observed during the determination of vitamin B1 in the presence of others components. Therefore, the present method is highly specific as well as selective for determination of vitamin B1 in food and water samples. The results, given in Table 2 and Table 3, of the present work have been validated by making a comparative analysis using a standard reported HPLC method [77] for analysis of vitamin B1 in food and environmental water samples.
Table 2.
Recovery percentage (%) for colorimetric determination of vitamin B1 using Cys-capped AgNPs in food and environmental water samples.
| Samples | Sample Sources | Standard Addition (μg mL−1) | Vitamin B1 Found (μg mL−1) | RSD (n = 4)% | Recovery (%) |
|---|---|---|---|---|---|
| Peas 1 | Atal Nagar | - | 16.4 ± 0.47 | 0.47 | - |
| 50 | 64.8 | - | 96.8 | ||
| 100 | 115.1 | - | 98.7 | ||
| Peas 2 | Atal Nagar | - | 15.2 ± 0.6 | 3.87 | - |
| 50 | 63.6 | - | 96.6 | ||
| 100 | 114.0 | - | 98.8 | ||
| Tomato 1 | Jashpur | - | 19.3 ± 0.38 | 2.0 | - |
| 50 | 68.4 | - | 98.2 | ||
| 100 | 115.2 | - | 95.9 | ||
| Tomato 2 | Jashpur | - | 16.0 ± 0.75 | 4.7 | - |
| 50 | 64.2 | - | 96.4 | ||
| 100 | 114.6 | - | 98.6 | ||
| Grape 1 | Dhamtari | - | 12.1 ± 0.40 | 3.3 | - |
| 50 | 60.8 | - | 97.4 | ||
| 100 | 110.1 | - | 98.0 | ||
| Grape 2 | Dhamtari | - | 10.9 ± 0.60 | 5.4 | - |
| 50 | 58.6 | - | 95.4 | ||
| 100 | 108.3 | - | 97.4 | ||
| River water | Raipur | - | 13.2 ± 0.64 | 4.8 | - |
| 50 | 62.7 | - | 99.0 | ||
| 100 | 112.8 | - | 99.6 | ||
| Sewage Water | Raipur | - | 16.75 ± 0.60 | 3.6 | - |
| 50 | 64.2 | - | 94.9 | ||
| 100 | 115.1 | - | 98.3 | ||
| Pond water | Raipur | - | 15.10 ± 0.71 | 4.7 | - |
| 50 | 64.6 | - | 99.0 | ||
| 100 | 113.8 | - | 98.7 |
Table 3.
Determination of vitamin B1 real samples analyzed by the present colorimetric method and reported HPLC.
|
Statistical Parameters |
Vitamin B1 |
|
|---|---|---|
| AgNPs-Colorimetry (Present method) | HPLC (Reference method) | |
| Linear range, (μg mL−1) | 25–500 | 0.25–10 |
| RSD, (%) | 5.0 | 6.4 |
| Correlation Coefficient, (R2) | 0.992 | 0.986 |
| Concentration (μg mL−1) | 10.9–115.1 | 2.5–100 |
| LOD, (μg mL−1) | 7.0 | 6.8 |
| LOQ, (μg mL−1) | 13.8 | ND |
| Recovery, (%) | 99.0 | 98.7 |
3.8. Comparison of AgNPs based colorimetric method and other reported methods for determination of vitamin B1
The linearity range, LOD, RSD and recovery % values obtained by newly developed Cys-capped AgNPs method were compared with other reported methods for the determination of vitamin B1 in different types of samples, Table 4. The LOD value obtained by present method was found lower than with fluorescence spectrometry, UV–Vis spectrophotometry, cyclic voltammetry, HPLC, electrochemistry, turbidimetry and atomic emission spectrometry (AES) [19, 78, 79, 80, 81, 82, 83, 84, 85]. These earlier reported methods require a time consuming sample preparation procedure, trained personnel and high cost chemical reagents. The present method based on AgNPs chemical sensor is very simple, sensitive, selective, rapid, and cost effective and also minimum quantity of chemical reagents are required as compared to column separation and chromatographic methods.
