Abstract
The ever-increasing prevalence of obesity and Type 2 diabetes has necessitated the development of newer and more effective approaches for achieving efficient glycemic control and weight loss. Conventional treatment methods often result in weight gain, further deteriorating the already impaired metabolic control in people with obesity/Type 2 diabetes. Alleviation of obesity and diabetes achieved after bariatric surgeries highlight the therapeutic importance of gut-brain axis and entails development of more patient-friendly approaches replicating the positive metabolic effects of bariatric surgery. Given the potential involvement of several gut hormones in the success of bariatric surgery, the therapeutic importance of synergistic interaction between these hormones for improved metabolism cannot be ignored. Many unimolecular multiagonist peptides are in preclinical and clinical trials as they maximize the combinatorial metabolic efficacy by concurrent activation of multiple gut hormone receptors. This review summarizes the ongoing developments of multiagonist peptides as novel therapeutic approaches against obesity-diabetes.
Keywords: Glucagon-like-peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), diabetes, obesity, hybrid, unimolecular, agonist
Introduction
The increasing prevalence and morbidity have made diabetes one of the biggest health care challenges of the 21st century.1 Both environmental (sedentary lifestyle, excessive calorie intake) and genetic factors contribute to this global epidemic.2 Therefore, lifestyle and pharmacological interventions are needed for management of diabetes and delaying progression of its related metabolic comorbidities. The undesirable side effects associated with oral glucose lowering medications3 and the progressive nature of Type 2 diabetes4 exacerbate the need for new treatment options with improved therapeutic profile. Although incretin mimetic drugs offer a broad range of benefits by targeting multiple tissues,5 their gastrointestinal side effects can limit clinical efficacy. In addition, the magnitude of antidiabetic efficacy demonstrated in preclinical trials has not fully translated to the clinical setting.6 Roux-en-Y gastric bypass (RYGB) surgery is now being regarded as the gold standard technique for obesity and diabetes treatment, resulting in approximately 88% diabetes remission, subsequent weight loss, and superior metabolic control than conventional pharmacological treatments.7-9 Ongoing research highlights the key role of gut hormones in improving metabolic dysfunction post bariatric surgeries.10,11 Therefore, it is suggested that synergistic manipulation of multiple gut hormone signalling pathways may offer similar therapeutic efficacy as surgical procedures, in turn providing obvious respite across several aspects of the metabolic syndrome.
Gut Hormones Interplay Post Bariatric Surgeries
Type 2 diabetes remission following bariatric surgery is independent of weight loss and includes mechanisms such as enhanced glycemic control,8 improved insulin sensitivity,12 augmented pancreatic beta-cell function,13 restored first-phase insulin secretion,14 and decreased truncal fat deposition.15 Altered secretion and action of gut-derived hormones such as peptide YY (PYY), glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), oxyntomodulin (OXM), cholecystokinin (CCK), and ghrelin after metabolic surgery is now being linked to Type 2 diabetes remission.11,16,17 This suggests that superior metabolic improvement post bariatric surgery is a result of interplay between gut hormones and subsequent re-programming of neuroendocrine signals. Despite these advantages, bariatric surgeries are invasive to patients, costly, and may result in complications such as kidney stones, abdominal pain, reduction of bone density, and vitamin deficiencies.18 Thus, non-invasive and novel antidiabetic therapies that target similar peptide hormone pathways, either through individual peptide analogue administration or more interestingly via a hybrid peptide approach, have distinct therapeutic utility for Type 2 diabetes. Indeed, coadministration of different gut hormones, such as GLP-1, GIP, PYY, and CCK alone and in combination with insulin, has previously been reported to maintain normoglycemia and metabolic homeostasis in diabetes.19-22 The clinical success of combined insulin and GLP-1 therapy (IDegLira) as a single-drug formulation in Type 1 diabetes patients further point to the importance of activating multiple signal transduction pathways for improved treatment outcomes.23
Unimolecular Poly-Agonist Approach
To combat reduced physiological concentrations of GLP-1 in Type 2 diabetes,24 various GLP-1 mimetics are currently clinically available.25 However, these mimetics target a single specific pathway and as such fail to achieve the superior glucose homeostasis, enhanced satiety and increased weight loss associated with bariatric surgeries, that are known to target numerous gut peptide–related cellular pathways.26,27 Therefore, strategies that can augment the antidiabetic actions of GLP-1 drugs are of key interest. In line with this, coadministration of various gut hormones with GLP-119-23 have shown therapeutic promise; however, their clinical application is often restricted due to dissimilar pharmacokinetic properties of the constituents and unwanted peptide–peptide interactions.28 Recent years have seen remarkable progress in the development of multitargeting peptides by combining the key bioactive domains of gastrointestinal hormones into a single molecule. These multifunctional molecules exhibit a single pharmacokinetic profile together with synergistic pharmacological action (Figure 1). Owing to its well-established mechanistic pathways, most of these multiagonists have centered their structures around GLP-1.29-31 In addition, knowledge of truncated bioactive forms32-34 of gut-derived peptides has also facilitated the cost-effective generation of hybrid peptides.
