Summary
Background
Georgia has a high prevalence of hepatitis C, with 5·4% of adults chronically infected. On April 28, 2015, Georgia launched a national programme to eliminate hepatitis C by 2020 (90% reduction in prevalence) through scaled-up treatment and prevention interventions. We evaluated the interim effect of the programme and feasibility of achieving the elimination goal.
Methods
We developed a transmission model to capture the hepatitis C epidemic in Georgia, calibrated to data from biobehavioural surveys of people who inject drugs (PWID; 1998–2015) and a national survey (2015). We projected the effect of the administration of direct-acting antiviral treatments until Feb 28, 2019, and the effect of continuing current treatment rates until the end of 2020. Effect was estimated in terms of the relative decrease in hepatitis C incidence, prevalence, and mortality relative to 2015 and of the deaths and infections averted compared with a counterfactual of no treatment over the study period. We also estimated treatment rates needed to reach Georgia's elimination target.
Findings
From May 1, 2015, to Feb 28, 2019, 54 313 patients were treated, with approximately 1000 patients treated per month since mid 2017. Compared with 2015, our model projects that these treatments have reduced the prevalence of adult chronic hepatitis C by a median 37% (95% credible interval 30–44), the incidence of chronic hepatitis C by 37% (29–44), and chronic hepatitis C mortality by 14% (3–30) and have prevented 3516 (1842–6250) new infections and averted 252 (134–389) deaths related to chronic hepatitis C. Continuing treatment of 1000 patients per month is predicted to reduce prevalence by 51% (42–61) and incidence by 51% (40–62), by the end of 2020. To reach a 90% reduction by 2020, treatment rates must increase to 4144 (2963–5322) patients initiating treatment per month.
Interpretation
Georgia's hepatitis C elimination programme has achieved substantial treatment scale-up, which has reduced the burden of chronic hepatitis C. However, the country is unlikely to meet its 2020 elimination target unless treatment scales up considerably.
Funding
CDC Foundation, National Institute for Health Research, National Institutes of Health.
Introduction
Hepatitis C virus (HCV) infection causes liver disease,1, 2 with 71 million people being infected globally in 2015 and 80% of them living in low-income and middle-income countries.3 HCV is primarily transmitted by injection drug use and unsafe medical procedures.4, 5, 6 The development of highly curative direct-acting antiviral treatments for HCV contributed to WHO's 2016 global strategy to eliminate hepatitis C.7
Hepatitis C prevalence is high in Georgia, with 150 000 adults (5·4% of the adult population) infected in 2015.8 Georgia launched the first national hepatitis C elimination programme in 2015, with donated treatments from Gilead Sciences and technical assistance from the US Centers for Disease Control and Prevention.9 This programme aims to reduce the prevalence of chronic hepatitis C infection by 90% through diagnosing 90% of infections, treating 95% of diagnosed infections, and curing 95% of treated individuals (90-95-95 target) by 2020.
A national survey done in 20158 found considerable variation in prevalence of chronic hepatitis C by gender and age. The highest prevalence of infection (15·7%) was among men aged 30–49 years, with much lower prevalence in adult women (2·2%). The high prevalence of chronic hepatitis C in men in this age bracket is thought to have resulted from extensive transmission after the collapse of the Soviet Union in 1991, when civil war and economic collapse10 resulted in considerable drug trafficking and injection drug use in Georgia.11 Although injection drug use has decreased since then, Georgia still has a high rate of injection drug use (2% of adults)12 compared with the global average (0·33%).4 Iatrogenic HCV transmission also occurred because of insufficient infection control practices and inadequately screened blood supply, which were not addressed until after 2009.8, 13 Prevention of these modes of transmission and improvements in harm-reduction interventions for people who inject drugs (PWID) are goals of the elimination programme, alongside HCV case-finding and treatment.14
Research in context.
Evidence before this study
We identified mathematical models of hepatitis C elimination by searching PubMed from database inception to May 1, 2019, using the terms “(“HCV” OR “Hepatitis C”) AND “elimination” AND (“model” OR “projection”)” in title and abstract fields. We identified several studies that project the scale-up of treatment of hepatitis C virus (HCV) infection required to eliminate hepatitis C within high-risk populations, such as people who inject drugs (PWID) or people living with HIV in subnational regions of the UK, Greece, Australia, and the USA, or nationally in Iceland, the USA, and Australia. We also identified models of hepatitis C elimination among the general population for subnational regions of the USA and Austria; at the national level for Switzerland, Australia, Italy, Greece, Belgium, Egypt, and Pakistan; regionally for the EU; and one global model. Of the national-level studies, only the general population models for Egypt and Pakistan, and the PWID-focused models in Iceland, Australia, and USA were based on dynamic HCV transmission models that account for the prevention impact of treatment on HCV incidence. No studies evaluated the interim effect of an ongoing HCV elimination programme.
Added value of this study
This study uses a dynamic model of HCV transmission among PWID and the general population to assess the interim effect of the first national-level HCV elimination programme in Georgia, a country with high HCV prevalence (5·4% in 2015). This study illustrates the importance of using modelling to assess the progress of ongoing elimination programmes. It suggests that a substantial effect (37% decrease in incidence and prevalence) has already been achieved by the Georgian HCV elimination programme, but that treatment rates either need to be increased dramatically (by four times) or the duration of the programme needs lengthening (from 2020 to 2026), to ensure it reaches its primary endpoint of a 90% reduction in HCV prevalence compared with the prevalence in 2015.
Implications of all the available evidence
Published data highlight that rapid and substantial treatment scale-up is required to reach HCV elimination targets set by WHO by 2030. This study shows that countries can achieve large increases in treatment, which should achieve substantial decreases in prevalence and incidence, but highlights the challenges of implementing sufficient scale-up to achieve elimination over a short timeframe even with a high level of government commitment.
