Abstract
Background
Etelcalcetide is an intravenous calcimimetic approved for treatment of secondary hyperparathyroidism (sHPT) in patients receiving hemodialysis. Besides lowering parathyroid hormone (PTH), etelcalcetide also significantly reduces fibroblast growth factor 23 (FGF23), but the mechanisms are unknown.
Methods
To investigate potential mediators of etelcalcetide-induced FGF23 reduction, we performed secondary analyses of the 26-week randomized trials that compared the effects on PTH of etelcalcetide (n = 509) versus placebo (n = 514) and etelcalcetide (n = 340) versus cinacalcet (n = 343) in adults with sHPT receiving hemodialysis. We analyzed changes in FGF23 in relation to changes in PTH, calcium, phosphate and bone turnover markers. We also investigated how concomitant treatments aimed at mitigating hypocalcemia altered the FGF23-lowering effects of etelcalcetide.
Results
Etelcalcetide reduced FGF23 [median % change (quartile 1–quartile 3)] from baseline to the end of the trial significantly more than placebo [–56% (–85 to –7) versus +2% (–40 to +65); P < 0.001] and cinacalcet [–68% (–87 to –26) versus –41% (–76 to +25); P < 0.001]. Reductions in FGF23 correlated strongly with reductions in calcium and phosphate, but not with PTH; correlations with bone turnover markers were inconsistent and of borderline significance. Increases in concomitant vitamin D administration partially attenuated the FGF23-lowering effect of etelcalcetide, but increased dialysate calcium concentration versus no increase and increased dose of calcium supplementation versus no increase did not attenuate the FGF23-lowering effects of etelcalcetide.
Conclusion
These data suggest that etelcalcetide potently lowers FGF23 in patients with sHPT receiving hemodialysis and that the effect remains detectable among patients who receive concomitant treatments aimed at mitigating treatment-associated decreases in serum calcium.
Keywords: calcimimetics, FGF23, hemodialysis, PTH, secondary hyperparathyroidism
INTRODUCTION
Circulating concentrations of fibroblast growth factor 23 (FGF23) are markedly elevated in patients with advanced chronic kidney disease (CKD) [1]. Increased FGF23 helps to maintain phosphate homeostasis in progressive CKD by stimulating renal phosphate excretion and suppressing circulating vitamin D levels, the latter of which promotes secondary hyperparathyroidism (sHPT) [2–8]. Although these effects of FGF23 mitigate hyperphosphatemia [1, 2, 9], elevated FGF23 is independently associated with increased risks of cardiovascular disease, infection, anemia and mortality [10–14].
Current guidelines for management of disordered mineral metabolism in patients receiving hemodialysis focus on achieving target concentrations of parathyroid hormone (PTH), calcium and phosphate [15]. Multiple combinations of therapeutic strategies are utilized, including dietary phosphate restriction, calcium-based and noncalcium-based phosphate binders, vitamin D preparations and calcimimetics [13, 16–22]. Different therapeutic approaches to sHPT have differential effects on FGF23. Cinacalcet tends to lower FGF23, vitamin D tends to increase FGF23 and calcium- and noncalcium-based phosphate binders have variable effects [13, 16, 23]. There are currently no therapeutic drugs specifically approved to target elevated FGF23 in patients with any stage of CKD.
Etelcalcetide (Parsabiv, Amgen, Thousand Oaks, CA, USA) is an intravenous calcimimetic recently approved for treatment of sHPT in patients receiving hemodialysis. In randomized controlled trials, etelcalcetide reduced PTH and FGF23 more than placebo or cinacalcet when added to standard-of-care treatment that included calcitriol or vitamin D analogs, phosphate binders and calcium supplementation administered at the discretion of individuals’ primary nephrologists [24, 25]. We conducted the current secondary analyses of these trials to further investigate potential mediators of etelcalcetide-induced reductions in FGF23. We tested the associations among changes in FGF23 and calcium, phosphate, PTH and markers of bone turnover and further tested whether concomitant changes in calcitriol or vitamin D analogs, dialysate calcium concentration or calcium supplementation altered the FGF23-lowering effects of etelcalcetide.