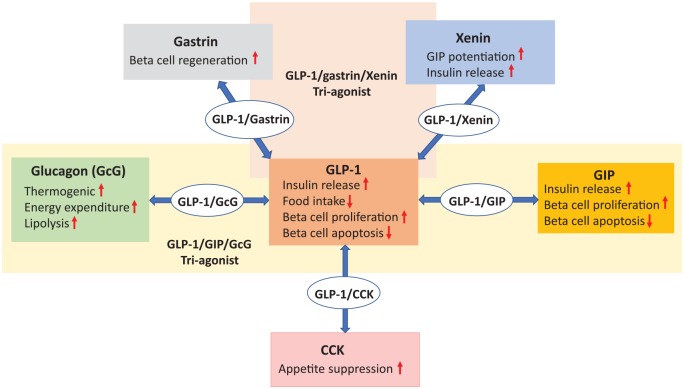
Figure 1.
This scheme demonstrates how peptide/peptide multiagonist approaches offer beneficial metabolic profile by targeting multiple receptor pathways and can be harnessed as obesity and diabetes therapeutics. CCK indicates cholecystokinin; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like-peptide-1.
Dual-agonist peptides
GLP-1/glucagon
GLP-1/glucagon hybrid peptides have been generated to incorporate the glycemic and appetite suppressive effects of GLP-135,36 with lipolytic and thermogenic properties of glucagon.37,38 In keeping with this, various GLP-1/glucagon coagonists have been reported to normalize body weights, improve glucose tolerance, lipid profile and liver steatosis in rodents and non-human primates.39-42 Furthermore, when given in combination with PEGylated-leptin, a GLP-1/glucagon hybrid synergistically lowered body weight by restoring leptin sensitivity in diet-induced obese (DIO) mice.43 Given their translational potential, several GLP-1/glucagon agonists with optimized pharmacokinetic profile are currently being evaluated in clinical trials.44 A recent success story is MEDI0382, a balanced GLP-1 and glucagon receptor dual-agonist that improved glycemic control and induced significantly higher reductions of bodyweight in obese patients with Type 2 diabetes when compared to placebo group (−3.8 kg mean for MEDI0382 vs −1.7 kg mean for placebo).45 More recently, SAR425899, another GLP-1/glucagon agonist, was reported to significantly reduce fasting glucose and HbA1c levels in patients with Type 2 diabetes. SAR425899 not only was well-tolerated but also promoted body weight reductions in healthy volunteers and in patients with Type 2 diabetes.46 In addition, GLP-1/glucagon agonism has also shown therapeutic potential in non-alcoholic steatohepatitis and liver regeneration in preclinical research.47 This indicates that by targeting multiorgan receptors, these peptides offer a more comprehensive beneficial profile in metabolic dysregulation.
GLP-1/GIP
Both GIP and GLP-1 are incretin hormones released postprandially to regulate blood glucose levels.48 Type 2 diabetes patients have an inherent incretin defect arising from decreased GLP-1 secretion and reduced insulinotropic action of GIP.49 It is primarily this reason that GIP analogues have been discounted as Type 2 diabetes therapy.48 In addition, the discrepancies seen in preclinical genetic knockout models and in the use of GIP receptor agonism or antagonism for metabolic regulation50 have further undermined the clinical utility of GIP analogues. In this regard, polypharmacy may help in unveiling the therapeutic potential of this rather ambiguous GIP system, as was seen previously with beneficial synergistic effects of GIP and GLP-1 coadministration.20 Glucagon-like-peptide-1/glucose-dependent insulinotropic polypeptide hybrid peptides activate both arms of the incretin axis (hence termed as Twincretins)28 and synergistically target the main hallmarks of metabolic syndrome including obesity, hyperglycemia, and dyslipidemia.28,51,52 Of mention is RG7697 (also known as NNC0090-2746), which was well-tolerated in Type 2 diabetes patients and displayed improved glycemic control and greater body weight reductions than placebo group.51,53 Another long-acting GIPR/GLP-1R agonist—LY3298176 (another name tirzepatide)—offers once-weekly administration and has been reported to concentration-dependently reduce HbA1c levels, body weight, fasting, and postprandial plasma glucose in Type 2 diabetes patients54,55 and has now progressed to long-term clinical safety trials. Interestingly, LY3298176 displayed a broader therapeutic window than that of a selective GLP-1 receptor agonist, as only the highest dose of LY3298176 (15 mg) showed greater gastrointestinal adverse events than dulaglutide (1.5 mg), and doses of 10 mg and less were not significantly different.55 In addition to regulating body weight and glucose metabolism, GLP-1/GIP coagonism with a single hybrid peptide improved memory function in DIO mice56 and displayed neuroprotective effects in animal models of brain injury, Parkinson and Alzheimer disease,57-59 further accentuating the superior metabolic profile associated with dual-receptor activation. Taken together, the strategy of harnessing synergistic antidiabetic effects of both arms of the incretin axis would help to sustain metabolic control by mimicking the native physiological incretin response.
GLP-1/CCK