We estimated the interim effect of the Georgian hepatitis C elimination programme using HCV transmission modelling with empirical treatment data and evaluated whether treatment needs scaling up to achieve the elimination target.
Methods
Model description
We developed a compartmental model of HCV transmission related to injection drug use and in the general population (iatrogenic and other risk factors) incorporating the changing demographics of PWID in Georgia (appendix pp 2–6). The model assumes susceptible (ie, uninfected) individuals can become infected, with some spontaneously clearing their infection and the remainder developing life-long chronic infection unless treated. Successful treatment leads to a sustained virologic response (ie, effective cure), which results in individuals becoming susceptible to re-infection. The model is stratified by HCV infection status (figure 1A), gender, age (figure 1C), liver disease progression (figure 1B), and injection drug use status (ie, PWID, people who have never injected drugs [non-PWID], and people who used to inject drugs; figure 1C).
Figure 1.
Schematics of state transitions in the model
(A) Infection compartments, (B) liver disease state compartments, (C) PWID and age compartments. Gender compartments are not shown. Dotted lines indicate transition to death. ex-PWID=people who used to inject drugs. Non-PWID=people who have never injected drugs. PWID=people who inject drugs.
Individuals enter the model at birth as susceptible non-PWID and transition through age categories, with some starting injection drug use at age-specific and gender-specific rates to match self-reported ages of initiation of injection drug use and proportion of female PWID (appendix p 4). Vertical HCV transmission is not included because few young women are infected (1%). Mortality of PWID is increased, compared with the general population, because of drug-related causes and this population ceases injecting at age-specific rates.
Susceptible individuals become infected at a rate proportional to Georgia's chronic hepatitis C prevalence, with a general transmission rate that applies to the whole population and an additional injection drug use-related transmission rate. Both transmission rates vary over time to account for changes in risk and harm-reduction intervention coverage. The model also allows for assortative mixing between younger (<30 years) and older (≥30 years) PWID.
Individuals with chronic infection progress through stages of liver disease (figure 1B). Individuals with decompensated cirrhosis and hepatocellular carcinoma have a heightened liver-related mortality. Treatment rates (ie, the number of individuals that initiate treatment per month) vary over time and by liver disease stage to match data from the elimination programme. Sustained virologic response halts disease progression for mild or moderate liver disease, whereas it continues at a decreased rate for more progressed disease.15 Individuals with hepatocellular carcinoma are not treated.
Model parameterisation and calibration
The model was parameterised and calibrated to the current HCV epidemic in Georgia, as described herein. We simulated a stable population approximating current demographic trends, within which we initiated injection drug use and HCV transmission in 1960. This time threshold was selected because individuals infected with HCV before this time are unlikely to be alive now and it enabled modelled HCV prevalences to reach equilibrium before changes in injection drug use were introduced. We modelled changes in injection drug use and associated HCV over time because evidence suggests it has shaped the Georgian HCV epidemic.11
Calibration and validation data
The model was calibrated to data on the prevalence of chronic hepatitis C from the 2015 national prevalence survey8 and seven biobehavioral surveys of PWID done during 1998–2015 (table 1; appendix pp 11).17, 18, 20, 21, 22, 23 The model was also calibrated to an observed ageing of PWID between 1998 and 2015, thought to be due to reductions in initiation of injection drug use (appendix p 14). Model projections were validated against empirical unpublished data for HCV incidence among PWID in 1997–2001 (appendix pp 7, 8), chronic hepatitis C prevalence data for PWID from five surveys (2001–12), and age-specific chronic hepatitis C prevalence data from the 2015 national prevalence survey not used for calibration.8, 17, 18, 20, 21, 22, 23
Table 1.
Key summary statistics used for calibrating the hepatitis C virus transmission model for Georgia
| Target value | Mean and range across baseline model fits | |
|---|---|---|
| Population of Georgia16 | 3·72 million | 3·73 million (3·35–4·10) |
| Hepatitis C prevalence in adult population8 | 5·4% | 5·4% (4·5–6·3) |
| Hepatitis C prevalence in adult women8 | 2·2% | 2·2% (1·6–2·9) |
| Hepatitis C prevalence in adult men8 | 9·0% | 9·7% (6·7–12·6) |
| Hepatitis C prevalence among PWID17 | 51·0% | 50·8% (45·4–66·3) |
| Hepatitis C prevalence in PWID aged 18–24 years17 | 15·5% | 36·1% (14·6–46·7) |
| Ratio of hepatitis C prevalence in PWID younger than 30 years in 1997 vs 201517, 18 | 0·5 | 0·81 (0·40–1·0) |
| PWID population size in Georgia,19 in 2014 | 49 700 | 83 999 (23 932–190 501) |
| Proportion of PWID that are female17 | 2·0% | 3·1% (0·1–8·0) |
| Proportion of PWID <30 years old,18 in 1998 | 63·2% | 62·4% (51·5–72·6) |
| Proportion of PWID <30 years old17 | 19·4% | 34·6% (20·7–46·0) |
Data refer to 2015 unless otherwise specified. References indicate where target values were obtained from. A full list of summary statistics is available in the appendix (p 11). Adults are defined as individuals aged 18 years or older. PWID=people who inject drugs.
Model parameterisation
Disease progression and HCV-related and injection drug use-related mortality were obtained from published literature,15, 24, 25, 26 whereas gender-specific and age-specific mortality were derived from life tables for Georgia27 (table 2; appendix pp 9–11). PWID recruitment and cessation parameters were estimated by fitting the model to the proportion of PWID that were aged 18–29 years and 30–49 years in 1998 and 2015, the estimated number of PWID in 2014, and their gender distribution (table 1; appendix p 11). The number of PWID in Georgia is thought to have increased dramatically after the fall of the Soviet Union, as suggested by an eight-fold increase in police records for people who used drugs over 1990–2004.10, 11 However, no PWID population size estimates exist over this time period,10 so we assumed a transient peak in the initiation of injection drug use, allowing uncertainty in its timing and magnitude (table 2; appendix pp 9, 10). The effect of assuming a peak in initiation of injecting was tested in our sensitivity analyses.
