Summary
The pathophysiological processes of Alzheimer’s dementia predate its clinical manifestation. Sleep disturbances can accelerate the aging process and are common features of dementia. This study examined whether quantitative sleep electroencephalogram changes predate the clinical development of mild cognitive impairment and/or incident dementia. We collected data from a nested case-control sample of women (mean age 83 years) from the Sleep and Cognition Study, an ancillary study to the longitudinal Study of Osteoporotic Fractures, who were characterized as cognitively normal at the time of a baseline polysomnography study (Study of Osteoporotic Fractures visit 8) based on a Mini-Mental Status Exam (MMSE) score >24. Cases (n = 85) were women who developed new mild cognitive impairment or dementia by objective cognitive testing 5 years after polysomnography. Controls were women with no mild cognitive impairment/dementia (n = 85) at baseline or at follow-up. Differences in electroencephalogram absolute and relative power density were observed between the two groups. Specifically, higher electroencephalogram power values were found in the dementia/mild cognitive impairment group, for the alpha (p = .01) and theta bands (p = .04) in non-rapid eye movement sleep, as well as alpha (p = .04) and sigma (p = .04) bands in rapid eye movement sleep. In contrast, there were no group differences in traditional polysomnography measures of sleep architecture and sleep stage distribution, as well as sleep apnea and periodic limb movement indices. Our results provide evidence for quantitative electroencephalogram changes, which precede the clinical onset of cognitive decline and the diagnosis of dementia in elderly women, and support the application of quantitative sleep electroencephalogram analysis as a promising biomarker for imminent cognitive decline.
Keywords: aging, longitudinal analysis, memory
1 |. INTRODUCTION
Alzheimer’s disease (AD) is estimated to affect 5 million people in the USA. The projected increase of the elderly population is expected to nearly triple this number by 2050 (Hebert, Weuve, Scherr, & Evans, 2013). This constitutes an urgent need to identify and test new prevention and treatment interventions. Evidence suggests that the pathophysiological process of AD can predate the clinical picture by years and even decades (Sperling et al., 2011). This long preclinical phase is a key prospect for disease-modifying interventions as significant neuronal injury has already occurred by the time clinical signs appear.
Sleep disturbances can accelerate the aging process, and can increase the risk for cognitive impairment (Diem et al., 2016; Liguori et al., 2014). Sleep and memory functions rely on similar network processes, and brain regions important for sleep and wake mechanisms are affected in early AD (Wang et al., 2015). Insufficient duration and poor quality of sleep have been associated with the risk of developing AD, even independently of sleep disorders such as sleep-disordered breathing (SDB; Blackwell et al., 2011; Lim et al., 2014). Research in animals and in normal humans supports the idea that poor sleep quality is associated with lower cognitive performance as well as greater beta amyloid (Aβ) burden (Spira, An, & Resnick, 2014). Aβ in brain interstitial fluid has been found to increase with time awake and decrease during sleep (Xie et al., 2013). Further, sleep has been shown to promote the removal of these waste products from the brain at a faster rate than the wake state, and may be of particular importance in preserving metabolic homeostasis. Neurodegenerative disorders are thus thought to be due to an impairment of Aβ clearance, resulting in its central nervous system accumulation (Mawuenyega et al., 2010). A recent study linked Aβ pathology with an impaired cortical generation of non-rapid eye movement (NREM) slow waves and the associated sleep-dependent memory consolidation, further supporting inter-relations between Aβ pathology, cognition and sleep (Mander et al., 2015).
There have been several studies looking at electroencephalogram (EEG) markers of dementia during wake and sleep states (Prinz, Larsen, Moe, & Vitiello, 1992; Van Der Hiele et al., 2007). Most studies have investigated group differences of different patient populations who already had manifested mild cognitive impairment (MCI) to look for EEG patterns predictive of further clinical deterioration (Brunovsky, Matousek, Edman, Cervena, & Krajca, 2003; Huang et al., 2000). One longitudinal study found quantitative EEG changes over a 7-year period between those subjects who cognitively declined and those who did not (Prichep et al., 2006). These studies have demonstrated correlations between wake EEG bands and global cognitive assessments. Changes in theta/gamma ratio and an increase of high alpha frequency were promising prognostic markers for those patients with MCI, who are more likely to convert to AD.
Quantitative EEG analysis provides insight into the dynamic neuronal processes during sleep and can provide complementary information indicating traits that are stable and highly heritable, and thus might help better characterize an EEG phenotype of prodromal MCI/dementia (Tafti, 2009). Spectral analysis comparing waking and rapid eye movement (REM) sleep has demonstrated EEG slowing in patients with amnestic MCI during wakefulness, which became more prominent in REM sleep (Brayet et al., 2016).
By contrast, studies applying quantitative sleep EEG during the preclinical phase of MCI or dementia have not been conducted so far. The current study is the first one to prospectively examine whether quantitative sleep EEG traits can precede the clinical manifestation of MCI and/or incident dementia.