MATERIALS AND METHODS
Study population
We assessed the effects of etelcalcetide and cinacalcet on circulating concentrations of FGF23 in a post hoc pooled analysis of Phase 3 trials that were designed to evaluate the efficacy of etelcalcetide to achieve PTH ≤300 pg/mL [24, 25]. The trials were approved by institutional review boards at participating trial sites and were conducted in accordance with the Declaration of Helsinki. Adult patients with sHPT receiving hemodialysis were randomized 1:1 to 26 weeks of treatment with etelcalcetide (n = 509) versus placebo (n = 514) or etelcalcetide (n = 340) versus cinacalcet (n = 343) added to the standard of care. All participants provided written informed consent. Etelcalcetide was administered intravenously at a starting dose of 5 mg three times weekly after hemodialysis and cinacalcet was taken orally at a starting dose of 30 mg/day. Etelcalcetide (and the corresponding intravenous placebo) could be titrated in increments of 2.5 or 5 mg and cinacalcet in increments of 30 mg (dose range 30–180 mg) at Weeks 5, 9, 13 and 17. With investigators blinded to PTH concentrations throughout the trial, calcimimetics were titrated by protocol using an interactive voice or web response system up to a maximum of 15 mg of etelcalcetide per dialysis session or 180 mg of cinacalcet per day to achieve target PTH levels between 100 and 300 pg/mL. The median average weekly calcimimetic doses during the efficacy assessment phase at Weeks 20–26 was 15.0 mg for etelcalcetide and the median average daily cinacalcet dose was 51.4 mg [interquartile range (IQR) 26.4–80.4] [25]. Dialysate calcium concentrations and specific types and doses of phosphate binders, calcium supplements and vitamin D preparations could be adjusted by individual investigators at their discretion. Due to difficulty in standardizing individual participants’ total calcium dose across the wide range of binders and supplements prescribed by investigators, we chose to dichotomize calcium supplementation into groups that either received any increase or no increase based on a comparison of their average doses during the efficacy assessment phase and their baseline doses. We used a similar strategy to dichotomize changes in vitamin D dose and dialysate calcium concentration according to whether individuals received an increase or no increase in these interventions.
Assays
Central laboratories (Covance Central Laboratory Services, Indianapolis, IN, USA; Meyrin, Switzerland; and Singapore) measured intact PTH (immunometric assay; ADVIA Centaur PTH Assay, Siemens Healthcare, Erlangen, Germany), calcium and phosphate in real time throughout the trials and stored and analyzed samples for measurements of intact FGF23 (Kainos ELISA kit, Kainos Labs, Tokyo, Japan), bone-specific alkaline phosphatase (BSAP) and C-telopeptide (CTX) at baseline and at the beginning of Week 27. Note that while treatment in both trials was 26 weeks, the final laboratory values were obtained at the beginning of Week 27. Albumin-corrected serum calcium is presented in all analyses.
Statistical analysis
Within the placebo-controlled trials, we stratified participants into three groups of sHPT severity according to baseline PTH (<600, 600–1000 and >1000 pg/mL) and investigated the effects of etelcalcetide versus placebo on the change in PTH from baseline to Week 27 within these groups. Within each PTH stratum we determined the mean baseline calcium, phosphate, calcium × phosphate product and FGF23 and the percent change from baseline following treatment with etelcalcetide or placebo during the efficacy assessment phase, which was Weeks 20–27.
We compared the percent change in FGF23 from baseline to Week 27 between etelcalcetide and placebo and etelcalcetide and cinacalcet using Wilcoxon rank sum tests. We calculated Pearson correlation coefficients for percent changes in FGF23, BSAP and CTX from the baseline to Week 27 measurements; for PTH, calcium, phosphate and calcium × phosphate, we calculated percent changes from baseline using the mean of all values during the efficacy assessment phase (Weeks 20–27). We analyzed the effects of an increase versus no increase in vitamin D dose, calcium supplementation and dialysate calcium concentration on randomized treatment-associated changes in FGF23 levels at Week 27 by testing the Spearman correlations of the within-group changes in FGF23 among participants in whom concomitant treatments were or were not increased. We analyzed all available data without imputation. Statistical significance was defined as P < 0.05 without adjusting for multiple comparisons. All analyses were performed using SAS software (SAS Institute, Cary, NC, USA).
Data-sharing agreement
There is a plan to share data. This may include deidentified individual patient data for variables necessary to address the specific research question in an approved data-sharing request; also related data dictionaries, study protocol, statistical analysis plan, informed consent form and/or clinical study report. Data-sharing requests relating to data in this article will be considered after the publication date and (i) this product and indication (or other new use) have been granted marketing authorization in both the USA and Europe or (ii) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data-sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of reevaluating safety and efficacy issues already addressed in the product labelling. A committee of internal advisors reviews requests. If not approved, then requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen’s sole discretion and without further arbitration. Upon approval, information necessary to address the research question will be provided under the terms of a data-sharing agreement. This may include anonymized individual patient data and/or available supporting documents containing fragments of analysis code where provided in analysis specifications. Further details are available at https://www.amgen.com/science/clinical-trials/clinical-data-transparency-practices/
RESULTS
Baseline data
Characteristics of the study population from the placebo-controlled trial of etelcalcetide are presented in Table 1 overall and according to baseline severity of sHPT, expressed in ascending strata of pretreatment PTH (<600, 600–1000 and >1000 pg/mL). Participants in the highest PTH stratum had the highest baseline serum concentrations of calcium, phosphate and FGF23 while the dose of calcitriol or vitamin D analogs at baseline was similar across strata (Table 1).
Table 1.
