Abstract
Objective
The purpose of this study was to understand the ethical, legal, and social issues described by parents of children with known or suspected genetic conditions that cause intellectual and developmental disabilities regarding research use of their child’s electronic health record (EHR).
Materials and Methods
We conducted 4 focus groups with parents of children with a known (n = 12) or suspected (n = 11) genetic condition, as well as 2 comparison groups with parents who had a child with no known genetic condition (n = 15). Focus group transcripts were coded and analyzed using directed content analysis.
Results
After weighing the risks and benefits, parents of children with known or suspected genetic conditions were willing to share their child’s EHR for research studies under certain conditions. Preferences were for studies conducted by universities or nonprofits that might benefit their child or others with the same condition. Parents also valued return of research results.
Discussion
Trust, transparency, altruism, and concerns about privacy emerged as factors that affect parents’ willingness to allow research use of their child’s EHR.
Conclusion
Researchers should consider how to build trust with parents by increasing transparency of the research process and explaining specifically how they will ensure the confidentiality of EHR data.
Keywords: electronic health records, human genetics, intellectual and developmental disability, biomedical ethics, clinical research
INTRODUCTION
The advent of electronic health records (EHRs) has ushered in a new era of health research, allowing researchers to more easily mine and aggregate clinical data. Research using EHRs has advanced knowledge about a multitude of health conditions.1–3 Combining EHRs with biospecimens has also led to discovery of genetic causes of disease4–7 and become a cornerstone of precision medicine.8 However, concerns about biomedical researchers using EHRs are pervasive, including privacy and security issues9–16 and issues surrounding return of research results.17–20 Despite this, studies have shown that most people perceive the benefits to outweigh the risks.11,13,16,21
To date, preferences about EHR research have been assessed mostly among the general public.9,11–13,15,16,22–24 However, with the push to include individuals with intellectual and developmental disabilities (IDDs) in precision medicine research,25–27 and given that an IDD often is the result of a genetic mutation,28 there is a need to better understand the preferences of individuals with IDDs and their families. Individuals with genetic conditions that result in IDDs have much to gain from advances in biomedical research, such as increased treatment efficacy or identification of subphenotypes. However, these individuals also may be at greater risk for loss of privacy, discrimination, or stigmatization.29
Fragile X syndrome (FXS) is the most common inherited cause of IDDs and causes a range of developmental or medical problems, such as anxiety, attention problems, aggression, and seizures.30 Autism spectrum disorder (ASD) is generally characterized by difficulty with communication and social interaction but may also result in IDDs.31 Depending on severity, individuals with FXS or ASD may need assistance into adulthood.
In this focus group study, we elicited the views of parents of children with a known (FXS) or suspected (ASD) genetic condition and parents of typically developing (TD) children on ethical, legal, and social issues related to sharing their child’s EHR for research. We hypothesized that parents of children with known or suspected genetic conditions may have different views about sharing their child’s EHR for research purposes than parents of TD children. In a complementary article, we summarize the perspectives of young adults with FXS or ASD. This information will be helpful as researchers begin to include these populations in precision medicine research.
MATERIALS AND METHODS
Participants
We conducted focus groups with 3 types of parents: parents of children with FXS, parents of children with ASD, and parents of TD children. Parents of children 14-17 years of age with FXS or ASD were eligible, as were legal guardians of an adult with ASD or FXS 18-40 years of age. Parents of TD children were eligible if they had a child 14-17 years of age who did not have IDDs or other chronic health conditions (eg, asthma, diabetes, epilepsy) and did not regularly see a medical specialist (eg, developmental or behavioral pediatrician, neurologist). All participants had to speak English.
We conducted 2 focus groups with each type of parent, with 5-8 participants per group. The number of focus groups was planned based on past research showing that 2-3 focus groups will likely capture at least 80% of themes on a topic.32 We found that we achieved thematic saturation by the conclusion of the 6 focus groups.33,34
We conducted half of the focus groups near Raleigh, North Carolina, and half near Baltimore, Maryland. We used a market research firm to recruit parents of TD children. Parents of children with FXS and ASD were a convenience sample recruited through research registries and university partners. A total of 38 parents participated (Table 1). Across all groups, most were women (71.1%), were married (84.2%), held at least a 4-year degree (76.3%), and had a household income of ≥$75 000 (69.4%).
Table 1.
