Abstract

The role that physicochemical properties play toward increasing the likelihood of toxicity findings in in vivo studies has been well reported, albeit sometimes with different conclusions. We decided to understand the role that physicochemical properties play toward the prediction of in vivo toxicological outcomes for Takeda chemistry using 284 internal compounds. In support of the previously reported “3/75 rule”, reducing lipophilicity of molecules decreases toxicity odds noticeably; however, we also found that the trend of toxicity odds is different between compounds classified by their ionization state. For basic molecules, the odds of in vivo toxicity outcomes were significantly impacted by both lipophilicity and polar surface area, whereas neutral molecules were impacted less so. Through an analysis of several project-related compounds, we herein demonstrate that the utilization of the 3/75 rule coupled with consideration of ionization state is a rational strategy for medicinal chemistry design of safer drugs.
Keywords: 3/75 rule, in vivo toxicological outcomes prediction, lipophilicity, polar surface area, ionization state
Limiting toxicity-driven attrition of drug candidates is a key challenge for pharmaceutical research and development. Nonclinical toxicology and clinical safety has been one of the largest sources of compound failure from the late 1990s to the present.1,2 The current cost of each new molecular entity has risen to approximately US$1.8 billion, ensuring that late stage drug attrition will have extensive impact on both patients and pharmaceutical companies alike.3 To address these safety issues, many approaches (i.e., in silico toxicity prediction by cheminformatic and bioinformatic strategies, the development of physiologically relevant in vitro toxicity assays, and biomarker utilization, including omics technologies) have been widely applied across the pharmaceutical industry.4 With respect to in silico methodologies, several scoring approaches have been reported that attempt to evaluate the probability of drug success in the CNS therapeutic area5 as well as the success rate of compound stage-gate transition (i.e., from the discovery stage through to clinical testing)6 and the druglikeness of a candidate compound.7 As a representative in silico toxicity prediction approach, Pfizer scientists carried out an analysis of 245 compounds with exploratory toxicology study data and suggested that drug candidates in the chemical space of low ClogP (less than 3) and high TPSA (more than 75) are considerably less likely to cause significant toxicological effects at total plasma concentrations below 10 μM (referred to as the 3/75 rule).8 They showed that compounds with this low-ClogP/high-TPSA profile are approximately 2.5 times more likely to be clean as to be toxic. This broad yet simple rule enables the prioritization of hit/lead compounds based on the prediction of in vivo toxicity occurrence and is therefore informative for medicinal chemistry design. Following this report, AbbVie reported a similar trend when using a total Cmax threshold at 5 μM for their analysis of an internal compound data set of 95 compounds.4 Eli Lilly also analyzed 485 internal compounds, and their result partially supported Pfizer’s analysis in that ClogP correlated with in vivo toxicity outcome whereas TPSA did not.9 When measuring a different compound-related outcome, such as failure to progress to the clinic, the 3/75 rule appears not to be applicable. For example, a study of preclinical vs Phase 1 survival at AstraZeneca identified that lipophilic compounds were more likely to be successful.10−12 In contrast, when four companies (AstraZeneca, Eli Lilly, GlaxoSmithKline, and Pfizer) partnered to evaluate a combined data set of 812 compounds, they showed that ClogP and TPSA did not influence the success rate of preclinical studies.1 Given that different results were observed between the smaller AstraZeneca’s study and the combined study, it is possible that the correlations that were observed for the smaller study are specific to the data set being modeled and may not be applicable to data sets with a structurally broad or larger applicability domain.
