Abstract

A series of 25 conjugates has been synthesized to evaluate their antiplasmodial potency and cytotoxicity against the chloroquine resistant (CQR) W2 strain of P. falciparum and Vero kidney cell lines, respectively. Most of the compounds showed IC50 values in the lower nM range and proved to be many fold more active than chloroquine (CQ). The studies were extended to decipher modes of action using techniques including UV–vis absorption, NMR titrations, and mass spectrometry, and conclusions were strengthened by docking and density functional theory (DFT) simulations. The most active compound, with IC50 15 nM and selectivity index >4000, proved to be an interesting template for antimalarial drug discovery. To the best of our knowledge this is the first report of a potent naphthalimide based antiplasmodial conjugate.
Keywords: 4-Aminoquinolines; 1,8-naphthalic anhydride; antiplasmodial; cytotoxicity; hemozoin inhibition; docking simulations
Malaria remains one of the most serious, life threatening infectious diseases. It is caused by Plasmodium species and transmitted by bites of infected female mosquitoes of the Anopheles genus.1 People residing in resource limited areas in Africa, Asia, and Central and South America bear the largest burden of malaria morbidity. The most severe disease is caused by P. falciparum. An estimated 219 million cases of malaria occurred worldwide in 2017 and resulted in almost half a million deaths.2 Past treatment for falciparum malaria included the use of quinine or CQ and mefloquine, which were once very effective antimalarial drugs.3 However, the emergence of CQR strains fuelled the need to introduce new drugs for malaria treatment.4 WHO now recommends artemisinin based combination therapy as first-line treatment for uncomplicated malaria and intravenous artesunate for treating severe malaria.5,6 However, no current first line antimalarials match the favorable efficacy, safety, and affordability once held by CQ.7,8 CQ (a 4-aminoquinoline based scaffold) imparts antimalarial activity by inhibiting hemozoin formation by complexing with free heme, which is released as a byproduct of hemoglobin degradation in the parasite digestive vacuole (DV); the resulting intraparasitic free heme results in parasite killing.9−11 CQ resistance is mediated by drug efflux from the DV due to mutations in the drug transporters PfCRT and PfMDR1.12−14
For the past few years, naphthalimide based moieties have received attention from medicinal chemists. Naphthalimide derivatives, originally developed as therapies for cancer (mitonafide, amonafide, and elinafide),15,16 demonstrate additional properties, including antibacterial, antifungal, antiviral, anti-inflammatory, and antidepressant activities.17 As CQ complexation with heme in P. falciparum primarily occurs via π–π stacking and electrostatic interactions between CQ and heme,9 the combining of naphthalimides with 4-aminoquinolines might improve existing π–π interactions. This concept was supported by the disclosure that new molecules designed with an additional π-system attached to aminoquinolines demonstrated favorable antiplasmodial results. For illustration (Figure 1), Sakata et al. reported that compounds (I) with two π-conjugated moieties at the terminal amino group of a triamine afforded antiplasmodial activity.18 Opsenica et al. showed that thiophene and furan based 4-amino-7-chloroquinolines (II) were potent antimalarials.19 A recent report by Kumar et al. showed the antiplasmodial potential of phthalimide–quinoline conjugates against CQR strains (III).20 Rathi et al. synthesized phthalimide derivatives functionalized with privileged scaffolds targetting parasitic enzymes and demonstrated potential for further development (IV).21 Encouraged by these reports and others,22−24 we anticipated antiplasmodial activity after combining 4-aminoquinolines with naphthalimides. In order to contemplate structure–activity-relationships (SAR) of the designed conjugates, varied spacer lengths were used to bind chosen pharmacophores, such as 4-aminoquinolines and functionalized naphthalic anhydride. Different secondary amines (piperidine, morpholine, hydroxyethyl piperazine) were also incorporated at the C-4 position of the naphthalimide core, with an aim to improve the basicity of the resulting scaffolds to promote concentration within the acidic DV of the parasite.25 Further, heme-binding studies using UV–vis absorption, NMR titrations, mass spectrometry, DFT studies with heme, and molecular docking to target peroxidase and plasmepsin aspartic proteases were performed in order to correlate results with biological data and to support the validity of the hybrid design concept.
Figure 1.