Baseline demographics and laboratory values by baseline PTH strata for etelcalcetide- or placebo-treated subjects
| Entire cohort |
PTH <600 pg/mL |
PTH 600–1000 pg/mL |
PTH >1000 pg/mL |
|||||
|---|---|---|---|---|---|---|---|---|
| ETL | Placebo | ETL | Placebo | ETL | Placebo | ETL | Placebo | |
| (n = 509) | (n = 514) | (n = 172) | (n = 169) | (n = 225) | (n = 233) | (n = 112) | (n = 112) | |
| Age (years) | 58.4 ± 14.6 | 58.1 ± 14.3 | 60.5 ± 13.8 | 59.4 ± 14.2 | 58.1 ± 14.4 | 58.2 ± 14.3 | 55.6 ± 15.8 | 55.8 ± 14.1 |
| Sex (female), n (%) | 196 (38.5) | 209 (40.7) | 68 (39.5) | 63 (37.3) | 83 (36.9) | 88 (37.8) | 45 (40.2) | 58 (51.8) |
| Race, n (%) | ||||||||
| White | 336 (66.0) | 344 (66.9) | 114 (66.3) | 101 (59.8) | 147 (65.3) | 160 (68.7) | 75 (67.0) | 83 (74.1) |
| Black | 136 (26.7) | 149 (29.0) | 48 (27.9) | 58 (34.3) | 59 (26.2) | 67 (28.8) | 29 (25.9) | 24 (21.4) |
| Asian | 18 (3.5) | 9 (1.8) | 2 (1.2) | 4 (2.4) | 11 (4.9) | 4 (1.7) | 5 (4.5) | 1 (0.9) |
| Other | 10 (2.0) | 6 (1.2) | 3 (1.7) | 3 (1.8) | 4 (1.8) | 0 (0.0) | 3 (2.7) | 3 (2.7) |
| Dialysis vintage (years), n (%) | ||||||||
| 0–1 | 60 (11.8) | 67 (13.0) | 27 (15.7) | 23 (13.6) | 29 (12.9) | 30 (12.9) | 4 (3.6) | 14 (12.5) |
| 1–5 | 247 (48.5) | 245 (47.7) | 92 (53.5) | 95 (56.2) | 105 (46.7) | 108 (46.4) | 50 (44.6) | 42 (37.5) |
| >5 | 202 (39.7) | 202 (39.3) | 53 (30.8) | 51 (30.2) | 91 (40.4) | 95 (40.8) | 58 (51.8) | 56 (50.0) |
| PTH (pg/mL) | 724 (552–949) | 716 (557–982) | 505 (459–552) | 500 (453–557) | 771 (671–877) | 759 (682–878) | 1281 (1113–1587) | 1244 (1094–1545) |
| Ca (mg/dL) | 9.6 ± 0.7 | 9.7 ± 0.7 | 9.6 ± 0.6 | 9.6 ± 0.5 | 9.6 ± 0.7 | 9.7 ± 0.7 | 9.7 ± 0.6 | 9.7 ± 0.7 |
| P (mg/dL) | 5.9 ± 1.6 | 5.8 ± 1.5 | 5.5 ± 1.4 | 5.4 ± 1.4 | 5.9 ± 1.7 | 5.9 ± 1.5 | 6.2 ± 1.7 | 6.3 ± 1.6 |
| Ca × P (mg2/dL2) | 56.3 ± 15.4 | 56.0 ± 15.2 | 52.7 ± 13.2 | 51.0 ± 13.7 | 57.2 ± 16.0 | 57.3 ± 14.5 | 60.2 ± 16.3 | 60.9 ± 16.6 |
| Vitamin D dose (μg/week) | 16.2 ± 13.7 | 15.3 ± 14.4 | 15.4 ± 13.0 | 15.7 ± 17.5 | 16.2 ± 13.6 | 15.2 ± 12.9 | 17.6 ± 15.4 | 14.8 ± 11.5 |
| BSAP (μg/L) | 30.4 ± 26.8 | 33.1 ± 33.6 | 20.3 ± 11.0 | 22.9 ± 11.8 | 28.6 ± 20.2 | 30.0 ± 17.1 | 48.7 ± 41.5 | 54.5 ± 60.9 |
| FGF23 (pg/mL) | 4206 (1070–15 061) | 3312 (816–12 431) | 2677 (744–10 711) | 1483 (616–6186) | 5437 (1101–18 301) | 3819 (1189–12 586) | 5844 (1842–21 061) | 5509 (1222–21 431) |
Values are reported as mean ± standard deviation or median (quartile 1, quartile 3) unless stated otherwise. Conversion factors for units: Ca in mg/dL to mmol/L × 0.2495; P in mg/dL to mmol/L × 0.3229.
PTH, parathyroid hormone; ETL, etelcalcetide; Ca, calcium; P, phosphate; BSAP, bone-specific alkaline phosphatase; FGF23, fibroblast growth factor-23.
Postrandomization data
The mean percent reduction in PTH with etelcalcetide treatment was similar regardless of baseline sHPT severity, ranging from –54% [95% confidence interval (CI) –59 to –50], to –58% (95% CI –62 to –54), to –55% (95% CI –62 to –49) from the lowest to the highest baseline PTH strata, despite the progressive increase in delivered dose of etelcalcetide across the ascending PTH strata (Table 2). In the full study populations, etelcalcetide decreased FGF23 from baseline to Week 27 significantly more than placebo [–56% (95% CI –85 to –7) versus +2% (–40 to +65); P < 0.001; Figure 1A] and significantly more than cinacalcet [–68% (95% CI –87 to –26) versus –41% (–76 to +25); P < 0.001; Figure 1B]. Although patients in the highest baseline PTH stratum in the placebo-controlled trial had the largest relative reduction in calcium, phosphate and FGF23 induced by etelcalcetide (Table 2), etelcalcetide (and cinacalcet)-induced reductions in FGF23 correlated strongly with concomitant reductions in calcium, phosphate and calcium × phosphate product, but not with changes in PTH (Table 3); changes in bone markers were less strongly correlated with change in FGF23.