In support, Naven and Louise-May suggested that the Pfizer data set was biased toward lipophilic basic molecules.13 Indeed, 73% of compounds within the data set that had values of ClogP > 3 and PSA < 75 were categorized as “basic molecule”. Ionization state is an important feature that influences the behavior of a small molecule in a physiological system, as highlighted by Gleeson where the ionization state was reported to have either a beneficial or a detrimental effect in multiple ADMET (absorption, distribution, metabolism, excretion, and toxicity) profiles.14 As most human tissues are of neutral pH, with the notable exception of the gastric and duodenal environment,15 volume of distribution (Vd) and absorption/clearance pathway of compounds are significantly influenced by their ionization state.16,17 In general, acidic molecules generally have lower Vd values, while basic molecules often have higher Vd values and neutral molecules tend to show low renal clearance.15 Ionization state also influences plasma protein binding (PPB). A study of 1,435 compounds at GSK indicated that acidic compounds tend to have higher PPB than those that are nonacidic, with basic molecules tending to have the lowest.18 Regarding toxicity profiles, basic molecules are known to possess several safety liabilities such as modulation of ion channel activity and lysosomal storage disorder.19 In addition, promiscuity against aminergic GPCRs is also problematic for highly basic amines.10 Ionization state can clearly influence the ADMET profile of drugs and is therefore expected to have significant impact on toxicity outcome. Consequently, we wanted to investigate the correlation between calculated physicochemical properties with in vivo toxicity outcome for each ionization state and to understand if the 3/75 rule is still a rational strategy for all types of compounds. This study endeavored to develop broad principles to guide safer drug design through the ionization-based analysis of 284 Takeda internal compounds and using in vivo outcome at 10 μM as a classification threshold.
Takeda in vivo toxicity studies performed in rats or mouse for which there was corresponding pharmacokinetic exposure data (Cmax) were included in this study. All compounds in the data set showed potent in vitro activities (EC50 or IC50 < 100 nM) against therapeutic target. Compounds that were administered orally and with a study duration from 3 to 28 days were selected for analysis. No distinction was made regarding the sex of the animals evaluated in these studies. Physicochemical properties of all compounds were calculated.20,21 For simplicity, we categorized compounds into 3 groups (highly lipophilic: ClogP > 3, TPSA < 75; nonlipophilic: ClogP < 3, TPSA > 75; and others). The data set consisted of 41 acidic, 112 basic, 120 neutral, and 11 zwitterionic compounds. Compounds were classified as “Tox” if any test article-related adverse events, as judged by an experienced toxicologist or pathologist, were observed below 10 μM Cmax (total) and “Clean” otherwise.8 A Toxicity odds was calculated as the number of “Tox” compounds divided by the number of “Clean” compounds. Similarly, a separate analysis classified compounds based on free concentration as “Clean” or “Tox” with respect to findings at the threshold of 1 μM Cmax (free). The number of compounds which was used for free concertation analysis was smaller due to lack of historical data on PPB for 30% of the compounds.
The profile of the Takeda data set with respect to calculated physicochemical properties is shown in Figure 1 along with the profile for the Pfizer data set for comparison. 29% of Takeda compounds are categorized nonlipophilic (Pfizer: 22%), and 21% are highly lipophilic (Pfizer: 41%) with relatively balanced coverage of ionization state. Target class is also diverse within this data set, including G protein-coupled receptors (GPCRs), nuclear hormone receptors (NHRs), enzymes, ion channels, kinases, chemokine, and transporters. GPCRs represented the most populous target class (29% of the compounds), with enzymes (27%), kinases (19%), and NHRs (11%) being the next most frequent (data not shown).
Figure 1.
Physicochemical property profile of Takeda’s and Pfizer’s data set.
First, we assessed whether the 3/75 rule is applicable to the Takeda internal compound data set without ionization state categorization. Similar with other investigations, the 3/75 rule is also applicable to the Takeda data set (Table 1a). Both ClogP and TPSA are correlated with toxicity odds with a value of 2.8-fold difference between nonlipophilic and high-lipophilic space. When compared with Pfizer’s result, the toxicity odds of compounds in the nonlipophilic category were somewhat higher (Pfizer: 0.39, Takeda: 0.61). We looked further into the data set and found that a large number of “Tox” compounds in nonlipophilic space were ATP-competitive kinase inhibitors. This finding is not surprising given that kinase inhibitors generally have lower ClogP and higher TPSA values owing to the necessity for hydrogen bond interaction(s) with hinge binding residues as well as other polar interactions. In fact, approximately half of the kinase inhibitors in our data set (18/41) are in this area of low lipophilicity and tend to show significant toxicity at lower concentrations, and this is likely due to promiscuity within the gene family. The toxicity odds for nonlipophilic compounds are significantly reduced by removing kinase inhibitors from the analysis, affording a 5.8-fold toxicity odds difference (Table 1b). We also demonstrated that the 3/75 rule is applicable for the prediction of significant in vivo findings at 1 μM Cmax (free), affording 10- to 20-fold toxicity odds difference between high-lipophilic or nonlipophilic compounds (Supporting Information Table 1).
