Abstract
Objective:
Social relationships can both influence and be influenced by immune processes. Past work implicates two distinct pathways along which this interaction may occur: inflammatory processes and antiviral processes. This article reviews how social behavior is modulated by these two immune processes and how such processes may in turn regulate social behavior.
Methods:
This narrative review outlines existing work on social behavior and both inflammatory and antiviral processes. We propose an evolutionary framework that aims to integrate these findings. Specifically, social isolation has evolutionarily increased the likelihood of wounding and therefore increased the need for inflammation, which works to promote healing. Conversely, broader social networks provide protection from physical threats but also lead to increased pathogen exposure, necessitating a more robust antiviral response.
Results:
This review highlights that social adversity, such as social exclusion or loneliness, is associated with increased inflammation, whereas social contact is associated with increased antiviral immunity. Furthermore, increased inflammation leads to sensitivity to social stimuli, presumably to avoid hostile conspecifics and approach allies who may provide care while vulnerable. Individuals with inadequate antiviral immunity engage in behaviors that minimize pathogen exposure, such as reduced affiliative behavior.
Conclusions:
This review suggests that adverse social experiences (social isolation, perceived social threat) may induce inflammatory responses while suppressing antiviral immunity, whereas positive experiences of social connection may reduce inflammation and bolster antiviral responses. Although acutely elevated inflammation would be adaptive under conditions where wounding is likely, chronic inflammation related to continued social adversity may have detrimental health consequences.
Keywords: antiviral, immune system, inflammation, social relationships
INTRODUCTION
Considerable research has shown that social relationships are important for physical health. Individuals who report feeling more socially connected have higher survival rates (1–3) and lower risk for a variety of diseases (4–6). A growing body of research has examined physiological and biological mechanisms underlying the link between social connection and physical health, and much of this work has specifically focused on immune-related processes. This past work implicates two distinct immune pathways along which this interaction may occur: inflammatory processes and antiviral processes. Each of these pathways affects and is affected by social relationships. Although a tight relationship between social connection and the activity of the immune system might seem a bit odd, it makes more sense when considering the ways in which social ties both (a) provide protection from harm and (b) expose us to disease.
For instance, although social connection provides protection from harm, a lack of social connection has evolutionarily led to increased exposure and vulnerability to physical attacks, making wounding from predators or hostile conspecifics more likely to occur. As a result, inflammatory processes, which promote wound healing and help combat subsequent infection, are more likely to be needed when there are insufficient social ties. On the other hand, our social ties also come repletewith disease and viruses that can be transmitted report feeling served transcriptional response to adversity (CTRA), a particular from person-to-person. Thus, increased social ties necessitate increased antiviral protection from such pathogen exposure (Figure 1). Indeed, in the first model of this evolutionary hypothesis, the conserved transcriptional response to adversity (CTRA), a particular gene expression profile hypothesized to emerge in the context of adverse social conditions or perceived social threat (e.g., social isolation or rejection) consists of both upregulation of genes involved in proinflammatory immune responding and downregulation of genes involved in antiviral immunity (7). As a result of the relationships between the social environment and the immune system, it has been suggested that the immune system may have evolved to be attuned to specific features of the social environment (such as low versus high social connection/integration), to anticipate what kinds of immune processes might be most needed at the present moment, and to redirect energy and resources to address those critical needs (8–10).
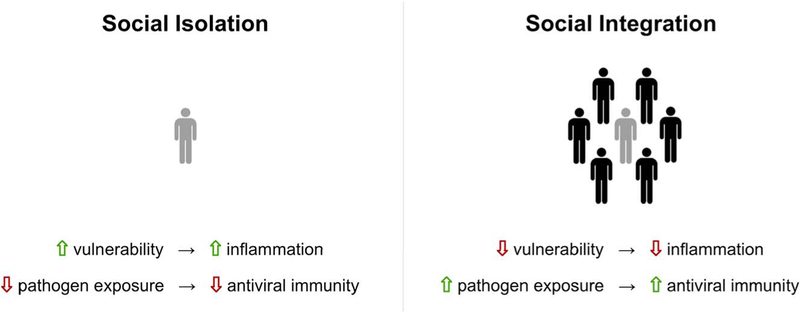
FIGURE 1.
Social modulation of inflammation and antiviral immunity. Social isolation has evolutionarily increased the likelihood of wounding (e.g., vulnerability) and therefore increased the need for inflammatory processes that work to promote healing. Conversely, broader social networks may provide protection from physical threats but also lead to increased pathogen exposure, necessitating a more robust antiviral response.
In addition to the fact that social ties can influence the immune system, the state of the immune system can influence subsequent social behavior, to either facilitate increased social interaction or withdrawal (11). For example, in times of sickness, one’s ability to fend off potential predators is reduced, and as such, one is in a particularly vulnerable state. As a result, high levels of inflammation, as might be induced in times of sickness, can promote social withdrawal from other potentially hostile conspecifics, in part to reduce the likelihood of encountering potential threats (12). However, simultaneously, in times of sickness, it would be advantageous to seek out others who may be able to provide care or protection in this vulnerable state. Overall, increases in inflammation may lead to differential sensitivity to social cues, subsequently facilitating discrimination between potentially threatening stimuli (e.g., strangers) and stimuli that might indicate safety (e.g., loved ones) (12).
Much less is known about how increases in antiviral immunity may relate to subsequent social behavior or experience in humans. However, recent work has provided evidence that manipulating vulnerability to pathogen threats (either via direct manipulation of antiviral immunity or via manipulation of perceptions of vulnerability to pathogen threats) leads to associated changes in social behavior, such that increased vulnerability to pathogen threats is associated with social avoidance. Such reductions in social contact seem to be prompted by decreases in antiviral protection, where reduced social contact is protective in the sense that it leads to reduced pathogen exposure.
This article reviews existing research supporting inflammatory and antiviral pathways in relation to social behavior, couched in an evolutionary framework. Although the inflammatory and antiviral pathways in this context are almost always studied in isolation, it is important to note that these two processes are typically working in concert. Generally speaking, impending or actual physical injury and bacterial challenges result in elevated circulating levels of inflammatory cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α, because they aid in healing damaged cells as a result of the injury, as well as preventing the spread of any microbial infection (13–15). On the other hand, upon a viral challenge, different types of cytokines known as interferons make up a key aspect of the subsequent antiviral response, primarily inhibiting viral replication (16,17). Despite these two distinct roles for inflammatory cytokines and interferons, complex interactions among these two systems have been documented(see (18,19) for recent reviews). For example, after a bacterial infection, interferons can promote either proinflammatory (20–22) or anti-inflammatory responses (23,24). Interestingly, in certain cases, inflammatory cytokines (such as TNF-α) seem able to take on important antiviral properties (25). The cross-talk that occurs between antiviral and inflammatory processes is undoubtedly complex and still not well understood.
