Abstract
Background
Euterpe oleracea Mart. (açaí) is a fruit with high antioxidant capacity and could be an adjuvant strategy to attenuate ischemia-reperfusion injury.
Objective
To evaluate the influence of açaí in global ischemia-reperfusion model in rats.
Methods
Wistar rats were assigned to 2 groups: Control (C: receiving standard chow; n = 9) and Açaí (A: receiving standard chow supplemented with 5% açaí; n = 10). After six weeks, the animals were subjected to the global ischemia-reperfusion protocol and an isolated heart study to evaluate left ventricular function. Level of significance adopted: 5%.
Results
There was no difference between the groups in initial body weight, final body weight and daily feed intake. Group A presented lower lipid hydroperoxide myocardial concentration and higher catalase activity, superoxide dismutase and glutathione peroxidase than group C. We also observed increased myocardial activity of b-hydroxyacyl coenzyme-A dehydrogenase, pyruvate dehydrogenase, citrate synthase, complex I, complex II and ATP synthase in the A group as well as lower activity of the lactate dehydrogenase and phosphofructokinase enzymes. The systolic function was similar between the groups, and the A group presented poorer diastolic function than the C group. We did not observe any difference between the groups in relation to myocardial infarction area, total and phosphorylated NF-kB, total and acetylated FOXO1, SIRT1 and Nrf-2 protein expression.
Conclusion
despite improving energy metabolism and attenuating oxidative stress, açai supplementation did not decrease the infarcted area or improve left ventricular function in the global ischemia-reperfusion model.
Keywords: Euterpe Olerácea, Oxidative Stress, Energy Metabolism, Chemical Reactions, Myocardial Ischemia, Myocardial Reperfusion Injury, Rats
Introduction
Although the mortality attributed to ischemic heart disease is declining in some countries, it still presents high morbidity, decreasing the quality of life and increasing healthcare spending.1 Cardiac ischemic events may be due to partial or total tissue ischemia, with reversible or irreversible myocardial dysfunction and cell death. Ischemic periods of more than 20 minutes cause irreversible damage of the cardiomyocytes and inability to functionally recover, even with the restoration of blood flow.2,3
During ischemia, to meet the myocardial energy demand, cellular ATP is generated by glycolysis, leading to intracellular pH reduction.1,4 In parallel, reduced ATP levels interrupt important active pumps in ionic homeostasis, which results in overloaded cytosolic Na+ and Ca2+, making cell repolarization unfeasible and leading to myocardial dysfunction. In addition, it is possible to observe elevated Ca2+ levels in cytosol, which activate enzymes (phospholipases, proteases, endonucleases and ATPases) associated with lipid peroxidation, production of reactive oxygen species (ROS), dysfunction of contractile proteins, and loss of cellular function.4
Although necessary to reverse ischemia, the restoration of blood flow may ultimately be more harmful than the ischemic process itself.1,4,5 During reperfusion, ischemia damages are worsened due to an additional discharge of ROS generated in the mitochondria by the restoration of oxygen flow.5,6 To improve myocardial protection during procedures involving reperfusion injury, attention has been focused on the research of drugs and substances that can prevent cardiac cell damage.7 In this context, we observe great interest in the antioxidant action of natural products such as açaí.8
Açaí (Euterpe oleracea Mart.) is a typical northern Brazilian fruit recently made popular for its high antioxidant capacity related to the presence of phenolic acids, flavonoids and anthocyanins.9-12Açaí pulp compounds consist of 31% flavonoids, 23% phenolic compounds, 11% lignoids and 9% anthocyanins.13 The main anthocyanins in açaí pulp are cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, which are responsible for the purple color of the fruit.13 Ferulic acid, p-hydroxybenzoic, gallic, protocatechuic, ellagic, vanillic, p-coumaric acids, and ellagic acid glycoside are the most abundant phenolic compounds.13,14
In experimental models, açaí supplementation reduced pulmonary9 and cerebral oxidative stress,15 reduced ROS formation in polymorphonuclear cells,11,16 decreased DNA damage, and presented anti-carcinogenic activity in bladder cancer.10 Oral açaí administration was able to attenuate hypertrophy and left ventricle dysfunction in rats subjected to myocardial infarction,17 but no studies on the effect of açaí in the global ischemia model or its effect on reperfusion injury were found.
The aim of our study was to evaluate the infarct area, left ventricle function, oxidative stress and the activity of enzymes involved in myocardial energy metabolism in the global ischemia-reperfusion model in rats after açaí supplementation.
Method
Study design
The experimental protocol of this study was approved by the Ethical Committee on the Use of Animals of the Botucatu Medical School (CEUA 1111/2014), and it is in accordance with the norms established by the National Council of Control of Animal Experimentation.
Twenty two-months-old male Wistar rats weighing 250-300 g were assigned to two groups: control (C; n = 10) and açaí (A; n = 10). Sample size was determined by convenience based on previous studies that used the same experimental model. Animals were kept in an environment with controlled temperature (23°C) and a 12-hour light-dark cycle in individual boxes to control feed intake. Group C received a standard chow, and the group A a standard one supplemented with 5% açaí18 for six weeks. After the supplementation period, all animals were anesthetized with sodium thiopental (80 mg/kg, IP ) to induce the global ischemia-reperfusion protocol, after which the heart was dissected. A sectional cut of the left ventricle was made to determine the infarct area, and the rest was stored in a freezer at -80°C for further analysis. One rat from the control group was lost due to technical problems during the ischemia-reperfusion protocol and the study was concluded with 9 rats in the control group and 10 rats in the açaí one.