Some previously reported scaffolds with good antiplasmodial potential against CQ resistant strains.
The synthesis of target scaffolds 3 involved the heating of 1,8-naphthalic anhydride, 1 (commercially available), with 4-diaminoquinolines 2, prepared by the reported methodology,26 at 150 °C using anhydrous N-methyl-2-pyrrolidone (NMP) as solvent. After completion of the reaction, the desired compounds were precipitated out on addition of ice cold water to the reaction mixture and were filtered (Scheme-1a). Among the conjugates 3, the one bearing a bromo group at the C-4 position of the naphthalimide core (3f–j) acted as a precursor for the synthesis of the rest of the target scaffolds 4. The heating of 3f–j with various cyclic secondary amines (piperidine, morpholine, and hydroxyethylpiperidine), at 180 °C in NMP as solvent, afforded the target conjugates 4a–o in good yields (Scheme 1b).27 The structures of the target conjugates were elucidated on the basis of spectral data and analytical evidence. For illustration, the conjugate 3b showed a molecular ion peak at m/z 416.1183 [M + H]+ in its HRMS (ESI). Its 1H NMR spectrum showed doublets at δ 6.57 (J = 6.1 Hz) and 8.24 (J = 9.1 Hz), corresponding to quinoline ring protons, and requisite peaks representing the naphthalimide core. Appearance of an absorption peak at δ 164.03, representing a carbonyl carbon, along with desired peaks in aliphatic and aromatic regions in the 13C NMR spectrum of 3b, further corroborated the assigned structure.
Scheme 1. Synthesis of 1,8-Naphthalimide-4-aminoquinoline Conjugates 3 and 4.
The synthesized series of 1,8-naphthalimide-4-aminoquinoline conjugates and their precursors were screened for in vitro activity against the CQR W2 strain of P. falciparum, with CQ as a reference (Table-1). Interestingly, 19 compounds among the synthesized series of 25 conjugates proved to be several fold more potent than CQ, with 12 conjugates exhibiting IC50 values <100 nM. In general, short spacer linked conjugates showed better results, while longer chain lengths proved to be detrimental to activity. The substitutions on the naphthalimide ring did not show remarkable effects, except for the hydroxyethyl piperazine substituted conjugates, that showed significant enhancement in activity. Among the conjugates having an unsubstituted naphthalimide core (R = H), 3a and 3c, with ethyl and butyl spacers, showed good antiplasmodial potency, with IC50 55.9 and 40.7 nM, respectively. On introducing a bromo substitutent at the C-4 position of the naphthalimide core, conjugates with propyl spacer also showed good antiplasmodial activity along with conjugates with ethyl and butyl spacers as evident from 3f, 3g, and 3h, with IC50 values 52.9, 59.7, and 53.2 nM, respectively. Further, on replacing the bromo substituent with piperidine, conjugates with propyl and butyl spacers, such as 4b (IC50 51.2 nM) and 4c (IC50 96.0 nM), showed better activities as compared to conjugates with shorter (n = 2) and longer (n = 6 and n = 8) spacer lengths. However, the inclusion of morpholine improved the activity in conjugates with longer (4i, n = 6, IC50 84.24 nM) or shorter alkyl chain length (4g, n = 3, IC50 66.88 nM). Exceptionally good results were obtained on introducing hydroxyethyl piperazine at the C-4 position of the naphthalimide core. Three conjugates (4k, 4l, and 4m with ethyl, propyl, and butyl as spacers, respectively) proved to be the most active among the synthesized series. Among these, 4k and 4m displayed IC50 values of 41.1 and 20.1 nM, respectively, while 4l, with a propyl chain length as linker, had an IC50 value of 15.4 nM, 15-fold more potent than CQ (IC50 value 232.6 nM) against the W2 strain. The inactivity of the naphthalimide based precursors 1a and 1b against P. falciparum, in contrast to the conjugated compounds, demonstrated the crucial importance of hybridization in the present set of scaffolds.