Table 2.
Effects of etelcalcetide versus placebo on mineral metabolites overall and by baseline PTH strata
| Mineral metabolites | Entire cohort |
PTH <600 pg/mL |
PTH 600–1000 pg/mL |
PTH >1000 pg/mL |
||||
|---|---|---|---|---|---|---|---|---|
| ETL | Placebo | ETL | Placebo | ETL | Placebo | ETL | Placebo | |
| (n = 509) | (n = 514) | (n = 172) | (n = 169) | (n = 225) | (n = 233) | (n = 112) | (n = 112) | |
| PTH (% change)a | −56.3 ± 1.4 | 13.4 ± 1.9 | −54.2 ± 2.3 | 17.1 ± 3.8 | −58.2 ± 1.9 | 14.0 ± 2.6 | −55.5 ± 3.2 | 5.9 ± 3.3 |
| Ca (% change)a | −7.0 ± 0.4 | 0.9 ± 0.2 | −5.1 ± 0.6 | 1.4 ± 0.4 | −7.5 ± 0.6 | 0.7 ± 0.3 | −8.9 ± 0.9 | 0.4 ± 0.4 |
| P (% change)a | −8.7 ± 1.4 | −1.5 ± 1.0 | −5.4 ± 1.9 | 3.1 ± 1.9 | −8.2 ± 2.4 | −3.6 ± 1.4 | −14.6 ± 2.3 | −4.6 ± 2.1 |
| Ca × P (% change)a | −15.1 ± 1.3 | −0.6 ± 1.0 | −10.2 ± 2.0 | 4.1 ± 1.9 | −15.3 ± 2.2 | −2.9 ± 1.4 | −22.1 ± 2.3 | −4.0 ± 2.1 |
| FGF23 (% change)b | −56.1 (−84.7, −7.1) | 2.1 (−40.1, 64.8) | −42.8 (−74.1, 17.0) | 5.2 (−44.3, 78.2) | −52.8 (−84.9, −15.5) | 5.1 (−35.9, 68.3) | −77.6 (−90.3, −44.9) | −1.0 (−45.5, 52.4) |
| ETL dose (mg/week)c | 21.5 ± 13.4 | — | 17.2 ± 11.7 | — | 22.3 ± 13.4 | — | 26.1 ± 13.8 | — |
Mean ± SE of change from baseline during the efficacy assessment period of Weeks 20–27.
Median (quartile 1, quartile 3) percent change from baseline at Week 27.
Mean ± SD during the efficacy assessment period. Percent and standard error values <0.5 were rounded to zero.
PTH, parathyroid hormone; ETL, etelcalcetide; Ca, calcium; P, phosphate; SE, standard error; FGF23, fibroblast growth factor-23.
FIGURE 1.
Changes in FGF23 in the randomized controlled trials of (A) etelcalcetide versus placebo and (B) etelcalcetide versus cinacalcet. Median percent change (quartile 1, quartile 3) = median percent change from baseline to Week 27.
Table 3.
Pearson correlations coefficients between percent changes in FGF23 and other bone mineral markers
| Bone mineral markers | Placebo-controlled |
Head-to-head |
||
|---|---|---|---|---|
| Etelcalcetide | Placebo | Etelcalcetide | Cinacalcet | |
| PTH | –0.08 | 0.01 | –0.02 | 0.13 |
| Calcium | 0.52 | 0.06 | 0.46 | 0.34 |
| Phosphate | 0.34 | 0.48 | 0.56 | 0.51 |
| Calcium × phosphate | 0.61 | 0.50 | 0.66 | 0.60 |
| Bone-specific alkaline phosphatase | –0.23 | –0.27 | –0.17 | –0.15 |
| Collagen type I cross-linked C-telopeptide | –0.11 | 0.00 | –0.02 | 0.02 |
Bold numbers connote statistical significance of P < 0.01. FGF23 = fibroblast growth factor-23; PTH, parathyroid hormone.