With these complications in mind, we attempt to summarize the existing research for each of these two pathways because they relate to social behavior. We first focus on the effect of psychological processes on the immune system, focusing on how social experiences and perceptions affect inflammatory and antiviral processes. This is followed by a discussion focusing on the effect of the immune system on psychological processes, specifically how inflammatory/antiviral processes serve to facilitate social approach and withdrawal. Finally, we discuss the implications of each pathway for understanding the interaction between the immune system and social relationships.
SOCIAL RELATIONSHIPS AND INFLAMMATION
A majority of work to date has focused on understanding how inflammatory processes may underlie links between social connection and health. As mentioned previously, the immune system may have evolved to respond to cues that signal that one is currently or at risk of becoming socially isolated, because social isolation makes one more vulnerable to physical attack, wounding, and thus infection. For example, in the course of human evolution, social isolation has related to increased vulnerability to attack by predators and hostile conspecifics. This vulnerability from external physical threats and attacks resulted in an increased likelihood of wounding and infection and a greater need for the inflammatory processes that typically work to promote healing after a physical attack. It follows that more socially isolated individuals would therefore have an upregulated inflammatory response, which is in line with a growing body of research (12,26,27).
Experiences of Social Isolation
Given the evolutionary association between social isolation and a greater likelihood of wounding, we would expect that isolated individuals would have increased inflammation, to prepare for potential wounding. In line with this, multiple longitudinal studies have now linked lack of social integration with increased inflammation. For example, in a national sample of more than 6000 individuals, a social network index measuring number of social ties and participation in social organizations was inversely associated with C-reactive protein (CRP) and fibrinogen (an inflammatory regulator), as well as a cumulative inflammation burden index (26). Similarly, other longitudinal studies have shown that lower levels of social integration were associated with elevated CRP and IL-6 in men (28,29). In addition, social isolation has been associated with increased IL-6 and CRP in men with depressed mood (30). Thus, individuals experiencing high levels of social isolation (e.g., few social contacts, limited social participation) tend to show increased levels of inflammation.
Regardless of one’s level of actual social isolation or integration, certain social experiences or perceptions may serve as early indicators of a potential physical attack. Thus, to the extent that negative social experiences, such as social rejection, social-evaluative threat, or subjective social isolation (i.e., loneliness), can indicate a current or potential future lack of social ties, such experiences may likewise indicate vulnerability to physical attacks and wounding, thus necessitating an increased inflammatory response.
Social Exclusion and Rejection
Experiences in which an individual is excluded, rejected, or otherwise ostracized by others indicate a severing of existing social ties and thus a loss of protection from predatory attacks (as well as potential physical attacks from the excluder). Thus, we would expect that experiences of social rejection would also be associated with increased inflammatory responses. Indeed, adolescent females who experienced a targeted social rejection showed upregulated inflammatory gene expression (31). Similarly, social rejection by peers during childhood is associated with increased inflammation in adulthood (32).
In addition to peers, strong ties with parental figures are particularly important in providing protection from outside attack for children who are especially vulnerable on their own. Thus, it is not surprising that parental separation or neglect is also associated with higher levels of IL-6 and CRP (33). Furthermore, a meta-analysis on the effects of childhood adversity on inflammation in adulthood revealed that adults exposed to childhood stressors such as parental neglect had significantly elevated baseline peripheral levels of CRP, IL-6, and TNF-α (34). Thus, social experiences in which one is ostracized from social ties who may otherwise offer physical protection from harm tend to be associated with increases in inflammation.
Social-Evaluative Threat
In instances of social rejection, a loss of social ties has readily occurred. However, even the mere possibility that social ties may be threatened, as is the case with social-evaluative threat, is sufficient to elicit an inflammatory response. Not surprisingly, this is what is observed in studies of traditional laboratory-based social stressors that involve social-evaluative threat. For example, the Trier Social Stress Test (TSST), in which participants perform a difficult mental arithmetic task and give a speech in front of a panel of critical judges, reliably elicits an elevated inflammatory response (see (35) for review). Importantly, when participants engage in the mental arithmetic and speech task portion of the TSST without a panel of critical judges (e.g., without a social-evaluative component), no inflammatory response is elicited in response to the stressor (15).Thus, the social-evaluative component of the TSST, rather than the nature of the arithmetic and speech portion, is critical in eliciting an inflammatory response.
Interpersonal stressors more broadly, such as arguments with family or peers, have also been associated with increased levels of inflammation in adolescents (36) and in adults (27). Arguments or conflicts with others may be associated with increased inflammation to the extent that they indicate a potential threat to the relationship or the possibility of a physical altercation and thus increased likelihood of wounding. In line with this, daily negative social experiences (e.g., interpersonal conflicts) and competitive social interactions have been associated with increased levels of inflammation (37).
Feelings and Perceptions Related to Social Isolation
Even in the absence of actual social isolation or threat, one may still appraise a situation as isolating or threatening. To the extent that such appraisals predict vulnerability to a physical attack, appraisals should directly relate to the strength of the resulting inflammatory response. The subjective perception that one is socially isolated or has insufficient social ties (regardless of objective measures of social integration), also referred to as loneliness, should therefore be associated with inflammation. Indeed, in older adults, loneliness is associated with increased expression of proinflammatory genes (38). Furthermore, although transcription factors involved in inducing proinflammatory genes are overexpressed in chronically lonely participants, genes regulating anti-inflammatory processes are underexpressed, compared with nonlonely participants (39).
Given that chronic loneliness may lead to enhanced sensitivity to social cues (40), chronically lonely individuals should display heightened inflammatory responses to social stressors. Indeed, greater loneliness is associated with larger increases in salivary levels of IL-6 and IL-1 receptor antagonist in response to a psychological stressor in women (41). In another study, individuals who reported more loneliness showed increased lipopolysaccharide-stimulated production of proinflammatory cytokines (TNF-α, IL-6) in response to acute stress relative to less lonely participants, suggesting that lonelier individuals tend to have a proinflammatory phenotype, which may put them at greater risk for disease (42). In addition, after being exposed to an acute stressor, more lonely breast cancer survivors tend to show a proinflammatory phenotype, in which they display increased lipopolysaccharide-stimulated production of proinflammatory cytokines (IL-6, IL-1β) relative to less lonely counterparts (42).
