Abstract
Tak-242 (resatorvid), a Toll-like Receptor 4 (TLR4) inhibitor, has been identified as a potent suppressor of innate inflammation. As a strategy to target Tak-242 to select tissue, four TLR4-inactive prodrugs were synthesized for activation via two different release mechanisms. Two nitrobenzyl Tak-242 prodrugs released the parent drug upon exposure to the exogenous enzyme nitroreductase, while the two propargyl prodrugs were converted to Tak-242 in the presence of Pd0.
Keywords: Prodrug, enzyme prodrug therapy, bioorthogonal organometallic activation, nitroreductase, TLR4
We have recently demonstrated that the potent toll-like receptor 4 (TLR4) inhibitor Tak-2421,2 can be utilized to significantly improve outcomes in islet transplantation. While the free drug protects pancreatic islets from TLR4-mediated innate inflammation during their isolation,3 the conjugation of a Tak-242 prodrug to islet surfaces using a slow-release linker provides localized and sustained protection of the islets after transplantation.4 Due to the promising outcomes from those studies, we have continued to explore strategies for the targeted delivery of Tak-242, and herein we report our work on the synthesis and characterization of two classes of Tak-242 prodrugs for localized delivery.
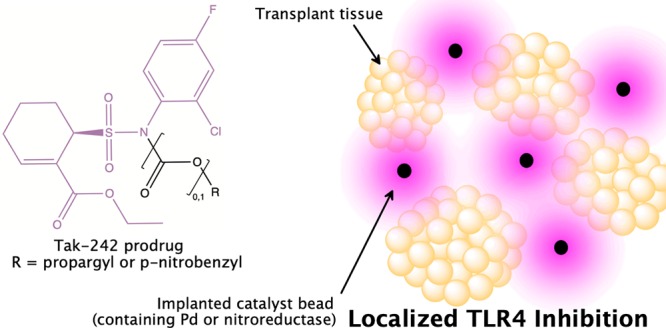
Prodrugs are inactive compounds that are converted to active drugs in vivo and are commonly developed to address suboptimal chemical or pharmacokinetic properties in the parent drugs.5,6 Site-selective prodrug activation, which can take advantage of inherent differences in the tissue of interest or rely upon the selective delivery of exogeneous prodrug activators, is able to provide drug localization and limit off-target effects. Two examples of prodrug targeting utilizing exogeneous activators are directed enzyme prodrug therapy (DEPT)7−13 and bioorthogonal organometallic (BOOM) drug activation.14−17 In DEPT, a prodrug-activating enzyme (with activity not found in “normal” tissue) is localized at the target tissue, often using antibodies. Unciti-Broceta’s BOOM strategy involves drug activation by localized biocompatible metal catalysts. Implanting solid-supported enzymes (DEPT) or metal catalysts (BOOM) in the vicinity of transplanted tissue has the potential to afford local prodrug activation with minimal systemic exposure (Figure 1).
Figure 1.
Co-transplantation of transplant tissue and immobilized catalysts provides drug activation localized to the vicinity of transplanted tissue.
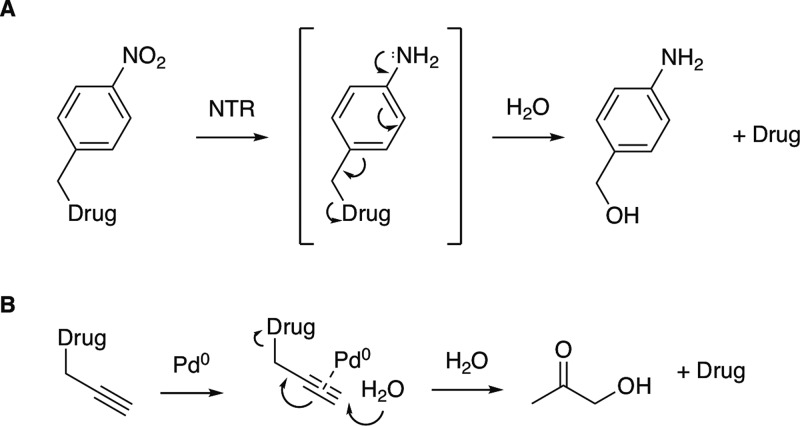
For enzymatic activation we chose to explore the use of nitroreductase, a bacterial enzyme commonly utilized in DEPT.18,19 This enzyme efficiently reduces aryl nitro groups, and p-nitrobenzyl-modified prodrugs can undergo this reduction followed by a 1,6-elimination to release active drug along with p-aminobenzylalcohol (Scheme 1A).20,21
Scheme 1. Release of Active Drugs: (a) a p-Nitrobenzyl Prodrug and Nitroreductase (NTR) and (b) a Propargyl Prodrug and Pd(0).