Table 2.
Selected parameters used in HCV transmission model for Georgia
| Prior range* | Posterior median (IQR) | |
|---|---|---|
| Average duration of injecting (years) among PWID aged <29 years | 5–50 | 17·3 (10·9–29·8) |
| Average duration of injecting (years) among PWID aged 30–49 years | 5–50 | 38·1 (30·6–44·3) |
| Average duration of injecting (years) among PWID aged ≥50 years | 5–50 | 29·5 (18·6–38·4) |
| Standardised mortality ratio for PWID26 | 7·2–11·3 | 9·0 (8·1–9·9) |
| Year that increase in PWID recruitment started10, 11 | 1980–95 | 1987 (1984–90) |
| Duration of period of increase in PWID recruitment (years) | 1–30 | 18·4 (13·0–22·4) |
| Year that decrease in general population transmission started13, 14 | 1994–200013, 14 | 1997 (1995–1998) |
| Relative risk of HCV transmission in general population after decrease | 0·01–0·50 | 0·22 (0·12–0·34) |
| Relative risk of HCV transmission on OST28 | 0·40–0·6322 | 0·52 (0·47–0·57) |
| Relative risk of PWID HCV transmission risk due to NSP from 2002† | 0·00–1·00 | 0·26 (0·14–0·42) |
| Relative risk of PWID HCV transmission risk due to NSP from 2012 | 0·00–1·00 | 0·19 (0·10–0·29) |
References indicate where prior ranges were obtained from. PWID=people who inject drugs. HCV=hepatitis C virus. OST=opioid substitution therapy. NSP=needle and syringe programmes.
All priors were uniformly distributed.
Needle and syringe programmes were initiated in Georgia in 2001 and opioid substitution therapy in 2005,29 with 4·5 million syringe kits distributed and 30 330 PWID reached by needle and syringe programmes in 2016, and 4775 PWID on opioid substitution therapy in the same year.20 The efficacy of opioid substitution therapy for reducing the risk of HCV acquisition (37–60) among PWID was obtained from a Cochrane review.28 Because of uncertainty in the efficacy of needle and syringe programmes and associated behavioural changes, we fitted the population-level effectiveness of needle and syringe programmes among PWID to capture an observed halving in HCV prevalence among young PWID (<30 years) over 1998–2006 (table 2; appendix p 15).
The general population HCV transmission rate was also allowed to reduce over 1994–2000 to account for reductions in medical risks coinciding with restructuring of the health system and the introduction of new regulations including blood donor screening from 1997.13, 14
Model calibration
We used a Markov Chain Monte Carlo Approximate Bayesian Computation (MCMC-ABC) approach to calibrate the model (appendix p 7).31 The method computes a probability distribution of model parameter values (the posterior) that constrain the initial prior ranges, producing model fits that incorporate uncertainty in the model parameters and calibration data. The parameter sets identified through MCMC-ABC were then filtered to only retain those within 95% CIs of the chronic hepatitis C prevalence for all adults (4·5–6·3) and adult women (1·6–2·9) from the 2015 national prevalence survey8 and for PWID (45·5–56·1) from the 2015 biobehavioral surveys.17 These filtered runs were termed the baseline model fits and were used to estimate the median and 95% credible interval (CrI) or central 95% range of all model projections.
Intervention analyses
We estimated the progress that Georgia has made toward its elimination goal by modelling the effect of all direct-acting antiviral treatments given from May 1, 2015, to Feb 28, 2019. The model used monthly treatment initiation data for the elimination programme, accounting for severity of liver disease and the initial targeting of patients with cirrhosis (table 3; appendix p 12).9 Adjusted cure rates were used, calculated separately for patients with or without cirrhosis. These cure rates assumed the per-protocol sustained virologic response rate (table 3) for the 78% of patients who completed treatment among those who initiated it, and a reduced sustained virologic response rate (55%) for the remaining individuals that did not complete treatment, based on studies of shorter treatment regimens (appendix p 7).32
Table 3.
Total treatment numbers and SVR rates for Georgia's hepatitis C elimination programme, by level of liver disease
| No, mild, or moderate liver disease | Cirrhosis or decompensated cirrhosis | |
|---|---|---|
| May 1, 2015–Feb 29, 2016 | ||
| Total number treated | 2800* | 3779† |
| Per-protocol SVR | 1395/1564 (89·2%) | 2245/2960 (75·8%) |
| Intention to treat SVR | 1395/2228 (62·6%) | 2245/4346 (51·7%) |
| Adjusted SVR‡ | 1765/2201 (80·2%) | 2963/4057 (73·0%) |
| March, 2016–February, 2019 | ||
| Total number treated | 41 474§ | 6259¶ |
| Per-protocol SVR | 25 954/26 314 (98·6%) | 4497/4665 (96·4%) |
| Intention-to-treat SVR | 25 954/34 024 (76·3%) | 4497/6738 (66·7%) |
| Adjusted SVR‡ | 30 104/33 826 (89·0%) | 5573/6467 (86·2%) |
From May 1, 2015, to Feb 29, 2016, patients were treated with sofosbuvir-based (with or without ribavirin) regimens and from March 1, 2016, to Feb 28, 2019, they were treated with ledipasvir-sofosbuvir combination-based regimens. SVR=sustained virological response.
68 patients with no or mild liver disease and 2732 patients with moderate liver disease.