CCK is an anorexigenic hormone secreted from enteroendocrine I-cells.60 CCK-8 is the major circulating form in plasma and inhibits food intake by binding to CCK-A receptors.60 In addition, benefits of GLP-1 at the level of the beta-cell have been suggested to be linked to a GLP-1/CCK intraislet loop.61 The combined treatment of CCK with GLP-1 analogues in rodent models significantly lowered circulating glucose levels, improved glucose tolerance, and induced greater weight loss and appetite suppressive effects when compared with individual treatments.62,63 In keeping with such evidence of synergistic metabolic actions, the potential of a novel hybrid peptide that incorporates the key bioactive regions of (pGlu-Gln)-CCK-8 and exendin-4 was investigated.31 By simultaneous activation of both GLP-1 and CCK-A receptors, this coagonist outperformed exendin-4 in terms of satiety and body weight reductions in DIO mice.31 Other beneficial effects of (pGluGln)-CCK-8/exendin-4 included prominent glucose homeostasis and reduced HbA1c and triglyceride levels, as well as improvements of insulin action and glucose disposal.31 In a recent study by Hornigold et al,64 another hybrid peptide, named C2816 generated by combining stabilized GLP-1R agonist (AC3174) and a CCK-AR-selective agonist (AC170222), exhibited superior weight reductions when compared with combined administration of the parent peptides in DIO mice. Although these initial observations clearly highlight the synergistic metabolic benefits of unimolecular approach, further preclinical studies are necessary for comprehensive functional characterization of these GLP-1/CCK coagonists, particularly the combined effects of GLP-1 and CCK on the gallbladder would need to be carefully evaluated.65
GLP-1/gastrin
Gastrin, a CCK homologous peptide, is secreted from gastric G-cells and binds to CCK-B receptors, with a potential role in beta-cell regeneration.66 Coadministration of gastrin and GLP-1 resulted in improved beta-cell mass and survival in non-obese diabetic mice,67 paving way for a GLP-1 and CCK-B receptor dual-agonist, ZP3022.68 ZP3022 reduced body weight, improved glucose tolerance, and increased beta-cell mass in db/db mice and Zucker diabetic fatty (ZDF) rats.68,69 While further studies are necessary to harness its potential as an antidiabetic pharmacotherapy, initial observations are encouraging.
GLP-1/xenin or GIP/xenin
Xenin is a 25-amino acid peptide hormone secreted into the bloodstream from the intestinal K-cells in response to food intake.70 Given the reduced insulinotropic effect of GIP, xenin is of key therapeutic significance for potentially overcoming GIP resistance in Type 2 diabetes patients.71,72 Indeed, glycemic normalization has been shown to restore GIP insulin-secretory function in Type 2 diabetes.73,74 GLP-1/xenin and GIP/xenin hybrids were generated by incorporating the key amino acid sequences of exendin-4 or (DAla)2 GIP with xenin-8-Gln,75,76 where xenin-8-Gln represents a stabilized fragment peptide of xenin that recapitulates the major biological functions of the parent peptide.77 Subchronic treatment with both xenin-based hybrid peptides displayed robust insulin secretory activity, improved glucose homeostasis, insulin sensitivity, and glucose tolerance in DIO mice.75,76 Notably, both hybrids augmented the biological response to native GIP in line with xenin’s GIP potentiating capabilities.75,76 Together with efficient glucose homeostasis, the superior efficacy of both hybrids was also evident from the improved lipid profile75 and preserved pancreatic islet architecture.76 It is suggested that xenin-mediated effects occur via activation of neuronal signalling69; therefore, further studies are still required to disentangle the contribution of individual components of gut-brain axis for these observed metabolic effects. Although both activation and inhibition of GIP is postulated as a possible treatment option for Type 2 diabetes and obesity,50,78-81 it is suggested from these studies that novel treatment options aimed at overcoming GIP resistance in Type 2 diabetes may have therapeutic potential.
GLP-1/amylin
The GLP-1/amylin hybrid peptide was engineered to combine the pronounced antidiabetic therapeutic capacity of GLP-1 mimetic-exendin-4,82 with the satiety and weight-lowering properties of stable amylin analogue, davalintide,83 within a single compound. These coagonists exhibited similar glycemic control as exenatide-treated group; however, their effects on body weight lowering were superior to exenatide or davalintide monotherapies in ob/ob mice and DIO rats.84 In a separate study, a PEGylated version of this hybrid with extended in vivo half-life showed enhanced glucose lowering and weight loss in rodents.85
Multiagonist peptides
The preclinical and clinical success of coagonist peptides discussed above has led to the development of novel triple-acting hybrids to achieve sustained metabolic improvements. One such example is GLP-1/Glucagon/GIP tri-agonists, where the main rationale was to incorporate the glucose lowering, insulinotropic, weight lowering and appetite suppressive effects of incretins with beneficial effects of glucagon on energy expenditure and lipid metabolism.37,38,48 The sequence similarities between the glucagon family of peptides and receptors86 led to development of triple agonist peptides, namely YAG/glucagon, [DAla2]GIP/Oxm, and [DAla2]GLP-1/glucagon hybrids.30,87,88 Using receptor transfected cells, receptor antagonists, and incretin receptor knockout mice, all 3 hybrids were reported to activate GLP-1, GIP, and glucagon-signalling pathways.30,87,88 Subchronic treatment with all 3 hybrid peptides displayed glucose lowering, improved glucose tolerance, and insulinotropic effects in DIO mice,30,87,88 but only [DAla2]GIP/Oxm and [DAla2]GLP-1/glucagon hybrids caused body weight reduction. [DAla2]GLP-1/glucagon also improved insulin sensitivity in DIO mice.87
