Coagulation disorders are a common complication in patients with chronic kidney disease (CKD), with thrombosis being the most common cause of death [1]. Recently Huang et al. [2] found that patients with CKD are characterized by increased factor VIII (FVIII) activity and endothelial dysfunction, as apparent from increased von Willebrand factor (VWF) antigen levels.
VWF exerts a procoagulant effect by carrying FVIII and mediating platelet adhesion and aggregation. Under physiological conditions, VWF adopts a globular conformation and is unable to interact spontaneously with platelets. Pathologically, high shear or binding to exposed subendothelial collagen at sites of vascular injury can result in unfolding, thereby exposing the VWF A1 domain [3]. This active conformation allows binding of VWF to the platelet glycoprotein Ib-IX-V receptor complex [4]. Additionally, ultralarge (UL)-VWF multimers are released from endothelial cells upon activation. These UL-VWF multimers are in their active, platelet-binding conformation, but are normally cleaved immediately by a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) [4].
Circulating active VWF is increased in various patient populations suffering from thrombotic complications [5]. Therefore we assessed if levels of active VWF are also affected by various stages of kidney disease.
Using an enzyme-linked immunosorbent assay [6], we determined active VWF levels in plasma from a cohort of patients (55% male) with renal disease. The cohort comprised patients not receiving haemodialysis [CKD, n = 25, mean age 61 ± 13 years, body mass index (BMI) 29.3 ± 6.6 kg/m2, estimated glomerular filtration rate (eGFR) 29 ± 14 mL/min/1.73 m2], patients with end-stage renal disease (ESRD) undergoing thrice weekly haemodialysis (HD-ESRD, n = 13, mean age 65 ± 15 years, BMI 26.6 ± 4.8 kg/m2) and patients with ESRD on peritoneal dialysis (PD-ESRD, n = 4, mean age 62 ± 20 years, BMI 26.1 ± 4.3 kg/m2). Macro-albuminuria [albumin:creatinine ratio (ACR): men >25 mg/mmol, women >35 mg/mmol] was unknown for HD-ESRD patients, but was present in 17 (68%) CKD patients and 3 (75%) PD-ESRD patients. A history of thrombosis was present in three (12%) CKD patients (one deep vein thrombosis, two ischaemic stroke), two (15%) HD-ESRD patients (one myocardial infarction, one ischaemic stroke) and one (25%) PD-ESRD patient (deep vein thrombosis). A group of healthy volunteers (n = 31, 36% male, mean age 50 ± 12 years, BMI 23.3 ± 2.5, eGFR > 60 mL/min/1.73 m2, no proteinuria and no history of thrombosis) served as unmatched controls. All participants gave full informed consent according to the 2013 Declaration of Helsinki and the study was approved by the Medical Ethical Committee of the Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands (study NL45975.008.14).
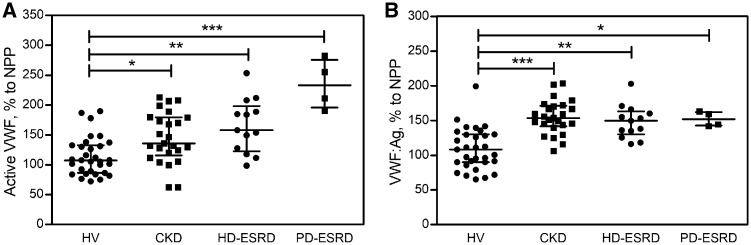
In the circulation of all patient groups, active VWF levels were elevated compared with healthy controls [median (Q1–Q3) 107.6% (86.4–132.8)] (Figure 1A). Although the differences between CKD [135.8% (115.8–179.6)], HD-ESRD [158.1% (122.7–198.4)] and PD-ESRD [233.3% (195.8–275.8)] patients were not significant, a trend towards increased active VWF with increased disease severity was observed. Of note, plasma levels of total VWF were also considerably higher in CKD [153.6% (141.7–171.3)], HD-ESRD [149.7% (130.2–163.2)] and PD-ESRD [152.0% (142.6–162.4)] patients than in healthy controls [108.2% (90.0–130.0)] (Figure 1B), corroborating previous reports [2, 7], but did not show the same trend with increased disease severity.
FIGURE 1.
