Abstract
The ability of generate new microvessels in desired numbers and at desired locations has been a long-sought goal in vascular medicine, engineering, and biology. Historically, the need to revascularize ischemic tissues non-surgically (so-called therapeutic vascularization) served as the main driving force for the development of new methods of vascular growth. More recently, vascularization of engineered tissues and the generation of vascularized microphysiological systems have provided additional targets for these methods, and have required adaptation of therapeutic vascularization to biomaterial scaffolds and to microscale devices. Three complementary strategies have been investigated to engineer microvasculature: angiogenesis (the sprouting of existing vessels), vasculogenesis (the coalescence of adult or progenitor cells into vessels), and microfluidics (the vascularization of scaffolds that possess the open geometry of microvascular networks). Over the past several decades, vascularization techniques have grown tremendously in sophistication, from the crude implantation of arteries into myocardial tunnels by Vineberg in the 1940s, to the current use of micropatterning techniques to control the exact shape and placement of vessels within a scaffold. This review provides a broad historical view of methods to engineer the microvasculature, and offers a common framework for organizing and analyzing the numerous studies in this area of tissue engineering and regenerative medicine.
INTRODUCTION
Before considering how to engineer microvasculature, it is useful to consider what microvessels are and why one would want to create them. Surprisingly, a universally accepted definition of “microvessel” does not exist. To the anatomist, a microvessel is any structure that lacks the well-organized elastic lamellae of arteries and the valves of veins; these constraints limit microvessels to diameters of less than ~100 μm (629, 630). In the lymphatic system, the initial capillaries and small collecting lymphatics are considered to be microvascular (496).
To the physiologist, a microvessel is defined by its primary function of regulating exchange of fluid, solutes, and cells between the lumen and the surrounding tissue (690). Arteries, veins, and collecting lymphatics serve mainly to conduct blood and lymph from one part of the vascular tree to another. In contrast, microvessels provide a large surface area-to-volume ratio that enables rapid exchange across the endothelial layer. With this focus on functionality, microvessels again refer to diameters less than ~100 μm, depending on the material to be exchanged.
To the engineer, the definition of a microvessel is quite broad. A microvessel can be much wider than 100 μm in diameter and does not even need to be lined by endothelial cells (107, 578). In fact, it appears that the only requirement for a microvessel is that it is able to conduct fluid.
To the surgeon, no strict definition of microvessel is noted. A practical requirement is that a microvessel lies beyond the ability of the surgeon to suture, which means that arteries narrower than ~500 μm in diameter qualify (45, 63). As microsurgical instruments and techniques become more refined (300), which structures are considered microvascular will become correspondingly more restricted. These perspectives from the fields of anatomy, physiology, engineering, and surgery should be kept in mind when assessing and comparing published results.
In this review, a microvessel will be defined by three main criteria: First, it should be able to conduct fluid; studies that show clear evidence of fluid flow within de novo tubes will take priority over studies that examine solid “cords” of endothelial cells (ECs) that lack a lumen. Second, it should be lined by ECs; immature endothelial tubes that lack a proper mural coat of pericytes or smooth muscle cells (SMCs) will also be considered. Third, it should generally have a diameter of at most ~100 μm; this requirement will be relaxed when discussing results primarily from the engineering community. These three criteria appear to be the bare minimum needed to define structures that are useful for applications in therapeutic vascularization and tissue engineering.
Objectives of vascularization
In contrast to the large vessels of the blood and lymphatic circulation, which exist to conduct fluid, the microvessels exist primarily to provide exchange of substances between the vascular lumen and the surrounding tissue. For solutes of small molecular weight, such as oxygen and glucose, the exchange is primarily diffusive. For larger solutes, advection across endothelial intercellular junctions and shuttling by caveolae play the more important role. For leukocytes, active mechanisms that involve adhesion to the endothelial surface and translocation across the endothelium guide the transport.
The motivation for engineering microvasculature stems mainly from three envisioned applications. First, new microvessels can be used to provide therapeutic vascularization (99). In patients with tissue ischemia, such as in the heart or limbs, revascularization through bypass vascular grafts may not be possible, often because the underlying occlusive disease is too diffuse. For these patients, one may be able to induce the formation of new microvessels that can provide an equivalent bypass. Therapeutic vascularization can also potentially provide perfusion to ischemic wounds and tissue flaps. In patients with lymphedema, a similar approach may be used to form lymphatic microvessels to help drain the edematous region.
Second, the ability to engineer microvasculature can provide a means to vascularize engineered tissues. In this application, a scaffold is typically used to contain the cells that will eventually form the volume of the engineered tissue. Without early perfusion in the scaffold, a densely seeded implant will develop a necrotic core, because the vast bulk of the cells will not sufficiently nourished, aside from a thin shell at the surface of the implant. If one wishes to develop highly cellularized tissues, then methods to form microvessels in a scaffold are needed.
Third, engineered microvasculature can be used to create microphysiological systems in vitro that serve as models of both the vessels themselves and vascularized tissues. These systems are not intended to treat any patient’s condition, but rather to understand the physiology of living tissues in the presence of perfusion and in the well-controlled in vitro environment. This objective is often coupled with traditional engineering approaches that use microlithography and other patterning techniques to create miniaturized “vessel-on-a-chip” or “organ-on-a-chip” devices that connect to external pumps, actuators, and sensors.
Vascularization strategies
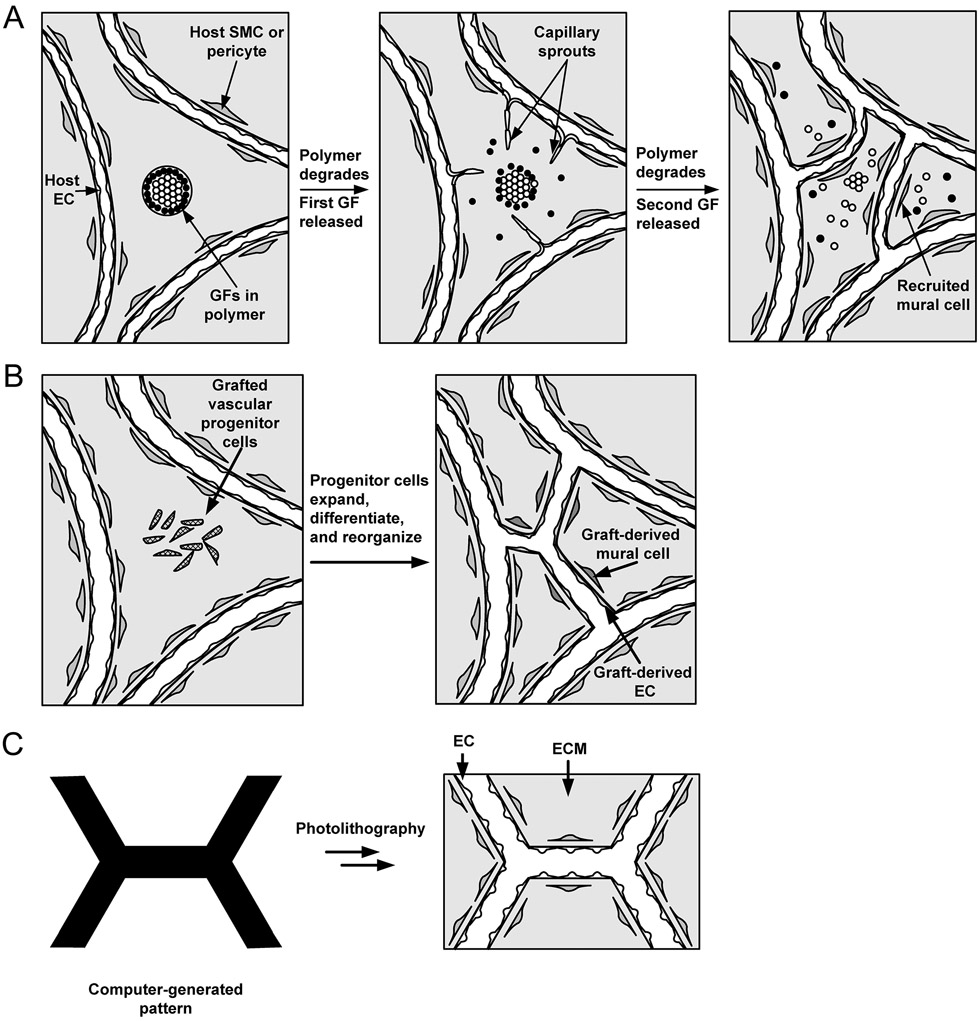
These three applications—therapeutic vascularization, engineering vascularized tissues, and creating vascularized microphysiological systems—have guided much of the efforts in engineering microvasculature for the last several decades. These efforts have focused on three complementary strategies for vascularization: those based on angiogenesis, vasculogenesis, or microfluidics (Fig. 1).
Figure 1.
Vascularization strategies based on (A) angiogenesis into a graft, (B) vasculogenesis within a graft, and (C) direct seeding and perfusion of a microfluidic scaffold. GF, growth factor; ECM, extracellular matrix. Adapted with permission from (584).
Angiogenesis is microvascular growth by sprouting, and is a normal process of vascular development in the developing human. When tissues grow in the adult, such as in a healing wound or a tumor, angiogenesis is the main mechanism for vascular expansion. The main issues in angiogenic vascularization concern which angiogenic molecules, cells, scaffold, or signals to use, how to deliver them, and in what doses to achieve results that are comparable to those that form naturally by angiogenesis (Fig. 1A).
Vasculogenesis is microvascular growth by the coalescence of individual vascular progenitor cells. Like angiogenesis, it is a normal developmental process. The earliest vascular networks in the embryo form by vasculogenesis, in which angioblasts align to form networked cords of cells that subsequently canalize to form lumens that can accept pressure-driven flow. It remains controversial whether vasculogenesis contributes to vascular growth in the adult; bona fide angioblasts almost certainly do not exist in the adult, but other cells may substitute for angioblasts in performing a vasculogenic function. For a vasculogenic approach, the main questions are which cell populations to use and how to coax them to form open networks in the adult (Fig. 1B).
Microfluidic vascularization has no natural biological analog and draws its inspiration from surgical approaches to tissue reconstruction. It refers to an artificial form of vascularization in which scaffolds are designed to contain perfusable channels that can subsequently support the growth of microvessels. The underlying motivation is to avoid the tubulogenesis that is needed in angiogenic and vasculogenic approaches. As an unnatural mode of vascularization, it relies heavily on engineering techniques for patterning biomaterials (Fig. 1C).
Although this review attempts to organize studies by application and vascularization approach, it is important to note that the distinctions are not so clear-cut. For instance, using injections of bone marrow to promote vascular growth undoubtedly has an angiogenic component from the elaboration of growth factors by the injected cells, but vasculogenesis may also contribute. Likewise, scaffolds are often used to deliver angiogenic factors and vasculogenic cells in the same volume. In these cases, the studies in question are discussed in the section that corresponds to the primary application and vascularization approach; to some extent, these choices are arbitrary.
Scope of review
This review is designed to provide an up-to-date (as of 2018) overview of efforts to engineer microvasculature in vivo and in vitro. It is intended to provide both newcomers and current researchers with a broad historical view of the fundamental issues in this field, how these issues were successfully addressed or not, and how other issues emerged. It is not intended to be topical, and the reader who is interested primarily in the most recent studies is pointed to further literature at the end of this article. As noted earlier, this review prioritizes the discussion of studies that create tubular structures that contain a complete endothelial lining and that sustain fluid flow; studies that result mainly in the formation of solid endothelial cords or that examine tubular structures that allow perfusion but lack endothelium will only be discussed briefly. The three sections on angiogenic, vasculogenic, and microfluidic vascularization are largely self-contained and can be understood out of sequence. Only studies that intend to create new vasculature are considered; although one might argue that the controlled regression or inhibition of vasculature [e.g., during tumor growth (214, 419)] is another form of microvascular “engineering”, such strategies are already reviewed thoroughly elsewhere (146, 240, 589) and are not discussed here.
CHARACTERIZATION OF ENGINEERED MICROVESSELS
Engineered microvessels are evaluated by their structure and function. Analysis of vascular structure uses well-described morphological and histological methods. Early studies simply counted the number of histologically identifiable vessels per tissue section. More refined approaches rely on staining the vasculature for EC-specific markers, such as CD31, VE-cadherin, Prox1 (for lymphatics), and LYVE-1 (for lymphatics), and using confocal microscopy to assess the three-dimensional distribution of these markers. To label vessels that are actually perfused, one can inject contrast agents, lectins, or fluorescent lipids into the circulation (342).
Functional assessments have focused on the transport properties of the engineered microvasculature. Many of these tests are qualitative and have relied on gross anatomy or histological images. For instance, to assess whether the microvessels assist in the delivery of oxygen, one can determine the fraction of tissue that is necrotic on histological sections. To assess whether lymphatics aid in resolving edema, one can visually determine whether the relevant tissue is swollen.
Quantitative tests of microvascular transport seek to provide normalized physiological measures that can be used to compare the transport properties in different vessels, organs, and subjects (108, 378, 665). These metrics are based on modeling the vascular wall as a uniformly permeable film that can be described by a hydraulic conductivity LP for the bulk flow of aqueous solution, and by a diffusional permeability coefficient Pd and osmotic reflection coefficient σ for each solute species. More complex models treat the vessel wall as an arrangement of pores that allow passage of small solutes but retard passage of large ones (465), or as a fibrous matrix that is tethered to the underlying endothelial monolayer (110). Organ-level measurements of hydraulic conductivity and reflection coefficients use Pappenheimer’s classic technique that plots filtration rates as a function of intravascular pressure (377, 428). Similarly, organ-level measurements of vascular permeability rely on the accumulation of a labeled solute in tissues (102, 379). Blood flow rates per tissue volume can be measured with magnetic resonance imaging and other non-invasive techniques, by tracking tissue signal as a function of time after bolus intravascular injection of a tracer (370, 686). Intravital microscopy with window chambers enables long-term observation of blood flow (93, 487, 488) and lymphatic drainage (94).
Physiological assays of individual vessels have also been developed (Fig. 2). Measurements of hydraulic conductivity apply Landis’s technique, in which a distal portion of the vessel is occluded and slow movement of tracer particles (such as red blood cells) in the proximal section is tracked (Fig. 2A) (313). Measurements of solute permeability coefficients in individual vessels require cannulation of the vessel and sudden introduction of labeled solute (Fig. 2B) (109, 227). Such methods are technically more demanding than organ-level assays, which may explain why they are less commonly seen in the microvascular engineering literature.
Figure 2.
Quantitative metrics of microvascular physiology. (A) Calculation of endothelial hydraulic conductivity relies on measurement of filtration speed as a function of vascular pressure. Reprinted with permission from (313). (B) Calculation of solute permeability relies on measurement of solute accumulation over time. Reproduced with permission from (227).
Assessment of the ability of engineered lymphatics to enhance solute drainage has relied on the injection of tracer solute into the tissue (481). The amount of solute that remains typically decays exponentially with time, and the rate constant or half-life of this decay provides a measure of drainage (386). Measurement of drainage constants through individual lymphatics is possible (581). Individual collecting lymphatics can be cannulated for measurement of their contraction amplitudes and frequencies (161, 667).
Although many of the quantitative assays of microvascular physiologists have been known for a long time, functional tests of engineered vessels remain less frequently used than structural characterization. Thus, it can be unclear to what degree engineered microvessels perform their intended physiological function, especially when compared with native microvessels.
ANGIOGENIC VASCULARIZATION
Angiogenic vascularization refers to the formation of capillaries by sprouting from existing ones. It is an “extrinsic” form of vascularization, in which the source of vessels resides outside of the original graft or injection site. Sprouting in the blood circulatory system occurs mainly from post-capillary venules, where the absence of a well-organized, layered vascular media facilitates the migration of ECs into the tissue space. In the lymphatic system, sprouting occurs from the initial lymphatic capillaries, which comprise the terminal ends of the lymphatic tree. In the adult, angiogenesis is primarily observed in healing wounds, inflamed tissues, and tumors. As a result, the materials that are used to engineer microvasculature by angiogenesis have consisted mainly of wound cells and tissue leukocytes, along with the soluble and insoluble molecules that they and tumor cells produce.
Angiogenic and lymphangiogenic factors
Blood vessel angiogenesis
In the 1960s and 1970s, the realization that tumors required a vascular supply before they could grow beyond a diffusion-limited size led to the isolation of tumor-derived angiogenic factors [reviewed in (148)]. These studies benefited from the development of in vivo angiogenesis assays that provided a clear view of growing capillary networks in the cornea and anterior eye chamber, in the hamster cheek pouch, and on the chick chorioallantoic membrane. Such assays provided a gold standard for testing angiogenic potency in vivo, in which the vascular growth could be direct (i.e., from effects of the added factor on ECs) or indirect (i.e., mediated by release of EC-specific factors by leukocytes and other non-endothelial cells) or both (30, 367). Careful attention to minimize tissue disruption was required so that direct angiogenic effects were not masked by inflammation. Later, the isolation of ECs from large vessels and from capillaries enabled the angiogenic process to be studied in vitro (147).
Blood vessel angiogenesis is driven mainly by a need for increased delivery of oxygen to a growing tissue. Insufficient oxygen delivery results in tissue hypoxia, which stabilizes hypoxia-inducible factors, particularly HIF1α, that act as transcription factors to stimulate the expression of target genes such as vascular endothelial growth factor-A (VEGF-A) and erythropoietin (501). Angiogenesis can also be stimulated by tissue injury, and here the fibroblast growth factors (FGFs) play a major role (96).
Overall, the regulation of angiogenesis is extremely complex. Even within a single family of angiogenic factors—the VEGFs—receptor binding is promiscuous (Fig. 3). For instance, VEGF-A binds VEGFR-1 and −2 and neuropilin-1 (Nrp-1); VEGF-B and placental growth factor (PlGF) bind VEGFR-1 and Nrp-1; VEGF-C and -D bind VEGFR-3, and when fully processed, VEGFR-2 as well; VEGF-E binds VEGFR-2 (247). Splice variants also differ in their receptor binding (537). Part of the challenge in harnessing angiogenesis to produce functional microvessels is to control the delivery of the appropriate factor(s) at the appropriate times and doses without inadvertently inducing bystander effects.
Figure 3.
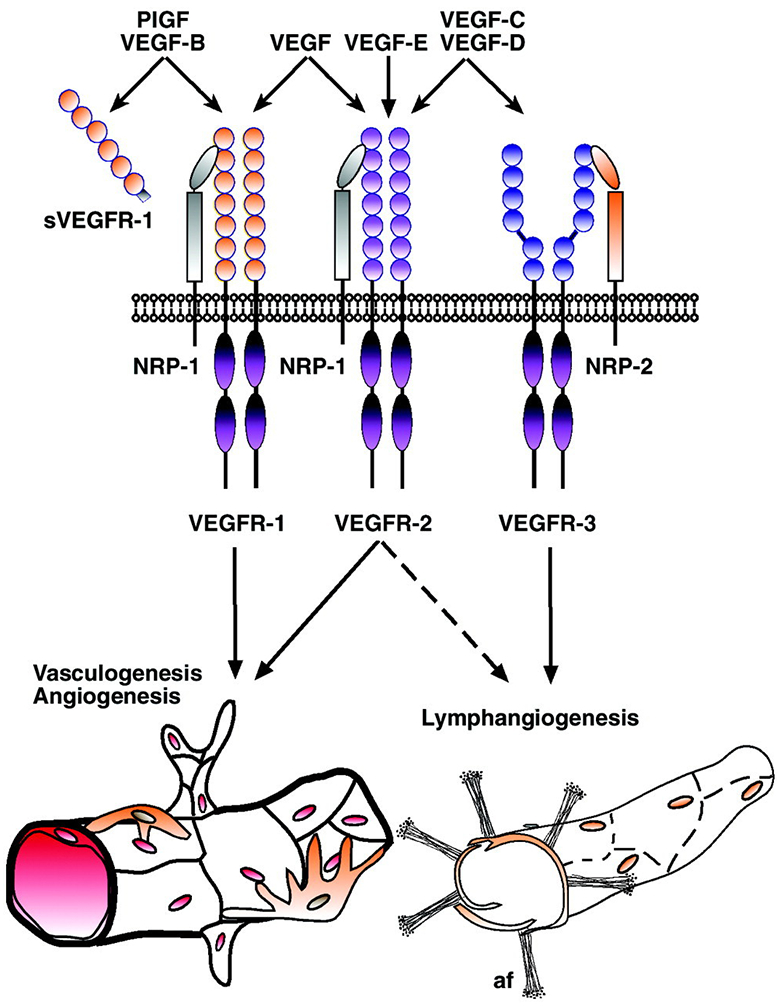
Binding of VEGFs to their receptors, the VEGFRs and neuropilins. Reproduced with permission from (247).
Whether mediated by VEGFs, FGFs, or some other signal, angiogenesis invariably requires degradation of the endothelial basement membrane, loosening of EC cell-cell contacts, loss of pericyte coverage, and migration of ECs into the tissue space. Endothelial proliferation is often observed, but not strictly required, for sprouting (519). Migrating cells tend to orient towards increasing concentrations of the angiogenic signal. Encounters between two capillary sprouts can lead to anastomosis, tubulogenesis, and establishment of flow, which delivers the blood cells that can relieve the hypoxia. Subsequent stabilization of immature capillaries requires pericyte coverage, which is mediated by perivascular gradients of platelet-derived growth factor (PDGF) (345).
The extracellular matrix also plays an important role in angiogenesis, in part by providing binding sites for growth factors and serving as a reservoir that can be mobilized by local proteolysis (231). Other substances, such as lipids, steroid derivatives, and divalent transition metal ions, can induce angiogenesis as well (683).
Lymphangiogenesis
Lymphangiogenesis follows a similar course of sprouting and migration, but the initiating signals are not as clear (10). Given that lymphatics drain tissues, one would expect tissue edema to result in increased lymphangiogenic signaling. Edema is accompanied by increased interstitial fluid pressure and accumulation of VEGF-C in the interstitial space (169). VEGF-C is the classic lymphangiogenic signal, which promotes proliferation and migration of lymphatic ECs (356). In transgenic mice, tissue-specific overexpression of VEGF-C causes persistent increases in lymphatic vessel density and diameter (242, 348), but can also lead to malformations such as lymphangiectasia that do not resolve even when the VEGF-C overexpression is transient (655). Little is known about how lymphatic ECs undergo tubulogenesis, which is required to establish drainage capacity to relieve the fluid congestion. To what extent increased interstitial pressure or changes in interstitial flow alter the response to VEGF-C, or whether these physical changes can provoke lymphangiogenesis independent of changes in VEGF-C levels, is still unclear.
Therapeutic angiogenesis and lymphangiogenesis
One of the earliest attempts at therapeutic angiogenesis was the development by Vineberg in the 1940s of a surgical procedure to revascularize the ischemic myocardium (614, 615). In contrast to arterial bypass grafting (which was to be developed later), the Vineberg procedure consisted of implanting the ligated end of a cut artery (typically, the internal mammary artery) into a tunnel within the myocardium (Fig. 4). The hope was that signals from the implanted artery would somehow initiate angiogenesis and ultimately grow new microvessels to bridge the gap between the implant and existing myocardial vessels within the ischemic region. Initial claims of revascularization were met with skepticism, and only after the invention of angiography was the presence of collateral bridges between the implanted artery and myocardial vessels definitively established [reviewed in (520)]. Of note, only the implantation of cut arteries resulted in anastomoses; placement of an intact artery with preserved blood flow in a myocardial tunnel did not allow anastomoses to form. Collaterals originated primarily from the distal third of the implant, and large ones formed only when the artery was implanted in ischemic tissue (48). Allowing intercostal branches to bleed freely into the myocardial tunnel appeared to help promote vascularization. If the implanted artery slipped out of the tunnel and adhered to the chest wall, then vessels were generated instead between the artery and the wall (615). In the dog heart, collaterals from the implant provided ~30% of the total flow conductance and ~10% of the total collateral flow to the ischemic zone (602, 603).
Figure 4.
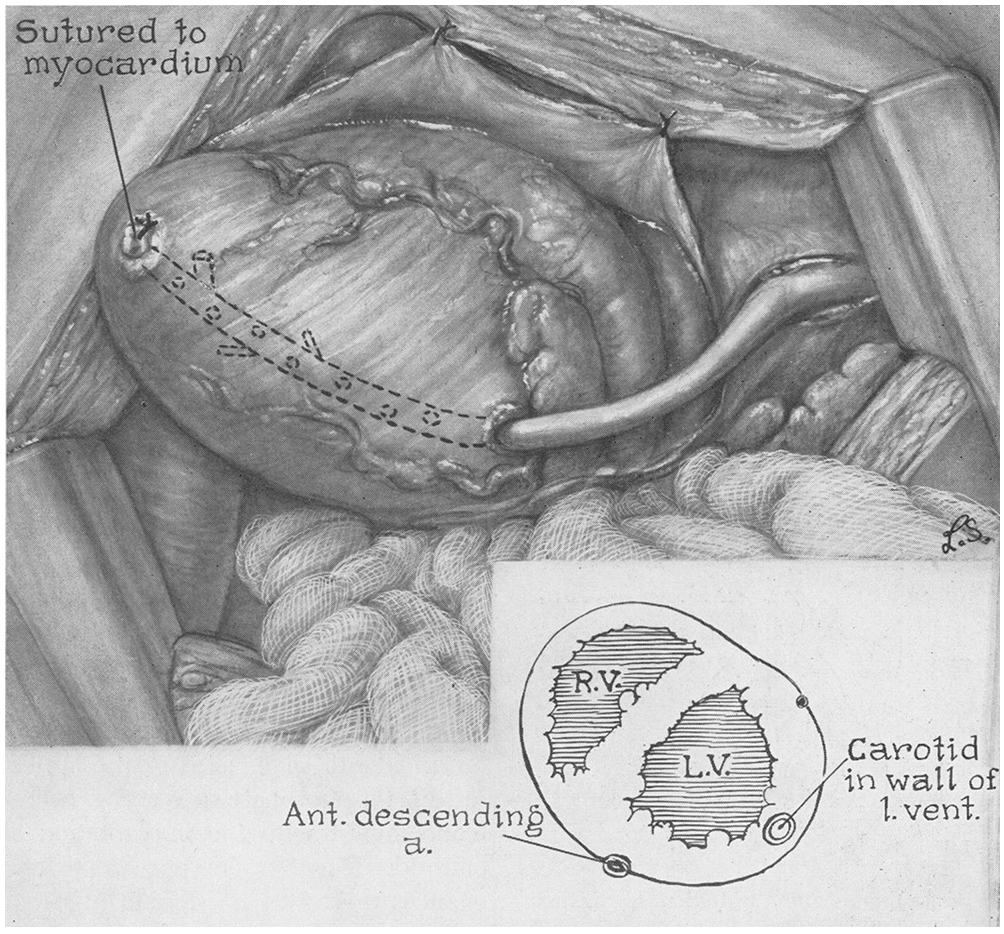
Diagram of the Vineberg procedure for vascularizing ischemic myocardium, using a carotid artery implant. RV, right ventricle; LV, left ventricle. Reproduced with permission from (479).
Later, Vineberg modified this procedure to include a free graft of omentum, which provided an additional source of ECs and angiogenic factors (616). Adipose tissue, particularly omental fat, can promote angiogenesis, as shown in the rabbit corneal pocket assay (527). Among the released angiogenic signals that have been attributed to intact fat are VEGF-A (673), prostaglandins (527), and a lipid factor, possibly 1-butyryl glycerol (118, 175).
Other arteries, such as the carotid, have also been successfully used to revascularize the heart. Attempts to generate connections between arterial implants and the vascular networks in other organs, such as kidney, skeletal muscle, and spleen, were unsuccessful (479); arteriovenous implants, which include venous return, successfully vascularized bone (215). Although the precise mechanisms that mediate angiogenesis in the Vineberg procedure still remain unclear, it has been suggested that blood flow that results from pressure oscillations between the implant lumen and myocardium may play a role (34).
Arteriovenous shunts have been shown to generate collateral vessels (180, 556). Although these shunts primarily act as large-bore vessels to revascularize distal ischemic tissues, they also result in the proximal formation of collaterals. Ligation of the shunt reduces blood flow to the most distal tissues but not to tissues near the shunt (180).
These early studies of surgically induced vascularization suggested that local tissue injury (induced by cutting a tissue or by creating an arteriovenous shunt) was needed for successful revascularization. In fact, epicardial injury by abrasion was sufficient to induce vascular growth at the site of the pericardial adhesions that subsequently developed (47). Possible initiators of angiogenesis include the local release of angiogenic factors from the cut surfaces of the tissue and the local deposition of blood cells and a provisional fibrin-based matrix. Similar principles underlie vascular growth from the cut ends of aortic and lymphatic ductal explants in vitro (412, 413). Subsequent studies thus focused on the precise angiogenic factors and cell types within these grafts that could promote angiogenesis.
Animal models
Studies of therapeutic angiogenesis for treatment of ischemic myocardium first used occlusion of coronary arteries in large animals, particularly the dog and pig, since the surgical techniques in these species were already mature. Findings in the dog were often critiqued because the dog myocardium appears to have a rich collateral circulation (599). The collateral circulation of pig myocardium, on the other hand, is less well-developed and thereby resembles that of the human. Techniques to reproducibly induce myocardial infarct and ischemia in smaller animals were more challenging to develop; local cryogenic injury to the myocardial wall, which generates a well-demarcated scar, served as an alternative to these techniques.
Models of limb ischemia have focused on occlusion or removal of hindlimb arteries and veins in small animals, particularly mice, rats, and rabbits. Rats and rabbits have the advantage of larger size, while mice provide the opportunity to use transgenic technologies to study genetic effects on angiogenesis. Similarly, models of ischemic wounds and surgical flaps have relied on small animals.
Direct injection of angiogenic factors
With the isolation of angiogenic growth factors and the cloning of their genes in the 1980s, it became feasible to deliver these factors to the ischemic regions directly, either as recombinant proteins or through gene therapy. In principle, this approach could afford better control over the angiogenic process, since the dose, timing, and identity of the added factors are not left to chance, as they would be in a Vineberg-type tissue implant (211). Moreover, combinations of angiogenic factors could optimize the functionality of the microvasculature that forms. For these reasons, by far the most effort in therapeutic angiogenesis has focused on the delivery of recombinant growth factors and plasmids that encode for them (143, 351, 530, 671). This section focuses on studies that use soluble growth factors to induce angiogenesis and lymphangiogenesis. Unless otherwise noted, all growth factors are for human isoforms.
FGFs.
As the first family of EC growth factors to be isolated and purified, the FGFs were the first ones to be applied towards therapeutic angiogenesis; of these, FGF2 (basic FGF) has been the main focus. The earliest study used soluble FGF2 to treat the infarcted dog myocardium (652). This work provided a template that was reapplied in numerous subsequent studies, in which one or a combination of growth factors (FGFs, VEGFs, etc.) was delivered with a variety of methods (bolus injection, constant delivery, etc.) at a variety of sites (intravascular, intramuscular, etc.) to treat a model of tissue ischemia (myocardial, limb, etc.), as assessed by a variety of readouts (tissue blood flow, capillary density, etc.). Here, intracoronary delivery of FGF2 increased microvascular density in the infarct. FGF2 also increased myocardial blood flow and caused acute vasodilation, although the role of vasodilation on the chronic increase in blood flow is unclear (490, 601).
Because repeated intracoronary infusion of growth factors is unlikely to be clinically practical, systemic delivery of FGF2 was studied (318). Injected FGF2 was removed from the circulation with a half-life of ~50 minutes, and injections were performed daily. FGF2-treated animals had higher blood flows than controls (no FGF) did, but only when treatment was initiated early (e.g., three days after occlusion); the flow-enhancing effects of FGF2 could be maintained for over two months and persisted even after discontinuation of therapy for four weeks. Side effects of chronic FGF2 administration consisted of anemia, thrombocytopenia, hypotension, and possibly intimal proliferation. Intravenous delivery of bovine FGF2 to ischemic pig heart increased angiographic scores, but did not change blood flow or cardiac function (490).
Pericardial delivery has been used to prolong the retention of FGF2 near ischemic tissue. In the dog, vascular and arteriolar densities in the outer half of at-risk myocardium increased to a greater extent compared to those in the inner half (596). Co-administration with heparan sulfate increased the effects of FGF2. In the pig, pericardial delivery of FGF (along with heparin) resulted in increased collateral indices and vascular densities compared to delivery of heparin or saline, and was accompanied by greater flow and myocardial function (310).
Intramuscular delivery of FGF2 rescued gross limb function and increased capillary density and capillaries per muscle fiber in the ischemic rabbit hindlimb, in a dose-dependent manner (33). High doses, however, led to muscle injury.
Topical application of FGF2 at the time of wounding increased vascular density by 30-50% one week later in rabbit ear skin wounds (404). The increase in vascular density was accompanied by increased epithelialization only in non-ischemic wounds (402, 404). In a hyperoxic chamber, treatment of ischemic wounds with FGF2 enhanced epithelialization and formation of granulation tissue (646). These findings suggest that, in ischemic wounds, the growth of new vessels is not the rate-limiting step in wound closure. Sensitivity to ischemia appears to be unique to FGF2 treatment, since FGF4 treatment was effective in both normal and ischemic wounds (646).
In bone grafts to the rabbit mandibular ramus, continuous intra-graft infusion with FGF2 accelerated vascularization by day 10, but did not increase the long-term steady-state vascular density over that obtained with infusion of saline (132). Vascular growth mainly occurred from the surrounding muscle rather than from bone.
Intracoronary administration of FGF1 (acidic FGF, or ECGF), the other major member of the FGF family, in the ischemic dog heart did not lead to greater blood flow than in control (600). The authors rationalized their result in several ways: First, both experimental and control infusions were performed with heparin, which helps to stabilize the FGFs (178) but is itself mildly angiogenic (603), and it is possible that the amount of added heparin already saturated the angiogenic response. Second, the infusion of FGF1 began more than a month after balloon constriction of the left circumflex coronary artery (LCCA) began; this late start of the angiogenic therapy, while perhaps more relevant to the setting of chronic human myocardial ischemia, may have weakened the responsiveness of the ischemic region to FGF. Third, the growth factor was delivered via the left main coronary artery, instead of the distal LCCA; as a result, it may not have been optimally delivered to the ischemic region that was originally perfused from the LCCA. As found in the ischemic hindlimb with FGF2, high doses of FGF1 to the myocardium led to muscle necrosis.
In the rabbit ischemic hindlimb, intramuscular bovine FGF1 (with heparin) led to growth of collaterals and improved perfusion (451). The effect of FGF depended on dose and route of administration (450). Even at the highest dose (4 mg), no inflammation accompanied the vascular growth. Systemic delivery of FGF by injection into the ipsilateral non-ischemic forelimb did not induce vascular growth in ischemic or non-ischemic muscle.
Other FGFs have been less commonly studied for therapeutic angiogenesis. FGF9 did not promote vascular growth when infused near the ischemic muscle of mouse hindlimb, but instead increased the fraction of vessels that were positive for smooth muscle actin (SMA) (150). This muscularization of microvessels was accompanied by improvement in limb function in nine days and by full restoration of blood flow in the ischemic limb in one month.
VEGFs and PlGF.
Because FGFs induce proliferation of many other cell types besides ECs, it is possible that FGF-based therapies can induce undesired sequelae that are unrelated to the angiogenic effects. Indeed, they can accelerate the progression of atherosclerosis (127). The discovery of the VEGFs, a family of EC-specific growth factors, led to the hope that these side effects could be avoided with VEGF-based therapies. In the ischemic dog heart, intracoronary VEGF-A (presumably the 165 kDa isoform) led to increased blood flows two weeks after growth factor infusion and a near-doubling of arteriolar density, but no increase in capillary density, similar to the results for FGF2 by the same research group (36).
When administered systemically via the left atrium and for only one week after initiation of coronary arterial constriction, VEGF-A did not improve relative blood flows or collateral flow resistance over control (319); in contrast, one-week administration of FGF2 led to increased blood flows and decreased collateral flow resistances. VEGF-A treatment led to acute hypotension, which resolved over a time-scale similar to the circulation half-life of ~50 minutes.
Sustained peri-myocardial delivery of VEGF-A165 (along with heparin) to the ischemic pig heart reduced the time delay for delivery of contrast agent to the ischemic region, compared to heparin-only controls (430); the time delay was confirmed to be inversely correlated with local blood flows based on microsphere deposition. VEGF treatment increased left ventricular ejection fractions and decreased the volume of infarcted and collateral-dependent regions. Peri-myocardial delivery also caused neovascularization primarily near the site of vascular occlusion (191); the induced collaterals were thin-walled with large lumens, a finding that resembles the enlarged, pericyte-poor “mother” vessels that have been found with VEGF treatment in other models (438).
In the ischemic rabbit hindlimb, intraarterial VEGF-A165 led to increased blood pressure ratios, angiographic scores, and capillary densities (570). It resulted in a modest increase in resting blood flow to the ischemic limb, but a large increase in papaverine-induced maximum flow. The thirty-day response of maximum flow rates to VEGF treatment was highest in animals that had the lowest maximum flow rates on day 0, which suggests that greater ischemia increases sensitivity to VEGF, most likely through an increase in expression of VEGFRs (195); a second VEGF injection or co-injection of heparin did not further increase the flow (43). VEGF-induced proliferation of ECs and SMCs localized largely in the “midzone” between the proximal and distal arteries by day 5 after injection (568), even though VEGF-A is not a direct growth factor for SMCs. The responses to VEGF depended on the administered dose, without any apparent limit in the increases in blood pressure ratios and angiographic scores; a large total dose of 1 mg did not cause muscle atrophy or skin necrosis (567). Similar results have been found when VEGF was delivered intravenously (44).