2 |. MATERIALS AND METHODS
2.1 |. Overview
Analyses were based on data derived from a subset of participants enrolled in the Sleep and Cognition Study, an ancillary study to the longitudinal Study of Osteoporotic Fractures (SOF), which is a multi-site, prospective, observational study of community-dwelling women aged 65 years and older, as described before (Cummings et al., 1990).
The Sleep and Cognition Study was initiated at the eighth clinic visit (January 2002-April 2004; Exam 8) at the clinical centres in Minneapolis and Pittsburgh. This baseline visit included in-home polysomnograms (PSG) in participants not reporting active treatment of sleep apnea, use of oxygen during sleep, or if they had an open tracheostomy. Of the 461 participants (all of which were Caucasians) who completed a PSG at visit 8, 305 returned to visit 9, which was carried out between November 2006 and September 2008 (median follow up = 5.0 years, range 3.4–6.2 years).
At each study visit, participants completed a questionnaire assessment of medical history and underwent a brief physical examination. Information collected included age, body mass index (BMI), education, and self-reported history of current smoking and alcohol consumption. In addition, a history of medical conditions was defined as a prior physician’s diagnosis of stroke, diabetes, Parkinson’s disease, cancer, chronic obstructive pulmonary disease (COPD), or coronary heart disease. Questionnaire data included the Geriatric Depression Scale, Functional Outcomes of Sleep and Epworth Sleepiness Scales, as well as the Pittsburgh Sleep Quality Index. Current medication use was verified by examination of pill bottles.
The institutional review boards at each clinic site approved the study, and written informed consent was obtained from all participants.
2.2 |. Characterization of cognitive status
Cognitive assessments at study visit 8 included the Mini-Mental and Short Mini-Mental Exams, and Trail Making B Test. Visit 9 included the California Verbal Fluency Test, Digit Span Forward and Backward, Short Mini-Mental Exam, Teng-Modified MMSE, Trails B test, and Verbal Category and Fluency Exams. Women were considered to screen positive for cognitive impairment at visit 9 if they met one or more of the following criteria: (1) score of less than 88 on the modified MMSE (compared with the Mini-Mental Exam, this task incorporates modifications to assess a broader variety of cognitive functions, and enhances reliability and validity; scores range from 0 to 100); (2) score of less than 4 (delayed recall) on the California Verbal Learning Test; (3) score of 3.6 or greater on the Informant Questionnaire on Cognitive Decline in the Elderly; (4) previous diagnosis of dementia or use of medication for dementia; or (5) nursing home residence (Yaffe, Middleton, et al., 2011).
Those women who screened positive underwent further clinical evaluation by a panel of clinical experts. These included a neurologist, two neuropsychologists and a geropsychologist. Assessment included the neuropsychological battery scores, prior cognitive test scores, demographics, medical history, medications, depression score and functional status. Average inter-rater reliability of diagnoses was 0.77 (95% confidence interval, 0.71–0.84), based on evaluation of 20 participants who screened positive for cognitive impairment. A diagnosis of dementia was made based on DSM-IV criteria. A diagnosis of MCI was made using modified Petersen criteria, which requires cognitive impairment that is insufficient to classify as dementia and a generally intact functional status. Those who did not meet criteria for MCI or dementia were classified as being cognitively normal.
2.3 |. PSG
Unattended in-home overnight PSG was obtained using the Compumedics Siesta Portable PSG (Abottsville, Australia), with collection of data from C3/A2 and C4/A1 EEGs (collected at 256 Hz); bilateral electrooculogram; a bipolar submental electromyogram; thoracic and abdominal excursions (Summit IP inductive plethysmography bands, with auto-calibrating sum channels); airflow (detected by a nasal-oral thermistor and nasal pressure with a nasal cannula connected to a pressure transducer); bilateral leg movements using piezoelectric sensors; oximetry (finger pulse oximetry); electrocardiogram (collected at 512 Hz); and body position (using a mercury gauge sensor). Sleep stages (REM, stages 1–4 NREM) were scored using standard criteria (Rechtschaffen & Kales, 1968; Sleep-Disorders-Atlas-Task-Force, 1992).
Apneas were defined by the absence or near absence of airflow on thermistor for ≥10 s with an oxygen desaturation of 3% or more. Hypopneas were defined by a decrease in breathing amplitude of ≥30% for ≥10 s with an oxygen desaturation of 3% or more. (Alternative indices were calculated based on events associated with a ≥4% desaturation.) The apnea-hypopnea index (AHI) was defined as apneas plus hypopneas per hour of sleep time.
All studies were scored by trained PSG technologists, blinded to group assignments of subjects according to standard criteria. Further information on PSG equipment and scoring algorithms has been published (Blackwell et al., 2008). Inter- and intra-scorer reliability of key PSG measurements exceeded 0.92.