Table 1. Toxicity Odds Associated with the Takeda Internal Dataset: (a) 10 μM Total Concentration Threshold of All Data Set; (b) 10 μM Total Concentration Threshold of Kinase Inhibitors Excluded Data Set.
| (a) Cmax (Total) analysis |
(b) Cmax (Total) analysisa |
||||
|---|---|---|---|---|---|
| Toxicity Odds at 10 μM | TPSA ≥ 75 | TPSA < 75 | Toxicity Odds at 10 μMa | TPSA ≥ 75 | TPSA < 75 |
| ClogP < 3 | 0.61 (66) | 0.75 (28) | ClogP < 3 | 0.26 (48) | 0.63 (26) |
| ClogP ≥ 3 | 1.16 (93) | 1.72 (49) | ClogP ≥ 3 | 1.03 (81) | 1.50 (40) |
Kinase inhibitors are excluded.
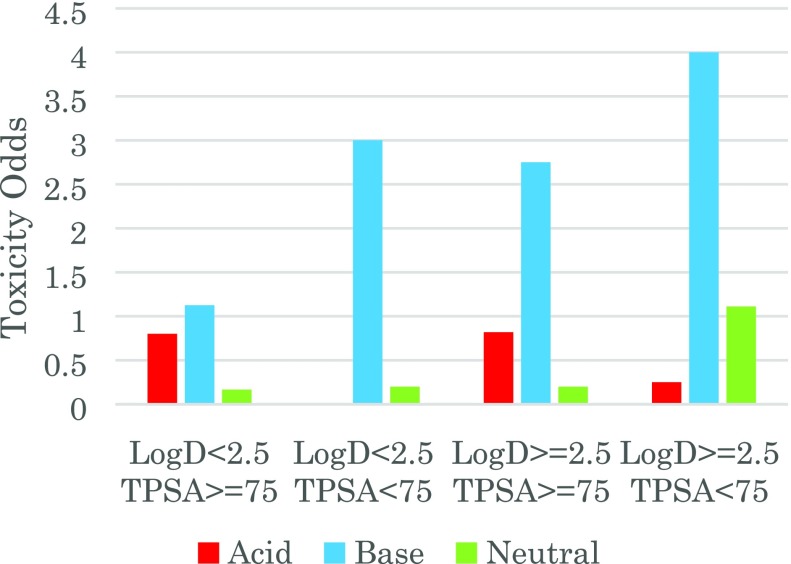
Following this analysis, we categorized compounds by their ionization state and investigated how ionization state influences the outcome prediction. The zwitterion category was excluded owing to the low number of molecules in the database. As shown in Figure 2 and Supporting Information Table 2, the correlation of toxicity odds with ClogP and TPSA had a different trend for each ionization state. For basic molecules, both increasing ClogP and decreasing TPSA contributed to the increase in toxicity odds. Basic compounds with high lipophilicity have a 20-fold toxicity occurrence risk when compared to nonlipophilic compounds at the 10 μM threshold. Regarding neutral molecules, highly lipophilic compounds have the highest toxicity odds but considerably less than basic molecules. Acidic molecules have no correlation between ClogP/TPSA with toxicity odds, although the number within the data set is limited.
Figure 2.
Toxicity odds based on ClogP/TPSA and ionization state category.