Similar to how perceiving oneself as isolated (e.g., lonely) suggests that one does not have adequate protection from harm, perceptions that existing social ties are not sufficient may also indicate that existing relationships do not provide sufficient protection from outside threats. Thus, perceptions of a lack of social support, for example, would be expected to be associated with inflammation. In adults, lower levels of social support from various sources are associated with higher levels of inflammation (27,43). Past work shows that lower levels of social support in cancer survivors were associated with increased levels of CRP (44). Similarly, low levels of social support before breast cancer treatment are longitudinally related to increased levels of IL-6 (45). Similarly, in patients with ovarian cancer, greater perceptions of closeness and intimacy in personal relationships (e.g., social attachment) are associated with lower levels of IL-6 (46).
Finally, certain trait factors that increase sensitivity to social stressors may also influence inflammatory responses. Individuals who are more sensitive to social stressors are more likely to appraise a situation as more stressful, even in the context of nonthreatening stimuli. As a result, such socially sensitive individuals should have an elevated inflammatory response to social stressors (47). In line with this, when individuals undergo a social stressor, greater reports of fear, anxiety, and perceived stress are associated with increased inflammatory responses (48–50). Individuals high in trait sensitivity to social disconnection (e.g., high in rejection sensitivity) show increased levels of proinflammatory cytokines (TNF-α, IL-6) in response to low-dose endotoxin (an inflammatory challenge), relative to those with low sensitivity to social disconnection (51). These socially sensitive individuals also show upregulated proinflammatory gene expression in response to endotoxin (51). Furthermore, in a neuroimaging study, increased activity in threat-related neural regions in response to social exclusion was associated with increases in the proinflammatory response to a social-evaluative threat (52), suggesting that sensitivity to social rejection is associated with increased inflammatory responding to a stressor.
Although most threats humans encounter in contemporary society are not physical, these subjective proxies for potential harm are still common and interpreted by the immune system as a correlate of physical harm.
Chronic Inflammation
We have suggested that increased inflammation in response to social adversity is adaptive in that it preemptively mobilizes the body for wound healing. However, in cases of chronic social adversity, levels of inflammation may remain elevated for an extended period. Although acute increases in inflammation would be adaptive under conditions where wounding is likely, chronic inflammation, as might be necessitated by continued social isolation, has been shown to have detrimental health consequences (53,54). For example, chronic inflammation has been linked with greater risk for mortality in a variety of patient (55–58) and healthy populations (59–61). Indeed, the link between persistent social adversity (e.g., chronic stress or loneliness) and poor health outcomes has been attributed to chronically elevated inflammation (26,62).
It is likely that an elevated inflammatory response evolved specifically to serve an adaptive function in situations of acute, but not chronic, social adversity. Even in cases of persistent social isolation or social adversity, when chronic inflammation may occur, an elevated inflammatory response in preparation of potential attacks would still be protective from likely wounding. This short-term survival utility may have been evolutionarily prioritized, despite the deleterious long-term health consequences, because immediate physical threats would be more critical for survival. Negative long-term health consequences would be irrelevant if an immediate threat (i.e., wounding) was not appropriately managed. In sum, although we propose an adaptive function for this inflammatory response, whether such a response propagates positive or negative health consequences is dependent on the chronicity of social adversity.
SOCIAL RELATIONSHIPS AND ANTIVIRAL PROCESSES
Although social isolation may increase the need for a proinflammatory response, it simultaneously leads to reduced exposure to viral pathogens that may be transmitted from person-to-person. Thus, more socially isolated individuals have a lesser need for a robust antiviral response. In contrast, individuals who are more socially connected require an upregulated antiviral response to compensate for the increased pathogen exposure. In addition, antiviral immunity during social stress may be downregulated to prioritize the proinflammatory response typically observed during social stress. Specifically, the potential wounding likely to occur in the context of social adversity or threat may be a more urgent concern for immediate survival.
It should be noted that work investigating how social behavior relates to antiviral processes is much more scarce than work examining how social behavior relates to inflammation. Thus, future work more closely examining this link is greatly needed to better understand the interplay between social processes and antiviral processes. However, a growing body of evidence supports a link between social integration and antiviral protection, as well as between social adversity and suppressed antiviral immunity.
Objective Measures of Social Integration
Individuals who have larger or more diverse social networks are exposed to a wider range of pathogens; thus, we would expect such individuals to have an upregulated antiviral response to protect from illness. In line with this, in a recently collected data set of breast cancer survivors, we found that women who lived with more individuals in their household had higher circulating levels of interferon γ (IFN-γ), a cytokine that plays a role in antiviral immunity (63–65; Leschak CJ, Dutcher JM, Haltom KEB, Bower JE, and Eisenberger NI: unpublished data). Moreover, women who scored higher on a measure of social integration also had higher levels of IFN-γ. Although these data are cross-sectional, it provides preliminary evidence of a link between increased social contact and increased antiviral immunity.
Early work by Cohen and colleagues (66), in which participants were inoculated with a rhinovirus, quarantined, and monitored for symptoms for upper respiratory illness, shows a similar pattern. Individuals who had more types of social ties (e.g., spouse, parent, friend, workmate) showed a greater resistance to developing the cold virus and also showed less severe symptoms (66).Interestingly, resistance to the rhinovirus was associated with social network diversity in a dose-dependent manner, such that the greater number of different types of social relationships, the greater the resistance to illness (66). Similarly, in a more recent study examining antibody responses to the influenza vaccine in college freshman, those with smaller social networks tended to have a poorer antibody response to one particular component of the vaccine (67).In another study, diverse social networks acted as a protective factor against developing cold symptoms for those with few stressful life events (68).
Aside from examining social networks as a whole, some studies have examined the effect of particular social roles, such as parenthood status, because they relate to antiviral immunity. For example, children’s frequent contact with a diverse network of peers leads to increased exposure to viral pathogens. Through interaction with their children, parents are in turn exposed to increased pathogen threats, which may serve to bolster parents’ acquired antiviral immunity. Among individuals exposed to a common cold virus, parents of children were less likely to develop colds in the 5 days after virus exposure, relative to nonparents (69). Such upregulation of antiviral immunity in parents may partially account for findings from longitudinal work, which has shown that parents have lower all-cause mortality relative to nonparents (70,71).