Preparation of the chow supplemented with açaí
Commercialized açaí pulp (Icefruit®) was defrosted and incorporated into crushed Nuvilab chow (Nuvital®). After homogenization, the chow was pelleted again, dried at 32°C and stored in a freezer at -20°C until the moment of use. The dose used in the study was 5%, as proposed by Fragoso et al.18
Induction of global ischemia, reperfusion and evaluation of cardiac function
The rats were anesthetized with thiopental sodium (80 mg/kg, IP), heparinized (2,000 IU, IP) and subjected to positive pressure ventilation with 100% oxygen. Then, median sternotomy was performed, and the ascending aorta was cannulated to start retrograde perfusion with a modified Krebs-Henseleit solution (NaCl 115 mmol/L, KCl 5.4 mmol/L, CaCl2 1.25 mmol/L; MgSO4 1.2 mmol/L, NaH2PO4 1.15 mmol/L, NaHCO3 25 mmol/L, 11 mmol/L glucose, and 8 mmol/L mannitol). The hearts were transferred to a Langendorff apparatus (Model 830 Hugo Sachs Eletronik, Germany) with perfusion pressure at 75 mmHg. The nutrient solution was constantly oxygenated with a gas mixture of 95% O2 and 5% CO2, and the temperature was maintained at 37°C. A pacemaker was used to maintain controlled heart rate (250 bpm).
Left atrium was removed, and a latex balloon was inserted into the left ventricular cavity. The balloon was coupled to a pressure transducer and to a syringe, which allowed variation in the volume of the balloon. After 10 minutes of stabilization, the hearts were subjected to a 30-minute period of global ischemia followed by 30 minutes of reperfusion.3 Global ischemia was induced by completely stopping the flow of Krebs-Henseleit solution to the heart.
After ischemia and reperfusion periods, an evaluation of left ventricular function was performed. The volume inside the balloon was progressively increased to obtain left ventricular diastolic pressure variation of 0 to 25 mmHg. In addition, for each increase in volume to the balloon, the diastolic and systolic pressure, the maximum left ventricular pressure development rate (+dP/dt) and the maximum left ventricular pressure decrease rate (-dP/dt) were recorded. Diastolic pressure-volume curves were constructed.
Analysis of infarcted myocardial area
A cross-sectional cut of the left ventricle (LV) was made - 5 mm from the apex, with a thickness of 2 mm - and incubated in phosphate buffer with 7.4 pH and 1% triphenyltetrazolium chloride (Sigma Aldrich) for 30 minutes at 37°C. After that, the sections were incubated in a 10% formaldehyde solution overnight. The ventricle sections were positioned between two glass slides and scanned to obtain the images.
Infarct area was measured through the ImageJ program by planimetry and expressed as the percentage of infarcted over total areas. In live cells the dye is reduced by dehydrogenases and appears with a dark red coloration. Dead cells lacking the enzymes are not stained and remain pale in color.19
Analysis of antioxidant enzymes and lipid hydroperoxide
Samples of approximately 100 mg of LV tissue were homogenized in a sodium phosphate buffer (0.01 M) with a pH of 7.4 and centrifuged for 30 minutes at -4°C; the total proteins in the samples were quantified by Bradford method. The activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase in the cardiac tissue were determined by spectrophotometry according to previously described methods.20,21 Lipid hydroperoxide concentration in the cardiac tissue was measured by the oxidation of ammoniacal ferrous sulfate and determined by spectrophotometry.22
Evaluation of energy metabolism
Samples of approximately 100 mg of LV tissue were homogenized in a sodium phosphate buffer (0.1 M, pH 7.0) and centrifuged. The supernatant was used to determine protein concentration and activity of the enzymes b-hydroxyacyl coenzyme A dehydrogenase, phosphofructokinase, lactate dehydrogenase, pyruvate dehydrogenase and citrate synthase.23 The pellet was re-suspended with sodium phosphate buffer (0.1 M) containing 250 mM sucrose and 2 mM EDTA and used to determine the activity of enzymatic complexes of the electron transport chain (complexes I, II and ATP synthase).24 Readings were performed on a microplate reader with controlGen5 2.0 software, and all reagents were obtained from the Sigma-Aldrich laboratory (Saint Louis, USA).
Western blot
LV samples (80 mg) were homogenized with 1 ml of radio immune precipitation assay extraction buffer (RIPA), centrifuged, and the supernatant was collected. The protein in the samples was quantified by the Bradford method; and the samples were used to determine total and phosphorylated nuclear factor signaling pathway kB (NF-kB), sirtuin 1 (SIRT1), and forkhead box protein O1 (FOXO1) protein expression. To determine the nuclear factor erithroid 2 (Nrf-2), LV samples were extracted with Nuclear Extraction Buffer.25 All samples were diluted in Laemmli buffer.