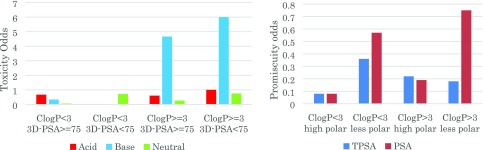
TPSA is simply calculated on the summation of tabulated surface contributions of 2D polar fragments without any consideration of molecular conformation.22 For example, the TPSA calculation does not take into account polar surface area arising from electronegative atoms, such as a halogen atom, or intramolecular hydrogen bonding.23 As the correlation of polar surface area with in vivo toxicity outcome has been proposed to be derived from molecular promiscuity,8 we hypothesized that a value for molecular surface area that has been derived from the van der Waals surface of a 3D molecular conformation (3D-PSA) could give a better prediction of toxicity outcome. We calculated the 3D-PSA value (QikProp v5.0, Schrödinger) of the data set by generation of 3D conformations (LigPrep, v40015, Schrödinger) and selection of the lowest energy conformations as the stable one for each compound. A value of 75 was set as the equivalent threshold for 3D-PSA.24 When multiple stable conformations were obtained, 3D-PSA values were averaged. As shown in Table 2, utilization of 3D-PSA as a polar surface area descriptor gave a similar odds-profile to TPSA, but with better separation of clean/tox compounds of data set than TPSA. Using this descriptor, compounds with high lipophilicity have over 7.2-fold toxicity risk compared to those with nonlipophilic area. For those compounds with ClogP < 3, there was a 5.4-fold toxicity odds for compounds with 3D-PSA < 75. This analysis clearly suggests that the 3/75 rule, with consideration of 3D conformation of molecules, is a better predictor of toxicity findings at 10 μM or below. We also confirmed that ionization-based analysis using 3D-PSA works well for basic molecules (Figure 3a and Supporting Information Table 3). Owing to the correlation of both these factors with in vivo toxicity, we also investigated the promiscuity of compounds in each physicochemical property category with respect to activity in various secondary pharmacology panels. Binding affinity in 70–80 secondary pharmacology assays was identified for 67 compounds from the Takeda data set. A hit was defined as >50% inhibition at 10 μM, and compounds that have >5% hit targets (i.e., more than 4 hits) were classified as promiscuous. Although the number of data points is relatively small, we found 3D-PSA is better correlated with promiscuity odds, particularly in high lipophilic physicochemical space (Figure 3b).
Table 2. Toxicity Odds Using 3D-PSA with ClogP (10 μM Cmax (Total) Threshold)a.
| Toxicity Odds at 10 μM | 3D-PSA ≥ 75 | 3D-PSA < 75 |
|---|---|---|
| ClogP < 3 | 0.24 (57) | 1.29 (16) |
| ClogP ≥ 3 | 1.02 (91) | 1.73 (30) |
Kinase inhibitors are excluded.
Figure 3.
(a) Toxicity odds based on ClogP/3D-PSA and ionization state category. (b) Promiscuity odds difference between descriptors of polar surface area.
Calculated ClogP values are commonly used, as opposed to measured values, in analyses describing the influence of lipophilicity on drug properties.25 A potential disadvantage of using ClogP is that there is a reasonable degree of error between ClogP with measured logD values. For example, AstraZeneca reported that the commercially available ClogP algorithm predicts a subset of neutral compounds measured in the logD assay with an average error of 0.87 (n = 4,564).25 Similarly, there is an average error of 1.46 (n = 276) between ClogP with measured logD in our data set. The difference could be derived from multiple factors, such as differences between 2D and 3D structure and/or the contribution of intramolecular hydrogen bonding. In this context, the analyses were performed using measured logD value, and using the median value in our data set of 2.5 as the threshold.26,27 As shown in Table 3, the toxicity odds difference is small among each physicochemical property category, although the actual trend is similar when using ClogP. With respect to each ionization state, either or both measured logD and TPSA contributed to the toxicity occurrence for the basic molecule, but both factors were needed for neutral molecules (Figure 4 and Supporting Information Table 4).
Table 3. Toxicity Odds Using Measured logD with TPSA (10 μM Cmax (Total) Threshold)a.
| Toxicity Odds at 10 μM | TPSA ≥ 75 | TPSA < 75 |
|---|---|---|
| LogD < 2.5 | 0.67 (60) | 0.93 (29) |
| LogD ≥ 2.5 | 0.73 (64) | 1.27 (34) |
Kinase inhibitors are excluded.
Figure 4.
Toxicity odds based on measured logD/TPSA and ionization state category.