In addition to directly measuring the extent of existing social ties or contact (e.g., social network indices, social roles), we might also expect that certain individual difference measures related to the tendency to seek out social ties may show similar effects. In line with this, after inoculation with a cold virus, those higher in extraversion, who likely spend more time interacting with others, tend to show less severe symptoms and less virus shedding (which is associated with a healthy immune system (72–75). Furthermore, higher trait sociability is associated with decreased probability of developing a cold, even after controlling for the actual number and quality of social interactions (4). Thus, it seems that those high in traits that may facilitate increased social contact (and thus increased exposure to pathogens) may also have increased antiviral immunity, perhaps as a type of preemptive protection.
Subjective Measures of Social Integration
There is increasing evidence that, in addition to the objective measures of social integration (e.g., diverse social networks), subjective perceptions of isolation (e.g., loneliness) are also related to antiviral immunity. This makes sense given that, to the extent that perceptions of isolation may predict actual social contact and thus pathogen exposure, antiviral immunity may be attuned accordingly. For example, feelings of loneliness have been found to predict self-reported cold symptoms after exposure to a cold virus (76), and in work examining the CTRA pattern of leukocyte gene expression, loneliness has been repeatedly associated with downregulated antiviral and antibody-related genes (38,39). Again, mirroring these findings, in our sample of breast cancer survivors, we found that individuals with higher levels of loneliness tended to show lower circulating levels of IFN-γ (Leschak CJ, Dutcher JM, Haltom KEB, Bower JE, and Eisenberger NI: unpublished data). Furthermore, in the previously mentioned study of antibody response to the influenza vaccine, elevated levels of self-reported loneliness were associated with lower levels of antibody titers at baseline and a poorer antibody response to the vaccine (even after controlling for baseline levels) (67). Interestingly, those who reported both high levels of loneliness and smaller social networks had the poorest antibody response (67), suggesting that subjective and objective indicators of social disconnection contribute somewhat independently to reduced antiviral immunity. Thus, cues signaling reduced pathogen exposure (such as smaller social networks or loneliness) seem to contribute to resistance to antiviral immunity.
Social Adversity
There are other social situations that do not involve social isolation or loneliness that may also lead to decreased antiviral responses but potentially through a different mechanism. Although low social integration may reduce the need for antiviral protection due to reduced pathogen exposure, social adversity may further suppress antiviral immunity as an adverse effect of increased inflammation. Upon physical injury, an inflammatory response ensues to begin healing the wounded area, while antiviral responses (e.g., IFN-γ) are decreased (77). Antiviral protection is temporarily downregulated because the inflammatory response is prioritized for wound healing. Thus, in social contexts that elicit an inflammatory response (e.g., social stress or adversity), we may expect to see compromised antiviral immunity as well.
In animals, social stress is often elicited via forced interactions with unfamiliar conspecifics, which often results in fighting and subsequent wounding. As a result, we would expect that animals undergoing such stress may display compromised antiviral immunity, particularly if wounded or fighting occurs. In line with this idea, mice exposed to social stress exhibited reduced antiviral immunity to an influenza virus, resulting in increased virus-related mortality, while simultaneously displaying elevated inflammatory activity (78). In another study, mice who underwent repeated social stress displayed impaired antiviral immunity in terms of antibody levels after inoculation with a herpes virus (79). Similarly, male pigs that were mixed with unfamiliar conspecifics displayed suppressed immune responding against a viral vaccine (80). In addition, rhesus macaques exposed to unstable social conditions (i.e., social stress) display enhanced density of neural fibers projecting from the sympathetic nervous system into tissues containing lymphocytes (81,82), leading to suppressed interferon gene expression (81) and production (82), thereby allowing enhanced viral replication.
Given that, in some situations, inflammatory activity can inhibit antiviral responses, it is not surprising that some effects of impaired antiviral immunity under conditions of social stress seem to be driven by the likelihood of being wounded. For example, only wounded mice displayed reduced antiviral immunity after herpes virus inoculation, exhibiting suppressed antiviral immunity more than 4-week poststressor(79). Although animals identified as “dominants” more frequently initiate confrontation or fighting with other conspecifics to maintain their status (83,84), lower status animals are more likely to leave the interaction wounded (85). Similar associations between low social status and reduced antiviral immunity have been observed in monkeys (86). Thus, lower status or submissive animals therefore may require upregulation of inflammatory responses, at the expense of antiviral protection. These findings further suggest that wounding during social stress may lead to inadequate antiviral immunity, possibly as an “undesirable adverse effect” of the required proinflammatory immune response that promotes wound healing ((79), p. 286).
Although most of the work in humans has focused on the link between social stress and inflammatory, rather than antiviral, responses, what is known regarding antiviral responses mirrors the animal findings. Thus, existing work that examines the CTRA gene expression profile supports an inverse association between inflammatory activity (upregulated) and antiviral immunity (downregulated) in the context of social adversity (10,38). In addition, chronically stressed caregivers of ill family members show suppressed genes related to interferon production and antiviral immunity (along with increased inflammatory activity) (87) and show low antibody production after a flu vaccine, suggesting that they were “nonresponders” to the vaccine (88). Among individuals exposed to a common cold virus, individuals reporting greater psychological stress (89,90), as well as those who reported experiencing chronic stressors (91) or more negative life events (90), were at an increased risk of developing an acute respiratory illness. Future work should include direct examinations of the antiviral consequences of social stress in humans, ideally in combination with inflammatory assessments, because this area is particularly lacking.
RECIPROCAL REGULATION OF SOCIAL RELATIONSHIPS AND THE IMMUNE SYSTEM
Thus far, we have reviewed evidence that adverse social experiences may induce inflammatory responses while suppressing antiviral immunity, whereas positive experiences of social connection may reduce inflammation and bolster antiviral responses. However, relationships between the immune system and the social environment are bidirectional and ongoing. As such, it is appropriate to consider how immune states and processes may in turn propagate systematic changes in social behavior and experience.