Effects of concomitant treatments
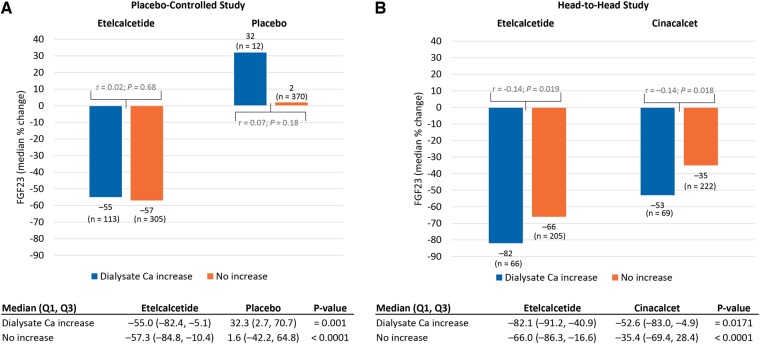
Etelcalcetide significantly decreased FGF23 compared with placebo, irrespective of whether the dose of vitamin D was increased (between-group comparisons; Figure 2A), but within the etelcalcetide group, the effect on FGF23 was partially attenuated among participants who received an increase versus no increase in their dose of calcitriol or vitamin D analog (median change –48% versus –62%; P = 0.045). In the active-controlled trials (Figure 2B), etelcalcetide decreased FGF23 significantly more than cinacalcet among participants whose calcitriol or vitamin D analog dose was not increased (–71% versus –41%; P < 0.001), but there was no significant difference among those in whom the calcitriol or vitamin D analog dose was increased (–60% versus –42%; P = 0.07); similar to the placebo-controlled trials, the etelcalcetide effect on FGF23 was partially attenuated among participants who received an increase versus no increase in their dose of calcitriol or vitamin D analog (–60% versus –71%; P = 0.004; Figure 2B). Etelcalcetide decreased FGF23 significantly more than placebo or cinacalcet regardless of whether dialysate calcium or calcium supplementation was increased (between-group comparisons; Figures 3 and 4). Compared with no increase, an increase in dialysate calcium concentration did not attenuate the FGF23-lowering effects of etelcalcetide in the placebo-controlled trials (–55% versus –57%; P = 0.68; Figure 3A) and was associated with significantly more FGF23 reduction in the active-controlled trials (–82% versus –66%; P = 0.02; Figure 3B). Likewise, compared with no increase, an increase in dose of calcium supplementation did not attenuate the FGF23-lowering effects of etelcalcetide in the placebo-controlled trials (–59% versus –53%; P = 0.24; Figure 4A) and was associated with significantly more FGF23 reduction in the active-controlled trials (–76% versus –64%; P = 0.03; Figure 4B).
FIGURE 2.
Changes in FGF23 according to concomitant vitamin D treatment in trials of (A) etelcalcetide versus placebo and (B) etelcalcetide versus cinacalcet. Median percent change (quartile 1–quartile 3) = median percent change from baseline to Week 27. Spearman correlation coefficients (r) and P-values on the figure panels summarize and compare the within-group associations between percent change from baseline to Week 27 in FGF23 according to whether vitamin D dose was or was not increased. Results below the figure panels summarize between-group comparisons stratified by the change in vitamin D dosing.
FIGURE 3.
Changes in FGF23 according to concomitant increases in dialysate calcium concentration in trials of (A) etelcalcetide versus placebo and (B) etelcalcetide versus cinacalcet. Median (quartile 1, quartile 3) = median percent change from baseline to week 27. Spearman correlation coefficients (r) and P-values on the figure panels summarize and compare the within-group associations between percent change from baseline to Week 27 in FGF23 according to whether dialysate calcium was or was not increased. Results below the figure panels summarize between-group comparisons stratified by the change in dialysate calcium.
FIGURE 4.
Changes in FGF23 according to concomitant increases in calcium supplementation in trials of (A) etelcalcetide versus placebo and (B) etelcalcetide versus cinacalcet. Median percent change (quartile 1, quartile 3) = median percent change from baseline to Week 27. Spearman correlation coefficients (r) and P-values on the figure panels summarize and compare the within-group associations between percent change from baseline to Week 27 in FGF23 according to whether calcium supplementation was or was not increased. Results below the figure panels summarize between-group comparisons stratified by the change in calcium supplementation.
DISCUSSION
This secondary analysis of etelcalcetide clinical trials offers new evidence relevant to our understanding of the pathogenesis and management of sHPT in patients receiving hemodialysis. Etelcalcetide markedly reduced FGF23 concentrations compared with placebo and also more than cinacalcet. The magnitude of the FGF23-lowering effect was largest among patients with the most severe sHPT at baseline, marked by their having the highest pretreatment PTH, calcium, phosphate and FGF23. Nevertheless, the degree of etelcalcetide-induced FGF23 reduction was most strongly associated with concomitant changes in calcium and phosphate rather than the change in PTH, which was reduced to a similar extent by etelcalcetide regardless of its baseline concentration.
The complex regulation of FGF23 in CKD involves competing effects of multiple intrinsic physiological factors and exogenous treatments. High serum concentrations of PTH, calcium and phosphate and reduced renal expression of Klotho each stimulate increases in FGF23 [6, 13, 26–32]. Therapeutically, phosphate binders can lower FGF23, presumably by reducing the total absorbed phosphate load; however, the effect depends on the type of binder. Whereas iron-based and other noncalcium-based binders lowered FGF23 in some but not all studies [33–36], calcium-based binders appear to raise FGF23 because calcium loading directly stimulates FGF23 independent of serum phosphate and despite calcium-induced lowering of PTH [37, 38]. PTH-lowering therapies also differ in their effects on FGF23. Activated vitamin D and its analogs stimulate FGF23 via direct effects on the vitamin D response element of the FGF23 gene promoter [3, 13] and perhaps also indirectly via increases in serum calcium and phosphate. In contrast, for a comparable magnitude of PTH reduction as activated vitamin D, calcimimetics lower FGF23, but the mechanisms are unknown [13, 16, 19, 22, 23].