As discussed above, the influence of the 3/75 rule is different between the ionization states of compounds. In particular, the rule is best applied to basic compounds, and a weaker trend is observed for neutral compounds. It is important to determine if these trends are applicable at the local project level, i.e. in the same chemical series related to the same therapeutic target. This is an important question to ask, because at the level of the project or chemotype, there may be other factors specific to that project which may influence the toxicity profile more. A clear example here is for the family of kinases, which by nature of their pharmacology in cell survival and homeostasis tend to possess higher toxicity odds at lower exposure levels than nonkinase targets. Toxicity in this group, may be more influenced by kinase selectivity, rather than physicochemical properties.
To understand the local applicability of this rule, we conducted a study based the on the mechanism of action (MOA) of compounds within the data set. In our data set, 34 projects had only 1 compound tested in vivo and so could not be used in the analysis. Of the remaining 31 projects, 16 projects contained only clean or only toxic compounds at 10 μM and 15 had more than 2 in vivo tested neutral or basic compounds with “Clean” or “Tox” at 10 μM. The resulting analysis is shown in Figure 5. Generally, the trend of toxicity odds obtained from our analysis rule works well for each chemical space; that is, nonlipophilic neutral molecules have low toxicity odds. There are exceptions, however, like MOA-6, which only has 1 clean compound with high lipophilicity and basic ionization state. These general findings indicate that the modulation of lipophilicity and basicity gives an impact for toxicity odds even within the same pharmacology. Combination with in vitro predictive tox assays or biodistribution investigation may give more precise results.
Figure 5.
Local applicability analysis of the 3/75 rule. MOA: mechanism of action. Blue: “Clean”, Red: “Tox”, the number in the circle means the compounds number.
For Takeda and other pharmaceutical companies, toxicity-driven drug attrition is one of the largest factors to hinder the delivery of novel medicines to the patient. Based on an internal analysis of discontinued compounds from 2002 to 2016, more than 50% of preclinical termination at Takeda was due to toxicity (data not published), while termination due to pharmacokinetics contributed approximately 5%. Among those terminated for safety reasons, around 80% were considered as compound-related or of unknown toxicity (i.e., not related to the pharmacological target). These findings emphasize the importance of early in silico/in vitro screening to identify chemotype-related safety liabilities in additional to a target-oriented derisking strategy. An in silico predictive toxicity model would be advantageous in early drug discovery as it can be applied before compound synthesis and therefore contribute to the reduction in the cost and cycle times. Herein, 284 preclinical compounds that were tested in vivo were investigated, and it was found that lipophilicity (as measured by both ClogP and measured logD) as well as polar surface area (as measured by TPSA and 3D-PSA) are correlated with toxicity occurrence outcome. We selected the threshold of 10 μM Cmax (total) as the definitive criteria of whether a compound is classified as “Clean” or “Tox”. Our analysis supported the 3/75 rule originally reported by Pfizer and subsequently by Eli Lilly and AbbVie. This support is strengthened by the knowledge that a patent analysis of Takeda’s compound library indicated that it has a different chemical space, as defined by various physicochemical and structural properties, when compared to those of Pfizer and Eli Lilly.28 The utility of the 3/75 rule is thus supported by this expansion of the applicability domain into Takeda’s chemical space. We also tried a best split of “Clean” and “Tox” compounds within the ranges of 2.5–4.5 of ClogP (median is 3.52) and 70–90 of TPSA (median is 83.7). This investigation afforded the combination of 3.07 of ClogP and 74.2 of TPSA is best split with 6.4-fold toxicity odds difference between nonlipophilic (toxicity odds: 0.25) and high-lipophilic area (toxicity odds: 1.6), indicating the odds ratio is similar to when “3/75 rule” is applied to our data set. In addition, by investigating the impact of ionization state, we found that toxicity odds were different among acidic, basic, and neutral molecules. In brief, the 3/75 rule works well for reducing the in vivo toxicity odds for basic molecules, i.e. increasing ClogP and reducing TPSA are risk factors for toxicity outcomes. Our studies also suggest that basic molecules tend to have higher toxicity odds than acidic and neutral molecules, with the exception for the low lipophilic space where acidic compounds have the highest, albeit low, toxicity odds. This result is consistent with several publications that have also demonstrated a link between the promiscuity of basic molecules in in vitro screening panels and their in vivo toxicity profile.29−33 Regarding the neutral state, only highly lipophilic molecules have slightly higher risk than others.