Similarly, another novel GLP-1/GIP/Glucagon hybrid was engineered based on the antidiabetic benefits of a previously validated GLP-1R/GIPR coagonist28 together with the notable metabolic advantages evoked through incorporation of a glucagon component. This triple-acting peptide retained balanced GLP-1, GIP, and glucagon receptor activity in rodent beta-cells, adipocytes, and hepatocytes, respectively, suggesting simultaneous multiorgan receptor agonism.29 Although clear reductions in body weight and hepatic steatosis were evident in DIO mice, this was not the case in lean mice, implicating that tri-agonist exerts benefits only when coupled with metabolic dysregulation.29 Treatment with GLP-1/GIP/Glucagon triagonist also reduced pancreatic alpha-cell invasion to the islet core, thereby preserving the islet cyto-architecture in ZDF rats and db/db mice.29 Notably, every other day treatment of HM15211, a long-acting tri-agonist peptide conjugated to the human aglycosylate Fc fragment, reduced body weight and glycemia and was more effective than daily administration of liraglutide in increasing energy expenditure in rodent models.89 Meanwhile, HM15211 and another triagonist, MAR423, have now entered phase 1 clinical trials.
To overcome the inherent GIP resistance in Type 2 diabetes condition, a triple agonist peptide was engineered by combining a previously characterized exendin-4/gastrin dual-agonist68 with xenin-8-Gln. Twice daily administration of exendin-4/gastrin/xenin-8-Gln decreased circulating glucose, increased plasma insulin, reduced body fat mass, and improved glucose tolerance, insulin sensitivity, lipid profile, and metabolic response to GIP in DIO mice.90 Moreover, a stable and long-acting fatty acid-derivatized form, namely exendin-4/gastrin/xenin-8-Gln-Lys27 PAL, was developed to extend its bioactive profile for up to 12 hours; however, the fatty acid acylation did not impart any additional therapeutic benefits as first hoped for.91
Taken together, the use of multitargeting hybrid peptides that incorporate a GLP-1 backbone structure, linked to the bioactive region(s) of various other regulatory hormones, have shown remarkable therapeutic efficacy and offer viable alternative to bariatric surgery. In addition, given the nexus of PYY in diabetes remission post bariatric surgeries92 and the success of PYY, GLP-1, and/or OXM coadministration,12,93 PYY/GLP-1-based coagonists may also be evaluated as a potent non-surgical therapy for diabetes and obesity.
Although these preclinical studies highlight the efficacy of multiagonist peptides, there is often insufficient evidence as to whether the purported efficacy is being fractionally contributed by more than one component. Therefore, a major requirement for preclinical studies is to scrutinize balanced activity at individual key receptors, necessary for quantifying selective agonism and averting any off-target adverse events. In addition, the animal studies cannot capture the heterogeneity of human disease and such safety is difficult to predict and must be established in large, diverse chronic human studies. Indeed, despite their pharmacological benefits, the reports of pancreatitis and thyroid cancer post incretin mimetic treatments have raised safety concerns for their clinical use.94,95 The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have since independently reviewed several clinical safety databases, pancreatic toxicology studies, and cardiovascular outcome trials, and both agencies continue to investigate the pancreatic safety associated with incretin-based drugs; however, in light of the current knowledge, these concerns have now been allayed.96,97 Therefore, an appropriate balance between potency and long-term safety must be established in clinical trials, before these multiaction peptides can be seriously considered as registered antidiabetic drugs.
Future Directions
Single-molecule drugs targeting 2 or more gut-derived peptide hormone receptors have shown tremendous therapeutic potential, but the macromolecular nature of these drugs raises the concerns of immunogenicity.98 Therefore, more extensive preclinical assessment of these unimolecular multiagonists is essential to establish their translational efficiency to clinical settings. While animal studies are indispensable for optimizing novel drug candidates and to ascertain their human translational potential, there have been several incidences where drugs with promising preclinical data have failed in clinical trials,99 hence a cautious interpretation of preclinical studies is required to ensure bench-to-bedside translation. A major challenge for translation of hybrid peptides from animal models to humans is the associated risk of cross-reactivity with other receptors leading to off-target effects.98 In this regard, receptor affinity studies in different aetiologies of diabetes are required to decipher the complete metabolic efficacy and safety profile of these multitargeting hybrid peptides. Although various chemical modifications are being used to increase receptor binding, specificity, solubility, and protracted action, further chemical optimizations are still needed to achieve a more desirable pharmacokinetic and pharmacodynamic profile of these multiacting peptides. Mechanistic and receptor balance studies are necessary to help fine tune the most potent synergistic effects in vivo. Despite these cautions, the concurrent activation of multiple receptors by single-molecular entities has shown superior or equivalent efficacy compared to single-peptide agonists (Table 1); however, species-specific receptor affinity and chronic safety need to be carefully evaluated before considering this multiple-targeting peptide strategy a viable therapeutic option for Type 2 diabetes.
Table 1.
Summary of multireceptor agonists currently in preclinical and clinical trials and their effect on metabolic regulation.
| Agonists | Receptors involved | Effect on glycemic control | Effect on appetite suppression | Effect on body weight | Effect on lipid metabolism | Effect on beta-cell proliferation/apoptosis | Other metabolic effects | Side effects |
|---|---|---|---|---|---|---|---|---|
| GLP-1/GcG39-43 | GLP-1R, GcGR |