Table 4.
Comparison of characteristic analytical features of different techniques used for the determination of vitamin B1.
| S/No. | Analytical techniques | Methods | Linear range (μg mL−1) | LOD (μg mL−1) | RSD (%) | Recovery (%) | References |
|---|---|---|---|---|---|---|---|
| 1. | Electrochemistry | Keineckate liquid membrane electrode | 3.0–350 | 0.3 | ND | 98.1 | [78] |
| 2. | Electrochemical luminescence | Rhodamine B | 0.1–2.0 | 80 | 3.2 and 2.1 | 99–102 | [79] |
| 3. | AES | Ammonium reineckate | 13.2–134.8 | ND | 0.26–1.64 | 100–101 | [80] |
| 4. | Turbidimetry | CTMAB–gelatin–BaSO4 | 40–200 | 40 | ND | ND | [81] |
| 5. | Phosphorescence method | Cu(II) (ultraviolet irradiation) | - | 60 | 1.4 | ND | [82] |
| 6. | Solid-Phase UV-spectrophotometry | Sephadex CMC-25 cation-exchange resin | 0.5–5.5 | 80 | 1.5 to 2.6 | ND | [19] |
| 7. | Chemiluminescence | Luminol–hydrogen peroxide system | 0.05–8.0 | 0.01 | 1.4 | ND | [83] |
| 8. | HPLC | Post-column photochemical derivatization, fluorescence detection |
0.033–10.0 | 3.8 | ND | ND | [84] |
| 9. | Spectrofluorimetry coupled with FIA | Dynamic liquid drops combined with flow injection-solid phase | 0.01–8.00 | 0.008 | 2.0–6.1 | 97–104 | [85] |
| 10. | UV-Visible spectroscopy | Cysteine capped silver nanoparticles | 25–500 | 7.0 | 5.0 | 95–99 | Present method |
ND = Not detected.
4. Conclusion
“In summary, we have developed a highly selective and sensitive colorimetric probe for the determination of vitamin B1 using Cys-capped AgNPs as a chemical sensor. The sensitivity of colorimetric assay is based on the LSPR band changes and aggregation of Cys-capped AgNPs upon the successive addition of vitamin B1. The proposed sensing system was also demonstrated towards its selectiveness for vitamin B1 in the presence of potential interfering chemical substances including other vitamins. The electrostatic interaction among negatively charged Cys-capped AgNPs and positively charged vitamin B1 was theoretically explored by density function theory (DFT) using Gaussian 09 (C.01) program. The synthesis process was fast and completed within a few minutes at room temperature without using heating sources and hazardous organic solvents.
This proposed work has provided new method for selective quantification of few selected biomolecules and has great potential for the inexpensive, rapid, simple and possibly highly sensitive in nature employing the basic and advanced logical concept derived from LSPR phenomenon based on colorimetric sensor. In the near future, the proposed method would be highly useful for monitoring of biomolecules in biological samples such as urine, blood and plasma. This work, however, has few limitations too as the scientific community has not validated these tests. The color change of NPs due to the aggregation of molecules over the surface of AgNPs were analysed only by localised surface plasmon based UV-vis spectrophotometry. This limitation mandates the need of a powerful tool to facilitate the applications of AgNPs. Therefore, other spectroscopic technique like Fourier transform infrared spectroscopy should be implied in such molecular structures and functional groups based qualitative and quantitative analysis of different composition and origin. The stability of Cys-capped AgNPs is for one month, this stability can be further improvised by optimizing conditions such as light and temperature. The amount of AgNPs used as a chemical sensor for the determination of the target analyte is about 1–2 ml. But due to the toxic nature of the silver ions, there is a need to minimize the amount of the nanoparticles to 5–10 μL which at present is a challenging job.”
Declarations
Author contribution statement
Manas Deb: Conceived and designed the experiments.
Beeta Rani Khalkho: Performed the experiments; Wrote the paper.
Ramsingh Kurrey, Santosh Singh Thakur: Analyzed and interpreted the data.