2.4 |. Quantitative EEG analysis
The sleep EEG recordings (C3/A2) performed at Exam 8 were subjected to an off-line spectral analysis based on a Fast-Fourier Transform (FFT) Routine (Vitascore, TEMEC). All recordings were inspected visually by a trained PSG research technician, and artefacts were manually excluded on a 4-s basis. Next, raw power spectra were calculated for 4-s epochs and a frequency range of 0.5–32.0 Hz by applying a 10% cosine-tapered window (Aeschbach & Borbely, 1993). These calculations resulted in power spectra with a quarter-hertz frequency resolution. Next, overlapping 4-s epochs were averaged to yield 30-s power spectra that were then matched with the 30-s sleep scores. Those 4-s sub-epochs with eye movements that resulted in unambiguous deflections in the EEG were discarded from further analysis. Data reduction steps that follow the FFT routine included the discarding of spectra above 25 Hz, and the averaging of power density into half-hertz bins. Finally, the data were reformatted to generate files in ASCII format that were then processed with commercial statistical software (SAS version 9.0).
Average absolute EEG spectra were computed for NREM and REM sleep separately, and transformed using log base 10 prior to statistical analysis. EEG analysis also included periods of wakefulness that were free of artefact.
2.5 |. Analytical sample
Of the 461 women who were characterized as cognitively normal based on cognitive assessments (MMSE of >24) at visit 8 and had PSG and neuropsychological testing performed, 305 returned for study visit 9. Our analytical sample included women with cognitive impairment (n = 49) or dementia (n = 36) at visit 9, which were considered “cases.” Of the remaining 220 women, who were cognitively normal at visit 9 and did not develop MCI or dementia, 85 were randomly chosen to serve as “controls.”
2.6 |. Statistical analysis
Baseline demographic variables as well as mean PSG and EEG variables were compared between the two groups (no MCI/dementia and MCI/dementia) using Student’s t-tests or Wilcoxon rank sum, if data were skewed, and Pearson’s chi square tests, as appropriate.
Correlations were performed between quantitative EEG and cognitive assessment measures at visit 8 prior to cognitive impairment, as well as cognitive measures at visit 9 and visit 8 EEG variables. Pearson or Spearman correlation coefficients were calculated depending on the normality of the data. Sensitivity analyses were performed by excluding those women who at visit 8 reported benzo-diazepine use and/or other medications likely to influence EEG activity, and rerunning the original analyses. All analyses were performed using SAS statistical software (version 9.2 SAS Institute, Cary, North Carolina, USA).
3 |. RESULTS
3.1 |. Demographic and questionnaire data
Characteristics of the entire SOF Sleep and Cognition population have been described before (Cummings et al., 1990; Yaffe, Laffan, et al., 2011). The baseline characteristics of cases and controls are shown in Table 1. Women who did and did not develop MCI or dementia were similar at baseline in respect to age, MMSE, BMI, education level, depression score, alcohol use, smoking habits, medication use, prevalent diabetes, hypertension and history of stroke. COPD was more common in the group that did not develop MCI or dementia (p = .05); however, the frequency was overall low. Impairment in activities of daily living was seen more in the MCI/dementia group. Groups did not differ in regards to self-reported sleepiness or sleep quality.
TABLE 1.
Baseline characteristics of participants
| Did not develop MCI/dementia (n = 85) | Developed MCI/dementia (n = 85) | p-value | |
|---|---|---|---|
| Age (years) | 83.0 (3.2) | 83.2 (3.1) | .64 |
| BMI (kg m−2) | 26.8 (4.0) | 27.5 (5.2) | .33 |
| Education | |||
| <High school | 13 (15.3) | 22 (25.9) | .17 |
| High school | 48 (56.5) | 38 (44.7) | |
| College/grad school | 24 (28.2) | 25 (29.4) | |
| Diabetes | 7 (8.2) | 11 (12.9) | .32 |
| Hypertension | 56 (65.9) | 47 (55.3) | .16 |
| Stroke | 12 (14.1) | 8 (9.4) | .34 |
| Geriatric Depression Scale (0–9) | 1.5 (1.7) | 2.1 (2.2) | .09 |
| COPD | 13 (15.3) | 5 (5.9) | .05 |
| 1 + IADL | 31 (36.5) | 47 (55.3) | .01 |
| Drinks per week | |||
| 0–2 | 78 (91.8) | 78 (91.8) | .81 |
| 3–13 | 6 (7.1) | 5 (5.9) | |
| 14+ | 1 (1.2) | 2 (2.4) | |
| Smoking | 1 (1.2) | 1 (1.2) | |
| Antidepressant use | 5 (5.9) | 8 (9.4) | .39 |
| Benzodiazepine use | 9 (10.6) | 6 (7.1) | .42 |
| Functional Outcomes of Sleep (5–20) | 19.2 (0.8) | 19.1 (1.0) | .95 |
| Epworth Sleepiness Scale (0–24) | 6.4 (3.5) | 5.4 (3.6) | .07 |
| Pittsburgh Sleepiness Index (0–21) | 6.6 (4.0) | 6.7 (3.6) | .89 |
M ± SD or N (%).
BMI, body mass index; COPD, chronic obstructive pulmonary disease; IADL, Instrumental Activities of Daily Living; MCI, mild cognitive impairment.