|

|

|

|
– | Non-alcoholic steatohepatitis | – |
| GLP-1/GIP28,51-59 | GLP-1R, GIPR |

|

|

|

|
– | Neuroprotective effects and memory function | Gastrointestinal side effects |
| GLP-1/CCK31,64 | GLP-1R, CCK-AR |

|

|

|

|
– | – | – |
| GLP-1/gastrin68,69 | GLP-1R, CCK-BR |

|
– |

|
– |

|
– | – |
| GLP-1/xenin or GIP/xenin75,76 | GLP-1R or GIPR, NTSR, muscarinic |

|
– | – |

|

|
GIP potentiation | – |
| GLP-1/amylin84,85 | GLP-1R, amylinR |

|
– |

|
– | – | – | – |
| GLP-1/GIP/Glucagon29,30,87-89 | GLP-1R, GIPR, GcGR |

|
– |

|

|

|
Fibrosis, non-alcoholic steatohepatitis, neuroprotection | – |
| GLP-1/gastrin/xenin90,91 | GLP-1R, CCK-BR, NTSR, muscarinic |

|
– | – |

|
– | GIP potentiation | – |
Abbreviations: CCK, cholecystokinin; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like-peptide-1.
Conclusion
The importance of combined actions of various regulatory peptide hormones for control of metabolism is slowly beginning to come to light, particularly given recent knowledge gained from certain types of bariatric surgeries. While unimolecular peptide approach seems to offer superior therapeutic efficacy, possibly reduced adverse effects, low dosage regimen when compared to individual peptide analogue administration, is patient friendly and may overcome the difficulties associated with solubility and dosage formulation of a mixture of 2 or more peptides when administered by single injection. However, careful outpatient clinical studies are still required to determine the sustainability, safety, and translational potential of these multiagonist drugs as novel class of antidiabetic and antiobesity medications. Nevertheless, regulation of energy balance is a complex process involving multiple central and systemic tissues, and these unimolecular peptides may offer a more patient-centric non-surgical breakthrough for the treatment of obesity-linked metabolic disorders.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AH: developed the structure of paper, wrote the manuscript, reviewed and approved the final manuscript.
ORCID iD: Annie Hasib  https://orcid.org/0000-0002-5388-6461
https://orcid.org/0000-0002-5388-6461
References
- 1. International Diabetes Federation. IDF Diabetes. 8th ed Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2. Ardisson Korat AV, Willett WC, Hu FB. Diet, lifestyle, and genetic risk factors for type 2 diabetes: a review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-up Study. Curr Nutr Rep. 2014;3:345-354. doi: 10.1007/s13668-014-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Dalem J, Brouwers MC, Stehouwer CD, et al. Risk of hypoglycaemia in users of sulphonylureas compared with metformin in relation to renal function and sulphonylurea metabolite group: population based cohort study. BMJ. 2016;354:i3625. doi: 10.1136/bmj.i3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn S. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3-19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 5. Gupta V. Pleiotropic effects of incretins. Indian J Endocrinol Metab. 2012;16:S47-S56. doi: 10.4103/2230-8210.94259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nauck MA, Baranov O, Ritzel RA, Meier JJ. Do current incretin mimetics exploit the full therapeutic potential inherent in GLP-1 receptor stimulation? Diabetologia. 2013;56:1878-1883. doi: 10.1007/s00125-013-2953-6. [DOI] [PubMed] [Google Scholar]
- 7. Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420-1428. doi: 10.2337/dc11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964-973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 9. Park JY, Heo Y, Kim YJ, et al. Long-term effect of bariatric surgery versus conventional therapy in obese Korean patients: a multicenter retrospective cohort study. Ann Surg Treat Res. 2019;96:283-289. doi: 10.4174/astr.2019.96.6.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol Regul Integr Comp Physiol. 2015;307:R1275-R1291. doi: 10.1152/ajpregu.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28-37. doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 12. Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15-23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025-2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 14. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375-380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;3636:2175-2182. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ionut V, Burch M, Youdim A, Bergman RN. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity (Silver Spring). 2013;21:1093-1103. doi: 10.1002/oby.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holst JJ, Madsbad S, Bojsen-Moller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14:708-714. doi: 10.1016/j.soard.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421. doi: 10.1007/s11695-015-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700-706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gault VA, Kerr BD, Harriott P, Flatt PR. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with type 2 diabetes and obesity. Clin Sci (Lond). 2011;121:107-117. doi: 10.1042/CS20110006. [DOI] [PubMed] [Google Scholar]