Kamlesh Shrivas, Shamsh Pervez, Vikas Kumar Jain: Contributed reagents, materials, analysis tools or data.
Funding statement
Beeta Rani Khalkho was supported by the Council of Scientific & Industrial Research (CSIR), New Delhi, India. This work was supported by Department of Science and Technology Fund for Improvement of S&T Infrastructure in Universities & Higher Educational Institutions [No.-SR/FST/CSI-259/2014(c)], New Delhi, India. This work was supported by the Chhattisgarh Council of Science & Technnology Raipur, India Endt. No. 2741/CCOST/MRP/2016 and 2236/CCOST/MRP/2015 for financial support.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to Prof. Shamsh Pervez, Head, School of Studies in Chemistry, Pt. Ravishankar Shukla University, Raipur for providing laboratory facilities.
References
- 1.Liu S., Zhang Z., Luo H., Kong L. Resonance Rayleigh-scattering method for the determination of vitamin B1 with methyl orange. Anal. Sci. 2002;44:971–976. doi: 10.2116/analsci.18.971. [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam A.B., Gudarzy F., Ganjkhanlou Y. A fluorescent probe for detecting thiamine using the luminescence intensity of nanoparticles. J. Fluoresc. 2014;24:1025–1030. doi: 10.1007/s10895-014-1377-0. [DOI] [PubMed] [Google Scholar]
- 3.Brahman P.K., Dar R.A., Pitre K.S. DNA-functionalized electrochemical biosensor for detection of vitamin B1 using electrochemically treated multiwalled carbon nanotube paste electrode by voltammetric methods. Sensor. Actuator. B Chem. 2013;177:807–812. [Google Scholar]
- 4.Li Y., Wang P., Wang X., Cao M., Xia Y.S., Cao C., Liu M.G., Zhu C.Q. An immediate luminescence enhancement method for determination of vitamin B1 using long-wavelength emitting water-soluble CdTe nanorods. Microchim. Acta. 2010;169:65–71. [Google Scholar]
- 5.Santos J., Mendiola J.A., Oliveira M.B.P.P., Ibnez E., Herrero M. Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A. 2012;1261:179–188. doi: 10.1016/j.chroma.2012.04.067. [DOI] [PubMed] [Google Scholar]
- 6.Lonsdale D. A Review of the biochemistry, metabolism and clinical benefits of thiamine (e) and its derivatives. Evid.Base Comple. Alternative. Med. 2006;3:49–59. doi: 10.1093/ecam/nek009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J., Li R., Jiang Z.T. Determination of thiamine (vitamin B1) in pharmaceutical tablets and human urine by titania-based ligand-exchange hydrophilic interaction chromatography. Anal. Methods. 2011;3:1568–1573. [Google Scholar]
- 8.Sun J., Liu L., Ren C., Chen X., Hu Z. A feasible method for the sensitive and selective determination of vitamin B1 with CdSe quantum dots. Microchim. Acta. 2008;163:271–276. [Google Scholar]
- 9.Pinto E., Pedersen M., Snoeijs P., Nieuwerburgh L.V., Colepicolo P. Simultaneous detection of thiamine and its phosphate esters from microalgae by HPLC. Biochem. Biophys. Res. Commun. 2002;291:344–348. doi: 10.1006/bbrc.2002.6438. [DOI] [PubMed] [Google Scholar]
- 10.Gupta V.K., Sethi B., Sharma R.A., Agarwal S., Bharti A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J. Mol. Liq. 2013;177:114–118. [Google Scholar]
- 11.Maleh H.K., Javazmi F.T., Atar N., Yola M.L., Gupta V.K., Ensafi A.A. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind. Eng. Chem. Res. 2015;54:3634–3639. [Google Scholar]
- 12.Srivastava S.K., Gupta V.K., Jain S. PVC-based 2,2,2-cryptand sensor for zinc ions. Anal. Chem. 1996;68:1272–1275. doi: 10.1021/ac9507000. [DOI] [PubMed] [Google Scholar]