3.2 |. Traditional measures obtained from PSG
There were no group differences in sleep architecture including total sleep time, sleep efficiency, as well as distributions of NREM, slow-wave sleep (SWS) and REM sleep (Table 2). In addition, there were no differences in measures of SDB, including the AHI, as well as percent time spent at oxygen saturations below 90%. Furthermore, there were no differences in other measures of sleep disturbances, including periodic limb movements (PLMs) and arousal indices.
TABLE 2.
Traditional PSG measures
| Did not develop MCI/dementia (n = 85) | Developed MCI/dementia (n = 85) | p-value | |
|---|---|---|---|
| Total sleep time (min) | 348.7 (72.4) | 354.5 (74.9) | .61 |
| WASO (min) | 91.1 (59.1) | 107.3 (66.0) | .09 |
| Sleep efficiency (%) | 75.3 (12.0) | 74.3 (13.3) | .62 |
| AHI (hypopneas with 3% desat) | 14.2 (13.8) | 14.3 (13.2) | .74 |
| AHI (hypopneas with 4% desat) | 8.6 (10.6) | 8.9 (10.6) | .51 |
| Percent sleep time SaO2 < 90% | 3.5 (6.8) | 3.2 (7.5) | .45 |
| Arousal index per hour of sleep | 20.5 (11.1) | 19.3 (10.4) | .45 |
| Time in stage 1 (%) | 5.3 (3.3) | 4.9 (2.7) | .42 |
| Time in stage 2 (%) | 55.6 (9.8) | 55.1 (13.5) | .76 |
| Time in stage ¾ (%) | 19.9 (9.8) | 22.0 (12.8) | .23 |
| Time in REM (%) | 19.2 (6.7) | 18.0 (7.7) | .29 |
| PLMS per hour of sleep | 26.6 (28.3) | 33.4 (35.7) | .21 |
| PLMS w/arousals per hour of sleep | 3.7 (6.8) | 4.1 (5.2) | .13 |
M ± SD.
AHI, apnea-hypopnea index; MCI, mild cognitive impairment; PLMS, periodic limb movements of sleep; REM, rapid eye movement; WASO, wake after sleep onset.
3.3 |. Quantitative EEG assessments
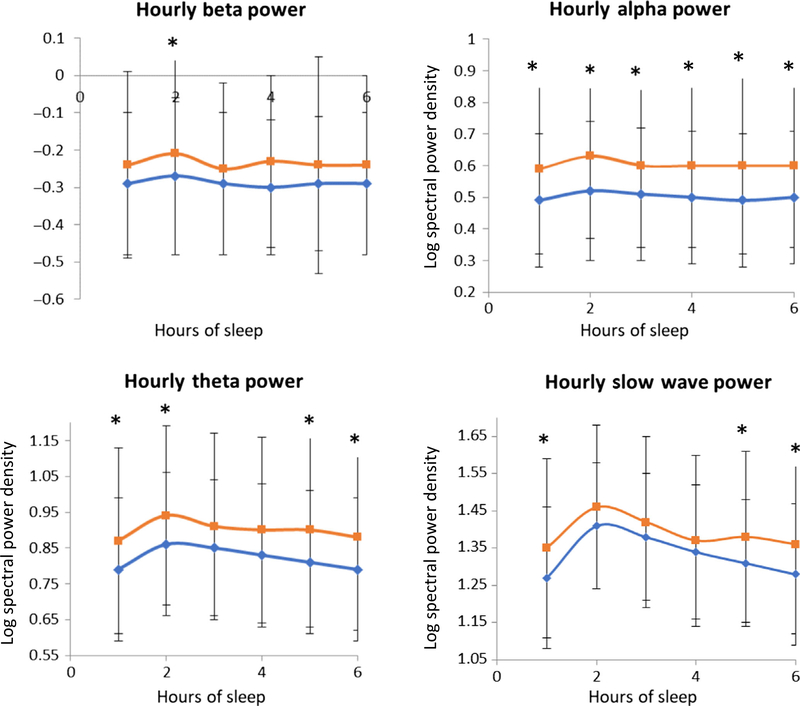
The average spectral logarithm in NREM and REM sleep was compared for each frequency band between the two groups, and adjusted for age, depression and education (Tables 3 and 4, absolute values; for relative power values, see Tables S1 and S2). Overall, those women who developed MCI and dementia demonstrated higher EEG power values across all frequency bands during NREM and REM sleep at the baseline visit when assessed to be cognitively normal using the MMSE (Figures 1, and S1 and S2). During NREM sleep, significant differences were seen in the alpha (p = .007) and theta (p = .04) bands after adjusting for age, education and depressive symptoms. Slow-wave activity (SWA) averages across the night did not show any differences (p = .14). We tested the possibility that the higher NREM alpha power was influenced by factors such as wake after sleep onset (WASO), PLM-arousal index and arousal index in the multivariable models, but found no differences in the results of models that included compared with models that did not include these co-variates. In REM sleep, adjusted analyses showed significant differences in the alpha (p = .04) and sigma frequency (p = .04) bands. Because of the known EEG dynamics across the night, we calculated the average spectral power density per hour of sleep during NREM sleep across the night for each frequency band. (Figure 1). Due to the age of our subjects and their considerable WASO, which limits the ability to define sleep cycles, we analysed consecutive hourly intervals as a means of displaying changes in the EEG dynamics of early versus late sleep.