We also investigated the utility of 3D PSA and measured logD values, which are often considered as more accurate descriptors of polar surface area and lipophilicity.34,35 As we expected, the use of the van der Waals surface-based polarity descriptor (3D-PSA) improved toxicity odds with or without combination with ClogP. This result is also supported by the comparison with molecular promiscuity, which shows that 3D-PSA is a better predictor of molecular promiscuity than TPSA (Figure 3b). On the other hand, when ClogP is replaced with measured logD, the odds difference of each property became small. This analysis was surprising to us, because measured logD values are often considered as more accurate indicators of molecule lipophilicity.35 Despite the lower toxicity odds, the toxicity occurrence analysis with measured logD values also gave the same trend with ClogP when the compounds were categorized based on ionization states.
In vivo systems are very complex, and numerous factors may contribute to the observation of toxicity, including physicochemical properties, on/off target effects, metabolism, and accumulation of drug and/or metabolites. Consequently, it is unlikely that the 3/75 rule will accurately predict the study outcome before in vivo exploratory toxicity study. In this context, improving toxicity odds prediction at the drug design phase would be a beneficial approach to reduce compound attrition. This study has shown that the influence of lipophilicity toward the prediction of in vivo toxicity is different for each ionization state, and the 3/75 rule works best for reducing the risk of basic molecules. As shown by our local, project-related compounds analysis, this observed trend was applicable for structurally related compounds within the same pharmacology space. These results provide support toward implementing the 3/75 rule as a useful “rule of thumb” for the design of safer drugs.
In addition, given the toxicity odds difference observed among the ionization states, it could be suggested that the difference in toxicity outcome profile between pharmaceutical companies may be due to the relative difference in compound ionization in their test sets. This highlights the importance of carrying out a deeper analysis that aims to understand the chemical space where in silico models may or may not be predictive. Such an analysis provides a better understanding as to where to apply predictive models and where there are knowledge gaps that need to be addressed. In the design of compounds, our analysis suggests that selecting a neutral chemotype or nonlipophilic basic compounds would be a rational strategy from a preclinical safety perspective.
This study is a critical analysis to understand the applicability domain of the 3/75 rule and support its application as a “rule of thumb” for medicinal chemistry design.
Acknowledgments
We thank Kazuko Yonemori and Hideto Hara for helpful discussions and Heather Estrella for scientific review. We express our appreciation to Yvonne Dragan for her encouragement and support.
Glossary
Abbreviations
- TPSA
topological polar surface area
- PSA
polar surface area
- ClogP
calculated log P
- log P
lipophilicity
- PPB
plasma protein binding
- Vd
volume of distribution
- GPCRs
G protein-coupled receptors
- NHRs
nuclear hormone receptors
- MOA
mechanism of action
- CNS
central nervous system
- ATP
adenosine triphosphate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00536.
