Abstract
Tau aggregate, which is the main component of the neurofibrillary tangle, is an attractive imaging target for diagnosing and monitoring the progression of Alzheimer’s disease (AD). In this study, we designed and synthesized six radioiodinated 6,5,6-tricyclic compounds to explore novel scaffolds for tau imaging probes. On in vitro autoradiography of AD brain sections, pyridoimidazopyridine and benzimidazopyrimidine derivatives ([125I]21 and [125I]22, respectively) showed selective binding affinity for tau aggregates, whereas carbazole, pyrrolodipyridine, and pyridoimidazopyrimidine derivatives ([125I]10, [125I]12, and [125I]23, respectively) bound β-amyloid aggregates. In a biodistribution study using normal mice, [125I]21 and [125I]22 showed high initial uptakes (5.73 and 5.66% ID/g, respectively, at 2 min postinjection) into and rapid washout (0.14 and 0.10% ID/g, respectively, at 60 min postinjection) from the brain. Taken together, two novel scaffolds including pyridoimidazopyridine and benzimidazopyrimidine may be applied to develop useful tau imaging probes.
Keywords: Tau imaging probe, Alzheimer’s disease, single photon emission computed tomography, tricyclic compound
Senile plaques and neurofibrillary tangles, which are mainly composed of β-amyloid (Aβ) aggregates and hyperphosphorylated tau protein, are the key pathological features of the Alzheimer’s disease (AD) brain.1,2 Since the deposition of senile plaques is considered to be the initial and a specific event in AD,3,4 many positron emission tomography (PET) and single photon emission computed tomography (SPECT) probes targeting Aβ aggregates have been developed for the diagnosis of AD.5,6 The accumulation of neurofibrillary tangles is closely associated with cognitive impairment of AD and is found in some other neurodegenerative diseases including progressive supranuclear palsy, corticobasal degeneration, and pick disease.7 Therefore, various PET and SPECT probes targeting tau aggregates have been developed.8−10 Among them, some PET probes such as [18F]AV-1451 (flortaucipir or T807), [18F]MK-6240, [18F]RO-948, [18F]PI-2620, and [18F]GTP1 have been tested clinically, and their feasibility has been demonstrated for imaging tau aggregates in AD brains.10−15 On the other hand, no SPECT probes have been tested clinically.
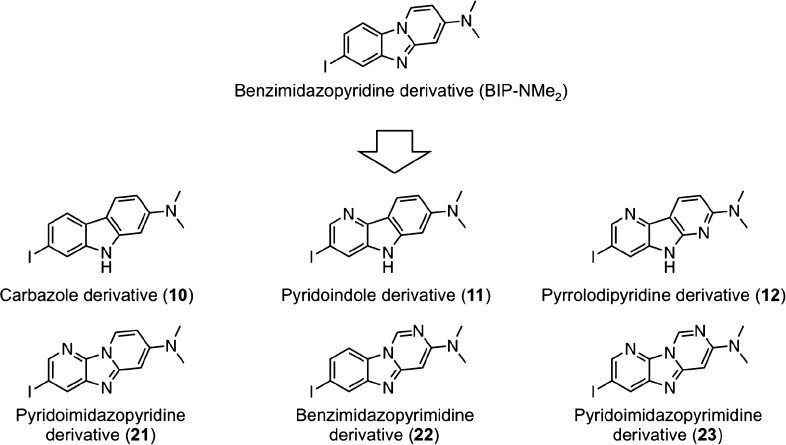
We recently developed benzimidazopyridine (BIP) derivatives (Figure 1) for the detection of tau aggregates in the brain.16−18 Almost all BIP derivatives displayed in vitro selective binding affinity for tau against Aβ aggregates in an autoradiographic study using AD brain sections. Among them, [125I]7-iodo-3-dimethylaminopyrido[1,2-α]benzimidazole ([125I]BIP-NMe2) showed the most favorable selective binding affinity for tau aggregates.
Figure 1.
Design strategy for this work.
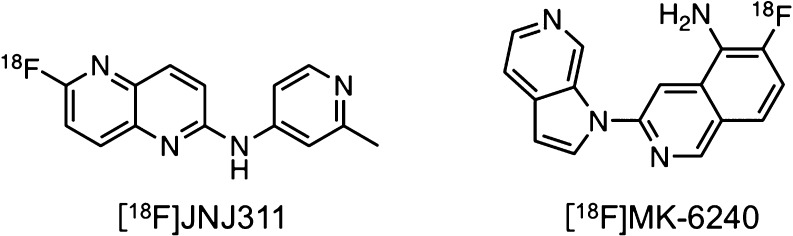
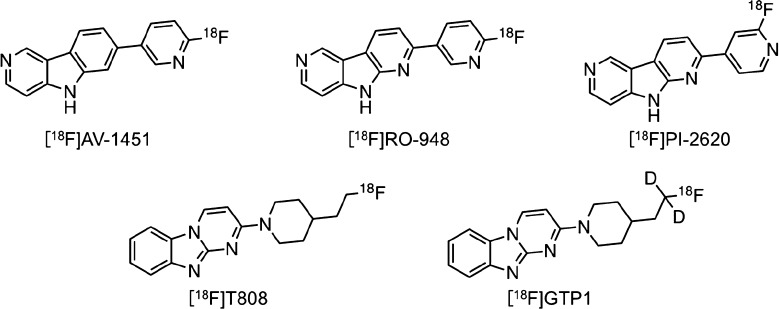
Recently, structure–activity relationship (SAR) studies to explore novel backbones of tau imaging probes have been reported.19−24 The results of SAR studies on the development of [18F]JNJ311 and [18F]MK-6240 (Figure 2) revealed that the position and number of nitrogen atoms affected the affinity for tau and Aβ aggregates.20,22,24 In addition, as well as [125I]BIP-NMe2, several PET probes, including [18F]AV-1451, [18F]RO-948, [18F]PI-2620, [18F]T808, and [18F]GTP1 (Figure 3), contain a 6,5,6-tricycle scaffold. These findings encouraged us to plan an SAR study of 6,5,6-tricyclic compounds based on BIP-NMe2 for developing novel tau imaging probes. In this study, we newly designed and synthesized six radioiodinated 6,5,6-tricyclic compounds, carbazole, pyridoindole, pyrrolodipyridine, pyridoimidazopyridine, benzimidazopyrimidine, and pyridoimidazopyrimidine with a dimethylamino group (Figure 1), and evaluated the affinity for tau and Aβ aggregates and in vivo pharmacokinetics in the murine brain.