- 13.Ulusoy S., Akçay M. Simultaneous determination of vitamins B1 and B2 in food samples by modified cloud point extraction method and HPLC-DAD. Food Anal. Methods. 2018;11:260–269. [Google Scholar]
- 14.Tyskiewicz K., Debczak A., Gieysztor R., Szymczak T., Roj E. Determination of fat- and water-soluble vitamins by supercritical fluid chromatography: a Review. J. Separ. Sci. 2017;41:336–350. doi: 10.1002/jssc.201700598. [DOI] [PubMed] [Google Scholar]
- 15.Dehghani M.H., Sanaei D., Ali I., Bhatnagar A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J. Mol. Liq. 2016;215:671–679. [Google Scholar]
- 16.Heudi O., Kilinc T., Fontannaz P. Separation of water-soluble vitamins by reversed-phase high performance liquid chromatography with ultra-violet detection: application to polyvitaminated premixes. J. Chromatogr. A. 2005;1070:49–56. doi: 10.1016/j.chroma.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Chen S., Gao H., Shen W., Lu C., Yuan Q. Colorimetric detection of cysteine using noncrosslinking aggregation of fluorosurfactant-capped silver nanoparticles. Sensor. Actuator. B Chem. 2014;190:673–678. [Google Scholar]
- 18.Dinc E., Kokdil G., Onur F. A comparison of matrix resolution method, ratio spectra derivative spectrophotometry and HPLC method for the determination of thiamine HCl and pyridoxine HCl in pharmaceutical preparation. J. Pharmaceut. Biomed. Anal. 2000;22:915–923. doi: 10.1016/s0731-7085(00)00308-3. [DOI] [PubMed] [Google Scholar]
- 19.Barrales P.O., Fernandez-de Cordova M.L., Diaz A.M. Micro determination of vitamin B1 in the presence of vitamins B2, B6, and B12 by solid-phase UV spectrophotometry. Anal. Chem. 1998;70:271–275. [Google Scholar]
- 20.Siddiqui I., Pitre K.S. Voltammetric determination of vitamins in a pharmaceutical formulation. J. Pharmaceut. Biomed. Anal. 2001;26:1009–1015. doi: 10.1016/s0731-7085(01)00466-6. [DOI] [PubMed] [Google Scholar]
- 21.Kasim E.A. Anodic adsorptive voltammetric determination of the vitamin B1 (thiamine) J. Pharmaceut. Biomed. Anal. 2000;22:1047–1054. doi: 10.1016/s0731-7085(99)00154-5. [DOI] [PubMed] [Google Scholar]
- 22.Brunetti B., Desimoni E. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J. Food Compos. Anal. 2014;33:155–160. [Google Scholar]
- 23.Ndaw S., Bergaentzle M., Werner D.A., Hasselmann C. Extraction procedures for the liquid chromatographic determination of thiamine, riboflavin and vitamin B6 in foodstuffs. Food Chem. 2000;71:129–138. [Google Scholar]
- 24.Klejdus B., Petrlova J., Potesil D., Adam V., Mikelova R., Vacek J., Kizek R., Kuban V. Simultaneous determination of water- and fat-soluble vitamins in pharmaceutical preparations by high-performance liquid chromatography coupled with diode array detection. Anal. Chim. Acta. 2004;520:57–67. [Google Scholar]
- 25.Gupta V.K., Mergu N., Kumawat L.K., Singh A.K. A reversible fluorescence “off–on–off” sensor for sequential detection of aluminium and acetate/fluoride ions. Talanta. 2015;144:80–89. doi: 10.1016/j.talanta.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 26.Halvatzis S.A., Potamia M.T. Kinetic-potentiometric determination of ascorbic acid, biotin, pyridoxine hydrochloride and thiamine hydrochloride with n bromosuccinimide. Anal. Chim. Acta. 1989;227:405–419. [Google Scholar]