Inflammatory Feedback Loop
A proinflammatory response initiates a cascade of “sickness behaviors” aimed at facilitating quick recovery from illness (92–94). Importantly, this recovery from illness is influenced by social factors. Although social withdrawal is a commonly observed sickness behavior in rodents (95,96), under certain conditions, inflammation can also promote social approach (11). Importantly, this more nuanced social behavior may ultimately facilitate more efficient recovery from illness. For example, while in a weakened or vulnerable state such as that associated with heightened inflammation, social withdrawal from unfamiliar others may be especially adaptive, in that it serves to avoid harm inflicted by potentially hostile strangers. On the other hand, social approach toward familiar others would allow an individual to receive additional care from close others. Indeed, rodents and other nonhuman animals also show increased preference for familiar conspecifics after an inflammatory challenge (97–99).
In recent years, controlled trials in which inflammation is experimentally induced has shed light on the link between proinflammatory effects and subsequent social behavior and experience in humans. In line with findings in animal models, human social behavior after an inflammatory challenge is nuanced in ways that seem to best support recovery from illness. Specifically, after exposure to an inflammatory challenge, individuals feel more socially disconnected, display deficits in social cognition, and are more sensitive to negative social stimuli (e.g., social rejection), both of which may serve to promote social withdrawal and avoidance of potential harm. At the same time, inflammation seems to induce heightened sensitivity to positive social stimuli (e.g., viewing close others, receiving positive social feedback), as well (100,101), which may serve to motivate social approach toward familiar or friendly others who may provide support during times of sickness (12).
For example, in response to heightened inflammation, individuals tend to experience feelings of social disconnection (102), loneliness (103), and social anhedonia (104). Such feelings may contribute to a motivational tendency to socially withdraw, thus avoiding potential harm from unfamiliar others. Given that deficits in social cognition could clearly lead to interpersonal difficulties, such deficits may also be a correlate of heightened inflammation and the corresponding social withdrawal. Indeed, participants exposed to endotoxin also showed several social deficits, including decreased performance on a theory of mind task, indicating impairments in the ability to perceive others’ emotional states (105).
Finally, inflammation-induced sensitivity to negative social stimuli may further prompt individuals to engage in social withdrawal from individuals likely to cause harm. For example, among those exposed to endotoxin, increases in inflammation (IL-6) were associated with increases in social pain-related neural activity during an experience of social exclusion, suggesting a greater inflammatory response prompted increased social sensitivity to negative social stimuli(106). Similarly, endotoxin has been shown to cause increased sensitivity to negative social feedback from an unfamiliar evaluator (101). Endotoxin has also been shown to induce increased amygdala activity in response to socially threatening images, and this amygdala activity is correlated with feelings of social disconnection (107), further suggesting a relation with social withdrawal. Thus, heightened inflammation seems to increase feelings of social disconnection, social cognitive deficits, and sensitivity to negative social stimuli, which may underlie the observed social withdrawal.
In addition to effects related to social withdrawal during sickness, there is evidence that inflammation may facilitate social approach, particularly toward individuals who are likely to provide care and support during sickness (e.g., close others). For example, past work in animals (rhesus monkeys, rats) has shown that an induced inflammatory response led to increases in clinging to or huddling near healthy familiar others (e.g., cage mates), who may be able to provide adequate care or protection (97,98). Aside from increases in physical contact with familiar others, increased affiliation toward potential mates may play a similar role, providing an additional support figure who may be equipped to provide care. After an inflammatory challenge, monogamous female prairie voles more quickly established a preference for a male vole (99).
Work in humans mirrors the previously mentioned patterns, suggesting that exposure to an inflammatory challenge increases social approach toward those likely to provide care. In one study, after exposure to an inflammatory challenge, individuals reported an increased desire to be near their social support figures (100). Furthermore, those exposed to the inflammatory challenge (versus placebo) showed increased reward-related neural activity when viewing photos of these social support figures (100). These findings make sense given that social support figures are some of the people most likely to provide care in times of need. In addition, others who may not be close others or support figures may still be potential providers of care, particularly if they engage in affiliative behaviors toward the injured or sick individual. For example, individuals exposed to an inflammatory challenge showed greater reward related activity to receiving positive feedback from an evaluator, compared with those who did not receive the inflammatory challenge (101). Taken together, these findings suggest that increased inflammation may prompt individuals to seek out those who are likely to provide care in times of sickness, such as close others.
Antiviral Feedback Loop
As with inflammation, changes in antiviral immune functioning also affect social behavior. Specifically, an upregulated antiviral response affords greater protection from potential threats and thus may facilitate increased affiliative or prosocial behavior. One could therefore engage in increased social behavior with limited additional risk of contracting contagious or infectious diseases. Some of the most compelling evidence that antiviral immunity may facilitate social behavior comes from recent work in mice. Mice deficient in T cells (which aid in antiviral immunity via detection of viruses (108,109), as well as production of cytokines important for antiviral immunity such as IFN-γ (110)) show deficiencies in social behavior, such that they prefer to spend time with an object instead of a novel mouse (111). A preference for nonsocial stimuli in animals is often considered typical of autistic phenotypes, for which deficits in social attention and lack of interest in social stimuli are a hallmark characteristic (112,113). After observing that IFN-γ–regulated genes were enriched in animals who had been exposed to social aggregation, Filiano and colleagues (111) assessed IFN-γ as a potential mediator of the effect of T cells on social behavior. In humans, IFN-γ is a cytokine that plays a major role in antiviral immunity (63,65,114). Mice deficient in IFN-γ, as well as mice deficient in the IFN-γ receptor showed social deficits similar to T-cell–deficient mice, showing no preference for social stimuli (111). Remarkably, after injecting IFN-γ–deficient mice with an injection of recombinant IFN-γ into the cerebrospinal fluid, social behavior was restored such that it was indistinguishable from wild type mice (with no immune deficiencies) (111).
Research, especially experimental data, examining how antiviral immunity affects subsequent social behavior and experience in humans is lacking. However, we have found that in breast cancer survivors, higher circulating levels of IFN-γ were associated with increased rates of prosocial giving behavior (Leschak CJ, Dutcher JM, Haltom KEB, Bower JE, and Eisenberger NI: unpublished data). While correlational, one potential interpretation of this effect is that increased antiviral protection (in the form of increased levels of IFN-γ) may facilitate social approach-related behaviors, such as increased contact with others or prosocial behavior.