As an alternative strategy for site-specific prodrug activation we chose to explore catalysis using Pd0 nanoparticles immobilized on TentaGel resins (Rapp Polymere GmbH). These catalysts have been used for the in vivo activation of prodrugs containing propargyl, allyl, and benzyl groups.22,23 Propargyl prodrugs have been reported to efficiently undergo hydrolysis to release the parent drug along with hydroxyacetone (Scheme 1B).24,25
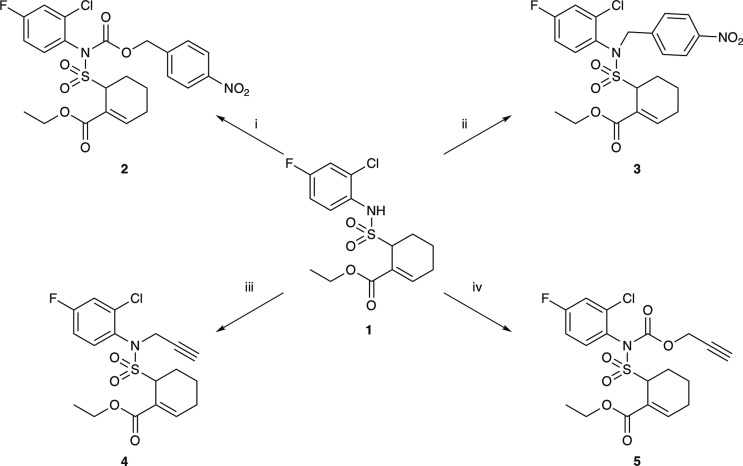
Since biologically inactive carbamates of Tak-242 could be readily synthesized from chloroformates,4 and as carbamates have been widely utilized in prodrug design,26 carbamate prodrugs 2 and 5 were chosen as the initial synthetic targets. Additionally, due to the relatively acidic sulfonamide N–H bond of Tak-242, we hypothesized that the drug itself could also serve as a leaving group without the assistance of a carbamate linkage and selected the alkylated compounds 3 and 4 as additional targets. In the event, racemic Tak-242 1 was readily converted to p-nitrobenzyl prodrugs 2 and 3 and propargyl prodrugs 4 and 5 (Scheme 2) with reasonable yields.
Scheme 2. Synthesis of Racemic Tak-242 Prodrugs 2–5.
(i) p-nitrobenzyl chloroformate, sodium carbonate (aq), EtOAc, 55% yield. (ii) p-nitrobenzyl chloride, TEA, DCM, 47% yield. (iii) propargyl bromide, potassium carbonate, MeCN, 83% yield. (iv) propargyl chloroformate, TEA, DMAP, DCM, 54% yield.