Toxicity odds associated with the Takeda internal data set by the threshold of 1 μM free concentration (Supplemental Table 1). The compound number of each ionization state with physicochemical properties: ClogP vs TPSA (Supplemental Table 2), ClogP vs 3D-PSA (Supplemental Table 3), and measured LogD vs TPSA (Supplemental Table 4). Experimental methods: Calculation methods of ClogP, TPSA, 3D-PSA, and pKa. Definition of ionization states. Experimental method of measured LogD. (PDF)
Internal data set table with ionization state, in vivo tox categorization, ClogP, measured LogD, TPSA, 3D-PSA, MOA categorization, and target class of our compounds. (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Waring M. J.; Arrowsmith J.; Leach A. R.; Leeson P. D.; Mandrell S.; Owen R. M.; Pairaudeau G.; Pennie W. D.; Pickett S. D.; Wang J.; Wallace O.; Weir A. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discovery 2015, 14 (7), 475–86. 10.1038/nrd4609. [DOI] [PubMed] [Google Scholar]
- Kola I.; Landis J. Can the pharmaceutical industry reduce attrition rates?. Nat. Rev. Drug Discovery 2004, 3 (8), 711–5. 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Paul S. M.; Mytelka D. S.; Dunwiddie C. T.; Persinger C. C.; Munos B. H.; Lindborg S. R.; Schacht A. L. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discovery 2010, 9 (3), 203–14. 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Blomme E. A.; Will Y. Toxicology Strategies for Drug Discovery: Present and Future. Chem. Res. Toxicol. 2016, 29 (4), 473–504. 10.1021/acs.chemrestox.5b00407. [DOI] [PubMed] [Google Scholar]
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1 (6), 435–49. 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52 (21), 6752–6. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Bickerton G. R.; Paolini G. V.; Besnard J.; Muresan S.; Hopkins A. L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4 (2), 90–8. 10.1038/nchem.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. D.; Blagg J.; Price D. A.; Bailey S.; Decrescenzo G. A.; Devraj R. V.; Ellsworth E.; Fobian Y. M.; Gibbs M. E.; Gilles R. W.; Greene N.; Huang E.; Krieger-Burke T.; Loesel J.; Wager T.; Whiteley L.; Zhang Y. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18 (17), 4872–5. 10.1016/j.bmcl.2008.07.071. [DOI] [PubMed] [Google Scholar]
- Sutherland J. J.; Raymond J. W.; Stevens J. L.; Baker T. K.; Watson D. E. Relating molecular properties and in vitro assay results to in vivo drug disposition and toxicity outcomes. J. Med. Chem. 2012, 55 (14), 6455–66. 10.1021/jm300684u. [DOI] [PubMed] [Google Scholar]
- Bowes J.; Brown A. J.; Hamon J.; Jarolimek W.; Sridhar A.; Waldron G.; Whitebread S. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat. Rev. Drug Discovery 2012, 11 (12), 909–22. 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- Luker T.; Alcaraz L.; Chohan K. K.; Blomberg N.; Brown D. S.; Butlin R. J.; Elebring T.; Griffin A. M.; Guile S.; St-Gallay S.; Swahn B. M.; Swallow S.; Waring M. J.; Wenlock M. C.; Leeson P. D. Strategies to improve in vivo toxicology outcomes for basic candidate drug molecules. Bioorg. Med. Chem. Lett. 2011, 21 (19), 5673–9. 10.1016/j.bmcl.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Muthas D.; Boyer S.; Hasselgren C. A critical assessment of modeling safety-related drug attrition. MedChemComm 2013, 4 (7), 1051–130. 10.1039/c3md00072a. [DOI] [Google Scholar]
- Naven R. T.; Louise-May S. Computational toxicology: Its essential role in reducing drug attrition. Hum. Exp. Toxicol. 2015, 34 (12), 1304–9. 10.1177/0960327115605440. [DOI] [PubMed] [Google Scholar]
- Gleeson M. P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51 (4), 817–34. 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- Charifson P. S.; Walters W. P. Acidic and basic drugs in medicinal chemistry: a perspective. J. Med. Chem. 2014, 57 (23), 9701–17. 10.1021/jm501000a. [DOI] [PubMed] [Google Scholar]
- Smith D. A.; Beaumont K.; Maurer T. S.; Di L. Volume of Distribution in Drug Design. J. Med. Chem. 2015, 58 (15), 5691–8. 10.1021/acs.jmedchem.5b00201. [DOI] [PubMed] [Google Scholar]
- Varma M. V.; Feng B.; Obach R. S.; Troutman M. D.; Chupka J.; Miller H. R.; El-Kattan A. Physicochemical determinants of human renal clearance. J. Med. Chem. 2009, 52 (15), 4844–52. 10.1021/jm900403j. [DOI] [PubMed] [Google Scholar]
- Gleeson M. P. Plasma protein binding affinity and its relationship to molecular structure: an in-silico analysis. J. Med. Chem. 2007, 50 (1), 101–12. 10.1021/jm060981b. [DOI] [PubMed] [Google Scholar]
- Price D. A.; Blagg J.; Jones L.; Greene N.; Wager T. Physicochemical drug properties associated with in vivo toxicological outcomes: a review. Expert Opin. Drug Metab. Toxicol. 2009, 5 (8), 921–31. 10.1517/17425250903042318. [DOI] [PubMed] [Google Scholar]
- The ClogP and TPSA were calculated using Daylight version 4.95 (Daylight Chemical Information Systems, Inc., Laguna Niguel, CA, USA: ). [Google Scholar]
- The pKa was calculated with ChemAxon 5.9. Acid/base classification was performed as described previously. The threshold was set at pKa = 7.