Characteristics of focus group participants
| TD Group | ASD Group | FXS Group | Total | |
|---|---|---|---|---|
| Sample | 15 (39.5) | 11 (28.9) | 12 (31.6) | 38 (100.0) |
| Sex | ||||
| Male | 7 (46.7) | 4 (36.4) | 0 (0.0) | 11 (28.9) |
| Female | 8 (53.3) | 7 (63.6) | 12 (100.0) | 27 (71.1) |
| Race/ethnicity | ||||
| Hispanic/Latino | 0 (0.0) | 2 (18.2) | 0 (0.0) | 2 (5.3) |
| White | 8 (53.3) | 6 (54.5) | 11 (91.7) | 25 (65.8) |
| Black/African American | 6 (40.0) | 3 (27.3) | 1 (8.3) | 10 (26.3) |
| Asian | 1 (6.7) | 2 (18.2) | 0 (0.0) | 3 (7.9) |
| Marital status | ||||
| Single/Never married | 2 (13.3) | 1 (9.1) | 0 (0.0) | 3 (7.9) |
| Married | 12 (80.0) | 8 (72.7) | 12 (100.0) | 32 (84.2) |
| Other | 1 (6.7) | 2 (18.2) | 0 (0.0) | 3 (7.9) |
| Education | ||||
| HS graduate/GED | 1 (6.7) | 0 (0.0) | 0 (0.0) | 1 (2.6) |
| Some college | 2 (13.3) | 2 (18.2) | 2 (16.7) | 6 (15.8) |
| 2-y degree or trade certification | 1 (6.7) | 0 (0.0) | 1 (8.3) | 2 (5.3) |
| 4-y degree or higher | 11 (73.3) | 9 (81.8) | 9 (75.0) | 29 (76.3) |
| Employment | ||||
| PT | 3 (20.0) | 1 (9.1) | 3 (25.0) | 7 (18.4) |
| FT | 9 (60.0) | 7 (63.6) | 2 (16.7) | 18 (47.4) |
| Not employed | 3 (20.0) | 3 (27.3) | 7 (58.3) | 13 (34.2) |
| Household incomea | ||||
| <$75 000 | 6 (40.0) | 2 (22.2) | 3 (25.0) | 11 (30.6) |
| ≥$75 000 | 9 (60.0) | 7 (77.8) | 9 (75.0) | 25 (69.4) |
| Total childrenb | 1.9 | 2.3 | 2.3 | 2.2 |
| Total affected childrenb | N/A | 1.2 | 1.3 | N/A |
| Age of oldestb,c | 15.5 | 17.2 | 24.4 | 19 |
Values are n (%) or mean.
ASD: autism spectrum disorder; FT: full-time; FXS: fragile X syndrome; HS: high school; N/A: not applicable; PT: part-time; TD: typically developing.
aNumbers reflect n = 9 for participants with ASD because 2 parents of participants with ASD did not respond.
bNumbers reflect n = 10 for participants with ASD because 1 parent of a participant with ASD did not respond. Total affected children not applicable to TD parents; by definition they did not have any affected children.
cFor parents of participants with ASD and FXS, age of oldest affected child for whom they are medical guardian.
We did not set recruitment targets based on demographic characteristics; however, we were intentional in trying to recruit as diverse a sample as possible across all groups. Nonetheless, parents of children with FXS or ASD were more educated and had a higher household income than the general U.S. population, and were not as racially or ethnically diverse.35
Data Collection
Focus groups were facilitated by a moderator and notetaker. Written consent was obtained. To start, we presented a brief PowerPoint (Microsoft Corporation, Redmond, WA) presentation that provided an overview of EHRs (eg, type of information in EHRs, who uses EHRs and how, privacy protections of EHRs) and how they can be used in research.
Next, the moderator led a discussion about different factors that could affect parents’ willingness to allow their child’s EHR to be used for research purposes, including what information from the EHR would be shared and with whom, how often they would want to be contacted about sharing the EHR, the duration for which the EHR could be used and for what kinds of research, whether or not results of the research would be returned, and perceived risks and benefits of sharing the EHR for research.
The semistructured moderator guide (Supplementary Appendix) was developed based on a scoping review of the ethical, legal, and social issues related to the research use of EHRs and input from a multidisciplinary team of investigators (M. Raspa et al, unpublished data, 2019). Each focus group lasted 90 minutes, and participants received $75. The Institutional Review Board of RTI International approved the data collection activities.
Analysis
Focus group transcripts were managed using NVivo 11 (QSR International, Melbourne, Australia) and analyzed using directed content analysis.36 Specifically, some codes (ie, descriptive labels assigned to segments of text) were designated a priori based on the moderator guide, while other codes emerged during coding. Development of the codebook was an iterative process. Initially, the study team drafted a list of codes based on the domains explored in the moderator guide and review of summary notes from the focus groups. Next, 2 team members refined the codebook by applying the codebook to specified portions of the first transcript, comparing coding, and making changes to the codebook as needed. When the codebook was finalized, the coders individually coded the first transcript, ran a statistical comparison of their coding using the kappa statistic, and discussed differences in coding. They repeated this process again until their kappa statistic for each coding category fell between 0.51 and 1.0, which is considered moderate-to-excellent agreement.37–40 Having established satisfactory interrater reliability, the coders divided up and coded the remaining 5 transcripts independently. Each then reviewed the other’s coding and reached consensus41 about the final coding of each transcript.