Table 1. In-Vitro Antimalarial Activity of the Synthesized Compounds along with Cytotoxicity Data against W2 Strain of P. falciparum and Vero Kidney Cell Lines, Respectively.
| S No. | Codes | IC50(nM)a± SDd | IC50(×103)b(nM) | SI | clogPc |
|---|---|---|---|---|---|
| 1 | 3a | 55.99 ± 13.5 | 248.84 | 4444.3 | 4.43 |
| 2 | 3b | 146.56 ± 74.9 | 3.137 | 21.404 | 4.70 |
| 3 | 3c | 40.725 ± 0.6 | 7.585 | 186.24 | 4.97 |
| 4 | 3d | 389.95 ± 75.4 | 218.364 | 559.97 | 5.98 |
| 5 | 3e | 357.5 ± 139.3 | 205.757 | 575.544 | 6.99 |
| 6 | 3f | 52.99 ± 7.2 | 7.794 | 147.084 | 5.19 |
| 7 | 3g | 59.705 ± 12.7 | 10.703 | 179.264 | 5.46 |
| 8 | 3h | 53.29 ± 0.9 | 13.946 | 261.700 | 5.73 |
| 9 | 3i | 161.75 ± 30.9 | 186.271 | 1151.59 | 6.74 |
| 10 | 3j | 562.4 ± 162.9 | 177.276 | 315.21 | 7.75 |
| 11 | 4a | 148.6 ± 33.1 | 126.458 | 850.995 | 5.39 |
| 12 | 4b | 51.2 ± 1.4 | 200.396 | 3913.9 | 5.66 |
| 13 | 4c | 96.08 ± 9.5 | 33.818 | 351.977 | 5.93 |
| 14 | 4d | 129.8 ± 5.2 | 184.812 | 1423.82 | 6.94 |
| 15 | 4e | 437.85 ± 147.4 | 175.666 | 401.20 | 7.95 |
| 16 | 4f | 121.1 ± 0.7 | 26.778 | 221.12 | 4.33 |
| 17 | 4g | 66.88 ± 6.4 | 69.024 | 1032.05 | 4.60 |
| 18 | 4h | 237.47 ± 276.9 | 12.492 | 52.604 | 4.87 |
| 19 | 4i | 84.24 ± 18.7 | 184.141 | 339.08 | 5.88 |
| 20 | 4j | 3968 ± 35.4 | 175.097 | 44.12 | 6.89 |
| 21 | 4k | 41.135 ± 8.1 | 45.224 | 1099.4 | 3.74 |
| 22 | 4l | 15.445 ± 1.7 | 64.755 | 4192.6 | 4.01 |
| 23 | 4m | 20.135 ± 1.1 | 31.913 | 1584.9 | 4.29 |
| 24 | 4n | 120.4 ± 36.5 | 13.109 | 108.87 | 5.29 |
| 25 | 4o | 214.8 ± 78.6 | 171.285 | 797.41 | 6.31 |
| 26 | 1a | >10000 | 504.617 | NDe | ND |
| 27 | 1b | >10000 | 360.919 | ND | ND |
| 28 | CQ | 232.65 ± 76.6 | ND | ND | 4.63 |
Antiplasmodial activity.
Cytotoxicity.
Values calculated from Molinspiration.
SD—Standard deviation.
ND—Not determined.
The synthesized series was then subjected to cytotoxicity evaluation against mammalian Vero kidney cell lines. All tested compounds were well tolerated by the tested cell line, with IC50 > 3000 nM. The most active scaffold 4l, displayed IC50 value 64.7 × 103 nM against Vero cells, far above its activity range (IC50 against P. falciparum 15 nM), Table-1. Selectivity indices (SI) were determined as SI = IC50(toxicity)/IC50(Pf. activity). Most of the compounds showed moderate to good SI, with the most active and noncytotoxic conjugate 4l with SI > 4000.