3757 patients with cirrhosis and 22 patients with decompensated cirrhosis.
The adjusted SVR assumes patients that completed treatment had the per-protocol SVR rate and that 55% of patients lost to follow up during treatment were cured on the basis of studies of shorter treatment regimens32 (appendix p 7).
21 608 patients with no or mild liver diseases and 19 866 with moderate liver disease.
5659 patients with cirrhosis and 601 patients with decompensated cirrhosis.
Effect was estimated in terms of the relative decrease in incidence and prevalence from Jan 1, 2015 (with treatment given from May 1, 2015), to Feb 28, 2019, and of the deaths and infections averted compared with a counterfactual of no treatment over this period. The future benefits of these treatments were also estimated up until 2030, assuming treatment stopped after Feb 28, 2019.
We then estimated the effect of either maintaining the current treatment rate (approximately 1000 patients treated per month from Aug 1, 2017, to Feb 28, 2019) or scaling-up treatment rates to achieve the 90-95-95 treatment target set by the Georgian Government (equivalent to treating 128 250 individuals during 2015–20). Lastly, we estimated the treatment rate required from the start of the programme and from March 1, 2019, to achieve the 90% reduction in prevalence set by the Georgian elimination target. For each strategy, we also estimated the effect on incidence and the number of prevented infections and deaths by the end of 2020.
Sensitivity analysis
In our baseline intervention scenarios, we assumed that all individuals eligible for treatment were equally likely to be treated from March 1, 2019. However, the degree to which PWID receive treatment and whether individuals with cirrhosis should be preferentially treated going forward is uncertain. We, therefore, did a sensitivity analysis to assess how the required treatment rate to achieve a 90% decrease in prevalence by 2020 would change if: individuals with cirrhosis are targeted (80% of infected individuals with cirrhosis are treated annually); PWID are not treated; or PWID are targeted for treatment at twice the rate of other groups.
We also did sensitivity analyses to assess how the treatment target would change if: the treatment programme achieved the upper bound (per protocol) or lower bound (intention to treat) sustained virologic response rates for all patients; existing needle and syringe programmes in Georgia had the effectiveness estimated for Europe by a recent Cochrane review (risk ratio 9–62% if on needle and syringe programmes);28 opioid substitution therapy coverage doubled from 2016, to 9000 PWID covered in 2019; no peak in PWID recruitment occurred; or treatment scale-up was delayed for 6 months. Lastly, we used analysis of covariance to calculate the variance in the number of treatments required to reach elimination that is explained by uncertainty in each parameter, for the baseline treatment scenario.
All analyses were done with Matlab version R2016b or R version 3.5.1.
Role of the funding source
The funders of the study played no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The Bayesian MCMC-ABC routine produced 554 baseline model fits that agreed well with general population and PWID demographic and chronic hepatitis C prevalence data (appendix pp 17, 18), with considerable uncertainty in the PWID population size, reflecting the uncertainty in the data described in the Methods. Fits to summary statistics and posterior distributions of fitted parameters are shown in the appendix (pp 19, 20).
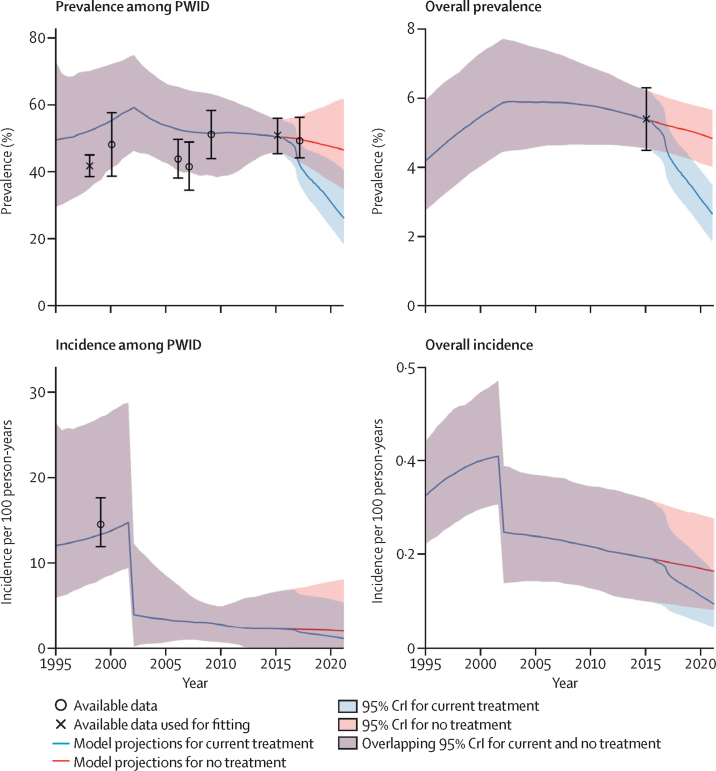
The baseline model fits project that the overall adult chronic hepatitis C prevalence and incidence have decreased since 2000, with both continuing to decline during 2015–20 in the absence of treatment by 11% (CrI 2–18; prevalence) and 14% (7–20; incidence; figure 2). These decreases imply a reduction in the number of new infections each year from 6700 (3542–11 076) to 5897 (3059–9920) during 2015–20. Conversely, over the same period, HCV-related mortality is expected to increase by 14% (7–25), from 590 (285–1001) deaths in 2015, to 676 (344–1091) deaths in 2020.
Figure 2.
Chronic hepatitis C prevalence and incidence among adult PWID and the overall adult population over time
Data are prevalence (95% CI) or incidence (95% CI). Model projections for current treatment (red line) incorporate actual treatment numbers from May 1, 2015, to Feb 28, 2019, and assume a treatment rate of 1000 individuals initiating treatment per month continuing from March, 2019. CrI=credible interval. HCV=hepatitis C virus. PWID=people who inject drugs.