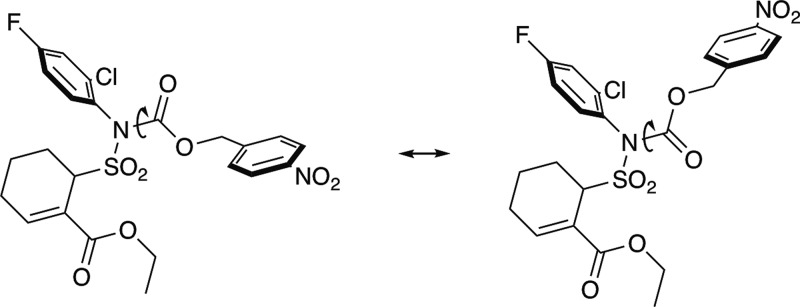
Initial examination of the proton NMR (27 °C; CDCl3) of prodrug 2 revealed two distinct sets of peaks at a ∼2:1 ratio (Scheme 3), which is not unexpected for this complex carbamate.27,28 In DMSO-d6 (27 °C) the proton NMR of compound 2 had broad peaks, while at 55 °C the proton NMR spectrum coalesced to a single set of clean signals (Figure 2). The proton NMR of carbamate 5 also exhibited two sets of peaks at an approximate 2:1 ratio at room temperature in CDCl3, but heating this NMR sample to 70 °C in DMSO-d6 only resulted in partial coalescence of the signals. The proton NMR spectrum of noncarbamate prodrug 3 only revealed one set of peaks, with the quartet and triplet associated with the ethyl ester showing up as broad singlets that resolve to the expected patterns at elevated temperature.
Scheme 3. Rotation of the N–C Bond Results in the Existence of Two Unique Conformers of Tak-242 Carbamate Prodrug 2.
Figure 2.
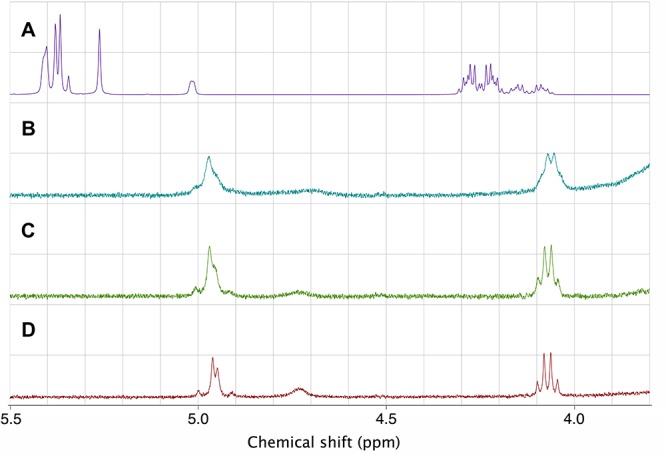
Stacked proton NMR spectra for compound 2 run in (a) chloroform-d at 27 °C, (b) DMSO-d6 at 27 °C, (c) DMSO-d6 at 40 °C, and (d) DMSO-d6 at 55 °C.
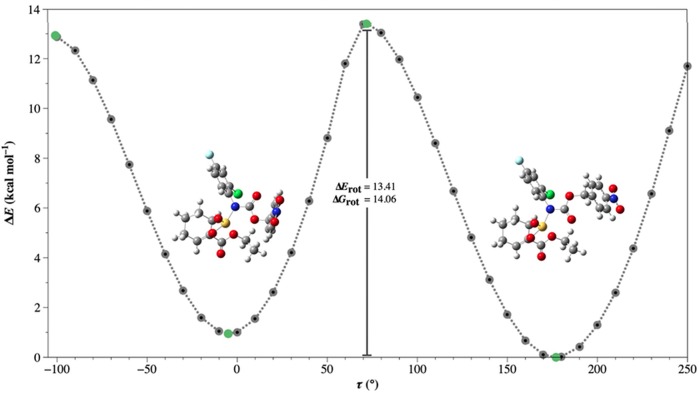
In order to better understand the conformational energetics of these prodrugs (2–5), each compound was probed through a set of relaxed torsional energy scans and subsequent full geometry optimizations and harmonic vibrational frequency computations using the hybrid B3LYP29−31 density functional in conjunction with a split-valence triple-ξ quality 6-311G(2df, 2pd) basis set32−35 within the Gaussian 09 software package.36 The cross sections of the potential energy surfaces of prodrugs 2 and 5 exhibit clear minima and relatively large energetic barriers of rotation (ΔErot = 13.41 kcal mol–1 and 12.99 kcal mol–1, respectively). Along with the similar energies calculated for the low energy rotamers of these carbamate prodrugs (ΔE < 2 kcal mol–1), these barriers are consistent with the observation of two distinct conformers in the experimental NMR spectra.37 As an example, the complete cross section of the potential energy surface for 2 is shown in Figure 3; the full results from these quantum chemical computations for all four compounds are found in the Supporting Information.
