Abstract
The main manifestation of acute interstitial nephritis (AIN) due to immune checkpoint inhibitors is acute kidney injury. We report here a biopsy-proven AIN revealed by tubular acidosis. This case highlights that immune checkpoint inhibitor prescribers must be aware of electrolytic disorders since tubular dysfunction can precede serum creatinine increase and reveal renal toxicity.
Keywords: distal tubular acidosis, immune checkpoint inhibitors, nephrotoxicity
BACKGROUND
Renal toxicity of immune checkpoint inhibitors is rare and mainly concerns tubulointerstitial disorders. Several cases have been reported in which acute interstitial nephritis (AIN) was revealed by an acute kidney injury (AKI) [1]. Renal tubular acidosis with AKI has been described, but not biopsy-proven [2]. We report here a biopsy-proven case of renal toxicity as an isolated distal tubular acidosis.
CASE REPORT
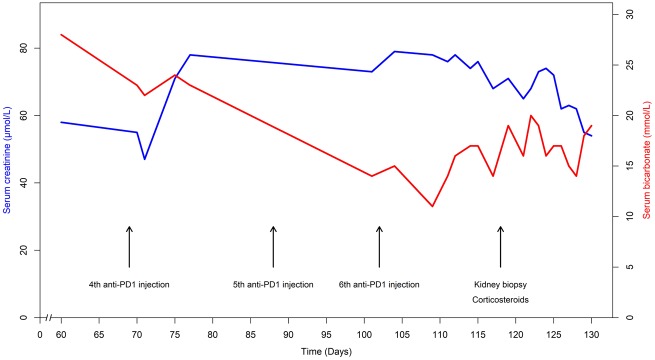
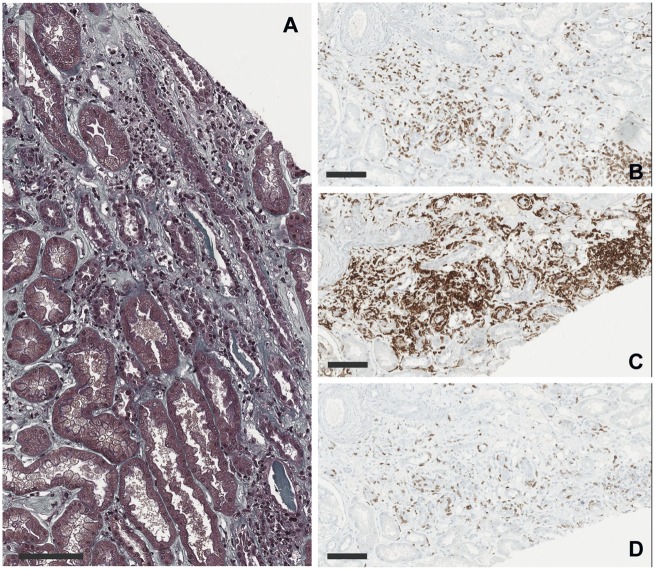
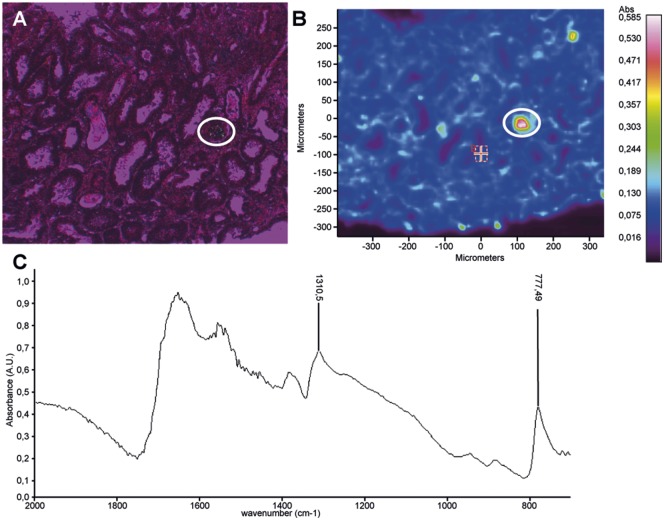
A 59-year-old man was hospitalized for metabolic acidosis. He had no medical history except for a Stage IV bronchopulmonary cancer, treated in first line by cisplatin and pemetrexed. Because of Programmed cell death-ligand 1 (PD-L1) over-expression in the tumour, an immune checkpoint inhibitor targeting PD-1 was initiated as a second line (five nivolumab injections, 3 mg/kg/15 days and a single injection of pembrolizumab 2 mg/kg). His baseline serum creatinine (SCr) was 55 µmol/L with normal estimated glomerular filtration rate at 108 mL/min/1.73 m2. Baseline serum bicarbonate and potassium were normal. After the sixth administration (Figure 1), he presented with fever and a maculopapular rash on the back. He did not report arthralgia or systemic dryness, and had no digestive troubles. Serum electrolytes showed an uncompensated metabolic hyperchloraemic acidosis with hypokalaemia without AKI: serum bicarbonate 11 mmol/L, chloride 113 mmol/L, potassium 3.3 mmol/L, pH 7.29, pCO2 21.8 mmHg and SCr 76 µmol/L. Urine anion gap was positive (+36 mEq/L) with an urine pH of 6. The initial urinary Na/K ratio was inferior to one. There was no proximal tubular disorder (no aminoaciduria, glycosuria or hypophosphataemia). Low tubular proteinuria was found (0.45 g/24 h of low molecular weight proteins) without leucocyturia or haematuria. The diagnosis of distal tubular acidosis was considered. Immunological tests were normal: no autoantibodies (antinuclear antibodies, anti-Ro/SSA, anti-La/SSB, anti-neutrophil cytoplasmic antibodies), complement not consumed (C3 1.92 g/L, C4 0.38 g/L, CH50 95 U/mL) and negative hepatitis B virus, hepatitis C virus and human immunodeficiency virus serologies. Renal ultrasonography was normal. Kidney biopsy (Figure 2) showed an interstitial oedematous fibrosis of 30–40% of the cortex with mononucleated cell infiltration [lymphocytes (CD4+ >CD8+), macrophages and rare eosinophils]. Tubulitis affected exclusively distal convoluted tubules and collecting ducts Supplementary data, Figure S1. Spectrophotometry identified monohydrated calcium oxalate crystals in some subcapsular tubes (Figure 3). Immunofluorescence analysis of the patient’s serum (dilutions 1:100 and 1:500) on control kidneys (human and rat) did not reveal any specific signal. The immune checkpoint inhibitor was stopped. Oral sodium bicarbonate alone was first given for 1 week and did not significantly improve acidosis. Then corticosteroids of 1 mg/kg/day were initiated: clinical symptoms disappeared and serum bicarbonate increased (Figure 1).