TABLE 3.
Quantitative absolute EEG power spectral measures (NREM)
| Did not develop MCI/dementia (n = 85) | Developed MCI/ dementia (n = 85) | p-value | |
|---|---|---|---|
| SWA (0.75–4.5 Hz) | 1.37 (1.33, 1.40) | 1.41 (1.37, 1.45) | .14 |
| Slow oscillations (0.5–1 Hz) | 2.33 (2.29, 2.37) | 2.36 (2.32, 2.40) | .33 |
| Theta power (4–8 Hz) | 0.83 (0.79, 0.88) | 0.91 (0.86, 0.95) | .04 |
| Alpha power (8–12 Hz) | 0.50 (0.45, 0.55) | 0.60 (0.55, 0.65) | .007 |
| Beta power (15–30 Hz) | −0.29 (−0.33, −0.25) | −0.24 (−0.28, −0.20) | .11 |
| Sigma frequency (12–15 Hz) | 0.13 (0.08, 0.17) | 0.19 (0.14, 0.23) | .08 |
| SWA in NREM sleep for consecutive hourly intervals | |||
| 0–1 | 1.28 (1.23, 1.32) | 1.35 (1.30, 1.40) | .04 |
| 1–2 | 1.40 (1.36, 1.45) | 1.46 (1.42, 1.50) | .08 |
| 2–3 | 1.38 (1.34, 1.43) | 1.42 (1.37, 1.46) | .26 |
| 3–4 | 1.34 (1.30, 1.39) | 1.37 (1.32, 1.42) | .46 |
| 4–5 | 1.31 (1.27, 1.36) | 1.38 (1.33, 1.42) | .05 |
Adjusted means (95% CI) for age, education and depression.
M ± SD, data are presented as log-transformed EEG power density (μV2 Hz−1). MCI, mild cognitive impairment; NREM, non-rapid eye movement; SWA, slow-wave activity.
TABLE 4.
Quantitative absolute EEG spectral power measures (REM)
| Did not develop MCI/dementia (n = 85) | Developed MCI/dementia (n = 85) | p-value | |
|---|---|---|---|
| SWA (0.75–4.5 Hz) | 0.97 (0.93, 1.01) | 1.02 (0.98, 1.06) | .14 |
| Slow oscillations |
1.99 (1.94, 2.05) | 2.01 (1.95, 2.06) | .71 |
| Theta power | 0.59 (0.54, 0.64) | 0.65 (0.60, 0.70) | .10 |
| Alpha power | 0.37 (0.31, 0.42) | 0.46 (0.40, 0.51) | .04 |
| Beta power | −0.22 (−0.27, −0.17) | −0.15 (−0.21, −0.10) | .09 |
| Sigma frequency (12–15 Hz) | 0.02 (−0.03, 0.08) | 0.11 (0.03, 0.05) | .04 |
Adjusted means (95% CI) for age, education and depression.
M ± SD, data are presented as log-transformed EEG power density (μV2 Hz−1).
MCI, mild cognitive impairment.
FIGURE 1.
Averaged spectral power density per hour of sleep (x-axis) for MCI/dementia (blue) and No MCI/dementia (red). The y-axes represent the values of the logarithm of the spectrum. * indicates p > .05
Significant differences were seen throughout hourly NREM sleep intervals for the alpha band. Further statistical analysis of group by time interaction did not reveal an effect for either SWA (p = .2) or theta (p = .43) bands.
In order to evaluate whether the EEG differences vary between MCI and dementia groups, we performed supplementary analyses that revealed that while SWA per hour of sleep and theta frequencies shows a more linear increase from healthy controls to MCI to dementia, alpha and sigma power are highest in the MCI group during NREM sleep (Tables S3 and S4).
To explore whether the increase in SWA seen in the MCI/dementia group was a reflection of generalized EEG slowing resulting in an overestimation, we also analysed waking EEG alpha background from a random sample of 10 studies from each group. The average alpha frequency for the control group was 9.0 Hz versus 9.25 Hz for the MCI/dementia group (p = .66).
3.4 |. Correlation with cognitive outcomes
We investigated the relationship between quantitative EEG measures and cognitive assessments obtained at visit 8 when both groups were assessed to be cognitively intact. There were no significant associations between any of the EEG frequency bands and the Mini-Mental and Short Mini-Mental Exams, and Trail Making B Test at visit 8.