Active VWF and total VWF are elevated in kidney disease compared with healthy controls. Levels of (A) active VWF and (B) total VWF, both normalized to levels in normal pooled plasma, were measured in patients with CKD, HD-ESRD or PD-ESRD and healthy volunteers. Bars represent the median and whiskers represent the first and third quartiles. P-values <0.05 were considered significant using Kruskal–Wallis test with post hoc Dunn’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. All statistical analyses were performed using Graphpad Prism version 7 (GraphPad Software, San Diego, CA, USA).
The underlying pathophysiological mechanism is likely related to chronic exposure of endothelium to inflammatory cytokines and oxidative stress [8]. As a result, with progressing stages of kidney disease, ongoing damage and/or activation of the endothelium lead to the release of increasingly large amounts of UL-VWF into the circulation. The degree of endothelial activation may also be related to the dialysis modality, as a trend towards higher active VWF levels in PD-ESRD compared with HD-ESRD patients can be observed. Additionally, a previous study [9] found substantially decreased ADAMTS13 levels in CKD patients compared with controls. This reduction in ADAMTS13 levels may be caused by increased loss via urine due to damage to the glomerular filtration membrane. Consequently, a proportion of UL-VWF will not be proteolysed, but remains in its active conformation. Interestingly, we observed a significant correlation (Spearman r = 0.390, P = 0.0021) between urinary protein (including ADAMTS13) loss (measured as ACR) and circulating active VWF levels in our study population.
In summary, circulating levels of total VWF and, even more pronounced, active VWF are increased in patients with CKD and ESRD compared with healthy controls. High plasma VWF levels were previously found to be a predictor of microalbuminuria, CVD and mortality in both diabetic and non-diabetic patients [10]. We hypothesize that active VWF, being the haemostatically active fraction of VWF, may be even more strongly associated with cardiovascular morbidity and mortality. However, follow-up studies with larger patient numbers are required to determine the mechanism behind increased active VWF in CKD, by examining the association between active VWF, circulating and urinary ADAMTS13 levels and the presence of an arteriovenous fistula. In addition, clinical follow-up data are required to unravel the relationship of active VWF and clinical outcome.
ACKNOWLEDGEMENTS
We thank V.J.F. Strijbis and R.M.W. Kremers for their assistance with the plasma samples.
AUTHORS’ CONTRIBUTIONS
L.N.V., D.L.A., H.C.H., R.V., P.L.R., R.v.H. and B.d.L. were involved in the conception and design of the experiments. D.L.A., R.V., P.L.R. and R.v.H. collected the samples. L.N.V. and A.V. collected, analysed and interpreted the data. L.N.V. drafted the manuscript. J.A.R., D.H., P.L.R. and B.d.L. critically revised the manuscript. All authors approved the final version of the article.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Wattanakit K, Cushman M, Stehman-Breen C. et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 2008; 19: 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang M-J, Wei R-B, Wang Y. et al. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open 2017; 7: e014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huizinga EG, Tsuji S, Romijn RAP. et al. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science 2002; 297: 1176–1179 [DOI] [PubMed] [Google Scholar]
- 4. Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res 2007; 120(Suppl 1): S5–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groot E, de Groot PG, Fijnheer R. et al. The presence of active von Willebrand factor under various pathological conditions. Curr Opin Hematol 2007; 14: 284–289 [DOI] [PubMed] [Google Scholar]
- 6. van der Vorm LN, Li L, Huskens D. et al. Analytical characterization and reference interval of an enzyme-linked immunosorbent assay for active von Willebrand factor. PLoS One 2019; 14: e0211961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pequeriaux NC, Fijnheer R, Gemen EF. et al. Plasma concentration of von Willebrand factor predicts mortality in patients on chronic renal replacement therapy. Nephrol Dial Transplant 2012; 27: 2452–2457 [DOI] [PubMed] [Google Scholar]
- 8. Merino A, Nogueras S, Buendía P. et al. Microinflammation and endothelial damage in hemodialysis. Contrib Nephrol 2008; 161: 83–88 [DOI] [PubMed] [Google Scholar]
- 9. Shen L, Lu G, Dong N. et al. Von Willebrand factor, ADAMTS13 activity, TNF-α and their relationships in patients with chronic kidney disease. Exp Ther Med 2012; 3: 530–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jager A, van Hinsbergh VWM, Kostense PJ. et al. Von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol 1999; 19: 3071–3078 [DOI] [PubMed] [Google Scholar]