Coded data were organized into a matrix42 to identify similarities and differences across the 3 groups of parents regarding factors that affected their willingness to share their child’s EHR for research purposes. The matrix was used to elucidate themes and collect supporting quotations.
RESULTS
Overview
Almost all parents, no matter their child’s genetic status, were willing to share their child’s EHR for research under certain conditions. Although participants were asked directly about the risks and benefits of sharing their child’s EHR, they discussed risks and benefits within the context of the other ethical, legal, and social issues. Thus, participants’ views about risks and benefits are embedded within each of the subheadings below.
Type of information
We asked parents about their willingness to share with researchers various types of information found in EHRs, such as medical history, mental health issues, DNA test results, personal and social information, and insurance information. Most parents were willing to share any data from their child’s EHR that were de-identified. Many parents were willing to share information from their child’s EHR even if it was only partially de-identified, particularly if social security numbers had been removed.
Apart from identifiable information, parents considered certain types of information, such as genetic and mental health information, to be “sensitive.” Parents of TD children were uncomfortable sharing sensitive information, and in some cases completely unwilling to do so. In contrast, parents of children with ASD and FXS were willing, and sometimes even eager, to share their child’s mental health and genetic information, which they considered highly relevant to the study of their child’s condition. Parents of children with ASD and FXS were most uncomfortable sharing information from their child’s EHR that they did not think was relevant to research about their child’s condition. For example, the parent of a child with ASD did not like the idea of socioeconomic information being available to researchers, out of concern that it could bias ASD research: “I feel as though you then become labeled as [this] particular group of people, because their income is maybe the working poor or middle class or upper class. You start putting the kids in different levels of even their care…It’s just like with those two famous stars that have kids allegedly with autism. Now, you know, everybody looked at theirs, but what about our kids?”
Risks
All parents expressed concerns about possible consequences if identifiable information from their child’s EHR was leaked, hacked, or used beyond the research to which they had consented. They thought this could increase the likelihood that their child would be stigmatized or discriminated against.
Benefits
Although security concerns were prevalent, many parents made caveats that they would share identifiable or sensitive information for altruistic purposes. Parents of children with ASD and FXS were particularly willing to share any information that could potentially help the ASD and FXS communities. This was the case for the parent of a child with FXS, who, when asked about her willingness to share information from her child’s EHR about mental health and aggression, said, “I would want to share it to help others…yeah, whatever they [researchers] need to know.”
Who is doing the research
We asked parents which researchers they would allow to access their child’s EHR. Across all groups, parents were most comfortable sharing their child’s EHR with researchers that they knew through their child’s clinical care, or from universities and other nonprofits. The main reason was that parents trusted these reputable, experienced entities to maintain security of their child’s data and use it appropriately. Parents in all groups were less willing to allow researchers from government agencies or for-profit companies (eg, pharmaceutical and insurance companies) to access their child’s EHR. The parent of a child with ASD said, “At least [university] researchers have like a level of ethics and an ethics board and really should be constrained by rules, where they, drug companies, not so much.”
Risks
The main risk most parents discussed was misuse of information by less trusted entities. For example, several stated concerns that information shared with government researchers could be used for nonresearch purposes. Parents also questioned the motives of pharmaceutical companies, suggesting they were more concerned about profit than research participants’ well-being. Parents of children with ASD worried about pharmaceutical companies pushing potentially unneeded drugs on their children, while parents of children with FXS feared these companies would ask their children to participate in research even though they did not have the cognitive ability to consent. The parent of a child with ASD explained, “For a prescription drug company to have access to all of this data, then they could in turn look at the data for all the wrong reasons for profit, medication targeted just for this, what they saw in the research study…yeah, I have some serious problems with that.” Parents in all groups said that their willingness to share their child’s EHR with a pharmaceutical company would depend on the details of the study, including what information in the EHR they would be accessing. All parents distrusted insurance companies, but parents of children with ASD and FXS were most worried about their children being denied coverage.