We then performed correlation analysis between cognitive measures at visit 9 and quantitative EEG measures for the MCI/dementia group, but did not find any associations between the California Verbal Fluency Test, Digit Span Forward and Backward, and Trails B test. To account for the dynamics in cognitive decline, we also correlated the change in Short Mini-Mental Exam and Trails B test scores, which were the only two tasks performed at both visits, with quantitative EEG measures. There were no associations for the Trails B test score changes and sleep EEG frequencies. However, a decline in Short Mini-Mental Exam scores was associated with higher values for slow oscillations (p = .016), SWA (p = .010) and theta frequencies (p = .043).
To evaluate the performance of quantitative sleep EEG in predicting cognitive impairment, we performed logistic regressions and generated c-statistics. In our population sample, age, education and depression did not predict the development of cognitive impairment (MCI/dementia) and nor did SWA. On the other hand, EEG alpha power was the most significant EEG predictor of MCI/dementia (p = .01), whereas theta power had a weaker association (p = .03).
4 |. DISCUSSION
We do not fully understand the complexity of pathways that eventually lead to AD, but there is emerging evidence supporting the role of sleep disturbances as an important component. While these network processes are deeply intertwined, little is known about the nature of their relationships (Lucey & Holtzman, 2015). Thus, this association has become an important research focus given that sleep quality and sleep disorders are modifiable.
Changes in quantitative EEG signals have been described in cross-sectional studies in patients with MCI and dementia, but their predictive role for cognitive decline has not yet been assessed. Our results demonstrate the novel finding that those women who are on the trajectory towards cognitive impairment show quantitative sleep EEG changes, which predate the clinical picture of MCI and dementia by at least 5 years. In particular, an analysis of carefully measured indices of EEG sleep power spectral data from a well-matched sample of elderly women, studied when assessed to be cognitively normal, identified an increase in sigma, alpha and theta absolute power in the group that subsequently developed MCI/dementia. Differences in quantitative EEG data were observed despite no differences in traditional measures of sleep architecture or sleep apnea.
The increase in alpha power observed in the MCI/dementia group is consistent with a publication linking an increase in EEG power of high-frequency alpha with greater cortical atrophy, and a way to identify individuals at risk for progression to AD (Moretti, 2015). Functional imaging studies in prodromal AD subjects have shown paradoxical hyperactivation rather than a loss of activity relative to baseline (Carrillo et al., 2009). Consistent with these findings, our observed increase in EEG power across certain frequency bands may represent hypersynchronization of brain areas as an early dysfunction of the larger pathological frameworks involved in the development of AD during its preclinical stage. Whether these paradoxical findings of hyperactivity are compensatory mechanisms to maintain memory performance or, instead, represent an imbalance of excitatory and inhibitory inputs remains unclear.
It is also possible that the higher alpha power could represent arousal-related occipital alpha activity. Given that the study was not designed to determine the topical distribution of alpha activity and distinguish occipital from fronto-central alpha, we cannot assess possible roles of sleep-maintaining or arousal-related mechanisms related to the increase in alpha power.
In general, SWS has been associated with the promotion of sleep-dependent memory processes. Both animal and human studies have provided evidence that SWS facilitates the consolidation of newly acquired information, in particular declarative, but also procedural memories (Aeschbach, Cutler, & Ronda, 2008; Landsness et al., 2009; Stickgold, 2005). For example, the low cholinergic tone during SWS is thought to enable the reactivation of memory representations after learning, resulting in stabilization and enhancement of these newly formed memories. Based on this concept, the age-related decline in SWS has been presumed to provide a potential mechanism for the memory decline in older adults (Backhaus et al., 2007).
However, the ubiquitous function of SWS towards cognition has been challenged, especially as it pertains to aging. A few studies have suggested that SWS may represent different phenomena in different people and settings. SWS disruption, while associated with sleepiness, has been shown to associate only weakly with cognition, with similar findings for younger and older age groups (Groeger, Stanley, Deacon, & Dijk, 2014). Furthermore, it is likely that the association between SWS and cognition across the lifespan is more complex, reflecting an age-related dissociation of the functional relationship between SWS and cognition. This relationship may be most applicable to young adults and dissociate in the older population in whom SWS might even be associated with negative outcomes in memory functions (Buechel et al., 2011; Scullin, 2013). It is also possible that large slow waves connote different neurophysiological processes and mechanisms across age.
Analogous to Buechel et al.’s (2011) conclusion that the nature of deep sleep in aged rats appears to be more disruptive to cognition than beneficial, we found a negative correlation between SWA and cognition based on performance of the Mini-Mental Exam.
Our study results were limited by the standard sleep EEG montage, which does not allow us to differentiate well whether the observed quantitative EEG changes are global or can be localized further.