Figure 3.
Cross section of the potential energy surface about torsional angle τ (C–N–C–O)° and the energetic barriers of rotation (ΔErot and ΔGrot) between the two minima of prodrug 2 computed at the B3LYP/6-311G(2df,2pd) level of theory. The green markers denote the fully optimized stationary points identified by the relaxed torsional energy scan.
In order to determine whether compounds 2–5 would function as inactive prodrugs, a TLR4 reporter cell assay was performed. All four racemic prodrugs demonstrated a significant reduction of TLR4 antagonism compared to the active drug (racemic Tak-242; Figure 4). Perhaps unsurprisingly, the simple propargyl-alkylated prodrug 4 was most Tak-242-like, inhibiting the TLR4 response to LPS stimulation with an IC50 approximately 10× the parent drug (Table 1).
Figure 4.
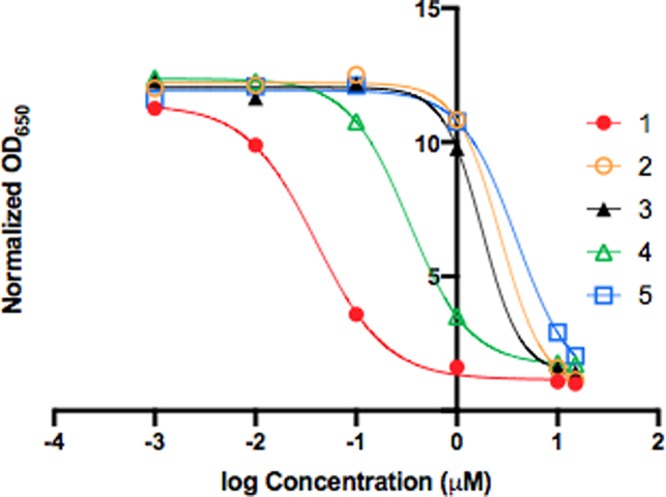
TLR4 reporter cell assay. HEK TLR4 reporter cells were treated with compounds 1–5 at various concentrations and stimulated with LPS. Complete suppression of TLR4 was defined as the optical density at 650 nm of cells incubated with racemic Tak-242 (1) at 15 μM.
Table 1. IC Values of Racemic Tak-242 and Racemic Tak-242 Prodrugs.
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| IC50(μM) | 0.040 | 2.701 | 1.785 | 0.334 | 3.897 |
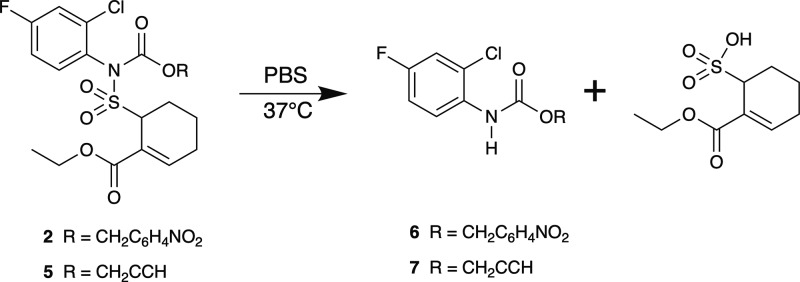
The aqueous stabilities of the Tak-242 prodrugs were then determined by incubation in phosphate buffered saline (PBS, pH 7.4 containing 5% DMSO) at 37 °C. Analysis by HPLC revealed that while the alkylated compounds 3 and 4 are stable under these conditions, the sulfonamide bonds of carbamates 2 and 5 slowly underwent hydrolysis (Scheme 4). This reactivity resembles the previously reported4 carbamate-functionalized prodrug/linker and confirms our suspicion that adding a carbamate functionality to the TAK-242 sulfonamide destabilizes it toward hydrolysis. While only ∼25% of 5 remained after 1 day of incubation, ∼85% of 2 remained at this point, presumably due to the increased steric bulk (see Supporting Information, Figure S14).