Benefits
Some parents were more open to studies in which pharmaceutical companies partnered with universities. Although they had more reservations about for-profit companies, parents of children with ASD and FXS also recognized that pharmaceutical companies may develop treatments that could benefit people in their communities, so they were not completely against sharing EHR information with them. The parent of a child with FXS said, “Pharmaceutical company, they’re in it for the profit but it could also benefit our kids. So I wouldn’t want to put that delineation on there [of being unwilling to allow pharmaceutical companies to use her child’s EHR].” Similarly, despite expressing distrust of government researchers, parents of children with ASD and FXS recognized that some government entities, such as the National Institutes of Health, fund and perform “good research,” some of which targets IDDs.
Duration of access and frequency of contact
We asked parents how long they would let researchers access their child’s EHR, and whether they wanted researchers to ask them before each time their child’s EHR was accessed. Most parents wanted to give access to their child’s EHR on a study-by-study basis or for a limited amount of time, rather than giving “blanket” access for future studies. It was important to them to know what their child’s EHR was being used for, to remain in control of access to their child’s EHR. One parent of a child with ASD said, “I mean, just tell me what’s going on…I do [want the information about each study], to make an informed decision. If I don’t like the study, what they’re trying to flesh out, then I would say no.”
A few parents said they would be willing to give access for an unlimited amount of time. However, for most, their willingness was conditional on more frequent contact from researchers, checking in with them periodically in case they had changed their mind and wanted to withdraw. On the other hand, a couple of parents of children with ASD or FXS said that they did not need to be recontacted each time researchers wanted to use their child’s EHR for a study, either because they did not want to slow the research or because they did not feel the need to continuously monitor the use of their child’s data by trusted researchers.
Risks
Some parents did not want to share their child’s EHR for an unlimited amount of time because they perceived a greater risk for data security breaches the longer researchers had access to the data. The parent of a child with FXS said, “Yeah, if you went for, if you went for, like, forever, you didn’t have a limit, I would wonder about accountability, who is keeping track of that organization and what happened to those records.”
Benefits
Parents of children with ASD and FXS recognized possible benefits of researchers having access to EHR data for a long time. Some thought this would increase the chances of researchers finding information that could help their children or others with FXS or ASD. The parent of a child with ASD said, “For me [researchers could have access to the EHR] as long as they wanted to. Maybe they find something, you know, that’s great. Like I said, what can we lose?” Additionally, a parent of a child with FXS said, “I would say [researchers can have access to EHR data] indefinitely, because I think it saves on a lot of time and paperwork and all of that to have to then go back to the families if something comes up in the future that it can be used for.”
Type of research
We asked parents for what type of research they would be willing to share their child’s EHR, such as research on types of treatments, research to develop or improve diagnostic tests, and research to learn more about a genetic condition. Parents across groups were enthusiastic about their child’s EHR being used for these purposes. Parents of children with ASD and FXS wanted to share their child’s EHR for research that was specifically focused on their child’s condition. A parent of a child with FXS said, “There’s a lot of cancer patients that could help with [cancer research], so I would personally rather our medical records go toward fragile X-related and not toward something that isn’t related.”
Risks
Some parents of TD children were concerned about genomic research, specifically the possibility of unintended results or the linkage of DNA data outside of the research context (eg, to criminal data).
Benefits
Parents of TD children said that they would share their child’s EHR for research that would benefit society, such as cures for diseases, development and improvement of treatments, and improvement of healthcare policies and practices. Parents of children with ASD and FXS were interested in benefits of the research to their own children or the ASD and FXS communities.
Return of results
We asked parents how important it is to receive the results of the research conducted using their child’s EHR. Most parents of TD children were less concerned about receiving results, and some questioned whether they would even understand them. In contrast, it was very important to parents of children with ASD and FXS to receive results from studies using their child’s EHR, and for some parents, return of results was a matter of respect, a way to build their trust and engage them in future research. A parent of a child with ASD said, “Returning [results] to me is like a courtesy that you would just do. Like is it too much trouble to keep track of who I am? You’re using all this data for your livelihood; let me know what you came up with, you know.” Still, most parents of children with FXS and some parents of children with ASD said that they would still consider sharing their child’s EHR for research even if results couldn’t be returned.
Risks
Some parents of children with ASD feared that researchers might be hiding something if they did not return results.
Benefits
Although they did not care to receive specific results, some parents of TD children were interested to know that the research helped people. For parents of children with ASD and FXS, return of research results was a direct benefit to themselves and their children, a way to learn more about ASD and FXS.