Projections suggest the PWID population peaked in 2002 (128 815, CrI 71 855–203 164) but declined to 64 420 (25 647–121 190) by 2018 (appendix p 21), with the HCV incidence among PWID decreasing by 76% (37–95) during 2000–05 (figure 2). These parallel decreases are required to ensure the model replicates the observed ageing among PWID and the decrease in HCV infection among young PWID. The HCV incidence among PWID decreased further, without treatment, from 2·4 new cases (0·19–6·8) per 100 person-years in 2015, to 2·2 new cases (0·15–8·0) in 2020 (figure 2).
Our model projects that the 54 313 treatments delivered between May 1, 2015, and Feb 28, 2019, have decreased the national prevalence of adult chronic hepatitis C by 37% (CrI 30–44), with incidence decreasing similarly (37%, 29–44; figure 3). This decrease prevented 252 (134–389) HCV-related deaths (mortality decrease by 14%, 3–30) and 3516 (1842–6250) new HCV infections by Feb 28, 2019, increasing to 3181 (1992–4393) the number of HCV-related deaths and to 20 907 (10 335–37 585) the number of HCV infections averted if benefits are tracked to 2030.
Figure 3.
Model projected interim effect at the end of February, 2019, and future effect of different treatment scenarios at the end of 2020
(A) Cumulative chronic hepatitis C treatments, (B) adult chronic hepatitis C prevalence, (C) adult hepatitis C incidence, and (D) annual hepatitis C mortality over time. x-axis tick marks indicate the beginning of each labelled year. CrI=credible interval.
Assuming all eligible individuals have equal access to treatment, continuing current treatment rates (1000 individuals initiating treatment per month) will halve chronic hepatitis C prevalence (decrease by 51%, CrI 42–61, to 2·7%, 1·9–3·5) and incidence (decrease by 51%, 40–62, to 0·097, 0·046–0·16) by 2020 (Figure 3, Figure 4), reaching a median 90% reduction in 2026, and mortality reaching a 65% reduction in 2028 (appendix p 25).
Figure 4.
Percent reduction in chronic hepatitis C prevalence (A), incidence (B), and mortality (C) from 2015, to the end of 2020, under different treatment strategies initiating in March 1, 2019
Data are median (credible interval). The no treatment (yellow) scenario (from 2015) is also shown, otherwise scenarios assume achieved treatment rates until February, 2019, followed by continuing treatment at indicated rate from March, 2019.
To reach Georgia's 90-95-95 treatment target by 2020, treatment rate needs to increase to 3361 individuals initiating treatment per month from March 1, 2019. This scale-up would achieve an 80% (CrI 68–96) reduction in prevalence and an 80% (66–96) reduction in incidence of chronic hepatitis C by 2020 (figure 4), a median reduction of 90% in 2021.
To reach a 90% reduction in prevalence by 2020, a monthly treatment rate of 2210 (CrI 1799–4000) individuals starting treatment per month would have been required over 2015–20. However, with the achieved treatment rates to Feb 28, 2019, treatment now needs to scale-up to a median of 4144 (2963–5322) individuals starting treatment per month from March 1, 2019, to reach the 90% reduction in prevalence by 2020. This scale-up would decrease HCV incidence by 90% (88–90) and chronic hepatitis C related mortality by 31% (CrI 18–46) by 2020, with mortality reaching a 65% reduction by 2025. Variability in the number of treatments required for achieving the 90% reduction in prevalence by 2020 is mainly due to uncertainty in the annual birth rate (35·9% of variation; appendix p 13) and parameters related to the transient peak in injection drug use initiation (49·0% of variation).
If, instead of equal access to treatment, individuals with cirrhosis are preferentially targeted from March 1, 2019 (80% of cirrhosis patients treated each year), then the same treatment rate (4144, CrI 2963–5323, individuals starting treatment per month; figure 5) would be needed to achieve a 90% reduction in prevalence by 2020 and the same decrease in mortality would occur (31% decrease, 18–46). If PWID are not treated from March 1, 2019, then a 90% decrease in prevalence will not be possible because current PWID make up a high proportion of prevalent infections (13–37%, in 2015). However, it makes little difference whether PWID are treated at a higher rate or equally to the rest of the population, with both scenarios requiring the same treatment rate to achieve a 90% reduction in prevalence (figure 5; appendix pp 22, 23).
Figure 5.
Sensitivity analysis of treatment rate needed under alternative model scenarios in comparison with baseline model to reach a 90% reduction in chronic hepatitis C prevalence by end of 2020
Data are median (credible interval). Treatment rates are for March, 2019, onwards except for the scenario, delay scale-up by 6 months, in which the treatment rate continues at 1000 individuals starting treatment per month until September, 2019, and then is scaled up. In scenario, exclude PWID, elimination is not possible at any level of treatment scale-up. NSP=needle and syringe programme. OST=opioid substitution therapy. PWID=people who inject drugs. SVR=sustained viral response.
The baseline projections assume an adjusted sustained virologic response rate (table 1) and a substantial effect of needle and syringe programmes. If, instead, the upper-bound, per-protocol, sustained virologic response rate is assumed, the monthly treatment rate from March 1, 2019, reduces to 3579 (CrI 2485–4650) individuals initiating treatment per month, whereas it increases to 5167 (3796–6519) individuals initiating treatment per month if the intention-to-treat, sustained virologic response rate is used (it assumes that only those not attending the sustained virologic response visit are not cured; figure 5). The necessary treatment rate only changes marginally if a reduced efficacy of needle and syringe programmes28 is used (4114, 2938–5734, individuals initiating treatment per month) or if opioid substitution therapy coverage is doubled (4141, 2952–5316). If no peak in PWID recruitment is included, then the required treatment rate increases slightly to 4443 (2941–6223) individuals initiating treatment per month, but the model no longer fits the calibration data well (appendix p 24). Lastly, if treatment scale-up is delayed by 6 months to Sept 1, 2019, the required monthly treatment rate increases to 5271 (3796–6519) individuals initiating treatment per month.