Figure 2.
Chemical structures of [18F]JNJ311 and [18F]MK-6240.
Figure 3.
Chemical structures of tau imaging probes based on 6,5,6-tricyclic scaffold.
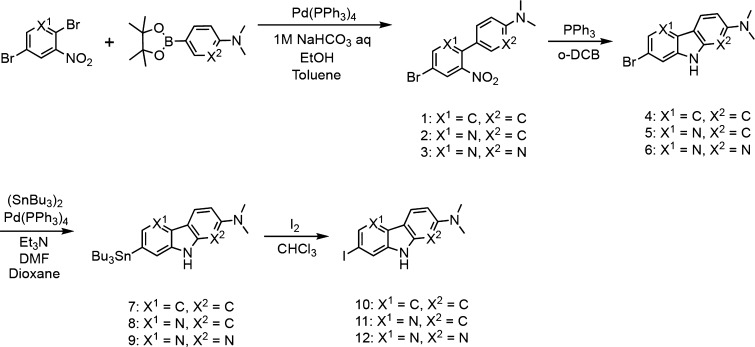
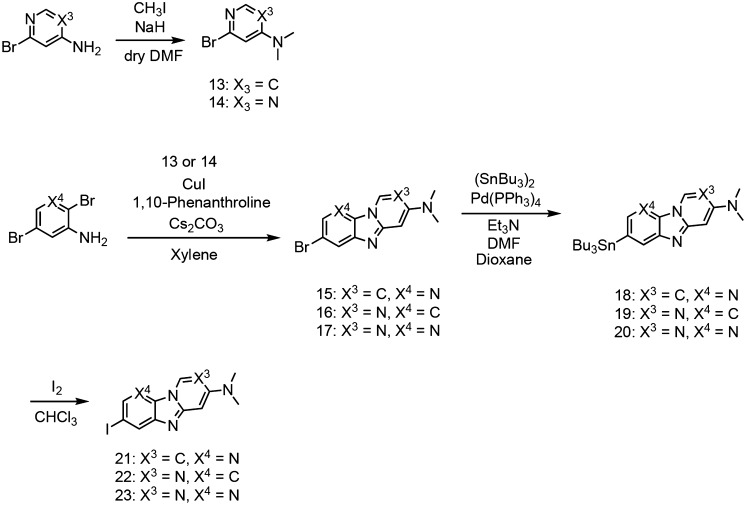
We synthesized 6,5,6-tricyclic compounds according to Schemes 1 and 2. The carbazole, pyridoindole, and pyrrolodipyridine scaffolds were prepared by reaction with 1, 2, or 3 in the presence of triphenylphosphine according to a method reported previously to yield bromo compounds (4, 5, and 6).25 The pyridoimidazopyridine, benzimidazopyrimidine, and pyridoimidazopyrimidine scaffolds were prepared by reaction with 13 or 14 in the presence of 1,10-phenanthroline and cesium carbonate, according to a method reported previously.26 Then, the tributyltin derivatives (7, 8, 9, 18, 19, and 20) were prepared from the corresponding bromo compounds (4, 5, 6, 15, 16, and 17) using a bromo to tributyltin exchange reaction catalyzed by Pd(0). These tributyltin derivatives were reacted with I2 in chloroform at room temperature to give iodo compounds (10, 11, 12, 21, 22, and 23).
Scheme 1. Synthetic Route of Carbazole, Pyridoindole, and Pyrrolodipyridine Derivatives.
Scheme 2. Synthetic Route of Pyridoimidazopyridine, Benzimidazopyrimidine, and Pyridoimidazopyrimidine Derivatives.