FIGURE 1.
Laboratory tests evolution. SCr and bicarbonate evolution over time under immune checkpoint inhibitor.
FIGURE 2.
Pathological findings on kidney biopsy. (A) Light microscopy showed interstitial oedema with mononuclear cells infiltrate and tubulitis (×200, Masson’s Trichrome, scale bar 100 µm). (B–D) Immunohistochemistry studies with CD3, CD4 and CD8 antibodies. Interstitial and intratubular inflammatory cells are mainly lymphocytes T 4 (×200, scale bars 100 µm).
FIGURE 3.
Spectrophotometry analysis. Calcium oxalate monohydrate infrared (IR) spectrum displays typical absorption bands at 1310 and 777 cm−1. (A) Optical image (circle: polarized crystals). (B) Absorption image. (C) Typical IR spectrum of calcium oxalate monohydrate in a protein tissue (renal biopsy).
DISCUSSION
Among the renal immune-related adverse events, tubular acidosis is not exceptional. Two cases have been reported in which AKI was associated with hypokalaemia and metabolic acidosis [2]. We report the first case of renal toxicity revealed by an isolated renal tubular acidosis without AKI. We provide a histological proof of isolated lesions of distal convoluted tubules. We also show additional crystals of monohydrated calcium oxalate by infrared spectrophotometry, probably secondary to hypovolaemia. The current Common Terminology Criteria for Adverse Events (fifth version) only consider SCr increase for renal toxicity. This case strengthens the fact that oncologists and nephrologists should be aware that tubular dysfunction could be the first sign of an immune checkpoint inhibitor-induced AIN, even in the absence of AKI.
Acquired distal tubular acidosis results from an alteration of H+-ATPase or Cl−/HCO3− pump in type A intercalated cells in collecting duct, of which autoimmunity is a frequent cause. In Sjögren’s syndrome, it has been proven that autoantibodies isolated from patients interact directly with one of these pumps [3]. We did not identify autoantibodies in our patient’s serum, but de novo organ-specific autoantibodies are rarely found under immune checkpoint inhibitors. If immune-related adverse events with systemic manifestations are more frequent in patients with pre-existing autoimmune disease, patients without autoimmune disease develop isolated organ lesions [4], as in the present case.
The PD-1/PD-L1 pathway is a late checkpoint that down regulates activated T lymphocytes. In the kidney during an inflammation condition, tubular epithelial cells increase PD-L1 expression as a protective mechanism [5]. Because PD-1/PD-L1 is non-specific of anti-tumour activity, immune checkpoint inhibitors allow a response directed against the own recipient’s organs. In the present case, this may explain the lymphocyte infiltrate and the sensitivity to corticosteroids. As lymphocytes affected exclusively distal tubular epithelial cells in our case, we postulate that distal and proximal tubular epithelial cells have a different arsenal of lymphocytic receptor ligands, and this difference imparts an immunological privilege to the proximal tubular epithelial cells during immune checkpoint inhibitors treatment.
In conclusion, distal tubular acidosis can be an isolated symptom of AIN induced by immune checkpoint inhibitors and must be known by oncologists and nephrologists.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Marie-Nathalie Kolopp-Sarda, MD, from the Department of Immunology, CHU Lyon Sud; Meghann Antoine and the other members of the Nephrology Department, CHU Lyon Sud; Guglielmo Schiano, from the Institute of Physiology, University of Zurich; Maurice Pérol, MD, from the Centre Léon Bérard, Lyon, for the help with patient management; and Denis Fouque, MD, PhD, from the Nephrology Department, CHU Lyon Sud, for his help in writing the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Wanchoo R, Karam S, Uppal NN. et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017; 45: 160–169 [DOI] [PubMed] [Google Scholar]
- 2. El Bitar S, Weerasinghe C, El-Charabaty E. et al. Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep Oncol Med 2018; 2018: 8408015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devuyst O, Lemaire M, Mohebbi N. et al. Autoantibodies against intercalated cells in Sjögren’s syndrome. Kidney Int 2009; 76: 229. [DOI] [PubMed] [Google Scholar]
- 4. Danlos F-X, Voisin A-L, Dyevre V. et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018; 91: 21–29 [DOI] [PubMed] [Google Scholar]
- 5. Starke A, Lindenmeyer MT, Segerer S. et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int 2010; 78: 38–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.