Discussion
Georgia has implemented an ambitious hepatitis C elimination programme which aims to reduce the prevalence of hepatitis C by 90% by 2020. Hepatitis C treatment has been scaled-up considerably since 2015, with more than 54 000 HCV-infected individuals treated from an estimated 150 000 infected individuals. Our model projections suggest this programme has reduced hepatitis C prevalence and incidence by 37%, since 2015, and will halve prevalence and incidence by 2020. However, the current treatment rate (approximately 1000 individuals initiating treatment per month) needs to be quadrupled to achieve the target of reducing prevalence by 90% by 2020. Strategies also need to maintain high rates of treatment completion, because decreased sustained virologic response rates will further increase the treatment rate required to reach the elimination target. In addition, PWID must be treated. Although PWID can be difficult to reach and face structural and social barriers to engagement in the elimination programme,33 ongoing efforts within the programme to decentralise care to harm-reduction centres and to follow up patients previously lost to follow-up are likely to increase treatment among PWID.14
In addition to Georgia's own elimination goals, WHO has set a global target to reduce HCV-related mortality by 65%. Even if hepatitis C treatment in Georgia is scaled up to reach the target of 90% prevalence reduction, it will still not meet the WHO mortality target by 2020. This result is caused by extensive liver damage among currently infected individuals (18% of those who initiated treatment had cirrhosis [unpublished data]), which limits the short-term mortality benefits of treatment. Nevertheless, Georgia is still on track to reach the WHO elimination target for mortality (and incidence) by 2030, confirming previous modelling projections.34
Importantly, our modelling suggests the prevalence and incidence of hepatitis C in Georgia were already in decline before the hepatitis C elimination programme began in 2015 (figure 2). The modelled decline was largely due to improvements in harm-reduction measures paired with a diminishing PWID population, which reduces the contribution of injection drug use to overall transmission. Because the epidemic is in decline, it is easier to achieve the elimination target, highlighting the important role that prevention interventions for PWID can have. In the general population, the risk of iatrogenic HCV transmission still persists. Developing infection control measures to reduce these risks is a key part of the elimination programme.35
Case-finding and linkage-to-care initiatives will be essential for reaching Georgia's elimination targets. These interventions might be difficult among PWID in particular, and although the contribution of PWID to the hepatitis C epidemic has declined, they still represent an important component of the chronic hepatitis C burden, so testing and treatment must be accessible to them. Increasing linkage to HCV treatment, in particular for PWID through harm-reduction interventions, is a goal of the elimination programme.14 HCV treatment at harm-reduction sites is being piloted,36 and HCV testing at these sites has increased by five times since the start of the elimination programme.36 In addition, a pilot programme in Tbilisi showed the feasibility of achieving high cure rates among PWID.37 Despite these positive signs, there are still barriers for PWID linking to care,33 and there is still uncertainty on the number of PWID being treated, because of their non-disclosure of national identity numbers required for linking screening and treatment data.36 This limits the evaluation of progress towards elimination.
This is the first study to evaluate the interim effect and treatment targets of an ongoing national-level hepatitis C elimination programme by using detailed modelling with in-depth data from the programme.38, 39, 40 A second serosurvey is planned for early 2020s to evaluate if the target effect has been achieved, the timing of which will be guided by modelling.
The main limitations of our analysis relate to small amount of data on how HCV transmission has changed over time, in the general population and because of injection drug use. Our model suggests a declining epidemic in terms of both prevalence and incidence, which fits available data (from the 2015 national prevalence survey) on reductions in chronic hepatitis C prevalence among new PWID over time and young male adults having a low chronic hepatitis C prevalence in 2015. However, the only available comparison of HCV prevalence in the general population (from a survey done in Tbilisi in 2001–02),41 suggests a stable or increasing prevalence of seropositivity (6·7%, 95% CI 5·7–7·9, in 2001–02 compared with 9·4%, 6·9–12·6, in Tbilisi according to the 2015 national survey). The Tbilisi survey was not included in our fitting process because of uncertainty in its comparability with the 2015 national survey, resulting from the clinic-based sampling methods used. If the epidemic is increasing as suggested by these data, then our projections (data not shown) suggest the treatment requirements for elimination will be higher than what we estimated (approximately 5000 individuals initiating treatment per month). Additionally, both the 2001–02 study and 2015 national survey were household-based surveys and, therefore, did not include prisoners or homeless people, potentially leading to an underestimation of the burden of chronic hepatitis C. It is important that further work evaluate the importance of this issue.
HCV-related mortality was not consistently recorded in Georgia before 2015, and although this recording is being improved as part of the elimination programme, complete data were not yet available for this analysis.14 New data will improve our model calibration. Furthermore, our model assumed a stable population for Georgia, despite projections suggesting it might decrease (it decreased by 5% during 2010–15).42 This decrease should not have an important effect on our projections because the changes are quite small.
Data had limitations on many other parameters, including being reliant on self-reported data about PWID demographics. To account for these limitations, we allowed for uncertainty in model parameters while remaining consistent with available data. In addition, we did sensitivity analyses that made alternative assumptions about the effectiveness of needle and syringe programmes or the degree to which PWID were treated; and although these changes did not affect our elimination projections, except if PWID were not treated at all, additional studies could still help reduce these uncertainties.