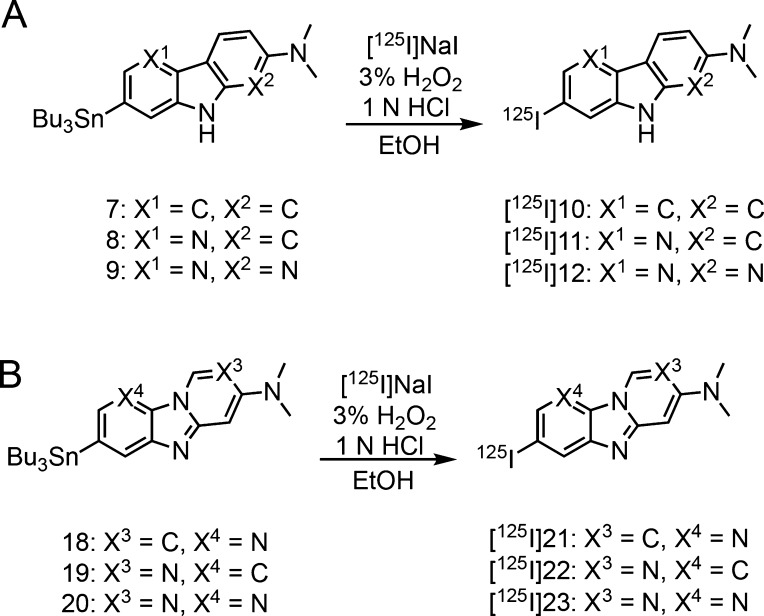
The radiosynthesis of [125I]10, [125I]11, [125I]12, [125I]21, [125I]22, and [125I]23 was accomplished by the iododestannylation reaction from the corresponding tributyltin derivatives using [125I]NaI and 3% hydrogen peroxide under acidic conditions (Scheme 3). After the reaction, the desirable radioiodinated compounds were purified by high performance liquid chromatography (HPLC). We verified the radiochemical identity of these compounds with HPLC by co-injection with nonradioactive compounds (Figure S1). The radioiodinated compounds were obtained at a radiochemical yield of 15–55% with a radiochemical purity of over 95%. The no-carrier-added derivatives were anticipated to result in a final product with a theoretical specific activity similar to that of 125I (81.4 TBq/mmol).
Scheme 3. Radioiodination Reaction of Carbazole, Pyridoindole, and Pyrrolodipyridine Derivatives (A) and Pyridoimidazopyridine, Benzimidazopyrimidine, and Pyridoimidazopyrimidine Derivatives (B).
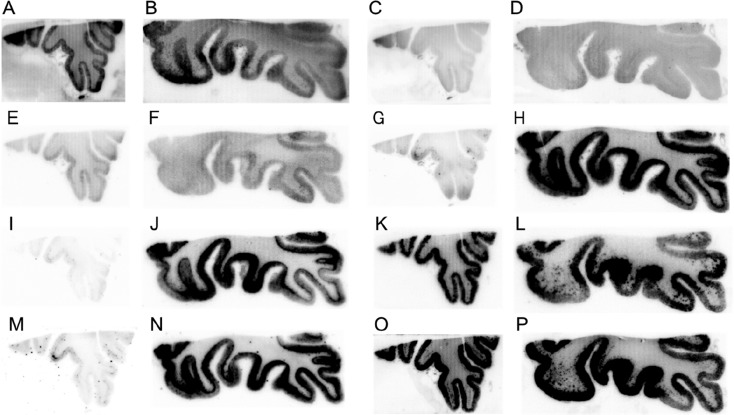
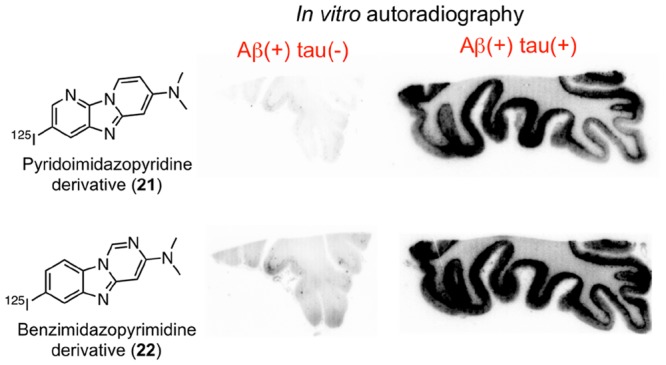
To assess selective binding affinity to tau against Aβ aggregates, we performed in vitro autoradiography (ARG) using AD brain sections. We used two different types of AD brain sections (Aβ(+)/tau(−) and Aβ(+)/tau(+)), which were confirmed by immunohistochemical staining with anti-Aβ and antiphosphorylated tau antibody (Figure S2). Figure 4 shows the results of the in vitro ARG of [125I]10, [125I]11, [125I]12, [125I]21, [125I]22, [125I]23, [125I]BIP-NMe2,16 and [125I]IMPY,27,28 which is well-known as an Aβ imaging probe for SPECT. Similarly to [125I]IMPY (Figure 4O and P), we observed the radioactivity accumulation of [125I]10, [125I]12, and [125I]23 in the cortex of both Aβ(+)/tau(−) and Aβ(+)/tau(+) brain sections (Figure 4A, B, E, F, K, and L). This result indicated that [125I]10, [125I]12, and [125I]23 bound to Aβ aggregates. In common with [125I]12, [18F]PI-2620 and [18F]RO-948 contain a pyrrolodipyridine scaffold, but these probes selectively bound to tau aggregates. The different position of a nitrogen atom in this scaffold ([125I]12; pyrrolo[2,3-b:4,5-b′]dipyridine, [18F]PI-2620 and [18F]RO-948; pyrrolo[2,3-b:4,5-c′]dipyridine) may be the reason for the opposite binding affinity to tau and Aβ aggregates. On the other hand, the radioactivity of [125I]21 and [125I]22 accumulated only in the cortex of Aβ(+)/tau(+) brain sections (Figure 4G–J). The accumulation pattern of these compounds corresponded well with that of [125I]BIP-NMe2 (Figure 4M and N) and the region of positive immunohistochemical staining with tau antibody (Figure S2D). These results suggest that [125I]21 and [125I]22 selectively bound to tau aggregates in the AD brain sections. [18F]T808 and [18F]GTP-1 also contain benzimidazopyrimidine, but the position of a nitrogen atom is different ([125I]22; benzo[4,5]imidazo[1,2-c]pyrimidine, [18F]T808 and [18F]GTP-1; benzo[4,5]imidazo[1,2-a]pyrimidine). Therefore, we identified the two novel scaffolds that have selective binding affinity to tau aggregates deposited in the AD brain. In contrast, [125I]11 showed very low