Malaria parasites degrade hemoglobin to provide amino acids for the growing parasite, and during this process large quantities of free heme (ferriprotoporphyrin-IX or Fp-IX), which is toxic to the parasite, are released. Fp-IX is detoxified within the DV of the parasite by crystallizing it to insoluble nontoxic material called hemozoin.28 4-Aminoquinolines are believed to act by inhibiting the process of hemozoin formation by making a complex with free heme using π–π interactions (between the quinoline ring and the porphyrin ring of heme).29 CQ complexation with FPIX in aqueous medium can be identified by changes in the UV–vis spectrum of aqueous hematin.30−33 On this basis, and in order to understand the primary mode of action of the synthesized hybrids, we conducted UV–vis spectrophotometry studies of the most potent compound, 4l, with monomeric heme. In brief, a solution of hemin in 40% DMSO shows a band at 401 nm corresponding to monomeric heme, and decrease in the intensity of this band after adding a drug is an indicator of drug–heme binding. The titrations of compound 4l with monomeric heme solutions at pH 7.4 (0.02 M HEPES buffer in aqueous DMSO) and pH 5.4 (0.02 M MES buffer in aqueous DMSO), corresponding to physiological pH and that of parasite DV, respectively, showed a significant decrease in the intensity of the band representing monomeric heme (Figure 2 and 3). The solvent (DMSO) used in the experiment had no effect on the association of 4l with heme at either pH (Figure S4, see Supporting Informtion). By analyzing the titration curves using HypSpec, a nonlinear least-squares fitting program, the values of binding constants at both pHs were determined. To compare values with those of the standard drug, CQ, the UV–vis experiment was conducted with CQ and monomeric heme under identical conditions (Figure S2 and S3, see Supporting Information). As evident from Table 2, the binding of 4l with heme was marginally better than that of CQ at both physiological as well as acidic pH.
Figure 2.
(A) Titration of monomeric heme (12 μM) at pH 7.4 (0.02 M HEPES buffer in aqueous DMSO solution) with increasing concentration of 4l (0–18 μM) in 0.02 M HEPES buffer in aqueous DMSO solution. (B) Linear dependence of absorption at 401 nm on heme–4l complex concentration.
Figure 3.
(A) Titration of monomeric heme (12 μM) at pH 5.6 (0.02 M MES buffer in aqueous DMSO solution) with increasing concentration of 4l (0–16 μM) in 0.02 M MES buffer in aqueous DMSO solution. (B) Linear dependence of absorption at 401 nm on heme–4l complex concentration.
Table 2. Binding Constant (log K) for 4l and CQ with Monomeric Heme.
| Entry | pH 5.6 (MES Buffer) | pH 7.4 (HEPES Buffer) |
|---|---|---|
| 4l | 6.06 | 6.33 |
| CQ | 5.18 | 5.10 |
1H NMR titrations and mass spectrometry were performed to offer complementary evidence for binding of 4l with monomeric heme. The 1H NMR spectrum of 4l was recorded after addition of heme (10 and 20 mol %) dissolved in DMSO. Shifting in all the peaks along with their broadening on addition of heme solutions was seen, clearly demonstrating complexation (Figure 4). Mass spectrometry was performed using an equimolar solution of hemin chloride and 4l and showed a molecular ion peak at m/z 1159.3267 Da (Figure 5), further confirming the molecular formula C64H62ClFeN9O7 with 1:1 complexation ratio.
Figure 4.
1H NMR spectral changes observed for 4l after addition of increasing amounts of heme [in DMSO-d6]: (A) 0 mol %, (B) 10 mol % [Δδ for peak: H-2 = 0.017, H-3 = 0.021, H-4 = 0.021, H-7 = 0.021, H-8 = 0.022, H-14 = 0.023, H-15 = 0.017, H-16 = 0.018, H-17= 0.019, H-18 = 0.030 ppm], and (C) 20 mol % [Δδ for peak: H-2 = 0.027, H-3 = 0.034, H-7 = 0.034, H-8 = 0.034, H-14 = 0.037, H-15 = 0.027, H-17 = 0.031 ppm].
Figure 5.
Solution phase HRMS (ESI) spectra of 4l (5 μmol) upon addition of monomeric heme (5 μmol) in aqueous DMSO solution.
The in silico inspection of the nature of interactions of these molecules with peroxidase, plasmepsin II, and plasmepsin IV was carried out. Peroxidase is an enzyme hosting heme as its cofactor. As our experiments showed the complexation of synthesized molecules with heme, the docking simulations targeting peroxidase offer further insights regarding these interactions. Some of the molecules include hydroxyethyl amine, which has been shown to target plasmepsins, a family of P. falciparum aspartic proteases that participate in a variety of cellular processes, including hemoglobin degradation. A study of interactions with peroxidase was carried out twice, first representing true protein–ligand interactions, with all the binding site residues of the protein preserved (whole peroxidase), and second with interactions of the binding site residues of the protein close to the surface of heme deleted (deleted-peroxidase), so that the ligands have free access to the surface of heme. The binding interaction results for the three ligands (3a, 4b, and 4l) against these enzymes are shown in Table S1 (see Supporting Information). The unique orientations of the ligands in their interactions with the three proteins are shown in Figure 6. Ligand 3a has a significantly different orientation from that of 4b and 4l in its interaction with plasmepsin II and plasmepsin IV, but it showed similarity to the interaction with peroxidase, because its three rings were found in the same location as that of 4b and 4l. The ligands 4b and 4l showed similar orientations in their interactions with plasmesin IV as in their interactions with plasmepsin II and peroxidase.