Protein electrophoresis was performed at 4°C on an 8 to 10% polyacrylamide gel (Mini-Protean 3 Electrophoresis Cell System, Bio-Rad, Hercules, USA). After electrophoresis, the gels were transferred to nitrocellulose membranes (Mini Trans-Blot system, Bio-Rad, Hercules, USA) in a wet transfer system followed by blocking with 5% skim milk powder solution. The membrane was washed, and primary antibodies were added (Santa Cruz Biotechnology, Inc., Europe). After overnight incubation, the membrane was washed with basal solution, and secondary antibodies were added (Santa Cruz Biotechnology, Inc., Europe). After 2 hours, the membrane was washed again in basal solution.
Immunodetection was performed with the chemiluminescence method using the SuperSignal West Pico Chemiluminescent Substrate Kit (ThermoScientific, USA). Photo documentation by ImageQuant LAS 4000 (General Eletrikcs) was used to generate images, which were analyzed in Gel-Pro 32 (Media Cybernetics, Rockville, USA). The results obtained for the target proteins were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression, and the same control animal was included in all electrophoreses for standardization between the experiments.
Statistical analysis
Values obtained are presented as the means ± standard deviation (variables with normal distribution) or median and 25 and 75% quartile (variables with non-normal distribution). Normality was verified by the Kolmogorov-Smirnov test. Comparisons between the groups were performed with a non-pared Student’s t-test (variables with normal distribution) or Mann-Whitney test (variables with non-normal distribution). Statistical analysis was carried out by use of the SigmaStat software, considering significance level at 5% for all analyses.
Results
General features
The animals’ initial and final body weight did not differ between groups. Additionally, the left and right ventricles, liver and lung weights were similar between groups. Daily food intake was approximately 26 g for both groups (Table 1).
Table 1.
Morphological variables and feed intake in rats submitted to global myocardial ischemia-reperfusion
| C (n = 9) | A (n = 10) | p-value | |
|---|---|---|---|
| Initial BW (g) | 274 ± 15 | 281 ± 11 | 0.274 |
| Final BW (g) | 468 ± 29 | 448 ± 40 | 0.225 |
| LV weight (g) | 1.03 ± 0.09 | 1.02 ± 0.07 | 0.934 |
| RV weight (g) | 0.30 ± 0.03 | 0.29 ± 0.03 | 0.431 |
| Liver weight (g) | 14.4 ± 1.6 | 12.9 ± 2.6 | 0.154 |
| Lung weight (g) | 1.62 ± 0.03 | 1.54 ± 0.18 | 0.359 |
| Daily chow intake (g) | 26.1 ± 2.2 | 26.6 ± 1.8 | 0.246 |
C: control group; A: açaí group; BW: body weight; LV: left ventricle; RV: right ventricle. Values are expressed as the means ± standard deviation; p-value: t test.
Infarcted myocardial area
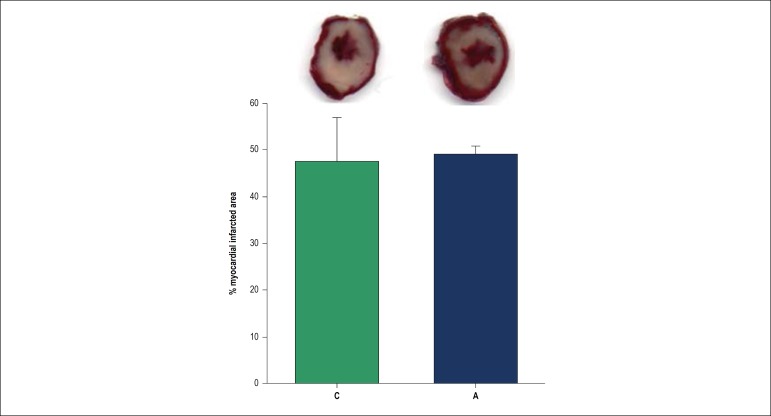
We observed no difference in the area of myocardial infarction between groups A and C, as observed in Figure 1.
Figure 1.
Myocardial infarcted area after global myocardial ischemia-reperfusion. Figure shows left ventricles sections stained with triphenyltetrazolium chloride 1%. White area represents the infarcted myocardium. C: control group; A: açaí group; There is no difference between groups (p = 0.710).
Oxidative stress and myocardial energy metabolism
Açaí supplementation promoted lower myocardial lipid hydroperoxide concentration, and greater activity of the enzymes catalase, SOD and GSH-Px in the myocardium of these rats was observed (Table 2).
Table 2.
Myocardial oxidative stress marker and antioxidant enzymes activity after global ischemia-reperfusion
| C (n = 9) | A (n = 10) | p-value | |
|---|---|---|---|
| LH (nmol/g tissue) | 330 ± 33 | 208 ± 22 | < 0.001 |
| CAT (µmol/g of tissue) | 58.5 ± 6.4 | 70.0 ± 8.9 | 0.005 |
| SOD (nmol/mg of protein) | 5.7 ± 0.4 | 8.1 ± 0.7 | < 0.001 |
| GSH-Px (nmol/mg of tissue) | 20.2 ± 3.3 | 32.8 ± 4.7 | < 0.001 |
C: control group; A: açaí group; LH: lipid hydroperoxide; CAT: catalase; SOD superoxide dismutase; GSH-Px: glutathione peroxidase. Values are expressed as the means ± standard deviation; p-value: t test.