accumulation in the cortex of both Aβ(+)/tau(−) and Aβ(+)/tau(+) brain sections (Figure 4C and D). In the presence of an excess of nonradioactive compounds, radioactivity accumulation of [125I]10, [125I]12, [125I]21, [125I]22, and [125I]23 in the gray matter was almost the same as that in white matter (Figure S3). This suggested that these probes specifically bound to Aβ or tau aggregates. Recent studies suggested that tau imaging probes should not show significant off-target binding to monoamine oxidase (MAO)-A and B.29−31 The number of nitrogen atoms affected not only the affinity for tau aggregates but also MAO-A,15 but [125I]21 and [125I]22 have not been evaluated regarding their off-target binding. In addition, we used 50% ethanol as a washing solution. Some studies on developing tau imaging probes also used a high ethanol concentration, but this condition may be effective at washing out nonspecific binding.22,23,32 Taken together, further studies of off-target binding are needed before clinical application.
Figure 4.
Comparison of in vitro autoradiography of [125I]10 (A and B), [125I]11 (C and D), [125I]12 (E and F), [125I]21 (G and H), [125I]22 (I and J), [125I]23 (K and L), [125I]BIP-NMe2 (M and N), and [125I]IMPY (O and P) in brain sections from an AD patient. A, C, E, G, I, K, M, and O show results in Aβ(+)/tau(−) brain sections. B, D, F, H, J, L, N, and P show results in Aβ(+)/tau(+) brain sections.
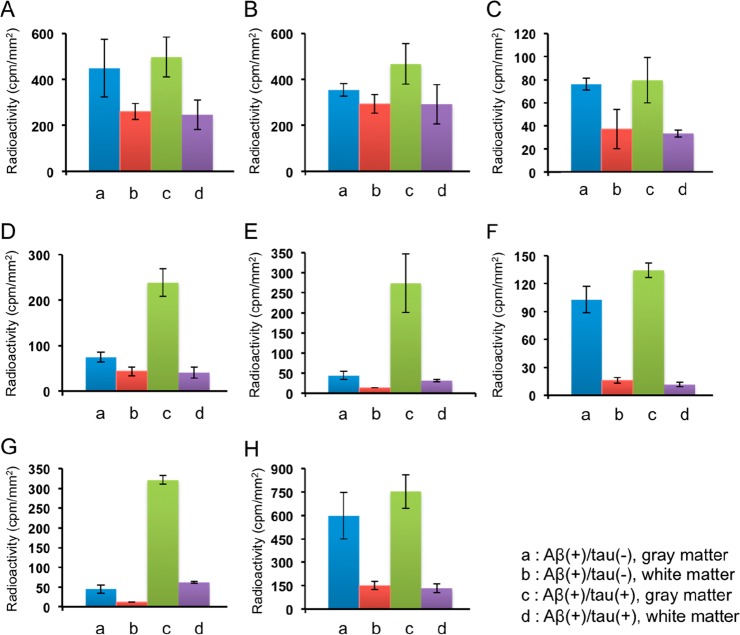
Furthermore, we performed quantitative analysis of the in vitro ARG. The regions of interest (ROIs) were set in the gray and white matter of both Aβ(+)/tau(−) and Aβ(+)/tau(+) brain sections, and the radioactivity accumulation (cpm/mm2) in each region was calculated (Figure 5). Since Aβ and tau mainly accumulated in gray matter (Figure S1), we calculated the ratio of radioactivity accumulation in the gray matter of Aβ(+)/tau(+) brain sections against the gray matter of Aβ(+)/tau(−) brain sections to evaluate whether 6,5,6-tricyclic compounds selectively bound to tau against Aβ aggregates (Table 1). The radioactivity accumulation in the gray matter of Aβ(+)/tau(+) brain section and the ratio of [125I]22 (273 ± 73 cpm/mm2 and 6.2, respectively) were similar to those of [125I]BIP-NMe2 (321 ± 11 cpm/mm2 and 7.1, respectively). [125I]21 also showed selective binding affinity for tau aggregates in the ARG image but a lower radioactivity accumulation and ratio (239 ± 31 cpm/mm2 and 3.2, respectively) than [125I]BIP-NMe2. These results suggest that [125I]22 showed higher potential as a selective tau imaging probe than [125I]21. [125I]10, [125I]11, [125I]12, and [125I]23 showed different values of radioactivity accumulation in all regions, but the ratios (1.1, 1.3, 1.0, and 1.3, respectively) were similar to those of [125I]IMPY (1.3), suggesting that these probes do not exhibit selective binding affinity for tau aggregates. Taken together, constancy of affinity for Aβ and tau aggregates of the 6,5,6-tricyclic structure is unclear, but we found that the position and number of nitrogen atoms and the condition of carbon–nitrogen bonds affect the binding affinity for Aβ and tau aggregates.