In addition, there is growing evidence that when microbial threats are made salient, individuals engage in behaviors that would serve to minimize exposure and contact with others. For example, after watching a slideshow of pictures and information regarding germs and transmission of contagious disease (versus architectural images), individuals scored lower on measures of extraversion, closely related to one’s self-perceived sociality (115). Furthermore, for participants who perceived themselves as highly vulnerable to disease, viewing the germ-related slideshow led them to view themselves as less open-minded to new people as well as less agreeable and cooperative toward others (115). In a separate study, participants who viewed a similar germ-related slideshow subsequently showed a tendency to avoid social stimuli, an effect that was particularly strong in individuals high in perceived disease vulnerability (115).These findings suggest that when a potential infectious threat is present, individuals may shift their self-perceptions and their behavior to best avoid social contact and therefore protect themselves from pathogen exposure.
Although these studies do not directly assess immune responding to cues of disease, they provide initial evidence that environmental cues that signal potential disease can lead to reduced affiliative tendencies. These findings are in line with theoretical accounts of a behavioral immune system, a behavioral defense system against pathogens propagated in part by psychological responses to disease cues (116). It is important to note that the few studies that have examined social approach or avoidance in the context of pathogen threats have primarily examined social behavior toward unfamiliar social stimuli (e.g., strangers). It is possible that, as has been shown in the inflammatory literature, approach or avoidance tendencies may be sensitive to the social target. For example, individuals exposed to a pathogen threat (e.g., exposure to a person with outward signs of disease) may be more likely to subsequently approach the infected target if it were a familiar other who may require care, while avoiding infected unfamiliar others, for whom motivation to help may be lower, or for whom providing care may be riskier because of a greater threat of foreign pathogen exposure (117). Although there is little existing research directly examining this premise, preliminary work suggests that feelings of disgust—theorized to underlie disease avoidance behaviors—are more readily elicited for disease cues associated with strangers relative to familiar others (118,119).
Future work will be critical in determining whether effects of antiviral threats on social behavior are differentially sensitive to social targets. Given the role that antiviral immunity plays in responding to the social environment to upregulate or downregulate protection from infection accordingly, it is possible that these psychological and behavioral reactions (e.g., reduced affiliative tendencies) to disease cues may be mediated by changes inimmune functioning specifically related to antiviral immunity. To directly address this possibility, future studies will need to specifically examine biomarkers of antiviral immunity in response to exposure to disease-related stimuli.
CONCLUSIONS
As described previously, social behavior is strongly related to inflammatory and antiviral processes. Specifically, we discussed how inflammatory processes may be upregulated to prepare for potential wounding in times of social disconnection, whereas antiviral processes tend to be upregulated in response to social exposure or connection to prepare an adequate antiviral defense to pathogens. In addition, existing immune processes, such as heightened inflammation or heightened levels of antiviral immunity, may in turn affect social behavior and experience in systematic and predictable ways. Importantly, these immune processes are critically tied to physical health and longevity throughout the lifespan (8,12,120,121) and thus may help explain the well-established link between social relationships and physical health benefits.
Acknowledgments
Source of Funding: This work has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Glossary
- CRP
C-reactive protein
- CTRA
conserved transcriptional response to adversity
- IFN-γ
interferon γ
- IL
interleukin
- IL-1β
interleukin 1β
- TNF-α
tumor necrosis factor α
- TSST
Trier Social Stress Test
Footnotes
Conflicts of Interest: The authors declared no conflicts of interest.
REFERENCES
- 1.Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med 2003;46:S39–52. [PubMed] [Google Scholar]
- 2.Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci 2015;10:227–37. [DOI] [PubMed] [Google Scholar]
- 3.Sugisawa H, Liang J, Liu X. Social networks, social support, and mortality among older people in Japan. J Gerontol 1994;49:S3–13. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Doyle WJ, Turner R, Alper CM, Skoner DP. Sociability and susceptibility to the common cold. Psychol Sci 2003;14:389–95. [DOI] [PubMed] [Google Scholar]
- 5.Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later. Arch Pediatr Adolesc Med 2006;160:805. [DOI] [PubMed] [Google Scholar]
- 6.Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: mediation by the autonomic nervous system. Biol Psychiatry 2003;54:1444–56. [DOI] [PubMed] [Google Scholar]
- 7.Slavich GM, Cole SW. The emerging field of human social genomics. Clin Psychol Sci 2013;1:331–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci 2012;15:669–74. [DOI] [PubMed] [Google Scholar]
- 9.Cole SW. Social regulation of human gene expression: mechanisms and implications for public health. Am J Public Health 2013;103:S84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole SW. Human social genomics. PLoS Genet 2014;10:e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennessy MB, Deak T, Schiml PA. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav Immun 2014;37:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR. In sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology 2017;42:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fullerton JN, Segre E, De Maeyer RP, Maini AA, Gilroy DW. Intravenous endotoxin challenge in healthy humans: an experimental platform to investigate and modulate systemic inflammation. J Vis Exp 2016; 53913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis 1999;179:1278–82. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychol Sci 2009;20:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karupiah G, Xie Q, Buller R, Nathan C, Duarte C, MacMicking J. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 1993;261:1445–8. [DOI] [PubMed] [Google Scholar]
- 17.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, Macdonald MR, Rice CM. Interferons alpha and gamma inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006;131:1887–98. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Liu J, Cao X. Regulation of type I interferon signaling in immunity and inflammation: a comprehensive review. J Autoimmun 2017;83:1–11. [DOI] [PubMed] [Google Scholar]
- 19.Kopitar-Jerala N The role of interferons in inflammation and inflammasome activation. Front Immunol 2017;8:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Miura M, Jung Y, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE interacting protease, is essential for the activation of ICE. Cell 1998;92:501–9. [DOI] [PubMed] [Google Scholar]