Regarding myocardium energy metabolism, açaí pulp supplementation promoted lower activity of the enzymes lactate dehydrogenase and phosphofructokinase and higher activity of the enzymes b-hydroxyacyl-coenzyme A dehydrogenase, citrate synthase and pyruvate dehydrogenase. In addition, we observed higher activity of complex I, complex II and ATP synthase in the group supplemented with açaí (Table 3).
Table 3.
Myocardial activity of enzymes related to energy metabolism after global ischemia-reperfusion protocol
| C (n= 9) | A (n=10) | p-value | |
|---|---|---|---|
| β-hydroxyacyl-CoA dehydrogenase (nmoL/mg protein) | 19.8 ± 3.3 | 49.8 ± 7.0 | < 0.001 |
| Phosphofructokinase (nmoL/g tissue) | 181 (160 - 228) | 73 (67 - 82) | < 0.001 |
| Lactate dehydrogenase (nmoL/mg protein) | 147 (145 - 167) | 70 (65 - 77) | < 0.001 |
| Pyruvate dehydrogenase (nmoL/g tissue) | 114 ± 11 | 176 ± 19 | < 0.001 |
| Citrate synthase (nmol/mg protein) | 30 (30 - 37) | 100 (81 - 117) | < 0.001 |
| Complex I (nmol/mg protein) | 3.7 ± 1.0 | 8.0 ± 1.7 | < 0.001 |
| Complex II (nmol/mg protein) | 2.1 ± 0.6 | 3.5 ± 0.5 | < 0.001 |
| ATP synthase (nmol/mg protein) | 22.0 ± 3.9 | 44.5 ± 6.6 | < 0.001 |
C: control group; A: açaí group. Values are expressed as the means ± standard deviation or median and 1st and 3rd quartile; p-value: t test or Mann-Whitney test.
Western blot
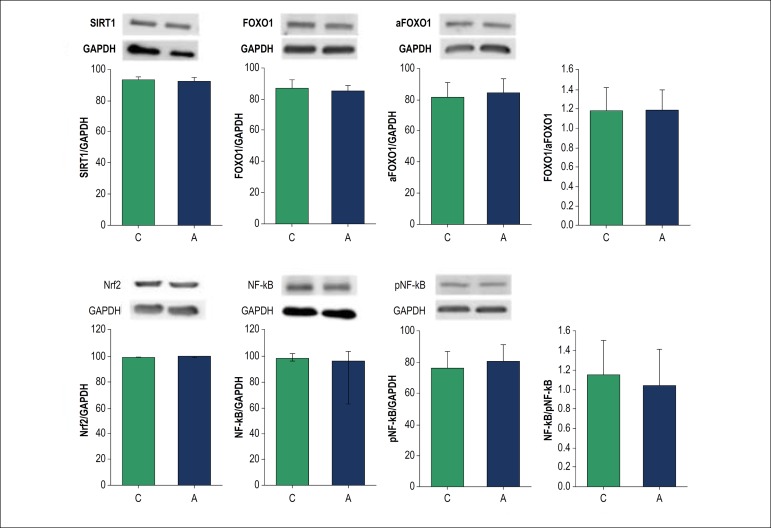
The expression of the proteins involved in the regulation of the oxidative stress pathway is shown in Figure 2. There was no difference in the expression of total and phosphorylated NF-kB, total and acetylated FOXO1, SIRT1 and Nrf-2. Also, no differences were observed in the NF-kB phosphorylated/total or the FOXO1 acetylated/total ratios.
Figure 2.
Protein expression evaluated by Western blot. C: control group; A: açaí group; SIRT1: silent information regulator 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; FOXO1: forkhead protein 1; aFOXO1: acetylated forkhead protein 1; Nrf-2: nuclear factor erythroid 2; NF-kB: nuclear factor kappa B; pNF-kB: phosphorylated nuclear factor kappa B. All proteins expressions were normalized by GAPDH. There are no differences between groups in protein expression (p > 0.05).
Isolated heart study
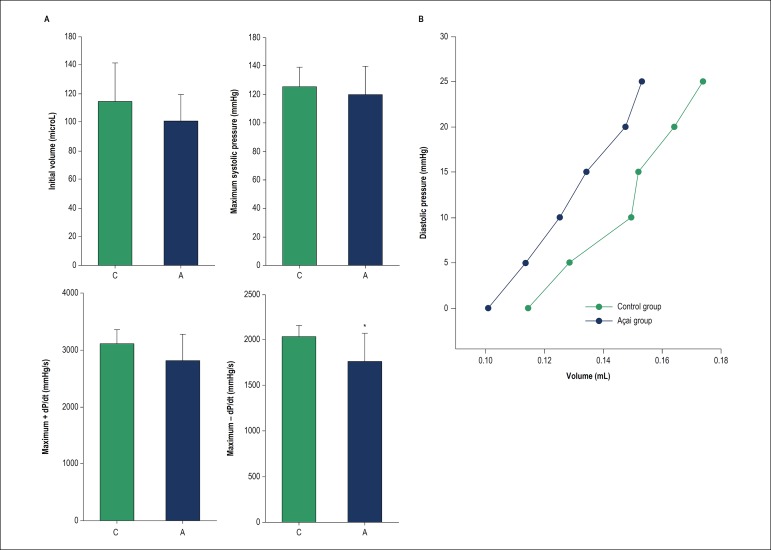
There was no difference between the groups regarding the initial volume of the balloon, the maximum systolic pressure reached, or +dP/dt, which represents systolic function. Group A presented worse -dP/dt than C, signifying impairment of the diastolic function in the rats that received açaí supplementation. The areas under the curves in the diastolic pressure-volume relationship did not differ between groups (Figure 3).