Figure 5.
Quantitative analysis of [125I]10 (A), [125I]11 (B), [125I]12 (C), [125I]21 (D), [125I]22 (E), [125I]23 (F), [125I]BIP-NMe2 (G), and [125I]IMPY (H) on in vitro autoradiography of AD brain sections.
Table 1. Ratio of Radioactivity Accumulation in the Gray Matter of Aβ(+)/tau(+) (c) against Aβ(+)/tau(−) Brain Sections (a).
| compd | c/a ratio |
|---|---|
| [125I]10 | 1.1 |
| [125I]11 | 1.3 |
| [125I]12 | 1.0 |
| [125I]21 | 3.2 |
| [125I]22 | 6.2 |
| [125I]23 | 1.3 |
| [125I]BIP-NMe2 | 7.1 |
| [125I]IMPY | 1.3 |
Since [125I]22 showed the most promising character as a tau imaging probe in vitro, we determined the Kd value for tau aggregates using AD brain sections (Figure S4). In this study, the Kd value for [125I]22 was determined to be 2.0 nM. However, because the amount of protein varies between brain sections, this assay is heterogeneous.33 Therefore, this value is difficult to compare with that of other probes.
Finally, we carried out a biodistribution study using normal mice to evaluate uptake into and wash out from the brain after the injection of radioiodinated 6,5,6-tricyclic compounds developed in this study (Tables 2 and S1). All probes except for [125I]10 displayed initial brain uptake (3.59–5.73% ID/g at 2 min postinjection) and rapid clearance (0.16–0.27% ID/g at 30 min postinjection and 0.09–0.16% ID/g at 60 min postinjection). Among them, [125I]21, [125I]22, and [125I]23 satisfied the criteria in mice for useful Aβ and tau imaging probes (>4% ID/g at 2 min postinjection and <1% ID/g at 30 min postinjection).34,35 Compared with [125I]BIP-NMe2 (3.98% ID/g),16 [125I]21 and [125I]22, which exhibit selective binding affinity for tau aggregates, showed superior brain uptake at 2 min postinjection (5.73 and 5.66% ID/g, respectively). In addition, the ratio of peak uptake divided by the value at 60 min of [125I]21 and [125I]22 (41.6 and 56.7, respectively) was higher than [125I]BIP-NMe2 (24.9), [18F]AV-1451 (6.8), [18F]PI-2620 (24.3), and [18F]RO948 (10.3),15 suggesting that [125I]21 and [125I]22 have favorable pharmacokinetics as tau imaging probes.
Table 2. Brain Uptake of Radioactivity after the Injection of Each 125I-Labeled 6,5,6-Tricyclic Compound Derivative in Normal Micea and the Ratio of Their Radioactivity Accumulation (2 min/30 and 2 min/60 min).
| %ID/g
in the brain |
ratio |
||||
|---|---|---|---|---|---|
| compd | 2 min | 30 min | 60 min | 2 min/30 min | 2 min/60 min |
| [125I]10 | 3.11 ± 0.49 | 0.95 ± 0.11 | 0.40 ± 0.06 | 3.3 | 7.8 |
| [125I]11 | 3.59 ± 0.32 | 0.27 ± 0.01 | 0.16 ± 0.01 | 13.2 | 22.4 |
| [125I]12 | 3.69 ± 0.52 | 0.16 ± 0.03 | 0.09 ± 0.01 | 23.0 | 41.2 |
| [125I]21 | 5.73 ± 0.21 | 0.21 ± 0.01 | 0.14 ± 0.02 | 27.6 | 41.6 |
| [125I]22 | 5.66 ± 0.34 | 0.19 ± 0.04 | 0.10 ± 0.01 | 30.2 | 56.7 |
| [125I]23 | 4.12 ± 0.68 | 0.20 ± 0.03 | 0.10 ± 0.01 | 20.5 | 41.8 |
| [125I]BIP-NMe2b | 3.98 ± 0.32 | 0.38 ± 0.03 | 0.16 ± 0.01 | 10.5 | 24.9 |
Each value represents the mean ± SD of five animals.
The data were reported previously.
[125I]21 and [125I]22 mainly accumulated in the liver (8.7 and 14.3% ID/g, respectively) and kidney (10.5 and 15.0% ID/g, respectively) at 2 min postinjection and were cleared with time. At 60 min postinjection, these probes accumulated in the intestine (7.3 and 15.7% ID/g, respectively) and stomach (27.9 and 12.2% ID, respectively). However, [125I]21 and [125I]22 showed very low accumulation in the thyroid (thyroid60 min = 0.04 and 0.02% ID, respectively), indicating that they show stability against in vivo deiodination until 60 min postinjection. Therefore, the increase in the radioactivity of the stomach may not be relevant to in vivo deiodination of the probes.