DISCUSSION
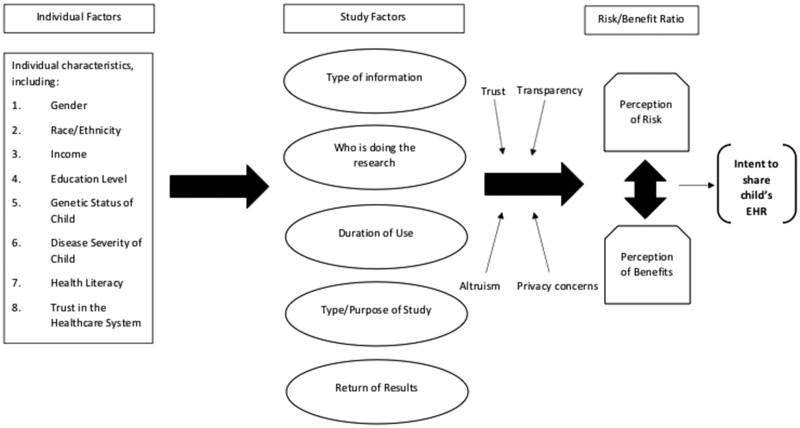
Although most parents supported research use of their child’s EHR, ultimately, parents’ intent was based on weighing the benefits and risks of research participation. To aid understanding of the focus group data and frame our discussion, we constructed a conceptual model to illustrate the way parents talked about their decision to share their child’s EHR (Figure 1). The degree to which EHR study characteristics (eg, type of information being accessed) influenced parents’ perception of risks and benefits was influenced by 4 factors: concerns about privacy, trust, transparency, and altruism. In the following paragraphs, we discuss each of these factors.
Figure 1.
Conceptual model for intent to share child’s electronic health record (EHR) for research purposes.
Concerns about privacy
Ultimately, the biggest risk parents identified was potential loss of privacy. This risk made parents less willing to share identifiable information, for fear that it could make their child vulnerable to identity theft or other data misuses. Parents also were concerned about the possibility of stigmatization if sensitive information about their child was leaked. These findings are consistent with a robust literature about preferences for sharing EHR data among those without genetic conditions or IDDs, which shows that prospective research participants are concerned about possible misuses of their EHR data.9–16,43 However, we found that parents of children with ASD or FXS were generally more willing to share any information contained in their child’s EHR if it advanced research for their child’s condition. Concerns about privacy were directly related to parents’ views about how long researchers could use their child’s EHR and how often they wanted to be contacted by researchers.
Trust
Parents’ trust in the researcher was a factor that could make them more willing to share their child’s EHR. Parents saw trusted researchers as more likely to preserve their child’s privacy and use their child’s data appropriately. If they trusted the researcher, some parents said that they were willing to share their child’s EHR for a longer duration and did not require recontact from researchers. This preference is supported by other literature showing that participants are less likely to want stringent control of their data if they trust the researchers who are handling it.9
In line with previous work, parents in this study described a hierarchy of trust: the most trusted entities were their child’s providers, followed by nonprofits and universities, for-profits (especially pharmaceutical companies) and government, and last, insurance companies.9,14,15,23,43,44 However, we recognize that researchers from any institution can foster or damage trust, and trust can change over time.
Transparency
As other populations have reported,11,14,22 the parents in our study wanted to know details about what information from their child’s EHR would be used, for what purpose, and for how long. If these details changed in any way, parents wanted to be informed. Most parents wanted researchers to recontact them if their child’s EHR was be used for a new study. This transparency on the part of the researcher could build parents’ trust and make them more likely to share their child’s EHR. Similarly, perceived lack of transparency was damaging to parents’ trust.
One key difference between parents of TD children and parents of children with ASD or FXS was that the latter saw return of research results as a reflection of transparency; lack of transparency about study results damaged these parents’ trust in researchers. There is a gap in the literature on return of results for EHR studies that do not involve linkage to biospecimens or other data sources. However, in a study on research use of biospecimens, participants who were concerned that they had a genetic condition were the most likely to endorse return of results.45
Altruism
Altruism was a powerful motivator to all parents when they considered whether they would share their child’s EHR for research purposes. Parents needed assurances about privacy, but many were willing to consider sharing their child’s EHR if convinced that it would benefit others. This finding is consistent with other literature showing that altruism outweighed the risks of sharing EHR data11,13,15,21 and that participants tolerated risk when they valued the purpose and potential outcomes of the research.9 Parents of children with ASD and FXS not only were eager to help others in the ASD and FXS communities, but also saw participating in EHR research as potentially benefitting their own child.
Weighing risks and benefits
Parents’ concerns about privacy, their trust in researchers, the transparency of the research process, and their sense of altruism influenced their perception of risks and benefits and, ultimately, their intent to share their child’s EHR. Within our framework, certain factors were stronger influencers for some parents than for others.