One of the main limitations for translating our results into recommendations for the Georgian HCV elimination programme is that the model does not incorporate case-finding, so it cannot identify what screening strategies are needed to achieve required rates of chronic hepatitis C treatment. In the early stages of the programme, many individuals with chronic hepatitis C were already aware of their infection and came forward for treatment.9 General population and targeted screening strategies are also underway, with 106 057 positive antibody screening tests done in health-care settings, harm-reduction services, designated public screening centres, and in prisons as of April 2019.34 Other screening and linkage-to-care strategies are also being piloted or scaled up to further increase the identification and treatment of undiagnosed infections, including treatment within harm-reduction services, door-to-door and workplace-based testing, simplification of the treatment pathway to encourage retention, and reducing the co-payment for patients.14 It is important that these and other possible strategies are evaluated to determine the most efficient way to achieve elimination,43 which could help other countries work toward their own elimination goals.
Georgia has committed to eliminating hepatitis C as a public health threat, with the ongoing national programme achieving high levels of treatment uptake. Data from the programme and our modelling indicate an urgent need to improve case-finding, referral, and treatment interventions for reaching Georgia's targets of hepatitis C elimination by 2020. Decision makers in Georgia will need to evaluate what is feasible for achieving hepatitis C elimination. This assessment will require considering what is currently limiting treatment numbers and how these issues can be remedied. Importantly, the treatments already achieved have had major effects on HCV transmission in Georgia, and even if the elimination targets are not feasible by 2020, Georgia will still be one of the first countries to eliminate HCV ahead of the WHO target. Lessons learnt from Georgia are transferable to other countries that are scaling up interventions to prevent hepatitis C.35 In particular, our study indicates the importance of identifying the characteristics and dynamics of an epidemic to make reliable impact projections.
Data sharing
Model code will be made available on request to the corresponding author.
Acknowledgments
Acknowledgments
JGW and PV were funded by Centers for Disease Control and Prevention Foundation. MH and PV are supported by the National Institute for Health Research (NIHR) Health Protection Research Units in Evaluation of Interventions at the University of Bristol in partnership with Public Health England (PHE). NKM acknowledges support from National Institute of Allergy and Infectious Diseases and National Institute on Drug Abuse (R01AI147490) and was additionally supported by the University of San Diego Center for AIDS Research, a National Institutes of Health funded programme (P30 AI036214). The views expressed in this publication are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention, the National Health Service, the NIHR, the University of Bristol, the Department of Health, or PHE.
Contributors
JGW and PV led the study. JGW developed the model on the basis of preliminary models developed by HF, AGL, NKM, and PV, did all modelling analyses, wrote the first draft of the paper with guidance from PV, and analysed data from behavioural surveys of people who inject drugs in Georgia. The concept for the study was developed with TK, MH, NKM, JM, FA, and MN, and PV. SS, LH, and LG analysed data from the Georgian hepatitis C virus programme. Data were contributed by MA, AA, DB, MB, IC, IKh, IKi, MHK, DO, LS, KS, TT, and MZ, and gathered under the supervision of DS, AG, and VK. All authors contributed to the interpretation of results and writing the report and approved the final version.
Declaration of interests
HF reports an honorarium from MSD. MH reports personal fees from Gilead, Abbvie, and MSD. NKM reports unrestricted research grants and honoraria from Gilead and Merck. PV has received unrestricted research grants from Gilead and honoraria from Gilead and AbbVie. All other authors declare no competing interests.
Supplementary Material
References
- 1.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Flaxman AD, Naghavi M. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res. 2015;119:89–96. doi: 10.1016/j.antiviral.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Peacock A, Colledge S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degenhardt L, Charlson F, Stanaway J. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385–1398. doi: 10.1016/S1473-3099(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 6.Trickey A, Fraser H, Lim AG. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4:435–444. doi: 10.1016/S2468-1253(19)30085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . World Health Organization; Geneva: 2016. Global health sector strategy on viral hepatitis 2016–2021. [Google Scholar]
- 8.Hagan LM, Kasradze A, Salyer SJ. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health. 2019;19:480. doi: 10.1186/s12889-019-6784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gvinjilia L, Nasrullah M, Sergeenko D. National progress toward hepatitis C elimination—Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep. 2016;65:1132–1135. doi: 10.15585/mmwr.mm6541a2. [DOI] [PubMed] [Google Scholar]
- 10.Stvilia K, Nizharadze G, Todadze K. United Nations Educational, Scientific and Cultural Organization; Paris: 2005. HIV and AIDS in Georgia: a socio-cultural approach. [Google Scholar]
- 11.Bouscaillou J, Champagnat J, Luhmann N. Hepatitis C among people who inject drugs in Tbilisi, Georgia: an urgent need for prevention and treatment. Int J Drug Policy. 2014;25:871–878. doi: 10.1016/j.drugpo.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Bemoni Public Union. Curatio International Foundation Population size estimation of people who inject drugs in Georgia. 2017. http://curatiofoundation.org/wp-content/uploads/2018/02/PWID-PSE-Report-2017-ENG.pdf
- 13.Richardson E, Berdzuli N, Durán A, Ensor T, Richardson E. Georgia: health system review. Health Syst Transit. 2017;19:1–90. [PubMed] [Google Scholar]