VEGF-A has also shown therapeutic vascularization potential in other settings. In skin wounds in diabetic (db/db) mice, topical VEGF-A165 greatly increased the number of ECs in the wound, increased blood flow, and hastened epithelialization (159). Topical VEGF-A121 and VEGF-A165 enhanced formation of granulation tissue in normal and ischemic wounds in the rabbit ear (100); in contrast, FGF2 was unable to improve ischemic wound healing (402, 404). Injection of VEGF-A165 beneath an ischemic skin wound caused greater vascularization of the wound skin, compared to saline controls and to non-ischemic wounds; it also hastened the recovery of tensile strength across the incision (670). Intraarterial injection of VEGF-A to an ischemic rat skin flap increased capillary density, blood flow, and flap survival over saline control (427). In a model of bronchopulmonary dysplasia, intramuscular injections of VEGF-A165 in neonatal rats after exposure to hyperoxia increased lung capillary densities beyond those of untreated normoxic lungs; these changes were accompanied by preservation of normal lung alveolar size and number (307).
A systematic comparison of different forms of VEGF administration in the setting of a rat skin flap showed that all surviving regions of VEGF-treated flaps had similar degrees of vascularization that were greater than that of control flaps (302). Of the VEGF treatments, repeated intravenous dosing resulted in the highest flap survival, and topical application had the lowest. In a rat myocutaneous composite flap, preoperative subcutaneous injection of VEGF protected the flap from ischemic necrosis; once a flap had been raised, however, neither systemic nor local intraarterial injection of VEGF improved flap survival (669). Preoperative VEGF caused vascular growth in the flap, compared to saline controls.
As with FGFs, VEGF-A can increase blood flow through mechanisms that are independent of angiogenesis, such as vasodilation. Studies to distinguish the contributions of angiogenesis and these other mechanisms towards improved tissue function are rare, and caution is warranted before claiming a causal link between vascular growth and improved tissue function.
VEGF-C, the other member of the VEGF family that has been extensively studied for therapeutic vascularization, induces lymphangiogenesis (242, 421). For instance, intradermal VEGF-C caused the growth of lymphatics and increased tissue drainage in the edematous rabbit ear (557).
Although VEGF-C is often viewed as a mainly lymphangiogenic factor, it also promotes therapeutically relevant angiogenesis. In the ischemic rabbit hindlimb, intraarterial VEGF-C had similar effects as VEGF-A165 did (639). Intradermal injection of VEGF-A or VEGF-C both led to local increases in vascular permeability that depended on nitric oxide production; the authors hinted that development of vascular permeability and angiogenesis may be linked.
Besides VEGF-A and -C, other members of the VEGF family have also been studied for their ability to promote therapeutic vascularization. Subcutaneous delivery of VEGF-B167 increased vascular density in mouse myocardial infarct (339). The VEGF-related proteins PlGF1 and PlGF2 both induced vascular growth in the ischemic mouse heart without causing edema or hypotension; PlGF2 induced arteriogenesis (the generation of collateral vessels) in the ischemic mouse hindlimb (354).
Other direct angiogenic factors.
EC migration and proliferation are early steps in angiogenesis, and the eventual maturation of newly formed, leaky capillaries into non-leaky microvessels requires interaction of mural cell-derived angiopoietin (specifically, Ang1) with the Tie2 receptor on ECs (114, 554). Because native Ang1 tends to oligomerize in solution and is difficult to purify in an active state, studies of recombinant Ang1 have typically modified the parent protein to promote stability. Modification of the amino terminus and mutation of Cys245 yields the variant Ang1* (developed by Regeneron Pharmaceuticals); replacement of the N-terminal portion with a short coiled-coil domain from cartilage oligomeric matrix protein yields the variant COMP-Ang1 (84).
Daily intravenous COMP-Ang1 for one week in the adult mouse resulted in reversible enlargement of tracheal microvessels, but not in angiogenesis (85). This finding is consistent with the developmental role of Ang1 in promoting vascular maturation rather than sprouting. Nevertheless, Ang1 derivatives (particularly, COMP-Ang1) have shown potential in generating new vessels in other settings. In a corneal pocket assay, COMP-Ang1 induced angiogenesis at a level similar to that achievable with VEGF, whereas native Ang1 had little effect (84). Compared to VEGF-induced vessels, the vessels that resulted from COMP-Ang1 were not leaky. When applied topically to healing wounds in diabetic (db/db) mice, COMP-Ang1 increased both blood and lymphatic vessel densities, increased blood flow to the wounds, and accelerated wound coverage (86).
The other major member of the angiopoietin family, Ang2, is believed to act primarily as a competitive inhibitor of Ang1-Tie2 binding, thereby delaying vascular maturation (157). As observed with Ang1, Ang2 did not promote angiogenesis in a corneal pocket assay (21). It is unclear whether intravenous or topical Ang2 (or its modified derivative COMP-Ang2) promote vascular growth, although results with adenoviral expression suggest that it would (271). Ang3 and Ang4 (mouse and human orthologs, respectively) promoted angiogenesis in a corneal pocket assay (324).
Intraarterial hepatocyte growth factor (HGF), followed by intravenous HGF, in the ischemic rabbit hindlimb led to even greater increases in vascularization and blood flow than an equivalent dose of VEGF-A165 did (608). Since HGF induces the secretion of VEGF-A by SMCs in vitro, the observed angiogenic effects in vivo may result from synergistic direct and indirect stimulation of ECs by HGF and HGF-induced VEGF, respectively.
Indirect factors.
In contrast to the FGFs and VEGFs, which act directly (but not necessarily solely, in the case of the FGFs) on ECs, a large number of angiogenic factors stimulate vascular growth mainly or only through paracrine mechanisms. Transforming growth factor β (TGFβ), tumor necrosis factor α (TNFα), and PDGF-BB are angiogenic when injected subcutaneously (61, 442, 470), but they do not stimulate EC proliferation directly. Instead, these growth factors recruit leukocytes and cause stromal fibroblasts to secrete extracellular matrix, which can result in formation of a neo-tissue that is similar to granulation tissue. Indeed, these growth factors are primarily known to enhance wound healing (280, 403), and at least one (PDGF-BB, as a topical gel) is already clinically approved for this application (632). Neo-tissue growth is invariably accompanied by vascular growth that is most likely stimulated by VEGF and other angiogenic factors that are elaborated by the recruited and/or activated non-endothelial cells. Along with indirect stimulation of angiogenesis, PDGF-BB can affect mural cell coverage of immature vessels (49). In contrast to FGF2, PDGF-BB increased granulation tissue in ischemic skin wounds (404). The less-studied PDGF-AB and -CC appear to act as indirect angiogenic factors in the aged myocardium and ischemic hindlimb, respectively (125, 338).
Combinations.
Whether direct or indirect, each angiogenic factor yields a distinct combination of vascular effects. It is natural to expect combinations of such factors to provide complementary improvements. Largely as a result of in vitro studies that provided evidence of synergy (434), initial application of a combinatorial strategy for therapeutic angiogenesis in vivo used two direct angiogenic factors, such as VEGF and FGF. Intraarterial VEGF and/or FGF2 for rabbit hindlimb ischemia showed synergistic improvements in capillary density, arterial diameter, and blood pressure ratio (20). As mechanistic understanding of the angiogenic cascade improved, growth factor combinations were chosen to promote distinct steps in angiogenesis. In general, a direct angiogenic factor (typically, VEGF) is used to induce endothelial sprouting, while the second factor (e.g., PDGF) is intended to modify the vascular growth. For instance, the combination of VEGF-A165 and the maturation factor Ang1* yielded increased vascular densities and diameters over VEGF alone in a corneal pocket assay (21). In the ischemic rat and rabbit hindlimbs, co-administration of FGF2 and PDGF-BB led to synergistic increases in vascular density and blood flow (69). Combinatorial strategies have also shown that ligands that do not promote angiogenesis by themselves can show angiogenic activity when co-administered with direct angiogenic factors. For instance, Ang2 has no observable angiogenic effect by itself in a corneal pocket assay, but in combination with VEGF-A165, it increased vessel lengths and densities (21).
A less common strategy to obtain complementary effects relies on the engineering of chimeric proteins, in which domains from two distinct ligands are combined to activate both of their respective receptors. Intramuscular injection of soluble VEGF-A/Ang1 chimera increased blood flow in the ischemic mouse hindlimb (15). Although such chimeras have shown promise and are perhaps more convenient for in vivo application (only one protein needs to be purified), they do not allow the relative levels of receptor activation to be tuned.
Hypoxia mimics.
Since hypoxia is the main physiological inducer of VEGF-A expression, pharmacological mimicry of hypoxia has been investigated for therapeutic angiogenesis. As a transcription factor, the hypoxia-inducible protein HIF1α would be difficult to deliver in a functional soluble form to an ischemic tissue. Instead, endogenous HIF1α is readily activated by inhibitors of prolyl hydroxylase domain (PHD) enzymes, such as cobalt ion and desferrioxamine (165, 167). Hypoxia mimics have been studied as potential inducers of vascular growth in the preterm neonatal lung, since normal vascular development in the lung occurs under hypoxic conditions. Intravenous delivery of a selective PHD inhibitor to preterm primates partially rescued the lung vascularization and VEGF deficit that is common in this population (27); similar results were found in mouse lung explants that were suffused with the less-specific inhibitor dimethyloxalylglycine (182). Nevertheless, these substances have not always led to expected results. For instance, cobalt and desferrioxamine both caused an increase in VEGF expression, but a decrease in vascularization, in developing mouse lungs (182). These discordant effects on vascularization, along with the ability of some hypoxia mimics to inhibit epithelial branching (182), warrants caution in the use of HIF-focused angiogenic strategies to treat bronchopulmonary dysplasia.
In the healing bone, local delivery of desferrioxamine increased vascular density and bone volume (623). In a model of progressive renal disease, cobalt administration preserved the density of glomerular capillaries and decreased the rarefaction of peritubular capillaries, in part through increased proliferation and decreased apoptosis of ECs; these vascular effects were accompanied by less tubulointerstitial injury (572). In the ischemic muscle, dimethyloxalylglycine increased vascular density (381).
Gene transfer
Injection of recombinant angiogenic growth factors has several disadvantages, including high cost and undesirable side effects, particularly when the factors are administered systemically. Moreover, plasma concentrations of VEGFs and FGFs spike immediately after injection and decay over a time-scale of ~1 hour to nearly background levels (319, 320); these large fluctuations in angiogenic signal are unlikely to be optimal for inducing the consistent and controlled growth of functional microvasculature. For these reasons, gene therapy of the same proteins has been envisioned as an alternative. In effect, the release of proteins by transduced cells can provide a therapy equivalent to slow constant infusion of protein, thereby addressing the issues of cost and dose. Plasmids can be introduced as virus or as naked DNA; the route of administration can be systemic (e.g., intravenous) or local [e.g., intramuscular (640)]. All plasmids described below encode for human protein, unless otherwise noted.
FGFs.
Intramuscular viral delivery of mouse FGF2 in the ischemic mouse hindlimb led to increased blood flow ratios and limb salvage (366). The beneficial effects of FGF2 were mediated via induction of endogenous VEGF, even though overexpression of exogenous VEGF did not provide the same improvement in limb function. Angiogenesis was accompanied by arteriogenesis, which relied on a VEGF-independent pathway that required the chemokine MCP-1 (154). VEGF and MCP-1 were required for limb salvage, and both are secreted by SMCs and fibroblasts upon addition of FGF2.
Subcutaneous injection of plasmid DNA for FGF2 with a type I collagen carrier increased vascularity in ischemic rat myocutaneous composite flaps (204); this increase, however, was not matched by an increase in blood flow nor by increased flap survival. This result mirrors those observed with topical soluble FGF2 on ischemic skin wounds, in which enhanced vascularization was not accompanied by improved wound healing (402, 404). Electroporation of FGF2 plasmid into rat subcutaneous muscle, followed by elevation of an overlying skin flap, resulted in higher levels of distal vascularization, larger vascular densities, and greater flap viability (152).
Intramuscular delivery of adenoviral FGF4 into the ischemic rabbit hindlimb resulted in vascularization that was accompanied by edema, likely because FGF4 induces expression of VEGF (467). In a pig model of myocardial ischemia, intracoronary delivery of adenoviral FGF5 led to improved myocardial function and blood flow (164). Capillary-to-fiber ratios also increased with FGF5, albeit modestly (an increase of ~10% in the ischemic region, compared with control adenovirus). The difference in physiological and anatomical effects suggests that angiogenesis-independent effects of FGF5 may also be important.
VEGFs and PlGF.
The most thoroughly studied growth factors for angiogenic gene therapy are the VEGFs, particularly VEGF-A and -C. Balloon delivery of naked plasmid VEGF-A121, VEGF-A165, VEGF-A189, or VEGF-C to rabbit iliac artery helped to revascularize ischemic hindlimb (569, 639); intramuscular delivery was effective as well (595). In incisional skin wounds in normal and diabetic mice, intradermal injection of viral VEGF-A165 resulted in increased vascularity, thicker granulation tissue, and stronger wounds (158). In the hyperoxic lung, intratracheal delivery of adenoviral VEGF-A145 increased lung capillarity and ameliorated alveolar loss and enlargement (577).
The vascular effects of VEGF-B gene therapy have been less clear. Electroporated intramuscular plasmid VEGF-B167 and VEGF-B186 appeared to improve vascularization in mouse ischemic hindlimb through a VEGFR-1 and nitric oxide-dependent mechanism (528). A later comparison of intramuscular adenoviral VEGF-A165, VEGF-B186, VEGF-B167, and PlGF2 showed benefit with VEGF-B186 (and to lesser extent, VEGF-B167) in pig myocardial infarct but not in rabbit ischemic hindlimb, possibly in part via indirect effects on cardiomyocytes and mural cells (312). VEGF-A165 was effective in both settings, while PlGF was effective in ischemic hindlimb only. Intravenous delivery of adenoviral VEGF-B167 increased vascular density in mouse myocardial infarct and border zone, but not in ischemic limb (339). Overall, it appears that VEGF-B promotes vascular growth selectively in the myocardium.
Naked plasmid VEGF-C induced lymphangiogenesis and reduced lymphedema severity in rabbit ear and mouse tail models; it did not increase angiogenesis (663). In contrast, VEGF-A165 induced angiogenesis but not lymphangiogenesis. This study found that it is important to introduce the VEGF therapy after an injury induces upregulation of VEGFRs, or the plasmid may not have much therapeutic effect.
A systematic comparison of adenoviral VEGFs (VEGF-A165, VEGF-B167, VEGF-C, the exclusively VEGFR-3 binding variant VEGF-C156S, VEGF-D, and the mature form VEGF-DΔNΔC) in rabbit ischemic hindlimb showed VEGF-A165 mainly induced angiogenesis (with a bit of lymphangiogenesis), VEGF-B167 had little effect, VEGF-C and -D induced lymphangiogenesis (with a bit of angiogenesis), VEGF-C156S induced lymphangiogenesis only, and VEGF-DΔNΔC was equally good at both (468). Strongest angiogenesis and lymphangiogenesis were with VEGF-DΔNΔC and VEGF-D, respectively. Angiogenesis, but not lymphangiogenesis, depended on nitric oxide. VEGF-A165 and VEGF-DΔNΔC caused edema and induced arteriogenesis. In normal mouse tissues, adenoviral VEGF-A induced angiogenesis, arteriogenesis, and lymphangiogenesis (405, 406, 438). Although the newly formed lymphatics persisted, they did not clear interstitial carbon particles well (405, 406). VEGF-C or VEGF-DΔNΔC gene therapy have been used to produce lymphatics that improve drainage to a transplanted lymph node (571), while adenoviral VEGF-C generated lymphatics that reestablished drainage across surgical wounds (476).
Since lymphatic vessels are critical to removal and presentation of antigen, induction of lymphangiogenesis may worsen chronic inflammation. Alternatively, lymphangiogenesis may alleviate inflammation, in part by reducing edema. Intraarticular injection of viral plasmid VEGF-C in a mouse model of chronic rheumatoid arthritis showed that the latter possibility was observed (680). Compared to controls, mice that were treated with VEGF-C plasmid demonstrated improved solute drainage, increased lymphatic vessel area, reduced synovial volume and cartilage erosion, and improved joint movement; no changes in blood vessel area were observed.
Intradermal injection of adenoviral mPlGF2 in the normal mouse ear led to long-lived (>1 year) vascular growth without edema or hemorrhage (354).
Although gene delivery of VEGF-A showed promising results in initial studies, several potential issues have arisen (71, 134, 329). First, VEGF-A promotes hyperpermeability, which can lead to edema and compromise of the ischemic tissue. In the ischemic mouse hindlimb, intramuscular injection of viral VEGF-A165 plasmid led to decreased blood flow ratios and eventual autoamputation of the limb, compared with injection of control plasmid (366). Second, the dose of VEGF appears to play an important role in the organization of the resulting microvessels. High doses can lead to the formation of vascular malformations, such as glomeruloid bodies that resemble vascular growth in tumors (438). Third, even though VEGF-A gene therapy induces angiogenesis, the higher vessel densities have not always translated into higher blood flows and can paradoxically lead to lower flows, and the neovessels often have lower pericyte coverage, compared to those induced by FGFs (366). These drawbacks have led to a reappraisal of therapies that are based purely on VEGF-A, and suggest that perturbations that focus on angiogenic “master switches” may yield more consistent benefits.
In contrast to VEGF-A, VEGF-E binds selectively to VEGFR-2, which indicates that it may be suited for avoiding deleterious inflammatory side-effects of VEGF-A therapy that are mediated by VEGFR-1 on leukocytes. Because VEGF-E is derived from the Orf virus and hence immunogenic, studies of its effect have used plasmids that encode for chimeric proteins of VEGF-E and human PlGF (233). Intramuscular injection of plasmid VEGF-A and two plasmids for viral VEGF-E/human PlGF showed that all three enhanced blood flow ratios in the ischemic rat hindlimb, but only the VEGF-E plasmids did not recruit tissue macrophages. Moreover, only VEGF-A treatment increased the expression of inflammatory cytokines IL-8 and TNFα.
Other direct angiogenic factors.
As observed with topical delivery of the recombinant protein, intravenous delivery of adenoviral COMP-Ang1 improved wound healing in the normal (274) and diabetic mouse and increased blood and lymphatic vessel densities (86). Sustained expression over weeks resulted in permanent microvascular enlargement in the normal adult mouse trachea, in contrast to the reversible change that occurred with short-term administration of recombinant protein (85). In healing wounds, the increases in blood vessel density were sustained for at least six weeks after injection of vector; in contrast, lymphatic densities were only transiently elevated (274).
Although the variant Ang1* showed no angiogenic effect when applied in soluble form in the corneal pocket (21), its expression via intramuscular delivery of naked plasmid into the ischemic rabbit hindlimb resulted in increased vascularization and blood flow (521). Because soluble Ang1* did increase vascular densities when co-administered with soluble VEGF in the corneal pocket (21), the efficacy of Ang1* gene therapy in ischemic tissue can be rationalized to result from induction of endogenous VEGF by ischemia.
Gene therapy of the other angiopoietins has been tried with some success. Intramuscular delivery of naked Ang2 plasmid did not result in any vascular changes in the ischemic rabbit hindlimb (521). In mouse skin wounds, adenoviral Ang2, Ang3, and Ang4 all resulted in sustained modest increases in blood vessel densities and transient increases in lymphatic densities (274). COMP-Ang2, an oligomeric variant of Ang2, showed indistinguishable angiogenic effects from COMP-Ang1 in healing mouse skin (271). This last result suggests that activation of the Tie2 receptor through oligomerization is sufficient to promote vascular growth.
Liposomal delivery of HGF plasmid to infarcted rat heart resulted in increased vascularization and blood flow (16).
Indirect factors.
Subcutaneous injection of plasmid DNA for PDGF-BB with a type I collagen carrier increased vascularity and blood flow in ischemic rat myocutaneous composite flap and led to a greater area of surviving tissue (204).
Combinations.
Angiogenic gene therapy has also benefited from combinatorial approaches. Intramuscular co-delivery of adenoviral VEGF-A165 and PDGF-B to rabbit ischemic hindlimb led to longer lasting improvements in perfusion, compared to delivery of VEGF or PDGF alone (299). Surprisingly, VEGF-A by itself led to increases in SMA-positive cells, while PDGF-B by itself had a modest effect; combination therapy resulted in loose pericyte coverage and more edema than VEGF-A alone. Co-delivery of plasmid VEGF-A165 and Ang1 resulted in capillary densities that were higher than those achieved with delivery of plasmid VEGF-A alone (75). Co-delivery of adenoviral COMP-Ang1 and Ang2 in mouse skin wounds resulted in an initially greater increase in blood vessel density (compared to COMP-Ang1 alone) but a smaller sustained increase (274). In the hyperoxic lung, co-delivery of adenoviral VEGF-A145 and Ang1* resulted in capillary growth, but not lung edema; in contrast, VEGF therapy by itself yielded fenestrated, leaky capillaries (577).
Expression of chimeric proteins has shown that a single vector can be used to obtain a combination of vascular effects. Intramuscular delivery of viral VEGF-A/Ang1 chimera in normal mouse skeletal muscle resulted in increased vascular coverage and blood flow that were similar to those achieved with VEGF-A alone (15); expression of the chimera did not yield the vascular malformations that were found with VEGF-A. In the ischemic hindlimb, the chimera led to lower vascular permeability and less tissue necrosis than VEGF-A did.
Hypoxia mimics.
Naked plasmid delivery of constitutively active HIF1α in the ischemic rabbit hindlimb resulted in increases in vascularization and blood flow that were at least as large as those induced by VEGF-A165 gene therapy (613).
Clinical trials
Phase I trials of intramuscular or intraarterial naked plasmid VEGF-A165 gene therapy in patients with critical limb ischemia showed that the therapy was well-tolerated, with mild edema in the ischemic limb as the main side effect (42, 234). As observed in animal studies, plasma concentrations of VEGF peaked 2-3 weeks after plasmid injection and then decreased to baseline. Ankle- and toe-brachial indices improved by eight weeks, and limb salvage occurred in some patients. Results with soluble recombinant VEGF were suggestive of a dose-dependent effect (199). Similarly promising results were found in patients with myocardial ischemia (352), and using other angiogenic factors (498, 500). Nevertheless, it has been challenging to obtain definitive proof of clinical efficacy versus placebo [reviewed in (187)].
No single cause of this discrepancy is apparent, and several possibilities have been suggested (311). First, some species (such as the dog) may be particularly well-suited for angiogenic therapies because they have well-developed collateral circulation. Second, even with species (such as the pig) whose collateral architectures more closely resemble that of humans, animal studies of therapeutic angiogenesis almost always apply an ischemic insult to young, otherwise healthy animals. In the clinic, however, the patient population is typically elderly, with substantial comorbidities (e.g., diabetes, obesity, hypercholesterolemia) that affect vascular response. Indeed, a comparison of topical TGFβ1 in rabbits of different ages showed that healing of ischemic wounds was negligible in old animals, even with application of growth factor (647); although this study did not analyze the effect of age on vascular growth, it suggests that responsiveness to growth factors may depend on patient age. In the ischemic hindlimbs of old rabbits, treatment with intraarterial VEGF was roughly equivalent to sham treatment in young rabbits; endogenous expression of VEGF was lower in older mice, but VEGFR-2 expression did not depend on age (469). Third, the endpoints in clinical trials are different, and often more stringent, than those in preclinical studies. Clinical trials of critical limb ischemia typically seek a reduction in amputation rate; in contrast, preclinical studies generally accept an increase in vascular density and/or blood flow as proof of efficacy. While the clinical endpoints are more relevant to the patient, they can depend on factors that are independent of vascular growth per se. For instance, as previously noted, topical FGF2 increased vascular density in ischemic wounds but did not improve wound healing (404). Fourth, placebo effects are surprisingly strong, which has made detection of a true therapeutic effect by angiogenic therapy more difficult. It is unclear why placebo treatment yields strong and sustained clinical improvement (456). Some researchers have suggested that patient selection bias and subsequent regression to the mean can partly account for the placebo effect (474). It is also possible that social and psychological improvements that result from participating in a clinical trial lead to true improvement. Regardless of the underlying causes, such results emphasize the requirement for double-blind randomized studies with long follow-up to ascertain the efficacy of therapeutic angiogenesis. Currently, no treatment based on therapeutic angiogenesis for ischemic conditions is clinically approved.
While angiogenic therapies appear to be well-tolerated, the long-term side-effects are unknown. Angiogenic factors could potentially trigger an angiogenic switch in latent tumors or induce vascular growth in atheroma (133). It is disconcerting that the list of angiogenic substances overlaps substantially with that of atherogenic ones (135). Because effects on tumor growth or atherosclerosis may require years before they are clinically apparent, well-powered trials to detect such effects are extremely costly.
Controlled release of growth factors
Even when injected directly into ischemic tissue, angiogenic growth factors have a limited half-life on the order of a few days at best (319, 320). Most of the injected material is quickly lost from the injection site, with typically <1% remaining after a few days (485); the growth factors are eventually cleared by the liver, kidneys, and lungs (320). As a result, therapeutic efficacy has required the application of large doses, typically well in excess of 100 μg for small-animal studies. Unfortunately, such large doses are accompanied by exposure outside the injection site, which can lead to undesirable side effects. Although the use of gene therapy has helped to prolong the time during which growth factor concentrations are in the desired range, even here the duration of plasmid expression is typically limited to a few weeks, particularly for viral delivery (636). The difficulty in maintaining a therapeutic concentration of growth factor at the desired site has been blamed for the modest clinical findings to date for angiogenic therapies.
To address these issues, numerous studies have examined the use of porous biomaterials to serve as carriers for the local, slow release of growth factors. As shown with FGF delivery to the heart, the use of carriers can increase the retention of injected material at the desired location by over two orders of magnitude (Table 1) (485). Moreover, carrier-mediated delivery allows for maintenance of spatial growth factor gradients, which can aid in the angiogenic response; superfusion with growth factor solution, which results in small or nonexistent concentration gradients, did not yield any change in vascular architecture in a rat skin chamber (432).
Table 1.
Fraction of FGF2 that was retained at myocardial infarct three days after administration. Unless noted, FGF was applied by injection from solution. Data are from (485).
| Route of administration | Fraction retained (%) |
|---|---|
| Intravenous | Less than 0.1 |
| Intracoronary | Less than 0.1 |
| Intramyocardial | 1.8 ± 0.9 |
| Intramyocardial with controlled release | 32.0 ± 5.2 |
Inert carriers.
In the simplest formulations, the carriers are passive; that is, they do not interact specifically with the growth factors. For instance, suspending crosslinked agarose microspheres in a solution of FGF2 results in diffusion of FGF into the pores of the microspheres (40). When FGF-loaded microspheres were injected into a pig coronary artery, they occluded the distal microvessels and reduced blood flow, but they also eventually resulted in larger microvascular densities in the myocardium, compared to injection of unloaded (FGF-free) microspheres. The vascular improvement is presumably the result of growth factor diffusing out of the microspheres to induce angiogenesis in surrounding tissue. (The increase in vascular density was not accompanied by improvements in ventricular function, another example of the disconnect between vascular growth and functional outcome.) Application of FGF2 to the rat cerebral cortex in an agarose gel carrier caused vascular growth (106).
Other types of porous carriers, such as sponges or films, can also serve as reservoirs of growth factor. Sponges of polytetrafluoroethylene (PTFE) were able to deliver FGF1 to the dog myocardium, and caused hyperplasia of vascular smooth muscle in infarcted tissue, which led to near-obliteration of lumens (35). Gelfoam, a gelatin sponge, served as a carrier of FGF1, which increased the angiographic signal and survival of ischemic rabbit skin flaps (213). Delivery of VEGF or the indirect angiogenic factor TNFα from a Gelfoam implant increased vascularization of ischemic rabbit skin flaps, but only VEGF increased flap survival; direct application of VEGF to the wound bed (i.e., without Gelfoam) had no effect (546).
Porous carriers have also been used for the controlled release of plasmids that encode for angiogenic proteins. Subcutaneous Matrigel or collagen released adenoviral VEGF-A, FGF1, or FGF2 and caused vascularization of the surrounding tissue (396, 397, 597). The ability of alginate gels to entrap plasmid DNA enabled the release of plasmid to be coupled to gel degradation (297). Mixtures of alginates allowed plasmid to be released over 2-3 weeks, which led to increased vascular density in the ischemic mouse hindlimb compared to injection of naked plasmid. Subcutaneous PLGA sponges released plasmid for PDGF-BB over several weeks in the rat, which resulted in increased vascularity in the surrounding granulation tissue 2-4 weeks after implantation (506). Although naked plasmid is readily taken up and expressed in skeletal muscle (640), extended exposure to low levels of plasmid may be helpful in tissues that take up minimal amounts of plasmid naturally, such as skin.
Reactive carriers.
While materials that passively store growth factor within pores can be effective reservoirs, they provide little versatility in tailoring the release kinetics, because the only mechanism of release is diffusion. Essentially all subsequent studies in controlled release have examined the development of carriers that not only provide pores for the uptake of growth factors, but that also interact with the proteins to slow their release. Given that many angiogenic factors (particularly, the FGFs and VEGF-A) bind strongly to heparin, it has been natural to use heparin-conjugated materials as a carrier for these factors. As seen during heparin affinity purification, heparin-Sepharose beads can be loaded simply by immersing the beads in growth factor solution. When loaded with FGF2 and injected around an occluded coronary artery, such beads (embedded within an alginate binder) induced collateral growth in the pig heart, increased flow, and improved myocardial function (Fig. 5) (350).
Figure 5.
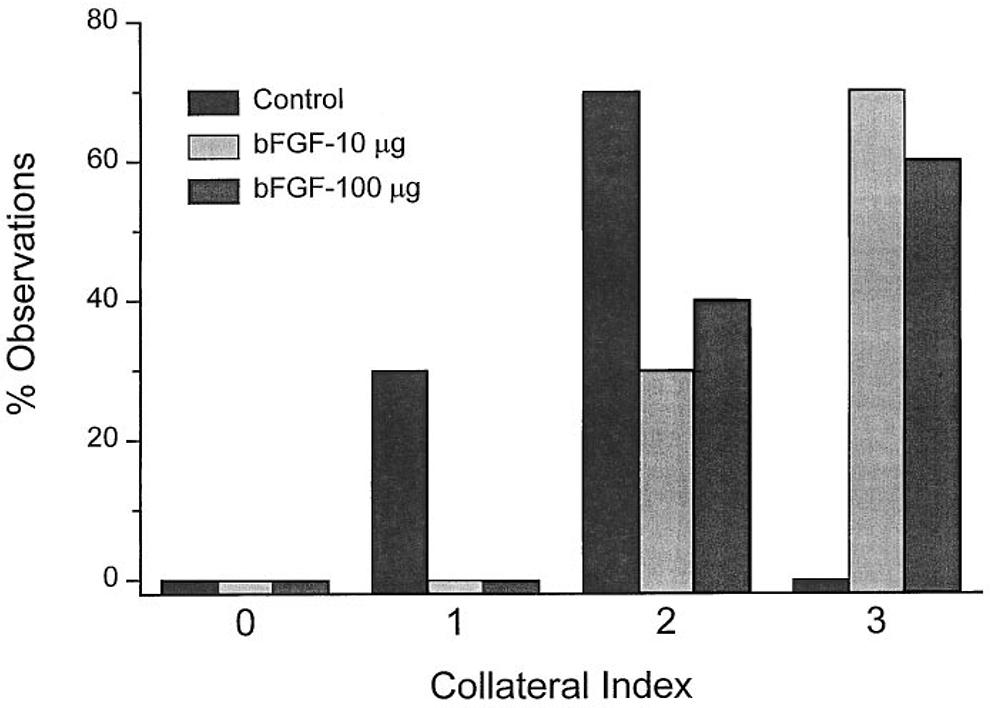
Dose-dependent collateral growth two months after implantation of FGF2-loaded heparin/alginate beads around occluded coronary arteries in the ischemic pig heart. bFGF, basic fibroblast growth factor (FGF2). Reproduced with permission from (350).
The highly anionic nature of heparin and the cationic properties of many angiogenic factors have motivated the use of other negatively charged biomaterials as carriers. In these materials, release of growth factors stems from diffusion, desorption from pores, and/or degradation of the carrier. An early example of anionic material for controlled release of angiogenic factors is sucralfate, a negatively charged derivative of sucrose; this material has been used extensively to test the angiogenic potential of compounds in the corneal pocket assay (111). Sucralfate is often delivered with the growth factor in a porous polymer; for instance, interposition of Gelfoam with FGF2 and sucralfate between an ischemic rat skin flap and the underlying wound bed resulted in increased numbers of collateral and SMA-positive vessels and increased flap survival (457).
Although Gelfoam itself appears to be near-neutral at physiological pH and thus acts mostly as a passive carrier (188), charged gelatin scaffolds can be synthesized. Crosslinked acidic gelatin hydrogels released FGF2 in two stages: a quick release over a few hours, followed by gradual incomplete release over several days (558-560, 564). In contrast, basic gelatin gels, which do not bind FGF2 electrostatically, released the growth factor in a single quick bolus (559, 560). The binding affinity of FGF2 to acidic gelatin was nearly a thousand-fold weaker than that to heparan sulfate (565). Nevertheless, release of the remaining FGF from acidic gels required degradation of the gels, a process that was faster in gels with a smaller solid content. When implanted subcutaneously in mice, 15% gelatin gels degraded in vivo with a half-life of nearly one month and induced a sustained increased in vascular volume in surrounding tissue (560). Extremely dense gels (~35% gelatin) led to a smaller increase in vascular volume, which suggests that optimal FGF carriers should have intermediate degradation times and pore sizes (558). Consistent with an electrostatic mechanism for FGF sorption, incorporation of the anionic polymer carboxymethylcellulose caused gelatin microspheres to release FGF more slowly; the slower release was accompanied by a delayed, but more sustained, increase in vascular volume around subcutaneous implants (559). In the ischemic rat skin flap, administration of FGF2-loaded acidic gelatin microspheres to the underlying muscle at the distal end of the flap (where ischemia is most severe) led to dose-dependent generation of capillaries and SMA-positive vessels in the flap, which was accompanied by improved flap survival (153); application of soluble FGF without gelatin carriers had no benefit. Intraarterial delivery of FGF2-loaded microspheres increased capillary densities in the ischemic hindlimb without compromising blood flow (216).
Alginate, a negatively charged polysaccharide, has also been heavily studied as a carrier for angiogenic factors. Growth factors are typically added to an alginate solution, which is subsequently gelled in the desired form by addition of calcium (435). Subcutaneous implants of alginate gel disks that contained VEGF-A or FGF2 demonstrated release over two weeks, and induced the formation of well-vascularized granulation tissue (327). The thickness of the vascularized region increased with VEGF dose, but vascular density did not.
Because alginates of different molecular weights and saccharide compositions are miscible and because they can be oxidized to hasten hydrolytic cleavage, they have been particularly suitable for generating customized carriers that release growth factor at defined rates. Mixtures of high- and low-molecular-weight oxidized alginates prolonged the release of VEGF-A to at least one month (524, 525). Intramuscular injections of VEGF-loaded alginate in the ischemic mouse hindlimb resulted in increased vascular densities that were independent of VEGF dose. Remarkably, the blood flow rate in the ischemic limb was restored to control (non-ischemic limb) levels one month post-implant. Two injections at different locations were more effective than a single injection of the same total gel volume (524).
Collagen-based materials have also been used for controlled delivery of angiogenic factors, although growth factor uptake is likely not purely electrostatic. Subcutaneous implants of crosslinked type I collagen sponges released VEGF-A over a few weeks; the more heavily crosslinked the matrix, the slower the release (562). As observed with FGF-loaded gelatin gels, VEGF-loaded collagen increased the local vascular volume fraction, with the most crosslinked implants resulting in the highest and most sustained increases. FGF2-loaded collagen microspheres released slowly in the ischemic mouse hindlimb, which led to increased capillary densities and sustained rescue of the perfusion defect one month after implant (253); of note, injection of FGF solution without collagen had no vascular or physiological effect. When introduced with a collagen suspension in a rabbit ear ulcer model, only PDGF and TGFβ accelerated the formation of granulation tissue and the accompanying vascular ingrowth; treatment with FGF2 resulted only in surprisingly modest increases in vascularity (402), which contrasts with the strong effect observed when FGF2 was delivered in the same model without a collagen carrier (441). Instillation of FGF2-containing collagen underneath a rat skin flap led to much higher vascular density in the flap midsection, compared with instillation of collagen only; this treatment resulted in improved survival of ischemic flaps but no change in the ability of flaps to survive transfer (268). Tight binding of angiogenic factors to collagen and other matrix-based scaffolds can be achieved by engineering the growth factor to contain matrix-binding domains, such as that from PlGF2 (362). Retention of VEGF-A165 after injection in the ischemic rat heart was enhanced by using a VEGF that was coupled to a collagen-binding domain; the fusion protein caused greater capillary growth and cardiac function, compared to native VEGF (672).
Although the efficacy of matrix- and alginate-based delivery can be attributed to the ability of these materials to bind and slowly release growth factors, another possibility is that these materials protect the growth factors against degradation. For instance, VEGF-A that was released from alginate beads showed 3- to 5-fold greater potency in an endothelial proliferation assay, compared to soluble VEGF that had not been exposed to alginate (435). Type I collagen protected FGF2 from tryptic digestion (253), and the ability of heparin to protect FGFs against denaturation is well-known (178).
Another large class of biomaterials that has proven effective as a vehicle for controlled release of angiogenic factors is synthetic biodegradable polymer, such as the copolymer of polylactic acid and polyglycolic acid (PLGA, copolymer of PLA and PGA). Because such polymers are typically not water soluble, special techniques are required to generate growth factor-loaded carriers. One approach is to emulsify aqueous growth factor solution with an organic polymer solution and then remove the organic solvent from the resulting water-oil-water double emulsions; the result are polymer microspheres that encapsulate growth factor (326). A second approach is to lyophilize growth factor solutions that contain a suspension of polymer particles. The latter approach usually adds a hydrophilic material, such as alginate or trehalose, as a co-carrier to stabilize the growth factor and promote its retention on the polymer surface (97, 436). Gas foaming of lyophilized materials with salt porogens and leaching of the salt is often used to generate pore space (192).