One of the primary aims of this study was to investigate the potential mediators through which calcimimetics reduce FGF23. The candidate factors we examined included PTH, calcium, phosphate and bone turnover markers. While PTH, calcium and phosphate were associated with baseline FGF23, etelcalcetide-mediated reductions in FGF23 were associated with reductions of calcium, phosphate and calcium × phosphate product, but, remarkably, not with changes in PTH; associations of FGF23 reduction with markers of bone turnover were of borderline and inconsistent significance. The signficant relations among changes in FGF23 with changes in calcium, phosphate and calcium × phosphate could be interpreted as suggesting that osteocytes may respond to total mineral ion load. This is supported by synergistic stimulation of FGF23 by calcium and phosphate in a double knockout mouse lacking both PTH and the calcium-sensing receptor [39–41]. The lack of association between PTH reduction and changes in FGF23 in the current analysis is also consistent with prior studies of cinacalcet in which reductions in FGF23 were unrelated to changes in PTH, but instead correlated with changes in calcium and phosphate [20, 22]. Furthermore, in patients receiving dialysis who underwent subtotal parathyroidectomy, which acutely and drastically reduces PTH, post-operative decreases in FGF23 were most closely related to changes in calcium × phosphate product [42]. In another study of total parathyroidectomy, FGF23 changed minimally in patients who were treated postoperatively with calcitriol and calcium to maintain serum calcium [43].
In aggregate, these data suggest that calcium and phosphate are more important drivers of calcimimetic-induced FGF23 reduction than PTH or bone turnover markers. However, alternative interpretations are also possible. For example, more biological variability in PTH versus calcium and phosphate and more laboratory imprecision in PTH assays relative to calcium and phosphate assays might have obscured a relation between the magnitude of PTH and FGF23 reduction. In support of this hypothesis, the highest PTH stratum did experience the greatest magnitude of FGF23 reduction (although this group also had the largest variations in serum calcium and phosphate). While it is presumed that PTH reduction is the mediator of etelcalcetide-induced reductions in calcium and phosphate, varying degrees of PTH resistance at the level of bone might also blur the relation between changes in PTH and changes in FGF23 [10, 27]. There also remains the possibility that calcimimetics might influence calcium and phosphate homeostasis (or FGF23 directly) via PTH-independent mechanisms mediated by direct effects on bone cells and bone turnover. Although the association with bone turnover markers was weak, this mechanism is plausible given that an investigational calcimimetic (AMG641) affected bone turnover in a uremic thyro-parathyroidectomized rat model in which PTH levels were maintained at a constant level by PTH infusion [44]. Finally, it is possible that while PTH is an important basal regulator of FGF23 [6, 26–29], its effects might be overwhelmed by concomitant changes in calcium and phosphate in the presence of a potent calcimimetic.
The second major aim of this study was to investigate the extent to which treatments that mitigate etelcalcetide-induced hypocalcemia might also lessen its efficacy to reduce FGF23 levels. In the trials, investigators were blinded to treatment and PTH levels but were able to monitor serial serum calcium levels for safety reasons. Treatment of calcium reduction was allowable but not mandated unless calcium decreased to <7.5 mg/dL or the patient developed symptomatic hypocalcemia. Potential interventions could include starting or increasing doses of calcitriol or vitamin D analogs, calcium supplements and calcium-based binders or increasing the dialysate calcium concentration. Overall, etelcalcetide reduced FGF23 by a median of ≥47%, regardless of whether participants underwent simultaneous increases in their doses of vitamin D. In contrast, etelcalcetide unexpectedly reduced FGF23 more in patients whose doses of calcium supplements or dialysate calcium increased—at least 52% and as high as 82%. This might have occurred because patients who required calcium-raising interventions were likely those who had the largest reductions in serum calcium, and thus FGF23, in response to etelcalcetide. These results have potentially important clinical implications. They suggest that if FGF23 reduction is advanced as a therapeutic goal in the future, use of etelcalcetide could be a potent therapeutic. While etelcalcetide is associated with high rates of hypocalcemia by virtue of its known mechanism of action to suppress PTH, our data suggest that ancillary treatments to abrogate symptomatic hypocalcemia will not fully offset the FGF23-lowering effects of etelcalcetide.