- 14.Ministry of Labour Health and Social Affairs Strategic plan for the elimination of hepatitis C virus in Georgia, 2016–2020. 2016. https://www.moh.gov.ge/uploads/files/2017/akordeoni/failebi/Georgia_HCV_Elimination_Strategy_2016-2020.pdf
- 15.van der Meer AJ, Veldt BJ, Feld JJ. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 16.GeoStat Population. 2015. https://www.geostat.ge/en/modules/categories/41/population
- 17.Chikovani I, Shengelia N, Sulaberidze L, Sirbiladze T, Tavzarashvili L. HIV risk and prevention behaviors among people who inject drugs in seven cities of Georgia. 2015. http://curatiofoundation.org/wp-content/uploads/2018/02/PWID-IBBS-Report-2017-ENG.pdf
- 18.Kuniholm MH, Aladashvili M, Del Rio C. Not all injection drug users are created equal: heterogeneity of HIV, hepatitis C virus, and hepatitis B virus infection in Georgia. Subst Use Misuse. 2008;43:1424–1437. doi: 10.1080/10826080802108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bemoni Public Union. Curatio International Foundation Population size estimation of people who inject drugs in Georgia 2014. 2015. http://curatiofoundation.org/wp-content/uploads/2016/05/PWIDs-PSE-Report-2015_ENG.pdf
- 20.Alavidze S, Duchidze N, Kirtadze I. The drug situation in Georgia—Annual report 2015. 2016. https://altgeorgia.ge/media/uploads/7_drug-report-en-2015.pdf
- 21.Javakhishvili J, Kariauli D, Lejava G, Stvilia K, Todadze K, Tsintsadze M. Southern Caucacus Anti Drug Programme National Focal Point; Tbilisi: 2006. Georgia Drug Situation 2005. [Google Scholar]
- 22.Shapatava E, Nelson KE, Tsertsvadze T, del Rio C. Risk behaviors and HIV, hepatitis B, and hepatitis C seroprevalence among injection drug users in Georgia. Drug Alcohol Depend. 2006;82:S35–S38. doi: 10.1016/s0376-8716(06)80006-2. [DOI] [PubMed] [Google Scholar]
- 23.Alavidze S, Balanchivadze N, Batselashvili L. Drug Situation in Georgia 2013. 2015. https://altgeorgia.ge/media/uploads/5_drug-report-eng-2013.pdf
- 24.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–205. doi: 10.3310/hta11110. [DOI] [PubMed] [Google Scholar]
- 25.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 26.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Global Health Observatory data. 2016. https://www.who.int/gho/en/
- 28.Platt L, Minozzi S, Reed J. Needle syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2017;113:545–563. doi: 10.1111/add.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javakhishvili J, Kariauli D, Lejava G, Stvilia K, Todadze K, Tsintsadze M. Southern Caucacus Anti Drug Programme National Focal Point; Tbilisi: 2006. Georgia Drug Situation 2005. [Google Scholar]
- 30.Dershem L, Tabatadze M, Sirbiladze T, Tavzarashvili L, Todadze K, Tsagareli T. US Agency for International Development; Washington (DC): 2007. Characteristics, high risk behaviors and knowledge of STI/HIV/AIDS, and prevalence of HIV, syphilis and hepatitis among injecting drug users in Tbilisi, Georgia 2006–2006.http://pdf.usaid.gov/pdf_docs/PNADK404.pdf [Google Scholar]
- 31.Wegmann D, Leuenberger C, Excoffier L. Efficient approximate Bayesian computation coupled with Markov chain Monte Carlo without likelihood. Genetics. 2009;182:1207–1218. doi: 10.1534/genetics.109.102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fawsitt C, Vickerman P, Cooke G, Welton NJ. A cost-effectiveness analysis of shortened direct-acting antiviral treatment in genotype 1 noncirrhotic treatment-naive patients with chronic hepatitis C virus. Value Health. 2019;22:693–703. doi: 10.1016/j.jval.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chikovani I, Ompad DC, Uchaneishvili M. On the way to hepatitis C elimination in the Republic of Georgia—barriers and facilitators for people who inject drugs for engaging in the treatment program: a formative qualitative study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Averhoff F, Lazarus JV, Sergeenko D. Excellence in viral hepatitis elimination—lessons from Georgia. J Hepatol. 2019;71:645–647. doi: 10.1016/j.jhep.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Nasrullah M, Sergeenko D, Gamkrelidze A, Averhoff F. HCV elimination—lessons learned from a small Eurasian country, Georgia. Nat Rev Gastroenterol Hepatol. 2017;14:447–448. doi: 10.1038/nrgastro.2017.100. [DOI] [PubMed] [Google Scholar]
- 36.Stvilia K, Spradling PR, Asatiani A. Progress in testing for and treatment of hepatitis C virus infection among persons who inject drugs—Georgia, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:637–641. doi: 10.15585/mmwr.mm6829a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikvidze T, Luhmann N, Avril E. Harm reduction-based and peer-supported hepatitis C treatment for people who inject drugs in Georgia. Int J Drug Policy. 2018;52:16–19. doi: 10.1016/j.drugpo.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Lim AG, Qureshi H, Mahmood H. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol. 2018;47:550–560. doi: 10.1093/ije/dyx270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott N, Ólafsson S, Gottfreðsson M. Modelling the elimination of hepatitis C as a public health threat in Iceland: a goal attainable by 2020. J Hepatol. 2018;68:932–939. doi: 10.1016/j.jhep.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Scott N, Doyle JS, Wilson DP. Reaching hepatitis C virus elimination targets requires health system interventions to enhance the care cascade. Int J Drug Policy. 2017;47:107–116. doi: 10.1016/j.drugpo.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Stvilia K, Tsertsvadze T, Sharvadze L. Prevalence of hepatitis C, HIV, and risk behaviors for blood-borne infections: a population-based survey of the adult population of T'bilisi, Republic of Georgia. J Urban Health. 2006;83:289–298. doi: 10.1007/s11524-006-9032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UN Department of Economic and Social Affairs Population Division World population prospects: the 2015 revision. 2015. https://population.un.org/wpp/
- 43.Nasrullah M, Sergeenko D, Gvinjilia L. The role of screening and treatment in national progress toward hepatitis C elimination—Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep. 2017;66:773–776. doi: 10.15585/mmwr.mm6629a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Model code will be made available on request to the corresponding author.