In conclusion, we newly designed and synthesized six radioiodinated 6,5,6-tricyclic compounds to identify scaffolds showing potential as backbones of tau imaging probes. This study revealed that the position and number of nitrogen atoms and the condition of carbon–nitrogen bonds affect the binding affinity for Aβ and tau aggregates. Among the six compounds, [125I]21 and [125I]22 showed selective binding affinity for tau aggregates and favorable pharmacokinetics in the brain. These results suggest that [125I]21 and [125I]22 showed feasibility as SPECT probes for imaging tau aggregates. In addition, pyridoimidazopyridine and benzimidazopyrimidine, which are the backbone structures of [125I]21 and [125I]22, respectively, may serve as novel scaffolds for the development of useful tau imaging probes.
Acknowledgments
This research was supported by JSPS KAKENHI Grant Numbers JP17H05092 and JP17H05694 and The Mochida Memorial Foundation for Medical and Pharmaceutical Research. We thank Kenji Matsumura (Kyoto University) for carrying out immunohistochemical staining and Masafumi Ihara (National Cerebral and Cardiovascular Center) for providing brain samples of AD cases.
Glossary
Abbreviations
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- BIP
benzimidazopyridine
- SAR
structure–activity relationship
- ARG
autoradiography
- HPLC
high-performance liquid chromatography
- o-DCB
o-dichlorobenzene
- Et3N
triethylamine
- DMF
N,N-dimethylformamide
- cpm
counts per minute
- %ID/g
percent injected dose per gram
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00456.
Full experimental methods, HPLC profile of all compounds, and results of immunohistochemical staining, in vitro blocking study, saturation binding assay, and biodistribution study for all organs (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Klunk W. E. Biological markers of Alzheimer’s disease. Neurobiol. Aging 1998, 19 (2), 145–147. 10.1016/S0197-4580(98)00013-X. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001, 81 (2), 741–766. 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Holtzman D. M.; Morris J. C.; Goate A. M. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 2011, 3 (77), 77sr1. 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R. Jr.; Knopman D. S.; Jagust W. J.; Petersen R. C.; Weiner M. W.; Aisen P. S.; Shaw L. M.; Vemuri P.; Wiste H. J.; Weigand S. D.; Lesnick T. G.; Pankratz V. S.; Donohue M. C.; Trojanowski J. Q. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12 (2), 207–216. 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M. Past and recent progress of molecular imaging probes for beta-amyloid plaques in the brain. Curr. Med. Chem. 2013, 21 (1), 82–112. 10.2174/09298673113209990216. [DOI] [PubMed] [Google Scholar]
- Villemagne V. L.; Dore V.; Burnham S. C.; Masters C. L.; Rowe C. C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 2018, 14 (4), 225–236. 10.1038/nrneurol.2018.9. [DOI] [PubMed] [Google Scholar]
- Villemagne V. L.; Fodero-Tavoletti M. T.; Masters C. L.; Rowe C. C. Tau imaging: early progress and future directions. Lancet Neurol. 2015, 14 (1), 114–124. 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]
- Watanabe H.; Ono M.; Saji H. Novel PET/SPECT probes for imaging of tau in Alzheimer’s disease. Sci. World J. 2015, 2015, 124192. 10.1155/2015/124192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada R.; Okamura N.; Furumoto S.; Tago T.; Yanai K.; Arai H.; Kudo Y. Characteristics of Tau and Its Ligands in PET Imaging. Biomolecules 2016, 6 (1), 7. 10.3390/biom6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A.; Chiotis K.; Lemoine L.; Gillberg P. G.; Almkvist O.; Rodriguez-Vieitez E.; Nordberg A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 2019, 24 (8), 1112–1134. 10.1038/s41380-018-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.; Wibom M.; Pawlik D.; Englund E.; Hansson O. Correlation of In Vivo [18F]Flortaucipir With Postmortem Alzheimer Disease Tau Pathology. JAMA Neurol 2019, 76 (3), 310–317. 10.1001/jamaneurol.2018.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betthauser T. J.; Cody K. A.; Zammit M. D.; Murali D.; Converse A. K.; Barnhart T. E.; Stone C. K.; Rowley H. A.; Johnson S. C.; Christian B. T. In Vivo Characterization and Quantification of Neurofibrillary Tau PET Radioligand 18F-MK-6240 in Humans from Alzheimer Disease Dementia to Young Controls. J. Nucl. Med. 2019, 60 (1), 93–99. 