Cognitive assessment at the baseline visit relied on two versions of the Mini-Mental Exam and differed from the evaluation that was done at visit 9, resulting in the possibility that, despite otherwise comparability, subtle cognitive group differences and a diagnosis of MCI could have been missed at the baseline visit. More detailed testing and a clinical adjudication process were performed to diagnose participants with MCI or dementia at the endpoint examination (visit 9). At visit 8, participants completed the Mini-Mental and Short Mini-Mental Exams. Both groups had similar baseline scores, the MCI/dementia group had an average MMSE score of 28.2 (±1.6), while the women who remained cognitively intact averaged 28.9 (±1) at visit 8. Studies using a MMSE cut-off point of 24, which was applied in our study population, have shown average sensitivities of 0.85 and specificities of 0.90 in diagnosing dementias (Creavin et al., 2016). Of key importance is that the entire subject pool received the same tests at baseline and follow-up. Our comparisons were focused on the differences in change in cognition between groups. Differences in sensitivity between the two batteries would not bias the reported between-group differences in “decline” over time.
Activities of Daily Living scores differed at baseline between the two groups and could indicate the presence of subtle differences at the baseline visit. On the other hand, depression and subjective scores of sleepiness, which are considered preclinical or early clinical features of AD, did not differ between the two groups (Donovan et al., 2014; Salcedo-Sora & Ward, 2013).
Given interest in exploring the role of multiple measurements of sleep and the correlation among many measurements, we did not correct for multiple testing. Some associations therefore may be spurious and should be replicated in independent cohorts.
Lastly, given that the SOF cohort consists mostly of white women, further studies are necessary to confirm that these results are generalizable.
Changes in quantitative EEG analysis in patients with MCI and dementia have been described (Vecchio et al., 2013). Experimental animal models have demonstrated changes in activity patterns, sleep architecture and overall EEG spectral power as prodromal markers prior to amyloid plaque formation, but this is the first longitudinal study to show that quantitative sleep EEG analysis has the potential as a highly sensitive biomarker that can help in distinguishing those individuals who are at risk for cognitive decline and the development of dementia (Platt, Welch, & Riedel, 2011).
Sleep and cognition are linked by sharing neuronal network mechanisms, but it remains unclear whether the decline in sleep and cognitive function is due to inter-relationships, or shares a common source and is the byproduct of structural degenerative changes. It also remains an open question whether sleep disturbances directly inhibit sleep-dependent memory consolidation or whether the neuroanatomical or neurochemistry changes as the result of aging and recurrent hypoxemia lead to atrophy and synaptic degeneration, which then results in poorer performance.
Further validation of these preclinical quantitative sleep EEG changes and examination of the pathophysiological processes behind them will elucidate fundamental questions concerning sleep disturbances as promising biomarkers and modifiable therapeutic targets that may aid in the prevention of cognitive decline.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brandon Lockyer for carrying out artifact detection and EEG spectral analysis.
Funding information
Center for Scientific Review, Grant/Award Number: K23HL103850, AG021918, AG026720, AG05394, AG0540
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
CONFLICT OF INTEREST
The authors report no disclosures relevant to the manuscript.
REFERENCES
- Aeschbach D, & Borbely AA (1993). All-night dynamics of the human sleep EEG. Journal of Sleep Research, 2, 70–81. 10.1111/j.1365-2869.1993.tb00065.x [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Cutler AJ, & Ronda JM (2008). A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. The Journal of Neuroscience, 28, 2766–2772. 10.1523/JNEUROSCI.5548-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, & Junghanns K. (2007). Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learning & Memory, 14, 336–341. 10.1101/lm.470507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Redline S, Ancoli-Israel S, Schneider JL, Surovec S, Johnson NL, … Study of Osteoporotic Fractures Research Group (2008). Comparison of sleep parameters from actigraphy and polysomnography in older women: The SOF study. Sleep, 31, 283–291. 10.1093/sleep/31.2.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, … Osteoporotic Fractures in Men Study Group. (2011). Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: The Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society, 59, 2217–2225. 10.1111/j.15325415.2011.03731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayet P, Petit D, Frauscher B, Gagnon JF, Gosselin N, Gagnon K, … Montplaisir J. (2016). Quantitative EEG of rapid-eye-movement sleep: A marker of amnestic mild cognitive impairment. Clinical EEG and Neuroscience, 47(2), 134–141. 10.1177/1550059415603050 [DOI] [PubMed] [Google Scholar]
- Brunovsky M, Matousek M, Edman A, Cervena K, & Krajca V. (2003). Objective assessment of the degree of dementia by means of EEG. Neuropsychobiology, 48, 19–26. 10.1159/000071824 [DOI] [PubMed] [Google Scholar]
- Buechel HM, Popovic J, Searcy JL, Porter NM, Thibault O, & Blalock EM (2011). Deep sleep and parietal cortex gene expression changes are related to cognitive deficits with age. PLoS ONE, 6, e18387. 10.1371/journal.pone.0018387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo MC, Blackwell A, Hampel H, Lindborg J, Sperling R, Schenk D, … Klunk W. (2009). Early risk assessment for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 5, 182–196. 10.1016/j.jalz.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Creavin ST, Wisniewski S, Noel-Storr AH, Trevelyan CM, Hampton T, Rayment D, … Cullum, S. (2016). Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations.The Cochrane Database of Systematic Reviews, CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, … Vogt TM (1990). Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA, 263, 665–668. 10.1001/jama.1990.03440050059033 [DOI] [PubMed] [Google Scholar]