- 27.Kumar M., Devi P., Kumar A. Structural analysis of PVP capped silver nanoparticles synthesized at room temperature for optical, electrical and gas sensing properties. J. Mater. Sci. Mater. Electron. 2017;28:5014–5020. [Google Scholar]
- 28.Qi W., Zhong-Fang L., Ling K., Shao-Pu L. Absorption and resonance Rayleigh scattering spectra of the interaction for copper nanoparticles with vitamin B1. Chin. J. Anal. Chem. 2007;35:365–369. [Google Scholar]
- 29.Filippo E., Manno D., Buccolieri A., Serra A. Green synthesis of sucralose-capped silver nanoparticles for fast colorimetric triethylamine detection. Sensor. Actuator. B Chem. 2013;178:1–9. [Google Scholar]
- 30.Setua P., Ghatak C., Rao V.G., Das S.K., Sarkar N. Dynamics of solvation and rotational relaxation of coumarin 480 in pure aqueous-AOT reverse micelle and reverse micelle containing different-sized silver nanoparticles inside its core: a comparative study. J. Phys. Chem. B. 2012;116:3704–3712. doi: 10.1021/jp203043k. [DOI] [PubMed] [Google Scholar]
- 31.Jiang C., Guan Z., Lim S.Y.R., Polavarapu L., Xu Q.H. Two-photon ratiometric sensing of Hg2+ by using cysteine functionalized Ag nanoparticles. Nanoscale. 2011;3:3316–3320. doi: 10.1039/c1nr10396b. [DOI] [PubMed] [Google Scholar]
- 32.Shen Z., Han G., Liu C., Wang X., Sun R. Green synthesis of silver nanoparticles with bagasse for colorimetric detection of cysteine in serum samples. J. Alloys Compd. 2016;686:82–89. [Google Scholar]
- 33.Yola M.L., Gupta V.K., Eren T., Sen A.E., Atar N. A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim. Acta. 2014;120:204–211. [Google Scholar]
- 34.Luo Y., Miao H., Yang X. Glutathione-stabilized Cu nanoclusters as fluorescent probes for sensing pH and vitamin B1. Talanta. 2015:488–495. doi: 10.1016/j.talanta.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Kunkalekar R.K., Naik M.M., Dubey S.K., Salker A.V. Antibacterial activity of silver-doped manganese dioxide nanoparticles on multidrug-resistant bacteria. J. Appl. Chem. Biotechnol. 2013;88:873–877. [Google Scholar]
- 36.Karthikeyan S., Gupta V.K., Boopathy R., Titus A., Sekaran G. A new approach for the degradation of high concentration of aromatic amine by heterocatalytic fenton oxidation: kinetic and spectroscopic studies. J. Mol. Liq. 2012;173:153–163. [Google Scholar]
- 37.Shanmugaraj K., Ilanchelian M. Colorimetric determination of sulfide using chitosan-capped silver nanoparticles. Microchim. Acta. 2016;183:1721–1728. [Google Scholar]
- 38.Khan U., Niaz A., Shah A., Zaman M.I., Ziac M.A., Iftikharb F.J., Nisard J., Ahmede M.N., Akhterf M.S., Shahg A.H. Thiamine functionalized silver nanoparticles for the highly selective and sensitive colorimetric detection of Hg2+ ions. New J. Chem. 2018;42:528–534. [Google Scholar]
- 39.Devi R., Batra B., Lata S., Yadav S., Pundir C.S. A method for determination of xanthine in meat by amperometric biosensor based on silver nanoparticles/cysteine modified Au electrode. Process Biochem. 2013;48:242–249. [Google Scholar]
- 40.Wang G., Wang W., Wu J., Liu H., Jiao S., Fang B. Self-assembly of a silver nanoparticles modified electrode and its electrocatalysis on neutral red. Microchim. Acta. 2009;164:149–155. [Google Scholar]