Regardless of the exact method used to load degradable polymers with angiogenic factors, numerous studies have shown the therapeutic efficacy of these materials. For instance, intracoronary infusion of poly(ethylene glycol-co-butylene terephthalate) microspheres that were loaded with VEGF-A165 increased capillary density in ischemic pig heart, although they did not improve cardiac function (598). In the ischemic mouse hindlimb, PLGA scaffolds that were loaded with VEGF-A165 released the growth factor over several weeks (551). This slow, sustained delivery resulted in near-normalization of perfusion and a doubling of capillary densities in the underlying muscle. Subcutaneous injection of VEGF-loaded PLGA microspheres in an alginate gel carrier resulted in increased vascular densities, compared with alginate-free injection of VEGF-loaded microspheres or with injection of VEGF solution (326). Intramuscular injection of the same formulation in the ischemic mouse hindlimb yielded similar results (325).
A third major class of angiogenic biomaterials is based on the covalent coupling of angiogenic factors to a degradable matrix. This design is intended to mimic the ability of native extracellular matrix to serve as a reservoir of growth factors that are mobilized upon proteolysis of the matrix. For instance, transglutaminase can crosslink VEGF variants that contain a transglutaminase recognition sequence to a fibrin scaffold (128, 688). Because tethered VEGF can only be released by local proteolysis, the concentration of free VEGF around an implant is only elevated where cells are active. Subcutaneously implanted VEGF-coupled fibrin gels induced the growth of non-leaky vessels in the surrounding tissue (128). It has been suggested that a bolus of residual unconjugated VEGF could provide an initial chemotactic signal for vessels to sprout towards an implant (688). Implantation of fibrin gels that were covalently linked to VEGF-C with a protease-sensitive linker in a mouse skin wound resulted in increased retention of VEGF (compared to fibrin gels that contained free VEGF) and lymphatic growth, although fluid and solute drainage did not increase (184). Similar results have been obtained with growth factor-coupled, protease-sensitive synthetic hydrogels (439).
Transglutaminase-mediated coupling has been applied to load plasmids into fibrin gels (593). A short recombinant peptide served as a linker to fibrin and as a cationic agent for DNA condensation. Topical application of fibrin gels that were coupled to a plasmid for HIF1α increased vascularization of mouse skin wounds. Notably, the fraction of vessels that was SMA-positive was much higher in HIF-treated wounds than in VEGF-treated ones.
As with delivery of soluble angiogenic factors and their plasmids, controlled release has been applied to multiple growth factors in a single implant. Intramuscular injection of collagen microspheres that contained FGF2 and/or HGF in the ischemic mouse hindlimb showed that dual release led to synergistic increases in capillary density and mural cell coverage; bolus injection of the same factors had no effect, even at higher doses (363). Alginate hydrogels that were loaded with VEGF-A and PDGF-BB tended to release VEGF earlier and PDGF later (190). Intramyocardial injection of such gels to the ischemic rat heart resulted in elevated capillary densities that were similar to those obtained with injection of VEGF-loaded alginate, but in arteriolar densities that were higher than those obtained with injection of VEGF- or PDGF-only gel. In the ischemic mouse hindlimb, average vascular diameters were nearly twice as large when VEGF and PDGF were co-released, compared to VEGF release only; co-release also led to a modest increase in hindlimb perfusion, compared to VEGF only (552). Co-delivery of VEGF-A and Ang1 from a hybrid PLGA/alginate scaffold to the ischemic mouse hindlimb yielded equivalent capillary densities compared to delivery of VEGF or Ang1 alone, but skewed the distribution towards higher vascular diameters (514). Co-delivery also led to a decrease in muscle fibrosis, equivalent to that achieved with VEGF alone and better than with Ang1 alone. Sustained delivery of two growth factors—VEGF-A165 to promote angiogenesis, insulin-like growth factor-1 (IGF1) to promote muscle regeneration—from intramuscular injection of loaded alginate gel resulted in fastest recovery from hindlimb ischemia in the mouse (56). Increases in vascular density depended only on the delivery of VEGF, and bolus co-injection of VEGF and IGF had no effect.
The most extreme example of multi-factor controlled release may be the use of platelet-derived factors to promote angiogenesis. The α granules of platelets are a rich source of direct and indirect angiogenic factors, including VEGF, FGF2, IGF1, PDGF-BB, and TGFβ (193). Exposure of platelet-rich plasma to gelatin microspheres resulted in platelet degranulation and the release and entrapment of growth factors within the microspheres (52, 308). Intramuscular injection of such loaded microspheres in the ischemic rat hindlimb increased the angiographic scores and blood flow, compared to injection of platelet-rich or platelet-poor plasma without the carrier (308). Similar results were found in the ischemic hindlimb of diabetic mice, where controlled release of growth factors from platelet-rich plasma increased both total and SMA-positive vessel densities; in contrast, controlled release of factors from platelet-poor plasma had no effect (52).
Nanoparticles have also served as effective delivery vehicles. Because microvessels in ischemic tissues are hyperpermeable, intravenously injected VEGF-conjugated gold nanoparticles accumulated into the muscle of ischemic mouse hindlimb (272). Alternatively, nanoparticles could be injected directly into the muscle (176). The resulting delivery of VEGF resulted in increased vessel density and normalization of blood flow; intravenous injection of free VEGF had no effect. In a more elaborate approach, the receptor-binding portion of an angiogenic protein can be coupled to a long hydrophobic tail that directs the self-assembly of cylindrical nanofibers in aqueous solution (306, 627). The surface of these fibers displays a high density of receptor-binding epitopes, which should result in activation of growth factor receptors by oligomerization. Indeed, injection of VEGF-mimetic nanofibers in the ischemic mouse hindlimb led to increased capillary densities, perfusion ratios, motor function, and tissue viability, compared to injection of scrambled nanofibers or unassembled VEGF-derived peptide (627). VEGF-mimetic nanofibers persisted at the injection site 2-4 weeks after delivery. Similar results were obtained with nanofibers that presented VEGF epitopes to promote angiogenic signaling and protease-sensitive sequences to allow degradation of the fiber (305).
Some extracellular matrix proteins promote angiogenesis independently of any added growth factors. The best-studied “angiomatrix” protein is Del-1, which promoted vascularization of the ischemic mouse hindlimb by activating αV integrins on ECs (208, 679).
Cells as source of angiogenic factors
Leukocytes.
In the 1970s, Polverini, Clark, and their colleagues showed that stimulated macrophages and their conditioned media could induce angiogenesis in a corneal pocket assay (95, 445). At about the same time, Schaper showed that monocytes were often found in the process of extravasating across the endothelium during the initial stages of collateral development (491). These early studies led to the idea that macrophages may secrete growth factors that induce angiogenesis and arteriogenesis. Of the blood-derived cell populations, macrophages appear to be the most effective at promoting vascular growth, and of these, only activated macrophages can do so (553). The arteriogenic chemokine MCP-1 is induced by acute elevation of endothelial shear stress and recruits circulating monocytes, which presumably secrete endothelial and other growth factors upon extravasation (492, 522).
Activated macrophages secrete a large variety of substances that affect cell growth, such as the direct angiogenic factors VEGF-A and FGF2, other growth factors, and interleukins (553). Angiotropin, a unique macrophage-derived ribonucleopeptide, increased capillary density in the normal rabbit ear, stimulated fibrovascular growth into an artificial dermal graft, and rescued an ischemic rabbit skin flap from necrosis (209, 210). How much the beneficial effect stems from direct activation of ECs versus indirect activation of neighboring mesenchymal cells, such as fibroblasts and SMCs, is unclear.
Other subsets of inflammatory cells may be angiogenic. The neutrophil chemoattractant f-Met-Leu-Phe (fMLP) is angiogenic in the corneal pocket assay, but not on the chick chorioallantoic membrane, which suggests that it acts indirectly through leukocyte recruitment (367). Whether neutrophils are the primary mediator of the vascular growth by fMLP is unclear. CD31+ T cells have been reported to be angiogenic (225).
Mesenchymal cells.
Non-inflammatory mesenchymal cells, particularly bone marrow-derived mesenchymal stem cells, have also been examined for their ability to promote angiogenesis. These effects, and the ability of such cells to form vasculature, are discussed in the section on vasculogenic approaches. Other types of mesenchymal cells, such as SMCs, fibroblasts, and cardiomyocytes, induced angiogenesis when transplanted into ischemic heart, albeit modestly (337, 657). Mouse preadipocytes induced angiogenesis in a skinfold chamber (156).
Transduced cells.
Given the effectiveness of angiogenic gene therapy in animal models of tissue ischemia, introduction of transduced cells has been explored. Intramyocardial injection of rat cardiomyocytes that were transfected with VEGF-A165 in the ischemic rat heart increased capillary densities and blood flow (657). Co-implantation of VEGF-transduced adipose-derived stem cells with free fat grafts generated twice the capillary density of control (non-transduced) implants and increased graft survival (353). Implants of FGF2-transduced myoblasts, but not VEGF-transduced ones, increased vascularization of ischemic skin flaps (464). In principle, injection of cells rather than plasmids allows for the pre-selection of cell populations that express the angiogenic gene(s) at the desired levels. By comparison, in vivo release of plasmids leads to uncontrolled uptake; some cells may contain many copies of the plasmid, while others may have a few or none.
A pre-selection strategy has been used to analyze the role of local growth factor levels on vascular growth (426, 619). Polyclonal populations of myoblasts that were transduced with retroviral mouse VEGF-A164 secreted VEGF at a broad range of rates per cell (426). Intramuscular implants of polyclonal populations led to formation of hemangiomas. Implants of myoblast clones that secreted VEGF at the same rate as the average from a polyclonal mixture resulted in vascular growth without hemangiomas. This result suggested that the local VEGF distribution controls the architecture of the induced vascular growth. Monoclonal implants avoid “hotspots” of high VEGF expression that are found with polyclonal implants, and can thus reduce the development of hemangiomas. The capillaries that developed from monoclonal implants that secreted VEGF at a low rate were resistant to VEGF deprivation. Similar results were found in the ischemic mouse hindlimb (619).
Use of bicistronic vectors enabled implantation of myoblasts that expressed mouse VEGF-A164 and human PDGF-BB at defined ratios (37). Remarkably, when VEGF and PDGF were expressed from the same vector, even a polyclonal implant resulted in hemangioma-free vascularization, despite the delivery of some cells that expressed high levels of VEGF (and PDGF). In contrast, co-injection of two separate polyclonal populations of myoblasts—one that secreted VEGF only, the other that secreted PDGF only—led to formation of hemangiomas. These results implied the local balance of VEGF and PDGF secretion is what determines whether a normal capillary bed forms. Findings from cell implants were confirmed with adenoviral delivery of the same bicistronic vector to the ischemic mouse hindlimb, which led to increased vessel densities and blood flow and decreased muscle damage without the development of hemangiomas; as expected with PDGF expression, newly formed capillaries were well-covered by pericytes. These results contrast with the current view that sequential, rather than simultaneous, presentation of VEGF and PDGF is needed for the formation of mature microvessels.
Myoblasts that overexpressed mouse VEGF-B167 were claimed to induce vessel growth in mouse myocardial infarct, but not in ischemic hindlimb (339).
Other inducers of angiogenesis
Heparin.
Compared with growth factors, heparin is modestly angiogenic at best. Intracoronary infusion of heparin in the ischemic dog heart led to increased collateral flow between an implanted artery and the myocardial circulation (603). Strangely, the total collateral flow did not change, which would imply that heparin reduced the development of collaterals that did not originate from the implanted artery. Pericardial injection of heparin did not improve collateral indices in the ischemic pig heart (310). Likewise, heparan sulfate did not improve vascular densities in the ischemic dog heart, although it did enhance the effect of FGF2 (596).
Notch inhibitors.
The Notch pathway modulates the emergence of “tip cells” at the forefront of nascent vascular sprouts (197); inhibitors of this pathway should promote angiogenesis. Indeed, the indirect Notch inhibitor DAPT increased the angiogenic response when co-released with VEGF-A from an alginate gel to the ischemic mouse hindlimb (67, 68). The highest levels of DAPT caused the highest capillary densities, but only weak improvements in blood flow. In the ischemic hindlimb of diabetic mice, sequential delivery of VEGF-A, DAPT, and PDGF-BB led to increased capillary and SMA-positive vascular densities, but did not show added benefit over delivery of VEGF and PDGF (67). Intradermal injection of viral plasmid for soluble Dll4, which serves as a competitive Notch inhibitor, resulted in lymphangiogenesis in the mouse ear; this effect synergized with that of VEGF-A gene therapy (676).
Lipids and related substances.
Early work by Goldsmith showed that omentum contained angiogenic activity in a chloroform-methanol lipid extract (175). This lipid fraction improved perfusion in ischemic cat hindlimb, regardless of whether intramuscular injection occurred locally or in a distant limb (174). Constant infusion of lipid fraction into demineralized bone allograft in a rat femoral defect led to vascular invasion throughout the entire graft by six weeks; blood flow also increased, but eventually returned to the level of controls after the lipid infusion was discontinued (417). Omental extract consists of a complex mixture of glycerides, glycolipids, cholesterol, and free fatty acids (368); the exact identity of the angiogenic lipid(s) in omentum remains unknown.
Other lipid-like molecules are angiogenic as well. Sphingosine 1-phosphate, a major lipid of platelets, induced vascular growth and recovery of blood flow when injected into ischemic mouse hindlimb (425). Retinoic acid induced lymphangiogenesis and restored drainage in a mouse tail lymphedema model (87). Slow release of prostaglandin E1 from polymer microspheres into the ischemic hindlimb increased vascular density in normal and diabetic mice (138, 220). Small doses of leukotriene B4 promoted lymphangiogenesis in the edematous mouse tail, while large doses inhibited it (582).
Small organic molecules.
As described earlier for chemical mimics of hypoxia, low-molecular-weight organic inhibitors and promoters of signaling pathways can be used to induce angiogenesis (499). Compared to growth factors, these small molecules are easier to synthesize and purify, but may be more difficult to deliver in a sustained fashion. Combinatorial screening of chemical libraries has been used to isolate small angiogenic molecules, such as phthalimide neovascular factor 1 (molecular weight 229 Da) for stimulation of rat mesentery microvasculature (631). A potential advantage of such compounds is that they may be easier to incorporate into polymeric scaffolds, since they cannot denature and are generally nonpolar; it remains to be seen to what extent such compounds can successfully promote therapeutic angiogenesis in ischemic tissues.
Transition metal ions.
Divalent cations can induce angiogenesis, through mechanisms that are not entirely clear. The species that has been most explored for engineering microvessels is Co2+, which can mimic the response to hypoxia (624). Controlled release of cobalt has been obtained with coated glass fibers, although the angiogenic efficacy of these materials in vivo is unclear (645). Other angiogenic cations, such as Cu2+ (and the copper-carrying protein ceruloplasmin), appear to act mainly through recruitment of inflammatory cells, since corticosteroids inhibit the angiogenic effect (367, 683).
Oxygen and nitric oxide.
Given the importance of hypoxia in inducing the expression of VEGF, it is counterintuitive that oxygen can promote angiogenesis (575). The inspiration of 100% O2 at hyperbaric pressures of 2.5 atm accelerated vascularization of elevated rat skin flaps, which was accompanied by higher tensile strength at the flap margins (373) and by less distal necrosis (359). Similarly, 100% O2 at 2.4 atm caused much greater vascular growth in irradiated bone and its surrounding soft tissue in the rabbit, compared to normobaric air (20% O2 at 1 atm) (365). In contrast, normobaric oxygen (100% O2 at 1 atm) yielded no change in vascular density over normobaric air.
Hyperbaric oxygen therapy works synergistically with application of soluble angiogenic factors. In ischemic rabbit skin ulcers, the combination of 100% O2 at 2 or 3 atm and topical PDGF-BB or TGFβ was able to increase vascularization and wound healing to levels seen in non-ischemic wounds (675). No added benefit was found in using O2 at 3 atm instead of 2 atm; 100% O2 at 1 atm had no effect over control. In ischemic myocutaneous rat flaps, the combination of 100% O2 at 2.5 atm and intraarterial FGF2 led to greater distal vascular densities, perfusion, and flap survival than either O2 or FGF delivery alone did (46).
In the ischemic mouse hindlimb, hyperbaric oxygen suppressed VEGF expression, but caused two- and twenty-fold increases in FGF2 and HGF expression, respectively (25). It appears that the net balance of the oxygen therapy is to increase the expression of angiogenic factors that overcompensate for the loss of VEGF. Oxygen therapy only showed these effects in the presence of existing tissue ischemia. Hyperbaric oxygen also increased the expression of the FGF2 receptor in ischemic hindlimb. It is important to recognize that the hyperbaric oxygen therapy is intermittent rather than continuous, and the duration of exposure is actually quite limited, on the order of 1-2 hours per day (365, 373). Some have hypothesized that the intermittency of hyperbaric exposure results in switching between hyperoxic and hypoxic conditions in the tissue, which may be important for the observed angiogenic effects (25).
The vasodilator nitric oxide mediates the angiogenic effect of VEGF but not FGF2 (155, 400, 684). Exogenous delivery of nitric oxide is typically achieved through systemic injection of a precursor compound, such as L-arginine. It remains to be seen whether site-specific controlled release of nitric oxide is a feasible approach to induce vascularization.
Angiogenesis to engineer vascularized tissues
The ability of angiogenic growth factors to promote vascular growth near the site of release led to the idea that those same factors could be used to promote angiogenesis into a newly formed tissue. While it is possible to induce the formation of new vascularized tissue by injection of a vulnerary growth factor (470), by sequential layering of thin tissues (512), or even by deliberate localized injury (433), nearly all studies that use angiogenesis to engineer vascularized tissues have relied on porous scaffolds to serve as templates in which the new tissue forms and into which new vessels extend (60). Many of the scaffolds that have been used for controlled release of growth factors in therapeutic angiogenesis have been repurposed for slow, controlled vascularization of the scaffolds.
Scaffolds that promote angiogenesis
Growth factor-free scaffolds.
By itself, implantation of a porous scaffold is often sufficient to induce eventual vascular ingrowth. For instance, fibrovascular tissue grew within 1-4 weeks into the pores of subcutaneous polyvinyl alcohol sponges (PVA; pore sizes of 60-350 μm) and mesenteric polylactic acid membranes (PLA; pore sizes of 90-500 μm) (504, 563, 621). Transcutaneous implants of polyhydroxyethylmethacrylate (polyHEMA; pore size of ~40 μm) in the pig also led to vascular ingrowth (638). The consensus among studies with growth factor-free scaffolds is that an optimal pore size—not too large, not too small—exists for vascularization. In templated polyHEMA gels, which can be synthesized with nearly uniform pores, vascular density within the scaffold at one month post-implant was the greatest at pore sizes of ~35 μm (Fig. 6) (361); surprisingly, vascularization did not depend on whether the scaffold was doped with collagen. For pore sizes of ~35 μm or less, vascular densities varied spatially, with largest densities at the surface of a scaffold and smallest densities at the core. Below a pore size of ~10 μm, essentially no vascular ingrowth occurred with PVA, cellulose, PTFE, and acrylic copolymer scaffolds (57, 505). Similar results were found in sponges of type I collagen and chondroitin sulfate, for which pore sizes of 20-100 μm resulted in the fastest wound healing, and presumably in fastest vascularization as well (653). In hydroxyapatite and porous polyethylene implants, fibrovascular tissue grew fastest in scaffolds with pore sizes on the order of 400 μm (473). In micropatterned collagen implants, in which the pore size and geometry was controlled, vascular ingrowth was highest in scaffolds with 100-μm-wide pores (678). Surprisingly, pre-filling the pores with a dilute collagen gel did not inhibit vascularization. In subcutaneous and intraperitoneal micropatterned poly(glycerol sebacate) implants, vascularization also occurred into 100-μm-wide pores (658).
Figure 6.

Density of vascular ingrowth into polyHEMA implants after one month. Reproduced with permission from (361).
The main stimulus for vascular ingrowth is most likely the influx of inflammatory cells that serves as the first stage of the foreign-body response. In scaffolds that permit vascularization, host inflammatory cells are often found within the scaffold pores, prior to vascular ingrowth (57). If the pores are too small to permit entry of leukocytes, then vascularization is invariably absent. With pore sizes of 1-10 μm, leukocytes penetrate into the scaffold even though vessels do not; it appears that leukocyte entry is necessary, but not sufficient, for vascularization. Not all inflammation is created equal, however: inflammation that accompanies infection of an implant can reduce vascularization (473).
A related approach is the use of lasers or needles to drill channels directly into an ischemic tissue; here, the tissue functions as the equivalent of a porous scaffold. Known as “transmyocardial revascularization” when applied to the myocardium, this technique is intended to provide channels for vascular ingrowth. Although some have found that lasing induced angiogenesis into the channels (120), the mechanisms and outcome remain controversial, as laser damage undoubtedly results in inflammation and a wound healing response [reviewed in (59)]. In particular, it is debatable whether laser-induced channels remain patent or are instead replaced by scar (91, 357).
Scaffolds for controlled release.
Although growth factor-free scaffolds can induce vascular ingrowth, the preclinical success of growth factor-based therapeutic angiogenesis naturally suggested that vascular ingrowth could be augmented if scaffolds released angiogenic factors. As seen with injection of vulnerary growth factors (PDGF, TGFβ, and FGF2), the controlled release of the same factors from subcutaneous porous chambers resulted in the formation of granulation tissue, but now within the pores of the chambers (Fig. 7) (541). Leukocytes and fibroblasts accumulated in the tissue ingrowth, and were accompanied by increases in capillary density, particularly when FGF was the released factor. FGF-releasing PVA sponges promoted more fibrovascular ingrowth into the scaffold pores than FGF-free implants did (563). Co-delivery of FGF2 and FGF9 from subcutaneous Matrigel scaffolds synergistically increased both total and SMA-positive vascular ingrowth (150).
Figure 7.

Cellularity of fibrovascular ingrowth into porous PTFE chambers in the presence of various growth factors after ten days. Reproduced with permission from (541).
Similarly, controlled release of VEGF increases vascular ingrowth. VEGF-releasing polymer matrices promoted vascular formation within subcutaneous implants in the mouse (436). PLGA scaffolds that were loaded with VEGF-A165 were invaded by 3- to 5-fold more capillaries than unloaded (VEGF-free) scaffolds were (131, 551). Remarkably, the density of SMA-positive vessels that developed within VEGF-releasing scaffolds not only exceeded that in unloaded scaffolds, but also that in the surrounding muscle (551). Nevertheless, the total vascular densities in the scaffolds were roughly an order of magnitude lower than those in the surrounding muscle (~100/mm2 versus ~800/mm2). The host response to sustained VEGF release from PLGA scaffolds depended on the implantation site and host immune competence (81). Subcutaneous Matrigel plugs that released VEGF-A165 or VEGF-B167 promoted vascularization of the gel (528). The result with VEGF-B is surprising, given that VEGF-B gene delivery has shown weak therapeutic effect (468); the presence of Matrigel may enhance the vascularization potential of VEGF-B, since Matrigel contains many angiogenic growth factors, including FGF2, PDGF, and TGFβ (283).
Scaffolds that incorporate heparin or heparan sulfate have been used to release heparin-binding growth factors, such as FGF2 and VEGF. Immersion of collagen-heparan sulfate matrices in a solution of rat FGF2 led to binding of the growth factor, which was slowly released over several weeks (440). Subcutaneous implants of collagen-heparan sulfate-FGF resulted in greater vascular ingrowth than implants without either FGF or heparan sulfate did. Similarly, collagen-heparin scaffolds can be loaded with VEGF by immersion in VEGF solution, and these implants led to greater vascular ingrowth than growth factor-free implants did (544). The incorporation of heparin by itself also led to greater vascularization of collagen scaffolds, perhaps by potentiating the effect of endogenous angiogenic factors. Complexation of anionic dextran sulfate with VEGF-A enabled loading and slow release from subcutaneous Matrigel and intraperitoneal PLGA implants, which yielded greater vascularization than uncomplexed VEGF did (117).
Angiogenic factors can be covalently coupled to scaffolds for subsequent release. Fibrin scaffolds in which VEGF-A121 was covalently coupled were invaded by a higher density of vessels than scaffolds with passively adsorbed VEGF were (129). Of note, all vascular densities returned to control levels (i.e., those of VEGF-free fibrin implants) after six weeks. Fibrin that was coupled to mouse VEGF164 and the protease inhibitor aprotinin to slow scaffold degradation led to stable vascularization after three months (480). Subcutaneous implants of polyurethane scaffolds that contained a protease-sensitive polymer gel to which VEGF-A was covalently coupled were vascularized after two weeks (687).
Multi-step polymer processing provides the ability to deliver multiple growth factors sequentially. Pre-encapsulation of PDGF-BB in PLGA microspheres, followed by immobilization of the microspheres with VEGF-A165, alginate, and more PLGA, generated scaffolds that released PDGF more slowly than VEGF (461). Sequential delivery of VEGF and PDGF resulted in the largest vascular density in the scaffold at four weeks post-implant, compared to sustained delivery of VEGF or PDGF only. In particular, sequential delivery yielded a density of SMA-positive microvessels that was nearly triple that achieved with single-factor delivery. In a note of caution, sequential delivery also resulted in the formation of multilayered and sinusoidal vessels. Scaffolds in which VEGF or PDGF were injected did not yield a sustained increase in vascular density.
The relative timing of signals that initiate angiogenesis versus those that mature newly formed capillaries is important in the final vascular outcome. PLGA scaffolds that released VEGF-A and Ang2 first, followed by PDGF-BB and Ang1, were invaded by greater densities of microvessels than scaffolds that released all four growth factors simultaneously were (62). Delayed release of PDGF and/or Ang1 yielded increased densities of SMA-positive vessels, compared to rapid release of VEGF and Ang2 only.
Sequential delivery of growth factors has also been achieved by using PLGA scaffolds that consist of spatially distinct VEGF- and PDGF-loaded layers (80). In the ischemic mouse hindlimb, scaffolds were invaded by higher densities of SMA-positive microvessels in the layers that contained PDGF. Surprisingly, PDGF-releasing scaffolds had larger SMA-positive microvessels, even in layers that did not contain PDGF. In principle, heterogeneous localization of VEGF and PDGF within a scaffold could be used to tailor the microvascular networks that develop in different regions of the scaffold. A similar strategy, using spatially distinct layers that released VEGF and antibody to VEGF, enabled the generation of pro- and anti-angiogenic regions in an implanted PLGA scaffold and in the surrounding muscle, which yielded tissues of higher and lower vascular densities (666).
Despite the advantage of sustained delivery over bolus injection of angiogenic factors, the durability of the induced microvessels remains an issue. For instance, despite continuous pumping of VEGF-A, FGF2, and/or PDGF into subcutaneously implanted PVA sponges for over one month, the vascular density in these scaffolds peaked around three weeks post-implant and then decreased back to control levels at five weeks post-implant (177). Continuous pumping of VEGF-A165 into subcutaneous implants of porous polyurethane for six weeks showed that delivery of 150 ng/day led to an increase in vascular density over control (no VEGF) implants at four months (113).
Other types of substances have been released from scaffolds to promote vascular ingrowth. Slow release of adenoviral PDGF-B, FGF2, or VEGF plasmid from collagen gels in a subcutaneous PVA sponge yielded fibrovascular ingrowth, with less matrix in implants that released FGF or VEGF plasmid (121). When released from a Matrigel implant, sphingosine 1-phosphate synergized with VEGF or FGF2 to induce vascular ingrowth; by itself, sphingosine 1-phosphate did not cause vascularization (328).
Cobalt has been incorporated into glass scaffolds for bone tissue engineering, with the idea that the HIF-activating ion would be slowly leached from the scaffolds to induce angiogenesis (645). A low level (2%) of cobalt increased expression of HIF1α and secretion of VEGF by bone marrow-derived stromal cells that were cultured on the scaffolds. Although a higher level (5%) of cobalt also increased HIF1α expression, it decreased cell proliferation and did not increase VEGF secretion. Whether release of cobalt can be used to increase vascular ingrowth into bioactive glass, which serves as an osteoconductive scaffold for bone engineering, is unclear. Other activators of HIF1α induced vascular ingrowth into subcutaneous Matrigel or polyurethane sponge implants (335, 626).
Cells as source of angiogenic factors
Cells that secrete angiogenic factors, or that induce neighboring cells to do so, have been used to promote angiogenesis within scaffolds. For instance, scaffolds that contained bone marrow-derived stromal cells that were treated with cobalt (to activate the HIF pathway) resulted in greater vascular densities in subcutaneous and skull defect implants, although the amount of bone that formed did not increase (139). Subcutaneous implants of Matrigel that contained FGF2-transfected fibroblasts displayed vascular ingrowth after a week (597).
The binding properties of a scaffold can tailor the response to implantation of growth factor-expressing cells. Because type I collagen binds poorly to VEGF-A, collagen sponges served as a “buffer” to blur spatial variations in growth factor secretion from VEGF-transduced adipose stem cells within the sponge (162). In contrast, ovalbumin scaffolds (which bind strongly to VEGF) limited the diffusion of VEGF; although this property caused greater amounts of VEGF to be trapped in ovalbumin scaffolds, the VEGF distribution was expected to be more heterogeneous than in collagen scaffolds. Indeed, abnormally large vascular structures were found to grow into subcutaneous ovalbumin, but not collagen, scaffolds that contained VEGF-transduced cells. If the cells were pre-selected to cull the ones that expressed VEGF at high levels before incorporation into ovalbumin scaffolds, then the implants yielded vessels of normal size distribution.
Cells can also promote angiogenesis by remodeling the scaffold. In Matrigel implants, recruited macrophages appeared to digest “tunnels” that served as routes for ingrowth of fibrovascular bundles (13, 14), while pericytes formed networks that appeared to facilitate ingrowth of vessels (585).
Tissues in angiogenic scaffolds
Angiogenic ingrowth into scaffolds is intended to provide the vascular support needed to sustain the metabolism of living tissues. Although vessels do invade growth factor-free scaffolds, this ingrowth is generally insufficient to engineer tissues of physiological cellularity. For instance, hepatocytes that were transplanted in PLGA or PLA sponges to the rat mesentery displayed very poor long-term viability, with <10% of the initial grafted cells surviving at two weeks post-implant (Fig. 8) (388). The surviving cells were clustered near the surface of the implants, where vascular densities were highest. When hepatocytes were transplanted in PLGA scaffolds that contained VEGF-A, the seven-day surviving fraction was double that for VEGF-free implants (535). Transplantation of hepatocytes in PLA discs that released FGF2 led to twice the level of cell engraftment as seen in FGF-free implants (323).
Figure 8.

Number of viable hepatocytes in PLGA sponge implants as a function of time. Hepatocytes were added directly to scaffolds before implantation; scaffolds were not pre-vascularized. Reproduced with permission from (388).
Although these results show the potential of growth factor-loaded scaffolds to increase the viability of engineered tissues, it must be noted that the surviving cell fractions were still quite low. For highly metabolic cells such as hepatocytes, the degree and rate of vascular ingrowth—even when enhanced with angiogenic factors—may not be sufficient to rescue most of the implanted cells from ischemic damage.
These limitations suggested that scaffolds should be “pre-vascularized” by prior implantation before adding cells. Subcutaneous or mesenteric implantation of PVA sponges in the rat allowed vascular ingrowth to take place for 5-7 days before grafting of hepatocytes (605). Approximately 10-20% of grafted cells remained viable after seven days. A subsequent study implanted PVA sponges, with and without FGF2, to the rat mesentery for seven-day pre-vascularization before grafting of hepatocytes (420). Long-term cell viability was 5-7% in FGF-loaded scaffolds, and <1% in control scaffolds. Pre-vascularization of myocardium with FGF2 enabled greater survival of injected cardiomyocytes (484). Nevertheless, it appears that grafting cells into scaffolds leads to modest cell viabilities, regardless of whether implants are pre-vascularized or not.
More promising results have been obtained by allowing the scaffolds to promote vascular and tissue ingrowth, such as for bone engineering. Coating of PLGA scaffolds with ceramic powder (Bioglass) made the scaffolds osteoconductive and osteoinductive (322). Implantation of Bioglass-coated scaffolds in a critical-sized rat skull defect led to generation of mineralized tissue after three months. Implants of scaffolds that were loaded with VEGF-A165 before Bioglass coating generated similar volumes of bony tissue, but with double the vascular density. In this case, vascularization did not augment tissue formation, probably because the scaffolds did not contain cells during implantation (in contrast to the hepatocyte-loaded scaffolds described above). Slow delivery of mouse VEGF from a PLA carrier into a femoral bone fracture induced vascularization of the surrounding soft tissue, but not existing bone; nevertheless, VEGF delivery enhanced repair of the fracture (550). Co-delivery of VEGF, the osteoinductive factor BMP4, and mesenchymal stem cells (MSCs) in PLGA sponges yielded the greatest level of bone formation in subcutaneous implants (219). Delivery of VEGF only or VEGF and MSCs led to ingrowth of fibrovascular tissue instead. Similarly, sequential delivery of VEGF and BMP2 led to formation of vascularized bone in rat subcutaneous implant and femoral defect models, with VEGF and BMP2 having largely independent effects on vascularization and osteogenesis, respectively (263). On the other hand, in irradiated cranial bone defects, delivery of VEGF increased sustained vascularization of PLGA scaffolds and led to increased scaffold mineralization, compared to VEGF-free implants (248). Whether VEGF is sufficient for mineralization appears to depend on the bone model used.
Subcutaneous injection of Matrigel with FGF1 or FGF2 led to vascularization of the injection site in one week (429), with subsequent conversion of FGF2-releasing implants into adipose tissue over the next several weeks (257). PDGF exerted similar effects on vascularization and adipose formation, when injected with Matrigel. When FGF2 was first sorbed into gelatin microspheres before incorporation into Matrigel, subcutaneous injection of the Matrigel/gelatin/FGF mixture resulted in even more robust adipose generation than injection of Matrigel/FGF mixture did (561). It is reasonable that the combination of Matrigel and angiogenic factors should yield vascularized tissue, but why adipose is the favored result remains unclear. Some have suggested that Matrigel may bind and thus protect growth factors that can promote angiogenesis and adipogenesis (257). Alternatively, Matrigel may release additional substances that synergize with FGF or PDGF. By itself, subcutaneous injection of Matrigel can induce adipogenesis, but only in young mice. Given that pericytes are multipotent cells that can differentiate into adipocytes (101), it is possible that angiogenesis supplies the adipose progenitors that contribute to subsequent adipogenesis.
Although controlled release of FGF2 from collagen implants has been reported to induce vascular growth (253), vascularization by itself appears to be insufficient to transform the collagen into adipose (257, 561). To form adipose within collagen scaffolds, it appears necessary to add both an angiogenic factor and adipose progenitors. Subcutaneous implantation of collagen sponges that contained human preadipocytes and FGF2-loaded gelatin microspheres generated adipose tissue within six weeks (279). Implants of collagen scaffolds that contained preadipocytes and free FGF2 also transformed into adipose, but to a lesser extent. The amount of adipose increased with number of preadipocytes, but not with FGF dose; in fact, high amounts of FGF (50 μg) caused local inflammation. Changing the FGF release rate by altering the degree of gelatin crosslinking did not affect the generation of adipose (278). When implanted in a rat fat pad, collagen scaffolds that contained FGF2-loaded gelatin microspheres were sufficient to generate adipose, presumably because the surrounding tissue served as an exogenous supply of preadipocytes (207). Intermediate FGF release rates yielded the greatest generation of adipose.
Controlled release of angiogenic factors has also been used to generate vascularized “bridges” to connect two tissues. For instance, implantation of an FGF1-loaded gelatin foam between two organs (e.g., liver and spleen) in the rat caused vascular ingrowth into the scaffold that connected the microcirculatory systems of the organs (580). Interposition of FGF1-loaded PTFE sponges (pre-coated with type I collagen) or fibrin glue between the thoracic aorta and ischemic myocardium in the rat induced vascular connections between the two tissues (141, 495). At extremely high doses (100-800 μg), however, FGF1 that was delivered from collagen or PTFE sponges did not induce vascular connections between the internal mammary artery and ischemic dog heart, and vascularization of the scaffolds was not observed (35). Instead, underlying vessels displayed smooth muscle hyperplasia that essentially obliterated the lumens of arterioles.
Intracranial delivery of VEGF- and heparin-releasing hyaluronan gels to a stroke lesion resulted in vascular and neuronal ingrowth that was accompanied by neurological improvement (414).
Arteriovenous bundles and loops
Flap prefabrication.
In the late 1970s, Erol discovered that overlaying a full-thickness skin graft onto an arteriovenous (AV) bundle of femoral vessels resulted in the generation of connections between the vascular system of the graft and the femoral vessels (136). After two weeks, the vascular connections were sufficiently numerous that the graft could be sustained purely by blood flow to the AV bundle. Subsequent work by Erol and Spira showed that a surgically constructed AV loop, such as a direct shunt or interpositional graft between femoral vessels, could also be used to generate new vascular growth that sustained skin grafts (Fig. 9) (137). These findings led to the development of reconstructive tissue flap “prefabrication”, in which a major vessel is mobilized to vascularize tissue that will eventually be transferred to another location in the body (186); a delay between implantation of the vessel and flap transfer is not absolutely required (618). Although flap prefabrication can be successful without the addition of angiogenic factors, these exogenous factors result in faster establishment of vascular connections between the flap tissue and the underlying pedicle (203). Addition of FGF2-containing heparin-Sepharose beads (in an alginate or ethylene-vinyl acetate binder) along a ligated vascular bundle enabled roughly half of the treated skin flaps to be perfused solely by the pedicle after one week; without FGF, the raised flaps were almost completely necrotic. Moreover, FGF treatment caused flaps to remain viable at four weeks even if their pedicles had thrombosed. Vascularization proceeded by angiogenesis from both the vascular bundle and existing vessels in the overlying skin. Only a small number of vascular connections was needed to maintain the viability of a raised flap. Similarly, instillation of TGFβ-containing collagen between skin and a vascular bundle accelerated the prefabrication of a skin flap that could be perfused solely by the pedicle (238); extensive vascular growth connected the pedicle and existing microvessels within the flap three days after delivery of growth factor. Introduction of PLGA microspheres that contained VEGF-A and/or FGF2 along an intramedullary vascular bundle in rat bone allotransplants caused increased capillary densities and faster bone formation; surprisingly, the combination of VEGF and FGF resulted in lower bone blood flow (314).