- 21. Irwin N, Hunter K, Montgomery I, Flatt PR. Comparison of independent and combined metabolic effects of chronic treatment with (pGlu-Gln)-CCK-8 and long-acting GLP-1 and GIP mimetics in high fat-fed mice. Diabetes Obes Metab. 2013;15:650-659. doi: 10.1111/dom.12079. [DOI] [PubMed] [Google Scholar]
- 22. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885-893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 23. Kapitza C, Bode B, Ingwersen SH, Jacobsen LV, Poulsen P. Preserved pharmacokinetic exposure and distinct glycemic effects of insulin degludec and liraglutide in IDegLira, a fixed-ratio combination therapy. J Clin Pharmacol. 2015;55:1369-1377. doi: 10.1002/jcph.549. [DOI] [PubMed] [Google Scholar]
- 24. Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609-613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 25. Trujillo JM, Nuffer W. GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy. 2014;34:1174-1186. doi: 10.1002/phar.1507. [DOI] [PubMed] [Google Scholar]
- 26. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 27. Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol. 2013;9:425-33. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- 28. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 29. Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27-36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 30. Bhat V, Kerr B, Vasu S, Flatt P, Gault V. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia. 2013;56:1417-1424. doi: 10.1007/s00125-013-2892-2. [DOI] [PubMed] [Google Scholar]
- 31. Irwin N, Pathak V, Flatt PR. A novel CCK-8/GLP-1 hybrid peptide exhibiting prominent insulinotropic, glucose-lowering, and satiety actions with significant therapeutic potential in high-fat-fed mice. Diabetes. 2015;64:2996-3009. doi: 10.2337/db15-0220. [DOI] [PubMed] [Google Scholar]
- 32. Hinke SA, Manhart S, Pamir N, et al. Identification of a bioactive domain in the amino-terminus of glucose-dependent insulinotropic polypeptide (GIP). Biochim Biophys Acta. 2001;1547:143-155. doi: 10.1016/s0167-4838(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 33. Irwin N, Frizelle P, O’Harte FP, Flatt PR. Metabolic effects of activation of CCK receptor signaling pathways by twice-daily administration of the enzyme-resistant CCK-8 analog, (pGlu-gln)-CCK-8, in normal mice. J Endocrinol. 2013;216:53-59. doi: 10.1530/JOE-12-0353. [DOI] [PubMed] [Google Scholar]
- 34. Martin CM, Parthsarathy V, Pathak V, Gault VA, Flatt PR, Irwin N. Characterisation of the biological activity of xenin-25 degradation fragment peptides. J Endocrinol. 2014;221:193-200. doi: 10.1530/JOE-13-0617. [DOI] [PubMed] [Google Scholar]
- 35. Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Nat Acad Sci U S A. 1987;84:3434-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turton M, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69-72. [DOI] [PubMed] [Google Scholar]
- 37. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol. 2019;10:413. doi: 10.3389/fphys.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salem V, Izzi-Engbeaya C, Coello C, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18:72-81. doi: 10.1111/dom.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749-757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 40. Henderson SJ, Konkar A, Hornigold DC, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab. 2016;18:1176-1190. doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pocai A, Carrington PE, Adams JR, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258-2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou J, Cai X, Huang X, et al. A novel glucagon-like peptide-1/glucagon receptor dual agonist exhibits weight-lowering and diabetes-protective effects. Eur J Med Chem. 2017;138:1158-1169. [DOI] [PubMed] [Google Scholar]
- 43. Clemmensen C, Chabenne J, Finan B, et al. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63:1422-1427. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- 44. Brandt SJ, Gotz A, Tschop MH, Muller TD. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides. 2018;100:190-201. doi: 10.1016/j.peptides.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607-2618. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
- 46. Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab. 2019;21:120-128. doi: 10.1111/dom.13494. [DOI] [PubMed] [Google Scholar]
- 47. Valdecantos MP, Pardo V, Ruiz L, et al. A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology. 2017;65:950-968. doi: 10.1002/hep.28962. [DOI] [PubMed] [Google Scholar]
- 48. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [DOI] [PubMed] [Google Scholar]
- 49. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Finan B, Muller TD, Clemmensen C, Perez-Tilve D, DiMarchi RD, Tschop MH. Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol Med. 2016;22:359-376. doi: 10.1016/j.molmed.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 51. Frias JP, Bastyr EJ, III, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343-352. doi: 10.1016/j.cmet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 52. Portron A, Jadidi S, Sarkar N, DiMarchi R, Schmitt C. Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects. Diabetes Obes Metab. 2017;19:1446-1453. doi: 10.1111/dom.13025. [DOI] [PubMed] [Google Scholar]