Scheme 4. Aqueous Hydrolysis of Sulfonamide Bonds in Carbamate Prodrugs 2 and 5.
The catalyzed conversion of each prodrug to the parent drug was then examined. Exposure of the nitrobenzyl prodrugs 2 and 3 to nitroreductase (from E. coli, 2 units/mL) and the reducing cofactor β-nicotinamide adenine dinucleotide (NADH; 1 mg/mL) in PBS (5% DMSO) resulted in the rapid consumption of the prodrugs and the efficient release of the parent drug 1 (Figure 5). The putative reduced amine intermediate of carbamate 2 was not observed, presumably due to a rapid 1,6-elimination and loss of CO2. Under the same activation conditions, the alkylated nitro-benzyl prodrug 3 was rapidly converted to the amine intermediate (visible in HPLC) which slowly underwent 1,6-elimination and release of compound 1 (Figure S13).
Figure 5.
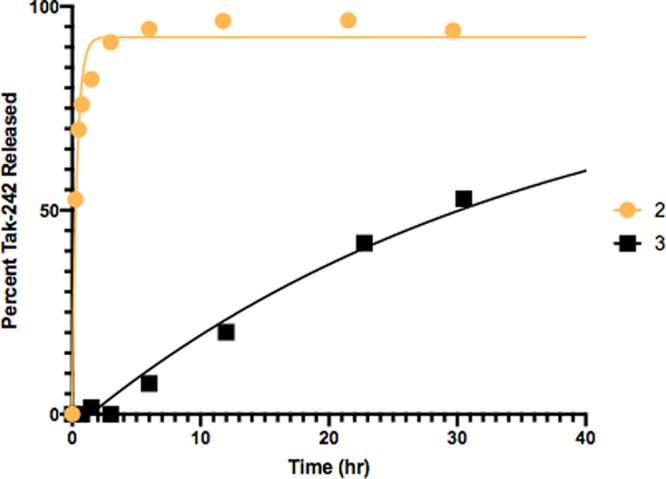
Tak-242 prodrugs 2 and 3 were incubated with nitroreductase (from E. coli; 2 units/mL) and NADPH (1 mg/mL) in PBS (pH 7.4) containing 5% DMSO and monitored by HPLC.
Similarly, the Pd0 catalyzed unmasking of propargyl prodrugs 4 and 5 were evaluated. Incubating the prodrugs in PBS (5% DMSO) with Pd0 immobilized on amino terminated polystyrene beads17 (30 μm diameter beads, 1 mg/mL; provided by Asier Unciti-Broceta, University of Edinburgh) led to the release of racemic 1 (Figure 6). Once again, the carbamate prodrug 5 released the free drug more rapidly than the alkylated prodrug 4.
Figure 6.
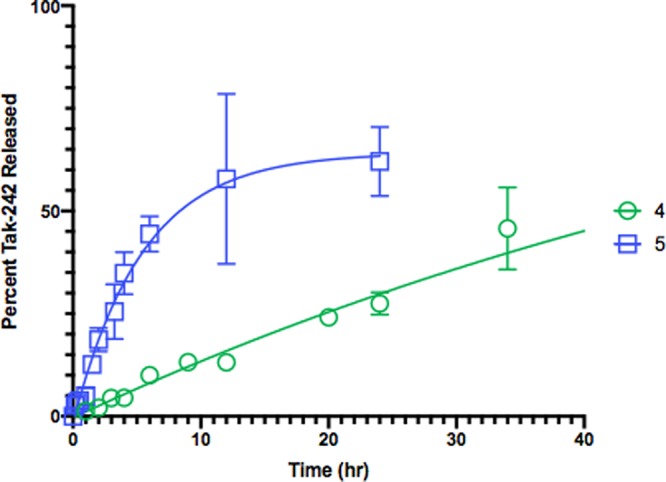
Tak-242 prodrugs 4 and 5 were incubated with Pd0 (on 30 μm beads; 1 mg/mL) in PBS (pH 7.4) containing 5% DMSO and monitored by LC-MS.