Figure 9.
Strategy for vascularization of grafts from a surgically constructed arteriovenous (AV) loop. Angiogenesis from the loop invades into overlying tissue. A, artery; V, vein. Reproduced with permission from (137).
Vascularized chambers.
In the above examples of flap prefabrication, angiogenesis occurs from both the implanted large vessels and surrounding tissue, and only the short distance between the two vascular systems needs to be bridged. When engineering vascularized tissue de novo, however, the vascular loop or bundle is the sole source of angiogenic growth into an initially avascular scaffold (656). Once vascularized, the scaffold can be left in place or transferred elsewhere by using the loop or bundle as an intact pedicle (574). Since angiogenesis must proceed throughout the scaffold to generate fully vascularized volume, this form of tissue generation is inherently slower than traditional flap prefabrication.
Comparison of different vessel configurations showed that vascular loops generated greater volumes of vascularized tissue than vascular bundles did (573). Although the underlying reasons are still unclear, it is likely that the extravasation of fibrinogen and blood cells at the suture holes that exist after construction of AV loops play a role in inducing angiogenesis. This possibility supports the earlier work of Vineberg, who found that wounding stimulated vascular growth from implanted arteries (614, 615). Ligated vascular bundles, however, generated smaller tissue volumes that were similar to those obtained with intact (“flow-through”) vessels. This latter finding suggests that the higher flows that result from shunting blood in AV loops may play a more important role than vascular injury in promoting angiogenesis. Indeed, thrombosis of ligated bundles eliminated their ability to generate new tissue (103). In vascular loops, new blood vessels formed from the adventitia of the femoral vessels and directly from the femoral vein, but not from the interpositional artery or vein graft (573). In ligated vascular bundles, capillaries sprouted mainly near the distal ends of the femoral vessels, again in accordance with Vineberg’s observations.
Regardless of the precise mechanism of angiogenesis, it is clear that the use of AV bundles and loops is a robust way to grow large volumes of vascularized tissue (Fig. 10). This strategy has been validated in rodents, large animals, and recently humans (393, 656); larger scaffolds resulted in greater final volumes of vascularized tissue (212). The type of tissue that results from an AV implant depends on the nature of the scaffold around the vessels. When an AV loop was implanted into a polycarbonate chamber without a scaffold, the chamber filled first with a provisional fibrin matrix that was then gradually infiltrated by fibrovascular tissue (349, 376). Similar fibrovascular growth was obtained when an AV loop was implanted in a PLGA scaffold (103). In contrast, implantation of an AV loop in Matrigel resulted in generation of adipose tissue. Unlike subcutaneous Matrigel implants [which require co-implantation of angiogenic factors to generate adipose (257)], inlaid AV loops caused Matrigel to be replaced by adipose in four weeks without any exogenous growth factors (103). AV bundles were much less effective in generating adipose, perhaps because vascular bundles are less angiogenic than loops; here, addition of FGF2 helped to increase the adipose volume fraction (622). FGF2, mouse VEGF-A120, and rat PDGF-BB synergistically increased adipogenesis by six weeks (471). Matrigel is not unique in its ability to be transformed into adipose by an AV bundle and slow-release FGF2, as type I collagen showed similar results (610). An exhaustive study of scaffolds and angiogenic factors showed that vascular and adipocyte volume fractions were poorly correlated (586). For some types of scaffolds (e.g., a matrix gel that was derived from skeletal muscle), the delivery of FGF, VEGF, and PDGF to the AV bundle resulted in decreased vascularization, compared to growth factor-free controls.
Figure 10.
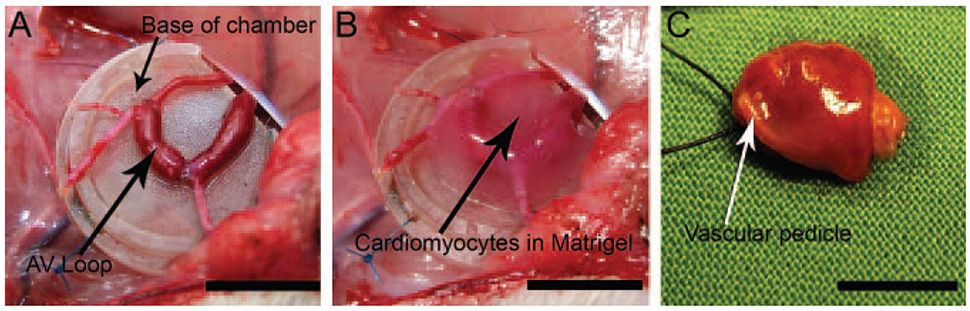
Generation of centimeter-scale tissue with an arteriovenous (AV) loop in a polycarbonate chamber. (A) AV loop overlaid on a polycarbonate base. (B) Addition of rat cardiomyocytes and Matrigel around the AV loop. (C) Explant of pedicled myocardium that formed by four weeks. Scale bar refers to 1 cm. Reproduced with permission from (394).
Because these experiments typically encased the scaffold within an impermable silicone or polymer chamber that only had holes for placement of the vascular pedicle, it was unclear where the adipose progenitors originated from. At first, local activation of preadipocytes was the favored mechanism; the existence of blood-borne adipose progenitors seemed unlikely. The loose perivascular adipose that surrounds the artery and vein in an AV bundle or loop may provide one source of progenitors; pericytes, such as those within the vasa vasorum, may be another source (140). It is also possible to provide adipose progenitors simply by implanting a pedicled fat flap into a rigid porous polymer chamber (119, 145, 393), or by implanting a free fat autograft with the AV bundle and FGF2-loaded Matrigel (262). The degree of vascularization was largely indifferent to the different chamber configurations (e.g., sealed or open, with or without fat graft), but adipogenesis was never observed when the pedicle was thrombosed (262). Angiogenesis was necessary, but not sufficient, for adipogenesis.
Addition of adipose-derived stem cells (ASCs) to the scaffold can help ensure that progenitors are well-distributed and ready to respond to vascular ingrowth. In a collagen-chitosan scaffold with a femoral AV bundle, addition of VEGF-loaded microspheres increased vascular density by two weeks, and ASCs increased the generation of adipose after four weeks (674). Just because ASCs led to adipogenesis, however, does not guarantee that the adipose was derived from those cells; in fact, it appears that most of the vascular and adipose growth was host- rather than graft-derived (549).
To date, a pilot study in humans has shown the feasibility of generating >200 cm3 of vascularized adipose tissue using AV bundles, but the success rate was low (393). The patient population (women who had undergone mastectomy several months to years prior) may have selected for co-morbidities or local tissue scarring that inhibited adipose growth.
Surprisingly, co-implantation of skeletal muscle with an AV loop also resulted in generation of adipose tissue (375). Implantation of non-viable, freeze-thawed muscle or muscle extract led to the same result. Only the implantation of cultured myoblasts avoided adipogenesis in the AV loop-containing chamber; in this case, skeletal muscle fibers were obtained. The abundance of basement membrane in skeletal muscle (living and non-viable), Matrigel, and muscle extract, and its absence from cultured cells, are consistent with the idea that all that is needed to form vascularized adipose is an AV loop and basement membrane. It is intriguing that adipose tissue appears to be the default outcome in both subcutaneous (257) and chamber-based implants of Matrigel; influx of macrophages is critical for this result (116). Implantation of rat cardiomyocytes and Matrigel with an AV loop generated spontaneously contracting heart tissue after several weeks (394).
Engineering bone with an AV loop or bundle has required the addition of separate signals for angiogenesis and osteogenesis (5). Subcutaneous insertion of an osteoconductive porous hydroxyapatite scaffold in rats led only to the ingrowth of fibrovascular tissue. Insertion of hydroxyapatite or bone-derived scaffold that contained a channel inlaid with a ligated epigastric bundle or a femoral AV loop resulted in vascularization throughout the scaffold, but no bone formation (5, 285). Incorporation of BMP2 into the scaffold promoted deposition of bone matrix; the effect of BMP2 addition was greatly enhanced in scaffolds that were vascularized by an implanted bundle (5). Surprisingly, the addition of FGF2 and BMP2 did not yield greater bone formation than BMP2 alone; while FGF release did increase vascular growth from the implanted epigastric vessels and osteoid deposition in the scaffold pores, it caused lower bone formation at four weeks (407, 408). When osteoblasts were injected into bone-derived scaffolds six weeks after the scaffolds had been implanted with or without an AV loop, cell viability was much higher in the samples that were prevascularized with the vascular loop; nevertheless, bone formation was rare in both types of implants (18). These studies suggested that vascular growth can compete with osteogenic cells for space within the scaffold pores. Thus, while angiogenesis is required for engineering bone, it is not always a positive factor; healing critical-sized bone defects with AV loop-containing scaffolds remains a challenge (19).
AV bundles have also been used to revascularize freeze-thawed (nonviable) bone allotransplants (634, 635), although the results are not as striking as those seen with viable allotransplants (314). Outgrowth from rat saphenous vessels that were placed in an intramedullary position in frozen bone allografts generated capillaries into the bone. The addition of PLGA microspheres that contained VEGF-A and FGF2 seemed to result in higher capillary densities, compared to PLGA alone. Sustained release of VEGF, with or without FGF, increased bone formation and blood flow.
Angiogenesis in microphysiological systems
Recent advances in materials engineering have enabled the development of microfluidic devices that contain microscale vascularized gels (641). These devices are commonly based on “soft lithography”, a collection of techniques that use crosslinked silicone elastomers to pattern biological materials (649). Selective delivery of angiogenic factors into different compartments of a device can expose ECs on the surface of a gel to growth factor gradients that promote angiogenesis (Fig. 11) (92). In many implementations, fibroblasts are placed in a nearby compartment and serve as the source of growth factors (277). Once ECs have invaded the gel (most commonly, fibrin) and reached its opposite end, flow can be established through the newly formed vessels. Delivery of solutes through the lumens has shown that the permeability of such vessels is similar to that of venules ex vivo.
Figure 11.
VEGF-driven angiogenesis into collagen gels in microfluidic devices. Treatment of devices with poly-D-lysine (PDL) enabled ECs to sprout from a monolayer towards a VEGF source. Arrows denote the direction of VEGF transport. Scale bars refer to 100 μm. EGM2mv, endothelial cell growth media. Reproduced with permission from (92).
Because microfluidic devices afford tight control over perfusion pressures and flows, such devices have been used to elucidate how mechanical stress affects angiogenesis. Negative trans-endothelial pressures, in which the basal pressure exceeds apical pressure, promote EC sprouting (276, 612). Shear stresses above ~10 dyn/cm2 also appear to promote angiogenesis (160), although contrary results have been reported (539). Increases in shear stress are often accompanied by a positive trans-endothelial pressure, which would attenuate the angiogenic response; indeed, when trans-endothelial pressures were held constant and close to zero, increased shear stress promoted sprouting (449). Elevated EC junctional shear stress may mediate the angiogenic effects of a negative trans-endothelial pressure (160).
Aside from investigation of the cell biology of angiogenesis, these devices can be used to study angiogenic vascularization of cell spheroids and other types of tissues (409).
Physical signals in angiogenic vascularization
Although the delivery of growth factors and their plasmids is by far the most popular strategy for angiogenic vascularization, it is also possible to use physical signals to induce angiogenesis in vivo. These signals include solid and fluid stresses, such as those imparted by tissue deformation and blood flow, and the stiffness of an implanted scaffold. Using such signals to control vascular growth is not as well-developed as methods based on biochemical factors, and how to best manipulate the physical environment to obtain vascular growth remains to be determined.
Over a century ago, Thoma recognized that increased blood flow led to generation of vascular networks (579). For instance, chronic vasodilation increases vascular densities in heart and skeletal muscle (682). Elevated shear stress is partly responsible for transducing the effects of increased flow into angiogenesis in vivo (222-224). Endothelial production of nitric oxide mediates this effect (41). In fact, the arteriolar growth that is promoted by VEGF gene therapy appears to result mainly from flow-mediated stimulation of upstream vessels, rather than from a direct effect of VEGF (466). Flow-mediated increase in VEGF expression may also partly account for the vascular growth (380).
In subcutaneous implants of collagen scaffolds, the angiogenic effect of scaffold crosslinking was found to be at least as strong as that of VEGF delivery (654). Caution is warranted in interpreting this result, however, as crosslinking also increased the scaffold pore size. A different mode of vascularization has been described when a mechanically compliant scaffold contracts, such as during wound healing; here, vessels are “pulled” by the retracting scaffold to fill the residual volume (269).
VASCULOGENIC VASCULARIZATION
Vasculogenic vascularization refers to the development of microvessels through the self-organization of individual ECs or progenitors. In contrast to angiogenesis, in which vessels invade a graft, the vessels that form in vasculogenesis develop within the graft; this type of vascularization is considered “intrinsic” to the graft. In reality, the distinction between the two processes is not so clear-cut, and vessels that form by vasculogenesis can subsequently undergo angiogenic sprouting as well. Although strictly speaking not vasculogenesis per se, the development of vascular networks from suspensions of microvascular fragments or preaggregated ECs (e.g., spheroids) is also a form of intrinsic vascularization and is thus discussed in this section.
Summary of developmental vasculogenesis
Formation of the embryonic blood vascular system begins when hemangioblasts, the common precursor to blood cells and ECs, organize into “blood islands” in the yolk sac by embryonic day 7 (389). Hemangioblasts are contained in the Brachyury-expressing VEGFR-2+ cell population in the mouse and human, and differentiate into hematopoietic stem cells and angioblasts, the true precursor to ECs (88, 142, 221, 264). Angioblasts, which share markers with hemangioblasts and are VEGFR-2+VE-cadherin+ cells (415, 651), organize into interconnected solid cords that subsequently undergo tubulogenesis to form the first vascular network by embryonic day 9 in the mouse (124, 389). Further growth of the vascular system proceeds by angiogenesis.
In contrast to the blood vascular system, the lymphatic system does not form from angioblast-like cells. Instead, lymphatic sacs form by budding of Prox1-expressing cells from the venous system on embryonic day 10-11 (633). These sacs then extend and grow by lymphangiogenesis (477, 478).
Therapeutic vasculogenesis
The challenge in harnessing developmental vasculogenesis for treatment of tissue ischemia is that true angioblasts are known to exist only in the early embryo and in small quantities, compared to the numbers anticipated for therapeutic benefit in the adult. As a result, essentially all studies of therapeutic vasculogenesis have examined to what extent readily available adult-derived ECs or progenitors can substitute for embryonic angioblasts. Early work focused on differentiated ECs, but it was quickly realized that blood- and bone marrow-derived endothelial progenitors held much greater promise. Because vasculogenic cells can both form new vasculature and secrete angiogenic factors, one of the main issues when interpreting studies of therapeutic vasculogenesis is the relative contributions of vasculogenesis versus angiogenesis to the observed vascular growth.
Differentiated endothelial cells
Injection of allogeneic or syngeneic aortic ECs into the cryo-injured rat myocardium resulted in modest levels of vascularization within the scar (270). Only cells that were grafted two weeks after injury were effective; when grafted earlier, ECs did not persist at the scar and were likely cleared by ongoing inflammation. When ECs were labeled with BrdU before injection, some vessels in the scar were found to contain labeled cells. VEGF levels within the scar were the same whether ECs or culture media was injected. These findings implied that the increase in vascular density resulted partly from vasculogenesis, but not from VEGF-induced angiogenesis. It is important to recognize, however, that the absolute vascular densities obtained with EC injection (~15 vessels/mm2) remained orders of magnitude lower than those in native myocardium. Other studies have found no increase in vascular densities from EC injection beyond those of controls (226, 252). When injected into ischemic hindlimb, ECs that were differentiated induced pluripotent stem cells (iPSCs) or reprogrammed from fibroblasts with defined factors increased blood flow and vascular densities, but with only <10% of injected ECs persisting at the injection site (189, 475); rescue occurred in roughly half of injected limbs (189, 446). Given the modest levels of vascularization and the sensitivity of EC suspensions to lack of matrix adhesion and blood flow, the injection of EC suspensions to treat ischemic tissues is currently out of favor; instead, these cells have been much more successfully applied when introduced within a scaffold for engineering vascularized tissues.
Endothelial progenitor cells
In 1997, Isner and co-workers reported that semi-purified CD34+ or VEGFR-2+ cells from human peripheral blood could differentiate on fibronectin-coated dishes into cells that resembled ECs in their molecular footprint [e.g., positive for VEGFR-2, CD31, and endothelial nitric oxide synthase (eNOS)] (23). In contrast with true ECs, however, these “adult angioblasts” or endothelial progenitor cells (EPCs) were round or spindly, did not form cobblestone monolayers in culture, and expressed the hematopoietic stem cell marker AC133 (Fig. 12A) (23, 431). EPCs grew as colonies that surrounded residual CD34−CD31+ T cells (225). When injected intravenously, lacZ-expressing or DiI-labeled EPCs homed to ischemic hindlimbs in mice and rabbits (23). Remarkably, these EPCs incorporated into capillaries, expressed CD31, and became histologically indistinguishable from ECs. Transfection with VEGF-A rendered EPCs even more effective in increasing vascular density and in restoring blood flow to ischemic tissue (235). Nevertheless, homing of EPCs is extremely inefficient: only ~3% of intravenously or intraventricularly injected cells deposited in the heart, even in the presence of ischemic injury; the vast majority of EPCs accumulated in the spleen (1).
Figure 12.

Derivation and characterization of EPCs. (A) Spindle-shaped EPCs that were derived from one-week-old cultures of CD34-enriched peripheral blood-derived mononuclear cells. Reproduced with permission from (23). (B) Flow cytometry of EPCs and monocytes. EPCs express blood cell markers, including CD45 and the monocyte activation marker CD11c. Adapted with permission from (459).
Although EPCs are mobilized into the blood after myocardial infarction and other ischemic episodes (515), the quantities are insufficient to restore the vascular function of the affected tissues, and methods to increase the level of mobilization are needed. Given the common embryonic origin of the hematopoietic and vascular systems, treatments that mobilize hematopoietic stem cells and/or progenitors into the circulation should have similar effects on EPCs. Indeed, administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) for one week resulted in increased counts of circulating EPCs in the rabbit (566). Induction of hindlimb ischemia led to a further increase in EPC mobilization and subsequent homing and differentiation into ECs in the ischemic tissue. Similar results were obtained with mobilization of CD34+ human EPCs by granulocyte colony-stimulating factor (G-CSF) and their homing and differentiation in the ischemic rat myocardium (290). The ability of EPCs to be mobilized by such factors implies these cells normally reside in bone marrow. Cord blood, which is a rich source of hematopoietic stem cells, is enriched in EPCs (401). Liver and intestine also appear to be a source of EPCs (3).
VEGF-A can mobilize EPCs into the blood and cause them to home to a distant organ (24, 250). This mobilization requires activity of eNOS and the resulting induction of the protease MMP-9 in bone marrow (2); similar mechanisms apply to mobilization of EPCs by estradiol (236). In skin wounds in the diabetic mouse, topical VEGF-A165 resulted in a 3- to 7-fold increase in EPCs in circulation (159). The EPCs integrated into newly formed vessels both in the treated wound and in adjacent ones that were not treated with VEGF. Thus, VEGF-mobilized EPCs provided a systemic benefit to wound healing that enhanced the angiogenic effects of local VEGF application. This result also implied that previous work in VEGF-induced therapeutic angiogenesis—and by extension, any angiogenic strategy based on mimicking hypoxia—should be re-evaluated for possible contributions from circulating EPCs.
Once mobilized, EPCs home to ischemic tissues using the same molecular signals that facilitate homing of leukocytes and other blood-borne cells to activated vessels during inflammation. EPCs bind to vascular surface VCAM-1, ICAM-1, and selectins with the ligands VLA-4 (α4β1 integrin), CD18 (β2 integrin), and PSGL-1, respectively (77, 245, 607, 661). Endogenous plasma and tissue levels of the chemokine SDF-1 were maximal six hours and three days after induction of ischemia, respectively, while levels in bone marrow reached a minimum after one day (115); these changes are consistent with SDF-mediated mobilization of EPCs from bone marrow to blood to ischemic tissue. Indeed, homing of EPCs to VEGF-releasing or ischemic tissues required expression of SDF-1 in the local tissue (74, 183). Intramuscular injection of SDF-1 into the ischemic mouse hindlimb increased the numbers of EPCs that incorporated into the muscle vasculature; of note, SDF-1 also increased VEGF-A expression in the ischemic tissue, which implies a positive feedback loop between the two signals (650). Controlled release of SDF-1 and VEGF from intramuscular injection of alginate gel into ischemic hindlimb promoted homing of EPCs to the same site, with subsequent increase in vascularization and recruitment of myeloid cells (12). Release of SDF-1 from a polymer chamber that contained an arteriovenous loop also resulted in homing of EPCs, but no increase in vascularization, perhaps because the loop-containing chamber is itself already highly angiogenic (529). Platelets that adhere to endothelium at sites of injury provide another source of SDF-1 to recruit EPCs (545). The proliferation rate of recruited EPCs appears to correlate with the degree of ischemia (576). Angiogenic therapies, such as transfection with COMP-Ang1, can indirectly upregulate SDF-1 and thus concentrate EPCs locally (664).
For clinical applications, mobilization of EPCs by cytokines may be insufficient, and methods to increase the concentration of EPCs even further may help. Human EPCs can be expanded in culture media that was originally designed for human microvascular ECs; this media contains a mixture of angiogenic and other growth factors, including VEGF, FGF2, IGF1, and epidermal growth factor (EGF) (251). Under optimized culture conditions, each milliliter of peripheral blood yields ~5000 EPCs that can be grown to yield ~35000 EPCs for injection. Expanded human EPCs homed to ischemic rat myocardium and incorporated into vessels there (258). Alternatively, EPCs can be directly injected at the ischemic site in large numbers. For instance, injection of CD34+ EPCs into a raised skin flap resulted in greater vascularization and flap survival, compared to injection of saline or ECs; EPCs, but not ECs, incorporated into capillaries (303). Intramyocardial injection of CD31+ autologous pig EPCs or CD34+ human EPCs into ischemic pig or rat myocardium, respectively, preserved heart function and generated EPC-incorporated capillaries (261). Surprisingly, intracardiac injection of cultured EPCs led to greater vascularization in the ischemic mouse hindlimb than injection of freshly isolated EPCs did, which suggests that other cells that co-purified in the initial isolation may hinder the vascularization process (251). Perhaps most importantly, injection of cultured EPCs restored the capillary density of the ischemic limb to that of the normal one (~500 vessel/mm2). EPCs can also be introduced by encapsulating them into an alginate implant that gradually releases VEGF and thereby promotes outward migration of cells into the surrounding ischemic tissue (523).
Endothelial outgrowth cells
CD34+ cells from peripheral blood contain at least two distinct populations that can differentiate into ECs (226). Culture on fibronectin-coated dishes yields spindle-shaped EPCs, as discussed above. Culture of cells that did not adhere by the first day and that were subsequently plated onto fibronectin/gelatin-coated dishes eventually yielded cobblestone monolayers that expressed typical EC markers (Fig. 13, left) (510). These endothelial “outgrowth” cells (EOCs) were morphologically indistinguishable from ECs, particularly those derived from large vessels. Like ECs and unlike EPCs, EOCs did not express the stem cell marker AC133 (398). Other methods for obtaining EOCs from peripheral blood have been developed, the simplest being to culture blood-derived mononuclear cells in endothelial growth media for several weeks (226). Intraventricular injection of EOCs and EPCs led to their incorporation into microvessels and equivalent levels of vascularization in the ischemic mouse hindlimb (~500 vessels/mm2); in contrast, injection of ECs or culture media led to much lower capillary densities. Moreover, EOCs and EPCs improved perfusion ratios and limb salvage by equivalent amounts, and their combination was synergistic (226, 662). Given the morphological and biochemical similarity between EOCs and ECs, it is surprising that EOCs induce such robust vascularization.
Figure 13.

Derivation and characterization of EOCs. (Left) Colony of cord blood-derived EOCs that emerged after mononuclear cells were cultured for nine days. (Right) Flow cytometry of EOCs. EOCs express EC markers, such as CD31 and CD144 (VE-cadherin), but do not express markers of blood cells, such as CD45 and the macrophage/monocyte marker CD14. Adapted with permission from (232).
The relationship, if any, between EOCs and EPCs remains unclear. Mononuclear blood cell cultures invariably yield EPCs in the first days, followed by EOCs over several weeks; this temporal sequence suggests that EOCs may derive from rare EPCs that lose their spindle shape and switch to EC-like spreading. In support of this view, DiI-labeled EPC cultures eventually yielded faintly labeled EOCs (226). On the other hand, the ability of EOCs to be derived from initially non-adherent blood cells and the expression of monocyte/macrophage markers and secretion of angiogenic factors by many EPCs and not by EOCs suggest that EPCs and EOCs do not share a common lineage (Fig. 12B and 13, right) (185, 459, 510, 604, 662). Although EPCs and macrophages share common surface markers, EPCs are not true macrophages, which display a much weaker vasculogenic potential (604). Further confusing the issue are that EPCs can also be derived from initially non-adherent cultures (660), and that “EPCs” is sometimes used to refer to cultures that display the cobblestone growth of EOCs (149, 372, 591). Moreover, a third source of vasculogenic cells—circulating ECs—exist in the blood and most likely originate by sloughing off from vascular walls (344). Even today, EPCs and EOCs are distinguished mainly by morphology and culture time. Until precise molecular-level definitions [akin to the clonogenic identification of hematopoietic stem cells (517)] become accepted, the relation between EPCs and EOCs will remain unclear.
Regardless of the true origin of EOCs (including the possibility that they are an artifact of in vitro cell culture), these cells have been isolated from many sources besides peripheral blood. As with hematopoietic stem cells, cord blood is a particularly rich source of EOCs (232).
Bone marrow
Arguably the most unsettled issue in therapeutic vasculogenesis is whether unpurified bone marrow (BM) can serve as an equivalent substitute for EPCs. Experiments with genetically labeled BM transplants showed that BM-derived endothelial progenitors incorporated into capillaries in mouse skin wounds and ischemic myocardium and expressed VEGFR-2 and Tie2 (Fig. 14) (22). These findings were consistent with the increased vascularization found after intramyocardial, intramuscular, or subcutaneous injection of autologous BM in animal models (122, 123, 230, 587). In rat myocardial scar and in mouse skin flaps, injected BM cells contributed modestly to new endothelium (228, 587). Intramyocardial injection of BM mononuclear cells or stromal cells into the ischemic pig heart resulted in their incorporation into 10-30% of vessels (252, 588). Intramuscular injection of BM-derived mononuclear cells into the ischemic rabbit hindlimb led to incorporation into vascular networks and increased angiographic scores, blood pressure ratios, and blood flow (516). In contrast, injection of bone marrow-derived fibroblasts had no effects beyond those of saline controls. When injected intramuscularly, BM-derived stromal cells contributed to both endothelial and smooth muscle layers in the ischemic rat hindlimb (6). Angiogenic gene therapy synergized with BM mononuclear cells to improve vascularization (260, 288).
Figure 14.
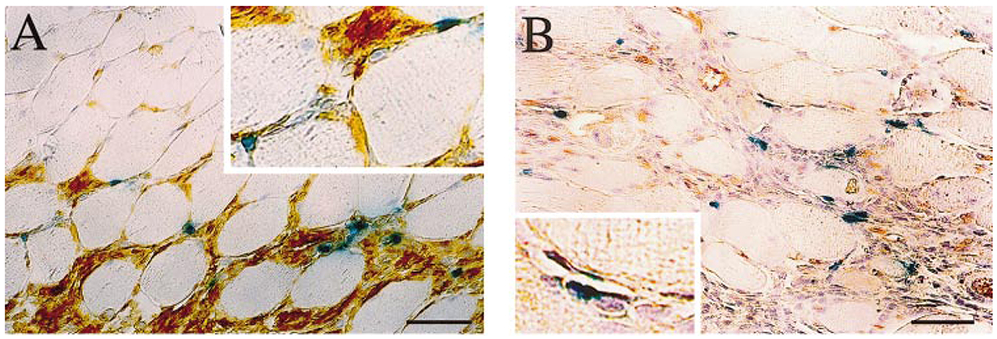
Incorporation of bone marrow-derived, lacZ-expressing EPCs at sites of ischemia. Sections were co-stained by X-gal (blue) and for lectin (A) and CD31 (B). Scale bar refers to 25 μm. Reproduced with permission from (22).
If the effects of BM injection result mainly from the EPC sub-population, then injection of EPCs alone or a greater number of BM cells that contain an equivalent number of EPCs should yield similar results. Experiments in the ischemic rat heart, however, showed that intramyocardial injection of human CD34+ EPCs increased capillary densities and decreased cardiac fibrosis, while BM mononuclear cells that contained the same number of EPCs did not (259). In fact, BM injection resulted in hemorrhagic infarction. Human ECs were present in the myocardium after injection of EPCs, but much less so after injection of unpurified BM. Given that <2% of BM consisted of CD34+ cells, the BM injection introduced a large excess of leukocytes that may have precipitated or exacerbated the hemorrhage. Indeed, injection of autologous BM in the ischemic rat myocardium greatly increased the tissue concentration of inflammatory cytokines IL-1β and CINC (289). Still, these results are difficult to reconcile with studies in which no adverse effects of local BM injection were noted.
Whether BM cells contribute to endothelium has also come into question. The initial studies were mirrored by several spectacular claims in the stem cell literature of BM transdifferentiation into cardiomyocytes, hepatocytes, neurons, skeletal myotubes, and many other non-hematopoietic cell types (9, 58, 144, 301, 422). Such claims were often based on detection of lacZ or GFP expression that could only have originated from graft-derived cells. The scrutiny that such claims engendered led to several counterclaims, in which the contribution of BM to non-hematopoietic tissues was declared to be minimal (73, 620). Several studies specifically examined the contribution of BM to endothelium and found it to be essentially nil (104, 179, 452, 685). BM-derived cells were found instead in a perivascular location and contributed to pericytes and tissue leukocytes (244, 452, 685). Vigorous debate about potential strengths and flaws of these studies even led some authors to question the correctness of the original series of EPC studies by Isner and co-workers (265). Vascular injury by the irradiation used in BM transplants appears to prime tissues for vasculogenesis from BM-derived cells (532).
Confounding the issue is the ability of BM cells—particularly, mesenchymal stem cells (MSCs)—to secrete angiogenic and vasculogenic factors. Compared to ECs, MSCs produce orders-of-magnitude higher amounts of VEGF-A, FGF2, PlGF, and the arteriogenic factor MCP-1 (281, 282); MSCs also secrete large amounts of SDF-1 (239). Injection of GFP- or lacZ-expressing MSCs into the ischemic mouse hindlimb improved blood flow and limb function, but did not result in colocalization of GFP or lacZ with vessels (282). Injection of MSC-conditioned media had similar effects, which implies that any direct contribution of MSCs to vessels is redundant (281). Indeed, intramyocardial injection of MSCs with or without EPCs did not lead to incorporation of MSCs into vascular endothelium (555), although contrary results have been reported (112, 526). BM-derived cells rescued a defect in PDGF-BB expression and vascularization in mouse heart grafts (126). Mononuclear cells secreted several angiogenic factors and inflammatory cytokines, including FGF2, VEGF, Ang1, IL-1β, and TNF-α, and comprised ~10% of macrophages after injection into ischemic pig heart (252). Subcutaneous injection of BM cells or MSCs restored lymphatic continuity in a mouse lymphedema model, but again, whether these cells contributed directly to endothelium is unclear (98, 513). Some studies have suggested that macrophages may transdifferentiate into lymphatic ECs in vivo at sites of injury (266, 364, 513).
Another issue is that some cell types seem able to form structures that resemble vessels and conduct blood but are not comprised of ECs, a process known as “vascular mimicry” (Fig. 15) (202, 358). Originally described in melanoma, vascular mimicry would enable a functional vasculogenesis that could increase blood supply without actually increasing the density of EC-lined vessels. As one might expect, the existence of such non-endothelial vasculogenesis is highly controversial (53, 201, 369).
Figure 15.

Vascular mimicry in uveal melanoma. Red blood cells (top, arrowheads) fill vessel-like structures that do not appear to be lined by ECs. Reproduced with permission from (358).
Other sources
Adipose-derived stem cells (ADSCs) have also been examined for their vasculogenic potential. In principle, these cells are easier to obtain than the BM-derived MSCs and display near-identical differentiability as MSCs do (689). Cells from cultured mouse adipose stromal-vascular fraction (SVF), a population that is enriched in ADSCs (but also contains ECs), enhanced vascularization to the same degree as BM-derived mononuclear cells did (444). Only SVF cells from white adipose tissue were effective; SVF cells from brown adipose were equivalent to saline controls. Cultured human SVF cells and dedifferentiated human adipocytes also increased vascular density and flow in the ischemic mouse hindlimb and incorporated into vessels (70, 444). As with BM, whether the vascularization potential of ADSCs or SVF cells is mainly the result of vasculogenesis or angiogenesis remains unclear. ADSCs secrete several angiogenic factors, including VEGF, HGF, TGFβ, and the lymphangiogenic factor VEGF-C (460, 513). Compared to MSCs and mature adipocytes, ADSCs secreted more SDF-1, which helped recruit EPCs to the ischemic rat heart and mouse hindlimb (229, 296). Half of injected ADSCs were already apoptotic by day 3, which would argue against a persistent vasculogenic benefit (229). In skin wounds, ADSCs synergized with platelet-rich plasma to induce vascularization, but resided only in a perivascular location (54). Subcutaneous injection of ADSCs reduced mouse tail lymphedema, but resulted in rare incorporation of ADSCs into regenerated lymphatics (513). On the other hand, injection of freshly harvested CD34+CD31− human ADSCs into the ischemic mouse hindlimb increased capillary densities, with ~8% of vessels showing endothelial incorporation of human cells (384). At bone fractures, ADSCs incorporated into vessels at early but not late stages of healing (518).
It has been claimed that blood and BM also contain circulating progenitors for SMCs (321, 482, 511, 531), although dissenting views certainly exist (489). Intravenous, intraventricular, or intramyocardial injection of human peripheral blood-derived CD34+ cells resulted in contribution of the cells to vascular smooth muscle in the ischemic rodent myocardium (237, 659). Cord blood-derived mononuclear cells differentiated into SMCs in vitro, and intravenous co-injection of SMCs and EOCs resulted in synergistic vascularization of ischemic hindlimb, in part via Ang1/Tie2 signaling; strangely, the injected SMCs were not observed to have incorporated into the vasculature (149). Although vasculogenic therapies do not require injected cells to contribute to the smooth muscle lineage, the ability of some cell populations to serve as smooth muscle progenitors (or as bipotent progenitors of both ECs and SMCs) may aid in the generation of mature vascular networks.
Clinical trials
If the 1990s can be considered the heyday of clinical trials for angiogenic therapies, then the 2000s holds the same status for trials of vasculogenesis (458). The association of higher EPC counts with spontaneous myocardial salvage in patients supported the idea that exogenous increases in EPCs could help repair ischemic tissue (418). As with angiogenesis, Phase I/II trials of vasculogenic therapies with EPCs, BM-derived mononuclear cells, AC133+ BM cells, and other cell populations showed safety of the approach and hints of clinical efficacy (543, 594). Initial enthusiasm was tempered by the inconsistent findings of angiogenic clinical trials; as Laham and Oettgen aptly stated, “[w]herever this field takes us, it is likely to follow the well-known pathway of incredible results in the setting of unrealistic expectations followed by disappointments and cautious optimism” (309). This statement proved to be prophetic, as randomized controlled trials of vasculogenic therapies have shown modest benefit over placebo [reviewed in (463) for critical limb ischemia].
The same sources of discrepancy between preclinical and clinical results for angiogenic therapies also hold for vasculogenic therapies. For instance, in contrast to animal studies, human trials use recipients with one or more comorbidities. The quantity and VEGF-induced migration of circulating EPCs in humans is inversely correlated with the number of risk factors for coronary arterial disease (206, 609) and the likelihood of future cardiovascular events (497, 628). Although the effects of these deficits may be minimized by collection, concentration, and direct injection of EPCs into the ischemic tissue, EPCs from patients in clinical trials are more senescent and are functionally impaired in their response to local growth factors (194, 206, 609). Old age, a major risk factor, impaired both the vasculogenic potency of donor BM-derived stromal cells and the responsiveness of the recipient, in a rat model of hindlimb ischemia (681). Notably, an old recipient of old cells (akin to the clinical situation) responded equivalently as a young recipient that was injected with culture medium only. In heart grafts into old rats, only BM from young rats was able to rescue aging-impaired angiogenesis and to incorporate into vessels (126). A second source of discrepancy is that clinical endpoints do not rely on vascularization per se, but rather than on improvements in blood flow and limb or heart function. In fact, it has been claimed any functional benefit of injected cells in the ischemic heart can largely be attributed to softening of the myocardium, rather than by any specific vasculogenic effect (50). As with angiogenic therapies, placebo effects are surprisingly strong, which make detection of a true clinical effect difficult.
Long-term side-effects of vasculogenic therapies are unknown. As seen with angiogenic factors, BM-derived cells promote vascularization and atherogenesis, in part through the induction of inflammatory mediators such as MCP-1 (135). Given the ability of BM-derived cells (particularly EPCs) to promote vasculogenesis within tumors, vasculogenic therapy may induce tumor progression (355). Currently, use of autologous cells that are processed on-site and then re-injected does not require clinical approval. No therapies with cultured autologous cells for therapeutic vascularization have been approved.
Vasculogenesis to engineer vascularized tissues
In the early 1980s, Montesano and co-workers showed that a monolayer of cultured bovine capillary ECs on a type I collagen gel could spontaneously reorganize to form tubes when overlaid by a second collagen gel (387). Later work found that initially dispersed ECs reassembled into “networks” on Matrigel and on laminin-supplemented collagen gels (304). Thin sections showed the presence of open lumens within the tubes, but evidence of fully interconnected networks was scant. Nevertheless, these studies led to the hope that ECs could be coaxed to undergo vasculogenesis if provided with an appropriate scaffold to reorganize on top of or within. In principle, incorporation of parenchymal cells and ECs within a scaffold could lead to generation of microvessels that enable perfusion of the developing construct. In contrast to angiogenesis, which starts at the surface of a scaffold and then invades deeper, vasculogenesis could result in synchronous vascularization throughout the scaffold and thereby decrease the ischemic time at the center of the tissue.