For most parents of TD children, concerns about privacy were tantamount. By definition, TD children do not have any known genetic or health conditions, so there is no obvious direct benefit for them to participate in EHR research. Although parents of TD children expressed a desire to help others, it was critical that they trust the researcher to maintain their child’s privacy and that the researcher be transparent about how, when, and why their child’s information would be used, particularly if identifiable data would be accessed. Even then, for some parents of TD children, the risk of loss of privacy outweighed the benefits.
In contrast, for parents of children with ASD and FXS, the potential benefit of sharing their child’s EHR was more tangible, and for many, outweighed concerns about privacy. Some parents of children with ASD or FXS were willing to share their child’s EHR with less trusted entities, specifically pharmaceutical companies, because of the potential for breakthroughs in treating ASD or FXS. However, in these situations, parents did desire a high degree of transparency.
These findings underscore the notion that parents of children with known or suspected genetic conditions like FXS and ASD have a complicated relationship with research and researchers. Their vested interest in research on their child’s condition, rooted in the desire to help their child and others in their community, can make them more vulnerable to negative consequences. For example, our data show that parents of children with known or suspected genetic disorders want to receive research results, which they see as a direct benefit to their children. However, to receive research results, the data cannot be fully de-identified, which opens them up to greater risk for loss of confidentiality. It is generally more difficult to maintain anonymity of research participants in the case of rare diseases, and individuals with known or suspected genetic conditions are more vulnerable to discrimination or stigmatization if anonymity is not maintained.29 In the case of genetic forms of IDDs, parents may be concerned about exploitation of their child into adulthood.46
Implications for researchers
These findings have implications for those who conduct EHR research on known or suspected genetic conditions. First, EHR researchers must work to build parents’ trust by being more transparent about the research before and during the informed consent process. For example, most parents in our study were not comfortable with a consent model in which they would give indefinite or unlimited access to their child’s EHR. Other work has supported this finding and also suggested that research participants, including parents consenting for their child, do not generally favor “opt-out” models.9,10,14,44,46
Consent models that may foster transparency and build trust among parents include “dynamic”21 or “flexible”9 consent in which individuals can change their consent preferences over time. Researchers can also build trust by increasing transparency about studies that are already underway. For example, Kraft et al14 advocated for the provision of ongoing information to research participants about where and how their data is being used as well as details about decision making, potentially through a website or newsletter. These models, although promising, are dependent of on the use of identifiable data.
Importantly, we found that, particularly in the case of the parents of children with known or suspected genetic conditions, the return of results is viewed as a valued form of transparency and a direct benefit. One practice that we have employed in our research is making online surveys anonymous by default but offering respondents the option of providing an email address to which research results can be sent. To engage this population and increase their trust and ongoing participation, researchers should consider study designs that allow them to, at minimum, return aggregated results. This practice would also serve to validate participants’ desire to be altruistic, as returning results gives participants a sense of the findings to which they have contributed.
Finally, existing literature suggests that researchers may build trust with parents by detailing security processes in the consent, enforcing security policies, and punishing violators of such policies.9,11,14,43 Information about security practices and how they will be enforced may reassure parents by conveying a sense of accountability for those who will be handling their child’s EHR data.9,11,14,43
CONCLUSION
Limitations
This study is not without its limitations. We recognize the possibility of selection bias; because some participants were recruited from research registries, they might have had different views of EHR research than did parents who have no research experience. Additionally, FXS and ASD are both spectrum disorders, so we cannot assume that this focus group study represents the views of all parents of children with FXS and ASD. Additionally, as noted in our methods, we recognize that our participants in the known and suspected genetic groups were not as diverse as the general population. All of these factors underscore the fact that our participants are not a representative sample of all parents, or even of parents of children with IDDs. Thus, our findings may not be generalizable. However, they do serve to identify preferences, priorities, and concerns of an important stakeholder group, and to highlight areas of focus for a larger, more representative study.
Regarding our conceptual model (Figure 1), we recognize that individual characteristics of participants (eg, sex, health literacy) likely inform their preferences surrounding research use of their child’s EHR, but our qualitative analyses did not measure this. We also acknowledge that there may be additional factors that influence parents’ perception of risk–benefit ratio that were not discussed by our participants.
Significance and future research
This work adds to the literature on ethical approaches to the research use of EHRs. The findings underscore the need for a large, representative quantitative assessment of the preferences of parents of children with genetic conditions to support these qualitative findings and test the conceptual model that we have proposed. Future work should explore ways to address research participants’ concerns about providing blanket consent and the desire of those with known or suspected genetic conditions to receive results even from EHR studies.
FUNDING
This work was funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R01HD086702-03).