- 53. Schmitt C, Portron A, Jadidi S, Sarkar N, DiMarchi R. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes Obes Metab. 2017;19:1436-1445. doi: 10.1111/dom.13024. [DOI] [PubMed] [Google Scholar]
- 54. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3-14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180-2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 56. Pathak NM, Pathak V, Gault VA, McClean S, Irwin N, Flatt PR. Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential. Biochem Pharmacol. 2018;155:264-274. doi: 10.1016/j.bcp.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 57. Tamargo IA, Bader M, Li Y, et al. Novel GLP-1R/GIPR co-agonist ‘twincretin’ is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp Neurol. 2017;288:176-186. doi: 10.1016/j.expneurol.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yuan Z, Li D, Feng P, et al. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol. 2017;812:82-90. doi: 10.1016/j.ejphar.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 59. Shi L, Zhang Z, Li L, Holscher C. A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer ICV. Behav Brain Res. 2017;327:65-74. doi: 10.1016/j.bbr.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 60. Karlsson S, Ahren B. CCK-8-stimulated insulin secretion in vivo is mediated by CCKA receptors. Eur J Pharmacol. 1992;213:145-146. doi: 10.1016/0014-2999(92)90245-y. [DOI] [PubMed] [Google Scholar]
- 61. Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB. Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol. 2015;29:978-987. doi: 10.1210/me.2015-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trevaskis J, Sun C, Athanacio J, D’Souza L, et al. Synergistic metabolic benefits of an exenatide analogue and cholecystokinin in diet-induced obese and leptin-deficient rodents. Diabetes Obes Metab. 2015;17:61-73. doi: 10.1111/dom.12390. [DOI] [PubMed] [Google Scholar]
- 63. Irwin N, Hunter K, Montgomery I, Flatt P. Comparison of independent and combined metabolic effects of chronic treatment with (pGlu-Gln)-CCK-8 and long-acting GLP-1 and GIP mimetics in high fat-fed mice. Diabetes Obes Metab. 2013;15:650-659. doi: 10.1111/dom.12079. [DOI] [PubMed] [Google Scholar]
- 64. Hornigold DC, Roth E, Howard V, et al. A GLP-1:CCK fusion peptide harnesses the synergistic effects on metabolism of CCK-1 and GLP-1 receptor agonism in mice. Appetite. 2018;127:334-340. doi: 10.1016/j.appet.2018.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rehfeld JF, Knop FK, Asmar A, Madsbad S, Holst JJ, Asmar M. Cholecystokinin secretion is suppressed by glucagon-like peptide-1: clue to the mechanism of the adverse gallbladder events of GLP-1-derived drugs. Scand J Gastroenterol. 2018;53:1429-1432. doi: 10.1080/00365521.2018.1530297. [DOI] [PubMed] [Google Scholar]
- 66. Rooman I, Lardon J, Bouwens L. Gastrin stimulates β-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes. 2002;51:686-690. doi: 10.2337/diabetes.51.3.686. [DOI] [PubMed] [Google Scholar]
- 67. Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57:3281-3288. doi: 10.2337/db08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dalboge LS, Almholt DL, Neerup TS, Vrang N, Jelsing J, Fosgerau K. The novel GLP – 1 – gastrin dual agonist ZP3022 improves glucose homeostasis and increases beta -cell mass without affecting islet number in db/db mice. J Pharmacol Exp Ther. 2014;350:353-360. doi: 10.1124/jpet.114.215293. [DOI] [PubMed] [Google Scholar]
- 69. Skarbaliene J, Secher T, Jelsing J, Ansarullah Neerup TS, Billestrup N, Fosgerau K. The anti-diabetic effects of GLP-1-gastrin dual agonist ZP3022 in ZDF rats. Peptides. 2015;69:47-55. doi: 10.1016/j.peptides.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 70. Anlauf M, Weihe E, Hartschuh W, Hamscher G, Feurle GE. Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J Histochem Cytochem. 2000;48:1617-1626. doi: 10.1177/002215540004801205. [DOI] [PubMed] [Google Scholar]
- 71. Wice BM, Wang S, Crimmins DL, et al. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem. 2010;285:19842-19853. doi: 10.1074/jbc.M110.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martin CM, Gault VA, McClean S, Flatt PR, Irwin N. Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol. 2012;84:312-319. doi: 10.1016/j.bcp.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 73. Piteau S, Olver A, Kim SJ, et al. Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat. Biochem Biophys Res Commun. 2007;362:1007-1012. doi: 10.1016/j.bbrc.2007.08.115. [DOI] [PubMed] [Google Scholar]