As with therapeutic vasculogenesis, many different types of ECs and progenitor cells have been studied for their ability to undergo vasculogenesis in scaffolds. In contrast to the use of spindle-shaped EPCs in therapeutic vasculogenesis, however, definitive ECs—isolated from existing vessels or differentiated from rare progenitors and grown as cobblestone monolayers—have been the main cell source when promoting vasculogenesis in engineered tissues.
Prevascularization of scaffolds by vasculogenesis
ECs and EOCs.
Embedding differentiated ECs into a collagen or Matrigel scaffold rapidly leads to apoptosis, and even the tubes that do form regress within a few days (494). ECs require survival signals from cell spreading, which is restricted within typical scaffolds of sub-micrometer pore size (78). Nevertheless, subcutaneous implantation of human umbilical vein EC (HUVEC)-containing collagen-fibronectin gels in the mouse yielded perfused, thin-walled microvessels after one month (Fig. 16A) (494). Virtually all the vessels were derived from HUVECs and not from the recipient mouse. Moreover, only gels that contained aggregated “cords” of ECs (the precursors to tubes) at the time of implant yielded perfused vessels. Transfection of ECs with a caspase-resistant Bcl-2 mutant before embedding in a collagen-fibronectin gel protected the ECs from apoptosis and increased vascular density nearly five-fold after implantation, compared to control transfection. These vessels became invested by a mouse-derived mural coat after one month, and matured to form hierarchical microvascular networks by two months (Fig. 16B, C) (130).
Figure 16.

Fine structure of vessels that formed from normal and Bcl2-expressing HUVECs in collagen-fibronectin gels one month after implantation. (A) Implants of normal HUVECs. (B, C) Implants of Bcl2-expressing HUVECs. The lumens are perfused with red blood cells (RBC); asterisks denote mural cells. Reproduced with permission from (494).
These findings served as a basic template for subsequent studies that varied the type of ECs, the method used to inhibit EC apoptosis, the scaffold composition, and the degree of in vitro culture (a form of “prevascularization”) before implantation. Since HUVECs are unlikely to be clinically obtainable for most patients, autologous ECs have been examined for vasculogenic potential in scaffolds. Adult ECs exhibit limited proliferation and resistance to apoptosis, and most attention has focused instead on ECs that are differentiated from progenitor cells in vitro. In particular, endothelial outgrowth cells (EOCs, also known as “endothelial colony-forming cells” or ECFCs) from bone marrow, peripheral blood, or cord blood have shown considerable promise in generating vascularized tissues (28, 660). Subcutaneous implants of collagen-fibronectin gels that contained human EOCs yielded human EC-lined vessels by four weeks; notably, similar implants with human EPCs showed virtually no vascularization (660). When co-implanted with 10T1/2 fibroblasts, cord blood-derived EOCs or iPSC-derived ECs organized into much longer-lived vessels than peripheral blood-derived EOCs did (28, 293, 486). When co-implanted with human SMCs in Matrigel, early-passage cord blood-derived EOCs yielded larger vascular densities (~100/mm2) than late-passage cord blood-derived EOCs, early-passage peripheral blood-derived EOCs, and microvascular ECs did (10-20/mm2); as expected, higher EOC concentrations resulted in higher vessel densities (372). Generation of perfused vessels in Matrigel occurred mainly between day 3 and 5 (255). Vascularization of Matrigel or gelatin implants with cord blood-derived EOCs and SMCs or MSCs required early infiltration of the scaffold by myeloid cells, particularly neutrophils (343, 371). Neutrophil recruitment and vascularization were more effective when EOCs and MSCs had yet to assemble into vascular networks before implantation (343). Ranked, the vasculogenic potentials of different ECs are: early-passage cord blood-derived EOCs > HUVECs > late-passage cord blood-derived EOCs ~ peripheral blood-derived EOCs ~ microvascular ECs (83, 372).
Lymphatic ECs have also shown vasculogenic capability in scaffolds (26, 360). Remarkably, co-implantation of blood- and lymphatic vessel-derived ECs in fibrin gels led to generation of distinct vascular networks, without co-mingling of the different ECs in the same vessel (Fig. 17) (360). The underlying mechanisms of this self-segregation are unclear, although platelet-derived signals appear to be important in the natural development of the two distinct vascular systems (72).
Figure 17.
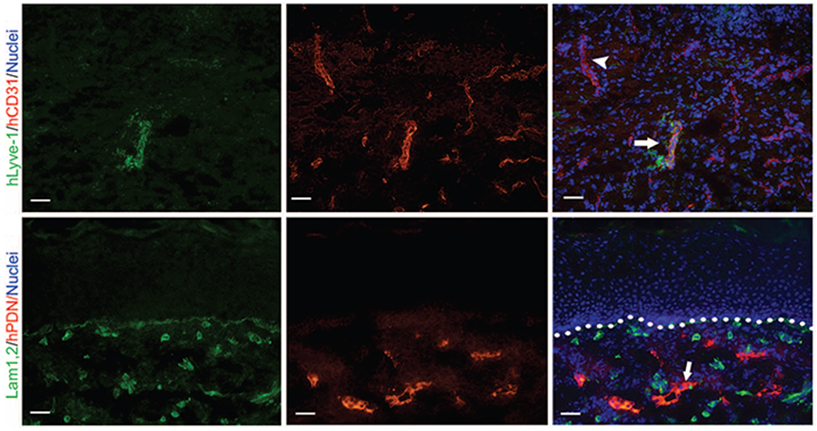
Organization of human microvascular ECs into blood and lymphatic vessels in a dermal graft after two weeks. Immunostains are shown for CD31, blood vessel marker laminin (Lam1,2), and lymphatic markers LYVE-1 and podoplanin (hPDN). Scale bars refer to 100 μm. Adapted with permission from (360).
Methods to promote EC survival within scaffolds have been developed. Incorporation of VEGF-releasing alginate microparticles in collagen-fibronectin gels that contained Bcl2-transduced HUVECs led to increased vascular densities (~100/mm2) and diameters in implants (241). Surprisingly, nearly all of the improved vascularization resulted from an increase in HUVEC-lined vessels, rather than from any VEGF-induced angiogenesis. Even more surprisingly, the addition of unloaded (i.e., VEGF-free) microparticles resulted in similar vascular densities, which suggests that the alginate may concentrate and slowly release local survival factors.
A different strategy to promote EC survival in implants is to provide mesenchymal cell support, as these cells produce numerous stabilizing factors, such as Ang1 (114). Numerous mesenchymal cell types, including fibroblasts, SMCs, BM-derived stromal cells, MSCs, and ADSCs, have been co-implanted with ECs (11, 28, 29, 181, 372, 591). Co-implantation of human saphenous vein SMCs with EOCs yielded perfused vessels in Matrigel, but EOCs by themselves did not (372). Human MSCs displayed the same potency as mouse 10T1/2 fibroblasts in stabilizing newly formed vessels from HUVECs in collagen-fibronectin implants (29). They resulted in a several-fold increase in vascular density in several types of implanted scaffolds (11). Likewise, ADSCs increased the total and SMA-positive vascular densities in cord blood-derived EOC-containing collagen-fibronectin implants (591). A feedback loop, in which ADSCs secreted VEGF to support EOCs or ECs that secreted PDGF-BB to support ADSCs, may underlie the enhanced vascularization (590, 591). Nitric oxide, likely EC-derived, mediates the association of mural cells with newly formed vessels (256). In HUVEC-containing fibrin implants, lung fibroblasts induced greater vessel densities than MSCs or ADSCs did; on the other hand, only MSCs and ADSCs generated vessels that expressed the smooth muscle marker calponin (181). The higher the density of fibroblasts, the greater the vascular density and perfusion (83). Invariably, the mesenchymal cells migrated to a perivascular location, with little evidence of transdifferentiation into ECs (29, 590). Implantation of mesenchymal cells in scaffolds without ECs resulted in minimal vascularization, which was recipient-derived (372, 591). Lymphatic ECs appear to be able to undergo vasculogenesis only in the presence of fibroblasts (26, 360).
Many types of scaffolds, including fibrin, Matrigel, biodegradable polymers, and a self-assembling peptide-based matrix (Puramatrix), have shown the ability to be vascularized by implanted ECs or EOCs (11, 416, 436). Regardless of the scaffold used, the resulting vascular densities are generally an order of magnitude lower than that of the surrounding native tissue.
EPCs and mesenchymal cells.
To what extent EPCs, hematopoietic cells, and mesenchymal stem cells from bone marrow (MSCs) or adipose tissue (ADSCs) contribute to vascular structures in scaffolds remains unclear. Some studies have claimed that these cells can differentiate into ECs and form perfused vessels within implants. In FGF2-loaded Matrigel implants, 6-26% of ECs in the vascular ingrowth were BM-derived (399). In subcutaneously implanted PVA sponges, ~10% of the ECs in the vascular ingrowth were BM-derived (104).
Other studies observed minimal incorporation of EPCs, MSCs, or ADSCs into newly formed vessels (162). Subcutaneous implants of EPC-containing collagen-fibronectin gels yielded minimal vessels (<1/mm2) by four weeks (660). Subcutaneous injections of MSC-containing Matrigel developed denser, more persistent, and better organized vascular networks than VEGF- or FGF2-loaded Matrigel did, with vascular densities of ~100/mm2 by four weeks (7). Although MSCs contributed both to endothelial and smooth muscle layers, the vast majority (~99%) of vessels in the Matrigel were derived from the recipient, which suggests that angiogenesis rather than vasculogenesis is the dominant effect of MSC therapy; consistent with this interpretation, VEGF antibodies reduced the vascular density in MSC-containing implants to control levels. Similar results were obtained for implants of collagen or fibrin gels that contained MSCs (11). In SVF-containing Matrigel implants, depletion of ECs from the SVF eliminated vasculogenesis, which implies that SVF-derived ADSCs are unable to form vessels (292).
Multicellular endothelial structures.
One way to hasten the generation of perfused microvessels in implanted scaffolds is to replace individual, dispersed ECs with multicellular structures that more closely resemble normal vascular networks. Allowing ECs to assemble into a network in vitro before implantation shortened the time to perfusion with blood in vivo by roughly a week; the total time between preparation of the EC-laden scaffold and perfusion remained ~2 weeks (82). Prevascularization of jejenum-based scaffolds for different culture times and subsequent abdominal implantation showed that longer times in vitro, up to three weeks, resulted in reduced vascular densities in vitro but greater densities in vivo (291). Thus, vessels that formed by vasculogenesis were not necessarily maintained in vivo. Other types of aggregated structures, such as EC spheroids, EC-coated microstructures, and patterned EC cords, have been used to generate vascular networks in vivo (Fig. 18) (4, 8, 38, 617).
Figure 18.
Transformation of endothelial aggregates into perfused vessels. (A) Random self-organized HUVEC networks and patterned HUVEC cords, before and after implantation. (B) Two-week-old implants after perfusion with species-specific lectins. Vessels in implants consist of ECs that are derived from graft (human, red) and host (mouse, green). Scale bars refer to 250 μm (A, upper left), 500 μm (A, upper middle and upper right), 100 μm (A, lower), and 150 μm (B). Reproduced with permission from (38).
A related strategy is to use freshly isolated microvascular fragments as the vascular source (Fig. 19). Here, the source tissue (primarily adipose tissue, as it is plentiful and accessible) is minced and digested to release microvessels that are collected and embedded within a scaffold. Subcutaneous, epicardial, or perimuscular implants of microvessel-containing collagen or polymer scaffolds showed perfusion near the scaffold surface after 1-3 days and deep within the scaffold after 10-14 days (316, 443, 507, 509). In vitro culture of microvessels before implantation appears to destabilize their mural coat and thereby render them more able to inosculate with host vessels upon implantation (317). Perfused vessels deep within a scaffold were almost completely derived from the implant, even after several weeks (507). Embedded microvessels secreted FGF2 and (to a lesser extent) VEGF, and the enhanced surface perfusion can be attributed partly to angiogenic ingrowth from the host (315, 316). Despite these promising results, the clinical potential of microvascular fragments is tempered by their reduced potency from older donors and a tendency to generate intra-scaffold hemorrhage (315).
Figure 19.

Increase in perfused vascular density over time in subcutaneous polymer implants that contained adipose-derived microvascular fragments (black circles) or that did not (clear circles). (Left) Vascular density near the surface of implants. (Right) Vascular density at the center of implants. Adapted with permission from (316).
Engineering tissues by vasculogenesis
Engineering vascularized tissues by vasculogenesis has consisted largely of supplementing a scaffold with ECs or EOCs, mesenchymal cells, and the parenchymal cells of interest. Culture of Bcl2-transduced HUVECs or EOCs and keratinocytes on an acellular dermal scaffold resulted in a vascularized skin equivalent (493, 508); similar results were obtained with HUVECs, fibroblasts, and keratinocytes in a collagen-chitosan scaffold (592). When grafted on a wound, the scaffolds integrated well, with primarily human microvessels at the epidermal-dermal interface at day 4; in contrast, EC-free scaffolds were vascularized by angiogenesis, with host-derived vessels reaching the epidermis only after two weeks. A similar approach, but with both lymphatic ECs and blood vessel-derived microvascular ECs, resulted in artificial skin that contained functional blood and lymphatic networks (360). Subcutaneous or intramuscular implants of PLA/PLGA scaffolds that were prevascularized with HUVECs, fibroblasts, and myoblasts yielded vascularized muscle constructs after two weeks (334); when implanted around the femoral vessels, such constructs incorporated the vessels and could subsequently be transposed as a pedicled muscle flap (503). HUVECs, fibroblasts, and cardiomyocytes generated vascularized myocardial patches after two weeks (Fig. 20) (332); this strategy was successful even when the scaffold was solely cell-generated (547). Implants of EOCs, ADSCs (as mesenchymal support), and pancreatic islets or adipocytes in collagen-fibronectin scaffolds yielded vascularized islets or adipose, respectively (591).
Figure 20.

Vascularized myocardial constructs that contained human cardiomyocytes, HUVECs, and mouse fibroblasts in a PLA/PLGA sponge after two weeks. (Left) Immunostain for human CD31 (red) and von Willebrand factor (green). (Right) Immunostain for human CD31 (brown). Reproduced with permission from (332).
Multicellular EC aggregates can also serve as the vasculogenic cells when engineering tissues. For instance, incorporation of microvascular fragments into an artificial skin (Integra) increased perfusion of the implant by day 6, generation of blood vessels (nearly all implant-derived) and lymphatic vessels (~60% from the implant), and epithelialization of the wound by day 10; focal hemorrhage occurred at day 6 but did not appear to impede generation of vascularized skin (151). Similarly, implantation of fibrin gels that contained hepatocyte/fibroblast spheroids and pre-aggregated EC cords yielded human hepatic tissue that grew in response to host (mouse) liver injury (548).
Regardless of the specific vasculogenic approach used, one must keep in mind that a vascularized tissue is viable, but not necessarily functional. For instance, co-implantation of myoblasts and microvascular fragments in a collagen scaffold into a full-thickness muscle defect resulted in a well-vascularized construct that still underwent fibrotic transformation (336). Moreover, although the initial vascular networks (particularly deep within a construct) are generated by vasculogenesis, these vessels are often eventually replaced by angiogenic ingrowth. In a subcutaneous implant of HUVEC-coated, islet-containing collagen gels, the vessels within the implant transitioned from initially human-derived to largely host (mouse)-derived over two weeks, although this change did not seem to hinder the restoration of normoglycemia (617).
Vasculogenesis in microphysiological systems
The development of microfluidic technologies has enabled the perfusion of self-organized EC networks in vitro (218, 277, 395). In these studies, suspensions of ECs or EOCs (with or without mesenchymal cells) are allowed to organize into open networks within microscale extracellular matrix gels in a microfluidic device (Fig. 21A). By applying pressure across the ends of each gel, perfusion can be initiated within the nascent lumens once they make contact with the microfluidic channels (Fig. 21B). Careful attention must be paid to interfaces within the device to minimize leakage, and a combination of vasculogenesis within the scaffold and angiogenesis from the microfluidic channels generated leak-free junctions (625).
Figure 21.

Perfused vascular networks in microscale fibrin gels within microfluidic devices. Fibroblasts, in the same or separate gel, were included to promote vasculogenesis. In (B), the resulting networks were perfused with a solution of fluorescent dextran. Reproduced with permission from (277, 395).
These perfused in vitro microvascular networks are primarily intended for microphysiological studies. For studies of cancer progression, tumor cells and candidate therapeutics can be delivered intravascularly in EC networks (79, 205, 243, 536). For studies of engineered tissues, parenchymal cells such as cardiomyocytes can be added to the self-organizing ECs and the resulting networks perfused to maintain tissue viability (483). The addition of C2C12 myoblasts or MSC-derived osteoblasts increased vascular permeability several-fold (243).
Physical signals in vasculogenic vascularization
The physical properties of a scaffold play an important role in determining the outcome of vasculogenesis in vivo and in vitro. Vasculogenesis in EC/EOC- and MSC-containing collagen and fibrin gels of different concentrations showed that intermediate scaffold concentrations of 5-10 mg/mL were optimal (11, 105, 286). Higher concentrations showed minimal vasculogenesis, and it appeared that these scaffolds resisted the remodeling needed to support EC migration and rearrangement. Lower concentrations resulted in vessels that could not resist hemorrhage, most likely because these scaffolds were mechanically weak. Correlation between vascular density and scaffold stiffness suggested that lower stiffness resulted in greater vascularization; it is not known whether a causal relation exists between the two, since it is difficult to vary stiffness independently of all other physical parameters.
Vasculogenesis in anisotropic environments invariably leads to generation of vessels that are preferentially oriented along the main axis of anisotropy. Such conditions can be induced by non-uniform contraction or strain of compliant scaffolds (298, 391, 472), by assembly of fibrous matrices in a magnetic field (392), by interstitial flow (198, 410), or by allowing EC self-organization to take place in a confined channel (455). The resulting aligned microvessels may prove to be advantageous when engineering tissues that contain highly aligned vascular networks, such as in heart, skeletal muscle, and kidney. Intramuscular implants that matched vascular and recipient tissue alignment yielded stronger grafts that those in which the vessels were perpendicular to the tissue fibers (472). On the other hand, isotropic vascular networks proved to be as effective as aligned ones in perfusing an epicardial patch (462).
As seen with angiogenic sprouting from flat EC monolayers in microfluidic devices, EC networks that are formed by vasculogenesis preferentially sprout against the direction of interstitial flow (275).
MICROFLUIDIC VASCULARIZATION
Microfluidic vascularization refers to the direct, physical construction of perfusable vessels in a graft or scaffold (583). Like vasculogenesis, this form of vascularization is intrinsic, since the vasculature is built into the graft or scaffold. Unlike vasculogenesis, however, no biological step of tubulogenesis is required for perfusable structures to form. Similarly, angiogenic sprouting is not required, although it can help enhance the ability of microfluidic structures to vascularize a region. Given that no natural biological analog of microfluidic vascularization exists, essentially all of the work in this area has originated from the surgical and engineering communities.
Flap-based revascularization
It has long been appreciated that the surgical vascular anastomosis of a single biological or prosthetic graft can bypass an arterial stenosis to revascularize an ischemic tissue. What is less appreciated is that tissue flaps, which contain intact microvascular networks, can perform the same function. Flaps based on the omentum through the gastroepiploic artery have been used to augment the circulation to the dog hindlimb (171) and brain (173); the flaps are partially transected to allow them to reach the extremities. In the ischemic human limb, omental flaps have been successfully used to salvage tissue that would otherwise be susceptible to amputation (172, 217). This procedure relies on growth of vessels to connect the flap vasculature to that of the ischemic tissue, a process that is similar to the vascular growth that underlies the Vineberg procedure for revascularization of ischemic myocardium.
Similar flap-based approaches have been applied for the treatment of lymphedema. The same pedicled omental flaps that can salvage ischemic tissues also reduced the severity of edema in 30-50% of patients (170). In mice and rats, myocutaneous flaps prevented the development of lymphedema after ligation of tail lymphatics (534). Lymphatics display a strong ability to regenerate; even in free flaps, which have no lymphatic continuity with host lymphatics after implantation, bridging between the lymphatics in the flap and those in the distal tissue occurs quickly to mitigate the development of lymphedema (533). Fibrosis at the flap border appears to be the main impediment to lymphatic regeneration (31).
Engineering microvessels with microfluidic scaffolds
To apply a microfluidic approach beyond the tissue flaps that are surgically accessible, the ability to generate microfluidic scaffolds (those that contain perfusable channels or networks) is critical. These perfusable structures can then serve as templates for the growth of open microvessels.
Generation of microfluidic scaffolds
Lithographic methods.
In 2000, Vacanti and colleagues proposed that the micropatterning techniques that were (and are still) widely used in the microelectronics industry could be adapted to the patterning of biomaterials (249). Initial work focused on silicon micromachining, in which planar, tree-like patterns on an etched silicon wafer provided a template for the growth of ECs (Fig. 22); once the cells formed a monolayer, they could be detached from the silicon surface for further processing.
Figure 22.
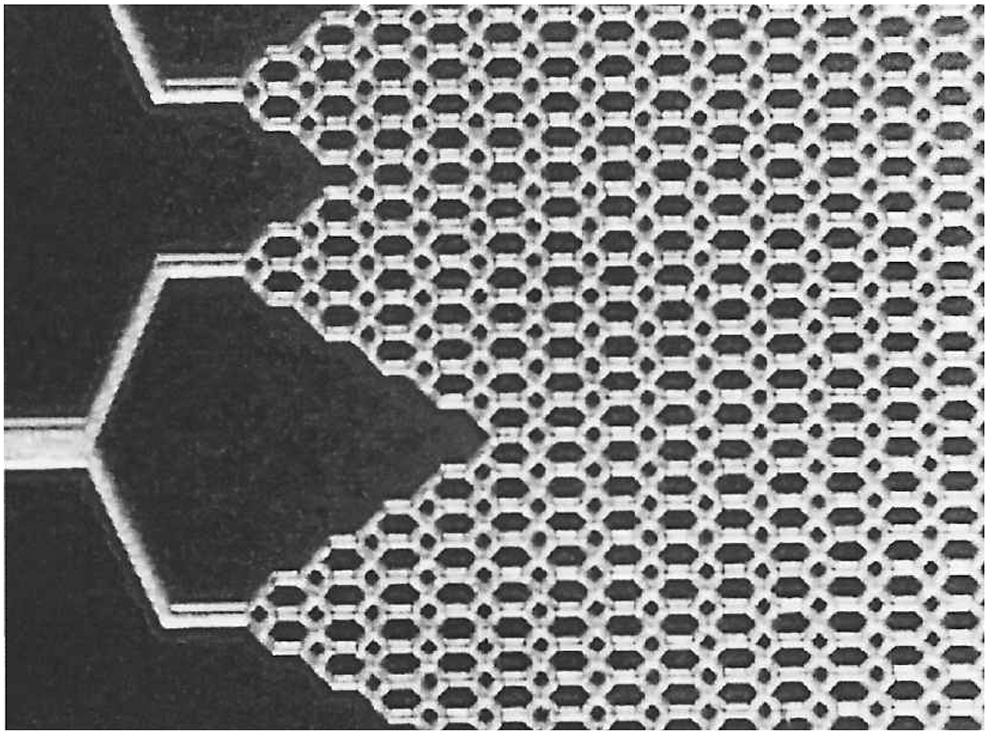
Branching vascular-like pattern that was etched into a silicon wafer. Such patterns could be seeded with ECs to generate patterned cultures. Feature widths were on the order of ~10 μm. Adapted with permission from (249).
Subsequent studies focused on developing similar micropatterning technologies to build perfusable, and not just patterned, structures in more biologically relevant materials (65, 90, 168). These studies required the adaptation of silicone-based micropatterning techniques, such as those used to create microfluidic devices. This so-called “soft lithography” proved to be particularly well-suited for patterning fragile biological materials, such as protein gels, because it takes place under ambient conditions without exposure to harsh chemical treatments (649).
One strategy for forming biomaterials that contain microfluidic networks (so-called “microfluidic biomaterials”) relies on additive processing, in which two or more distinct parts are combined to form the microfluidic material. The earliest example of this strategy used micropatterned molds to form an alginate hydrogel on which a negative pattern was replicated (65, 89). Coupling the patterned gel to a second, flat gel generated a composite that contained internal channels through which fluid could be pumped. Since alginate is not adhesive to ECs, these channels were left un-vascularized, but they were able to sustain the metabolism of bovine chondrocytes that were embedded within the alginate (89). Similar avascular microfluidic hydrogels provided exchange of solutes with an underlying porous substrate (66). Adaptation of the same design to type I collagen gels and agarose-gelatin gels required the development of methods to secure two separate gels so that they would not detach under perfusion (448, 453, 677). When seeded into the collagen channels, individual ECs rapidly attached and proliferated to line the channels with a monolayer of cells (Fig. 23).
Figure 23.

Vascularization of a microfluidic type I collagen scaffold that was formed by combining a micropatterned and planar gel. Both en face and reconstructed 3D views of HUVEC-seeded structures are shown. Scale bar refers to 100 μm. Reproduced with permission from (677).
A second strategy is based on negative processing, in which material is removed to leave behind channels. Many removable materials have been investigated, including waxes (578, 611), gelatin (168), thermoreversible polymers (648), and metals (90). The negative material is first patterned, either by micromolding or direct printing, before being encapsulated in a scaffold that is compatible with EC adhesion. Removal of the patterned material (e.g., by raising the temperature to melt the material and allow it to be flushed out) results in a scaffold that contains channels. In scaffolds that are made of extracellular matrix proteins, seeded ECs grow to line the channels (Fig. 24). Alternatively, vascular cells can be grown on matrix fibers that are later digested to yield open lumens (39).
Figure 24.

Vascularization of a microfluidic type I collagen scaffold that was formed around a removable needle. (Top) Unseeded scaffold. (Bottom) Scaffold that was seeded with HUVECs. Insets show cross-sectional views. Scale bar refers to 100 μm. Reproduced with permission from (90).
The widespread availability of laser and inkjet printing, and recent advances in three-dimensional (3D) printing technologies, have led to the exploration of methods that can directly draw 3D scaffolds that contain channels for vascularization (385). Early work retrofitted commercial inkjet cartridges so that they contained hydrogel precursor solution with or without ECs (55, 637). Printing an “ink” that consisted of thrombin droplets onto a solution of fibrinogen caused local polymerization of fibrin; rasterizing the ink ejection point in 3D yielded fibrin structures that contained channels (107). The resolution of these structures was low, and channels had corners and were bowed inward, in part because the inks tended to diffuse quickly in the medium that they were printed into. Printing inks of higher viscosity allowed the printed structures to hold their shape while the surrounding material set in place (51, 648). Switching between different ink cartridges, each of which contains a unique solution, allowed 3D printing of different cell types and materials (254). As long as one of the inks is compatible with EC adhesion, it is possible to generate perfused microvessels in 3D-printed scaffolds (with the caveat that “microvessel” often refers to structures much wider than the 100 μm limit typically encountered in the microvascular physiology community); sacrificial inks to define the vascular architecture include gelatin, Pluronic block co-polymers, and glassy mixtures of saccharides (Fig. 25) (294, 295, 330, 331, 382). In general, the versatility of 3D printing is unmatched, and it excels at drawing complex customized shapes at the scale of whole organs. Nevertheless, the resolution and speed of printing techniques remain below those of micromolding approaches; in particular, routine construction of interconnected channels with appropriate cross-sectional shape for microvascular engineering remains a challenge.
Figure 25.

Vascularization of a microfluidic fibrin scaffold that was formed around a sacrificial 3D-printed network of sugar-based fibers. Channels were seeded with HUVECs (red), and the scaffold bulk contained 10T1/2 mouse fibroblasts (green). Scale bar refers to 1 mm. Reproduced with permission from (382).
Optical methods have also been explored for generating microfluidic scaffolds in photosensitive materials. The material can either be photopolymerized to create solid regions that together encompass the desired channels, or photodegraded to create channels within a preformed solid. For instance, polyethylene glycol gels that contained photodegradable acrylates in the polymer backbone enabled complete degradation of the gel with UV light (284). Focusing and scanning a UV source into a preformed gel with a two-photon microscope allowed channels and other complex structures to be drawn into the gel. At a sufficiently high intensity, such as that available with pulsed lasers, channels can be ablated into collagen (17). As with 3D printing, the pixel-by-pixel drawing of patterns into or onto photosensitive materials can be slow. Seeding of optically patterned scaffolds with ECs yielded microvascular networks (Fig. 26) (196).
Figure 26.
Vascularization of a microfluidic polyethylene glycol gel that was patterned by photodegradation. (Left) Visualization of interconnected channels by perfusion with fluorescent dextran. (Right) 3D confocal reconstruction of a vascularized channel that was stained for ZO-1 (green). Reproduced with permission from (196).
Decellularization of organs.
An entirely different method of creating microfluidic scaffolds treats tissues with specialized solutions that decellularize the tissue, leaving the original extracellular matrix intact (32). These solutions typically contain detergents to solubilize cell membranes, chaotropic agents to disrupt intermolecular bonds, and enzymes to digest DNA and RNA. Early work used decellularization mainly to reduce the antigenicity of xenografts, such as pig heart valves (163). While such decellularized tissues retained the geometry of any vascular networks that they contained, it was not obvious how these channels could be accessed, given the low mechanical stiffness of decellularized tissue. Moreover, because these tissues were decellularized by immersion in the appropriate solution, diffusion-limited transport meant that decellularization was practical for small tissues only, on the order of one centimeter in size and smaller.
The potential of decellularization to generate microfluidic scaffolds for vascularization was only realized in 2008, when Ott and Taylor showed that an entire heart could be perfused with decellularization solutions (Fig. 27) (424). As with small tissues, decellularization of the heart yielded the extracellular matrix, but now as a perfusable structure and with organ-scale size and shape. It is important to appreciate that the architectural complexity of this type of 3D microfluidic scaffold is far beyond anything that can be engineered by lithography. Subsequent work demonstrated that other whole organs and large tissues—lung (423, 437), liver (606), kidney (538), pancreas (166), small intestine (374), and skin flap (200)—could be perfusion-decellularized.
Figure 27.
Perfusion-decellularization of whole hearts preserves the vascular architecture. (A) Corrosion casts of native and decellularized hearts. Scale bars refer to 1 mm (top) and 250 μm (bottom). (B) Transplanted decellularized heart before and after re-establishment of blood flow. The recipient animal was heparinized to minimize thrombosis. Reproduced with permission from (424).
Vascularization of microfluidic scaffolds
Whether created by lithography or decellularization, microfluidic scaffolds are vascularized by perfusing a suspension of ECs into the channels in vitro. ECs are able to distribute through channels that are wider than ~30 μm and grow to form perfusable vessels (90). In narrower channels, ECs can form plugs, which require additional signals to promote cell migration along the channels (346). Seeding lymphatic ECs into blind-ended channels generated lymphatics (581). An alternate strategy used electrical desorption to transfer a monolayer of ECs from a thin metal rod onto a channel in collagen gel (502).
When formed in microscale extracellular matrix gels, these microvessels have been used in microphysiological systems (641). Since the vessel wall can be viewed in side profile and at high resolution, such vessels are particularly well-suited for studies of vascular permeability, inflammation, and angiogenesis (90, 273, 411, 447, 677). Solute permeability tends to be comparable to those of explanted microvessels and up to an order-of-magnitude higher than those observed in vivo. Vessels in these gels can also be used to perfuse microscale biomaterials and tissues (64, 246, 340). Skin equivalents that incorporate vessels can be used to study vascular absorption of topically applied substances (390). The addition of adipocytes to the gel increased vascular permeability (340).
Application of such vessels in vivo has been challenging. Subcutaneous implants of vascularized microfluidic gels were able to provide perfusion to the ischemic hindlimb; surprisingly, only channels of diameter 400 μm were effective (383). Because these implants were not surgically anastomosed to the recipient circulation, perfusion relied on angiogenic sprouting and inosculation with host vessels, and the advantage over implants of EC cords or microvessel fragments is unclear. The near-impossibility of suture anastomosis with vessels of ~100 μm diameter has led to the exploration of ways to couple these vessels to larger ones that can be more easily stitched (341, 540, 668).
Perfusion of decellularized organs with ECs has yielded vascular networks in all organs tested in vitro. Methods to enhance EC coverage include pre-coating the decellularized channels with antibodies to CD31 (287), or changing the position of the organ during seeding (542). In general, EC coverage does not appear to be complete, but is sufficient to sustain blood flow in vivo for several hours. Orthotopic implants of revascularized lungs provided functional gas exchange in rats for two hours, albeit with intra-alveolar hemorrhage (437).
Physical signals in microfluidic vascularization
Because the vascular geometry can be readily controlled in microfluidic scaffolds, these structures enable routine study of how physical signals affect vascularization. Long-term maintenance of the EC lining appears to depend on the mechanical stress at the EC-scaffold interface. Signals that reduce this stress—increased vascular pressure, decreased scaffold pore pressure, reduced EC contractility, decreased vascular permeability—promote vascular stability (Fig. 28A) (333, 449, 642, 644). Scaffold crosslinking also increases vascular stability, in part by reducing the amount of elastic energy available to detach the EC layer (Fig. 28B) (76). High flow promoted vascularization by decreasing vascular permeability and increasing trans-endothelial pressure (449).
Figure 28.
Physical mechanisms for stable vascularization of microfluidic scaffolds. Maintenance of vascular adhesion can be viewed as (A) a balance of outward (stabilizing) and inward (destabilizing) stresses, including pressures, contractile stress, and adhesion stress, or as (B) a balance of adhesion (stabilizing) and stored elastic (destabilizing) energies. Adapted with permission from (643).
CONCLUSIONS
Methods for microvascular engineering are now quite sophisticated and have led to numerous positive preclinical findings, although translating those studies to clinical practice remains a challenge. Each decade has introduced a new approach: angiogenesis in the 1980s, vasculogenesis in the 1990s, and microfluidic vascularization in the 2000s. History has shown that roughly a decade of preclinical development takes place before clinical trials begin; thus, one expects the first trials of microfluidic scaffolds to be proposed soon.
Several themes have emerged from these decades of research. First, combinatorial approaches work better than single strategies. Synergies between direct and indirect angiogenic factors, between vasculogenic and stromal cells, and between angiogenesis and vasculogenesis have shown that using multiple ways to form microvessels usually leads to more robust results. These synergies suggest that it may be just as fruitful to design therapies based on the removal of factors that inhibit vascularization, rather than on the addition of ones that promote it. The recent development of microfluidic vascularization will suggest new combination therapies.
Second, vascular persistence is not guaranteed. Whether formed by angiogenesis or vasculogenesis, microvessels tend to regress over time in vivo and become replaced by host-derived vessels. In microfluidic scaffolds, which provide a template for vascular growth, the vessels must receive additional perfusion- or scaffold-derived signals to be stable over the long term in vitro. Since vascular densities are tightly matched to metabolic needs in vivo, engineering persistent vascularization may be easier in the presence of metabolically active parenchymal cells. The challenge is that parenchymal cells need vessels to survive, so which component to make first poses a tough dilemma. Perhaps an iterative process of growing vessels and parenchyma in alternating stages would yield more durable vascularization, compared to forming vessels exclusively first.
Third, co-morbidities affect the vascular outcome. Old age, and the chronic conditions that often accompany it, greatly reduces the effectiveness of vascularization therapies. To increase chances of clinical success, trials in younger and/or healthier patients may be advantageous.
Fourth, vascularization is necessary, but can be insufficient, for tissue functionality. Having a sufficient vascular density only enables survival of a tissue. For simple tissues, such as skin and adipose, creating vascularized “flesh” is enough. For more complex tissues, such as muscle, bone, liver, and kidney, the vessels provide functions beyond perfusion. In many tissues, the vessels display organ-specific fenestra, discontinuities, or tight junctions, and organ-specific 3D architectures (267); the endothelium can secrete soluble factors that regulate parenchymal function (454).
How to obtain tissue-specific vascular architecture and function remains a mystery. It seems likely that the use of pluripotent stem cell-derived ECs will increase, especially since such stem cells may provide a route to obtain tissue-specific ECs, which tend with dedifferentiate upon extended culture in vitro. A very recent study demonstrated that iPSCs can be differentiated into ECs that form perfusable vessels with a blood-brain barrier (347). Robust methods to generate other types of tissue-specific ECs and to preserve their phenotype upon assembly into vessels are sorely needed. Greater emphasis on the entire suite of microvascular functions, including autoregulation of blood flow, antigen presentation, and enzymatic processing of hormones, may help shed light on this issue.
DIDACTIC SYNOPSIS.
Major Teaching Points
The ability to engineer microvasculature is crucial to the success of therapeutic vascularization and tissue engineering.
Currently, the main strategies for engineering microvasculature are based on angiogenesis, vasculogenesis, and microfluidics.
Engineered vasculature can be formed in an intrinsic or extrinsic manner.
Local and sustained delivery of vascularizing signals usually leads to more robust formation of vascular networks, compared to systemic and transient delivery of the same signals.
Biomaterial scaffolds can provide a means for local, controlled release of vascularizing signals and a space for vascular ingrowth.
Widespread success of vascularization methods in laboratory and animal studies has yet to lead to clinically relevant therapies in humans.
ACKNOWLEDGMENTS
This review is dedicated to the late David Shepro, Professor Emeritus of Biology and Surgery at Boston University, a pioneer in the study of microvascular biology and physiology and a greatly appreciated mentor who long ago helped guide my approach to problems in this field. Support during the preparation of this article was provided by the National Institute of Biomedical Imaging and Bioengineering (award EB024660) and the National Cancer Institute (award CA214292).