Figure 6.
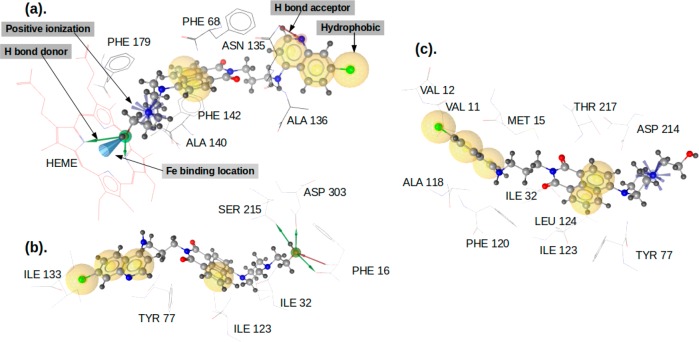
Orientations of molecules 3a (blue), 4b (red), and 4l (green) in their inhibitory interaction with (a) peroxidase, (b) plasmepsin II, and (c) plasmepsin IV.
Among all three ligands, 4l showed the most promising interaction with peroxidase, plasmepsin II, and plasmepsin IV. Also, in terms of interaction with heme in both limited-heme (retaining all the protein catalytic residues) and free-heme (removing the catalytic residues around heme) considerations, molecule 4l showed the best interaction. The order of the predicted inhibitory activities of the ligands in their interaction with the three selected proteins is 4l > 4b > 3a (Table S1, Supporting Information), which is the order of their in vitro antimalarial activities (Table 1). The predicted activities of 4l against the three proteins follow the order of peroxidase > plasmepsin II > plasmepsin IV. The features of the interaction of 4l with the three proteins are shown in Figure 7, while details regarding the type of interactions are in the Supporting Information (under the section of Computational Studies).
Figure 7.
Interaction of 4l with (a) peroxidase, (b) plasmepsin II, and (c) plasmepsin IV showing the interacting residues and the type of interactions.
Further interaction of the best docking pose for the three ligands with the surface of heme was analyzed using quantum methods (DFT). The interactions of the three molecules 3a, 4b, and 4l, with the modeled heme surfaces hemin, hematin, and dihematin (see Figure S1, Supporting Information), were computed twice as ΔEbind and ΔENEDA, and the values are shown in Tables S2 and S3 (Supporting Information), respectively. The results of both ΔEbind and ΔENEDA for the free surface of the heme derivative fail to reproduce the observed experimental order (4l> 4b > 3a), which is an indication that the true interaction of the inhibitor with the heme surface is limited, and the concept of free access to the whole surface of heme, as reported in the literature,18 will result in a false prediction of the interaction energy. Therefore, the interaction energies ΔEbind and ΔENEDA will be discussed with respect to the limited surface, as it is found to give better experimental correlation. The computed interaction energy ΔEbind is limited, as it only reproduces the experimental order of the inhibitory strength of the ligands in their interaction with hemin, but not with hematin and dihematin (Table S2, Supporting Information).
This is an indication that other properties besides the polar and steric features are contributing. Results that agree with the experimental results were obtained when considering the ΔENEDA type of interaction energy (Table S3, Supporting Information) for the interaction of inhibitors with both hemin and hematin (dihematin was not computed because it is computationally expensive with this method).
Thus, the results of the docking interaction of the ligands with peroxidase, plasmepsin II, and plasmepsin IV agreed with the experimental order of the inhibitory activities of 4l> 4b > 3a. Moreover, the interaction energy computed using NEDA analysis suggested that interaction with the heme surface plays a significant role. Also, inhibitory activities of 4l against the three proteins follow the order peroxidase > plasmepsin II > plasmepsin IV due to the different types and number of residue–ligand interactions observed. It is obvious that heme surface interaction is limited in access to any ligand, since heme resides as cofactor in a protein. Thus, any attempt to interact the ligand with the whole surface of heme will result in false interaction energy.