Figure 3.
Isolated heart study after global myocardial ischemia-reperfusion. Panel A: C: control group; A: açaí group. Initial volume represents the volume inside the balloon when diastolic pressure was zero; +dP/dt: left ventricular pressure development rate; -dP/dt: left ventricular pressure decrease rate; *different from group C (p = 0.025). Panel B: Relationship between diastolic pressure and volume. The area under the curve and inclination were similar between the groups.
Discussion
Myocardial ischemia-reperfusion injury is one of the leading causes of death worldwide, and it remains a situation for which current clinical therapies are surprisingly deficient.26 Irreversible myocardial injury progresses with increased duration of ischemia; therefore, rapid restoration of blood flow to the ischemic area is essential to save the viable myocardium.1 Reperfusion, however, can induce death of cardiomyocytes regardless of the ischemic episode by a process known as reperfusion injury.1,5,27 Decreased reperfusion injury is a key target in the battle to preserve cardiac function in patients with acute myocardial infarction.
During ischemia-reperfusion, changes such as the release of cytokines, interaction between leukocytes and endothelial cells and production of reactive nitrogen/oxygen species and free radicals (ERs)27 occur in cellular metabolism, which can lead to oxidative damage. ERs are generated from various sources, and the energetic metabolism, more precisely the mitochondrial electron transport chain (ETC), is one of the most important generators of these radicals.5
The continuous production of ERs during metabolic processes is regulated by an antioxidant defense system, which limits the intracellular levels and controls the occurrence of cellular damage. The antioxidant defense system can be enzymatic and non-enzymatic.6,7,28 Non-enzymatic defense system includes antioxidant compounds of dietary origin, like vitamins, mineral and phenolic compounds. Flavonoids and other compounds present in açaí can act as non-enzymatic antioxidant, inactivating reactive species.29
During ischemia, there is an increase in nicotinamide adenine dinucleotide oxidase, nitric oxide synthase, xanthine oxidase, cytochrome P450 and cyclooxygenase,8,30 which may result in increased ER generation; likewise, ischemia is associated with a decrease in different antioxidant enzymes. All these changes may result in oxidative stress. In this study, group A presented higher antioxidants enzymes activity in addition to lower concentration of myocardial lipid hydroperoxide. Lipid hydroperoxide is a marker of oxidative damage and is originated from the oxidative lesion of membrane lipids. Membrane lesion can lead to disturbances in permeability, alteration of the ionic flow and DNA, and impairment of extracellular matrix components.31
In relation to the proteins involved in the regulation of the oxidative stress pathway, the mechanisms by which phenolic dietary compounds act in the prevention of degenerative pathologies have been partially studied. The complex interactions between these dietary molecules and their molecular targets activate signaling pathways of the cellular response, including the NF-kB, Nrf2, SIRT1, and FOXO1.32-34
NF-kB is considered an important transcriptional factor related to oxidative stress. Açaí treatment inhibited NF-kB activation in astrocytes cell culture,35,36 inhibited NF-kB phosphorylation in microglial cells37 and down-regulated gene expression of NF-kB in colon myofibroblasts cell culture.38 Similarly, Nrf2 is an important regulator of antioxidant enzymes production. In response to oxidative stress, Nrf2 dissociates from the Keap1 protein and migrates to the cell nucleus, where it stimulates the production of antioxidant enzymes.39 Myocardial ischemia promotes increased protein expression of Nrf240 and the effect of açaí on Nrf2 expression was verified in an astrocyte culture study in which the fruit reduced the protein expression of Nrf2.41 However, in another study, also with astrocyte culture, açaí administration increased Nrf2 expression,36 showing that the effects of açaí on Nrf2 are not completely understood.
Another cellular balance regulator is the SIRT1 protein, which can act on apoptosis, mitochondrial biogenesis, inflammation, glucose and lipid metabolism, autophagy and adaptations to cellular stress through the deacetylation of target proteins such as NF-kB and FOXO1. When acetylated by SIRT1, FOXO1 leads to increased expression of gluconeogenic genes.33 Increased transcriptional activity of FOXO1 also increases the gene expression of catalase and superoxide dismutase.34 To the best of our knowledge, there are no studies that evaluated the effect of açai on FOXO1 and SIRT1 protein expression.
In the normal adult heart, approximately 95% of ATP production is derived from mitochondrial oxidative phosphorylation. The adult heart normally obtains 50-70% of its ATP from fatty acid b-oxidation instead of glucose oxidation.42 The flavin adenine dinucleotide and nicotinamide adenine dinucleotide produced during fatty acid oxidation are used in mitochondrial ATP synthesis via oxidative phosphorylation.43 This is critically dependent on the maintenance of an electrochemical proton gradient across the inner mitochondrial membrane generated by the extrusion of protons from the matrix to the intermembrane space by complexes I, III and IV, which form the ETC.44
In our study, açaí supplementation led to higher activity of b-hydroxyacyl CoA-dehydrogenase and citrate synthase enzymes, which can characterize higher fatty acid oxidation. Moreover, there was lower activity of phosphofructokinase, the enzyme for glycolysis. This shows that açai supplementation altered the substrate selection for mitochondrial oxidation in reperfusion from glucose to fatty acids, maintaining energy metabolism closer to a physiological situation, as showed previously in an experimental model of renal ischemia-reperfusion.45 Additionally,açaí supplementation increased the activity of complexes I, II and ATP synthase by protecting the damage to mitochondrial complexes. However, fatty acid oxidation decreases the metabolic efficiency of injured hearts.