- 21.Ucia C, Roux-Lombard P, Fey S, Dayer JM, Mach B. Interferon gamma drastically modifies the regulation of interleukin 1 genes by endotoxin inU937cells. J Clin Investig 1990;85:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujisawa H, Wang B, Sauder DN, Kondo S. Effects of interferons on the production of Interleukin-6 and Interleukin-8 in human keratinocytes. J Interferon Cytokine Res 1997;17:347–53. [DOI] [PubMed] [Google Scholar]
- 23.Benveniste EN, Qin H. Type I interferons as anti-inflammatory mediators. Sci STKE 2007;2007:pe70. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G Type I interferon: friend or foe? J Exp Med 2010;207:2053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenzavirus effects in lung epithelial cells. J Virol 2002;76:1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang YC, McClintock MK, Kozloski M, Li T. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J Health Soc Behav 2013;54:182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang YC, Schorpp K, Harris KM. Social support, social strain and inflammation: evidence from a national longitudinal study of U.S. adults. Soc Sci Med 2014;107:124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loucks EB, Berkman LF, Gruenewald TL, Seeman TE. Relation of social integration to inflammatory marker concentrations in men and women 70 to 79 years. Am J Cardiol 2006;97:1010–6. [DOI] [PubMed] [Google Scholar]
- 29.Ford ES, Loucks EB, Berkman LF. Social integration and concentrations of C-reactive protein among US adults. Ann Epidemiol 2006;16:78–84. [DOI] [PubMed] [Google Scholar]
- 30.Häfner S, Emeny RT, Lacruz ME, Baumert J, Herder C, Koenig W, Thorand B,Ladwig KH. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: results from the MONICA/KORA study. Brain Behav Immun 2011;25:1701–7. [DOI] [PubMed] [Google Scholar]
- 31.Murphy ML, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clin Psychol Sci 2013;1:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacey RE, Kumari M, Bartley M. Social isolation in childhood and adult inflammation: evidence from the National Child Development Study. Psychoneuroendocrinology 2014;50:85–94. [DOI] [PubMed] [Google Scholar]
- 33.Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology 2013;38:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 2016;21:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007;21:901–12. [DOI] [PubMed] [Google Scholar]
- 36.Fuligni AJ, Telzer EH, Bower JE, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosom Med 2009;71:329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proc Natl Acad Sci 2012;109:1878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole SW, Levine ME, Arevalo JM, Ma J, Weir DR, Crimmins EM. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology 2015;62:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol 2007;8:R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner WL, Pickett CL, Jefferis V, Knowles M. On the outside looking in: loneliness and social monitoring. Pers Soc Psychol Bull 2005;31:1549–60. [DOI] [PubMed] [Google Scholar]
- 41.Hackett RA, Hamer M,Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology 2012;37:1801–9. [DOI] [PubMed] [Google Scholar]
- 42.Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Loneliness promotes inflammation during acute stress. Psychol Sci 2013;24:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McHugh Power J, Carney S, Hannigan C, Brennan S, Wolfe H, Lynch M, Kee F, Lawlor B. Systemic inflammatory markers and sources of social support among older adults in the Memory Research Unit cohort. J Health Psychol 2016; 135910531667633. [DOI] [PubMed] [Google Scholar]
- 44.Muscatell KA, Eisenberger NI, Dutcher JM, Cole SW, Bower JE. Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain Behav Immun 2016;53:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes S, Jaremka LM, Alfano CM, Glaser R, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE, Malarkey WB, Kiecolt-Glaser JK. Social support predicts inflammation, pain, and depressive symptoms: longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology 2014; 42:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky JI, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer 2005;104:305–13. [DOI] [PubMed] [Google Scholar]
- 47.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull2004;130:355–91. [DOI] [PubMed] [Google Scholar]
- 48.Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain Behav Immun 2010;24:215–9. [DOI] [PubMed] [Google Scholar]
- 49.Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immunity 2011;25:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M,Kaneko H, Ohira H. Transient responses of inflammatory cytokines in acute stress. Biol Psychol 2009;82:25–32. [DOI] [PubMed] [Google Scholar]
- 51.Moieni M, Irwin MR, Jevtic I, Breen EC, Cho HJ, Arevalo JM, Ma J, Cole SW, Eisenberger NI. Trait sensitivity to social disconnection enhances proinflammatory responses to a randomized controlled trial of endotoxin. Psychoneuroendocrinology 2015;62:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci 2010;107:14817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000;51:245–70. [DOI] [PubMed] [Google Scholar]
- 54.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999;47:639–46. [DOI] [PubMed] [Google Scholar]
- 55.Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, Rindi P, Donati G, Antonelli A, Panicucci E, Tripepi G, Tetta C, Palla R. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant 2008;23:2337–43. [DOI] [PubMed] [Google Scholar]
- 56.Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all-cause mortality: a Glasgow Inflammation Outcome Study. PLoS One 2015;10:e0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raffetti E, Donato F, Casari S, Castelnuovo F, Sighinolfi L, Bandera A,Maggiolo F, Ladisa N, di Pietro M, Fornabaio C, Digiambenedetto S, QuirosR-oldan E. Systemic inflammation-based scores and mortality for all causes in HIV-infected patients: a MASTER cohort study. BMC Infect Dis 2017;17:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sin DD, Man SF. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol 2007;85:141–7. [DOI] [PubMed] [Google Scholar]
- 59.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica 2016;101:1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE.Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 2002;50:638–44. [DOI] [PubMed] [Google Scholar]
- 61.Harris TB, Ferrucci L, Tracy RP, Chiara Corti M, Wacholder S, Ettinger WH Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am JMed 1999; 106:506–12. [DOI] [PubMed] [Google Scholar]
- 62.Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress related diseases. Front Hum Neurosci 2017;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol 1999;73:3418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilček J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science 1993;259:1742–5. [DOI] [PubMed] [Google Scholar]
- 65.Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science 1994;264:1918–21. [DOI] [PubMed] [Google Scholar]
- 66.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr. Socialties and susceptibility to the common cold. JAMA 1997;277:1940–4. [PubMed] [Google Scholar]
- 67.Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol 2005;24:297–306. [DOI] [PubMed] [Google Scholar]
- 68.Hamrick N, Cohen S, Rodriguez MS. Being popular can be healthy or unhealthy: stress, social network diversity, and incidence of upper respiratory infection. Health Psychol 2002;21:294–8. [PubMed] [Google Scholar]