- Caron G.; Ermondi G. Molecular descriptors for polarity: the need of going beyond polar surface area. Future Med. Chem. 2016, 8 (17), 2013–6. 10.4155/fmc-2016-0165. [DOI] [PubMed] [Google Scholar]
- Kuhn B.; Mohr P.; Stahl M. Intramolecular hydrogen bonding in medicinal chemistry. J. Med. Chem. 2010, 53 (6), 2601–11. 10.1021/jm100087s. [DOI] [PubMed] [Google Scholar]
- Ertl P.; Rohde B.; Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43 (20), 3714–7. 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Lipophilicity in drug discovery. Expert Opin. Drug Discovery 2010, 5 (3), 235–48. 10.1517/17460441003605098. [DOI] [PubMed] [Google Scholar]
- Katsuhiko Y.; Yukihiro I. Kinetic solubility and lipophilicity evaluation connecting formulationtechnology strategy perspective. J. Drug Delivery Sci. Technol. 2016, 33, 13–18. 10.1016/j.jddst.2016.03.002. [DOI] [Google Scholar]
- Log D7.4, which is the partition coefficient of the compounds between 1-octanol and aqueous buffer at pH 7.4, was measured using a chromatographic procedure. The instruments utilized were a Waters Alliance 2795 HPLC system and a 2996 UV–vis detector (Milford, MA, USA).
- Leeson P. D.; St-Gallay S. A. The influence of the ’organizational factor’ on compound quality in drug discovery. Nat. Rev. Drug Discovery 2011, 10 (10), 749–65. 10.1038/nrd3552. [DOI] [PubMed] [Google Scholar]
- Lee E. C.; Steeno G.; Wassermann A. M.; Zhang L.; Shah F.; Price D. A. Amine promiscuity and toxicology analysis. Bioorg. Med. Chem. Lett. 2017, 27 (3), 653–657. 10.1016/j.bmcl.2016.11.085. [DOI] [PubMed] [Google Scholar]
- Lounkine E.; Keiser M. J.; Whitebread S.; Mikhailov D.; Hamon J.; Jenkins J. L.; Lavan P.; Weber E.; Doak A. K.; Cote S.; Shoichet B. K.; Urban L. Large-scale prediction and testing of drug activity on side-effect targets. Nature 2012, 486 (7403), 361–7. 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. U.; Hert J.; Bissantz C.; Hillebrecht A.; Gerebtzoff G.; Bendels S.; Tillier F.; Migeon J.; Fischer H.; Guba W.; Kansy M. Can we discover pharmacological promiscuity early in the drug discovery process?. Drug Discovery Today 2012, 17 (7–8), 325–35. 10.1016/j.drudis.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Leeson P. D.; Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discovery 2007, 6 (11), 881–90. 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- Sameshima T.; Yukawa T.; Hirozane Y.; Yoshikawa M.; Katoh T.; Hara H.; Yogo T.; Miyahisa I.; Okuda T.; Miyamoto M.; Naven R. Small-Scale Panel Comprising Diverse Gene Family Targets To Evaluate Compound Promiscuity. Chem. Res. Toxicol. 2020, 33 (1), 154–61. 10.1021/acs.chemrestox.9b00128. [DOI] [PubMed] [Google Scholar]
- Prasanna S.; Doerksen R. J. Topological polar surface area: a useful descriptor in 2D-QSAR. Curr. Med. Chem. 2009, 16 (1), 21–41. 10.2174/092986709787002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott J. A.; Planey S. L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discovery 2012, 7 (10), 863–75. 10.1517/17460441.2012.714363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.