In summary, we have successfully synthesized four novel prodrugs of Tak-242 for the localized inhibition of TLR4-induced inflammation. While the propargyl-substituted prodrugs 4 and 5 are readily converted to the parent drug by Pd0, they suffer from residual TLR4 activity (for the alkylated derivative 4) or marginal aqueous stability (for carbamate 5). On the other hand, nitrobenzyl prodrugs 2 and 3 were found to have low TLR4 activity as well as good aqueous stability and provide dramatically different release kinetics affording rapid or slow delivery of the parent Tak-242. We are presently exploring the application of the prodrugs to the protection of transplanted pancreatic islets from acute peri-transplant inflammation, are evaluating solid supports and conjugation methods for generating biocompatible nitroreductase beads, and are developing second-generation prodrugs to address the increased hydrophobicity of compounds 2 and 3.
Acknowledgments
This research was supported by JDRF (Grant 1-INO-2019-787-S-B) and Baylor University (Faculty Research Investment Program and the Vice Provost for Research). OPO, TLE, and KLS were supported by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy under Award Number DE-SC0019327. The authors thank Asier Unciti-Broceta for helpful conversations and for providing Pd0-resins.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00518.
Full experimental details for the synthesis of prodrugs 2–5, computational modeling of prodrug conformers, HPLC measurement of prodrug hydrolysis, catalytic activation and release of parent drug, and TLR4 reporter cell assay (PDF)
Author Contributions
MAP synthesized and characterized the prodrugs, performed the kinetic analyses, and produced the manuscript. AA performed the reporter-cell assays and contributed to the manuscript preparation. OPO, TLE, and KLS computed the rotamer energetics and contributed to the manuscript preparation. RRK guided the experimentation and edited the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ii M.; Matsunaga N.; Hazeki K.; Nakamura K.; Takashima K.; Seya T.; Hazeki O.; Kitazaki T.; Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol. 2006, 69 (4), 1288–1295. 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- Yamada M.; Ichikawa T.; Ii M.; Sunamoto M.; Itoh K.; Tamura N.; Kitazaki T. Discovery of Novel and Potent Small-molecule inhibitors of NO and cytokine production as antisepsis agents: Synthesis and biological activity of alkyl 6-(N-substituted sulfamoyl)cyclohex-1-ene-1-carboxylate. J. Med. Chem. 2005, 48 (23), 7457–7467. 10.1021/jm050623t. [DOI] [PubMed] [Google Scholar]
- Chang C. A.; Murphy K.; Kane R. R.; Lawrence M. C.; Naziruddin B. Early TLR4 blockade attenuates sterile inflammation-mediated stress in islets during isolation and promotes successful transplant outcomes. Transplantation 2018, 102 (9), 1505–1513. 10.1097/TP.0000000000002287. [DOI] [PubMed] [Google Scholar]
- Chang C. A.; Akinbobuyi B.; Quintana J. M.; Yoshimatsu G.; Naziruddin B.; Kane R. R. Ex-vivo generation of drug-eluting islets improves transplant outcomes by inhibiting TLR4-Mediated NF kappa B upregulation. Biomaterials 2018, 159, 13–24. 10.1016/j.biomaterials.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Huttunen K. M.; Raunio H.; Rautio J. Prodrugs—from serendipity to rational design. Pharmacol. Rev. 2011, 63 (3), 750–771. 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- Rautio J.; Meanwell N. A.; Di L.; Hageman M. J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discovery 2018, 17, 559–587. 10.1038/nrd.2018.46. [DOI] [PubMed] [Google Scholar]