Interestingly, açaí pulp is composed predominantly by lipids that correspond to 48%.46 Offering more lipids could contribute to increased b-hydroxyacyl CoA-dehydrogenase activity in the treated açaí group. This pattern was observed in the experimental myocardial infarction model that administered a chow rich in lipids.47
Regarding the infarcted area, as expected for the 30-minute period of ischemia, we observed a large infarcted area in our study, approximately 50% in both groups. Previously, supplementation of a diet rich in anthocyanins in an experimental model of ischemia-reperfusion decreased the myocardial infarcted area.48 However, in the present study, açaí supplementation did not reduce infarct size.
Large infarctions usually present with important left ventricular dysfunction resulting from the impairment of cellular processes and changes in cardiac morphology. Increased oxidative stress compromises the plasma membrane permeability of myocytes, the activity of ionic pumps in the plasma membrane and in the sarcoplasmic reticulum, and intracellular calcium transit, compromising both systole and diastole.7 It would be expected that the attenuation of oxidative stress would be followed by an improvement in left ventricular function, as showed in different myocardial damage models.25,49,50 However, this study evidenced that the administration of açaí worsened the diastolic function after cardiac ischemia-reperfusion. This leads us to infer that the ventricular dysfunction observed in this model depends on mechanisms other than oxidative damage. Curiously, a study performed to investigate the effect of anthocyanin extract in global rat heart ischemia-reperfusion suggests that anthocyanin was cardioprotective in low doses and could be cardiotoxic in high ones.51
Additionally, the change in energetic metabolism that occurs in stress situations has a protective role in the myocardium.42 The fact that açai supplementation prevents the use of glucose and favors the maintenance of myocardial metabolism close to normal may have negatively interfered with this adaptive protective mechanism.
Whereas ischemia induces morphological and functional alterations and reperfusion injury can exacerbate these features, the discovery of new drugs, substances or strategies that can minimize cardiac damage is essential. The quantity of açaí that rats took was equivalent to 600 mg for a 60 kg human,52 which is feasible human açaí ingestion. Therefore, açaí could be a potential strategy to attenuate ischemia-reperfusion injury in clinical scenario.
However, there are some limitations that difficult the translational impact of these results. First, rats share several metabolic pathways with humans, but the organisms are quite different. Second, in this study we evaluated a global myocardial ischemia that is not the routine in clinical practice. The ischemic process in humans is the result of a long inflammatory process that can modulate completely different metabolic pathways from our model.53
Conclusion
Açaí supplementation did not decrease the infarcted area nor improve the left ventricular function in the global ischemia-reperfusion model despite improving cardiac energy metabolism and attenuating myocardial oxidative stress, suggesting that these mechanisms may not be the main determinants of the worsened diastolic left ventricular function observed after ischemia and reperfusion with açaísupplementation.
Footnotes
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of master submitted by Patricia Alegre, from pela Universidade Estadual Paulista, Faculdade de Medicina de Botucatu.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of the Faculdade de Medicina de Botucatu under the protocol number 1111/2014. All the procedures in this study were in accordance with the 1975 Helsinki Declaration, updated in 2013.
Author contributions
Conception and design of the research: Zornoff L, Paiva SAR, Polegato BF; Acquisition of data: Alegre P, Mathias L, Lourenço MA, Gonçalves A, Fernandes AA; Analysis and interpretation of the data: Alegre P, Santos PP, Gaiolla PSA, Minicucci MF, Zornoff L, Paiva SAR, Polegato BF; Statistical analysis: Minicucci MF, Paiva SAR, Polegato BF; Writing of the manuscript: Alegre P, Santos PP, Polegato BF; Critical revision of the manuscript for intellectual content: Alegre P, Mathias L, Lourenço MA, Santos PP, Gonçalves A, Fernandes AA, Gaiolla PSA, Minicucci MF, Zornoff L, Paiva SAR, Polegato BF.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65(14):1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Kals J, Starkopf J, Zilmer M, Pruler T, Pulges K, Hallaste M, et al. Antioxidant UPF1 attenuates myocardial stunning in isolated rat hearts. Int J Cardiol. 2008;125(1):133–135. doi: 10.1016/j.ijcard.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BS, Hadziahmetovic M, Veci T, Angelos MG. Global ischemic duration and reperfusion function in the isolated perfused rat heart. Resuscitation. 2004;62(1):97–106. doi: 10.1016/j.resuscitation.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Borges JP, Lessa MA. Mechanisms involved in exercise-induced cardioprotection: a systematic review. Arq Bras Cardiol. 2015;105(1):71–81. doi: 10.5935/abc.20150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018 Mar;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Martins GF, Siqueira Filho AG, Santos JB, Assuncao CR, Bottino F, Carvalho KG, et al. Trimetazidine on ischemic injury and reperfusion in coronary artery bypass grafting. Arq Bras Cardiol. 2011;97(3):209–216. doi: 10.1590/s0066-782x2011005000079. [DOI] [PubMed] [Google Scholar]