AUTHOR CONTRIBUTIONS
MR and ACW conceptualized the study design. SA, MR, AE, RM, LT-B, LW, AA-R, MKF, and ACW created data collection tools and/or collected the data. SA, MR, AE, RM, LW, AA-R, and MKF participated in data analysis. SA drafted the manuscript. MR, AE, RM, LT-B, LW, MKF, and ACW reviewed 1 or more of SA's drafts of the manuscript and contributed important intellectual content. SA, MR, AE, RM, LT-B, LW, AA-R, MKF, and ACW gave final approval of the version of the article to be published. SA, MR, AE, RM, LT-B, LW, AA-R, MKF, and ACW agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
References
- 1. Corvino FA, Oliveri D, Phillips AL.. The association of timing of disease-modifying drug initiation and relapse in patients with multiple sclerosis using electronic health records. Curr Med Res Opin 2017; 33 (6): 1127–32. [DOI] [PubMed] [Google Scholar]
- 2. Matthews A, Scanlan J, Kirkby KC.. Increasing knowledge of mental illness through secondary research of electronic health records: opportunities and challenges AU-Spiranovic, Caroline. Adv Ment Health 2016; 14 (1): 14–25. [Google Scholar]
- 3. Wu LT, Ghitza UE, Batch BC, et al. Substance use and mental diagnoses among adults with and without type 2 diabetes: results from electronic health records data. Drug Alcohol Depend 2015; 156: 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet 2011; 43 (5): 436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ritchie MD, de Andrade M, Kuivaniemi H.. The foundation of precision medicine: integration of electronic health records with genomics through basic, clinical, and translational research. Front Genet 2015; 6: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolford BN, Willer CJ, Surakka I.. Electronic health records: the next wave of complex disease genetics. Hum Mol Genet 2018; 27 (R1): R14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohane IS. Using electronic health records to drive discovery in disease genomics. Nat Rev Genet 2011; 12 (6): 417–28. [DOI] [PubMed] [Google Scholar]
- 8. Sitapati A, Kim H, Berkovich B, et al. Integrated precision medicine: the role of electronic health records in delivering personalized treatment. Syst Biol Med 2017; 9 (3): e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aitken M, de St Jorre J, Pagliari C, Jepson R, Cunningham-Burley S.. Public responses to the sharing and linkage of health data for research purposes: a systematic review and thematic synthesis of qualitative studies. BMC Med Ethics 2016; 17 (1): 73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caine K, Hanania R.. Patients want granular privacy control over health information in electronic medical records. J Am Med Inform Assoc 2013; 20 (1): 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clerkin P, Buckley BS, Murphy AW, MacFarlane AE.. Patients' views about the use of their personal information from general practice medical records in health research: a qualitative study in Ireland. Fam Pract 2013; 30 (1): 105–12. [DOI] [PubMed] [Google Scholar]
- 12. Dimitropoulos L, Patel V, Scheffler SA, Posnack S.. Public attitudes toward health information exchange: perceived benefits and concerns. Am J Manag Care 2011; 17 (12 Spec No): Sp111–6. [PubMed] [Google Scholar]
- 13. Kim KK, Sankar P, Wilson MD, Haynes SC.. Factors affecting willingness to share electronic health data among California consumers. BMC Med Ethics 2017; 18 (1): 25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraft SA, Cho MK, Gillespie K, et al. Beyond consent: building trusting relationships with diverse populations in precision medicine research. Am J Bioeth 2018; 18 (4): 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perera G, Holbrook A, Thabane L, Foster G, Willison DJ.. Views on health information sharing and privacy from primary care practices using electronic medical records. Int J Med Inform 2011; 80 (2): 94–101. [DOI] [PubMed] [Google Scholar]
- 16. Platt J, Kardia S.. Public trust in health information sharing: implications for biobanking and electronic health record systems. J Pers Med 2015; 5 (1): 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Secretary’s Advisory Committee on Human Research Protections. SACHRP Letter to the HHS Secretary, Attachment B: Return of Individual Research Results. https://www.hhs.gov/ohrp/sachrp-committee/recommendations/attachment-b-return-individual-research-results/index.html Accessed July 21, 2016.