- 69.Sneed RS, Cohen S, Turner RB, Doyle WJ. Parenthood and host resistance to the common cold. Psychosom Med 2012;74:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lund E, Arnesen E, Borgan JK. Pattern of childbearing and mortality in married women—a national prospective study from Norway. J Epidemiol Community Health 1990;44:237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grundy E, Kravdal Ø. Reproductive history and mortality in late middle age among Norwegian men and women. Am J Epidemiol 2007;167:271–9. [DOI] [PubMed] [Google Scholar]
- 72.Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J Infect Dis 2006;193:760–4. [DOI] [PubMed] [Google Scholar]
- 73.Boivin G, Goyette N, Bernatchez H. Prolonged excretion of amantadine-resistantinfluenza A virus quasi species after cessation of antiviral therapy in an immunocompromised patient. Clin Infect Dis 2002;34:e23–5. [DOI] [PubMed] [Google Scholar]
- 74.Totman R, Kiff J, Reed SE, Craig JW. Predicting experimental colds in volunteers from different measures of recent life stress. J Psychosom Res 1980; 24:155–63. [DOI] [PubMed] [Google Scholar]
- 75.Broadbent DE, Broadbent MH, Phillpotts RJ, Wallace J. Some further studies on the prediction of experimental colds in volunteers by psychological factors. J Psychosom Res 1984;28:511–23. [DOI] [PubMed] [Google Scholar]
- 76.LeRoy AS, Murdock KW, Jaremka LM, Loya A, Fagundes CP. Loneliness predicts self-reported cold symptoms after a viral challenge. Health Psychol 2017; 36:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller CH, Quattrocchi KB, Frank EH, Issel BW, Wagner FC Jr. Humoral and cellular immunity following severe head injury: review and current investigations. Neurol Res 1991;13:117–24. [DOI] [PubMed] [Google Scholar]
- 78.Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci 2000;917:894–905. [DOI] [PubMed] [Google Scholar]
- 79.De Groot J, Boersma WJ, Scholten JW, Koolhaas JM. Social stress in male mice impairs long-term antiviral immunity selectively in wounded subjects. Physiol Behav 2002;75:277–85. [DOI] [PubMed] [Google Scholar]
- 80.De Groot J, Ruis MA, Scholten JW, Koolhaas JM, Boersma WJ. Long-term effects of social stress on antiviral immunity in pigs. Physiol Behav 2001;73:145–58. [DOI] [PubMed] [Google Scholar]
- 81.Capitanio JP, Cole SW. Social instability and immunity in rhesus monkeys: therole of the sympathetic nervous system. Philos Trans R Soc Lond B Biol Sci 2015;370:20140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci 2007;27:8857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meese GB, Ewbank R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim Behav 1973;21:326–34. [Google Scholar]
- 84.Bernstein IS. Dominance: the baby and the bathwater. Behav Brain Sci 1981;4:419–29. [Google Scholar]
- 85.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR.Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 1995;20:117–34. [DOI] [PubMed] [Google Scholar]
- 86.Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom Med 1997;59:213–21. [DOI] [PubMed] [Google Scholar]
- 87.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol Psychiatry 2008;64:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiecolt-Glaser JK, Glasert R, Gravenstein S, Malarkeyt WB, Sheridanii J.Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A 1996;93:3043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med 1991;325:606–12. [DOI] [PubMed] [Google Scholar]
- 90.Cohen S, Tyrrell DA, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Pers Soc Psychol 1993;64:131–40. [DOI] [PubMed] [Google Scholar]
- 91.Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol 1998;17:214–23. [DOI] [PubMed] [Google Scholar]
- 92.Dantzer R Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci 2001;933:222–34. [DOI] [PubMed] [Google Scholar]
- 93.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 2002;25:154–9. [DOI] [PubMed] [Google Scholar]
- 94.Hart BL. The behavior of sick animals. Vet Clin North Am Small Anim Pract 1991;21:225–37. [DOI] [PubMed] [Google Scholar]
- 95.Bluthé R-M, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 1994; 19:197–207. [DOI] [PubMed] [Google Scholar]
- 96.Bluthé R-M, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagoniston the behavioral effects of lipopolysaccharide inrat. Brain Res 1992;573:318–20. [DOI] [PubMed] [Google Scholar]
- 97.Yee JR, Prendergast BJ. Sex-specific social regulation of inflammatory responses and sickness behaviors. Brain Behav Immun 2010;24:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behav Immun 2007;21:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bilbo SD, Klein SL, Devries AC, Nelson RJ. Lipopolysaccharide facilitates partner preference behaviors in female prairie voles. Physiol Behav 1999;68:151–6. [DOI] [PubMed] [Google Scholar]
- 100.Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun 2015;44:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muscatell KA, Moieni M,Inagaki TK, Dutcher JM, JevticI, Breen EC,IrwinMR, Eisenberger NI. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun 2016;21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 2010;24:558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 2015; 40:1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav Immun 2011;25:256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moieni M, Irwin MR, Jevtic I, Breen EC, Eisenberger NI. Inflammation impairssocial cognitive processing: a randomized controlled trial of endotoxin. Brain Behav Immun 2015;48:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage 2009;47:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 2012;59:3222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med 1999;189:423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sant AJ, McMichael A. Revealing the role of CD4+ T cells in viral immunity.J Exp Med 2012;209:1391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol 1998;16:365–96. [DOI] [PubMed] [Google Scholar]
- 111.Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC,Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 2016;535:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Werner E, Dawson G, Osterling JA, Dinno N. Brief report: recognition of Autism Spectrum Disorder before one year of age: a retrospective study based on home videotapes. J Autism Dev Disord 2000;30:18–23. [DOI] [PubMed] [Google Scholar]
- 113.Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol 2002;14:239–51. [DOI] [PubMed] [Google Scholar]
- 114.Huang JY, Sedlovskaya A, Ackerman JM, Bargh JA. Immunizing against prejudice: effects of disease protection on attitudes toward out-groups. Psychol Sci 2011;22:1550–6. [DOI] [PubMed] [Google Scholar]
- 115.Mortensen CR, Becker DV, Ackerman JM, Neuberg SL, Kenrick DT. Infection breeds reticence: the effects of disease salience on self-perceptions of personality and behavioral avoidance tendencies. Psychol Sci 2010;21:440–7. [DOI] [PubMed] [Google Scholar]
- 116.Schaller M, Duncan LA. The behavioral immune system: its evolution and social psychological implications In: Forgas JP, Haselton MG, Hippel Wvon, editors. Evolution and the Social Mind: Evolutionary Psychology and Social Cognition. New York: Routledge/Taylor & Francis Group; 2007; 293–307. [Google Scholar]
- 117.Kurzban R, Leary MR. Evolutionary origins of stigmatization: the functions of social exclusion. Psychol Bull 2001;127:187–208. [DOI] [PubMed] [Google Scholar]
- 118.Curtis V, Aunger R, Rabie T. Evidence that disgust evolved to protect from risk of disease. Proc Biol Sci 2004;271:S131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peng M, Chang L, Zhou R. Physiological and behavioral responses to strangers compared to friends as a source of disgust. Evol Human Behav 2013;34:94–8. [Google Scholar]
- 120.Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev 2010;35:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med 2006;29:377–87. [DOI] [PubMed] [Google Scholar]