- 7.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27(5):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoudabady M, Haghshenas M, Niazmand S. Extract from Teucrium polium L. protects rat heart against oxidative stress induced by ischemic-reperfusion injury. Adv Biomed Res. 2018 Jan 30;7:15–15. doi: 10.4103/abr.abr_218_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moura RS, Ferreira TS, Lopes AA, Pires KM, Nesi RT, Resende AC, et al. Effects of Euterpe oleracea Mart. (AÇAÍ) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine. 2012;19(3-4):262–269. doi: 10.1016/j.phymed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Fragoso MF, Prado MG, Barbosa L, Rocha NS, Barbisan LF. Inhibition of mouse urinary bladder carcinogenesis by açaí fruit (Euterpe oleraceae Martius) intake. Plant Foods Hum Nutr. 2012;67(3):235–241. doi: 10.1007/s11130-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 11.Guerra JF, Magalhaes CL, Costa DC, Silva ME, Pedrosa ML. Dietary acai modulates ROS production by neutrophils and gene expression of liver antioxidant enzymes in rats. J Clin Biochem Nutr. 2011;49(3):188–194. doi: 10.3164/jcbn.11-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassimotto NM, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem. 2005;53(8):2928–2935. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi KK, Pereira LF, Lamarão CV, Lima ES, da Veiga-Junior VF. Amazon acai: chemistry and biological activities: a review. Food Chem. 2015 Jul 15;179:137–151. doi: 10.1016/j.foodchem.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 14.da Silveira TFF, Godoy HT. Non-anthocyanin phenolic compounds in açaí (Euterpe oleracea Mart.) juice by ultrahigh-performance liquid chromatography-diode array detector (UHPLC-DAD): a multivariate optimization. J Chromatogr Sci. 2019;57(2):139–148. doi: 10.1093/chromsci/bmy095. [DOI] [PubMed] [Google Scholar]
- 15.Spada PD, Dani C, Bortolini GV, Funchal C, Henriques JA, Salvador M. Frozen fruit pulp of Euterpe oleraceae Mart. (Acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J Med Food. 2009;12(5):1084–1088. doi: 10.1089/jmf.2008.0236. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Schauss AG. Mitigation of inflammation with foods. J Agric Food Chem. 2012;60(27):6703–6717. doi: 10.1021/jf3007008. [DOI] [PubMed] [Google Scholar]
- 17.Zapata-Sudo G, da Silva JS, Pereira SL, Souza PJ, de Moura RS, Sudo RT. Oral treatment with Euterpe oleracea Mart. (acai) extract improves cardiac dysfunction and exercise intolerance in rats subjected to myocardial infarction. BMC Complement Altern Med. 2014 Jul 08;14:227–227. doi: 10.1186/1472-6882-14-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fragoso MF, Romualdo GR, Ribeiro DA, Barbisan LF. Acai (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem Toxicol. 2013 Aug;58:68–76. doi: 10.1016/j.fct.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yuan B, Cheng B, Liu Y, Zhang B, Lin X, et al. Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of MIR-34A/SIRT1/NRF2 pathway. Shock. 2019;51(1):123–130. doi: 10.1097/SHK.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura A, Minakami S. Synthesis of catalase in liver slices from aminotriazole-pretreated rats. J Biochem. 1974;75(6):1373–1375. doi: 10.1093/oxfordjournals.jbchem.a130523. [DOI] [PubMed] [Google Scholar]
- 21.Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem. 1995;232(2):243–248. doi: 10.1006/abio.1995.0014. [DOI] [PubMed] [Google Scholar]
- 22.Jiang ZY, Woollard AC, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26(10):853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- 23.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 24.Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328(2):309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 25.Lustosa BB, Polegato B, Minicucci M, Rafacho B, Santos PP, Fernandes AA, et al. Green tea (Cammellia sinensis) attenuates ventricular remodeling after experimental myocardial infarction. Int J Cardiol. 2016 Dec 15;225:147–153. doi: 10.1016/j.ijcard.2016.09.092. [DOI] [PubMed] [Google Scholar]
- 26.Lefer DJ, Marbán E. Is cardioprotection dead? Circulation. 2017;136(1):98–109. doi: 10.1161/CIRCULATIONAHA.116.027039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pell VR, Chouchani ET, Frezza C, Murphy MP, Krieg T. Succinate metabolism: a new therapeutic target for myocardial reperfu. [DOI] [PubMed] [Google Scholar]
- 28.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47(3):446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 29.Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, et al. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56(17):7796–7802. doi: 10.1021/jf8007037. [DOI] [PubMed] [Google Scholar]