- Geresu M. A.; Sultan A. F.; Ahmed S. K.; Kassa G. M. Immunotherapy against cancer: A comprehensive review. J. Cancer Res. Exp. Oncol. 2016, 8 (2), 15–25. 10.5897/JCREO2015.0124. [DOI] [Google Scholar]
- Walther R.; Rautio J.; Zelikin A. N. Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv. Drug Delivery Rev. 2017, 118, 65–77. 10.1016/j.addr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Deckert P. M.; Renner C.; Cohen L. S.; Jungbluth A.; Ritter G.; Bertino J. R.; Old L. J.; Welt S. A33scFv–cytosine deaminase: a recombinant protein construct for antibody-directed enzyme-prodrug therapy. Br. J. Cancer 2003, 88, 937–939. 10.1038/sj.bjc.6600751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-h.; Aloysius H.; Inoyama D.; Chen Y.; Hu L.-q. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharm. Sin. B 2011, 1 (3), 143–159. 10.1016/j.apsb.2011.08.001. [DOI] [Google Scholar]
- Kucerova L.; Durinikova E.; Toro L.; Cihova M.; Miklikova S.; Poturnajova M.; Kozovska Z.; Matuskova M. Targeted antitumor therapy mediated by prodrug-activating mesenchymal stromal cells. Cancer Lett. 2017, 408, 1–9. 10.1016/j.canlet.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Sagnou M. J.; Howard P. W.; Gregson S. J.; Eno-Amooquaye E.; Burke P. J.; Thurston D. E. Design and synthesis of novel pyrrolobenzodiazepine (PBD) prodrugs for ADEPT and GDEPT. Bioorg. Med. Chem. Lett. 2000, 10 (18), 2083–2086. 10.1016/S0960-894X(00)00404-2. [DOI] [PubMed] [Google Scholar]
- Park J. I.; Cao L.; Platt V. M.; Huang Z.; Stull R. A.; Dy E. E.; Sperinde J. J.; Yokoyama J. S.; Szoka F. C. Antitumor therapy mediated by 5-fluorocytosine and a recombinant fusion protein containing TSG-6 hyaluronan binding domain and yeast cytosine deaminase. Mol. Pharmaceutics 2009, 6 (3), 801–812. 10.1021/mp800013c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-López A. M.; Rubio-Ruiz B.; Sebastián V.; Hamilton L.; Adam C.; Bray Thomas L.; Irusta S.; Brennan Paul M.; Lloyd-Jones Guy C.; Sieger D.; Santamaría J.; Unciti-Broceta A. Gold-triggered uncaging chemistry in living systems. Angew. Chem., Int. Ed. 2017, 56 (41), 12548–12552. 10.1002/anie.201705609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unciti-Broceta A.; Johansson E. M. V.; Yusop R. M.; Sánchez-Martín R. M.; Bradley M. Synthesis of polystyrene microspheres and functionalization with Pd0 nanoparticles to perform bioorthogonal organometallic chemistry in living cells. Nat. Protoc. 2012, 7, 1207–1218. 10.1038/nprot.2012.052. [DOI] [PubMed] [Google Scholar]
- Yusop R. M.; Unciti-Broceta A.; Johansson E. M. V.; Sánchez-Martín R. M.; Bradley M. Palladium-mediated intracellular chemistry. Nat. Chem. 2011, 3, 239–243. 10.1038/nchem.981. [DOI] [PubMed] [Google Scholar]
- Bray T. L.; Salji M.; Brombin A.; Pérez-López A. M.; Rubio-Ruiz B.; Galbraith L. C. A.; Patton E. E.; Leung H. Y.; Unciti-Broceta A. Bright insights into palladium-triggered local chemotherapy. Chemical Science 2018, 9 (37), 7354–7361. 10.1039/C8SC02291G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Voak A.; Wilkinson S. R.; Hu L. Design, synthesis, and evaluation of potential prodrugs of DFMO for reductive activation. Bioorg. Med. Chem. Lett. 2012, 22 (21), 6583–6586. 10.1016/j.bmcl.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Johansson E.; Parkinson G. N.; Denny W. A.; Neidle S. Studies on the nitroreductase prodrug-activating system. crystal structures of complexes with the inhibitor dicoumarol and dinitrobenzamide prodrugs and of the enzyme active form. J. Med. Chem. 2003, 46 (19), 4009–4020. 10.1021/jm030843b. [DOI] [PubMed] [Google Scholar]