In summary, we have reported a series of functionalized naphthalimide-4-aminoquinolines and evaluated their antiplasmodial activity against the CQ-resistant W2 strain of P. falciparum. The designed hybrids exhibited moderate to high in vitro activities in the nanomolar range. The most potent of the synthesized hybrids displayed ∼15-fold greater activity compared to CQ, with a selectivity index of >4000. Heme binding experiments revealed high affinity for heme, allowing inhibition of hemozoin formation. This class of hybrids also showed high selectivity indices and low toxicity against mammalian cell lines along with favorable predicted bioavailability. The experimental findings along with docking and DFT studies validated our hybrid design concept.
Acknowledgments
We are grateful to Laurent Kremer and Matt D. Johansen (IRIM, France) for cytotoxic studies. Parvesh Singh would like to thank the Centre for High Performance Computing based in Cape Town for access to computational resources.
Glossary
Abbreviations
- CQ
chloroquine
- CQR
chloroquine resistant
- DV
digestive vacuole
- IC50
inhibitory concentration
- SAR
structure activity relationship
- DFT
density functional theory.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00521.
Synthesis and spectral data of the synthesized hybrids (3a–j and 4a–o), materials and methods, computational studies, and scanned copies of 1H and 13C NMR spectra of a few representative compounds, viz. 3c, 3d, 3e, 3i, 3j, 4c, 4e, 4g, 4j, 4l, and 4n. (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Department of Science and Technology (DST), New Delhi, India, under INSPIRE program (Shalini IF160180), has been gratefully acknowledged for a fellowship. VK acknowledges the Science and Engineering Research Board (SERB), New Delhi, for providing financial support [EMR/2015/001687].
The authors declare no competing financial interest.
Supplementary Material
References
- Phillips M. A.; Burrows J. N.; Manyando C.; van Huijsduijen R. H.; Van Voorhis W. C.; Wells T. N. C. Malaria. Nat. Rev. Dis. Primers. 2017, 3 (Article N0.17050), 1–24. 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- World Malaria Report; WHO: Geneva, November 2018.
- Teixeira C.; Vale N.; Ṕerez B.; Gomes A.; Gomes J. R. B.; Gomes P. Recycling classical drugs for malaria. Chem. Rev. 2014, 114 (22), 11164–11220. 10.1021/cr500123g. [DOI] [PubMed] [Google Scholar]
- Corey V. C.; Lukens A. K.; Istvan E. S.; Lee M. C. S.; Franco V.; Magistrado P.; Coburn-Flynn O.; Sakata-Kato T.; Fuchs O.; Gnädig N. F.; Goldgof G.; Linares M.; Gomez-Lorenzo M. G.; De Cózar C.; Lafuente-Monasterio M. J.; Prats S.; Meister S.; Tanaseichuk O.; Wree M.; Zhou Y.; Willis P. A.; Gamo F. J.; Goldberg D. E.; Fidock D. A.; Wirth D. F.; Winzeler E. A. A broad analysis of resistance development in the malaria parasite. Nat. Commun. 2016, 7, 11901–11909. 10.1038/ncomms11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony M. P.; Burrows J. N.; Duparc S.; JMoehrle J.; Wells T. N. The global pipeline of new medicines for the control and elimination of malaria. Malar. J. 2012, 11 (1), 316–341. 10.1186/1475-2875-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T. N. C.; Alonso P. L.; Gutteridge W. E. New medicines to improve control and contribute to the eradication of malaria. Nat. Rev. Drug Discovery 2009, 8 (11), 879–891. 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- Okell L. C.; Cairns M.; Griffin J. T.; Ferguson N. M.; Tarning J.; Jagoe G.; Hugo P.; Baker M.; D’Alessandro U.; Bousema T.; Ubben D.; Ghani A. C. Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat. Commun. 2014, 5 (1), 5606–5616. 10.1038/ncomms6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhi B.; Patyar S.; Rao R. S.; Byrav D. S. P.; Prakash A. Pharmacokinetic and toxicological profile of artemisinin compounds: an update. Pharmacology 2009, 84 (6), 323–332. 10.1159/000252658. [DOI] [PubMed] [Google Scholar]