- 30.Raedschelders K, Ansley DM, Chen DD. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012;133(2):230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, et al. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic Biol Med. 2007;42(12):1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 33.Albertoni G, Schor N. Resveratrol plays important role in protective mechanisms in renal disease-mini-review. J Bras Nefrol. 2015;37(1):106–114. doi: 10.5935/0101-2800.20150015. [DOI] [PubMed] [Google Scholar]
- 34.Ma Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther. 2010;125(3):376–393. doi: 10.1016/j.pharmthera.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Noratto GD, Angel-Morales G, Talcott ST, Mertens-Talcott SU. Polyphenolics from açaí (Euterpe oleracea Mart.) and red muscadine grape (Vitis rotundifolia) protect human umbilical vascular Endothelial cells (HUVEC) from glucose- and lipopolysaccharide (LPS)-induced inflammation and target microRNA-126. J Agric Food Chem. 2011;59(14):7999–8012. doi: 10.1021/jf201056x. [DOI] [PubMed] [Google Scholar]
- 36.Ajit D, Simonyi A, Li R, Chen Z, Hannink M, Fritsche KL, et al. Phytochemicals and botanical extracts regulate NF-kB and Nrf2/ARE reporter activities in DI TNC1 astrocytes. Neurochem Int. 2016 Jul;97:49–56. doi: 10.1016/j.neuint.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulose SM, Fisher DR, Larson J, Bielinski DF, Rimando AM, Carey AN, et al. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem. 2012;60(4):1084–1093. doi: 10.1021/jf203989k. [DOI] [PubMed] [Google Scholar]
- 38.Dias MM, Martino HS, Noratto G, Roque-Andrade A, Stringheta PC, Talcott S, et al. Anti-inflammatory activity of polyphenolics from açai (Euterpe oleracea Martius) in intestinal myofibroblasts CCD-18Co cells. Food Funct. 2015;6(10):3249–3256. doi: 10.1039/c5fo00278h. [DOI] [PubMed] [Google Scholar]
- 39.Bai H, Wang Z, Cui J, Yun K, Zhang H, Liu RH, et al. Synergistic radiation protective effect of purified Auricularia auricular-judae polysaccharide (AAP IV) with grape seed procyanidins. Molecules. 2014;19(12):20675–20694. doi: 10.3390/molecules191220675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Sano M, Shinmura K, Tamaki K, Katsumata Y, Matsuhashi T, et al. 4-hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol Cell Cardiol. 2010;49(4):576–586. doi: 10.1016/j.yjmcc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 41.da Silva Santos V, Bisen-Hersh E, Yu Y, Cabral IS, Nardini V, Culbreth M, et al. Anthocyanin-rich açaí (Euterpe oleracea Mart.) extract attenuates manganese-induced oxidative stress in rat primary astrocyte cultures. J Toxicol Environ Health A. 2014;77(7):390–404. doi: 10.1080/15287394.2014.880392. [DOI] [PubMed] [Google Scholar]
- 42.Azevedo PS, Minicucci MF, Santos PP, Paiva SAR, Zornoff LAM. Energy metabolism in cardiac remodeling and heart failure. Cardiol Rev. 2013;21(3):135–140. doi: 10.1097/CRD.0b013e318274956d. [DOI] [PubMed] [Google Scholar]
- 43.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 44.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2005;1706(1-2):1–11. doi: 10.1016/j.bbabio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 45.El Morsy EM, Ahmed MA, Ahmed AA. Attenuation of renal ischemia/reperfusion injury by acai extract preconditioning in a rat model. Life Sci. 2015 Feb 15;123:35–42. doi: 10.1016/j.lfs.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Alessandra-Perini J, Rodrigues-Baptista KC, Machado DE, Nasciutti LE, Perini JA. Anticancer potential, molecular mechanisms and toxicity of Euterpe oleracea extract (açaí): a systematic review. PLoS One. 2018;13(7):e0200101. doi: 10.1371/journal.pone.0200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ardisson LP, Minicucci MF, Azevedo PS, Chiuso-Minicucci F, Matsubara BB, Matsubara LS, et al. Influence of AIN-93 diet on mortality and cardiac remodeling after myocardial infarction in rats. Int J Cardiol. 2012;156(3):265–269. doi: 10.1016/j.ijcard.2010.10.128. [DOI] [PubMed] [Google Scholar]
- 48.Toufektsian MC, de Lorgeril M, Nagy N, Salen P, Donati MB, Giordano L, et al. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr. 2008;138(4):747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho PB, Gonçalves AF, Alegre PH, Azevedo PS, Roscani MG, Bergamasco CM, et al. Pamidronate attenuates oxidative stress and energetic metabolism changes but worsens functional outcomes in acute doxorubicin-induced cardiotoxicity in rats. Cell Physiol Biochem. 2016;40(3-4):431–442. doi: 10.1159/000452558. [DOI] [PubMed] [Google Scholar]
- 50.Zornoff LA, Matsubara LS, Matsubara BB, Okoshi MP, Okoshi K, Dal Pai-Silva M, et al. Beta-carotene supplementation attenuates cardiac remodeling induced by one-month tobacco-smoke exposure in rats. Toxicol Sci. 2006;90(1):259–266. doi: 10.1093/toxsci/kfj080. [DOI] [PubMed] [Google Scholar]
- 51.Ziberna L, Lunder M, Moze S, Vanzo A, Tramer F, Passamonti S, et al. Acute cardioprotective and cardiotoxic effects of bilberry anthocyanins in ischemia-reperfusion injury: beyond concentration-dependent antioxidant activity. Cardiovasc Toxicol. 2010;10(4):283–294. doi: 10.1007/s12012-010-9091-x. [DOI] [PubMed] [Google Scholar]
- 52.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 53.Garcia LR, Polegato BF, Zornoff LAM. Challenges of translational science. Arq Bras Cardiol. 2017;108(5):388–389. doi: 10.5935/abc.20170061. [DOI] [PMC free article] [PubMed] [Google Scholar]