REFERENCES
- 1.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 107: 2134–2139, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res 100: 581–589, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Akahori T, Kobayashi A, Komaki M, Hattori H, Nakahama K-I, Ichinose S, Abe M, Takeda S, Morita I. Implantation of capillary structure engineered by optical lithography improves hind limb ischemia in mice. Tissue Eng A 16: 953–959, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Akita S, Tamai N, Myoui A, Nishikawa M, Kaito T, Takaoka K, Yoshikawa H. Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng 10: 789–795, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg 75: 204–209, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther 10: 621–629, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K, Korff T, Zentgraf H, Obodozie C, Graeser R, Christian S, Finkenzeller G, Stark GB, Héroult M, Augustin HG. Spheroid-based engineering of a human vasculature in mice. Nat Methods 5: 439–445, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature 406: 257, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med 5: e74–e86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson EM, Kwee BJ, Lewin SA, Raimondo T, Mehta M, Mooney DJ. Local delivery of VEGF and SDF enhances endothelial progenitor cell recruitment and resultant recovery from ischemia. Tissue Eng A 21: 1217–1227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in Matrigel: implications for a role in neovascularization. Stem Cells Dev 13: 665–676, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol 168: 529–541, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anisimov A, Tvorogov D, Alitalo A, Leppänen V-M, An Y, Han EC, Orsenigo F, Gaál EI, Holopainen T, Koh YJ, Tammela T, Korpisalo P, Keskitalo S, Jeltsch M, Ylä-Herttuala S, Dejana E, Koh GY, Choi C, Saharinen P, Alitalo K. Vascular endothelial growth factor-angiopoietin chimera with improved properties for therapeutic angiogenesis. Circulation 127: 424–434, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, Nakamura T, Kaneda Y, Higaki J, Ogihara T. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther 7: 417–427, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Applegate MB, Coburn J, Partlow BP, Moreau JE, Mondia JP, Marelli B, Kaplan DL, Omenetto FG. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc Natl Acad Sci USA 112: 12052–12057, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arkudas A, Beier JP, Heidner K, Tjiawi J, Polykandriotis E, Srour S, Sturzl M, Horch RE, Kneser U. Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng 13: 1549–1560, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Arkudas A, Lipp A, Buehrer G, Arnold I, Dafinova D, Brandl A, Beier JP, Körner C, Lyer S, Alexiou C, Kneser U, Horch RE. Pedicled transplantation of axially vascularized bone constructs in a critical size femoral defect. Tissue Eng A 24: 479–492, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 92: II365–II371, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 83: 233–240, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asano T, Kaneko E, Shinozaki S, Imai Y, Shibayama M, Chiba T, Ai M, Kawakami A, Asaoka H, Nakayama T, Mano Y, Shimokado K. Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression, and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ J 71: 405–411, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Asano Y, Shimoda H, Matsusaki M, Akashi M. Transplantation of artificial human lymphatic vascular tissues fabricated using a cell-accumulation technique and their engraftment in mouse tissue with vascular remodelling. J Tissue Eng Regen Med 12: e1501–e1510, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol 291: L588–L595, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 111: 1302–1305, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111: 4551–4558, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem 49: 32–40, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg 124: 438–450, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13: 27–53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg 16: 181–191, 1992. [PubMed] [Google Scholar]
- 34.Baird RJ. The reasons for patency and anastomosis formation of the internal mammary artery implant. Ann R Coll Physicians Surg Can 2: 172–190, 1969. [Google Scholar]
- 35.Banai S, Jaklitsch MT, Casscells W, Shou M, Shrivastav S, Correa R, Epstein SE, Unger EF. Effects of acidic fibroblast growth factor on normal and ischemic myocardium. Circ Res 69: 76–85, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 89: 2183–2189, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Banfi A, von Degenfeld G, Gianni-Barrera R, Reginato S, Merchant MJ, McDonald DM, Blau HM. Therapeutic angiogenesis due to balanced single-vector delivery of VEGF and PDGF-BB. FASEB J 26: 2486–2497, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, Yang MT, Miller JS, Bhatia SN, Chen CS. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci USA 110: 7586–7591, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barreto-Ortiz SF, Fradkin J, Eoh J, Trivero J, Davenport M, Ginn B, Mao H-Q, Gerecht S. Fabrication of 3-dimensional multicellular microvascular structures. FASEB J 29: 3302–3314, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battler A, Scheinowitz M, Bor A, Hasdai D, Vered Z, Di Segni E, Varda-Bloom N, Nass D, Engelberg S, Eldar M, Belkin M, Savion N. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol 22: 2001–2006, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Baum O, Da Silva-Azevedo L, Willerding G, Wöckel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol 287: H2300–H2308, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 97: 1114–1123, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Bauters C, Asahara T, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Physiological assessment of augmented vascularity induced by VEGF in ischemic rabbit hindlimb. Am J Physiol 267: H1263–H1271, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Bauters C, Asahara T, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Site-specific therapeutic angiogenesis after systemic administration of vascular endothelial growth factor. J Vasc Surg 21: 314–324; discussion 324-325, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Baxter TJ, O’Brien BM, Henderson PN, Bennett RC. The histopathology of small vessels following microvascular repair. Br J Surg 59: 617–622, 1972. [DOI] [PubMed] [Google Scholar]
- 46.Bayati S, Russell RC, Roth AC. Stimulation of angiogenesis to improve the viability of prefabricated flaps. Plast Reconstr Surg 101: 1290–1295, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Beck CS, Tichy VL, Moritz AR. Production of a collateral circulation to the heart. Proc Soc Exp Biol Med 32: 759–761, 1935. [Google Scholar]
- 48.Bellman S, Frank HA. Vascular channels established by implantation of a systemic artery into the myocardium. Ann Surg 147: 425–442, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290: H2196–H2203, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14: 2202–2211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bir SC, Esaki J, Marui A, Sakaguchi H, Kevil CG, Ikeda T, Komeda M, Tabata Y, Sakata R. Therapeutic treatment with sustained-release platelet-rich plasma restores blood perfusion by augmenting ischemia-induced angiogenesis and arteriogenesis in diabetic mice. J Vasc Res 48: 195–205, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Bissell MJ. Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch. A rose by any other name? Am J Pathol 155: 675–679, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanton MW, Hadad I, Johnstone BH, Mund JA, Rogers PI, Eppley BL, March KL. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg 123: 56S–64S, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec A 272: 497–502, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA 107: 3287–3292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res 29: 1517–1524, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Brazelton TR, Rossi FMV, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290: 1775–1779, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Bridges CR. Myocardial laser revascularization: the controversy and the data. Ann Thorac Surg 69: 655–662, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Briquez PS, Clegg LE, Martino MM, Mac Gabhann F, Hubbell JA. Design principles for therapeutic angiogenic materials. Nat Rev Mater 1: 1–15, 2016. [Google Scholar]
- 61.Brown DM, Hong SP, Farrell CL, Pierce GF, Khouri RK. Platelet-derived growth factor BB induces functional vascular anastomoses in vivo. Proc Natl Acad Sci USA 92: 5920–5924, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 34: 9201–9209, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buncke HJ Jr., McLean DH, George PT, Creech BJ, Chater NL, Commons GW. Thumb replacement: great toe transplantation by microvascular anastomosis. Br J Plast Surg 26: 194–201, 1973. [DOI] [PubMed] [Google Scholar]
- 64.Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthcare Mater 6: 1700015, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cabodi M, Choi NW, Gleghorn JP, Lee CS, Bonassar LJ, Stroock AD. A microfluidic biomaterial. J Am Chem Soc 127: 13788–13789, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Cabodi M, Cross VL, Qu Z, Havenstrite KL, Schwartz S, Stroock AD. An active wound dressing for controlled convective mass transfer with the wound bed. J Biomed Mater Res B 82: 210–222, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Cao L, Arany PR, Kim J, Rivera-Feliciano J, Wang Y-S, He Z, Rask-Madsen C, King GL, Mooney DJ. Modulating Notch signaling to enhance neovascularization and reperfusion in diabetic mice. Biomaterials 31: 9048–9056, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao L, Arany PR, Wang Y-S, Mooney DJ. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials 30: 4085–4093, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med 9: 604–613, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332: 370–379, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Carmeliet P VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med 6: 1102–1103, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Carramolino L, Fuentes J, García-Andrés C, Azcoitia V, Riethmacher D, Torres M. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res 106: 1197–1201, 2010. [DOI] [PubMed] [Google Scholar]
- 73.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science 297: 1299, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol 20: 2573–2578, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Chan KLS, Khankhel AH, Thompson RL, Coisman BJ, Wong KHK, Truslow JG, Tien J. Crosslinking of collagen scaffolds promotes blood and lymphatic vascular stability. J Biomed Mater Res A 102: 3186–3195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of β2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med 201: 63–72, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 276: 1425–1428, 1997. [DOI] [PubMed] [Google Scholar]
- 79.Chen MB, Whisler JA, Jeon JS, Kamm RD. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr Biol 5: 1262–1271, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 24: 258–264, 2007. [DOI] [PubMed] [Google Scholar]
- 81.Chen RR, Snow JK, Palmer JP, Lin AS, Duvall CL, Guldberg RE, Mooney DJ. Host immune competence and local ischemia affects the functionality of engineered vasculature. Microcirculation 14: 77–88, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CCW, George SC. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng A 15: 1363–1371, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen X, Aledia AS, Popson SA, Him L, Hughes CCW, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng A 16: 585–594, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho C-H, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim K-T, Kim I, Choi H-H, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA 101: 5547–5552, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho C-H, Kim KE, Byun J, Jang H-S, Kim D-K, Baluk P, Baffert F, Lee GM, Mochizuki N, Kim J, Jeon BH, McDonald DM, Koh GY. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res 97: 86–94, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Cho C-H, Sung H-K, Kim K-T, Cheon HG, Oh GT, Hong HJ, Yoo O-J, Koh GY. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA 103: 4946–4951, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi I, Lee S, Kyoung Chung H, Suk Lee Y, Eui Kim K, Choi D, Park EK, Yang D, Ecoiffier T, Monahan J, Chen W, Aguilar B, Lee HN, Yoo J, Koh CJ, Chen L, Wong AK, Hong Y-K. 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation 125: 872–882, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development 125: 725–732, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater 6: 908–915, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 71: 185–196, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Chu VF, Giaid A, Kuang J, McGinn AN, Li CM, Pelletier MP, Chiu RC-J. Angiogenesis in transmyocardial revascularization: comparison of laser versus mechanical punctures. Ann Thorac Surg 68: 301–307; discussion 307-308, 1999. [DOI] [PubMed] [Google Scholar]
- 92.Chung S, Sudo R, Zervantonakis IK, Rimchala T, Kamm RD. Surface-treatment-induced three-dimensional capillary morphogenesis in a microfluidic platform. Adv Mater 21: 4863–4867, 2009. [DOI] [PubMed] [Google Scholar]
- 93.Clark ER, Clark EL. Observations on living preformed blood vessels as seen in a transparent chamber inserted into the rabbit’s ear. Am J Anat 49: 441–477, 1932. [Google Scholar]
- 94.Clark ER, Clark EL. Observations on the new growth of lymphatic vessels as seen in transparent chambers introduced into the rabbit’s ear. Am J Anat 51: 49–87, 1932. [Google Scholar]
- 95.Clark RA, Stone RD, Leung DYK, Silver I, Hohn DC, Hunt TK. Role of macrophages in wound healing. Surg Forum 27: 16–18, 1976. [PubMed] [Google Scholar]
- 96.Clark RAF. Wound repair: basic biology to tissue engineering In: Principles of Tissue Engineering, edited by Lanza R, Langer R, Vacanti JP. San Diego, CA: Academic Press, 2014, p. 1595–1617. [Google Scholar]
- 97.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(D,L-lactide-coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Release 72: 13–24, 2001. [DOI] [PubMed] [Google Scholar]
- 98.Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ, Ott HC, Jauch K-W, Bruns CJ. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation 119: 281–289, 2009. [DOI] [PubMed] [Google Scholar]
- 99.Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res 116: 1561–1578, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg 134: 200–205, 1999. [DOI] [PubMed] [Google Scholar]
- 101.Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P-N, Traas J, Schugar R, Deasy BM, Badylak S, Bühring H-J, Giacobino J-P, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. [DOI] [PubMed] [Google Scholar]
- 102.Crone C, Levitt DG. Capillary permeability to small solutes In: Handbook of Physiology; Section 2: The Cardiovascular System, edited by Renkin EM, Michel CC. Bethesda, MD: American Physiological Society, 1984, p. 411–466. [Google Scholar]
- 103.Cronin KJ, Messina A, Knight KR, Cooper-White JJ, Stevens GW, Penington AJ, Morrison WA. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast Reconstr Surg 113: 260–269, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res 87: 728–730, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31: 8596–8607, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cuevas P, Giménez-Gallego G, Carceller F, Cuevas B, Crespo A. Single topical application of human recombinant basic fibroblast growth factor (rbFGF) promotes neovascularization in rat cerebral cortex. Surg Neurol 39: 380–384, 1993. [DOI] [PubMed] [Google Scholar]
- 107.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30: 6221–6227, 2009. [DOI] [PubMed] [Google Scholar]
- 108.Curry FE. Mechanics and thermodynamics of transcapillary exchange In: Handbook of Physiology; Section 2: The Cardiovascular System, edited by Renkin EM, Michel CC. Bethesda, MD: American Physiological Society, 1984, p. 309–374. [Google Scholar]
- 109.Curry FE, Huxley VH, Sarelius IH. Techniques in the microcirculation: measurement of permeability, pressure and flow In: Techniques in the Life Sciences; Physiology Section, edited by Linden RJ. New York, NY: Elsevier, 1983, p. 1–34. [Google Scholar]
- 110.Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res 20: 96–99, 1980. [DOI] [PubMed] [Google Scholar]
- 111.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91: 4082–4085, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Hervé P, Etievent J-P, Kantelip J-P. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation 108: II-253–II-258, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Davies N, Dobner S, Bezuidenhout D, Schmidt C, Beck M, Zisch AH, Zilla P. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials 29: 3531–3538, 2008. [DOI] [PubMed] [Google Scholar]
- 114.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87: 1161–1169, 1996. [DOI] [PubMed] [Google Scholar]
- 115.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104: 3472–3482, 2004. [DOI] [PubMed] [Google Scholar]
- 116.Debels H, Galea L, Han X-L, Palmer J, van Rooijen N, Morrison W, Abberton K. Macrophages play a key role in angiogenesis and adipogenesis in a mouse tissue engineering model. Tissue Eng A 19: 2615–2625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.des Rieux A, Ucakar B, Mupendwa BPK, Colau D, Feron O, Carmeliet P, Préat V. 3D systems delivering VEGF to promote angiogenesis for tissue engineering. J Control Release 150: 272–278, 2011. [DOI] [PubMed] [Google Scholar]
- 118.Dobson DE, Kambe A, Block E, Dion T, Lu H, Castellot JJ Jr., Spiegelman BM. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell 61: 223–230, 1990. [DOI] [PubMed] [Google Scholar]
- 119.Dolderer JH, Abberton KM, Thompson EW, Slavin JL, Stevens GW, Penington AJ, Morrison WA. Spontaneous large volume adipose tissue generation from a vascularized pedicled fat flap inside a chamber space. Tissue Eng 13: 673–681, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Domkowski PW, Biswas SS, Steenbergen C, Lowe JE. Histological evidence of angiogenesis 9 months after transmyocardial laser revascularization. Circulation 103: 469–471, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Doukas J, Chandler LA, Gonzalez AM, Gu D, Hoganson DK, Ma C, Nguyen T, Printz MA, Nesbit M, Herlyn M, Crombleholme TM, Aukerman SL, Sosnowski BA, Pierce GF. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gene Ther 12: 783–798, 2001. [DOI] [PubMed] [Google Scholar]
- 122.Doulet M Revascularization in ischemic heart with autologous bone marrow transplantation. Jpn J Cardiovasc Surg 26: 248–253, 1997. [Google Scholar]
- 123.Doulet M, Noishiki Y, Yamane Y, Takahashi K, Makoto M, Kondo J, Matsumoto A. Angiogenesis with bone marrow transplantation in subcutaneous layer. Jpn J Artif Organs 25: 486–489, 1996. [Google Scholar]
- 124.Downs K Florence Sabin and the mechanism of blood vessel lumenization during vasculogenesis. Microcirculation 10: 5–25, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Edelberg JM, Lee SH, Kaur M, Tang L, Feirt NM, McCabe S, Bramwell O, Wong SC, Hong MK. Platelet-derived growth factor-AB limits the extent of myocardial infarction in a rat model: feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation 105: 608–613, 2002. [DOI] [PubMed] [Google Scholar]
- 126.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res 90: E89–E93, 2002. [DOI] [PubMed] [Google Scholar]
- 127.Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest 89: 465–473, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, Wood J, Burri PH, Hubbell JA, Zisch AH. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res 94: 1124–1132, 2004. [DOI] [PubMed] [Google Scholar]
- 129.Ehrbar M, Zeisberger SM, Raeber GP, Hubbell JA, Schnell C, Zisch AH. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials 29: 1720–1729, 2008. [DOI] [PubMed] [Google Scholar]
- 130.Enis DR, Shepherd BR, Wang Y, Qasim A, Shanahan CM, Weissberg PL, Kashgarian M, Pober JS, Schechner JS. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci USA 102: 425–430, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A 79: 176–184, 2006. [DOI] [PubMed] [Google Scholar]
- 132.Eppley BL, Doucet M, Connolly DT, Feder J. Enhancement of angiogenesis by bFGF in mandibular bone graft healing in the rabbit. J Oral Maxillofac Surg 46: 391–398, 1988. [DOI] [PubMed] [Google Scholar]
- 133.Epstein SE, Fuchs S, Zhou YF, Baffour R, Kornowski R. Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc Res 49: 532–542, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation 104: 115–119, 2001. [DOI] [PubMed] [Google Scholar]
- 135.Epstein SE, Stabile E, Kinnaird T, Lee CW, Clavijo L, Burnett MS. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation 109: 2826–2831, 2004. [DOI] [PubMed] [Google Scholar]
- 136.Erol OÖ. The transformation of a free skin graft into a vascularized pedicled flap. Plast Reconstr Surg 58: 470–477, 1976. [DOI] [PubMed] [Google Scholar]
- 137.Erol OO, Spira M. New capillary bed formation with a surgically constructed arteriovenous fistula. Plast Reconstr Surg 66: 109–115, 1980. [DOI] [PubMed] [Google Scholar]
- 138.Esaki J, Sakaguchi H, Marui A, Bir SC, Arai Y, Huang Y, Tsubota H, Kanaji T, Ikeda T, Sakata R. Local sustained release of prostaglandin E1 induces neovascularization in murine hindlimb ischemia. Circ J 73: 1330–1336, 2009. [DOI] [PubMed] [Google Scholar]
- 139.Fan W, Crawford R, Xiao Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials 31: 3580–3589, 2010. [DOI] [PubMed] [Google Scholar]
- 140.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110: 2226–2232, 2004. [DOI] [PubMed] [Google Scholar]
- 141.Fasol R, Schumacher B, Schlaudraff K, Hauenstein K-H, Seitelberger R. Experimental use of a modified fibrin glue to induce site-directed angiogenesis from the aorta to the heart. J Thorac Cardiovasc Surg 107: 1432–1439, 1994. [PubMed] [Google Scholar]
- 142.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130: 4217–4227, 2003. [DOI] [PubMed] [Google Scholar]
- 143.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 5: 1359–1364, 1999. [DOI] [PubMed] [Google Scholar]
- 144.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279: 1528–1530, 1998. [DOI] [PubMed] [Google Scholar]
- 145.Findlay MW, Dolderer JH, Trost N, Craft RO, Cao Y, Cooper-White J, Stevens G, Morrison WA. Tissue-engineered breast reconstruction: bridging the gap toward large-volume tissue engineering in humans. Plast Reconstr Surg 128: 1206–1215, 2011. [DOI] [PubMed] [Google Scholar]
- 146.Folkman J Angiogenesis. Annu Rev Med 57: 1–18, 2006. [DOI] [PubMed] [Google Scholar]
- 147.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature 288: 551–556, 1980. [DOI] [PubMed] [Google Scholar]
- 148.Folkman J, Klagsbrun M. Angiogenic factors. Science 235: 442–447, 1987. [DOI] [PubMed] [Google Scholar]
- 149.Foubert P, Matrone G, Souttou B, Leré-Déan C, Barateau V, Plouët J, Le Ricousse-Roussanne S, Lévy BI, Silvestre J-S, Tobelem G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res 103: 751–760, 2008. [DOI] [PubMed] [Google Scholar]
- 150.Frontini MJ, Nong Z, Gros R, Drangova M, O’Neil C, Rahman MN, Akawi O, Yin H, Ellis CG, Pickering JG. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol 29: 421–427, 2011. [DOI] [PubMed] [Google Scholar]
- 151.Frueh FS, Später T, Lindenblatt N, Calcagni M, Giovanoli P, Scheuer C, Menger MD, Laschke MW. Adipose tissue-derived microvascular fragments improve vascularization, lymphangiogenesis, and integration of dermal skin substitutes. J Invest Dermatol 137: 217–227, 2017. [DOI] [PubMed] [Google Scholar]
- 152.Fujihara Y, Koyama H, Nishiyama N, Eguchi T, Takato T. Gene transfer of bFGF to recipient bed improves survival of ischemic skin flap. Br J Plast Surg 58: 511–517, 2005. [DOI] [PubMed] [Google Scholar]
- 153.Fujihara Y, Koyama H, Ohba M, Tabata Y, Fujihara H, Yonehara Y, Takato T. Controlled delivery of bFGF to recipient bed enhances the vascularization and viability of an ischemic skin flap. Wound Repair Regen 16: 125–131, 2008. [DOI] [PubMed] [Google Scholar]
- 154.Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, Takehara T, Inoue M, Hasegawa M, Kuwano H, Sueishi K. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol 26: 2483–2489, 2006. [DOI] [PubMed] [Google Scholar]
- 155.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun C-O, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98: 2604–2609, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93: e88–e97, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 3: 411–423, 2002. [DOI] [PubMed] [Google Scholar]
- 158.Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, Torre V, Giacca M, Squadrito F. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia 46: 546–555, 2003. [DOI] [PubMed] [Google Scholar]
- 159.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 164: 1935–1947, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Galie PA, Nguyen D-HT, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci USA 111: 7968–7973, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann NY Acad Sci 1131: 100–109, 2008. [DOI] [PubMed] [Google Scholar]
- 162.Gaudiello E, Melly L, Cerino G, Boccardo S, Jalili-Firoozinezhad S, Xu L, Eckstein F, Martin I, Kaufmann BA, Banfi A, Marsano A. Scaffold composition determines the angiogenic outcome of cell-based vascular endothelial growth factor expression by modulating its microenvironmental distribution. Adv Healthcare Mater 6: 1700600, 2017. [DOI] [PubMed] [Google Scholar]
- 163.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 27: 3675–3683, 2006. [DOI] [PubMed] [Google Scholar]
- 164.Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, Mathieu-Costello O, Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med 2: 534–539, 1996. [DOI] [PubMed] [Google Scholar]
- 165.Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am J Physiol 268: C1362–C1368, 1995. [DOI] [PubMed] [Google Scholar]
- 166.Goh S-K, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34: 6760–6772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem 269: 4355–4359, 1994. [PubMed] [Google Scholar]
- 168.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 7: 720–725, 2007. [DOI] [PubMed] [Google Scholar]
- 169.Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowski JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol 292: H2176–H2183, 2007. [DOI] [PubMed] [Google Scholar]
- 170.Goldsmith HS. Long term evaluation of omental transposition for chronic lymphedema. Ann Surg 180: 847–849, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goldsmith HS. Omental transposition for peripheral vascular insufficiency. Rev Surg 24: 379–380, 1967. [PubMed] [Google Scholar]
- 172.Goldsmith HS. Salvage of end stage ischemic extremities by intact omentum. Surgery 88: 732–736, 1980. [PubMed] [Google Scholar]
- 173.Goldsmith HS, Chen W-F, Duckett SW. Brain vascularization by intact omentum. Arch Surg 106: 695–698, 1973. [DOI] [PubMed] [Google Scholar]
- 174.Goldsmith HS, Griffith AL, Catsimpoolas N. Increased vascular perfusion after administration of an omental lipid fraction. Surg Gynecol Obstet 162: 579–583, 1986. [PubMed] [Google Scholar]
- 175.Goldsmith HS, Griffith AL, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. JAMA 252: 2034–2036, 1984. [PubMed] [Google Scholar]
- 176.Golub JS, Kim Y, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, Weiss D, Robert Taylor W, Guldberg RE. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol Heart Circ Physiol 298: H1959–H1965, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gosain A, Matthies AM, Dovi JV, Barbul A, Gamelli RL, DiPietro LA. Exogenous pro-angiogenic stimuli cannot prevent physiologic vessel regression. J Surg Res 135: 218–225, 2006. [DOI] [PubMed] [Google Scholar]
- 178.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol 128: 475–484, 1986. [DOI] [PubMed] [Google Scholar]
- 179.Göthert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Göttgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood 104: 1769–1777, 2004. [DOI] [PubMed] [Google Scholar]
- 180.Graham AM, Sniderman A, Jothy S, Homan J, Symes JF. Staged reversal of venous flow for revascularization of the severely ischemic limb. J Surg Res 35: 11–20, 1983. [DOI] [PubMed] [Google Scholar]
- 181.Grainger SJ, Carrion B, Ceccarelli J, Putnam AJ. Stromal cell identity influences the in vivo functionality of engineered capillary networks formed by co-delivery of endothelial cells and stromal cells. Tissue Eng A 19: 1209–1222, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Groenman FA, Rutter M, Wang J, Caniggia I, Tibboel D, Post M. Effect of chemical stabilizers of hypoxia-inducible factors on early lung development. Am J Physiol Lung Cell Mol Physiol 293: L557–L567, 2007. [DOI] [PubMed] [Google Scholar]
- 183.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175–189, 2006. [DOI] [PubMed] [Google Scholar]
- 184.Güc E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, Swartz MA. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 131: 160–175, 2017. [DOI] [PubMed] [Google Scholar]
- 185.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93: 1023–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 186.Guo L, Pribaz JJ. Clinical flap prefabrication. Plast Reconstr Surg 124: 340e–350e, 2009. [DOI] [PubMed] [Google Scholar]
- 187.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res 105: 724–736, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hämäläinen KM, Määttä E, Piirainen H, Marianne S, Väisänen A, Ranta V-P, Urtti A. Roles of acid/base nature and molecular weight in drug release from matrices of gelfoam and monoisopropyl ester of poly(vinyl methyl ether-maleic anhydride). J Control Release 56: 273–283, 1998. [DOI] [PubMed] [Google Scholar]
- 189.Han J-K, Chang S-H, Cho H-J, Choi S-B, Ahn H-S, Lee J, Jeong H, Youn S-W, Lee H-J, Kwon Y-W, Cho H-J, Oh B-H, Oettgen P, Park Y-B, Kim H-S. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation 130: 1168–1178, 2014. [DOI] [PubMed] [Google Scholar]
- 190.Hao X, Silva EA, Månsson-Broberg A, Grinnemo K-H, Siddiqui AJ, Dellgren G, Wärdell E, Brodin LA, Mooney DJ, Sylvén C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res 75: 178–185, 2007. [DOI] [PubMed] [Google Scholar]
- 191.Harada K, Friedman M, Lopez JJ, Wang SY, Li J, Prasad PV, Pearlman JD, Edelman ER, Sellke FW, Simons M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am J Physiol 270: H1791–H1802, 1996. [DOI] [PubMed] [Google Scholar]
- 192.Harris LD, Kim B-S, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res 42: 396–402, 1998. [DOI] [PubMed] [Google Scholar]
- 193.Harrison P, Cramer EM. Platelet α-granules. Blood Rev 7: 52–62, 1993. [DOI] [PubMed] [Google Scholar]
- 194.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 109: 1615–1622, 2004. [DOI] [PubMed] [Google Scholar]
- 195.Heidenreich R, Murayama T, Silver M, Essl C, Asahara T, Röcken M, Breier G. Tracking adult neovascularization during ischemia and inflammation using Vegfr2-LacZ reporter mice. J Vasc Res 45: 437–444, 2008. [DOI] [PubMed] [Google Scholar]
- 196.Heintz KA, Bregenzer ME, Mantle JL, Lee KH, West JL, Slater JH. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Adv Healthcare Mater 5: 2153–2160, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hellström M, Phng L-K, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson A-K, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. [DOI] [PubMed] [Google Scholar]
- 198.Helm C-LE, Fleury ME, Zisch AH, Boschetti F, Swartz MA. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci USA 102: 15779–15784, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO. Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation 101: 118–121, 2000. [DOI] [PubMed] [Google Scholar]
- 200.Henderson PW, Nagineni VV, Harper A, Bavinck N, Sohn AM, Krijgh DD, Jimenez N, Weinstein AL, Spector JA. Development of an acellular bioengineered matrix with a dominant vascular pedicle. J Surg Res 164: 1–5, 2010. [DOI] [PubMed] [Google Scholar]
- 201.Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3: 411–421, 2003. [DOI] [PubMed] [Google Scholar]
- 202.Hendrix MJC, Seftor EA, Meltzer PS, Gardner LMG, Hess AR, Kirschmann DA, Schatteman GC, Seftor REB. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 98: 8018–8023, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hickey MJ, Wilson Y, Hurley JV, Morrison WA. Mode of vascularization of control and basic fibroblast growth factor-stimulated prefabricated skin flaps. Plast Reconstr Surg 101: 1296–1304; discussion 1305-1306, 1998. [PubMed] [Google Scholar]
- 204.Hijjawi J, Mogford JE, Chandler LA, Cross KJ, Said H, Sosnowski BA, Mustoe TA. Platelet-derived growth factor B, but not fibroblast growth factor 2, plasmid DNA improves survival of ischemic myocutaneous flaps. Arch Surg 139: 142–147, 2004. [DOI] [PubMed] [Google Scholar]
- 205.Hikimoto D, Nishiguchi A, Matsusaki M, Akashi M. High-throughput blood- and lymph-capillaries with open-ended pores which allow the transport of drugs and cells. Adv Healthcare Mater 5: 1969–1978, 2016. [DOI] [PubMed] [Google Scholar]
- 206.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003. [DOI] [PubMed] [Google Scholar]
- 207.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng 12: 1475–1487, 2006. [DOI] [PubMed] [Google Scholar]
- 208.Ho H-KV, Jang JJ, Kaji S, Spektor G, Fong A, Yang P, Hu BS, Schatzman R, Quertermous T, Cooke JP. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: its role in ischemia. Circulation 109: 1314–1319, 2004. [DOI] [PubMed] [Google Scholar]
- 209.Höckel M, Burke JF. Angiotropin treatment prevents flap necrosis and enhances dermal regeneration in rabbits. Arch Surg 124: 693–698, 1989. [DOI] [PubMed] [Google Scholar]
- 210.Höckel M, Jung W, Vaupel P, Rabes H, Khaledpour C, Wissler JH. Purified monocyte-derived angiogenic substance (angiotropin) induces controlled angiogenesis associated with regulated tissue proliferation in rabbit skin. J Clin Invest 82: 1075–1090, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Höckel M, Schlenger K, Doctrow S, Kissel T, Vaupel P. Therapeutic angiogenesis. Arch Surg 128: 423–429, 1993. [DOI] [PubMed] [Google Scholar]
- 212.Hofer SOP, Knight KM, Cooper-White JJ, O’Connor AJ, Perera JM, Romeo-Meeuw R, Penington AJ, Knight KR, Morrison WA, Messina A. Increasing the volume of vascularized tissue formation in engineered constructs: an experimental study in rats. Plast Reconstr Surg 111: 1186–1192; discussion 1193-1194, 2003. [DOI] [PubMed] [Google Scholar]
- 213.Hom DB, Baker SR, Graham LM, McClatchey KD. Utilizing angiogenic agents to expedite the neovascularization process in skin flaps. Laryngoscope 98: 521–526, 1988. [DOI] [PubMed] [Google Scholar]
- 214.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science 296: 2404–2407, 2002. [DOI] [PubMed] [Google Scholar]
- 215.Hori Y, Tamai S, Okuda H, Sakamoto H, Takita T, Masuhara K. Blood vessel transplantation to bone. J Hand Surg 4: 23–33, 1979. [DOI] [PubMed] [Google Scholar]
- 216.Hosaka A, Koyama H, Kushibiki T, Tabata Y, Nishiyama N, Miyata T, Shigematsu H, Takato T, Nagawa H. Gelatin hydrogel microspheres enable pinpoint delivery of basic fibroblast growth factor for the development of functional collateral vessels. Circulation 110: 3322–3328, 2004. [DOI] [PubMed] [Google Scholar]
- 217.Hoshino S, Hamada O, Iwaya F, Takahira H, Honda K. Omental transplantation for chronic occlusive arterial diseases. Int Surg 64: 21–29, 1979. [PubMed] [Google Scholar]
- 218.Hsu Y-H, Moya ML, Hughes CCW, George SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 13: 2990–2998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang Y-C, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res 20: 848–857, 2005. [DOI] [PubMed] [Google Scholar]
- 220.Huang Y, Marui A, Sakaguchi H, Esaki J, Arai Y, Hirose K, Bir SC, Horiuchi H, Maruyama T, Ikeda T, Tabata Y, Komeda M. Sustained release of prostaglandin E1 potentiates the impaired therapeutic angiogenesis by basic fibroblast growth factor in diabetic murine hindlimb ischemia. Circ J 72: 1693–1699, 2008. [DOI] [PubMed] [Google Scholar]
- 221.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432: 625–630, 2004. [DOI] [PubMed] [Google Scholar]
- 222.Hudlicka O What makes blood vessels grow? J Physiol 444: 1–24, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hudlická O Capillary growth: role of mechanical factors. News Physiol Sci 3: 117–120, 1988. [Google Scholar]
- 224.Hudlická O Development of microcirculation: capillary growth and adaptation In: Handbook of Physiology; Section 2: The Cardiovascular System, edited by Renkin EM, Michel CC. Bethesda, MD: American Physiological Society, 1984, p. 165–215. [Google Scholar]
- 225.Hur J, Yang H-M, Yoon C-H, Lee C-S, Park K-W, Kim J-H, Kim T-Y, Kim J-Y, Kang H-J, Chae I-H, Oh B-H, Park Y-B, Kim H-S. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation 116: 1671–1682, 2007. [DOI] [PubMed] [Google Scholar]
- 226.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24: 288–293, 2004. [DOI] [PubMed] [Google Scholar]
- 227.Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: α-lactalbumin transport. Am J Physiol 252: H188–H197, 1987. [DOI] [PubMed] [Google Scholar]
- 228.Ichioka S, Kudo S, Shibata M, Ando J, Sekiya N, Nakatsuka T. Bone marrow cell implantation improves flap viability after ischemia-reperfusion injury. Ann Plast Surg 52: 414–418, 2004. [DOI] [PubMed] [Google Scholar]
- 229.Ii M, Horii M, Yokoyama A, Shoji T, Mifune Y, Kawamoto A, Asahi M, Asahara T. Synergistic effect of adipose-derived stem cell therapy and bone marrow progenitor recruitment in ischemic heart. Lab Invest 91: 539–552, 2011. [DOI] [PubMed] [Google Scholar]
- 230.Ikenaga S, Hamano K, Nishida M, Kobayashi T, Li T-S, Kobayashi S, Matsuzaki M, Zempo N, Esato K. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J Surg Res 96: 277–283, 2001. [DOI] [PubMed] [Google Scholar]
- 231.Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell 58: 803–805, 1989. [DOI] [PubMed] [Google Scholar]
- 232.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells utilizing human peripheral and umbilical cord blood. Blood 104: 2752–2760, 2004. [DOI] [PubMed] [Google Scholar]
- 233.Inoue N, Kondo T, Kobayashi K, Aoki M, Numaguchi Y, Shibuya M, Murohara T. Therapeutic angiogenesis using novel vascular endothelial growth factor-E/human placental growth factor chimera genes. Arterioscler Thromb Vasc Biol 27: 99–105, 2007. [DOI] [PubMed] [Google Scholar]
- 234.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 348: 370–374, 1996. [DOI] [PubMed] [Google Scholar]
- 235.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 105: 732–738, 2002. [DOI] [PubMed] [Google Scholar]
- 236.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation 113: 1605–1614, 2006. [DOI] [PubMed] [Google Scholar]
- 237.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 113: 1311–1325, 2006. [DOI] [PubMed] [Google Scholar]
- 238.Iwasawa M Accelerated maturation in prefabricated flaps by transforming growth factor-β: an experimental study in the rabbit. Ann Plast Surg 31: 72–75, 1993. [PubMed] [Google Scholar]
- 239.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 66: 543–551, 2005. [DOI] [PubMed] [Google Scholar]
- 240.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62, 2005. [DOI] [PubMed] [Google Scholar]
- 241.Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J 22: 2949–2956, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276: 1423–1425, 1997. [DOI] [PubMed] [Google Scholar]