- Weissbuch I.; Leiserowitz L. Interplay between malaria, crystalline hemozoin formation, and antimalarial drug action and design. Chem. Rev. 2008, 108 (11), 4899–4914. 10.1021/cr078274t. [DOI] [PubMed] [Google Scholar]
- Buller R.; Peterson M. L.; Almarsson Ö.; Leiserowitz L. Quinoline binding site on malaria pigment crystal: A rational pathway for antimalaria drug design. Cryst. Growth Des. 2002, 2 (6), 553–562. 10.1021/cg025550i. [DOI] [Google Scholar]
- Olafson K. N.; Nguyen T. Q.; Rimer J. D.; Vekilov P. G. Antimalarials inhibit hematin crystallization by unique drug–surface site interactions. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (29), 7531–7536. 10.1073/pnas.1700125114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A.; Lehane A. M.; Clain J.; Fidock D. A. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012, 28 (11), 504–514. 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray P. G.; Janneh O.; Ward S. A. Chloroquine uptake and activity is determined by binding to ferriprotoporphyrin IX in Plasmodium falciparum. Novartis Found. Symp. 1999, 226, 252–260. [DOI] [PubMed] [Google Scholar]
- Cooper R. A.; Hartwig C. L.; Ferdig M. T. Pfcrt is more than the Plasmodium falciparum chloroquine resistance gene: A functional and evolutionary perspective. Acta Trop. 2005, 94 (3), 170–180. 10.1016/j.actatropica.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Banerjee S.; Veale E. B.; Phelan C. M.; Murphy S. A.; Tocci G. M.; Gillespie L. J.; Frimannsson D. O.; Kelly J. M.; Gunnlaugsson T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42 (4), 1601–1618. 10.1039/c2cs35467e. [DOI] [PubMed] [Google Scholar]
- Seliga R.; Pilatova M.; Sarissky M.; Viglasky V.; Walko M.; Mojzis J. Novel naphthalimide polyamine derivatives as potential antitumor agents. Mol. Biol. Rep. 2013, 40 (6), 4129–4137. 10.1007/s11033-013-2523-5. [DOI] [PubMed] [Google Scholar]
- Gong H. H.; Addla D.; Lv J. S.; Zhou C. H. Heterocyclic naphthalimides as new skeleton structure of compounds with increasingly expanding relational medicinal applications. Curr. Top. Med. Chem. 2016, 16 (28), 3303–3364. 10.2174/1568026616666160506145943. [DOI] [PubMed] [Google Scholar]
- Sakata Y.; Yabunaka K.; Kobayanshi Y.; Omiya H.; Umezawa N.; Kim H. S.; Wataya Y.; Tomita Y.; Hisamatsu Y.; Kato N.; Yagi H.; Satoh T.; Kato K.; Ishikawa H.; Higuchi T. Potent antimalaria activity of two arenes linked with triamine designed to have multiple interactions with heme. ACS Med. Chem. Lett. 2018, 9 (10), 980–985. 10.1021/acsmedchemlett.8b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsenica I. M.; Verbić T. Ž.; Tot M.; Sciotti R. J.; Pybus B. S.; Djurković-Djoković O.; Slavić K.; Šolaja B. A. Investigation into novel thiophene- and furan-based 4-amino-7-chloroquinolines afforded antimalarials that cure mice. Bioorg. Med. Chem. 2015, 23 (9), 2176–2186. 10.1016/j.bmc.2015.02.061. [DOI] [PubMed] [Google Scholar]
- Rani A.; Singh A.; Gut J.; Rosenthal P. J.; Kumar V. Microwave-promoted facile access to 4-aminoquinoline-phthalimides: Synthesis and anti-plasmodial evaluation. Eur. J. Med. Chem. 2018, 143, 150–156. 10.1016/j.ejmech.2017.11.033. [DOI] [PubMed] [Google Scholar]