- 41.Bamdad F., Khorram F., Samet M., Bamdad K., Sangi M.R., Allahbakhshi F. Spectrophotometric determination of L-cysteine by using polyvinylpyrrolidone-stabilized silver nanoparticles in the presence of barium ions, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016;161:52–57. doi: 10.1016/j.saa.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Kumar N., Upadhyay L.S.B. Facile and green synthesis of highly stable l-cysteine functionalized copper nanoparticles. Appl. Surf. Sci. 2016;385:225–233. [Google Scholar]
- 43.Mulpur P., Kurdekar A., Podila R., Rao A.M., Kamisetti V. Surface plasmon coupled emission as a novel analytical platform for the sensitive detection of cysteine. Nanotechnol. Rev. 2015;4:393–400. [Google Scholar]
- 44.Li J., Chen L., Lou T., Wang Y. Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl. Mater. Interfaces. 2011;3:3936–3941. doi: 10.1021/am200810x. [DOI] [PubMed] [Google Scholar]
- 45.Asfaram A., Ghaedi M., Agarwal S., Tyagi I., Gupta V.K. Removal of basic dye auramine-O by ZnS: Cu nanoparticles loaded on activated carbon optimization of parameters using response surface methodology with central composite design. RSC Adv. 2015;5:18438–18450. [Google Scholar]
- 46.Li H., Cui Z., Han C. Glutathione-stabilized silver nanoparticles as colorimetric sensor for Ni2+ ion. Sensor. Actuator. B Chem. 2009;143:87–92. [Google Scholar]
- 47.Wiesner M.R., Lowry G.V., Jones K.L., Hochella M.F., Di Giulio R.T., Casman E., Bernhardt E.S. Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials. Environ. Sci. Technol. 2009;43:6458–6462. doi: 10.1021/es803621k. [DOI] [PubMed] [Google Scholar]
- 48.Song K.C., Lee S.M., Park T.S., Lee B.S. Preparation of colloidal silver nanoparticles by chemical reduction method. Kor. J. Chem. Eng. 2009;26:153–155. [Google Scholar]
- 49.Alqadi M.K., Noqtah O.A.A., Alzoubi F.Y., Alzouby J., Aljarrah K. pH effect on the aggregation of silver nanoparticles synthesized by chemical reduction. Mater Sci-Poland. 2014;32:107–111. [Google Scholar]
- 50.Shrivas K., Nirmalkar N., Thakur S.S., Deb M.K., Shinde S.S., Shankar Ravi. Sucrose capped gold nanoparticles as a plasmonic chemical sensor based on non-covalent interactions: application for selective detection of vitamins B1 and B6 in brown and white rice food samples. Food Chem. 2018;250:14–21. doi: 10.1016/j.foodchem.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Gupta V.K., Singh L.P., Singh R., Upadhyay N., Kaur S.P., Sethi B. A novel copper (II) selective sensor based on Dimethyl 4,4′-(o-phenylene)-bis(3-thioallophanate) in PVC matrix. J. Mol. Liq. 2012;174:11–16. [Google Scholar]
- 52.Gupta V.K., Kumar P. Cadmium (II)-selective sensors based on dibenzo-24-crown-8 in PVC matrix. Anal. Chim. Acta. 1999;389:205–212. [Google Scholar]
- 53.Sarkar D. Probing the interaction of a globular protein with a small fluorescent probe in the presence of silver nanoparticles: spectroscopic characterization of its domain specific association and dissociation. RSC Adv. 2013;3:24389–24399. [Google Scholar]
- 54.Srivastava S.K., Gupta V.K., Jain S. Determination of lead using a poly(viny1 chloride)-based crown ether membrane. Analyst. 1995;120:495–498. [Google Scholar]
- 55.Lebiedzinska A., Marszałł M.L., Kuta J., Szefer P. Reversed-phase high-performance liquid chromatography method with coulometric electrochemical and ultraviolet detection for the quantification of vitamins B1 (thiamine), B6 (pyridoxamine, pyridoxal and pyridoxine) and B12 in animal and plant foods. J. Chromatogr. A. 2007;1173:71–80. doi: 10.1016/j.chroma.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 56.Jain K., Gupta V.K., Sahoo B.B., Singh L.P. Copper(II)-selective electrodes based on macrocyclic compounds. Anal. Proc. 1995;32:99–101. [Google Scholar]