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Tranah G, Cauley JA, … Ensrud KE (2016). Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. The American Journal of Geriatric Psychiatry, 24, 248–258. 10.1016/j.jagp.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, … Rentz DM (2014). Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. The American Journal of Geriatric Psychiatry, 22, 1642–1651. 10.1016/j.jagp.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger JA, Stanley N, Deacon S, & Dijk DJ (2014). Dissociating effects of global SWS disruption and healthy aging on waking performance and daytime sleepiness. Sleep, 37, 1127–1142. 10.5665/sleep.3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wahlund L, Dierks T, Julin P, Winblad B, & Jelic V. (2000). Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clinical Neurophysiology, 111, 1961–1967. 10.1016/S1388-2457(00)00454-5 [DOI] [PubMed] [Google Scholar]
- Landsness EC, Crupi D, Hulse BK, Peterson MJ, Huber R, Ansari H, … Tononi G. (2009). Sleep-dependent improvement in visuomotor learning: A causal role for slow waves. Sleep, 32, 1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, … Placidi F. (2014). Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurology, 71, 1498–1505. 10.1001/jamaneurol.2014.2510 [DOI] [PubMed] [Google Scholar]
- Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, … Saper CB (2014). Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain, 137, 2847–2861. 10.1093/brain/awu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, & Holtzman DM (2015). How amyloid, sleep and memory connect. Nature Neuroscience, 18, 933–934. 10.1038/nn.4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, … Walker MP (2015). Beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nature Neuroscience, 18, 1051–1057. 10.1038/nn.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, … Bateman RJ (2010). Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science, 330, 1774 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti DV (2015). Theta and alpha EEG frequency interplay in subjects with mild cognitive impairment: Evidence from EEG, MRI, and SPECT brain modifications. Frontiers in Aging Neuroscience, 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Welch A, & Riedel G. (2011). FDG-PET imaging, EEG and sleep phenotypes as translational biomarkers for research in Alzheimer’s disease. Biochemical Society Transactions, 39, 874–880. 10.1042/BST0390874 [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER, Ferris SH, Rausch L, Fang Z, Cancro R, … Reisberg B. (2006). Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiology of Aging, 27, 471–481. 10.1016/j.neurobiolaging.2005.07.021 [DOI] [PubMed] [Google Scholar]
- Prinz PN, Larsen LH, Moe KE, & Vitiello MV (1992). EEG markers of early Alzheimer’s disease in computer selected tonic REM sleep. Electroencephalography and Clinical Neurophysiology, 83, 36–43. 10.1016/0013-4694(92)90130-A [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, & Kales A. (Eds.) (1968). A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington DC: National Institutes of Health, NIH Publication 204. [Google Scholar]
- Salcedo-Sora JE, & Ward SA (2013). The folate metabolic network of Falciparum malaria. Molecular and Biochemical Parasitology, 188, 51–62. 10.1016/j.molbiopara.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Scullin MK (2013). Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychology and Aging, 28, 105–114. 10.1037/a0028830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep-Disorders-Atlas-Task-Force. (1992). EEG arousals: Scoring rules and examples: A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep, 15, 173–184. [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, An Y, & Resnick SM (2014). Self-reported sleep and beta-amyloid deposition in older adults-reply. JAMA Neurology, 71, 651–652. 10.1001/jamaneurol.2014.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. (2005). Sleep-dependent memory consolidation. Nature, 437, 1272–1278. 10.1038/nature04286 [DOI] [PubMed] [Google Scholar]
- Tafti M. (2009). Genetic aspects of normal and disturbed sleep. Sleep Medicine, 10(Suppl 1), S17–S21. 10.1016/j.sleep.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Van Der Hiele K, Vein AA, Reijntjes RH, Westendorp RG, Bollen EL, van Buchem MA, … Middelkoop HA (2007). EEG correlates in the spectrum of cognitive decline. Clinical Neurophysiology, 118, 1931–1939. 10.1016/j.clinph.2007.05.070 [DOI] [PubMed] [Google Scholar]
- Vecchio F, Babiloni C, Lizio R, Fallani Fde V, Blinowska K, Verrienti G, … Rossini PM (2013). Resting state cortical EEG rhythms in Alzheimer’s disease: Toward EEG markers for clinical applications: A review. Supplements to Clinical Neurophysiology, 62, 223–236. 10.1016/B978-0-7020-5307-8.00015-6 [DOI] [PubMed] [Google Scholar]
- Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, … Saper CB (2015). Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Annals of Neurology, 78, 317–322. 10.1002/ana.24432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, … Nedergaard M. (2013). Sleep drives metabolite clearance from the adult brain. Science, 342, 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, … Stone KL (2011). Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA, 306, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Middleton LE, Lui LY, Spira AP, Stone K, Racine C, … Kramer JH (2011). Mild cognitive impairment, dementia, and their subtypes in oldest old women. Archives of Neurology, 68, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.