- Singh A. K.; Rathore S.; Tang Y.; Goldfarb N. E.; Dunn B. M.; Rajendran V.; Ghosh P. C.; Singh N.; Latha N.; Singh B. K.; Rawat M.; Rathi B. Hydroxyethylamine based phthalimides as new class of plasmepsin hits: Design, synthesis and antimalarial evaluation. PLoS One 2015, 10 (10), e0139347-e0139366 10.1371/journal.pone.0139347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani A.; Legac J.; Rosenthal P. J.; Kumar V. Substituted 1,3-dioxoisoindoline-4-aminoquinolines coupled via amide linkers: Synthesis, antiplasmodial and cytotoxic evaluation. Bioorg. Chem. 2019, 88, 102912–102922. 10.1016/j.bioorg.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Singh A.; Gut J.; Rosenthal P. J.; Kumar V. 4-Aminoquinoline-ferrocenyl-chalcone conjugates: Synthesis and anti-plasmodial evaluation. Eur. J. Med. Chem. 2017, 125, 269–277. 10.1016/j.ejmech.2016.09.044. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Saini A.; Gut J.; Rosenthal P. J.; Raj R.; Kumar V. 4-Aminoquinoline-chalcone/N-acetylpyrazoline conjugates: Synthesis and antiplasmodial evaluation. Eur. J. Med. Chem. 2017, 138, 993–1001. 10.1016/j.ejmech.2017.07.041. [DOI] [PubMed] [Google Scholar]
- Kelly J. X.; Smilkstein M. J.; Brun R.; Wittlin S.; Cooper R. A.; Lane K. D.; Janowsky A.; Johnson R. A.; Dodean R. A.; Winter R.; Hinrichs D. J.; Riscoe M. K. Discovery of dual function acridones as a new antimalarial chemotype. Nature 2009, 459 (7244), 270–273. 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De D.; Krogstad F. M.; Byers L. D.; Krogstad D. J. Structure-activity relationships for antiplasmodial activity among 7-substituted 4-aminoquinolines. J. Med. Chem. 1998, 41 (25), 4918–4926. 10.1021/jm980146x. [DOI] [PubMed] [Google Scholar]
- Shalini; Johansen M. D.; Kremer L.; Kumar V. Variedly connected1,8-naphthalimide-7-chloroquinoline conjugates: Synthesis, anti-mycobacterial and cytotoxic evaluation. Bioorg. Chem. 2019, 92, 103241–103253. 10.1016/j.bioorg.2019.103241. [DOI] [PubMed] [Google Scholar]
- Egan T. J. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J. Inorg. Biochem. 2008, 102 (5–6), 1288–1299. 10.1016/j.jinorgbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Foley M.; Tilley L. Quinoline antimalarials: Mechanisms of action and resistance. Int. J. Parasitol. 1997, 27 (2), 231–240. 10.1016/S0020-7519(96)00152-X. [DOI] [PubMed] [Google Scholar]
- Cohen S. N.; Phifer K. O.; Yielding K. L. Complex formation between chloroquine and ferrihmic acid in vitro and its effect on the antimalarial action of chloroquine. Nature 1964, 202 (4934), 805–806. 10.1038/202805a0. [DOI] [PubMed] [Google Scholar]
- Dascombe M. J.; Drew M. G. B.; Morris H.; Wilairat P.; Auparakkitanon S.; Moule W. A.; Alizadeh-Shekalgourabi S.; Evans P. G.; Lloyd M.; Dyas A. M.; Carr P.; Ismail F. M. D. Mapping antimalarial pharmacophores as a useful tool for the rapid discovery of drugs effective in vivo: Design, construction, characterization, and pharmacology of metaquine. J. Med. Chem. 2005, 48 (17), 5423–5436. 10.1021/jm0408013. [DOI] [PubMed] [Google Scholar]
- Egan T. J.; Mavuso W. W.; Ross D. C.; Marques H. M. Thermodynamic factors controlling the interaction of quinoline antimalarial drugs with ferriprotoporphyrin IX. J. Inorg. Biochem. 1997, 68 (2), 137–145. 10.1016/S0162-0134(97)00086-X. [DOI] [PubMed] [Google Scholar]
- Egan T. J.; Hunter R.; Kaschula C. H.; Marques H. M.; Misplon A.; Walden J. Structure-Function relationships in aminoquinolines: Effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of β-hematin formation, and antiplasmodial activity. J. Med. Chem. 2000, 43 (2), 283–291. 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.