- 57.Gupta V.K., Singh A.K., Kumawat L.K. Thiazole schiff base turn-on fluorescent chemosensor for Al3+ ion. Sensor. Actuator. B Chem. 2014:98–108. [Google Scholar]
- 58.Siriwardana K., Wang A., Gadogbe M., Collier W.E., Fitzkee N.C., Zhang D. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. J. Phys. Chem. C. 2015;119:2910–2916. doi: 10.1021/jp512440z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shrivas K., Sahu B., Deb M.K., Thakur S.S., Sahu S., Kurrey R., Kant T., Patle T.K., Jangde R. Colorimetric and paper-based detection of lead using PVA capped silver nanoparticles: experimental and theoretical approach. Microchem. J. 2019;150:1–10. [Google Scholar]
- 60.Gupta V.K., Kumar S., Singh R., Singh L.P., Shoora S.K., Sethi B. Cadmium(II) ion sensing through p-tert-butyl calix[6]arene based potentiometric sensor. J. Mol. Liq. 2014;195:65–68. [Google Scholar]
- 61.Gupta V.K., Ganjali M.R., Norouzi P., Khani H., Nayak A., Agarwal S. Electrochemical analysis of some toxic metals by ion–selective electrodes. Crit. Rev. Anal. Chem. 2011;41:282–313. doi: 10.1080/10408347.2011.589773. [DOI] [PubMed] [Google Scholar]
- 62.Gupta V.K., Nayak A., Agarwal S., Singhal B. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb. Chem. High Throughput Screen. 2011;14:284–302. doi: 10.2174/138620711795222437. [DOI] [PubMed] [Google Scholar]
- 63.Gupta V.K., Mergu N., Kumawat L.K., Singh A.K. Selective naked-eye detection of magnesium (II) ions using acoumarin-derived fluorescent probe. Sensor. Actuator. B Chem. 2015;207:216–223. [Google Scholar]
- 64.Kurrey R., Deb M.K., Shrivas K. Surface enhanced infra-red spectroscopy with modified silver nanoparticles (AgNPs) for detection of quaternary ammonium cationic surfactants. New J. Chem. 2019 [Google Scholar]
- 65.Kurrey R., Deb M.K., Shrivas K., Khalkho B.R., Nirmalkar J., Sinha D., Jha S. Citrate-capped gold nanoparticles as a sensing probe for determination of cetyltrimethylammonium surfactant using FTIR spectroscopy and colorimetry. Anal. Bioanal. Chem. 2019:1–16. doi: 10.1007/s00216-019-02067-8. [DOI] [PubMed] [Google Scholar]
- 66.Nidya M., Umadevi M., Rajkumar B.J.M. Structural, morphological and optical studies of L-cysteine modified silver nanoparticles and its application as a probe for the selective colorimetric detection of Hg2+ Spectrochim. Acta Mol. Biomol. Spectrosc. 2014;133:265–271. doi: 10.1016/j.saa.2014.04.193. [DOI] [PubMed] [Google Scholar]
- 67.Gupta V.K., Karimi-Maleh H., Sadegh R. Simultaneous Determination of hydroxylamine, phenol and sulfite in water and waste water samples using a voltammetricnanosensor. Int. J. Electrochem. Sci. 2015;10:303–316. [Google Scholar]
- 68.Khan M.M., Kalathil S., Lee J., Cho M.H. Synthesis of cysteine capped Silver nanoparticles by electrochemically active biofilm and their antibacterial activities. Bull. Kor. Chem. Soc. 2012;33:2592–2596. [Google Scholar]
- 69.Rajamanikandan R., Ilanchelian M. Simple and visual approach for highly selective biosensing of vitamin B1 based on glutathione coated silver nanoparticles as a colorimetric probe. Sensor. Actuator. B Chem. 2017;244:380–386. [Google Scholar]