- 74. Hojberg PV, Vilsboll T, Rabol R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199-207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 75. Hasib A, Ng MT, Khan D, Gault VA, Flatt PR, Irwin N. A novel GLP-1/xenin hybrid peptide improves glucose homeostasis, circulating lipids and restores GIP sensitivity in high fat fed mice. Peptides. 2018;100:202-211. doi: 10.1016/j.peptides.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 76. Hasib A, Ng MT, Gault VA, et al. An enzymatically stable GIP/xenin hybrid peptide restores GIP sensitivity, enhances beta cell function and improves glucose homeostasis in high-fat-fed mice. Diabetologia. 2017;60:541-552. doi: 10.1007/s00125-016-4186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin CM, Parthsarathy V, Hasib A, et al. Biological activity and antidiabetic potential of C-terminal octapeptide fragments of the gut-derived hormone xenin. PLoS ONE. 2016;11:e0152818. doi: 10.1371/journal.pone.0152818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Irwin N, Flatt PR. Therapeutic potential for GIP receptor agonists and antagonists. Best Pract Res Clin Endocrinol Metab. 2009;23:499-512. doi: 10.1016/j.beem.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 79. Killion EA, Wang J, Yie J, et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med. 2018;10:eaat3392. doi: 10.1126/scitranslmed.aat3392. [DOI] [PubMed] [Google Scholar]
- 80. Gasbjerg LS, Gabe MB, Hartmann B, et al. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides. 2018;100:173-181. doi: 10.1016/j.peptides.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 81. Mroz PA, Finan B, Gelfanov V, et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metab. 2019;20:51-62. doi: 10.1016/j.molmet.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arakawa M, Ebato C, Mita T, et al. Effects of exendin-4 on glucose tolerance, insulin secretion, and beta-cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem Biophys Res Commun. 2009;390:809-814. doi: 10.1016/j.bbrc.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 83. Mack CM, Soares CJ, Wilson JK, et al. Davalintide (AC2307), a novel amylin -mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes (Lond). 2010;34:385-395. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- 84. Trevaskis JL, Mack CM, Sun C, et al. Improved glucose control and reduced body weight in rodents with dual mechanism of action peptide hybrids. PLoS ONE. 2013;8:e78154. doi: 10.1371/journal.pone.0078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sun C, Trevaskis JL, Jodka CM, et al. Bifunctional PEGylated exenatide -amylinomimetic hybrids to treat metabolic disorders: an example of long -acting dual hormonal therapeutics. J Med Chem. 2013;56:9328-9341. doi: 10.1021/jm401418s. [DOI] [PubMed] [Google Scholar]
- 86. Brubaker P, Drucker D. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Recept Chan. 2002;8:179-188. [PubMed] [Google Scholar]
- 87. Gault VA, Bhat VK, Irwin N, Flatt PR. A novel glucagon-like peptide-1 (GLP-1)/glucagon hybrid peptide with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptors and therapeutic potential in high fat-fed mice. J Biol Chem. 2013;288:35581-35591. doi: 10.1074/jbc.M113.512046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bhat VK, Kerr BD, Flatt PR, Gault VA. A novel GIP-oxyntomodulin hybrid peptide acting through GIP, glucagon and GLP-1 receptors exhibits weight reducing and anti-diabetic properties. Biochem Pharmacol. 2013;85:1655-1662. doi: 10.1016/j.bcp.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 89. Kim JK. Therapeutic effect of a novel long-acting GLP-1/GIP/Glucagon triple agonist (HM15211) in NASH and fibrosis animal models. Paper presented at: EASD annual meeting; October, 2018; Berlin, Germany http://www.hanmipharm.com/ehanmi/img/rnd/pipeline/Oral_Presentation_at_EASD_in_2018(HM15211).pdf. Accessed September 2, 2019. [Google Scholar]
- 90. Hasib A, Ng MT, Khan D, Gault VA, Flatt PR, Irwin N. Characterisation and antidiabetic utility of a novel hybrid peptide, exendin-4/gastrin/xenin-8-Gln. Eur J Pharmacol. 2018;834:126-135. doi: 10.1016/j.ejphar.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 91. Hasib A, Ng MT, Tanday N, et al. Exendin-4 (Lys27PAL)/gastrin/xenin-8-Gln: a novel acylated GLP-1/gastrin/xenin hybrid peptide that improves metabolic status in obese-diabetic (ob/ob) mice. Diabetes Metab Res Rev. 2019;35:e3106. doi: 10.1002/dmrr.3106. [DOI] [PubMed] [Google Scholar]
- 92. Guida C, Stephen SD, Watson M, et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine. 2019;40:67-76. doi: 10.1016/j.ebiom.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tan T, Behary P, Tharakan G, et al. The effect of a subcutaneous infusion of GLP-1, OXM, and PYY on energy intake and expenditure in obese volunteers. J Clin Endocrinol Metab. 2017;102:2364-2372. doi: 10.1210/jc.2017-00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nauck MA, Friedrich N. Do GLP-1–based therapies increase cancer risk? Diabetes Care. 2013;36:S245-S252. doi: 10.2337/dcS13-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care. 2013;36:2118-2125. doi: 10.2337/dc12-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36:2126-2132. doi: 10.2337/dc12-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Engl J Med. 2014;370:794-797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
- 98. Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on dual incretin receptor activation: ‘twincretins’. Diabetes Obes Metab. 2016;18:847-854. doi: 10.1111/dom.12685. [DOI] [PubMed] [Google Scholar]
- 99. Jain M, Carlson G, Cook W, et al. Randomised, phase 1, dose-finding study of MEDI4166, a PCSK9 antibody and GLP-1 analogue fusion molecule, in overweight or obese patients with type 2 diabetes mellitus. Diabetologia. 2019;62:373-386. doi: 10.1007/s00125-018. [DOI] [PubMed] [Google Scholar]