The strengths of this study include its large and diverse clinical trial populations, frequent central measurements of PTH, calcium, phosphate and markers of bone turnover and protocol-directed titration of the interventions. In addition to these strengths, certain limitations may influence our interpretation of the data. Although PTH, calcium and phosphate were assessed at several points throughout the trials, we only measured FGF23 at baseline and at Week 27. It is also unclear how the results would have differed had we included a population with shorter dialysis vintage and less severe sHPT at baseline. Due to the complexity of standardizing individual patients’ total calcium dose across the multiple different preparations they could have received, we chose to dichotomize calcium supplementation and the other concomitant medications into two groups defined by whether their dose was or was not increased during follow-up. As a result, we could not perform more nuanced investigations of potential dose-dependent effects of these treatments, which are needed. Furthermore, use of concomitant calcium-raising therapies were not randomized or administered by protocol, which further limits inferences that can be drawn from the concomitant treatment analyses. However, variability in the use of these strategies to raise serum calcium enabled our hypothesis-generating analyses that otherwise would have been limited had these strategies been tightly regulated by protocol rather than at the discretion of investigators.
The evaluation of changes in FGF23 was a prespecified exploratory analysis of the placebo-controlled and active-controlled etelcalcetide clinical trials, but the studies were not primarily designed to explore the effect of etelcalcetide on FGF23. We speculate that etelcalcetide could induce even more pronounced reductions in FGF23 if drug titration were motivated by FGF23 rather than PTH targets. Since our results focused only on biochemical endpoints, additional studies are also needed to determine the effect on clinical outcomes of etelcalcetide-induced reduction in FGF23.
ACKNOWLEDGEMENTS
This manuscript was prepared in collaboration with Charles Henley, PhD (funded by Amgen) and William W. Stark, Jr, PhD (employee and stockholder, Amgen), who provided assistance in editing the manuscript. All authors contributed to the development of the manuscript and approved the final version in accordance with International Committee of Medical Journal Editors criteria for authorship.
FUNDING
The study was funded and conducted by Amgen (Thousand Oaks, CA). The authors, in collaboration with Amgen, collected and interpreted the data, wrote the report and made the decision to submit the article for publication. The studies were funded and conducted by Amgen (Amgen studies 20120229 [NCT01785849], 20120230 [NCT01788046] and 20120360 [NCT1896232]).
AUTHORS’ CONTRIBUTIONS
M.W., G.A.B., G.M.C., K.C., B.F., S.M.M., Y.S., H.T., M.V. and R.O. were involved in conception and study design. G.A.B., G.M.C., S.M.M. and R.O. acquired the data. G.A.B., G.M.C, K.C., B.F., S.M.M., Y.S., H.T. and R.O. analysed and interpreted the data.
Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
M.W. has received consultancy fees from Akebia, AMAG Pharmaceuticals, Amgen, Diasorin and Sanofi and grants from Shire. G.B. has received consultancy fees from Akebia, Amgen, AstraZeneca, Daiichi-Sankyo, Keryx, Kirin, Omeros, Ono and OPKO; participated in the speakers bureau for OPKO and received research grants from Keryx. G.C. is on the Board of Directors of Satellite Healthcare; owns stock/stock options in Ardelyx, Cricket Health, Durect, DxNow, Eliaz Therapeutics, Outset Medical, Physiowave and Puracath Medical; has received an institutional grant from Amgen and Janssen; has received consulting fees from AMAG Pharmaceuticals, Gilead and Sanafit; has Data and Safety Monitoring Board membership for Bayer, Bristol-Myers Squibb and ReCor and is on the Trial Steering Committee for Akebia and AstraZeneca. S.M.M. reports grants from Chugai, the National Institutes of Health and Department of Veterans Affairs and personal fees from Sanofi/Genzyme and Amgen. H.T. was an employee and stockholder of Amgen at the time the study was conducted and during initial drafting of the manuscript. M.V. has received grants from Amgen, AbbVie, FMC and Sanofi and personal fees from Amgen, Baxter, FMC and Otsuka. R.O. has received grants from Amgen, Chiesi, Fresenius and Sandoz and participated in speakers bureaus for Amgen, Astellas, Chiesi, Neovii and Sandoz. B.F. and K.C. are employees and stockholders of Amgen. Y.S. is an employee of Amgen.