- 243.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci USA 112: 214–219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jin DK, Shido K, Kopp H-G, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 12: 557–567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, Varner J. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest 116: 652–662, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jusoh N, Oh S, Kim S, Kim J, Jeon NL. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab Chip 15: 3984–3988, 2015. [DOI] [PubMed] [Google Scholar]
- 247.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev 82: 673–700, 2002. [DOI] [PubMed] [Google Scholar]
- 248.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res 21: 735–744, 2006. [DOI] [PubMed] [Google Scholar]
- 249.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng 6: 105–117, 2000. [DOI] [PubMed] [Google Scholar]
- 250.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi S-I, Isner JM, Asahara T. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 86: 1198–1202, 2000. [DOI] [PubMed] [Google Scholar]
- 251.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97: 3422–3427, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104: 1046–1052, 2001. [DOI] [PubMed] [Google Scholar]
- 253.Kanematsu A, Marui A, Yamamoto S, Ozeki M, Hirano Y, Yamamoto M, Ogawa O, Komeda M, Tabata Y. Type I collagen can function as a reservoir of basic fibroblast growth factor. J Control Release 99: 281–292, 2004. [DOI] [PubMed] [Google Scholar]
- 254.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34: 312–319, 2016. [DOI] [PubMed] [Google Scholar]
- 255.Kang K-T, Allen P, Bischoff J. Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood 118: 6718–6721, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest 115: 1816–1827, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA 95: 1062–1066, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kawamoto A, Gwon H-C, Iwaguro H, Yamaguchi J-I, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103: 634–637, 2001. [DOI] [PubMed] [Google Scholar]
- 259.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 114: 2163–2169, 2006. [DOI] [PubMed] [Google Scholar]
- 260.Kawamoto A, Murayama T, Kusano K, Ii M, Tkebuchava T, Shintani S, Iwakura A, Johnson I, von Samson P, Hanley A, Gavin M, Curry C, Silver M, Ma H, Kearney M, Losordo DW. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation 110: 1398–1405, 2004. [DOI] [PubMed] [Google Scholar]
- 261.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107: 461–468, 2003. [DOI] [PubMed] [Google Scholar]
- 262.Kelly JL, Findlay MW, Knight KR, Penington A, Thompson EW, Messina A, Morrison WA. Contact with existing adipose tissue is inductive for adipogenesis in Matrigel. Tissue Eng 12: 2041–2047, 2006. [DOI] [PubMed] [Google Scholar]
- 263.Kempen DHR, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJA. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30: 2816–2825, 2009. [DOI] [PubMed] [Google Scholar]
- 264.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109: 2679–2687, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kerbel RS, Benezra R, Lyden DC, Hattori K, Heissig B, Nolan DJ, Mittal V, Shaked Y, Dias S, Bertolini F, Rafii S. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci USA 105: E54; author reply E55, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Kröber SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med 12: 230–234, 2006. [DOI] [PubMed] [Google Scholar]
- 267.Kessel RG, Kardon RH. Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy. San Francisco, CA: W. H. Freeman, 1979. [Google Scholar]
- 268.Khouri RK, Brown DM, Leal-Khouri SM, Tark KC, Shaw WW. The effect of basic fibroblast growth factor on the neovascularisation process: skin flap survival and staged flap transfers. Br J Plast Surg 44: 585–588, 1991. [DOI] [PubMed] [Google Scholar]
- 269.Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med 15: 657–664, 2009. [DOI] [PubMed] [Google Scholar]
- 270.Kim EJ, Li R-K, Weisel RD, Mickle DAG, Jia Z-Q, Tomita S, Sakai T, Yau TM. Angiogenesis by endothelial cell transplantation. J Thorac Cardiovasc Surg 122: 963–971, 2001. [DOI] [PubMed] [Google Scholar]
- 271.Kim H-Z, Jung K, Kim HM, Cheng Y, Koh GY. A designed angiopoietin-2 variant, pentameric COMP-Ang2, strongly activates Tie2 receptor and stimulates angiogenesis. Biochim Biophys Acta 1793: 772–780, 2009. [DOI] [PubMed] [Google Scholar]
- 272.Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ. Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis. Nano Lett 11: 694–700, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kim JA, Kim HN, Im S-K, Chung S, Kang JY, Choi N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 9: 024115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kim KE, Cho C-H, Kim H-Z, Baluk P, McDonald DM, Koh GY. In vivo actions of angiopoietins on quiescent and remodeling blood and lymphatic vessels in mouse airways and skin. Arterioscler Thromb Vasc Biol 27: 564–570, 2007. [DOI] [PubMed] [Google Scholar]
- 275.Kim S, Chung M, Ahn J, Lee S, Jeon NL. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab Chip 16: 4189–4199, 2016. [DOI] [PubMed] [Google Scholar]
- 276.Kim S, Chung M, Jeon NL. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials 78: 115–128, 2016. [DOI] [PubMed] [Google Scholar]
- 277.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13: 1489–1500, 2013. [DOI] [PubMed] [Google Scholar]
- 278.Kimura Y, Inamoto T, Tabata Y. Adipose tissue formation in collagen scaffolds with different biodegradabilities. J Biomater Sci Polym Ed 21: 463–476, 2010. [DOI] [PubMed] [Google Scholar]
- 279.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials 24: 2513–2521, 2003. [DOI] [PubMed] [Google Scholar]
- 280.Kingsnorth AN, Slavin J. Peptide growth factors and wound healing. Br J Surg 78: 1286–1290, 1991. [DOI] [PubMed] [Google Scholar]
- 281.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94: 678–685, 2004. [DOI] [PubMed] [Google Scholar]
- 282.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109: 1543–1549, 2004. [DOI] [PubMed] [Google Scholar]
- 283.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15: 378–386, 2005. [DOI] [PubMed] [Google Scholar]
- 284.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324: 59–63, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kneser U, Polykandriotis E, Ohnolz J, Heidner K, Grabinger L, Euler S, Amann KU, Hess A, Brune K, Greil P, Stürzl M, Horch RE. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng 12: 1721–1731, 2006. [DOI] [PubMed] [Google Scholar]
- 286.Kniazeva E, Kachgal S, Putnam AJ. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng A 17: 905–914, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, Zimmerman C, Clouse C, Zhao W, Shupe TD, Soker S, Yoo JJ, Atala A. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials 40: 72–79, 2015. [DOI] [PubMed] [Google Scholar]
- 288.Kobayashi K, Kondo T, Inoue N, Aoki M, Mizuno M, Komori K, Yoshida J, Murohara T. Combination of in vivo angiopoietin-1 gene transfer and autologous bone marrow cell implantation for functional therapeutic angiogenesis. Arterioscler Thromb Vasc Biol 26: 1465–1472, 2006. [DOI] [PubMed] [Google Scholar]
- 289.Kobayashi T, Hamano K, Li T-S, Katoh T, Kobayashi S, Matsuzaki M, Esato K. Enhancement of angiogenesis by the implantation of self bone marrow cells in a rat ischemic heart model. J Surg Res 89: 189–195, 2000. [DOI] [PubMed] [Google Scholar]
- 290.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7: 430–436, 2001. [DOI] [PubMed] [Google Scholar]
- 291.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci USA 108: 14789–14794, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Koh YJ, Koh BI, Kim H, Joo HJ, Jin HK, Jeon J, Choi C, Lee DH, Chung JH, Cho C-H, Park WS, Ryu J-K, Suh JK, Koh GY. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol 31: 1141–1150, 2011. [DOI] [PubMed] [Google Scholar]
- 293.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Creation of long-lasting blood vessels. Nature 428: 138–139, 2004. [DOI] [PubMed] [Google Scholar]
- 294.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA 113: 3179–3184, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26: 3124–3130, 2014. [DOI] [PubMed] [Google Scholar]
- 296.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol 29: 61–66, 2009. [DOI] [PubMed] [Google Scholar]
- 297.Kong HJ, Kim ES, Huang Y-C, Mooney DJ. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res 25: 1230–1238, 2008. [DOI] [PubMed] [Google Scholar]
- 298.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci 112: 3249–3258, 1999. [DOI] [PubMed] [Google Scholar]
- 299.Korpisalo P, Karvinen H, Rissanen TT, Kilpijoki J, Marjomäki V, Baluk P, McDonald DM, Cao Y, Eriksson U, Alitalo K, Ylä-Herttuala S. Vascular endothelial growth factor-A and platelet-derived growth factor-B combination gene therapy prolongs angiogenic effects via recruitment of interstitial mononuclear cells and paracrine effects rather than improved pericyte coverage of angiogenic vessels. Circ Res 103: 1092–1099, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Koshima I, Yamamoto T, Narushima M, Mihara M, Iida T. Perforator flaps and supermicrosurgery. Clin Plast Surg 37: 683–689, 2010. [DOI] [PubMed] [Google Scholar]
- 301.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105: 369–377, 2001. [DOI] [PubMed] [Google Scholar]
- 302.Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ. The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg 53: 234–239, 2000. [DOI] [PubMed] [Google Scholar]
- 303.Kubota Y, Kishi K, Satoh H, Tanaka T, Nakajima H, Nakajima T. Transplanted endothelial progenitor cells augment the survival areas of rat dorsal flaps. Cell Transplant 12: 647–657, 2003. [DOI] [PubMed] [Google Scholar]
- 304.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 107: 1589–1598, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kumar VA, Liu Q, Wickremasinghe NC, Shi S, Cornwright TT, Deng Y, Azares A, Moore AN, Acevedo-Jake AM, Agudo NR, Pan S, Woodside DG, Vanderslice P, Willerson JT, Dixon RA, Hartgerink JD. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials 98: 113–119, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kumar VA, Taylor NL, Shi S, Wang BK, Jalan AA, Kang MK, Wickremasinghe NC, Hartgerink JD. Highly angiogenic peptide nanofibers. ACS Nano 9: 860–868, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 289: L529–L535, 2005. [DOI] [PubMed] [Google Scholar]
- 308.Kurita J, Miyamoto M, Ishii Y, Aoyama J, Takagi G, Naito Z, Tabata Y, Ochi M, Shimizu K. Enhanced vascularization by controlled release of platelet-rich plasma impregnated in biodegradable gelatin hydrogel. Ann Thorac Surg 92: 837–844; discussion 844, 2011. [DOI] [PubMed] [Google Scholar]
- 309.Laham RJ, Oettgen P. Bone marrow transplantation for the heart: fact or fiction? Lancet 361: 11–12, 2003. [DOI] [PubMed] [Google Scholar]
- 310.Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, Simons M, Hung D. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther 292: 795–802, 2000. [PubMed] [Google Scholar]
- 311.Lähteenvuo J, Ylä-Herttuala S. Advances and challenges in cardiovascular gene therapy. Hum Gene Ther 28: 1024–1032, 2017. [DOI] [PubMed] [Google Scholar]
- 312.Lähteenvuo JE, Lähteenvuo MT, Kivelä A, Rosenlew C, Falkevall A, Klar J, Heikura T, Rissanen TT, Vähäkangas E, Korpisalo P, Enholm B, Carmeliet P, Alitalo K, Eriksson U, Ylä-Herttuala S. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation 119: 845–856, 2009. [DOI] [PubMed] [Google Scholar]
- 313.Landis EM. Micro-injection studies of capillary permeability. II. The relation between capillary pressure and the rate at which fluid passes through the walls of single capillaries. Am J Physiol 82: 217–238, 1927. [Google Scholar]
- 314.Larsen M, Willems WF, Pelzer M, Friedrich PF, Yaszemski MJ, Bishop AT. Augmentation of surgical angiogenesis in vascularized bone allotransplants with host-derived a/v bundle implantation, fibroblast growth factor-2, and vascular endothelial growth factor administration. J Orthop Res 28: 1015–1021, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Laschke MW, Grässer C, Kleer S, Scheuer C, Eglin D, Alini M, Menger MD. Adipose tissue-derived microvascular fragments from aged donors exhibit an impaired vascularisation capacity. Eur Cell Mater 28: 287–298, 2014. [DOI] [PubMed] [Google Scholar]
- 316.Laschke MW, Kleer S, Scheuer C, Schuler S, Garcia P, Eglin D, Alini M, Menger MD. Vascularisation of porous scaffolds is improved by incorporation of adipose tissue-derived microvascular fragments. Eur Cell Mater 24: 266–277, 2012. [DOI] [PubMed] [Google Scholar]
- 317.Laschke MW, Mussawy H, Schuler S, Kazakov A, Rücker M, Eglin D, Alini M, Menger MD. Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue. Tissue Eng A 17: 841–853, 2011. [DOI] [PubMed] [Google Scholar]
- 318.Lazarous DF, Scheinowitz M, Shou M, Hodge E, Rajanayagam S, Hunsberger S, Robison WG Jr., Stiber JA, Correa R, Epstein SE, Unger EF. Effects of chronic systemic administration of basic fibroblast growth factor on collateral development in the canine heart. Circulation 91: 145–153, 1995. [DOI] [PubMed] [Google Scholar]
- 319.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, Epstein SE, Unger EF. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation 94: 1074–1082, 1996. [DOI] [PubMed] [Google Scholar]
- 320.Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E, Unger EF. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res 36: 78–85, 1997. [DOI] [PubMed] [Google Scholar]
- 321.Le Ricousse-Roussanne S, Barateau V, Contreres J-O, Boval B, Kraus-Berthier L, Tobelem G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc Res 62: 176–184, 2004. [DOI] [PubMed] [Google Scholar]
- 322.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 27: 3249–3255, 2006. [DOI] [PubMed] [Google Scholar]
- 323.Lee H, Cusick RA, Browne F, Ho Kim T, Ma PX, Utsunomiya H, Langer R, Vacanti JP. Local delivery of basic fibroblast growth factor increases both angiogenesis and engraftment of hepatocytes in tissue-engineered polymer devices. Transplantation 73: 1589–1593, 2002. [DOI] [PubMed] [Google Scholar]
- 324.Lee HJ, Cho C-H, Hwang S-J, Choi H-H, Kim K-T, Ahn SY, Kim J-H, Oh J-L, Lee GM, Koh GY. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J 18: 1200–1208, 2004. [DOI] [PubMed] [Google Scholar]
- 325.Lee J, Bhang SH, Park H, Kim B-S, Lee KY. Active blood vessel formation in the ischemic hindlimb mouse model using a microsphere/hydrogel combination system. Pharm Res 27: 767–774, 2010. [DOI] [PubMed] [Google Scholar]
- 326.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res 26: 1739–1744, 2009. [DOI] [PubMed] [Google Scholar]
- 327.Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release 87: 49–56, 2003. [DOI] [PubMed] [Google Scholar]
- 328.Lee M-J, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312, 1999. [DOI] [PubMed] [Google Scholar]
- 329.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 102: 898–901, 2000. [DOI] [PubMed] [Google Scholar]
- 330.Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo S-S, Vincent PA, Dai G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35: 8092–8102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lee VK, Lanzi AM, Ngo H, Yoo S-S, Vincent PA, Dai G. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng 7: 460–472, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng A 16: 115–125, 2010. [DOI] [PubMed] [Google Scholar]
- 333.Leung AD, Wong KHK, Tien J. Plasma expanders stabilize human microvessels in microfluidic scaffolds. J Biomed Mater Res A 100: 1815–1822, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23: 879–884, 2005. [DOI] [PubMed] [Google Scholar]
- 335.Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M. PR39, a peptide regulator of angiogenesis. Nat Med 6: 49–55, 2000. [DOI] [PubMed] [Google Scholar]
- 336.Li M-T, Ruehle MA, Stevens HY, Servies N, Willett NJ, Karthikeyakannan S, Warren GL, Guldberg RE, Krishnan L. Skeletal myoblast-seeded vascularized tissue scaffolds in the treatment of a large volumetric muscle defect in the rat biceps femoris muscle. Tissue Eng A 23: 989–1000, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Li R-K, Mickle DAG, Weisel RD, Mohabeer MK, Zhang J, Rao V, Li G, Merante F, Jia Z-Q. Natural history of fetal rat cardiomyocytes transplanted into adult rat myocardial scar tissue. Circulation 96: II-179–II-186; discussion II-186-II-187, 1997. [PubMed] [Google Scholar]
- 338.Li X, Tjwa M, Moons L, Fons P, Noel A, Ny A, Zhou JM, Lennartsson J, Li H, Luttun A, Pontén A, Devy L, Bouché A, Oh H, Manderveld A, Blacher S, Communi D, Savi P, Bono F, Dewerchin M, Foidart J-M, Autiero M, Herbert J-M, Collen D, Heldin C-H, Eriksson U, Carmeliet P. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J Clin Invest 115: 118–127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Li X, Tjwa M, Van Hove I, Enholm B, Neven E, Paavonen K, Jeltsch M, Juan TD, Sievers RE, Chorianopoulos E, Wada H, Vanwildemeersch M, Noel A, Foidart J-M, Springer ML, von Degenfeld G, Dewerchin M, Blau HM, Alitalo K, Eriksson U, Carmeliet P, Moons L. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol 28: 1614–1620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Li X, Xia J, Nicolescu CT, Massidda MW, Ryan TJ, Tien J. Engineering of microscale vascularized fat that responds to perfusion with lipoactive hormones. Biofabrication 11: 014101, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Li X, Xu J, Nicolescu CT, Marinelli JT, Tien J. Generation, endothelialization, and microsurgical suture anastomosis of strong 1-mm-diameter collagen tubes. Tissue Eng A 23: 335–344, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Li Y, Song Y, Zhao L, Gaidosh G, Laties AM, Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc 3: 1703–1708, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lin R-Z, Lee CN, Moreno-Luna R, Neumeyer J, Piekarski B, Zhou P, Moses MA, Sachdev M, Pu WT, Emani S, Melero-Martin JM. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat Biomed Eng 1: 0081, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105: 71–77, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellström M, Bäckström G, Fredriksson S, Landegren U, Nyström HC, Bergström G, Dejana E, Östman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17: 1835–1840, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Linville RM, Boland NF, Covarrubias G, Price GM, Tien J. Physical and chemical signals that promote vascularization of capillary-scale channels. Cell Mol Bioeng 9: 73–84, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Linville RM, DeStefano JG, Sklar MB, Xu Z, Farrell AM, Bogorad MI, Chu C, Walczak P, Cheng L, Mahairaki V, Whartenby KA, Calabresi PA, Searson PC. Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials 190-191: 24–37, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lohela M, Heloterä H, Haiko P, Dumont DJ, Alitalo K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am J Pathol 173: 1891–1901, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lokmic Z, Stillaert F, Morrison WA, Thompson EW, Mitchell GM. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J 21: 511–522, 2007. [DOI] [PubMed] [Google Scholar]
- 350.Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Caputo RP, Carrozza JP, Douglas PS, Sellke FW, Simons M. Basic fibroblast growth factor in a porcine model of chronic myocardial ischemia: a comparison of angiographic, echocardiographic and coronary flow parameters. J Pharmacol Exp Ther 282: 385–390, 1997. [PubMed] [Google Scholar]
- 351.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 109: 2487–2491, 2004. [DOI] [PubMed] [Google Scholar]
- 352.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 98: 2800–2804, 1998. [DOI] [PubMed] [Google Scholar]
- 353.Lu F, Li J, Gao J, Ogawa R, Ou C, Yang B, Fu B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg 124: 1437–1446, 2009. [DOI] [PubMed] [Google Scholar]
- 354.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert J-M, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840, 2002. [DOI] [PubMed] [Google Scholar]
- 355.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7: 1194–1201, 2001. [DOI] [PubMed] [Google Scholar]
- 356.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 20: 4762–4773, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Malekan R, Reynolds C, Narula N, Kelley ST, Suzuki Y, Bridges CR. Angiogenesis in transmyocardial laser revascularization: a nonspecific response to injury. Circulation 98: II62–II65; discussion II66, 1998. [PubMed] [Google Scholar]
- 358.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe’er J, Trent JM, Meltzer PS, Hendrix MJC. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155: 739–752, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Manson PN, Im MJ, Myers RAM, Hoopes JE. Improved capillaries by hyperbaric oxygen in skin flaps. Surg Forum 31: 564–566, 1980. [Google Scholar]
- 360.Marino D, Luginbühl J, Scola S, Meuli M, Reichmann E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci Transl Med 6: 221ra214, 2014. [DOI] [PubMed] [Google Scholar]
- 361.Marshall AJ, Irvin CA, Barker T, Sage EH, Hauch KD, Ratner BD. Biomaterials with tightly controlled pore size that promote vascular in-growth. Polym Prepr 45: 100–101, 2004. [Google Scholar]
- 362.Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Müller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343: 885–888, 2014. [DOI] [PubMed] [Google Scholar]
- 363.Marui A, Kanematsu A, Yamahara K, Doi K, Kushibiki T, Yamamoto M, Itoh H, Ikeda T, Tabata Y, Komeda M. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J Vasc Surg 41: 82–90, 2005. [DOI] [PubMed] [Google Scholar]
- 364.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 115: 2363–2372, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg 160: 519–524, 1990. [DOI] [PubMed] [Google Scholar]
- 366.Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, Nakagawa K, Hou X, Nagai Y, Hasegawa M, Sugimachi K, Sueishi K. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res 90: 966–973, 2002. [DOI] [PubMed] [Google Scholar]
- 367.McAuslan BR, Reilly WG, Hannan GN, Gole GA. Angiogenic factors and their assay: activity of formyl methionyl leucyl phenylalanine, adenosine diphosphate, heparin, copper, and bovine endothelium stimulating factor. Microvasc Res 26: 323–338, 1983. [DOI] [PubMed] [Google Scholar]
- 368.McCluer RH, Evans JE, Williams M, Griffith AL, Catsimpoolas N. Characterization of feline omentum lipids. Lipids 22: 229–235, 1987. [DOI] [PubMed] [Google Scholar]
- 369.McDonald DM, Munn L, Jain RK. Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol 156: 383–388, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol 6: 731–744, 1954. [DOI] [PubMed] [Google Scholar]
- 371.Melero-Martin JM, De Obaldia ME, Allen P, Dudley AC, Klagsbrun M, Bischoff J. Host myeloid cells are necessary for creating bioengineered human vascular networks in vivo. Tissue Eng A 16: 2457–2466, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109: 4761–4768, 2007. [DOI] [PubMed] [Google Scholar]
- 373.Meltzer T, Myers B. The effect of hyperbaric oxygen on the bursting strength and rate of vascularization of skin wounds in the rat. Am Surg 52: 659–662, 1986. [PubMed] [Google Scholar]
- 374.Mertsching H, Schanz J, Steger V, Schandar M, Schenk M, Hansmann J, Dally I, Friedel G, Walles T. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation 88: 203–210, 2009. [DOI] [PubMed] [Google Scholar]
- 375.Messina A, Bortolotto SK, Cassell OCS, Kelly J, Abberton KM, Morrison WA. Generation of a vascularized organoid using skeletal muscle as the inductive source. FASEB J 19: 1570–1572, 2005. [DOI] [PubMed] [Google Scholar]
- 376.Mian R, Morrison WA, Hurley JV, Penington AJ, Romeo R, Tanaka Y, Knight KR. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng 6: 595–603, 2000. [DOI] [PubMed] [Google Scholar]
- 377.Michel CC. Fluid movements through capillary walls In: Handbook of Physiology; Section 2: The Cardiovascular System, edited by Renkin EM, Michel CC. Bethesda, MD: American Physiological Society, 1984, p. 375–409. [Google Scholar]
- 378.Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999. [DOI] [PubMed] [Google Scholar]
- 379.Miles AA, Miles EM. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol 118: 228–257, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation 8: 229–241, 2001. [DOI] [PubMed] [Google Scholar]
- 381.Milkiewicz M, Pugh CW, Egginton S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J Physiol 560: 21–26, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11: 768–774, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, Chen CS. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat Biomed Eng 1: 0083, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110: 349–355, 2004. [DOI] [PubMed] [Google Scholar]
- 385.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol 21: 157–161, 2003. [DOI] [PubMed] [Google Scholar]
- 386.Modi S, Stanton AWB, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: a critical review of lymphoscintigraphy. Lymphat Res Biol 5: 183–202, 2007. [DOI] [PubMed] [Google Scholar]
- 387.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol 97: 1648–1652, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, Langer R. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J Biomed Mater Res 37: 413–420, 1997. [DOI] [PubMed] [Google Scholar]
- 389.Moore MAS, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol 18: 279–296, 1970. [DOI] [PubMed] [Google Scholar]
- 390.Mori N, Morimoto Y, Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 116: 48–56, 2017. [DOI] [PubMed] [Google Scholar]
- 391.Morin KT, Smith AO, Davis GE, Tranquillo RT. Aligned human microvessels formed in 3D fibrin gel by constraint of gel contraction. Microvasc Res 90: 12–22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Morin KT, Tranquillo RT. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials 32: 6111–6118, 2011. [DOI] [PubMed] [Google Scholar]
- 393.Morrison WA, Marre D, Grinsell D, Batty A, Trost N, O’Connor AJ. Creation of a large adipose tissue construct in humans using a tissue-engineering chamber: a step forward in the clinical application of soft tissue engineering. EBioMedicine 6: 238–245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation 115: 353–360, 2007. [DOI] [PubMed] [Google Scholar]
- 395.Moya ML, Hsu Y-H, Lee AP, Hughes CCW, George SC. In vitro perfused human capillary networks. Tissue Eng C 19: 730–737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mühlhauser J, Merrill MJ, Pili R, Maeda H, Bacic M, Bewig B, Passaniti A, Edwards NA, Crystal RG, Capogrossi MC. VEGF165 expressed by a replication-deficient recombinant adenovirus vector induces angiogenesis in vivo. Circ Res 77: 1077–1086, 1995. [DOI] [PubMed] [Google Scholar]
- 397.Mühlhauser J, Pili R, Merrill MJ, Maeda H, Passaniti A, Crystal RG, Capogrossi MC. In vivo angiogenesis induced by recombinant adenovirus vectors coding either for secreted or nonsecreted forms of acidic fibroblast growth factor. Hum Gene Ther 6: 1457–1465, 1995. [DOI] [PubMed] [Google Scholar]
- 398.Mund JA, Estes ML, Yoder MC, Ingram DA Jr., Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol 32: 1045–1053, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol 30: 967–972, 2002. [DOI] [PubMed] [Google Scholar]
- 400.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 105: 1527–1536, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest 87: 694–703, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-β. Science 237: 1333–1336, 1987. [DOI] [PubMed] [Google Scholar]
- 404.Mustoe TA, Tae Ahn S, Tarpley JE, Pierce GF. Role of hypoxia in growth factor responses: differential effects of basic fibroblast growth factor and platelet-derived growth factor in an ischemic wound model. Wound Repair Regen 2: 277–283, 1994. [DOI] [PubMed] [Google Scholar]
- 405.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 196: 1497–1506, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Manseau EJ, Dvorak AM, Dvorak HF. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol 67: 227–237, 2002. [DOI] [PubMed] [Google Scholar]
- 407.Nakasa T, Ishida O, Sunagawa T, Nakamae A, Yasunaga Y, Agung M, Ochi M. Prefabrication of vascularized bone graft using a combination of fibroblast growth factor-2 and vascular bundle implantation into a novel interconnected porous calcium hydroxyapatite ceramic. J Biomed Mater Res A 75: 350–355, 2005. [DOI] [PubMed] [Google Scholar]
- 408.Nakasa T, Ishida O, Sunagawa T, Nakamae A, Yokota K, Adachi N, Ochi M. Feasibility of prefabricated vascularized bone graft using the combination of FGF-2 and vascular bundle implantation within hydroxyapatite for osteointegration. J Biomed Mater Res A 85: 1090–1095, 2008. [DOI] [PubMed] [Google Scholar]
- 409.Nashimoto Y, Hayashi T, Kunita I, Nakamasu A, Torisawa Y, Nakayama M, Takigawa-Imamura H, Kotera H, Nishiyama K, Miura T, Yokokawa R. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr Biol 9: 506–518, 2017. [DOI] [PubMed] [Google Scholar]
- 410.Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res 68: 258–264, 2004. [DOI] [PubMed] [Google Scholar]
- 411.Nguyen D-HT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci USA 110: 6712–6717, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Nicosia RF. Angiogenesis and the formation of lymphaticlike channels in cultures of thoracic duct. In Vitro Cell Dev Biol 23: 167–174, 1987. [DOI] [PubMed] [Google Scholar]
- 413.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest 63: 115–122, 1990. [PubMed] [Google Scholar]
- 414.Nih LR, Gojgini S, Carmichael ST, Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat Mater 17: 642–651, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Nishikawa S-I, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125: 1747–1757, 1998. [DOI] [PubMed] [Google Scholar]
- 416.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest 81: 453–463, 2001. [DOI] [PubMed] [Google Scholar]
- 417.Nottebaert M, Lane JM, Juhn A, Burstein A, Schneider R, Klein C, Sinn RS, Dowling C, Cornell C, Catsimpoolas N. Omental angiogenic lipid fraction and bone repair. An experimental study in the rat. J Orthop Res 7: 157–169, 1989. [DOI] [PubMed] [Google Scholar]
- 418.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation 114: I114–I119, 2006. [DOI] [PubMed] [Google Scholar]
- 419.O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 2: 689–692, 1996. [DOI] [PubMed] [Google Scholar]
- 420.Ogawa K, Asonuma K, Inomata Y, Kim I, Ikada Y, Tabata Y, Tanaka K. The efficacy of prevascularization by basic FGF for hepatocyte transplantation using polymer devices in rats. Cell Transplant 10: 723–729, 2001. [PubMed] [Google Scholar]
- 421.Oh S-J, Jeltsch MM, Birkenhäger R, McCarthy JEG, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol 188: 96–109, 1997. [DOI] [PubMed] [Google Scholar]
- 422.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001. [DOI] [PubMed] [Google Scholar]
- 423.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16: 927–933, 2010. [DOI] [PubMed] [Google Scholar]
- 424.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 14: 213–221, 2008. [DOI] [PubMed] [Google Scholar]
- 425.Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, Takuwa Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc Res 78: 301–307, 2008. [DOI] [PubMed] [Google Scholar]
- 426.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 113: 516–527, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Padubidri A, Browne E Jr. Effect of vascular endothelial growth factor (VEGF) on survival of random extension of axial pattern skin flaps in the rat. Ann Plast Surg 37: 604–611, 1996. [DOI] [PubMed] [Google Scholar]
- 428.Pappenheimer JR, Soto-Rivera A. Effective osmotic pressure of the plasma proteins and other quantities associated with the capillary circulation in the hindlimbs of cats and dogs. Am J Physiol 152: 471–491, 1948. [DOI] [PubMed] [Google Scholar]
- 429.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 67: 519–528, 1992. [PubMed] [Google Scholar]
- 430.Pearlman JD, Hibberd MG, Chuang ML, Harada K, Lopez JJ, Gladstone SR, Friedman M, Sellke FW, Simons M. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med 1: 1085–1089, 1995. [DOI] [PubMed] [Google Scholar]
- 431.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95: 952–958, 2000. [PubMed] [Google Scholar]
- 432.Peirce SM, Price RJ, Skalak TC. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1*. Am J Physiol Heart Circ Physiol 286: H918–H925, 2004. [DOI] [PubMed] [Google Scholar]
- 433.Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AMJ. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 33: 518–523, 2015. [DOI] [PubMed] [Google Scholar]
- 434.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 189: 824–831, 1992. [DOI] [PubMed] [Google Scholar]
- 435.Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci Polym Ed 9: 1267–1278, 1998. [DOI] [PubMed] [Google Scholar]
- 436.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res 60: 668–678, 2002. [DOI] [PubMed] [Google Scholar]
- 437.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science 329: 538–541, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 80: 99–115, 2000. [DOI] [PubMed] [Google Scholar]
- 439.Phelps EA, Landázuri N, Thulé PM, Taylor WR, García AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci USA 107: 3323–3328, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJA, Brouwer LA, Veerkamp JH, van Kuppevelt TH. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res 62: 185–194, 2002. [DOI] [PubMed] [Google Scholar]
- 441.Pierce GF, Tarpley JE, Yanagihara D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-β1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol 140: 1375–1388, 1992. [PMC free article] [PubMed] [Google Scholar]
- 442.Piguet PF, Grau GE, Vassalli P. Subcutaneous perfusion of tumor necrosis factor induces local proliferation of fibroblasts, capillaries, and epidermal cells, or massive tissue necrosis. Am J Pathol 136: 103–110, 1990. [PMC free article] [PubMed] [Google Scholar]
- 443.Pilia M, McDaniel JS, Guda T, Chen XK, Rhoads RP, Allen RE, Corona BT, Rathbone CR. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur Cell Mater 28: 11–23; discussion 23-24, 2014. [DOI] [PubMed] [Google Scholar]
- 444.Planat-Benard V, Silvestre J-S, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109: 656–663, 2004. [DOI] [PubMed] [Google Scholar]
- 445.Polverini PJ, Cotran RS, Gimbrone MA Jr., Unanue ER. Activated macrophages induce vascular proliferation. Nature 269: 804–806, 1977. [DOI] [PubMed] [Google Scholar]
- 446.Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, Fett A, Gupta M, Rapp BM, Saadatzadeh MR, Ginsberg M, Elemento O, Lee Y, Voytik-Harbin SL, Chung HM, Hong KS, Reid E, O’Neill CL, Medina RJ, Stitt AW, Murphy MP, Rafii S, Broxmeyer HE, Yoder MC. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol 32: 1151–1157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Price GM, Chrobak KM, Tien J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res 76: 46–51, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Price GM, Chu KK, Truslow JG, Tang-Schomer MD, Golden AP, Mertz J, Tien J. Bonding of macromolecular hydrogels using perturbants. J Am Chem Soc 130: 6664–6665, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Price GM, Wong KHK, Truslow JG, Leung AD, Acharya C, Tien J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31: 6182–6189, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Pu L-Q, Sniderman AD, Arekat Z, Graham AM, Brassard R, Symes JF. Angiogenic growth factor and revascularization of the ischemic limb: evaluation in a rabbit model. J Surg Res 54: 575–583, 1993. [DOI] [PubMed] [Google Scholar]
- 451.Pu L-Q, Sniderman AD, Brassard R, Lachapelle KJ, Graham AM, Lisbona R, Symes JF. Enhanced revascularization of the ischemic limb by angiogenic therapy. Circulation 88: 208–215, 1993. [DOI] [PubMed] [Google Scholar]
- 452.Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 105: 6620–6625, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, Mannino RG, Ciciliano JC, Lamb TJ, Joiner CH, Ofori-Acquah SF, Lam WA. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat Biomed Eng 2: 453–463, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Rafii S, Butler JM, Ding B-S. Angiocrine functions of organ-specific endothelial cells. Nature 529: 316–325, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng A 16: 2255–2263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Rana JS, Mannam A, Donnell-Fink L, Gervino EV, Sellke FW, Laham RJ. Longevity of the placebo effect in the therapeutic angiogenesis and laser myocardial revascularization trials in patients with coronary heart disease. Am J Cardiol 95: 1456–1459, 2005. [DOI] [PubMed] [Google Scholar]
- 457.Rashid MA, Akita S, Razzaque MS, Yoshimoto H, Ishihara H, Fujii T, Tanaka K, Taguchi T. Coadministration of basic fibroblast growth factor and sucrose octasulfate (sucralfate) facilitates the rat dorsal flap survival and viability. Plast Reconstr Surg 103: 941–948, 1999. [DOI] [PubMed] [Google Scholar]
- 458.Raval Z, Losordo DW. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res 112: 1288–1302, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003. [DOI] [PubMed] [Google Scholar]
- 460.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109: 1292–1298, 2004. [DOI] [PubMed] [Google Scholar]
- 461.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol 19: 1029–1034, 2001. [DOI] [PubMed] [Google Scholar]
- 462.Riemenschneider SB, Mattia DJ, Wendel JS, Schaefer JA, Ye L, Guzman PA, Tranquillo RT. Inosculation and perfusion of pre-vascularized tissue patches containing aligned human microvessels after myocardial infarction. Biomaterials 97: 51–61, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res 120: 1326–1340, 2017. [DOI] [PubMed] [Google Scholar]
- 464.Rinsch C, Quinodoz P, Pittet B, Alizadeh N, Baetens D, Montandon D, Aebischer P, Pepper MS. Delivery of FGF-2 but not VEGF by encapsulated genetically engineered myoblasts improves survival and vascularization in a model of acute skin flap ischemia. Gene Ther 8: 523–533, 2001. [DOI] [PubMed] [Google Scholar]
- 465.Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 74: 163–219, 1994. [DOI] [PubMed] [Google Scholar]
- 466.Rissanen TT, Korpisalo P, Markkanen JE, Liimatainen T, Ordén M-R, Kholová I, de Goede A, Heikura T, Gröhn OH, Ylä-Herttuala S. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation 112: 3937–3946, 2005. [DOI] [PubMed] [Google Scholar]
- 467.Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Närvänen O, Taipale P, Kauppinen RA, Rubanyi GM, Ylä-Herttuala S. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J 17: 100–102, 2003. [DOI] [PubMed] [Google Scholar]
- 468.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholová I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Ylä-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res 92: 1098–1106, 2003. [DOI] [PubMed] [Google Scholar]
- 469.Rivard A, Fabre J-E, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation 99: 111–120, 1999. [DOI] [PubMed] [Google Scholar]
- 470.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83: 4167–4171, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Rophael JA, Craft RO, Palmer JA, Hussey AJ, Thomas GPL, Morrison WA, Penington AJ, Mitchell GM. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am J Pathol 171: 2048–2057, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Rosenfeld D, Landau S, Shandalov Y, Raindel N, Freiman A, Shor E, Blinder Y, Vandenburgh HH, Mooney DJ, Levenberg S. Morphogenesis of 3D vascular networks is regulated by tensile forces. Proc Natl Acad Sci USA 113: 3215–3220, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Rubin PAD, Popham JK, Bilyk JR, Shore JW. Comparison of fibrovascular ingrowth into hydroxyapatite and porous polyethylene orbital implants. Ophthalmic Plast Reconstr Surg 10: 96–103, 1994. [DOI] [PubMed] [Google Scholar]