- 18. Haga SB, Beskow LM.. Ethical, legal, and social implications of biobanks for genetics research. Adv Genet 2008; 60: 505–44. [DOI] [PubMed] [Google Scholar]
- 19. Fullerton SM, Wolf WA, Brothers KB, et al. Return of individual research results from genome-wide association studies: experience of the Electronic Medical Records and Genomics (eMERGE) Network. Genet Med 2012; 14 (4): 424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brelsford KM, Spratt SE, Beskow LM.. Research use of electronic health records: patients' perspectives on contact by researchers. J Am Med Inform Assoc 2018; 25 (9): 1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spencer K, Sanders C, Whitley EA, Lund D, Kaye J, Dixon WG.. Patient perspectives on sharing anonymized personal health data using a digital system for dynamic consent and research feedback: a qualitative study. J Med Internet Res 2016; 18 (4): e66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA.. Patients, privacy and trust: patients' willingness to allow researchers to access their medical records. Soc Sci Med 2007; 64 (1): 223–35. doi: 10.1016/j.socscimed.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 23. Grande D, Mitra N, Shah A, Wan F, Asch DA.. Public preferences about secondary uses of electronic health information. JAMA Intern Med 2013; 173 (19): 1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robling MR, Hood K, Houston H, Pill R, Fay J, Evans HM.. Public attitudes towards the use of primary care patient record data in medical research without consent: a qualitative study. J Med Ethics 2004; 30 (1): 104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabatello M. Precision medicine, health disparities, and ethics: the case for disability inclusion. Genet Med 2018; 20 (4): 397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spong CY, Bianchi DW.. Improving public health requires inclusion of underrepresented populations in research. JAMA 2018; 319 (4): 337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabatello M, Chen Y, Zhang Y, Appelbaum PS.. Disability inclusion in precision medicine research: a first national survey. Genet Med 2019; 21 (10): 2319–27. doi: 10.1038/s41436-019-0486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaufman L, Ayub M, Vincent JB.. The genetic basis of non-syndromic intellectual disability: a review. J Neurodev Disord 2010; 2 (4): 182–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Griggs RC, Batshaw M, Dunkle M, et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab 2009; 96 (1): 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raspa M, Wheeler AC, Riley C. Public Health Literature Review of Fragile X Syndrome. Pediatrics. 2017;139(Suppl 3):S153–S171. doi:10.1542/peds.2016-1159C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholas JS, Charles JM, Carpenter LA, King LB, Jenner W, Spratt EG. Prevalence and characteristics of children with autism-spectrum disorders. Annals of epidemiology. 2008;18(2):130–6. doi 10.1016/j.annepidem.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 32. Guest G, Namey E, McKenna K.. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods 2017; 29 (1): 3–22. [Google Scholar]
- 33. Fusch P, Ness L.. Are we there yet? Data saturation in qualitative research. Qual Rep 2015; 20: 1408–16. [Google Scholar]
- 34. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018; 52 (4): 1893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Census Bureau. QuickFacts table. 2018. https://www.census.gov/quickfacts/fact/table/US/PST045218 Accessed October 15, 2019.
- 36. Hsieh HF, Shannon SE.. Three approaches to qualitative content analysis. Qual Health Res 2005; 15 (9): 1277–88. [DOI] [PubMed] [Google Scholar]
- 37. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994; 6 (4): 284–90. [Google Scholar]
- 38. Hruschka DJ, Schwartz D, St.John DC, Picone-Decaro E, Jenkins RA, Carey JW.. Reliability in coding open-ended data: lessons learned from HIV behavioral research. Field Methods 2004; 16 (3): 307–31. [Google Scholar]
- 39.QSR International. Running a coding comparison query. http://help-nv11.qsrinternational.com/desktop/procedures/run_a_coding_comparison_query.htm Accessed January 19, 2017.
- 40. Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics 1977; 33 (1): 159–74. [PubMed] [Google Scholar]
- 41. Hill CE, Knox S, Thompson BJ, Williams EN, Hess SA, Ladany N.. Consensual qualitative research: an update. J Couns Psychol 2005; 52 (2): 196. [Google Scholar]
- 42. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res 2002; 12 (6): 855–66. [DOI] [PubMed] [Google Scholar]
- 43. Harle CA, Golembiewski EH, Rahmanian KP, et al. Patient preferences toward an interactive e-consent application for research using electronic health records. J Am Med Inform Assoc 2018; 25 (3): 360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones RD, Sabolch AN, Aakhus E, Spence RA, Bradbury AR, Jagsi R.. Patient perspectives on the ethical implementation of a rapid learning system for oncology care. J Oncol Pract 2017; 13 (3): e163–75. [DOI] [PubMed] [Google Scholar]
- 45. Hoop JG, Roberts LW, Hammond KA.. Genetic testing of stored biological samples: views of 570 U.S. workers. Genet Test Mol Biomarkers 2009; 13 (3): 331–7. [DOI] [PubMed] [Google Scholar]
- 46. McCormack P, Kole A, Gainotti S, et al. ‘ You should at least ask’. The expectations, hopes and fears of rare disease patients on large-scale data and biomaterial sharing for genomics research. Eur J Hum Genet 2016; 24 (10): 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.