- 70.Gupta V.K., Atar N., Yola M.L., Ustundag Z., Uzun L. A novel magnetic Fe@Aucoreeshell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 2014;48:210–217. doi: 10.1016/j.watres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 71.Liu S., Chen Y., Liu Z., Hu X., Wang F. A highly sensitive resonance Rayleigh scattering method for the determination of vitamin b1 with gold nanoparticles probe. Microchim. Acta. 2006;154:87–93. [Google Scholar]
- 72.Srivastava S.K., Gupta V.K., Dwivedi M.K., Jain S. Caesium PVC-crown (dibenzo-24-crown-8) based membrane sensor. Anal. Proc. 1995;32:21–23. [Google Scholar]
- 73.Jain K., Gupta V.K., Singh L.P. Neutral carrier and organic resin based membranes as sensors for uranyl ions. Anal. Proc. 1995;32:263–266. [Google Scholar]
- 74.Kurrey R., Mahilang M., Deb M.K., Nirmalkar J., Shrivas K., Pervez S., Rai M.K., Rai J. A direct DRS-FTIR probe for rapid detection and quantification of fluoroquinolone antibiotics in poultry egg-yolk. Food Chem. 2019;270:459–466. doi: 10.1016/j.foodchem.2018.07.129. [DOI] [PubMed] [Google Scholar]
- 75.Kurrey R., Mahilang M., Deb M.K., Shrivas K. Analytical approach on surface active agents in the environment and challenges. Trends Environ. Anal. Chem. 2018 [Google Scholar]
- 76.Kurrey R., Deb M.K., Shrivas K. Methyl orange paired microextraction and diffuse reflectance- Fourier transform infrared spectral monitoring for improved signal strength of total mixed cationic surfactants. J.Surfact.Deterg. 2018;21:197–208. [Google Scholar]
- 77.Machado D.I.S., Cervantes J.L., Hernandez J.L., Losada P.P. Simultaneous determination of thiamine and riboflavin in edible marine seaweeds by high performance liquid chromatography. J. Chromatogr. Sci. 2004;42:117–119. doi: 10.1093/chromsci/42.3.117. [DOI] [PubMed] [Google Scholar]
- 78.M Saad S., Eman E. Thiamine-reineckate liquid membrane electrode for the selective determination of thiamine (vitamin B1) in pharmaceutical preparations. Analyst. 1989;114:735–737. [Google Scholar]
- 79.Zhang C., Zhou G., Zhang Z., Aizawa M. Highly sensitive electrochemical luminescence determination of thiamine. Anal. Chim. Acta. 1999;394:165–170. [Google Scholar]
- 80.Issa Y.M., Shoukry A.F., Ibrahim H., Mohamed S.K. Atomic emission spectrometric determination of antazoline, hydralazine, amiloride, thiamine and quinine based on formation of ion associates with ammonium reineckate. Anal. Lett. 1994;27:731–742. [Google Scholar]
- 81.Wang Q.L., Chen M.D. Determination of the V B1 content in V B1-containing medicines by the turbidimetric method of the reaction system of BaSO4-gelatine-CTMAB. Phy. Testing Chem. Anal. B Chem. Anal. 1995;31:285–297. [Google Scholar]
- 82.Huang R.H. Phosphorescence of thiamine after ultraviolet irradiation, China. J. Anal. Chem. 1998;26:1349–1355. [Google Scholar]
- 83.Du J., Li Y., Lu J. Flow injection chemiluminescence determination of thiamine based on its enhancing effect on the luminol-hydrogen peroxide system. Talanta. 2002;57:661–665. doi: 10.1016/s0039-9140(02)00079-6. [DOI] [PubMed] [Google Scholar]
- 84.Ren Y.P., Chen H.W., Chen Q.J., Bao L.B., Huang B.F., He Q.H. Determination of thiamine in foods by high performance liquid chromatography with post-column photochemical derivatization and fluorescence detection. Chin. J. Anal. Chem. 2000;28:548–554. [Google Scholar]
- 85.Feng F., Wang K., Chen Z., Chen Q., Lin J., Huang S. Flow injection renewable drops spectrofluorimetry for sequential determinations of vitamins B1, B2 and B6. Anal. Chim. Acta. 2004;527:187–193. [Google Scholar]