REFERENCES
- 1. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012; 82: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gutierrez O, Isakova T, Rhee E. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 2005; 16: 2205–2215 [DOI] [PubMed] [Google Scholar]
- 3. Liu S, Tang W, Zhou J. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 2006; 17: 1305–1315 [DOI] [PubMed] [Google Scholar]
- 4. Stubbs J, Liu S, Quarles LD.. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dial 2007; 20: 302–308 [DOI] [PubMed] [Google Scholar]
- 5. Gattineni J, Bates C, Twombley K. et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 2009; 297: F282–F291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zisman AL, Wolf M.. Recent advances in the rapidly evolving field of fibroblast growth factor 23 in chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19: 335–342 [DOI] [PubMed] [Google Scholar]
- 7. Gattineni J, Alphonse P, Zhang Q. et al. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Renal Physiol 2014; 306: F351–F358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Razzaque MS. Bone-kidney axis in systemic phosphate turnover. Arch Biochem Biophys 2014; 561: 154–158 [DOI] [PubMed] [Google Scholar]
- 9. Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 2004; 44: 250–256 [DOI] [PubMed] [Google Scholar]
- 10. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kendrick J, Cheung AK, Kaufman JS. et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isakova T, Xie H, Yang W. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sprague SM, Wetmore JB, Gurevich K. et al. Effect of cinacalcet and vitamin D analogs on fibroblast growth factor-23 during the treatment of secondary hyperparathyroidism. Clin J Am Soc Nephrol 2015; 10: 1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pichler G, Haller MC, Kainz A. , et al. Prognostic value of bone- and vascular-derived molecular biomarkers in hemodialysis and renal transplant patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2017; 32: 1566–1578 [DOI] [PubMed] [Google Scholar]
- 15. Ketteler M, Block GA, Evenepoel P. et al. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what's changed and why it matters . Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 16. Cozzolino M, Ketteler M, Martin KJ. et al. Paricalcitol- or cinacalcet-centred therapy affects markers of bone mineral disease in patients with secondary hyperparathyroidism receiving haemodialysis: results of the IMPACT-SHPT study. Nephrol Dial Transplant 2014; 29: 899–905 [DOI] [PubMed] [Google Scholar]
- 17. Hansen D, Rasmussen K, Pedersen SM. et al. Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol Dial Transplant 2012; 27: 2263–2269 [DOI] [PubMed] [Google Scholar]
- 18. Hryszko T, Brzosko S, Rydzewska-Rosolowska A. et al. Cinacalcet lowers FGF-23 level together with bone metabolism in hemodialyzed patients with secondary hyperparathyroidism. Int Urol Nephrol 2012; 44: 1479–1486 [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Kim H, Shin N. et al. Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: a randomized controlled study. BMC Nephrol 2013; 14: 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koizumi M, Komaba H, Nakanishi S. et al. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant 2012; 27: 784–790 [DOI] [PubMed] [Google Scholar]
- 21. Wesseling-Perry K, Pereira RC, Sahney S. et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 2011; 79: 112–119 [DOI] [PubMed] [Google Scholar]
- 22. Wetmore JB, Liu S, Krebill R. et al. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol 2010; 5: 110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ketteler M, Martin KJ, Wolf M. et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study. Nephrol Dial Transplant 2012; 27: 3270–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Block GA, Bushinsky DA, Cunningham J. et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017; 317: 146–155 [DOI] [PubMed] [Google Scholar]
- 25. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 26. Burnett-Bowie SM, Henao MP, Dere ME. et al. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res 2009; 24: 1681–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lavi-Moshayoff V, Wasserman G, Meir T. et al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 2010; 299: F882–F889 [DOI] [PubMed] [Google Scholar]
- 28. Lopez I, Rodriguez-Ortiz ME, Almaden Y. , et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 2011; 80: 475–482 [DOI] [PubMed] [Google Scholar]
- 29. Rhee Y, Bivi N, Farrow E. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 2011; 49: 636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nabeshima Y. Discovery of α-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Clin Calcium 2008; 18: 923–934 [PubMed] [Google Scholar]
- 31. Olauson H, Lindberg K, Amin R. et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 2012; 23: 1641–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erben RG, Andrukhova O.. FGF23-Klotho signaling axis in the kidney. Bone 2017; 100: 62–68 [DOI] [PubMed] [Google Scholar]
- 33. Soriano S, Ojeda R, Rodriguez M. et al. The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol 2013; 80: 17–22 [DOI] [PubMed] [Google Scholar]
- 34. Koiwa F, Kazama JJ, Tokumoto A. et al. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 2005; 9: 336–339 [DOI] [PubMed] [Google Scholar]
- 35. Lin HH, Liou HH, Wu MS. et al. Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum Klotho levels in chronic haemodialysis patients. Nephrology (Carlton) 2014; 19: 672–678 [DOI] [PubMed] [Google Scholar]
- 36. Iguchi A, Yamamoto S, Yamazaki M. , et al. Effect of ferric citrate hydrate on FGF23 and PTH levels in patients with non-dialysis-dependent chronic kidney disease with normophosphatemia and iron deficiency. Clin Exp Nephrol 2018; 22: 789–796 [DOI] [PubMed] [Google Scholar]
- 37. Cancela AL, Oliveira RB, Graciolli FG. et al. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract 2011; 117: c74–c82 [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez-Ortiz ME, Lopez I, Munoz-Castaneda JR. et al. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol 2012; 23: 1190–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinn SJ, Thomsen AR, Pang JL, et al. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol Metab 2013; 304: E310–E320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wesseling-Perry K, Harkins GC, Wang. et al. The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7-84). J Clin Endocrinol Metab 2010; 95: 2772–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meir T, Durlacher K, Pan Z. et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int 2014; 86: 1106–1115 [DOI] [PubMed] [Google Scholar]
- 42. Sato T, Tominaga Y, Ueki T. et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis 2004; 44: 481–487 [PubMed] [Google Scholar]
- 43. Liao SC, Moi SH, Chou FF. et al. Changes in serum concentrations of fibroblast growth factor 23 and soluble klotho in hemodialysis patients after total parathyroidectomy. Biomed Res Int 2016; 2016: 6453803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tocados JMD, Ortiz MER, Almaden Y. et al. In uremic rats, the calcimimetic maintains bone turnover in a parathyroid hormone-independent manner. Annual Congress of the American Society of Nephrology 2016 [Google Scholar]