10.2967/jnumed.118.209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. F.; Comley R. A.; Kuwabara H.; Rosenberg P. B.; Resnick S. M.; Ostrowitzki S.; Vozzi C.; Boess F.; Oh E.; Lyketsos C. G.; Honer M.; Gobbi L.; Klein G.; George N.; Gapasin L.; Kitzmiller K.; Roberts J.; Sevigny J.; Nandi A.; Brasic J.; Mishra C.; Thambisetty M.; Mogekar A.; Mathur A.; Albert M.; Dannals R. F.; Borroni E. Characterization of 3 Novel Tau Radiopharmaceuticals, 11C-RO-963, 11C-RO-643, and 18F-RO-948, in Healthy Controls and in Alzheimer Subjects. J. Nucl. Med. 2018, 59 (12), 1869–1876. 10.2967/jnumed.118.209916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria Bohorquez S.; Marik J.; Ogasawara A.; Tinianow J. N.; Gill H. S.; Barret O.; Tamagnan G.; Alagille D.; Ayalon G.; Manser P.; Bengtsson T.; Ward M.; Williams S. P.; Kerchner G. A.; Seibyl J. P.; Marek K.; Weimer R. M. [18F]GTP1 (Genentech Tau Probe 1), a radioligand for detecting neurofibrillary tangle tau pathology in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (10), 2077–2089. 10.1007/s00259-019-04399-0. [DOI] [PubMed] [Google Scholar]
- Kroth H.; Oden F.; Molette J.; Schieferstein H.; Capotosti F.; Mueller A.; Berndt M.; Schmitt-Willich H.; Darmency V.; Gabellieri E.; Boudou C.; Juergens T.; Varisco Y.; Vokali E.; Hickman D. T.; Tamagnan G.; Pfeifer A.; Dinkelborg L.; Muhs A.; Stephens A. Discovery and preclinical characterization of [18F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (10), 2178–2189. 10.1007/s00259-019-04397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M.; Watanabe H.; Kitada A.; Matsumura K.; Ihara M.; Saji H. Highly Selective Tau-SPECT Imaging Probes for Detection of Neurofibrillary Tangles in Alzheimer’s Disease. Sci. Rep. 2016, 6, 34197. 10.1038/srep34197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaide S.; Ono M.; Watanabe H.; Kitada A.; Yoshimura M.; Shimizu Y.; Ihara M.; Saji H. Structure-Activity Relationships of Radioiodinated Benzoimidazopyridine Derivatives for Detection of Tau Pathology. ACS Med. Chem. Lett. 2018, 9 (5), 478–483. 10.1021/acsmedchemlett.8b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaide S.; Watanabe H.; Shimizu Y.; Tatsumi H.; Iikuni S.; Nakamoto Y.; Togashi K.; Ihara M.; Saji H.; Ono M. 18F-labeled benzimidazopyridine derivatives for PET imaging of tau pathology in Alzheimer’s disease. Bioorg. Med. Chem. 2019, 27 (16), 3587–3594. 10.1016/j.bmc.2019.06.039. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Ono M.; Kitada A.; Watanabe H.; Yoshimura M.; Iikuni S.; Kimura H.; Okamoto Y.; Ihara M.; Saji H. Structure-Activity Relationship Study of Heterocyclic Phenylethenyl and Pyridinylethenyl Derivatives as Tau-Imaging Agents That Selectively Detect Neurofibrillary Tangles in Alzheimer’s Disease Brains. J. Med. Chem. 2015, 58 (18), 7241–7257. 10.1021/acs.jmedchem.5b00440. [DOI] [PubMed] [Google Scholar]
- Walji A. M.; Hostetler E. D.; Selnick H.; Zeng Z.; Miller P.; Bennacef I.; Salinas C.; Connolly B.; Gantert L.; Holahan M.; O’Malley S.; Purcell M.; Riffel K.; Li J.; Balsells J.; JA O. B.; Melquist S.; Soriano A.; Zhang X.; Ogawa A.; Xu S.; Joshi E.; Della Rocca J.; Hess F. J.; Schachter J.; Hesk D.; Schenk D.; Struyk A.; Babaoglu K.; Lohith T. G.; Wang Y.; Yang K.; Fu J.; Evelhoch J. L.; Coleman P. J. Discovery of 6-(Fluoro-18F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([18F]-MK-6240): A Positron Emission Tomography (PET) Imaging Agent for Quantification of Neurofibrillary Tangles (NFTs). J. Med. Chem. 2016, 59 (10), 4778–4789. 10.1021/acs.jmedchem.6b00166. [DOI] [PubMed] [Google Scholar]
- Gobbi L. C.; Knust H.; Korner M.; Honer M.; Czech C.; Belli S.; Muri D.; Edelmann M. R.; Hartung T.; Erbsmehl I.; Grall-Ulsemer S.; Koblet A.; Rueher M.; Steiner S.; Ravert H. T.; Mathews W. B.; Holt D. P.; Kuwabara H.; Valentine H.; Dannals R. F.; Wong D. F.; Borroni E. Identification of Three Novel Radiotracers for Imaging Aggregated Tau in Alzheimer’s Disease with Positron Emission Tomography. J. Med. Chem. 2017, 60 (17), 7350–7370. 10.1021/acs.jmedchem.7b00632. [DOI] [PubMed] [Google Scholar]