- Bae J.; McNamara L. E.; Nael M. A.; Mahdi F.; Doerksen R. J.; Bidwell G. L.; Hammer N. I.; Jo S. Nitroreductase-triggered activation of a novel caged fluorescent probe obtained from methylene blue. Chem. Commun. 2015, 51 (64), 12787–12790. 10.1039/C5CC03824C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B.; Hu W.; Sun J.; Chi S.; Lei Y.; Zhang F.; Zhong C.; Liu Z. A two-photon fluorescent probe for nitroreductase imaging in living cells, tissues and zebrafish under hypoxia conditions. Analyst 2017, 142 (9), 1545–1553. 10.1039/C7AN00058H. [DOI] [PubMed] [Google Scholar]
- Weiss J. T.; Dawson J. C.; Macleod K. G.; Rybski W.; Fraser C.; Torres-Sánchez C.; Patton E. E.; Bradley M.; Carragher N. O.; Unciti-Broceta A. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 2014, 5 (11), 3277. 10.1038/ncomms4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Ruiz B.; Weiss J. T.; Unciti-Broceta A. Efficient palladium-triggered release of Vorinostat from a bioorthogonal precursor. J. Med. Chem. 2016, 59 (21), 9974–9980. 10.1021/acs.jmedchem.6b01426. [DOI] [PubMed] [Google Scholar]
- Weiss J. T.; Dawson J. C.; Fraser C.; Rybski W.; Torres-Sánchez C.; Bradley M.; Patton E. E.; Carragher N. O.; Unciti-Broceta A. Development and bioorthogonal activation of palladium-labile prodrugs of Gemcitabine. J. Med. Chem. 2014, 57 (12), 5395–5404. 10.1021/jm500531z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam C.; Pérez-López A. M.; Hamilton L.; Rubio-Ruiz B.; Bray T. L.; Sieger D.; Brennan P. M.; Unciti-Broceta A. Bioorthogonal uncaging of the active metabolite of Irinotecan by palladium-functionalized microdevices. Chem. - Eur. J. 2018, 24 (63), 16783–16790. 10.1002/chem.201803725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Brindisi M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58 (7), 2895–2940. 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Est-Stammer R.; Engberts J. B. F. N. Hindered internal rotation in carbamates: An NMR study of the conformations of alkyl and aryl N-(alkylsulfonylmethyl)-N-methylcarbamates and aryl N-(arylsulfonylmethyl)-N-methylcarbamates. Recueil des Travaux Chimiques des Pays-Bas 1971, 90 (12), 1307–1319. 10.1002/recl.19710901204. [DOI] [Google Scholar]
- Smith B. D.; Goodenough-Lashua D. M.; D’Souza C. J. E.; Norton K. J.; Schmidt L. M.; Tung J. C. Substituent effects on the barrier to carbamate C–N rotation. Tetrahedron Lett. 2004, 45 (13), 2747–2749. 10.1016/j.tetlet.2004.02.037. [DOI] [Google Scholar]
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98 (7), 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Miehlich B.; Savin A.; Stoll H.; Preuss H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157 (3), 200–206. 10.1016/0009-2614(89)87234-3. [DOI] [Google Scholar]
- Bauernschmitt R.; Ahlrichs R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256 (4), 454–464. 10.1016/0009-2614(96)00440-X. [DOI] [Google Scholar]
- Frisch M. J.; Pople J. A.; Binkley J. S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80 (7), 3265–3269. 10.1063/1.447079. [DOI] [Google Scholar]
- Krishnan R.; Binkley J. S.; Seeger R.; Pople J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72 (1), 650–654. 10.1063/1.438955. [DOI] [Google Scholar]
- McLean A. D.; Chandler G. S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72 (10), 5639–5648. 10.1063/1.438980. [DOI] [Google Scholar]
- Francl M. M.; Pietro W. J.; Hehre W. J.; Binkley J. S.; Gordon M. S.; DeFrees D. J.; Pople J. A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77 (7), 3654–3665. 10.1063/1.444267. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. J. A.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Stewart W. E.; Siddall T. H. Nuclear magnetic resonance studies of amides. Chem. Rev. 1970, 70 (5), 517–551. 10.1021/cr60267a001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.