- 474.Rück A, Sylvén C. “Improvement” in the placebo group could be due to regression to the mean as well as to sociobiologic factors. Am J Cardiol 97: 152–153, 2006. [DOI] [PubMed] [Google Scholar]
- 475.Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial cells derived from human iPSCs increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol 31: e72–e79, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Saaristo A, Tammela T, Timonen J, Yla-Herttuala S, Tukiainen E, Asko-Seljavaara S, Alitalo K. Vascular endothelial growth factor-C gene therapy restores lymphatic flow across incision wounds. FASEB J 18: 1707–1709, 2004. [DOI] [PubMed] [Google Scholar]
- 477.Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat 1: 367–389, 1902. [Google Scholar]
- 478.Sabin FR. The Origin and Development of the Lymphatic System. Baltimore, MD: The Johns Hopkins Press, 1913. [Google Scholar]
- 479.Sabiston DC Jr., Fauteux JP, Blalock A. An experimental study of the fate of arterial implants in the left ventricular myocardium; with a comparison of similar implants in other organs. Ann Surg 145: 927–938; discussion, 938-942, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Sacchi V, Mittermayr R, Hartinger J, Martino MM, Lorentz KM, Wolbank S, Hofmann A, Largo RA, Marschall JS, Groppa E, Gianni-Barrera R, Ehrbar M, Hubbell JA, Redl H, Banfi A. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci USA 111: 6952–6957, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Sacks GA, Sandler MP, Born ML, Clanton JA, Franklin JD, Partain CL. Lymphoscintigraphy as an adjunctive procedure in the perioperative assessment of patients undergoing microlymphaticovenous anastomoses. Clin Nucl Med 8: 309–311, 1983. [DOI] [PubMed] [Google Scholar]
- 482.Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med 7: 382–383, 2001. [DOI] [PubMed] [Google Scholar]
- 483.Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, Okano T. In vitro engineering of vascularized tissue surrogates. Sci Rep 3: 1316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Sakakibara Y, Nishimura K, Tambara K, Yamamoto M, Lu F, Tabata Y, Komeda M. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg 124: 50–56, 2002. [DOI] [PubMed] [Google Scholar]
- 485.Sakakibara Y, Tambara K, Sakaguchi G, Lu F, Yamamoto M, Nishimura K, Tabata Y, Komeda M. Toward surgical angiogenesis using slow-released basic fibroblast growth factor. Eur J Cardiothorac Surg 24: 105–111; discussion 112, 2003. [DOI] [PubMed] [Google Scholar]
- 486.Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, Buecker C, Schäfer R, Han X, Au P, Scadden DT, Duda DG, Fukumura D, Jain RK. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA 110: 12774–12779, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Sandison JC. Observations on the growth of blood vessels as seen in the transparent chamber introduced into the rabbit’s ear. Am J Anat 41: 475–496, 1928. [Google Scholar]
- 488.Sandison JC. The transparent chamber of the rabbit’s ear, giving a complete description of improved technic of construction and introduction, and general account of growth and behavior of living cells and tissues as seen with the microscope. Am J Anat 41: 447–473, 1928. [Google Scholar]
- 489.Sata M, Tanaka K, Nagai R. Origin of smooth muscle progenitor cells: different conclusions from different models. Circulation 107: e106; author reply e106-e107, 2003. [DOI] [PubMed] [Google Scholar]
- 490.Sato K, Laham RJ, Pearlman JD, Novicki D, Sellke FW, Simons M, Post MJ. Efficacy of intracoronary versus intravenous FGF-2 in a pig model of chronic myocardial ischemia. Ann Thorac Surg 70: 2113–2118, 2000. [DOI] [PubMed] [Google Scholar]
- 491.Schaper J, König R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined SEM and TEM study. Virchows Arch A Pathol Anat Pathol 370: 193–205, 1976. [DOI] [PubMed] [Google Scholar]
- 492.Schaper W Collateral circulation: past and present. Basic Res Cardiol 104: 5–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Schechner JS, Crane SK, Wang F, Szeglin AM, Tellides G, Lorber MI, Bothwell ALM, Pober JS. Engraftment of a vascularized human skin equivalent. FASEB J 17: 2250–2256, 2003. [DOI] [PubMed] [Google Scholar]
- 494.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, Lorber MI, Tellides G, Kashgarian M, Bothwell AL, Pober JS. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA 97: 9191–9196, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Schlaudraff K, Schumacher B, von Specht BU, Seitelberger R, Schlosser V, Fasol R. Growth of “new” coronary vascular structures by angiogenetic growth factors. Eur J Cardiothorac Surg 7: 637–643; discussion 643-644, 1993. [DOI] [PubMed] [Google Scholar]
- 496.Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev 70: 987–1028, 1990. [DOI] [PubMed] [Google Scholar]
- 497.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111: 2981–2987, 2005. [DOI] [PubMed] [Google Scholar]
- 498.Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation 97: 645–650, 1998. [DOI] [PubMed] [Google Scholar]
- 499.Sefcik LS, Petrie Aronin CE, Botchwey EA. Engineering vascularized tissues using natural and synthetic small molecules. Organogenesis 4: 215–227, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg 65: 1540–1544, 1998. [DOI] [PubMed] [Google Scholar]
- 501.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15: 551–578, 1999. [DOI] [PubMed] [Google Scholar]
- 502.Seto Y, Inaba R, Okuyama T, Sassa F, Suzuki H, Fukuda J. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials 31: 2209–2215, 2010. [DOI] [PubMed] [Google Scholar]
- 503.Shandalov Y, Egozi D, Koffler J, Dado-Rosenfeld D, Ben-Shimol D, Freiman A, Shor E, Kabala A, Levenberg S. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci USA 111: 6010–6015, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res 37: 401–412, 1997. [DOI] [PubMed] [Google Scholar]
- 505.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res 40: 586–597, 1998. [DOI] [PubMed] [Google Scholar]
- 506.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol 17: 551–554, 1999. [DOI] [PubMed] [Google Scholar]
- 507.Shepherd BR, Chen HY, Smith CM, Gruionu G, Williams SK, Hoying JB. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol 24: 898–904, 2004. [DOI] [PubMed] [Google Scholar]
- 508.Shepherd BR, Enis DR, Wang F, Suarez Y, Pober JS, Schechner JS. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J 20: 1739–1741, 2006. [DOI] [PubMed] [Google Scholar]
- 509.Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng 13: 2871–2879, 2007. [DOI] [PubMed] [Google Scholar]
- 510.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood 92: 362–367, 1998. [PubMed] [Google Scholar]
- 511.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med 7: 738–741, 2001. [DOI] [PubMed] [Google Scholar]
- 512.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J 20: 708–710, 2006. [DOI] [PubMed] [Google Scholar]
- 513.Shimizu Y, Shibata R, Shintani S, Ishii M, Murohara T. Therapeutic lymphangiogenesis with implantation of adipose-derived regenerative cells. J Am Heart Assoc 1: e000877, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Shin S-H, Lee J, Ahn D-G, Lee KY. Co-delivery of vascular endothelial growth factor and angiopoietin-1 using injectable microsphere/hydrogel hybrid systems for therapeutic angiogenesis. Pharm Res 30: 2157–2165, 2013. [DOI] [PubMed] [Google Scholar]
- 515.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103: 2776–2779, 2001. [DOI] [PubMed] [Google Scholar]
- 516.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 103: 897–903, 2001. [DOI] [PubMed] [Google Scholar]
- 517.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med 56: 509–538, 2005. [DOI] [PubMed] [Google Scholar]
- 518.Shoji T, Ii M, Mifune Y, Matsumoto T, Kawamoto A, Kwon S-M, Kuroda T, Kuroda R, Kurosaka M, Asahara T. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest 90: 637–649, 2010. [DOI] [PubMed] [Google Scholar]
- 519.Sholley MM, Ferguson GP, Seibel HR, Montour JL, Wilson JD. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest 51: 624–634, 1984. [PubMed] [Google Scholar]
- 520.Shrager JB. The Vineberg procedure: the immediate forerunner of coronary artery bypass grafting. Ann Thorac Surg 57: 1354–1364, 1994. [DOI] [PubMed] [Google Scholar]
- 521.Shyu K-G, Manor O, Magner M, Yancopoulos GD, Isner JM. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation 98: 2081–2087, 1998. [DOI] [PubMed] [Google Scholar]
- 522.Shyy Y-J, Hsieh H-J, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci USA 91: 4678–4682, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Silva EA, Kim E-S, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA 105: 14347–14352, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 31: 1235–1241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost 5: 590–598, 2007. [DOI] [PubMed] [Google Scholar]
- 526.Silva GV, Litovsky S, Assad JAR, Sousa ALS, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RVC, Oliveira EM, He R, Geng Y-J, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111: 150–156, 2005. [DOI] [PubMed] [Google Scholar]
- 527.Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun 153: 347–352, 1988. [DOI] [PubMed] [Google Scholar]
- 528.Silvestre J-S, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, Lévy BI. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res 93: 114–123, 2003. [DOI] [PubMed] [Google Scholar]
- 529.Simcock JW, Penington AJ, Morrison WA, Thompson EW, Mitchell GM. Endothelial precursor cells home to a vascularized tissue engineering chamber by application of the angiogenic chemokine CXCL12. Tissue Eng A 15: 655–664, 2009. [DOI] [PubMed] [Google Scholar]
- 530.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov 2: 863–871, 2003. [DOI] [PubMed] [Google Scholar]
- 531.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation 106: 1199–1204, 2002. [DOI] [PubMed] [Google Scholar]
- 532.Singhal M, Liu X, Inverso D, Jiang K, Dai J, He H, Bartels S, Li W, Abdul Pari AA, Gengenbacher N, Besemfelder E, Hui L, Augustin HG, Hu J. Endothelial cell fitness dictates the source of regenerating liver vasculature. J Exp Med 215: 2497–2508, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Slavin SA, Upton J, Kaplan WD, Van den Abbeele AD. An investigation of lymphatic function following free-tissue transfer. Plast Reconstr Surg 99: 730–741; discussion 742-743, 1997. [DOI] [PubMed] [Google Scholar]
- 534.Slavin SA, Van den Abbeele AD, Losken A, Swartz MA, Jain RK. Return of lymphatic function after flap transfer for acute lymphedema. Ann Surg 229: 421–427, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Smith MK, Peters MC, Richardson TP, Garbern JC, Mooney DJ. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue Eng 10: 63–71, 2004. [DOI] [PubMed] [Google Scholar]
- 536.Sobrino A, Phan DTT, Datta R, Wang X, Hachey SJ, Romero-López M, Gratton E, Lee AP, George SC, Hughes CCW. 3D microtumors in vitro supported by perfused vascular networks. Sci Rep 6: 31589, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Soker S, Miao H-Q, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem 85: 357–368, 2002. [DOI] [PubMed] [Google Scholar]
- 538.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19: 646–651, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA 108: 15342–15347, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Sooppan R, Paulsen SJ, Han J, Ta AH, Dinh P, Gaffey AC, Venkataraman C, Trubelja A, Hung G, Miller JS, Atluri P. In vivo anastomosis and perfusion of a three-dimensionally-printed construct containing microchannel networks. Tissue Eng C 22: 1–7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Sprugel KH, McPherson JM, Clowes AW, Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol 129: 601–613, 1987. [PMC free article] [PubMed] [Google Scholar]
- 542.Stabler CT, Caires LC Jr., Mondrinos MJ, Marcinkiewicz C, Lazarovici P, Wolfson MR, Lelkes PI. Enhanced re-endothelialization of decellularized rat lungs. Tissue Eng C 22: 439–450, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Stamm C, Westphal B, Kleine H-D, Petzsch M, Kittner C, Klinge H, Schümichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 361: 45–46, 2003. [DOI] [PubMed] [Google Scholar]
- 544.Steffens GC, Yao C, Prével P, Markowicz M, Schenck P, Noah EM, Pallua N. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng 10: 1502–1509, 2004. [DOI] [PubMed] [Google Scholar]
- 545.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 117: 206–215, 2008. [DOI] [PubMed] [Google Scholar]
- 546.Stepnick DW, Peterson MK, Bodgan C, Davis J, Wasman J, Mailer K. Effects of tumor necrosis factor α and vascular permeability factor on neovascularization of the rabbit ear flap. Arch Otolaryngol Head Neck Surg 121: 667–672, 1995. [DOI] [PubMed] [Google Scholar]
- 547.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA 106: 16568–16573, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, Xiao JW, Fung C, Mirabella T, Chen AX, McCue MG, Yang MT, Fleming HE, Chung K, de Jong YP, Chen CS, Rice CM, Bhatia SN. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci Transl Med 9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Stillaert F, Findlay M, Palmer J, Idrizi R, Cheang S, Messina A, Abberton K, Morrison W, Thompson EW. Host rather than graft origin of Matrigel-induced adipose tissue in the murine tissue-engineering chamber. Tissue Eng 13: 2291–2300, 2007. [DOI] [PubMed] [Google Scholar]
- 550.Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr., Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RAD, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 99: 9656–9661, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res 22: 1110–1116, 2005. [DOI] [PubMed] [Google Scholar]
- 552.Sun Q, Silva EA, Wang A, Fritton JC, Mooney DJ, Schaffler MB, Grossman PM, Rajagopalan S. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm Res 27: 264–271, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Sunderkötter C, Goebeler M, Schulze-Osthoff K, Bhardwaj R, Sorg C. Macrophage-derived angiogenesis factors. Pharmacol Ther 51: 195–216, 1991. [DOI] [PubMed] [Google Scholar]
- 554.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996. [DOI] [PubMed] [Google Scholar]
- 555.Suuronen EJ, Price J, Veinot JP, Ascah K, Kapila V, Guo X-W, Wong S, Mesana TG, Ruel M. Comparative effects of mesenchymal progenitor cells, endothelial progenitor cells, or their combination on myocardial infarct regeneration and cardiac function. J Thorac Cardiovasc Surg 134: 1249–1258, 2007. [DOI] [PubMed] [Google Scholar]
- 556.Symes JF, Graham AM, Stein L, Sniderman AD. Salvage of a severely ischemic limb by arteriovenous revascularization: a case report. Can J Surg 27: 274–277, 1984. [PubMed] [Google Scholar]
- 557.Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985–1987, 2002. [DOI] [PubMed] [Google Scholar]
- 558.Tabata Y, Hijikata S, Ikada Y. Enhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogels. J Control Release 31: 189–199, 1994. [Google Scholar]
- 559.Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed 10: 79–94, 1999. [DOI] [PubMed] [Google Scholar]
- 560.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials 20: 2169–2175, 1999. [DOI] [PubMed] [Google Scholar]
- 561.Tabata Y, Miyao M, Inamoto T, Ishii T, Hirano Y, Yamaoki Y, Ikada Y. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng 6: 279–289, 2000. [DOI] [PubMed] [Google Scholar]
- 562.Tabata Y, Miyao M, Ozeki M, Ikada Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J Biomater Sci Polym Ed 11: 915–930, 2000. [DOI] [PubMed] [Google Scholar]
- 563.Tabata Y, Miyao M, Yamamoto M, Ikada Y. Vascularization into a porous sponge by sustained release of basic fibroblast growth factor. J Biomater Sci Polym Ed 10: 957–968, 1999. [DOI] [PubMed] [Google Scholar]
- 564.Tabata Y, Nagano A, Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng 5: 127–138, 1999. [DOI] [PubMed] [Google Scholar]
- 565.Tabata Y, Nagano A, Muniruzzaman M, Ikada Y. In vitro sorption and desorption of basic fibroblast growth factor from biodegradable hydrogels. Biomaterials 19: 1781–1789, 1998. [DOI] [PubMed] [Google Scholar]
- 566.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999. [DOI] [PubMed] [Google Scholar]
- 567.Takeshita S, Pu L-Q, Stein LA, Sniderman AD, Bunting S, Ferrara N, Isner JM, Symes JF. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation 90: II228–II234, 1994. [PubMed] [Google Scholar]
- 568.Takeshita S, Rossow ST, Kearney M, Zheng LP, Bauters C, Bunting S, Ferrara N, Symes JF, Isner JM. Time course of increased cellular proliferation in collateral arteries after administration of vascular endothelial growth factor in a rabbit model of lower limb vascular insufficiency. Am J Pathol 147: 1649–1660, 1995. [PMC free article] [PubMed] [Google Scholar]
- 569.Takeshita S, Tsurumi Y, Couffinahl T, Asahara T, Bauters C, Symes J, Ferrara N, Isner JM. Gene transfer of naked DNA encoding for three isoforms of vascular endothelial growth factor stimulates collateral development in vivo. Lab Invest 75: 487–501, 1996. [PubMed] [Google Scholar]
- 570.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu L-Q, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis: a single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93: 662–670, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Tammela T, Saaristo A, Holopainen T, Lyytikkä J, Kotronen A, Pitkonen M, Abo-Ramadan U, Ylä-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13: 1458–1466, 2007. [DOI] [PubMed] [Google Scholar]
- 572.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 85: 1292–1307, 2005. [DOI] [PubMed] [Google Scholar]
- 573.Tanaka Y, Sung K-C, Tsutsumi A, Ohba S, Ueda K, Morrison WA. Tissue engineering skin flaps: which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast Reconstr Surg 112: 1636–1644, 2003. [DOI] [PubMed] [Google Scholar]
- 574.Tanaka Y, Tsutsumi A, Crowe DM, Tajima S, Morrison WA. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. Br J Plast Surg 53: 51–57, 2000. [DOI] [PubMed] [Google Scholar]
- 575.Tandara AA, Mustoe TA. Oxygen in wound healing--more than a nutrient. World J Surg 28: 294–300, 2004. [DOI] [PubMed] [Google Scholar]
- 576.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105: 1068–1077, 2005. [DOI] [PubMed] [Google Scholar]
- 577.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005. [DOI] [PubMed] [Google Scholar]
- 578.Therriault D, Shepherd RF, White SR, Lewis JA. Fugitive inks for direct-write assembly of three-dimensional microvascular networks. Adv Mater 17: 395–399, 2005. [Google Scholar]
- 579.Thoma R Über die histomechanik des gefäßsystems und die pathogenese der angiosklerose. Virchows Arch 204: 1–74, 1911. [Google Scholar]
- 580.Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, Anderson WF, Maciag T. Site-directed neovessel formation in vivo. Science 241: 1349–1352, 1988. [DOI] [PubMed] [Google Scholar]
- 581.Thompson RL, Margolis EA, Ryan TJ, Coisman BJ, Price GM, Wong KHK, Tien J. Design principles for lymphatic drainage of fluid and solutes from collagen scaffolds. J Biomed Mater Res A 106: 106–114, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Tian W, Rockson SG, Jiang X, Kim J, Begaye A, Shuffle EM, Tu AB, Cribb M, Nepiyushchikh Z, Feroze AH, Zamanian RT, Dhillon GS, Voelkel NF, Peters-Golden M, Kitajewski J, Dixon JB, Nicolls MR. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med 9: eaal3920, 2017. [DOI] [PubMed] [Google Scholar]
- 583.Tien J Microfluidic approaches for engineering vasculature. Curr Opin Chem Eng 3: 36–41, 2014. [Google Scholar]
- 584.Tien J, Golden AP, Tang MD. Engineering of blood vessels In: Microvascular Research: Biology and Pathology, edited by Shepro D, D’Amore PA. San Diego, CA: Elsevier Academic Press, 2006, p. 1087–1093. [Google Scholar]
- 585.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development 135: 523–532, 2008. [DOI] [PubMed] [Google Scholar]
- 586.Ting ACH, Craft RO, Palmer JA, Gerrand Y-W, Penington AJ, Morrison WA, Mitchell GM. The adipogenic potential of various extracellular matrices under the influence of an angiogenic growth factor combination in a mouse tissue engineering chamber. Acta Biomater 10: 1907–1918, 2014. [DOI] [PubMed] [Google Scholar]
- 587.Tomita S, Li R-K, Weisel RD, Mickle DAG, Kim E-J, Sakai T, Jia Z-Q. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100: II247–II256, 1999. [DOI] [PubMed] [Google Scholar]
- 588.Tomita S, Mickle DAG, Weisel RD, Jia Z-Q, Tumiati LC, Allidina Y, Liu P, Li R-K. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg 123: 1132–1140, 2002. [DOI] [PubMed] [Google Scholar]
- 589.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 5: 423–435, 2005. [DOI] [PubMed] [Google Scholar]
- 590.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102: 77–85, 2008. [DOI] [PubMed] [Google Scholar]
- 591.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res 104: 1410–1420, 2009. [DOI] [PubMed] [Google Scholar]
- 592.Tremblay P-L, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant 5: 1002–1010, 2005. [DOI] [PubMed] [Google Scholar]
- 593.Trentin D, Hall H, Wechsler S, Hubbell JA. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1α variant for local induction of angiogenesis. Proc Natl Acad Sci USA 103: 2506–2511, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Tse H-F, Kwong Y-L, Chan JKF, Lo G, Ho C-L, Lau C-P. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 361: 47–49, 2003. [DOI] [PubMed] [Google Scholar]
- 595.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation 94: 3281–3290, 1996. [DOI] [PubMed] [Google Scholar]
- 596.Uchida Y, Yanagisawa-Miwa A, Nakamura F, Yamada K, Tomaru T, Kimura K, Morita T. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J 130: 1182–1188, 1995. [DOI] [PubMed] [Google Scholar]
- 597.Ueno H, Li J-J, Masuda S, Qi Z, Yamamoto H, Takeshita A. Adenovirus-mediated expression of the secreted form of basic fibroblast growth factor (FGF-2) induces cellular proliferation and angiogenesis in vivo. Arterioscler Thromb Vasc Biol 17: 2453–2460, 1997. [DOI] [PubMed] [Google Scholar]
- 598.Uitterdijk A, Springeling T, van Kranenburg M, van Duin RWB, Krabbendam-Peters I, Gorsse-Bakker C, Sneep S, van Haeren R, Verrijk R, van Geuns R-JM, van der Giessen WJ, Markkula T, Duncker DJ, van Beusekom HMM. VEGF165A microsphere therapy for myocardial infarction suppresses acute cytokine release and increases microvascular density but does not improve cardiac function. Am J Physiol Heart Circ Physiol 309: H396–H406, 2015. [DOI] [PubMed] [Google Scholar]
- 599.Unger EF. Experimental evaluation of coronary collateral development. Cardiovasc Res 49: 497–506, 2001. [DOI] [PubMed] [Google Scholar]
- 600.Unger EF, Banai S, Shou M, Jaklitsch M, Hodge E, Correa R, Jaye M, Epstein SE. A model to assess interventions to improve collateral blood flow: continuous administration of agents into the left coronary artery in dogs. Cardiovasc Res 27: 785–791, 1993. [DOI] [PubMed] [Google Scholar]
- 601.Unger EF, Banai S, Shou M, Lazarous DF, Jaklitsch MT, Scheinowitz M, Correa R, Klingbeil C, Epstein SE. Basic fibroblast growth factor enhances myocardial collateral flow in a canine model. Am J Physiol 266: H1588–H1595, 1994. [DOI] [PubMed] [Google Scholar]
- 602.Unger EF, Sheffield CD, Epstein SE. Creation of anastomoses between an extracardiac artery and the coronary circulation. Proof that myocardial angiogenesis occurs and can provide nutritional blood flow to the myocardium. Circulation 82: 1449–1466, 1990. [DOI] [PubMed] [Google Scholar]
- 603.Unger EF, Sheffield CD, Epstein SE. Heparin promotes the formation of extracardiac to coronary anastomoses in a canine model. Am J Physiol 260: H1625–H1634, 1991. [DOI] [PubMed] [Google Scholar]
- 604.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation 108: 2511–2516, 2003. [DOI] [PubMed] [Google Scholar]
- 605.Uyama S, Kaufmann P-M, Takeda T, Vacanti JP. Delivery of whole liver-equivalent hepatocyte mass using polymer devices and hepatotrophic stimulation. Transplantation 55: 932–935, 1993. [DOI] [PubMed] [Google Scholar]
- 606.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16: 814–820, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, Vestweber D, Hatzopoulos AK. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med 197: 1755–1765, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation 97: 381–390, 1998. [DOI] [PubMed] [Google Scholar]
- 609.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: e1–e7, 2001. [DOI] [PubMed] [Google Scholar]
- 610.Vashi AV, Abberton KM, Thomas GP, Morrison WA, O’Connor AJ, Cooper-White JJ, Thompson EW. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng 12: 3035–3043, 2006. [DOI] [PubMed] [Google Scholar]
- 611.Vernon RB, Gooden MD, Lara SL, Wight TN. Native fibrillar collagen membranes of micron-scale and submicron thicknesses for cell support and perfusion. Biomaterials 26: 1109–1117, 2005. [DOI] [PubMed] [Google Scholar]
- 612.Vickerman V, Kamm RD. Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr Biol 4: 863–874, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Vincent KA, Shyu K-G, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation 102: 2255–2261, 2000. [DOI] [PubMed] [Google Scholar]
- 614.Vineberg AM. Development of an anastomosis between the coronary vessels and a transplanted internal mammary artery. Can Med Assoc J 55: 117–119, 1946. [PubMed] [Google Scholar]
- 615.Vineberg AM, Jewett BL. Development of an anastomosis between the coronary vessels and a transplanted internal mammary artery. Can Med Assoc J 56: 609–614, 1947. [PMC free article] [PubMed] [Google Scholar]
- 616.Vineberg AM, Shanks J, Pifarre R, Criollos R, Kato Y. Combined internal mammary artery implantation and free omental graft operation: a highly effective revascularization procedure (a study of 17 cases). Can Med Assoc J 90: 717–722, 1964. [PMC free article] [PubMed] [Google Scholar]
- 617.Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci USA 114: 9337–9342, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Vogt PM, Steinau HU, Spies M, Kall S, Steiert A, Boorboor P, Vaske B, Jokuszies A. Outcome of simultaneous and staged microvascular free tissue transfer connected to arteriovenous loops in areas lacking recipient vessels. Plast Reconstr Surg 120: 1568–1575, 2007. [DOI] [PubMed] [Google Scholar]
- 619.von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, Merchant MJ, Cooke JP, Blau HM. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J 20: 2657–2659, 2006. [DOI] [PubMed] [Google Scholar]
- 620.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297: 2256–2259, 2002. [DOI] [PubMed] [Google Scholar]
- 621.Wake MC, Patrick CW Jr., Mikos AG. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant 3: 339–343, 1994. [DOI] [PubMed] [Google Scholar]
- 622.Walton RL, Beahm EK, Wu L. De novo adipose formation in a vascularized engineered construct. Microsurgery 24: 378–384, 2004. [DOI] [PubMed] [Google Scholar]
- 623.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc Natl Acad Sci USA 105: 686–691, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90: 4304–4308, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Wang X, Phan DTT, Sobrino A, George SC, Hughes CCW, Lee AP. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16: 282–290, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Warnecke C, Griethe W, Weidemann A, Jürgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt K-U. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J 17: 1186–1188, 2003. [DOI] [PubMed] [Google Scholar]
- 627.Webber MJ, Tongers J, Newcomb CJ, Marquardt K-T, Bauersachs J, Losordo DW, Stupp SI. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc Natl Acad Sci USA 108: 13438–13443, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005. [DOI] [PubMed] [Google Scholar]
- 629.Wiedeman MP. Architecture In: Handbook of Physiology; Section 2: The Cardiovascular System, edited by Renkin EM, Michel CC. Bethesda, MD: American Physiological Society, 1984, p. 11–40. [Google Scholar]
- 630.Wiedeman MP. Patterns of the arteriovenous pathways In: Handbook of Physiology; Section 2: Circulation, edited by Hamilton WF. Bethesda, MD: American Physiological Society, 1963, p. 891–933. [Google Scholar]
- 631.Wieghaus KA, Capitosti SM, Anderson CR, Price RJ, Blackman BR, Brown ML, Botchwey EA. Small molecule inducers of angiogenesis for tissue engineering. Tissue Eng 12: 1903–1913, 2006. [DOI] [PubMed] [Google Scholar]
- 632.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 21: 822–827, 1998. [DOI] [PubMed] [Google Scholar]
- 633.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999. [DOI] [PubMed] [Google Scholar]
- 634.Willems WF, Larsen M, Friedrich PF, Shogren KL, Bishop AT. Induction of angiogenesis and osteogenesis in surgically revascularized frozen bone allografts by sustained delivery of FGF-2 and VEGF. J Orthop Res 30: 1556–1562, 2012. [DOI] [PubMed] [Google Scholar]
- 635.Willems WF, Larsen M, Giusti G, Friedrich PF, Bishop AT. Revascularization and bone remodeling of frozen allografts stimulated by intramedullary sustained delivery of FGF-2 and VEGF. J Orthop Res 29: 1431–1436, 2011. [DOI] [PubMed] [Google Scholar]
- 636.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med 334: 1185–1187, 1996. [DOI] [PubMed] [Google Scholar]
- 637.Wilson WC Jr., Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec A 272: 491–496, 2003. [DOI] [PubMed] [Google Scholar]
- 638.Winter GD. Transcutaneous implants: reactions of the skin-implant interface. J Biomed Mater Res Symp 8: 99–113, 1974. [DOI] [PubMed] [Google Scholar]
- 639.Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu J-S, Isner JM. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol 153: 381–394, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 247: 1465–1468, 1990. [DOI] [PubMed] [Google Scholar]
- 641.Wong KHK, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng 14: 205–230, 2012. [DOI] [PubMed] [Google Scholar]
- 642.Wong KHK, Truslow JG, Khankhel AH, Chan KLS, Tien J. Artificial lymphatic drainage systems for vascularized microfluidic scaffolds. J Biomed Mater Res A 101: 2181–2190, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Wong KHK, Truslow JG, Khankhel AH, Tien J. Biophysical mechanisms that govern the vascularization of microfluidic scaffolds In: Vascularization: Regenerative Medicine and Tissue Engineering, edited by Brey EM. Boca Raton, FL: CRC Press, 2014, p. 109–124. [Google Scholar]
- 644.Wong KHK, Truslow JG, Tien J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31: 4706–4714, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Wu C, Zhou Y, Fan W, Han P, Chang J, Yuen J, Zhang M, Xiao Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 33: 2076–2085, 2012. [DOI] [PubMed] [Google Scholar]
- 646.Wu L, Pierce GF, Ladin DA, Zhao LL, Rogers D, Mustoe TA. Effects of oxygen on wound responses to growth factors: Kaposi’s FGF, but not basic FGF stimulates repair in ischemic wounds. Growth Factors 12: 29–35, 1995. [DOI] [PubMed] [Google Scholar]
- 647.Wu L, Xia Y-P, Roth SI, Gruskin E, Mustoe TA. Transforming growth factor-β1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age. Am J Pathol 154: 301–309, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv Mater 23: H178–H183, 2011. [DOI] [PubMed] [Google Scholar]
- 649.Xia Y, Whitesides GM. Soft lithography. Angew Chem Int Ed 37: 551–575, 1998. [DOI] [PubMed] [Google Scholar]
- 650.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107: 1322–1328, 2003. [DOI] [PubMed] [Google Scholar]
- 651.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S-I. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408: 92–96, 2000. [DOI] [PubMed] [Google Scholar]
- 652.Yanagisawa-Miwa A, Uchida Y, Nakamura F, Tomaru T, Kido H, Kamijo T, Sugimoto T, Kaji K, Utsuyama M, Kurashima C, Ito H. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science 257: 1401–1403, 1992. [DOI] [PubMed] [Google Scholar]
- 653.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA 86: 933–937, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Yao C, Markowicz M, Pallua N, Noah EM, Steffens G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials 29: 66–74, 2008. [DOI] [PubMed] [Google Scholar]
- 655.Yao L-C, Testini C, Tvorogov D, Anisimov A, Vargas SO, Baluk P, Pytowski B, Claesson-Welsh L, Alitalo K, McDonald DM. Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ Res 114: 806–822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Yap KK, Yeoh GC, Morrison WA, Mitchell GM. The vascularised chamber as an in vivo bioreactor. Trends Biotechnol 36: 1011–1024, 2018. [DOI] [PubMed] [Google Scholar]
- 657.Yau TM, Fung K, Weisel RD, Fujii T, Mickle DAG, Li R-K. Enhanced myocardial angiogenesis by gene transfer with transplanted cells. Circulation 104: I-218–I-222, 2001. [DOI] [PubMed] [Google Scholar]
- 658.Ye X, Lu L, Kolewe ME, Park H, Larson BL, Kim ES, Freed LE. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials 34: 10007–10015, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Yeh ETH, Zhang S, Wu HD, Körbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 108: 2070–2073, 2003. [DOI] [PubMed] [Google Scholar]
- 660.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Yoon C-H, Hur J, Oh I-Y, Park K-W, Kim T-Y, Shin J-H, Kim J-H, Lee C-S, Chung J-K, Park Y-B, Kim H-S. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 26: 1066–1072, 2006. [DOI] [PubMed] [Google Scholar]
- 662.Yoon C-H, Hur J, Park K-W, Kim J-H, Lee C-S, Oh I-Y, Kim T-Y, Cho H-J, Kang H-J, Chae I-H, Yang H-K, Oh B-H, Park Y-B, Kim H-S. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 112: 1618–1627, 2005. [DOI] [PubMed] [Google Scholar]
- 663.Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 111: 717–725, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Youn S-W, Lee S-W, Lee J, Jeong H-K, Suh J-W, Yoon C-H, Kang H-J, Kim H-Z, Koh G-Y, Oh B-H, Park Y-B, Kim H-S. COMP-Ang1 stimulates HIF-1α-mediated SDF-1 overexpression and recovers ischemic injury through BM-derived progenitor cell recruitment. Blood 117: 4376–4386, 2011. [DOI] [PubMed] [Google Scholar]
- 665.Yuan SY, Rigor RR. Regulation of Endothelial Barrier Function. San Rafael, CA: Morgan & Claypool Life Sciences, 2011. [PubMed] [Google Scholar]
- 666.Yuen WW, Du NR, Chan CH, Silva EA, Mooney DJ. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci USA 107: 17933–17938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol 7: 87–96, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 15: 669–678, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Zhang F, Fischer K, Komorowska-Timek E, Guo M, Cui D, Dorsett-Martin W, Buncke HJ, Lineaweaver WC. Improvement of skin paddle survival by application of vascular endothelial growth factor in a rat TRAM flap model. Ann Plast Surg 46: 314–319, 2001. [DOI] [PubMed] [Google Scholar]
- 670.Zhang F, Lei MP, Oswald TM, Pang Y, Blain B, Cai ZW, Lineaweaver WC. The effect of vascular endothelial growth factor on the healing of ischaemic skin wounds. Br J Plast Surg 56: 334–341, 2003. [DOI] [PubMed] [Google Scholar]
- 671.Zhang F, Waller W, Lineaweaver WC. Growth factors and flap survival. Microsurgery 24: 162–167, 2004. [DOI] [PubMed] [Google Scholar]
- 672.Zhang J, Ding L, Zhao Y, Sun W, Chen B, Lin H, Wang X, Zhang L, Xu B, Dai J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation 119: 1776–1784, 2009. [DOI] [PubMed] [Google Scholar]
- 673.Zhang Q-X, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res 67: 147–154, 1997. [DOI] [PubMed] [Google Scholar]
- 674.Zhang Q, Hubenak J, Iyyanki T, Alred E, Turza KC, Davis G, Chang EI, Branch-Brooks CD, Beahm EK, Butler CE. Engineering vascularized soft tissue flaps in an animal model using human adipose-derived stem cells and VEGF+PLGA/PEG microspheres on a collagen-chitosan scaffold with a flow-through vascular pedicle. Biomaterials 73: 198–213, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Zhao LL, Davidson JD, Wee SC, Roth SI, Mustoe TA. Effect of hyperbaric oxygen and growth factors on rabbit ear ischemic ulcers. Arch Surg 129: 1043–1049, 1994. [DOI] [PubMed] [Google Scholar]
- 676.Zheng W, Tammela T, Yamamoto M, Anisimov A, Holopainen T, Kaijalainen S, Karpanen T, Lehti K, Ylä-Herttuala S, Alitalo K. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118: 1154–1162, 2011. [DOI] [PubMed] [Google Scholar]
- 677.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 109: 9342–9347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Zheng Y, Henderson PW, Choi NW, Bonassar LJ, Spector JA, Stroock AD. Microstructured templates for directed growth and vascularization of soft tissue in vivo. Biomaterials 32: 5391–5401, 2011. [DOI] [PubMed] [Google Scholar]
- 679.Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau N, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest 112: 30–41, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang Y-J, Schwarz EM, Xing L. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum 63: 2318–2328, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Zhuo Y, Li S-H, Chen M-S, Wu J, Kinkaid HYM, Fazel S, Weisel RD, Li R-K. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg 139: 1286–1294, 2010. [DOI] [PubMed] [Google Scholar]
- 682.Ziada AMAR, Hudlicka O, Tyler KR, Wright AJA. The effect of long-term vasodilatation on capillary growth and performance in rabbit heart and skeletal muscle. Cardiovasc Res 18: 724–732, 1984. [DOI] [PubMed] [Google Scholar]
- 683.Ziche M, Jones J, Gullino PM. Role of prostaglandin E1 and copper in angiogenesis. J Natl Cancer Inst 69: 475–482, 1982. [PubMed] [Google Scholar]
- 684.Ziche M, Morbidelli L, Choudhuri R, Zhang H-T, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99: 2625–2634, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 94: 230–238, 2004. [DOI] [PubMed] [Google Scholar]
- 686.Zierler KL. Circulation times and the theory of indicator-dilution methods for determining blood flow and volume In: Handbook of Physiology; Section 2: Circulation, edited by Hamilton WF. Bethesda, MD: American Physiological Society, 1963, p. 585–615. [Google Scholar]
- 687.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmökel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J 17: 2260–2262, 2003. [DOI] [PubMed] [Google Scholar]
- 688.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF-fibrin matrices for endothelialization. J Control Release 72: 101–113, 2001. [DOI] [PubMed] [Google Scholar]
- 689.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228, 2001. [DOI] [PubMed] [Google Scholar]
- 690.Zweifach BW. Functional Behavior of the Microcirculation. Springfield, IL: Charles C. Thomas, 1961. [Google Scholar]
FURTHER READING
- 1.Briquez PS, Clegg LE, Martino MM, Mac Gabhann F, Hubbell JA. Design principles for therapeutic angiogenic materials. Nat Rev Mater 1: 1–15, 2016. [Google Scholar]
- 2.Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res 116: 1561–1578, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lähteenvuo J, Ylä-Herttuala S. Advances and challenges in cardiovascular gene therapy. Hum Gene Ther 28: 1024–1032, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 6: 1316–1320, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KM, Gerecht S. Harnessing developmental processes for vascular engineering and regeneration. Development 141: 2760–2769, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tien J Microfluidic approaches for engineering vasculature. Curr Opin Chem Eng 3: 36–41, 2014. [Google Scholar]
- 7.Yap KK, Yeoh GC, Morrison WA, Mitchell GM. The vascularised chamber as an in vivo bioreactor. Trends Biotechnol 36: 1011–1024, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124: 878–887, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
CROSS-REFERENCES
- 1.Cardiovascular Physiology: Overview of the microcirculation (legacy)
- 2.Cardiovascular Physiology: Physiology and pathobiology of microvascular endothelium (legacy)
- 3.Cardiovascular Physiology: Angiogenesis
- 4.Cardiovascular Physiology: Development of a vascular network
- 5.Cardiovascular Physiology: Lymphatics