- Rombouts F. J. R.; Declercq L.; Andres J. I.; Bottelbergs A.; Chen L.; Iturrino L.; Leenaerts J. E.; Marien J.; Song F.; Wintmolders C.; Wuyts S.; Xia C. A.; Te Riele P.; Bormans G.; Vandenberghe R.; Kolb H.; Moechars D. Discovery of N-(4-[18F]Fluoro-5-methylpyridin-2-yl)isoquinolin-6-amine (JNJ-64326067), a New Promising Tau Positron Emission Tomography Imaging Tracer. J. Med. Chem. 2019, 62 (6), 2974–2987. 10.1021/acs.jmedchem.8b01759. [DOI] [PubMed] [Google Scholar]
- Tago T.; Furumoto S.; Okamura N.; Harada R.; Adachi H.; Ishikawa Y.; Yanai K.; Iwata R.; Kudo Y. Structure-Activity Relationship of 2-Arylquinolines as PET Imaging Tracers for Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2016, 57 (4), 608–614. 10.2967/jnumed.115.166652. [DOI] [PubMed] [Google Scholar]
- Rombouts F. J.; Andres J. I.; Ariza M.; Alonso J. M.; Austin N.; Bottelbergs A.; Chen L.; Chupakhin V.; Cleiren E.; Fierens K.; Fontana A.; Langlois X.; Leenaerts J. E.; Marien J.; Martinez Lamenca C.; Salter R.; Schmidt M. E.; Te Riele P.; Wintmolders C.; Trabanco A. A.; Zhang W.; Macdonald G.; Moechars D. Discovery of N-(Pyridin-4-yl)-1,5-naphthyridin-2-amines as Potential Tau Pathology PET Tracers for Alzheimer’s Disease. J. Med. Chem. 2017, 60 (4), 1272–1291. 10.1021/acs.jmedchem.6b01173. [DOI] [PubMed] [Google Scholar]
- Freeman A. W.; Urvoy M.; Criswell M. E. Triphenylphosphine-mediated reductive cyclization of 2-nitrobiphenyls: a practical and convenient synthesis of carbazoles. J. Org. Chem. 2005, 70 (13), 5014–5019. 10.1021/jo0503299. [DOI] [PubMed] [Google Scholar]
- Wu Z. Q.; Huang Q.; Zhou X. G.; Yu L. T.; Li Z. K.; Wu D. Synthesis of Pyrido[1,2-a]benzimidazoles through a Copper-Catalyzed Cascade C-N Coupling Process. Eur. J. Org. Chem. 2011, 2011 (27), 5242–5245. 10.1002/ejoc.201100834. [DOI] [Google Scholar]
- Kung M. P.; Hou C.; Zhuang Z. P.; Zhang B.; Skovronsky D.; Trojanowski J. Q.; Lee V. M.; Kung H. F. IMPY: an improved thioflavin-T derivative for in vivo labeling of β-amyloid plaques. Brain Res. 2002, 956 (2), 202–10. 10.1016/S0006-8993(02)03436-4. [DOI] [PubMed] [Google Scholar]
- Zhuang Z. P.; Kung M. P.; Wilson A.; Lee C. W.; Plossl K.; Hou C.; Holtzman D. M.; Kung H. F. Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting β-amyloid plaques in the brain. J. Med. Chem. 2003, 46 (2), 237–43. 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- Ng K. P.; Pascoal T. A.; Mathotaarachchi S.; Therriault J.; Kang M. S.; Shin M.; Guiot M. C.; Guo Q.; Harada R.; Comley R. A.; Massarweh G.; Soucy J. P.; Okamura N.; Gauthier S.; Rosa-Neto P. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimer's Res. Ther. 2017, 9 (1), 25. 10.1186/s13195-017-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake L. R.; Pham J. M.; Desmond T. J.; Mossine A. V.; Lee S. J.; Kilbourn M. R.; Koeppe R. A.; Brooks A. F.; Scott P. J. H. Identification of AV-1451 as a Weak, Nonselective Inhibitor of Monoamine Oxidase. ACS Chem. Neurosci. 2019, 10 (8), 3839–3846. 10.1021/acschemneuro.9b00326. [DOI] [PubMed] [Google Scholar]
- Murugan N. A.; Chiotis K.; Rodriguez-Vieitez E.; Lemoine L.; Agren H.; Nordberg A. Cross-interaction of tau PET tracers with monoamine oxidase B: evidence from in silico modelling and in vivo imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (6), 1369–1382. 10.1007/s00259-019-04305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquie M.; Normandin M. D.; Vanderburg C. R.; Costantino I. M.; Bien E. A.; Rycyna L. G.; Klunk W. E.; Mathis C. A.; Ikonomovic M. D.; Debnath M. L.; Vasdev N.; Dickerson B. C.; Gomperts S. N.; Growdon J. H.; Johnson K. A.; Frosch M. P.; Hyman B. T.; Gomez-Isla T. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol. 2015, 78 (5), 787–800. 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C. F.; Arteaga J.; Chen G.; Gangadharmath U.; Gomez L. F.; Kasi D.; Lam C.; Liang Q.; Liu C.; Mocharla V. P.; Mu F.; Sinha A.; Su H.; Szardenings A. K.; Walsh J. C.; Wang E.; Yu C.; Zhang W.; Zhao T.; Kolb H. C. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimer's Dementia 2013, 9 (6), 666–676. 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Mason N. S.; Mathis C. A.; Klunk W. E. Positron emission tomography radioligands for in vivo imaging of Aβ plaques. J. Labelled Compd. Radiopharm. 2013, 56 (3–4), 89–95. 10.1002/jlcr.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N.; Harada R.; Furumoto S.; Arai H.; Yanai K.; Kudo Y. Tau PET imaging in Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2014, 14 (11), 500. 10.1007/s11910-014-0500-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.